Introduction
Extrahepatic bile duct carcinoma (EHBDC) is an
epithelial tumor that forms in the bile ducts outside the liver,
and its incidence ranges from 0.53 to 2.0 per 100,000 in the
western world, but can be much higher (0.97 to 85.0) in Asian
countries including Thailand, Republic of Korea and China (1). Despite recent advances in diagnostic
and therapeutic techniques, complete surgical resection of the
tumor remains the best treatment for EHBDC. However, EHBDC patients
are often diagnosed at an advanced stage. Even in patients who have
undergone therapeutic resection, the prognosis is dismal due to the
high recurrence rate of this tumor (2,3). A
late diagnosis and frequent recurrence in EHBDC compromise surgical
resection, the only potentially curative treatment. Current
chemotherapeutic drugs for these EHBDC patients have a limited
efficacy because more than 50% have relapsed early (4,5).
Recently, with the advent of immunotherapy, studies
for the interactive relationships between tumor cells and the
peritumoral immune cells (tumor microenvironment) (TME) have
received much attention. However, difficult obstacles remain for
deciphering TME because of tumor heterogeneity and immune system
complexity, especially in immune suppression mechanisms. Local
immune suppression of the tumor microenvironment is crucial for
cancer development, metastasis, and even tumor immune escape
(6,7). Therefore, elucidation of the immune
suppression mechanisms involved in the carcinogenesis, cancer
progression, and metastasis can improve the accuracy of predicting
its prognosis and provide a target for therapeutic intervention,
contributing to the improvement of survival rate.
IDO1 has been emerging as an important biomarker
associated with the immunosuppressive mechanism in addition to
PD-L1/PD-1 and B7/CTLA-4 interactions. IDO1 is a 403 amino acid and
cytosolic heme enzyme encoded by the INDO gene on human chromosome
8p22 that catalyzes the initial and rate-limiting steps in the
kynurenine (Kyn) pathway (8). It
is expressed ubiquitously in various normal tissues including the
small intestine, epididymis, lungs, female genital tract, and
placenta as well as various types of solid tumors (8–10).
IDO1 is known to be involved in maternal-fetal immune tolerance in
the mouse placenta (9) and has
been shown to be linked in a mechanism of response to tumor immune
evasion through tumor infiltrating lymphocyte (TIL) inhibition
(11). To date, contradictory
results for prognostic implication of IDO1 expression has been
reported in various carcinomas (12–19),
but there is no study in EHBDC.
In this study, we investigated intratumoral IDO1
expression in epithelial tumor cells in 76 patients with EHBDC who
had undergone surgical resection. In addition, we defined the
association between CD8+ TILs and IDO1 expression and
examined prognostic differences between subgroups by performing
stratification combining CD8 TIL status and IDO1 expression.
Materials and methods
Patients and clinicopathological
data
This study was approved by the Institutional Review
Board of Gangneung Asan Hospital. A total of 76 consecutive
patients with bile duct carcinoma between January 2001 and December
2017 were collected at Gangneung Asan Hospital. In detail,
inclusion criteria were as follows: i) surgical resection specimen
with enough tumor cells, ii) obtaining each patient's informed
consent for use of their clinical records and pathological
specimen, and iii) without previous history of a cancer other than
bile duct carcinoma and chemo-or radiotherapy. Exclusion criteria
were as follows: i) histological diagnosis of a tumor type other
than bile duct carcinoma, ii) inappropriate amount of tumor sample,
iii) insufficient preservation of paraffin blocks for tissue
microarray (TMA) construction, iv) Follow-up loss, and v) failure
to obtain informed consent.
The patients' medical records were reviewed for
clinical information, and histological parameters were evaluated on
hematoxylin & eosin (H&E)-stained slides. Clinical data
included the patient's sex and age, tumor location, operation date,
American Joint Committee on Cancer (AJCC) TNM stage (20), most recent follow-up date,
recurrence, and survival status. Pathological data included the
tumor size, depth of invasion, tumor location, histologic subtype,
tumor differentiation, nodal metastasis, and perineural or
lymphovascular invasion.
Tissue microarray (TMA)
Tumor samples collected from clinical cases were
fixed with 10% formalin at room temperature for 24 h.
Formalin-fixed, paraffin-embedded tissue samples of randomly
selected bile duct adenocarcinoma (n=76) were obtained and arrayed
using a tissue-arraying instrument (Quick-Ray, Unitma Co., Ltd.).
Briefly, areas with invasive adenocarcinomas were identified on the
corresponding H&E-stained slides, and sections were indicated
as representative tumors. Three cores were sampled from the center
and border of invasive areas in each representative tumor block
using a 2.0-mm punch. Four-µm-thick slides were cut from the TMA
blocks for immunohistochemical and H&E staining. H&E stains
for TMA blocks were performed by automated DAKO CoverStainer (Dako
Korea Co., Ltd.; temperature, 4°C; duration, 46 min) to correlate
with the immunohistochemical TMA slides.
Immunohistochemical staining and
interpretation
Five-µm thick sections were cut from the
formalin-fixed, paraffin-embedded tumor samples and stained with
Leica auto-stainer Bond Max using the Bond Polymer Refine Detection
System (Leica Biosystems Newcastle Ltd.) according to the
manufacturer's protocols. Briefly, the sections were deparaffinized
by Bond Dewax Solution (Leica Biosystems Newcastle Ltd.), followed
by heat-induced antigen retrieval using Bond Epitope retrieval
solution (Leica Biosystems Newcastle Ltd.) for 20 min at 100°C. The
endogenous peroxidase was quenched by incubation with hydrogen
peroxide for 15 min. Sections were incubated for 15 min at ambient
temperature with primary mouse monoclonal anti-IDO1 antibody
(Abcam; Ab13248, 1:100) and CD8 (SP16; Thermo Fisher Scientific;
1:100). Bound primary antibodies were visualized using a
biotin-free polymeric horseradish peroxidase-linker antibody
conjugate system (Bond Polymer Refine Detection; ready-to-use
dilution; cat. no. DS9800; Leica Biosystems, Inc.) in a Bond-Max
automatic slide stainer (Leica Biosystems Melbourne Pty. Ltd.). The
nuclei of these sections were counterstained with hematoxylin
(Biocare, cat: NM-HEM-M) for 4 min at 25°C in the Bond Polymer
detection kit (Leica Biosystems).
The tumor epithelial immunoreactivity for IDO1 was
semi quantitatively interpreted by means of light microscopic
examination and evaluated without prior knowledge of the
clinicopathological data. Cytoplasmic immunostaining was evaluated
as the percentage and intensity of positive epithelial cells, as
previously described (21).
Staining intensity was graded as follows: 0, negative; 1, weak; 2,
moderate; and 3, strong. The percentage of positively stained cells
was graded as 1 (1–24%), 2 (25–49%), 3 (50–74%), or 4 (≥75%). The
final immunohistochemical score was calculated by multiplying these
2 grades to yield a score ranging from 0 to 12. We defined high
IDO1 expression as a total score >3. The cut-point value is
determined using the AUC (area under the ROC (receiver operating
characteristic) curve) and Youden's index for patient's survival
(22).
CD8 was estimated for TILs in the tumor bed
including the tumor epithelium and intratumoral stroma using an
automatic image analysis software (https://oncoimmunoquantifier.com). The images of each
case were captured using a digital camera (Jenoptik ProgRes
speedXT) attached to an Olympus light microscope (BX51; Olympus).
To reduce the effect of sampling error, whole areas of 2-mm TMA
cores were included in analysis using a low power view (×4)
according to recommendations of the software guide. CD8-positive
cells were automatically detected, segmented, and counted. The
cut-off numbers (176.5/mm2) were used to determine high
expression of CD8 in TILs. This cut-off point is referred to as the
optimal value that is calculated using AUC and Youden's index for
patient's survival (23).
Statistical analysis
Statistical analyses were performed with SPSS
software (version 23.0; SPSS Inc., Chicago, IL, USA). The average
values were utilized as cut-off points for age and tumor size.
Categorical data were assessed using Pearson's χ2 or Fisher's exact
tests. The mean number of CD8+ TILs was examined using
an unpaired Student's t-test. Overall survival (OS) was measured by
the amount of time the patient is alive after the primary
treatment. Disease-free survival (DFS) was referred to as the
length of time that the patient survives without any signs or
symptoms of that cancer after primary treatment for a cancer ends.
In univariate analyses for survival, survival curves were
illustrated and the relationship between survival rates and various
clinicopathological factors were compared using the Kaplan-Meier
method with the log-rank test. In multivariate analyses for
survival, we investigated the prognostic significance using Cox
proportional hazards modelling. P-values less than 0.05 were
considered to denote statistical significance.
Results
Clinicopathological
characteristics
The demographics and clinicopathological correlation
of IDO1 expression are summarized in Tables I and II, respectively. The series consisted of
54 men and 23 women with an average age of 70 years (range, 40–87
years). The carcinomas were located in the proximal bile duct (26
cases), including 6 cases in the perihilar area, and in the distal
(intrapancreatic head) bile duct (45 cases). Histologically, 73
cases were adenocarcinoma, not otherwise specified (NOS), and four
cases were classified as adenocarcinoma arising in intraductal
papillary neoplasm of the bile duct. Two cases were associated with
a congenital biliary abnormality. Surgical resection and regional
lymph node dissection were dependent on the location of the primary
tumor. Pancreaticoduodenectomy or pylorus-preserving
pancreaticoduodenectomy was performed in 45 patients, bile duct
resection in 26 patients, and combined hepatectomy with bile duct
resection in six patients. Forty-seven patients (61.0%) showed
clear resection margins. In 18 patients (23.4%), resection margins
were involved by carcinoma. In addition, low-and high-grade
dysplasia at resection margins were identified in 5 (6.5%) and 7
(9.1%) patients, respectively. Thirty-one patients received
postoperative chemotherapy. The range of follow-up was from 0.3
months to 96.0 months (median: 20.9 months). No follow-up loss is
identified.
 | Table I.Patient demographics. |
Table I.
Patient demographics.
| Parameters | Grouping | N (%) |
|---|
| Age, years | <70 | 37 (48.7) |
|
| >70 | 39 (51.3) |
| Sex | Male | 53 (69.7) |
|
| Female | 23 (30.3) |
| Size, cm | <2.3 | 40 (52.6) |
|
| >2.3 | 36 (47.4) |
| Location | Distal | 45 (59.2) |
|
| Proximal | 31 (40.8) |
|
Differentiationa | Well | 21 (27.6) |
|
| Moderately | 47 (61.8) |
|
| Poorly | 6 (7.9) |
| LVIa | Absent | 49 (64.5) |
|
| Present | 23 (30.3) |
| PNIa | Absent | 15 (19.7) |
|
| Present | 53 (69.7) |
| LN metastasis | Absent | 47 (61.8) |
|
| Present | 29 (38.2) |
| pT
stageb | pT1 | 12 (15.8) |
|
| pT2 | 48 (63.2) |
|
| pT3 | 16 (21.1) |
| pN
stageb | pN0 | 47 (61.8) |
|
| pN1 | 22 (28.9) |
|
| pN2 | 7 (9.2) |
| pTNM
stageb | I | 9 (11.8) |
|
| II | 60 (78.9) |
|
| III | 7 (9.2) |
| Recurrence | Negative | 23 (30.3) |
|
| Positive | 53 (69.7) |
| Death | Alive | 24 (31.6) |
|
| Dead | 52 (68.4) |
 | Table II.Clinicopathological correlation of
IDO1 expression. |
Table II.
Clinicopathological correlation of
IDO1 expression.
|
|
| IDO1 expression
(%) |
|
|---|
|
|
|
|
|
|---|
| Parameters | Grouping | Low | High | P-value |
|---|
| Age, years | <70 | 25 (67.6) | 12 (32.4) | 0.933 |
|
| >70 | 26 (66.7) | 13 (33.3) |
|
| Sex | Male | 34 (64.2) | 19 (35.8) | 0.441 |
|
| Female | 17 (73.9) | 6 (26.1) |
|
| Size, cm | <2.3 | 28 (70.0) | 12 (30.0) | 0.571 |
|
| >2.3 | 23 (63.9) | 13 (36.1) |
|
| Location | Distal | 28 (62.2) | 17 (37.8) | 0.200 |
|
| Proximal | 23 (74.2) | 8 (25.8) |
|
|
Differentiation | Well | 15 (71.4) | 6 (28.6) | 0.618 |
|
| Moderately | 31 (66.0) | 16 (34.0) |
|
|
| Poorly | 3 (50.0) | 3 (50.0) |
|
| LVI | Absent | 35 (71.4) | 14 (28.6) | 0.422 |
|
| Present | 14 (60.9) | 9 (39.1) |
|
| PNI | Absent | 9 (60.0) | 6 (40.0) | 0.555 |
|
| Present | 36 (67.9) | 17 (32.1) |
|
| LN metastasis | Absent | 35 (74.5) | 12 (25.5) | 0.082 |
|
| Present | 16 (55.2) | 13 (44.8) |
|
| pT
stagea | pT1 | 10 (83.3) | 2 (16.7) | 0.384 |
|
| pT2 | 30 (62.5) | 18 (37.5) |
|
|
| pT3 | 11 (68.8) | 5 (31.3) |
|
| pN
stagea | pN0 | 35 (74.5) | 12 (25.5) | 0.007 |
|
| pN1 | 15 (68.2) | 7 (31.8) |
|
|
| pN2 | 1 (14.3) | 6 (85.7) |
|
| pTNM
stagea | I | 9 (100) | 0 (0) | 0.001 |
|
| II | 41 (68.3) | 19 (31.9) |
|
|
| III | 1 (14.3) | 6 (85.7) |
|
| CD8+
TIL/mm2 | Mean | 214.78 | 98.38 | 0.008 |
| Recurrence | Negative | 20 (87.0) | 3 (13.0) | 0.018 |
|
| Positive | 31 (58.5) | 22 (41.5) |
|
| Death | Alive | 20 (83.3) | 4 (16.7) | 0.065 |
|
| Death | 31 (59.6) | 21 (40.4) |
|
IDO1 expression was evaluated in 76 cases except for
one case with no assessable staining due to loss of the cores in
the TMA block. IDO1 was expressed in the cytoplasm and/or nuclei of
the tumor epithelium and stromal mononuclear immune cells. The
epithelial IDO1-expressing tumors were grouped into two categories
of high and low expression according to the proportion and
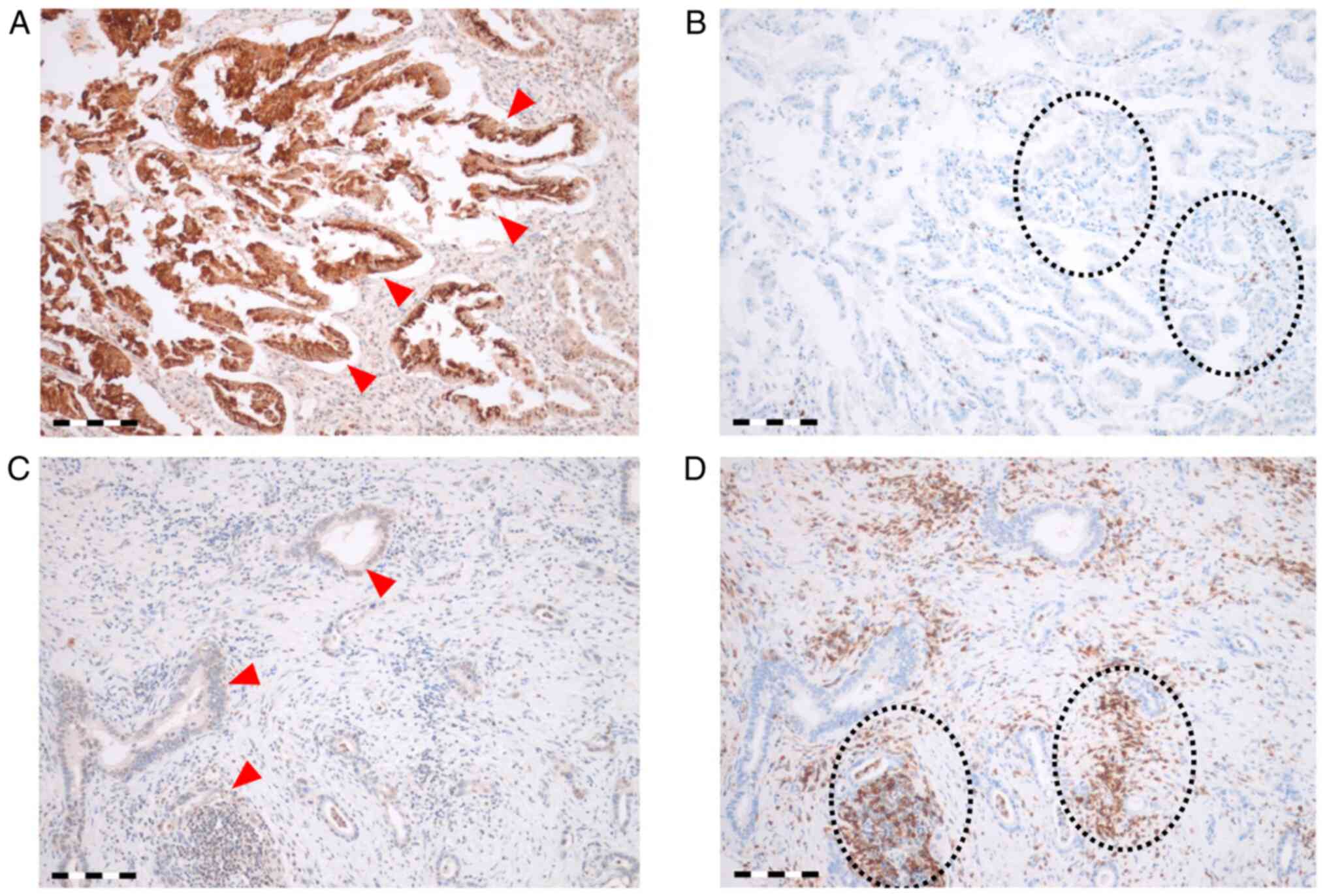
intensity score (Fig. 1). IDO1 was
highly expressed in 25 of 76 (32.9%) tumor specimens. In 51 of 76
(67.1%) cases, IDO1 expression is low. IDO1-high expressing tumors
(Fig. 1A) exhibited a
significantly low proportion of intratumoral CD8+ cells (mean ±
standard error (SE), 98.38/mm2±100.26; Fig. 1B), whereas IDO1-low expressing
tissue samples (Fig. 1C)
demonstrated high CD8+ cells (214.78/mm2±268.26; Fig. 1D) (P=0.008). Comparing IDO1
expression with CD8 expression in TILs, IDO1 expression was
inversely associated with the number of CD8+ TILs.
High IDO1 expression was associated with a higher pN
category (P=0.007), an advanced overall TNM stage (P=0.001), and
more frequent lymph node metastasis with marginal statistical
significance (P=0.082). Patients with high IDO1 expression
experienced more frequent recurrence (P=0.018). There was
non-significant relationship between IDO1 overexpression and
disease-specific death (P=0.065) (Table II).
CD8 expression in TILs was evaluated in all 76
cases. Although the difference was not statistically significant,
high CD8 expression in TILs tended to be associated with less
frequent recurrence (61.5%) and a lower disease-specific death rate
(64.1%) than low CD8 expression (78.4 and 73.0%, respectively).
Univariate and multivariate analyses
for overall Survival and disease-free survival
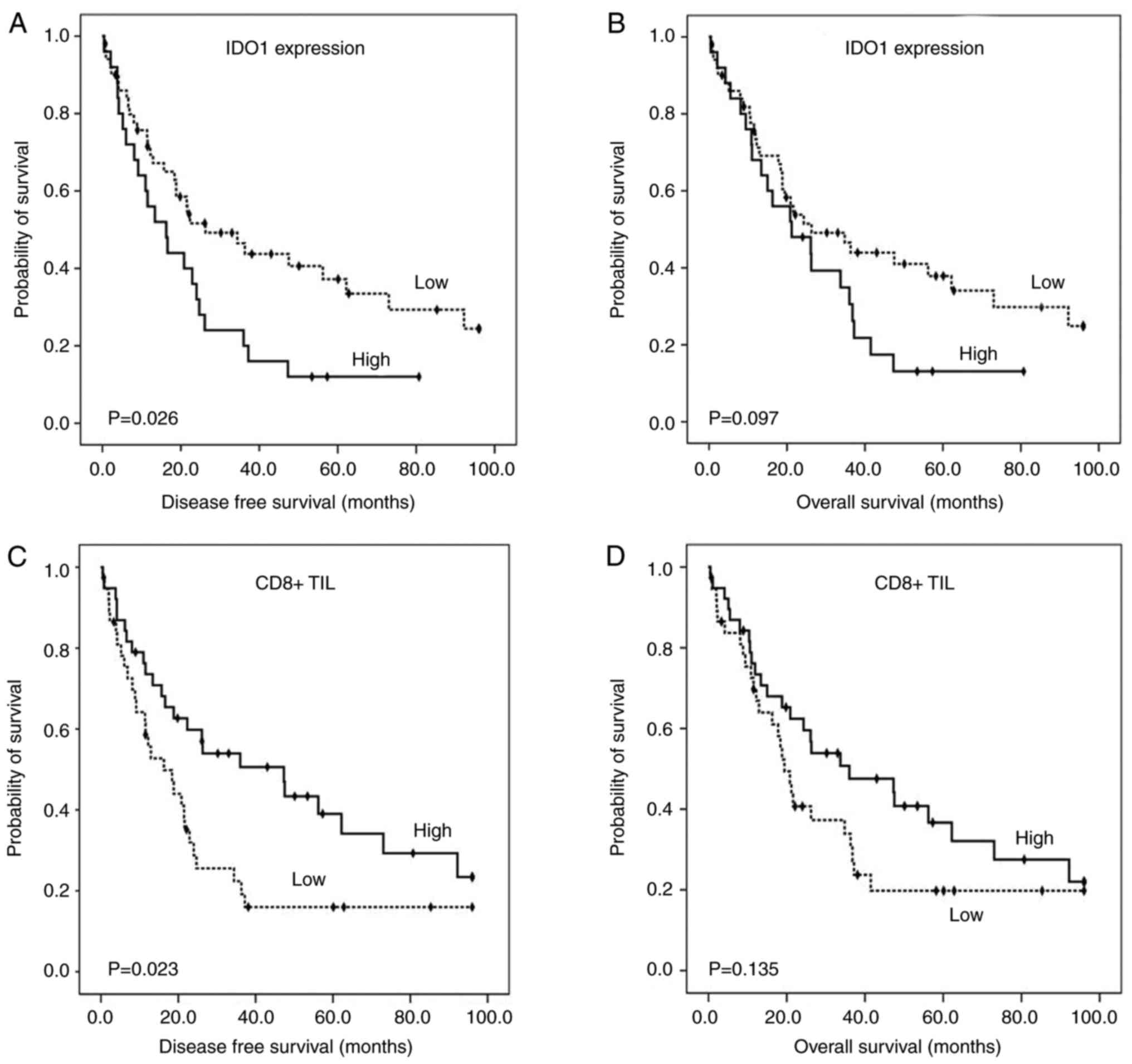
High IDO1 expression was significantly associated
with shorter disease-free survival (median time: 16.30 months vs.
26.30 months, P=0.026; Fig. 2A)
and had a non-significant trend to decreased overall survival
(median time: 21.20 months vs. 26.30 months, P=0.097; Fig. 2B). Patients with high
CD8+ TILs showed longer disease-free survival (median
survival time: 47.30 months vs. 16.30 months, P=0.023; Fig. 2C) than those with low
CD8+ TILs. No significant relationship between high
CD8+ TILs and overall survival (median survival time:
36.00 months vs. 19.4 months, P=0.135; Fig. 2D) was illustrated.
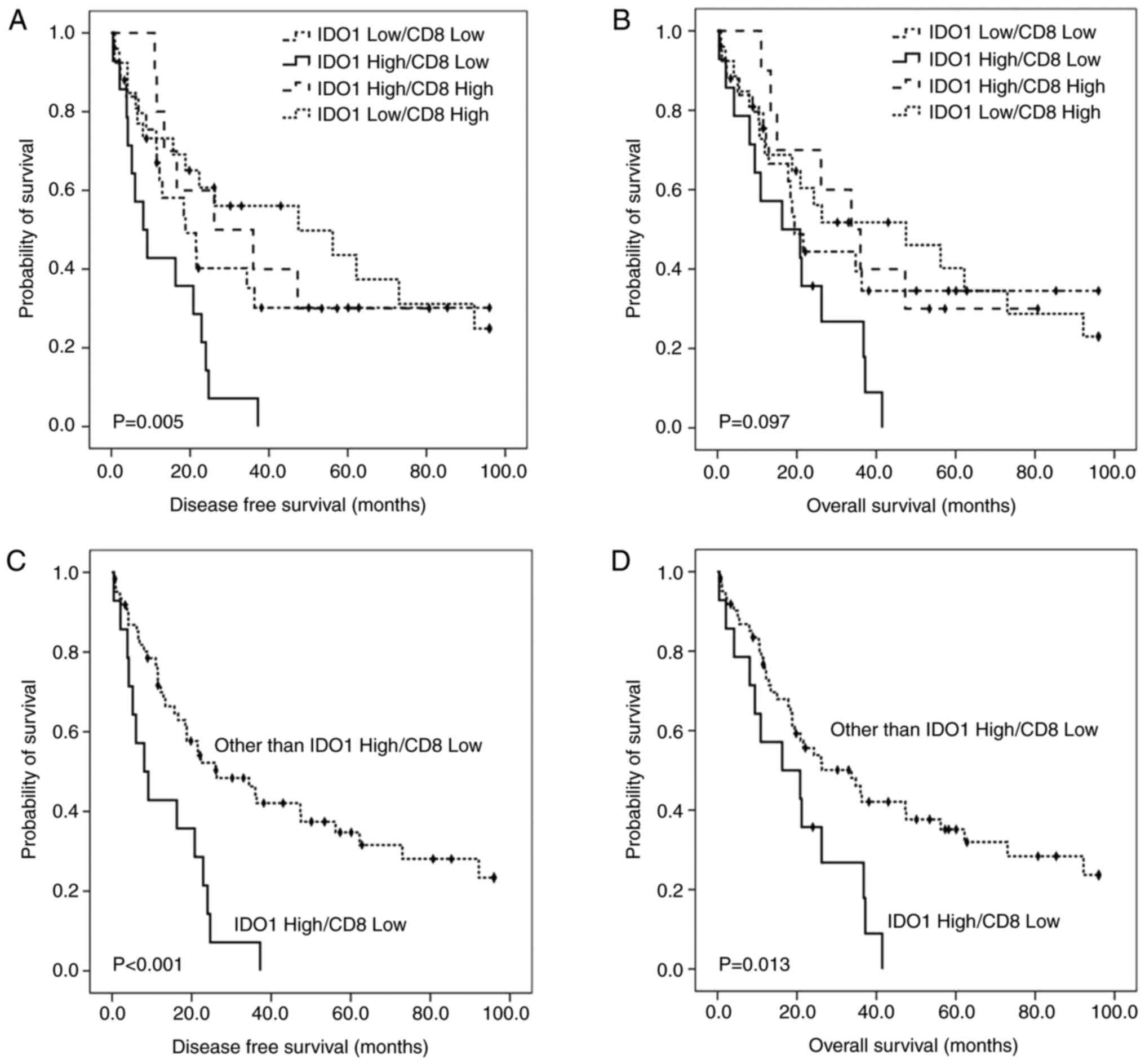
Patient survival was further stratified according to
four expression combinations of IDO1 and CD8 arranged in order of
adverse prognostic value as follows (Fig. 3):
IDO1high/CD8low (18.4%, 14/76);
IDO1low/CD8low (32.9%, 25/76);
IDO1high/CD8high (13.2%, 10/76);
IDO1low/CD8high (35.5%, 27/76). The patients
whose carcinomas were IDO1high/CD8low showed
the worst disease-free survival times (Fig. 3A and C) and median overall
(Fig. 3B and D) (8.10 and 16.30
months, respectively). The median overall survival time of patients
with IDO1low/CD8low and
IDO1high/CD8high expression were 19.40 months
and 33.70 months, respectively. The median disease-free survival
time of patients with IDO1low/CD8low and
IDO1high/CD8high expression were 18.80 months
and 26.1 months, respectively. Finally, patients with
IDO1low/CD8high expression revealed the best
survival (medial overall survival, 47.50 months; disease-free
survival, 47.50 months) (Table
III).
 | Table III.Univariate analyses (log-rank test)
for overall and disease-free survival. |
Table III.
Univariate analyses (log-rank test)
for overall and disease-free survival.
| A, Overall
survival |
|---|
|
|---|
|
|
|
| CI (95%) |
|
|---|
|
|
|
|
|
|
|---|
| Parameters |
| Mean survival
(months) | Lower | Upper | P-value |
|---|
| Age, years | <70 | 36.8 | 8.41 | 65.19 | 0.056 |
|
| >70 | 20.9 | 10.67 | 31.13 |
|
| Sex | Male | 20.9 | 9.54 | 32.26 | 0.769 |
|
| Female | 26.1 | 9.11 | 43.09 |
|
| Size, cm | <2.3 | 47.3 | 13.54 | 81.16 | 0.03 |
|
| >2.3 | 20.8 | 17.56 | 24.04 |
|
| Location | Distal | 36.3 | 23.55 | 49.05 | 0.007 |
|
| Proximal | 13.4 | 4.39 | 22.41 |
|
|
Differentiation | Well | 20.9 | 0 | 44.36 | 0.303 |
|
| Moderately | 26.2 | 11.5 | 40.9 |
|
|
| Poorly | 8.1 | 4.14 | 12.06 |
|
| LVI | Absent | 26.2 | 8.09 | 44.31 | 0.392 |
|
| Present | 26.1 | 13.7 | 38.49 |
|
| PNI | Absent | 56.2 | 7.56 | 104.84 | 0.026 |
|
| Present | 20.9 | 14.84 | 26.96 |
|
| LN metastasis | Absent | 36 | 21.29 | 50.71 | 0.1 |
|
| Present | 20.8 | 12.76 | 28.84 |
|
| pT
stagea | pT1 | 41.5 | 0 | 115.37 | 0.439 |
|
| pT2 | 24.3 | 17.87 | 30.73 |
|
|
| pT3 | 18.4 | 10.95 | 25.85 |
|
| pN
stagea | pN0 | 36 | 21.29 | 50.71 | 0.092 |
|
| pN1 | 20.9 | 17.61 | 24.19 |
|
|
| pN2 | 10.9 | 3.75 | 18.09 |
|
| pTNM
stagea | I | 73 | 7.37 | 138.63 | 0.112 |
|
| II | 26.1 | 11.68 | 40.52 |
|
|
| III | 10.9 | 3.72 | 18.09 |
|
| Postoperative
chemotherapy | No | 26.1 | 10.54 | 41.67 | 0.705 |
|
| Yes | 24.3 | 15.16 | 33.44 |
|
| IDO1
expression | Low | 26.3 | 7.94 | 44.67 | 0.097 |
|
| High | 21.2 | 5.68 | 36.72 |
|
| CD8+
TIL | Low | 19.4 | 15.42 | 23.38 | 0.135 |
|
| High | 36 | 8.2 | 63.8 |
|
|
IDO1/CD8+ TIL, 2 tiers | Other than
High/Low | 33.7 | 17.5 | 49.9 | 0.013 |
|
| High/Low | 16.3 | 0 | 34.45 |
|
|
IDO1/CD8+ TIL, 4 tiers | Low/Low | 19.4 | 14.5 | 24.3 | 0.097 |
|
| High/Low | 16.3 | 0 | 34.45 |
|
|
| High/High | 33.7 | 18.36 | 49.04 |
|
|
| Low/High | 47.5 | 5.98 | 89.02 |
|
|
| B, Disease-free
survival |
|
|
|
|
| CI
(95%) |
|
|
|
|
|
|
|
|
Parameters |
| Mean survival
(months) | Lower | Upper | P-value |
|
| Age, years | <70 | 24.7 | 4.43 | 44.97 | 0.082 |
|
| >70 | 18.8 | 12.13 | 25.47 |
|
| Sex | Male | 20.8 | 11.26 | 30.34 | 0.827 |
|
| Female | 21.6 | 15.91 | 27.09 |
|
| Size, cm | <2.3 | 47.3 | 8.15 | 86.45 | 0.011 |
|
| >2.3 | 18.4 | 8.45 | 28.35 |
|
| Location | Distal | 26.1 | 9.43 | 42.77 | 0.007 |
|
| Proximal | 12.9 | 10.31 | 15.49 |
|
|
Differentiation | Well | 20.8 | 12.35 | 29.26 | 0.184 |
|
| Moderately | 26.1 | 15.1 | 37.1 |
|
|
| Poorly | 8.1 | 2.1 | 14.1 |
|
| LVI | Absent | 24.7 | 7.98 | 41.42 | 0.151 |
|
| Present | 15.7 | 9.76 | 21.64 |
|
| PNI | Absent | 92.2 | 38.03 | 146.37 | 0.005 |
|
| Present | 16.6 | 6.22 | 26.98 |
|
| LN metastasis | Absent | 36 | 19.53 | 52.47 | 0.012 |
|
| Present | 11.4 | 1.55 | 21.25 |
|
| pT
stagea | pT1 | 20.8 | 0 | 95 | 0.573 |
|
| pT2 | 22.9 | 14.2 | 31.61 |
|
|
| pT3 | 18.4 | 4.09 | 32.71 |
|
| pN
stagea | pN0 | 36 | 19.53 | 52.47 | 0.017 |
|
| pN1 | 15.7 | 7.92 | 23.48 |
|
|
| pN2 | 8.1 | 0.66 | 15.54 |
|
| pTNM
stagea | I | 73 | 0 | 150.25 | 0.063 |
|
| II | 22.3 | 16.17 | 28.43 |
|
|
| III | 8.1 | 0.66 | 15.42 |
|
| Postoperative
chemotherapy | No | 21.5 | 13.6 | 29.4 | 0.728 |
|
| Yes | 22.3 | 10.58 | 34.02 |
|
| IDO1
expression | Low | 26.3 | 9.23 | 43.87 | 0.026 |
|
| High | 16.3 | 7.98 | 24.62 |
|
| CD8+
TIL | Low | 16.3 | 7.03 | 25.57 | 0.023 |
|
| High | 47.3 | 20.93 | 73.67 |
|
|
IDO1/CD8+ TIL, 2 tiers | Other than
High/Low | 26.3 | 10.19 | 42.42 | <0.001 |
|
| High/Low | 8.1 | 2.42 | 13.78 |
|
|
IDO1/CD8+ TIL, 4 tiers | Low/Low | 18.8 | 5.75 | 31.85 | 0.005 |
|
| High/Low | 8.1 | 2.42 | 13.78 |
|
|
| High/High | 26.1 | 0 | 56.16 |
|
|
| Low/High | 47.5 | 0 | 97.81 |
|
In univariate analyses (Table III), larger tumor size (P=0.030),
proximal location (P=0.007), perineural invasion (P=0.026), and the
IDO1high/CD8low subgroup (P=0.013) revealed
significantly shorter overall survival. Similarly, worse
disease-free survival was significantly related to proximal
location (P=0.007), perineural invasion (P=0.005), lymph node
metastasis (P=0.012), higher pN stage (P=0.017), high IDO1
expression (P=0.026), low CD8+ TILs (P=0.023), and the
IDO1high/CD8low expression subgroup
(P<0.001). Multivariate statistical analyses of the
IDO1high/CD8low expression subgroup were
performed with the significant prognostic valuables examined by
univariate analyses. As shown in Table IV,
IDO1high/CD8low expression (P=0.025, Cox
hazard ratio=2.168) in addition to tumor proximal location and
perineural invasion was an independent prognostic factor for
overall survival. Furthermore,
IDO1high/CD8low expression (P=0.015, Cox
hazard ratio=2.460) with tumor proximal location, lymph node
metastasis, and perineural invasion was an independent
prognosticator for disease-free survival.
 | Table IV.Multivariate analyses (Cox
proportional hazards model). |
Table IV.
Multivariate analyses (Cox
proportional hazards model).
|
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|
|---|
| Parameter | Comparison | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Location | Proximal vs.
Distal | 1.349 | 1.004-1.812 | 0.047 | 1.449 | 1.073-1.957 | 0.016 |
| PNI | Presence vs.
Absence | 2.159 | 0.984-4.737 | 0.055 | 2.486 | 1.060-5.830 | 0.036 |
| LN metastasis | Presence vs.
Absence | - | - | - | 1.956 | 1.026-3.732 | 0.042 |
|
IDO1/CD8+ TIL |
IDO1high/CD8low vs.
Others | 2.168 | 1.1-4.272 | 0.025 | 2.460 | 1.195-5.065 | 0.015 |
Discussion
Since the immunosuppressive effects of IDO1 were
discovered, IDO1 has been reported to be highly expressed and
associated with clinical outcomes in a variety of solid tumors
showing contradictory biologic behaviors. IDO1 expression is a
predictor of poor clinical outcomes in many kinds of solid tumors,
such as ovarian adenocarcinomas, colorectal adenocarcinomas,
laryngeal squamous cell carcinomas, and endometrial and esophageal
cancers (12–17). In contrast, patients with high IDO1
expression in some tumors, such as basal cell-like breast
carcinoma, hepatocellular carcinoma, and renal cell carcinoma, have
increased survival (13,18,19).
In the present study of 76 EHBDC surgical specimens, we
demonstrated that high IDO1 expression in the tumor epithelial
cells was positively correlated with tumor recurrence and poor
patient survival. As is well known, some stromal mononuclear immune
cells are also positive for IDO1; however, this expression was not
correlated with patient survival (data not shown).
The role of CD8+ T lymphocytes in tumor
progression has been examined in a variety of human malignancies
(24–28). Most research has revealed a
beneficial prognostic effect of high intratumoral/intraepithelial
CD8+ T lymphocyte infiltration. In accordance with our
results, there are a few reports of a favorable prognostic effect
of CD8+ TILs in patients with EHBDC (24,29,30).
IDO1 expression in tumor epithelial cells was inversely associated
with number of CD8+ TILs in the present study. Similar
to our results, Ino et al reported that tumoral IDO1
expression was correlated with a reduced number of TILs and natural
killer (NK) cells in endometrial cancer, possibly contributing to
disease progression and poor clinical outcomes (14). Brandacher et al also
reported that high IDO1 expression was associated with a
significant reduction in CD3+ TILs in colon cancer as
compared to tumor samples with low IDO1 expression (31).
IDO1 is an immunomodulatory enzyme that catalyzes
the degradation of tryptophan (Trp) to Kyn. The depletion of Trp
and accumulation of Kyn have been reported to induce effector
T-cell apoptosis/dysfunction and generate immunosuppressive
regulatory T cells (32).
Recently, functional inactivation of tumor-reactive T cells has
been considered to be an essential mechanism of tumor immune
evasion (33). The upregulation of
IDO1 occurs in tumor cells in response to interferon-γ
(IFN-γ) secreted by CD8+ T cells (34) while the increased expression of
IDO1 suppresses the CD8+ T cell response, resulting in
tumor immune evasion and tumor growth, suggesting a possible
negative feedback loop to regulate T-cell activation (5). Since both PD-L1 and IDO1 are
increased in tumor cells by IFN-γ induced by CD8+
T cells (35), IDO1-and
PD-L1-expressing tumors are expected to have similar clinical
significance (36). However, IDO1
expression may have different clinicopathological implications from
PD-L1 expression because there is the isolated mechanism to promote
IDO1 secretion by activation pathway of RAS and PAMP
(pathogen-associated molecular pattern) that is not shared with
PD-L1 (37). In this regard,
further research to elucidate the relationship between IDO1 and
PD-L1 is needed in the future. These suggest that a more complex
mechanism than previously evaluated acts between IDO1 expression
and the immune microenvironment. Considering that single-agent
treatments with IDO1 enzyme inhibitors have a negligible effect on
decreasing the established cancer burden, a combination of select
therapies with IDO1 blockade for a synergistic benefit against
tumor growth is likely needed. Capitalizing on this background and
the negative association between IDO1 expression and
CD8+ TILs as shown in our study, patients with
IDO1high/CD8low or
IDO1low/CD8high subgroups can represent a
stronger interaction between IDO1-expressing tumor and
CD8+ TILs compared to other subgroups. Therefore, IDO1
blockers are expected to be more effective for the
IDO1high/CD8low subgroup with worst prognosis
by inhibiting a patent link between IDO1-expressing tumor and
CD8+ TILs.
This is the first study to demonstrate the detailed
clinicopathological and prognostic impact of IDO1 expression
associated with decreased numbers of CD8+ TILs. In the
future, our results can be utilized as a novel candidate biomarker
in EHBDC for the development of immunotherapeutic drugs by
augmenting our understanding of complex immune mechanisms.
The limitations of this study include the
retrospective nature of the study design and the relatively small
number of cases. In addition, the fundamental problem in TMA
studies, such as tumor heterogeneity, remains unresolved.
In conclusion, IDO1 was highly expressed in the
tumor epithelial cells in approximately one-third of cases of
EHBDC. High expression of IDO1 was associated with decreased
numbers of CD8+ TILs, an increased pN category, an
advanced overall stage, and frequent recurrence. When further
stratified by combining IDO1 expression with CD8+ TIL
status, the IDO1high/CD8low subgroup had the
worst prognosis for overall survival and disease-free survival.
High IDO1 expression with decreased numbers of CD8+ TILs
is an independent prognostic indicator, and expected to be an IDO1
blocker-targetable candidate in patients with EHBDC.
Acknowledgements
Not applicable.
Funding
Professor Dae-Woon Eom received a research grant (grant no.
NRF-2020R1F1A1067158) of the Basic Science Research Program from
the National Research Foundation of Korea funded by the Ministry of
Science and ICT, and a grant (grant no. 2020-IB008) from the
Gangneung Asan Hospital Biomedical Research Center Promotion
Fund.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BJN, GMC, HJJ, CHM, HSO, MK and DWE conceived and
designed the research. BJN and DWE performed the experiments,
analysed the data and wrote the manuscript. GMC, HJJ, CHM, HSO and
MK reviewed the manuscript and approved the final version. All
authors have read and approved the final manuscript. BJN and DWE
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Gangneung Asan Hospital (approval no. GNAH 2020-06-018).
All procedures were in accordance with the ethical standards of the
responsible committee on human experimentation (institutional and
national) and with the Helsinki Declaration of 1964 and later
versions. Informed consent to be included in the study, or the
equivalent, was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bridgewater JA, Goodman KA, Kalyan A and
Mulcahy MF: Biliary tract cancer: Epidemiology, radiotherapy, and
molecular profiling. Am Soc Clin Oncol Educ Book. 35:e194–e203.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rizvi S and Gores GJ: Pathogenesis,
diagnosis, and management of cholangiocarcinoma. Gastroenterology.
145:1215–1229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagino M, Ebata T, Yokoyama Y, Igami T,
Sugawara G, Takahashi Y and Nimura Y: Evolution of surgical
treatment for perihilar cholangiocarcinoma: A single-center 34-year
review of 574 consecutive resections. Ann Surg. 258:129–140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malenica I, Donadon M and Lleo A:
Molecular and immunological characterization of biliary tract
cancers: A paradigm shift towards a personalized medicine. Cancers
(Basel). 12:21902020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lou Q, Liu R, Yang X, Li W, Huang L, Wei
L, Tan H, Xiang N, Chan K, Chen J and Liu H: miR-448 targets IDO1
and regulates CD8(+) T cell response in human colon cancer. J
Immunother Cancer. 7:2102019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harden JL and Egilmez NK: Indoleamine
2,3-dioxygenase and dendritic cell tolerogenicity. Immunol Invest.
41:738–764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moon YW, Hajjar J, Hwu P and Naing A:
Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J
Immunother Cancer. 3:512015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brochez L, Chevolet I and Kruse V: The
rationale of indoleamine 2,3-dioxygenase inhibition for cancer
therapy. Eur J Cancer. 76:167–182. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Munn DH, Zhou M, Attwood JT, Bondarev I,
Conway SJ, Marshall B, Brown C and Mellor AL: Prevention of
allogeneic fetal rejection by tryptophan catabolism. Science.
281:1191–1193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu CP, Fu SF, Chen X, Ye J, Ye Y, Kong LD
and Zhu Z: The clinicopathological and prognostic significance of
IDO1 expression in human solid tumors: Evidence from a systematic
review and meta-analysis. Cell Physiol Biochem. 49:134–143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frumento G, Rotondo R, Tonetti M, Damonte
G, Benatti U and Ferrara GB: Tryptophan-derived catabolites are
responsible for inhibition of T and natural killer cell
proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med.
196:459–468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soliman H, Rawal B, Fulp J, Lee JH, Lopez
A, Bui MM, Khalil F, Antonia S, Yfantis HG, Lee DH, et al: Analysis
of indoleamine 2–3 dioxygenase (IDO1) expression in breast cancer
tissue by immunohistochemistry. Cancer Immunol Immunother.
62:829–837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riesenberg R, Weiler C, Spring O, Eder M,
Buchner A, Popp T, Castro M, Kammerer R, Takikawa O, Hatz RA, et
al: Expression of indoleamine 2,3-dioxygenase in tumor endothelial
cells correlates with long-term survival of patients with renal
cell carcinoma. Clin Cancer Res. 13:6993–7002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ino K, Yoshida N, Kajiyama H, Shibata K,
Yamamoto E, Kidokoro K, Takahashi N, Terauchi M, Nawa A, Nomura S,
et al: Indoleamine 2,3-dioxygenase is a novel prognostic indicator
for endometrial cancer. Br J Cancer. 95:1555–1561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao YF, Peng RQ, Li J, Ding Y, Zhang X, Wu
XJ, Pan ZZ, Wan DS, Zeng YX and Zhang XS: The paradoxical patterns
of expression of indoleamine 2,3-dioxygenase in colon cancer. J
Transl Med. 7:712009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inaba T, Ino K, Kajiyama H, Shibata K,
Yamamoto E, Kondo S, Umezu T, Nawa A, Takikawa O and Kikkawa F:
Indoleamine 2,3-dioxygenase expression predicts impaired survival
of invasive cervical cancer patients treated with radical
hysterectomy. Gynecol Oncol. 117:423–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye J, Liu H, Hu Y, Li P, Zhang G and Li Y:
Tumoral indoleamine 2,3-dioxygenase expression predicts poor
outcome in laryngeal squamous cell carcinoma. Virchows Arch.
462:73–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jacquemier J, Bertucci F, Finetti P,
Esterni B, Charafe-Jauffret E, Thibult ML, Houvenaeghel G, Van den
Eynde B, Birnbaum D, Olive D and Xerri L: High expression of
indoleamine 2,3-dioxygenase in the tumour is associated with
medullary features and favourable outcome in basal-like breast
carcinoma. Int J Cancer. 130:96–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishio T, Goto S, Tahara K, Tone S, Kawano
K and Kitano S: Immunoactivative role of indoleamine
2,3-dioxygenase in human hepatocellular carcinoma. J Gastroenterol
Hepatol. 19:319–326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th edition.
Springer; New York, USA: pp. 317–325. 2017
|
|
21
|
Ferdinande L, Decaestecker C, Verset L,
Mathieu A, Moles Lopez X, Negulescu AM, Van Maerken T, Salmon I,
Cuvelier CA and Demetter P: Clinicopathological significance of
indoleamine 2,3-dioxygenase 1 expression in colorectal cancer. Br J
Cancer. 106:141–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma W, Duan H, Zhang R, Wang X, Xu H, Zhou
Q and Zhang L: High expression of indoleamine 2, 3-dioxygenase in
adenosquamous lung carcinoma correlates with favorable patient
outcome. J Cancer. 10:267–276. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lugli A, Karamitopoulou E, Panayiotides I,
Karakitsos P, Rallis G, Peros G, Iezzi G, Spagnoli G, Bihl M,
Terracciano L and Zlobec I: CD8+ lymphocytes/tumour-budding index:
An independent prognostic factor representing a ‘pro-/anti-tumour’
approach to tumour host interaction in colorectal cancer. Br J
Cancer. 101:1382–1392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oshikiri T, Miyamoto M, Shichinohe T,
Suzuoki M, Hiraoka K, Nakakubo Y, Shinohara T, Itoh T, Kondo S and
Katoh H: Prognostic value of intratumoral CD8+ T lymphocyte in
extrahepatic bile duct carcinoma as essential immune response. J
Surg Oncol. 84:224–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fukunaga A, Miyamoto M, Cho Y, Murakami S,
Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y,
et al: CD8+ tumor-infiltrating lymphocytes together with CD4+
tumor-infiltrating lymphocytes and dendritic cells improve the
prognosis of patients with pancreatic adenocarcinoma. Pancreas.
28:e26–e31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mahmoud SM, Paish EC, Powe DG, Macmillan
RD, Grainge MJ, Lee AH, Ellis IO and Green AR: Tumor-infiltrating
CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin
Oncol. 29:1949–1955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mlecnik B, Tosolini M, Kirilovsky A,
Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman
WH, Pagès F and Galon J: Histopathologic-based prognostic factors
of colorectal cancers are associated with the state of the local
immune reaction. J Clin Oncol. 29:610–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kitano Y, Okabe H, Yamashita YI, Nakagawa
S, Saito Y, Umezaki N, Tsukamoto M, Yamao T, Yamamura K, Arima K,
et al: Tumour-infiltrating inflammatory and immune cells in
patients with extrahepatic cholangiocarcinoma. Br J Cancer.
118:171–180. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goeppert B, Frauenschuh L, Zucknick M,
Stenzinger A, Andrulis M, Klauschen F, Joehrens K, Warth A, Renner
M, Mehrabi A, et al: Prognostic impact of tumour-infiltrating
immune cells on biliary tract cancer. Br J Cancer. 109:2665–2674.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brandacher G, Perathoner A, Ladurner R,
Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G,
Weiss HG, Göbel G, et al: Prognostic value of indoleamine
2,3-dioxygenase expression in colorectal cancer: Effect on
tumor-infiltrating T cells. Clin Cancer Res. 12:1144–1151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Munn DH and Mellor AL: IDO in the tumor
microenvironment: Inflammation, counter-regulation, and tolerance.
Trends Immunol. 37:193–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: From immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maggi E, Giudizi MG, Biagiotti R,
Annunziato F, Manetti R, Piccinni MP, Parronchi P, Sampognaro S,
Giannarini L, Zuccati G and Romagnani S: Th2-like CD8+ T cells
showing B cell helper function and reduced cytolytic activity in
human immunodeficiency virus type 1 infection. J Exp Med.
180:489–495. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ludovini V, Bianconi F, Siggillino A,
Vannucci J, Baglivo S, Berti V, Tofanetti FR, Reda MS, Bellezza G,
Mandarano M, et al: High PD-L1/IDO-2 and PD-L2/IDO-1 Co-expression
levels are associated with worse overall survival in resected
non-small cell lung cancer patients. Genes (Basel). 12:2732021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang ML, Kem M, Mooradian MJ, Eliane JP,
Huynh TG, Iafrate AJ, Gainor JF and Mino-Kenudson M: Differential
expression of PD-L1 and IDO1 in association with the immune
microenvironment in resected lung adenocarcinomas. Mod Pathol.
32:511–523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Prendergast GC, Malachowski WP, DuHadaway
JB and Muller AJ: Discovery of IDO1 inhibitors: From bench to
bedside. Cancer Res. 77:6795–6811. 2017. View Article : Google Scholar : PubMed/NCBI
|