Introduction
In 2020, 417,367 novel cases of endometrial cancer
were diagnosed worldwide, with a cumulative risk of 1.6% (1). In Japan, 17,089 new cases of
endometrial cancer were diagnosed in 2018 and 2,597 deaths
associated with endometrial cancer were reported in 2019 (2).
Risk factors for uterine cancer include high
estrogen levels (due to obesity, diabetes and high-fat diet),
menarche at a young age, non-menarche, menopause at an older age,
Lynch syndrome, older age (>55 years) and use of tamoxifen
(3). The incidence of uterine
cancer is increasing owing to increased life expectancy and obesity
(3). The medical cost of uterine
cancer is increasing year on year and early detection is important
for the associated medical economic effects (3,4).
To the best of our knowledge, there are currently no
useful screening methods for early detection of uterine cancer in
asymptomatic individuals based on European Society for Medical
Oncology (ESMO) and National Comprehensive Cancer Network (NCCN)
guidelines (3,5). According to the Japanese guidelines,
uterine cancer may be suspected in women with irregular vaginal
bleeding and endometrial thickening ≥20 mm or ≥5 mm on transvaginal
ultrasonography before or after menopause, respectively (6). The ESMO guidelines state that
endometrial thickening >11 mm on transvaginal ultrasonography
should be managed on a case-by-case basis (5).
Endometrial carcinoma is classified as either type
I, which is estrogen-dependent, or type II, which is
estrogen-independent. Type I is a histological type of endometrioid
carcinoma with a clinical background of obesity and dyslipidemia
and typically develops following emergence of precancerous lesions,
such as endometrial hyperplasia. Type II adenocarcinoma is caused
by mismatch repair gene abnormality and multistep carcinogenesis,
characterized by mutations in KRAS and PTEN genes.
Moreover, type II endometrial cancer can be of multiple unique
histological types, including serous and clear cell carcinoma, and
are associated with a poor prognosis. Overexpression of human
epidermal growth factor receptor-2/neu and mutations in tumor
protein P53 (TP53) gene are carcinogenesis-associated
factors (7). In an integrated
analysis of endometrial carcinoma in The Cancer Genome Atlas (TCGA)
database, four novel categories were proposed as follows: i)
Ultra-mutated polymerase ε (POLE); ii) hypermutated
microsatellite instability; iii) low copy number (endometrioid) and
iv) high copy number (serous-like) (7). Furthermore, endometrial carcinoma is
divided into four molecular subtypes according to the World Health
Organization (WHO) classification system as follows: i)
POLE-ultra-mutated; ii) mismatch repair-deficient and iii)
p53-mutant endometrioid carcinoma and iv) no specific molecular
profile (8). The molecular
features of WHO classification subgroups correspond to those of the
TCGA genomic classification system (8).
Identification of biomarkers based on molecular
genetic characteristics may contribute to elucidation of mechanisms
of uterine carcinogenesis and development of methods for early
diagnosis. Notably, Matsuura et al (9) reported that the diagnostic yield of
uterine cancer may be improved by combining endometrial cytology
with next-generation sequencing (NGS) analysis of liquefied
endometrial cytology specimens: The sensitivity was reported as
88.5%, however there were numerous false-positive cases (25.2%).
The diagnostic criteria and specimen standardization are
insufficient for early detection of endometrial cancer (5,9).
Furthermore, endometrial cytology is not currently included in ESMO
or NCCN guidelines (3,4). In Japan, endometrial cytology is
routinely used to test for uterine cancer (10). Moreover, it has been recognized
worldwide that cervical cytology is a useful tool for the
prevention and early detection of cervical cancer (11). Endometrial cytology can be painful
and cannot be performed if the cervix is narrow. The detection
sensitivity of cervical cytology for uterine cancer is ~45%
(12). Therefore, it was
hypothesized that an approach combining cervical cytology and NGS
analysis of cervical liquid-based cytology (LBC) samples for
detection of tumor cells migrating to the cervix may improve
diagnostic rate. Therefore, the sensitivity of cervical cytology
combined with NGS analysis of cervical LBC specimens for early
detection of uterine cancer was evaluated.
Patients and methods
Patients and clinical information
The present study was approved by the institutional
review board of Mie University Hospital (approval no. H2020-075)
and performed according to the ethical standards of The Declaration
of Helsinki, revised in 2001. A total of 30 female patients
diagnosed with endometrial cancer from October 2019 to November
2020 at the Department of Obstetrics and Gynecology, Mie University
Hospital, was included. The median age of patients was 55.7 years
(range, 36–74 years). Written informed consent was obtained from
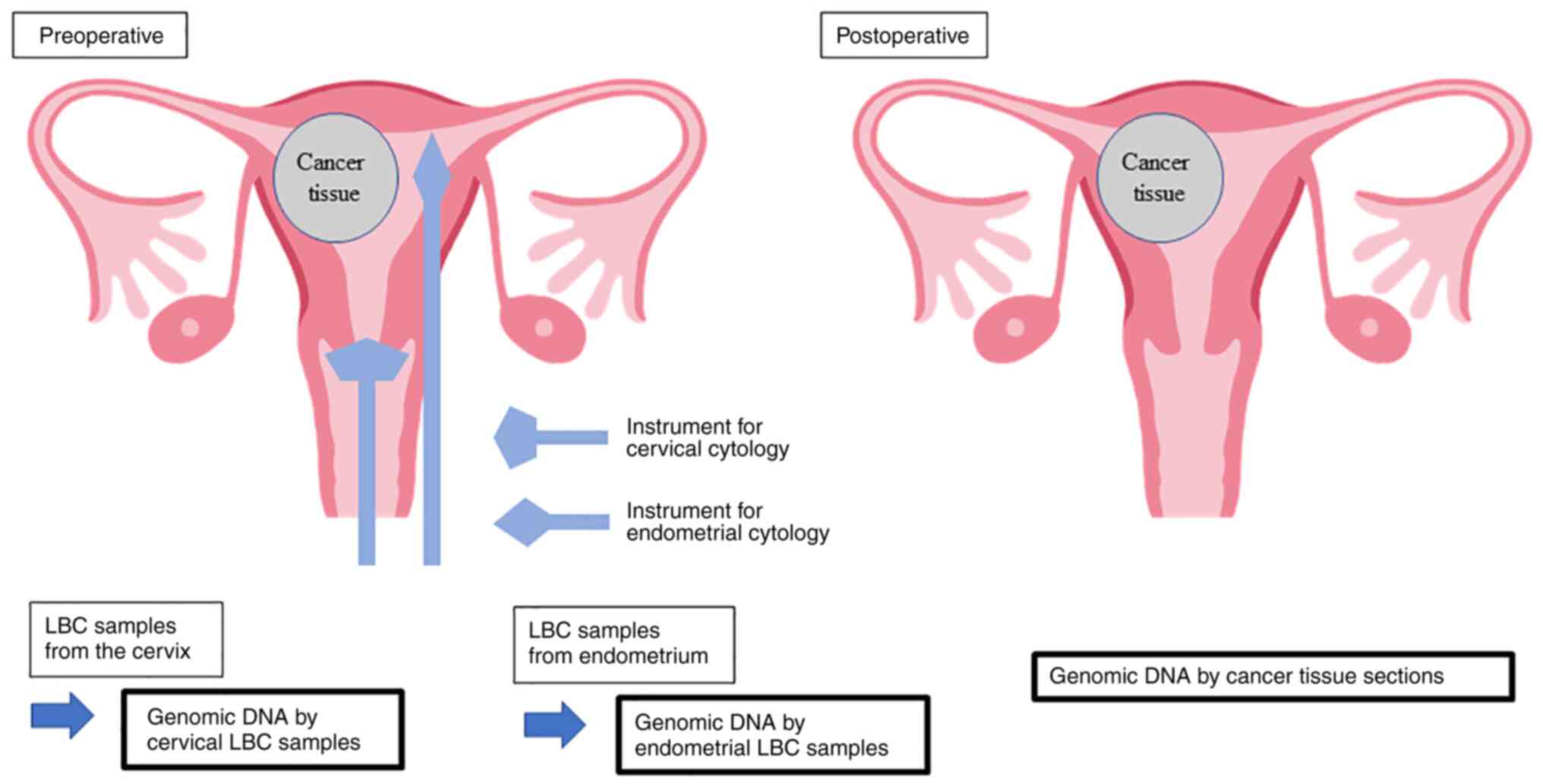
all patients. A schematic representation of the present study is
presented in Fig. 1. LBC specimens
of the uterine cervix and endometrium were collected prior to
surgery. They were processed using SurePath kits (Becton-Dickinson
and Company) according to manufacture's protocol, and cytologically
diagnosed using light microscopy after Papanicolaou staining (Leica
Autostainer XL; Leica Biosystems Nussloch GmbH). Briefly, samples
underwent fixation with 100% ethanol for 10 min, 70% ethanol for 40
sec, 50% ethanol for 60 sec, rinse in tap water, Gill hematoxylin
for 100 sec, rinse in tap water, 70% ethanol with 0.5% hydrochloric
acid for 30 sec, rinse in tap water, 70% ethanol for 50 sec, 95%
ethanol for 60 sec, OG-6 for 3 min, 95% ethanol for 60 sec twice,
EA-50 for 4 min, 95% ethanol for 55 sec, 100% ethanol for 30 sec ×3
times then 60 sec, Hemo-De® (a xylene substitute for
low-toxicity, Falma Inc.) for 5 min ×4 times, all processes were
performed at room temperature. Surgically resected organs were
fixed in 10% buffered formalin at room temperature for 24 to 48 h,
the tissue specimens were formalin-fixed and paraffin-embedded by
automated tissue processor (Tissue-Tek® VIP6; Sakura
Finetek Japan Co., Ltd.). FFPE specimens were thin sectioned at 4
µm, stained with hematoxylin and eosin with autostainer (Leica
Autostainer XL) as follows; Clear Plus (a xylene substitute for
low-toxicity; Falma Inc.) for 5 min ×3 times, 100% ethanol for 3
min ×3 times, rinse in tap water, distilled water for 2 min, Mayer
hematoxylin for 7 min, rinse in tap water, wash in warm tap water
for 3 min, 100% ethanol for 60 sec, eosin Y for 5 min, 100% ethanol
for 30 sec ×2 times, 2 min ×1, 4 min ×1, then Clear Plus for 5 min
×3 times, all processes were performed at room temperature except
for warm tap water. Tissue slides were pathologically examined
under light microscopy. Furthermore, endometrial thickness on
transvaginal ultrasonography (6 MHz) was measured to investigate
the sensitivity and specificity of primary screening.
The primary endpoint was genetic analysis of the
cervical cytology specimens and LBC for detection of endometrial
cancer. The secondary endpoints were concordance rates of gene
mutations in cervical and endometrial cytology and surgically
removed cancer tissue sections. The median age of patients with
endometrial thickness ≥11 and <11 mm and cervical cytology test
results were compared using Mood's median test. The reproducibility
of endometrial thickening by transvaginal ultrasonography, cervical
cytology and genetic analysis were assessed using Cohen's κ
coefficient.
Statistical analysis
Student's unpaired t-test and Mood's median test
were used for comparison between endometrial thickness and cervical
cytology test results and age. Data are presented as the mean ± SD.
All statistical tests were performed using SPSS (version 27.0; IBM
Corp.). P<0.05 was considered to indicate a statistically
significant difference.
DNA extraction from formalin-fixed
paraffin-embedded (FFPE) samples and LBC specimens
FFPE tissue blocks were serially sectioned at 10 µm
thickness. Mucosal endometrial tissue was micro-dissected and
genomic DNA was extracted using the QIAmp DNA FFPE Tissue kit
(Qiagen, Inc.) according to the manufacturer's protocol. Genomic
DNA was extracted from LBC and paraffin-embedded surgical tissue
samples using QIAmp DNA Mini kits (Qiagen, Inc.), according to the
manufacturer's protocol. The quality and quantity of each DNA
sample were assessed using Qubit® dsDNA (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Next-generation sequencing (NGS) of 50
cancer-associated genes
NGS of genomic DNA from each sample was performed
using the Ion Ampliseq™ Cancer Hotspot Panel v2 (cat.
no. 4475346; Thermo Fisher Scientific, Inc.), which covers ~2,800
mutational hotspot regions from 50 cancer-associated genes, as
previously described (13–15). Endometrioid carcinoma is
preferentially associated with mutations in PTEN, KRAS,
AT-rich interaction domain 1A (ARID1A), catenin β1
(CTNNB1) and PI3K catalytic subunit α (PIK3CA) as
well as microsatellite instability, whereas serous
(non-endometrioid, type II) carcinoma exhibits HER2
amplification and TP53 and PPP2R1A mutations
(16), which were assessed in the
present study. Briefly, 10 ng genomic DNA extracted from FFPE or
LBC specimens was used to construct barcoded DNA libraries
utilizing an Ion Ampliseq Library kit Plus (cat. no. A38875; Thermo
Fisher Scientific, Inc.). The obtained libraries were purified
using AMPure XP Reagent (Beckman Coulter, Inc.) and sequenced using
an Ion Personal Genome Machine® or Ion
GeneStudio™ S5 platform (Thermo Fisher Scientific,
Inc.). Sequencing nucleotide is single end. Sequence kit names are
Ion PGM Hi-Q View Chef Kit (A30798) and Ion 510™ &
Ion 520™ & Ion 530™ Kit-Chef (A34461).
The loading concentration of the library is 25 pM. The sequencing
reads were aligned to the reference genome GRCh37 and converted
into binary alignment and map files using Torrent Suite 5.12.3
Software (Thermo Fisher Scientific, Inc.). Sequence variants were
called using Ion Reporter™ 5.12 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols. The
mean read depth of coverage in DNA sequencing was
>1,500-fold.
Classification of oncogenic
mutations
Somatic mutations were called based on all criteria
as follows: i) Variant allele frequency of somatic mutations in
tumor tissue >5%; ii) variant allele frequency of somatic
mutations in LBC >0.1% and iii) registration of mutations as
‘pathogenic/likely pathogenic variants’ in the ClinVar database
(https://www.ncbi.nlm.nih.gov/clinvar/).
Results
Background and clinical
characteristics
Patient background and clinical characteristics are
presented in Table I. The mean age
of patients was 58.1±8.92 years (range, 45–74 years) and the mean
body mass index (BMI) was 24.2±8.12 kg/m2 (range, 18–47
kg/m2). Obesity is a risk factor for uterine cancer; one
of the ten patients was considered to be obese (BMI ≥30) (3). The mean endometrial thickness was
13.6±8.92 mm (range, 4–22 mm). A total of seven of ten patients
were postmenopausal and six of ten patients presented with
endometrial thickness of ≥11 mm. The age of patients with
endometrial thickness ≥11 mm (56.70±8.80 years) and <11 mm
(60.25±8.67 years) were not significantly different. Cervical
cytology demonstrated adenocarcinoma in five of ten patients. Age
of patients in adenocarcinoma and negative for intraepithelial
lesion or malignancy (NILM) groups as assessed using cervical
cytology was not significantly.
 | Table I.Patient background and clinical
characteristics. |
Table I.
Patient background and clinical
characteristics.
| Case | Age, years | Number of births | BMI | Menopausal
status | Endometrium
thickness, mm | Cervical
cytology |
|---|
| 1 | 45 | 0 | 29 | Premenopausal | 21 | NILM |
| 2 | 50 | 0 | 21 | Premenopausal | 9 | NILM |
| 3 | 59 | 3 | 20 | Postmenopausal | 6 | Adenocarcinoma |
| 4 | 51 | 2 | 47 | Premenopausal | 20 | NILM |
| 5 | 58 | 2 | 24 | Postmenopausal | 4 | NILM |
| 6 | 71 | 2 | 20 | Postmenopausal | 13 | Adenocarcinoma |
| 7 | 52 | 2 | 18 | Postmenopausal | 11 | Adenocarcinoma |
| 8 | 65 | 0 | 20 | Postmenopausal | 21 | Adenocarcinoma |
| 9 | 56 | 1 | 22 | Postmenopausal | 22 | NILM |
| 10 | 74 | 2 | 21 | Postmenopausal | 9 | Adenocarcinoma |
Cytology and genetic analysis
The pathogenic variants detected in cancer tissue
sections and cervical and endometrial LBC specimens are presented
in Fig. 2. A total of eight of the
ten cases was assessed to be endometrioid and two were serous
carcinoma. Histopathological examination demonstrated no double
cervical cancer cases. In seven of ten cases, pathogenic variants
detected in cancer tissue sections were also detected in cervical
and/or endometrial LBC specimens. Variants detected in ≥2 types of
sample are presented in Table II.
Pathogenic variants were called in PTEN in four cases,
TP53, PIK3CA and fibroblast growth factor receptor 2
(FGFR2) in two cases each and APC regulator of WNT signaling
pathway (APC), KRAS and CTNNB1 in one case
each.
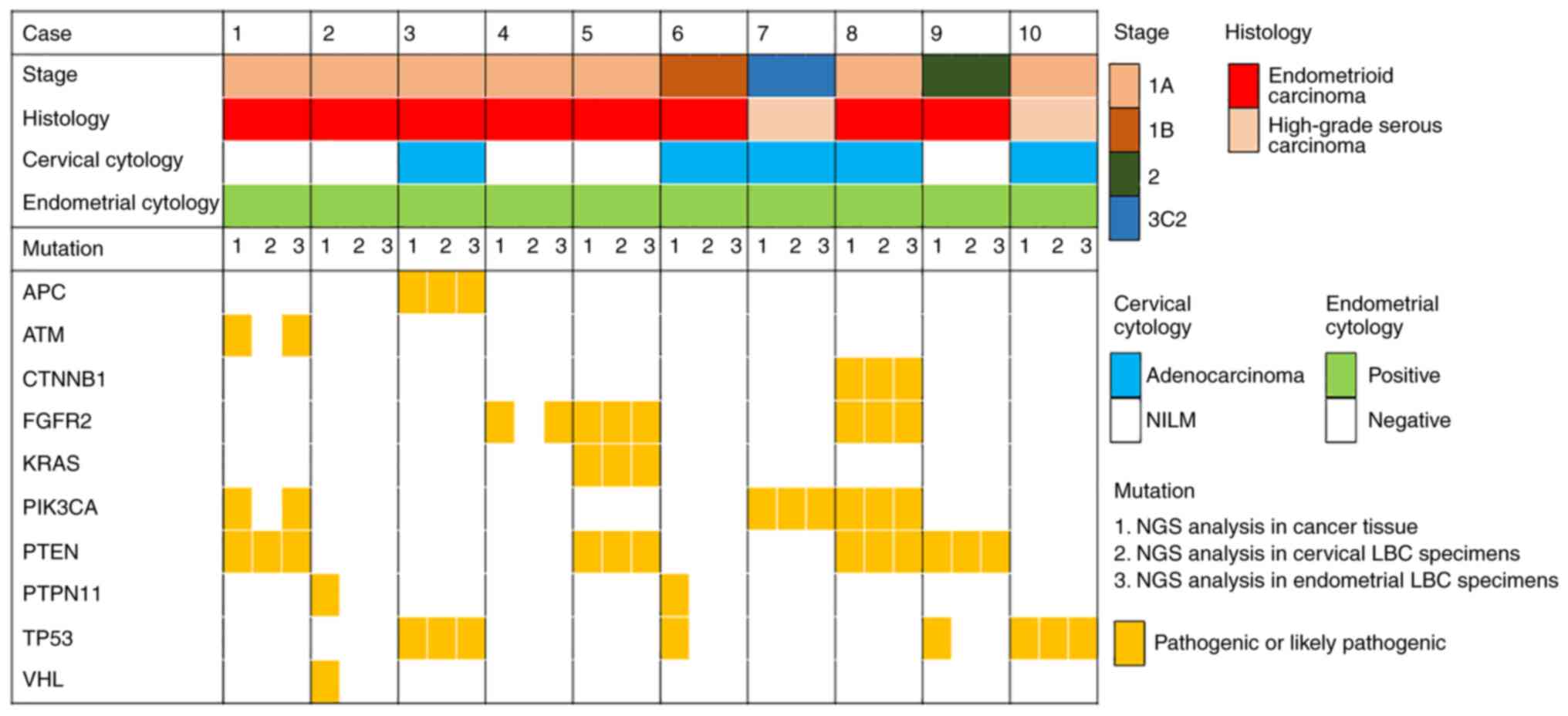 | Figure 2.Pathogenic variants detected in cancer
tissue and cervical and endometrial LBC specimens and association
with World Health Organization stage classification, histology,
cervical cytology and endometrial cytology. LBC, liquid-based
cytology; NILM, negative for intraepithelial lesion or malignancy;
NGS, next-generation sequencing; APC, APC regulator of WNT
signaling pathway; ATM, ATM serine/threonine kinase; CTNNB1,
catenin β1; FGFR2, fibroblast growth factor receptor 2; PIK3CA,
PI3K catalytic subunit α; PTPN11, protein tyrosine phosphatase
non-receptor type 11; TP53, tumor protein P53; VHL, Von
Hippel-Lindau tumor suppressor. |
 | Table II.Variants detected in ≥2 sample types
from cancer tissue sections and cervical and endometrial
liquid-based cytology specimens. |
Table II.
Variants detected in ≥2 sample types
from cancer tissue sections and cervical and endometrial
liquid-based cytology specimens.
| Gene | Variant | Classification | Case |
|---|
| PTEN | c.388C>G
(p.Arg130Gly) | Pathogenic | 1 |
|
| c.518G>A
(p.Arg173His) | Pathogenic | 5 |
|
| c.697C>T
(p.Arg233Ter) | Pathogenic | 5 |
|
| c.388C>G
(p.Arg130Gly) | Pathogenic | 8 |
|
| c.209+5G>A | Pathogenic | 9 |
| TP53 | c.818G>T
(p.Arg273Leu) | Pathogenic | 3 |
|
| c.818G>A
(p.Arg273His) | Pathogenic | 10 |
| PIK3CA | c.1357G>A
(p.Glu453Lys) | Likely
pathogenic | 7 |
|
| c.1053T>A
(p.Asn345Lys) | Likely
pathogenic | 8 |
| FGFR2 | c.758C>G
(p.Pro253Arg) | Pathogenic | 5 |
|
| c.755C>G
(p.Ser252Trp) | Pathogenic | 8 |
| APC | c.4671delT
(p.lle1557MetfsTer8) | Pathogenic | 3 |
| KRAS | c.175G>A
(p.Ala59Thr) | Pathogenic | 5 |
| CTNNB1 | c.110C>T
(p.Ser37Phe) | Pathogenic | 8 |
LBC specimens were obtained from the cervix and
endometrium before surgery in patients with pre-operatively
diagnosed endometrial cancer. A total of three of seven cases with
pathogenic variants detected in ≥2 types of sample were negative
for intraepithelial lesions (NILM) or malignancy using cervical
cytology (cervical and endometrial cytology is presented in
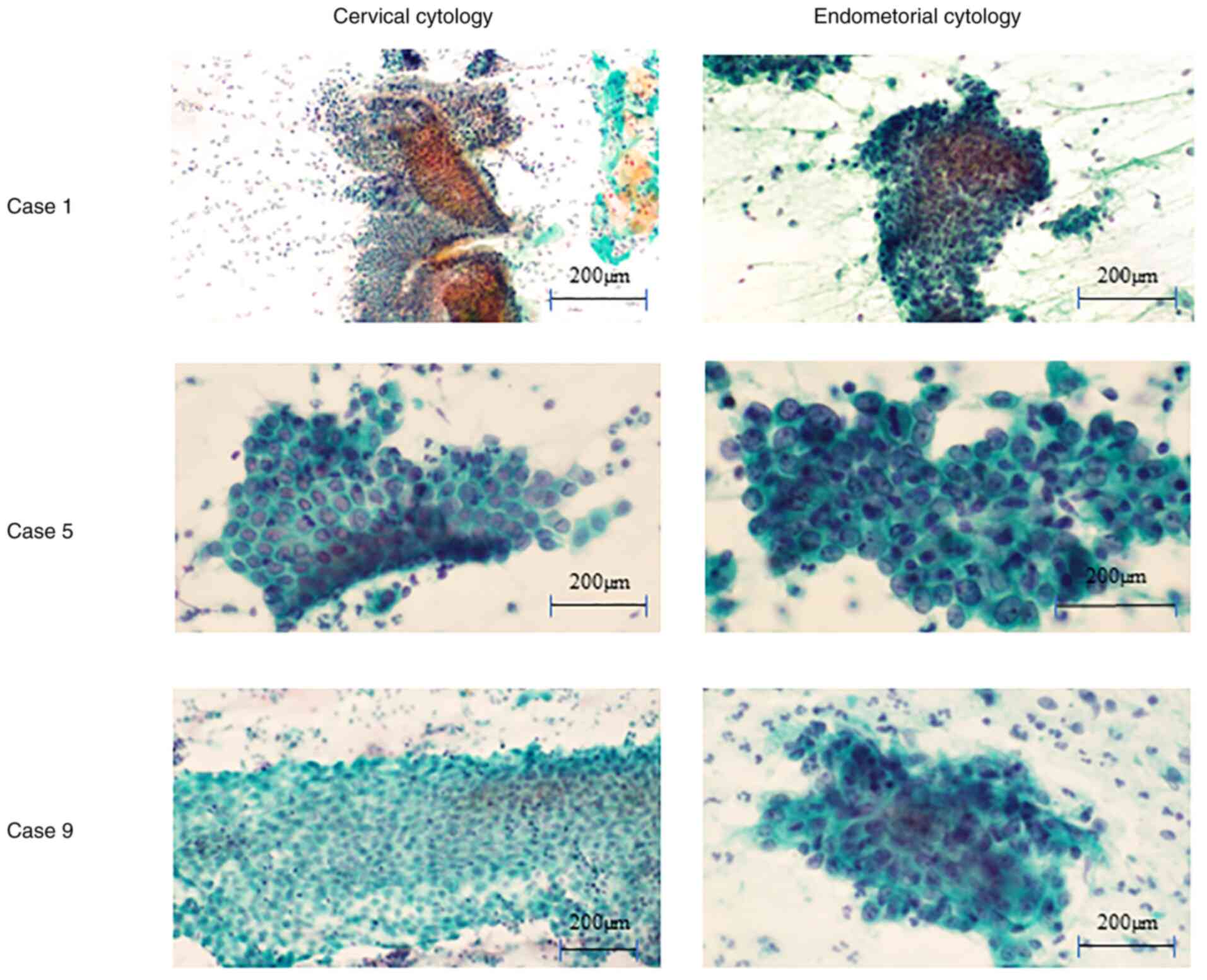
Fig. 3). Case 1 demonstrated no
endometrial cells using cervical cytology. Cases 5 and 9
demonstrated benign endometrial cells using cervical cytology. The
sheet patterns were accompanied by stromal cells and nuclear
overlapping was limited to <3 layers. All ten cases demonstrated
malignant endometrial cells using endometrial cytology. Notable
nuclear overlapping was observed in the cluster and nuclei
demonstrated enlarged and varied shapes. In eight of ten cases,
pathogenic variants detected in cancer tissue sections were also
detected in endometrial LBC specimens. Detected pathogenic variants
were PTEN in four cases, FGFR2 in three cases,
TP53 and PIK3CA in two cases each and APC,
KRAS and CTNNB1 in one case each.
Sensitivity of the combination of
tests
Endometrial thickening (>11 mm) on transvaginal
ultrasonography was present in 60% of cases and adenocarcinoma on
cervical cytology was present in 50% of cases. The concordance of
cervical LBC specimen and genetic analysis results was 70%.
Furthermore, combined cervical cytology and genetic analysis showed
a sensitivity of 80%, whereas combined endometrial thickening
assessed using transvaginal ultrasonography, cervical cytology and
genetic analysis had a sensitivity of 90% (Fig. 4).
The reproducibility of endometrial thickening
assessment using transvaginal ultrasonography, cervical cytology
and genetic analysis was analyzed using Cohen's κ coefficient
(Table III). All tests had a
reproducibility of ≤0.2, which indicated that each test may be used
independently to diagnose endometrial cancer. These tests may be
considered to be an effective combination.
 | Table III.Cohen's κ coefficient for endometrial
thickening using transvaginal ultrasonography, cervical cytology
and genetic analysis. |
Table III.
Cohen's κ coefficient for endometrial
thickening using transvaginal ultrasonography, cervical cytology
and genetic analysis.
| Test | κ coefficient | P-value |
|---|
| Transvaginal
ultrasonography-cervical cytology | 0.000 | 1.000 |
| Transvaginal
ultrasonography-genetic analysis | 0.097 | 0.778 |
| Cervical
cytology-genetic analysis | 0.200 | 0.490 |
Discussion
NCCN guidelines recommend endometrial biopsy for the
diagnosis of endometrial cancer, which has a sensitivity of 90% and
a false-negative rate of 10% (3).
In the present study, the combination of assessment of endometrial
thickening using transvaginal ultrasonography, cervical cytology
and genetic analysis demonstrated a sensitivity of 90%, which was
equivalent to that of endometrial biopsy. Furthermore, comparable
sensitivity to endometrial biopsy was achieved by combining these
three minimally invasive tests, indicating that this method may
alleviate the need for highly invasive endometrial biopsy. Methods
of testing for uterine cancer include blood sampling (tumor
marker), CT and MRI; however, it can be difficult to perform these
tests on the same day. However, it is not difficult to perform
transvaginal ultrasonography, cervical cytology and genetic
analysis in the same day. Matsuura et al (9) reported the use of endometrial
cytology to test for uterine cancer, however endometrial cytology
is not used worldwide. By contrast, cervical cytology is used
worldwide, has excellent versatility and is convenient (3). Certain pathogenic variants were not
detected compared with endometrial cytology, however most
pathogenic variants were identified by cervical cytology in the
present study. Furthermore, if TCGA categories can be determined
pre-operatively following early diagnosis using NGS analysis,
prognosis could be estimated before treatment and choice of
treatment strategy could be more effective, such as deciding
whether a more aggressive surgical approach is necessary, whether
referral to a high-volume center should be considered and how long
the patient can wait until starting treatment (7).
In three cases (cases 2, 4 and 6), pathological gene
mutations detected in cancer tissue sections were not be detected
in LBC specimens from the cervix. In case 6, the cervical cytology
was adenocarcinoma and endometrial cells with malignant findings
were demonstrated using cervical cytology, however no pathological
genetic mutations were detected. In case 2 and 4, the cervical
cytology was NILM and endometrial cells were not demonstrated using
cervical cytology.
There were certain limitations to the present study.
First, the sensitivity of cervical cytology for detecting uterine
cancer was only 45%. Furthermore, the present approach requires
multiple tests to be combined to achieve high sensitivity.
Therefore, future studies are required to increase sample size,
establish early screening methods for uterine cancer and plan
treatment strategies using NGS data before treatment. A further
limitation of the present study was that no healthy subjects were
assessed. It would have been desirable to assess the specificity
and false-positive and -negative rates by including normal cases or
to evaluate only Stage 1A cases. However, owing to lack of funding,
it was decided to conduct a balanced analysis of advanced stages
and histological types using these 10 cases to assess the
usefulness of the present method. In the present study, 30
endometrial cancer cases were diagnosed; 25 cases were stage I, one
was stage II and four were stage III. The histological types were
endometrial carcinoma in 26 cases and serous carcinoma in 4 cases.
A total of 10 cases were selected for inclusion in the present
study. Furthermore, the present study was performed to detect
specific somatic variants for each patient. The specificity was
assessed to be high. To the best of our knowledge, however, an
optimal endometroid cancer biomarker has not yet been widely
reported; therefore, further studies are needed, including a
broader panel and a panel that includes not only the genome but
also the epigenome.
In the present study, the combination of the
assessment of endometrial thickening using transvaginal
ultrasonography, cervical cytology and genetic analysis resulted in
a high sensitivity of 90% for the detection of endometrial cancer.
The three tests reported here are more expensive than conventional
diagnosis methods; however, delayed detection of uterine cancer
requires multidisciplinary treatment, which increases healthcare
costs. Increased spending on early detection of uterine cancer is
better economically and may improve patient quality of life.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the SAMD00511249-SAMD00511278
repository, ddbj.nig.ac.jp/search.
Authors' contributions
EK, RN and YO conceptualized the study. RN, EK, KY,
MKK, MNi, MI, MNa, HI, YO, KN and TI performed experiments and
collected data. EK, KY and HI analyzed data. MI and MNa performed
NGS. KN designed the study methodology. MNi and MKK performed study
administration. EK provided software. KN supervised the study. EK
and KY validated the results. TI produced visualization. RN and EK
wrote the first draft. EK and MI confirm the authenticity of all
the raw data. All authors reviewed and edited the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
review board of Mie University Hospital (approval no. H2020-075).
Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviation
Abbreviations:
|
LBC
|
liquid-based cytology
|
References
|
1
|
Bray F, Colombet M, Mery L, Piñeros M,
Znaor A, Zanetti R and Ferlay J: Cancer incidence in five
continents, Vol XI (electronic version). International Agency for
Research on Cancer Lyon; 2017, Available from:. https://ci5.iarc.fr/CI5-XI/Default.aspxAccessed
November 14. 2021
|
|
2
|
National Cancer Center Japan, Cancer
Statistics, endometrial cancer, . Available from. https://ganjoho.jp/reg_stat/statistics/stat/cancer/18_corpus_uteri.htmlApril
20–2022
|
|
3
|
National Comprehensive Cancer Network
[NCCN guidelines], . Cervical cancer version 1.2021, 2021.
Available from. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1426November
14–2021
|
|
4
|
Kosa SD, Ferguson SE, Panzarella T, Lau S,
Abitbol J, Samouëlian V, Giede C, Steed H, Renkosinski B, Gien LT
and Bernardini MQ: A prospective comparison of costs between
robotics, laparoscopy, and laparotomy in endometrial cancer among
women with class III obesity or higher. J Surg Oncol. 125:747–753.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Colombo N, Creutzberg C, Amant F, Bosse T,
González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza
MR, et al: ESMO-ESGO-ESTRO consensus conference on endometrial
cancer: Diagnosis, treatment and follow-up. Int J Gynecol Cancer.
26:2–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawaguchi R, Matsumoto K, Ishikawa T,
Ishitani K, Okagaki R, Ogawa M, Oki T, Ozawa N, Kawasaki K,
Kuwabara Y, et al: Guideline for gynecological practice in Japan:
Japan society of obstetrics and gynecology and Japan association of
obstetricians and gynecologists 2020 edition. J Obstet Gynaecol
Res. 47:5–25. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cancer Genome Atlas Research Network, .
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
WHO Classification of Tumors Editorial
Board, . Female genital tumors. WHO classification of tumours. 5th
edition. Vol 4. International Agency for Research on Cancer; pp.
252–255. 2020
|
|
9
|
Matsuura M, Yamaguchi K, Tamate M,
Satohisa S, Teramoto M, Iwasaki M, Sugita S, Hasegawa T, Koubo R,
Takane K, et al: Efficacy of liquid-based genetic diagnosis of
endometrial cancer. Cancer Sci. 109:4025–4032. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujiwara H, Takahashi Y, Takano M,
Miyamoto M, Nakamura K, Kaneta Y, Hanaoka T, Ohwada M, Sakamoto T,
Hirakawa T, et al: Evaluation of endometrial cytology:
Cytohistological correlations in 1,441 cancer patients. Oncology.
88:86–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamashima C, Aoki D, Miyagi E, Saito E,
Nakayama T, Sagawa M, Saito H and Sobue T; Japanese Research Group
for Development of Cervical Cancer Screening Guidelines, : The
Japanese guideline for cervical cancer screening. Jpn J Clin Oncol.
40:485–502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frias-Gomez J, Benavente Y, Ponce J,
Brunet J, Ibáñez R, Peremiquel-Trillas P, Baixeras N, Zanca A,
Piulats JM, Aytés Á, et al: Sensitivity of cervico-vaginal cytology
in endometrial carcinoma: A systematic review and meta-analysis.
Cancer Cytopathol. 128:792–802. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ab Mutalib NS, Syafruddin SE, Md Zain RR,
Mohd Dali AZ, Mohd Yunos RI, Saidin S, Jamal R and Mokhtar NM:
Molecular characterization of serous ovarian carcinoma using a
multigene next generation sequencing cancer panel approach. BMC Res
Notes. 7:8052014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watanabe T, Nanamiya H, Kojima M, Nomura
S, Furukawa S, Soeda S, Tanaka D, Isogai T, Imai JI, Watanabe S and
Fujimori K: Clinical implication of oncogenic somatic mutations in
early-stage cervical cancer with radical hysterectomy. Sci Rep.
10:187342020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watanabe T, Nanamiya H, Endo Y, Kojima M,
Nomura S, Furukawa S, Soeda S, Tamura H, Ryufuku M, Tanaka D, et
al: Identification and clinical significance of somatic oncogenic
mutations in epithelial ovarian cancer. J Ovarian Res. 14:1292021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murali R, Soslow RA and Weigelt B:
Classification of endometrial carcinoma: More than two types.
Lancet Oncol. 15:e268–e278. 2014. View Article : Google Scholar : PubMed/NCBI
|