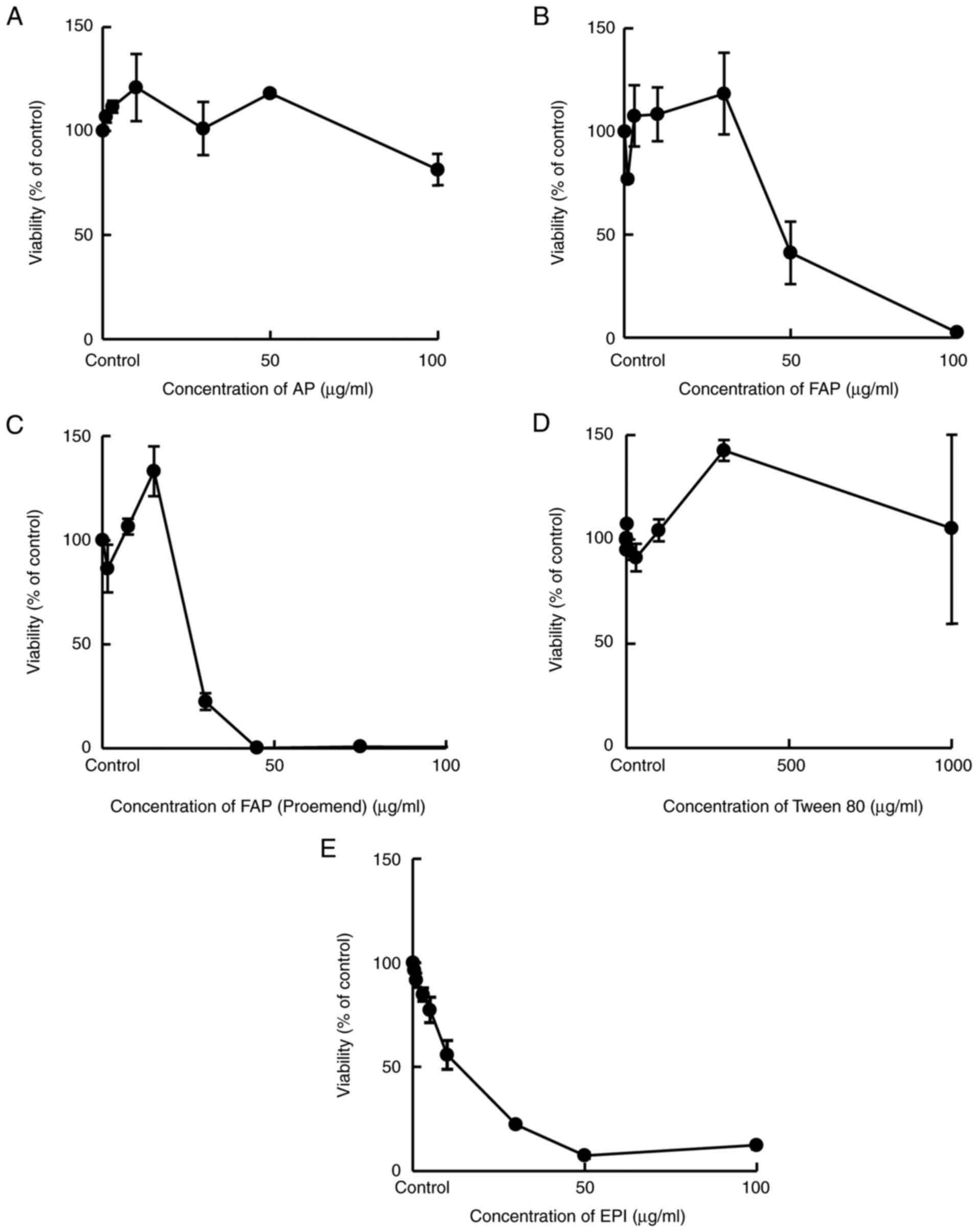
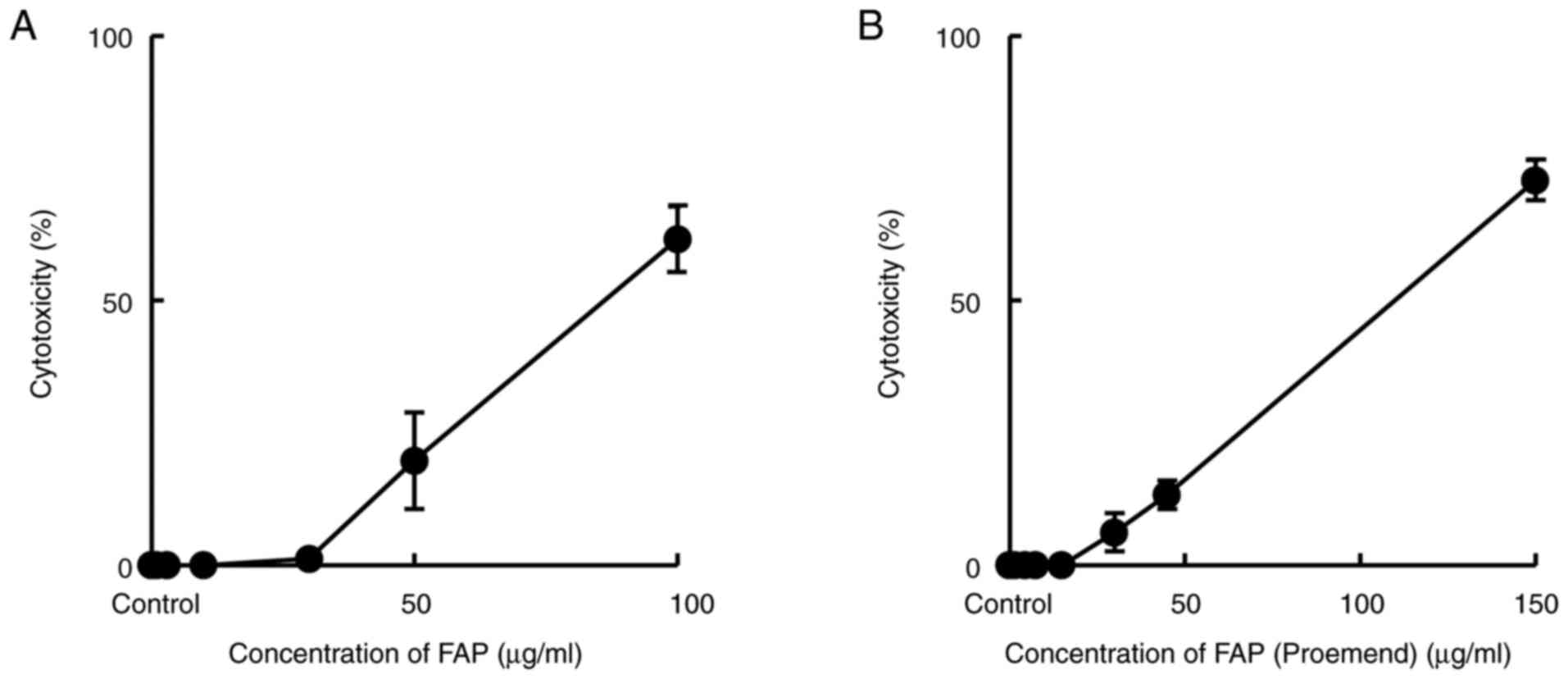
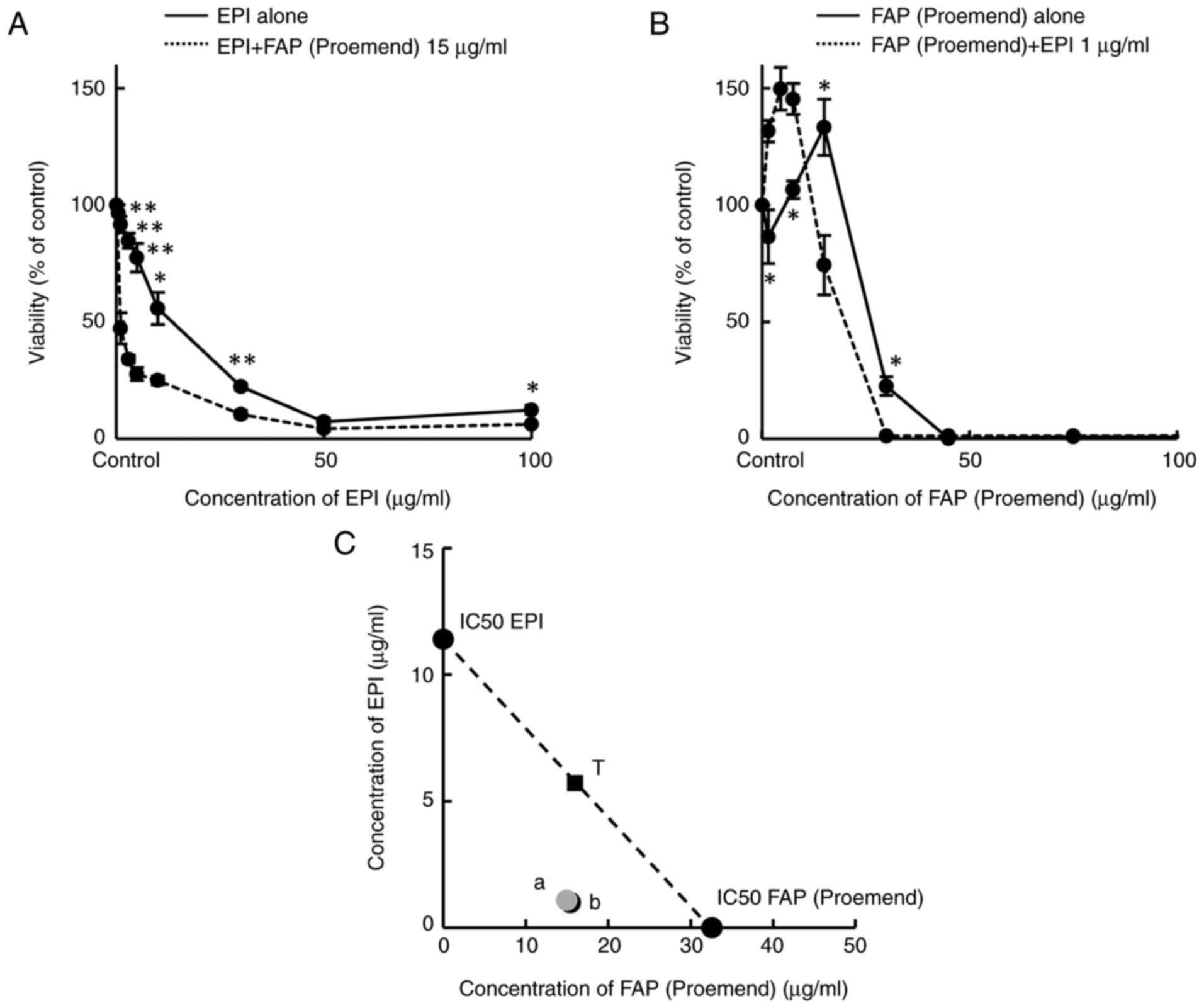
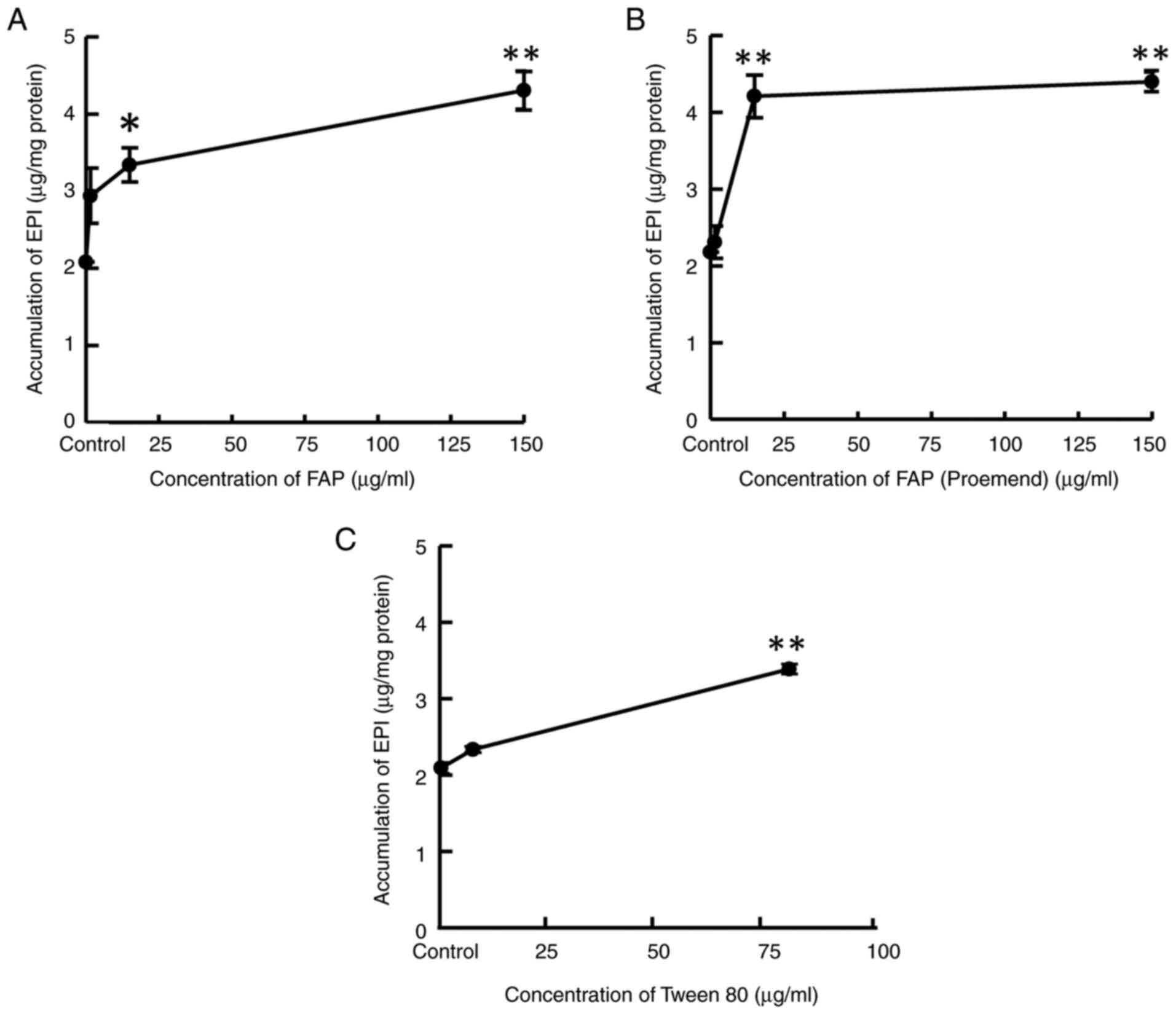
|
1
|
Pritchett W and Kinsley K: Benefits and
Risks of fosaprepitant in patients receiving emetogenic regimens.
Clin J Oncol Nurs. 20:555–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sato Y, Kondo M, Inagaki A, Komatsu H,
Okada C, Naruse K, Sahashi T, Kuroda J, Ogura H, Uegaki S, et al:
Highly frequent and enhanced injection site reaction induced by
peripheral venous injection of fosaprepitant in
anthracycline-treated patients. J Cancer. 5:390–397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujii T, Nishimura N, Urayama KY, Kanai H,
Ishimaru H, Kawano J, Takahashi O, Yamauchi H and Yamauchi T:
Differential impact of fosaprepitant on infusion site adverse
events between cisplatin- and anthracycline-based chemotherapy
regimens. Anticancer Res. 35:379–383. 2015.PubMed/NCBI
|
|
4
|
Hegerova LT, Leal AD, Grendahl DC, Seisler
DK, Sorgatz KM, Anderson KJ, Hilger CR and Loprinzi CL: An analysis
of fosaprepitant-induced venous toxicity in patients receiving
highly emetogenic chemotherapy. Support Care Cancer. 23:55–59.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boccia R, Geller RB, Clendeninn N and
Ottoboni T: Hypersensitivity and infusion-site adverse events with
intravenous fosaprepitant after anthracycline-containing
chemotherapy: A retrospective study. Future Oncol. 15:297–303.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lundberg JD, Crawford BS, Phillips G,
Berger MJ and Wesolowski R: Incidence of infusion-site reactions
associated with peripheral intravenous administration of
fosaprepitant. Support Care Cancer. 22:1461–1466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chau E, Lundberg J, Phillips G, Berger M
and Wesolowski R: Updated report on incidence of infusion-site
reactions associated with peripheral intravenous administration of
fosaprepitant. J Oncol Pharm Pract. 25:1053–1057. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ottoboni T, Keller MR, Cravets M,
Clendeninn N and Quart B: Bioequivalence of HTX-019 (aprepitant IV)
and fosaprepitant in healthy subjects: A phase I, open-label,
randomized, two-way crossover evaluation. Drug Des Devel Ther.
12:429–435. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ottoboni T, Lauw M, Keller MR, Cravets M,
Manhard K, Clendeninn N and Quart B: Safety of HTX-019 (intravenous
aprepitant) and fosaprepitant in healthy subjects. Future Oncol.
14:2849–2859. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamasaki M, Kimura R, Mayahara S, Maeda Y,
Takahashi M, Nishida T, Oda K and Murakami T: Study on the
infusion-site adverse events and vascular distribution of
epirubicin in chemotherapy with epirubicin and fosaprepitant. Mol
Clin Oncol. 11:43–49. 2019.PubMed/NCBI
|
|
11
|
Oda K, Umakoshi T, Mori N, Kasai R and
Murakami T: Biopharmaceutical properties of tubeimoside-1: A
cytotoxic amphipathic cyclic bisdesmoside. Int J Clin Pharmacol
Pharmacother. 2:1262017. View Article : Google Scholar
|
|
12
|
Varela-Castillo O, Cordero P,
Gutiérrez-Iglesias G, Palma I, Rubio-Gayosso I, Meaney E,
Ramirez-Sanchez I, Villarreal F, Ceballos G and Nájera N:
Characterization of the cytotoxic effects of the combination of
cisplatin and flavanol (−)-epicatechin on human lung cancer cell
line A549. An isobolographic approach. Exp Oncol. 40:19–23. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chuang SH and Reddy DS: Isobolographic
analysis of antiseizure activity of the GABA type A
receptor-modulating synthetic neurosteroids brexanolone and
ganaxolone with tiagabine and midazolam. J Pharmacol Exp Ther.
372:285–298. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teno N, Iguchi Y, Oda K, Yamashita Y,
Masuda A, Fujimori K, Une M and Gohda K: Discovery of orally active
and nonsteroidal farnesoid X receptor (FXR) antagonist with
propensity for accumulation and responsiveness in ileum. ACS Med
Chem Lett. 12:420–425. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Olver IN: Prevention of
chemotherapy-induced nausea and vomiting: Focus on fosaprepitant.
Ther Clin Risk Manag. 4:501–506. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fotakis G and Timbrell JA: In vitro
cytotoxicity assays: Comparison of LDH, neutral red, MTT and
protein assay in hepatoma cell lines following exposure to cadmium
chloride. Toxicol Lett. 160:171–17. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eccardt AM, Bell TP, Mattathil L, Prasad
R, Kelly SC and Fisher JS: Trans-plasma membrane electron transport
and ascorbate efflux by skeletal muscle. Antioxidants (Basel).
6:892017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scarcello E, Lambremont A, Vanbever R,
Jacques PJ and Lison D: Mind your assays: Misleading cytotoxicity
with the WST-1 assay in the presence of manganese. PLoS One.
15:e02316342020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Zou M, Piao H, Liu Y, Tang B, Gao
Y, Ma N and Cheng G: Characterization and pharmacokinetic study of
aprepitant solid dispersions with soluplus®. Molecules.
20:11345–11356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cosgun ZC, Fels B and Kusche-Vihrog K:
Nanomechanics of the endothelial glycocalyx: From structure to
function. Am J Pathol. 190:732–741. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurbanoglu S, Palabiyik BB, Gumustas M,
Şanlı S and Uslu B; Ozkan Ankara University SA, : Development and
validation of a stability-indicating RP-LC method for the
determination of anticancer drug epirubicin in pharmaceuticals. J
Liq Chromatogr Relat Technol. 37:1583–1596. 2014. View Article : Google Scholar
|
|
22
|
Yata N, Toyoda T, Murakami T, Nishiura A
and Higashi Y: Phosphatidylserine as a determinant for the tissue
distribution of weakly basic drugs in rats. Pharm Res. 7:1019–1025.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murakami T and Yumoto R: Role of
phosphatidylserine binding in tissue distribution of
amine-containing basic compounds. Expert Opin Drug Metab Toxicol.
7:353–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wotton K, Gassner LA and Ingham E:
Flushing an i.v. line: A simple but potentially costly procedure
for both patient and health unit. Contemp Nurse. 17:264–273. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flint A, McIntosh D and Davies MW:
Continuous infusion versus intermittent flushing to prevent loss of
function of peripheral intravenous catheters used for drug
administration in newborn infants. Cochrane Database Syst Rev.
CD0045932005.PubMed/NCBI
|
|
26
|
Perez A, Feuz I, Brotschi B and Bernet V:
Intermittent flushing improves cannula patency compared to
continuous infusion for peripherally inserted venous catheters in
newborns: Results from a prospective observational study. J Perinat
Med. 40:311–314. 2012.PubMed/NCBI
|
|
27
|
Kovacevich DS, Corrigan M, Ross VM,
McKeever L, Hall AM and Braunschweig C: American society for
parenteral and enteral nutrition guidelines for the selection and
care of central venous access devices for adult home parenteral
nutrition administration. JPEN J Parenter Enteral Nutr. 43:15–31.
2019. View Article : Google Scholar : PubMed/NCBI
|