Introduction
In chemotherapy for breast cancer patients, FEC or
EC regimen is widely used, and these regimens contain epirubicin
(EPI), an anthracycline-based antineoplastic drug, as follows: FEC
regimen consists of fluorouracil, EPI and cyclophosphamide, and EC
regimen consists of EPI and cyclophosphamide. Oral aprepitant (AP),
which is a neurokinin 1 (NK1) antagonist, is ingested once before
and twice after EPI treatment, once/day for 3 days, mostly for
inpatients, and intravenous Proemend® containing
fosaprepitant (FAP) meglumine, a phosphoryl prodrug for AP, and
Tween 80, a non-ionic surfactant, is administered intravenously
through an intravenous (IV) tube once by the constant-rate infusion
of more than 30 min just before the EPI treatment mostly for
outpatients (1). The combined use
of FAP and EPI, however, can cause infusion-site adverse events
such as primarily edema/swelling, erythema or dermatitis, in
addition to individual hypersensitivity systemic reactions
(2–5). The risk for the incidence of
infusion-site reactions with intravenous FAP before the
administration of chemotherapy drugs involves the following three
factors: age, location of IV line, and simultaneous maintenance IV
fluid rate of <100 ml/h (6).
The incidence of infusion-site reactions decreased to 5.74% from
28.7% when the intravenous FAP vial was diluted to FAP 150 mg/250
ml from FAP 150 mg/150 ml, and it was infused for more than 30 min
(7). Additionally, the use of
HTX-019 130 mg was reported as an alternative to FAP 150 mg.
HTX-019 130 mg is a polysorbate 80- and synthetic surfactant-free
AP injectable emulsion. It was reported that the number of
treatment-emergent adverse events was lower with HTX-019 130 mg
(30-min infusion) than with FAP 150 mg (20- or 30-min infusion)
(8,9). We also studied the method to avoid
adverse events induced by the combined use of intravenous FAP
followed by EPI based on the viewpoint of the perivascular tissue
distribution of EPI, because the infusion-site adverse events in
breast cancer chemotherapy with the FEC regimen combined with
Proemend® for I.V. Infusion containing FAP and Tween 80
were sometimes observed in the hospital (Chugoku Rosai Hospital)
(10). In rats, the administration
of FAP and EPI using the same IV tube exhibited significantly
higher perivascular tissue distribution of EPI compared to that
administered from different peripheral veins, and the higher EPI
distribution caused more severe infusion-site adverse events. Based
on these findings, we suggested that the infusion of FAP and EPI
from different peripheral veins (right and left) can avoid the
infusion-site adverse events greatly (10). In the present study, we further
studied the method to avoid infusion-site adverse events in the
chemotherapy with FEC or EC regimen combined with the intravenous
infusion of FAP by employing immortalized human umbilical vein
endothelial (HUEhT-1) cells.
Materials and methods
Materials
AP, FAP and EPI were obtained from Combi-Blocks (San
Diego, USA), Sigma-Aldrich Japan (Tokyo, Japan), and Toronto
Research Chemicals (Toronto, Canada), respectively. Separately,
Proemend I.V. Infusion containing FAP meglumine 243.3 mg
(corresponding to FAP 150 mg) and Tween 80 (polysorbate 80) 78.8 mg
was obtained from Ono Pharmaceutical Co., Ltd. (Osaka, Japan) and
used by diluting with culture medium appropriately. The diluted
Proemend solution containing FAP meglumine and Tween 80 was
described as FAP (Proemend) to distinguish it from FAP alone, and
the concentration of FAP (Proemend) shown in the figures refers to
the concentration of FAP within Proemend. Tween 80 was obtained
from MP Biochemicals, LLC (Santa Ana, USA). Regents used for cell
culture were obtained as follows: culture medium, Endothelial Cell
Growth Medium (EGM) from Takara Bio (Siga, Japan), fetal bovine
serum (FBS) from Moregate Biotech (Bulimba, Australia), and
Endothelial cell growth SupplementMix from Takara Bio (Siga,
Japan). Other chemicals such as acetic acid and acetonitrile used
for high-performance liquid chromatography (HPLC) analysis were of
the highest grade available.
Cell culture
HUEhT-1 cells, an immortalized human umbilical vein
endothelial cell line (HUVEC) with cell No. JCRB1458 established by
electroporation of pIRES-hTERT-hygr, between passages 6 and 20 were
obtained from JCRB Cell Bank, National Institute of Biomedical
Innovation, Health and Nutrition (Osaka, Japan). For cytotoxicity
experiments, HUEhT-1 cells were seeded at a density of
10×104 cells/100 µl/well in a 96-well plate (Corning
Japan KK, Tokyo, Japan). For the intracellular accumulation study
of EPI, HUEhT-1 cells were seeded at a density of 5×104
cells/well on 12 well collagen I coated plates (Corning Japan KK,
Tokyo, Japan). These cells were cultured for 72 h in EGM medium
supplemented with 10% fetal bovine serum and 2% EGM SupplementMix
under 5% CO2−95% air at 37°C according to the indication
by JCRB Cell Bank of cells as preincubation before experiments.
Cell culture experiments were repeated four times for each test
sample independently.
Cytotoxicity of AP, FAP, FAP
(Proemend), Tween 80, and EPI alone (evaluated by viability)
HUEhT-1 cells were incubated with a culture medium
containing either AP, FAP, FAP (Proemend), Tween 80, or EPI with
different concentrations (AP: 0, 1, 3, 10, 30, 50, 100 µg/ml; FAP:
0, 1, 3, 10, 30, 50, 100 µg/ml; FAP (Proemend): 0, 1.5, 7.5, 15,
30, 45, 75, 150 µg/ml; Tween 80: 0, 0.3, 1, 3, 10, 30, 100, 300,
1,000 µg/ml; EPI: 0, 0.5, 1, 3, 5, 10, 30, 50, 100 µg/ml) to
evaluate the cytotoxicity of each test compound. After 24
h-incubation at 37°C, the cell viability was estimated by WST-1
assay according to the manufacturer's protocol (Dojindo, Kumamoto,
Japan) in the same manner as reported previously (11). Briefly, each culture medium
containing the test compound was discarded and WST-1 reaction
mixture (100 µl) containing WST-1
[2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt, a cell proliferation reagent] and 1-methoxy
phenazinium methylsulfate was added according to the manufacture's
protocol (Dojindo, Kumamoto, Japan). After 60 min-incubation, the
ultraviolet (UV) absorbance at 450 nm was measured with a
microplate reader (SpectraMax Plus 384, Molecular Devices Japan).
The UV absorbance of samples that were cultured with a culture
medium alone was regarded as a control (100%).
Cytotoxicity of FAP and FAP (Proemend)
(evaluated by LDH leakage)
HUEhT-1 cells were incubated for 24 h with a culture
medium containing FAP or FAP (Proemend) at different concentrations
at follows: FAP; 0, 1, 3, 10, 30, 50, 100 µg/ml; FAP (Proemend); 0,
1.5, 4.5, 7.5, 15, 30, 45, 150 µg/ml. After 24 h-incubation, the
concentrations of lactate dehydrogenase (LDH) leaked from the cells
into the incubation medium were determined using LDH Cytotoxicity
Assay Kit (Nacalai Tesque, Kyoto, Japan). Separately, cells
incubated with a culture medium alone were mixed with Triton-X
(final concentration, 1%) to estimate the maximal leakage of LDH
(100%).
Cytotoxicity of combined use of FAP
and EPI (evaluated by viability)
HUEhT-1 cells were incubated for 24 h with a culture
medium containing EPI alone at different concentrations (0, 0.5, 1,
3, 5, 10, 30, 50, 100 µg/ml) or a combination of EPI at different
concentrations and FAP (Proemend) 15 µg/ml (a non-cytotoxic
concentration). Similarly, HUEhT-1 cells were incubated for 24 h
with a culture medium containing FAP (Proemend) alone at different
concentrations (0, 1.5, 4.5, 7.5, 15, 30, 45, 75, 150 µg/ml) or a
combination of FAP (Proemend) at different concentrations and EPI
1.0 µg/ml (a non-cytotoxic concentration). Cell viability was
estimated after 24 h-incubation by WST-1 assay. In this study, the
values of half maximal inhibitory concentration (IC50) of each
compound (EPI, FAP (Proemend) and combinations of EPI and FAP
(Proemend) for the viability of HUEhT-1 cell were estimated to
evaluate the synergic cytotoxicity of EPI and FAP (Proemend) in a
quantitative manner. Estimation of IC50 values for isobolographic
analysis was made by using concentration-viability curves and
ImageJ, a Java program inspired by NIH Image that runs on Windows
<https://imagej.nih.gov/ij/> in the same manner as reported
previously (12–14).
Effect of FAP, FAP (Proemend) and
Tween 80 on the intracellular accumulation of EPI
HUEhT-1 cells precultured on 12 well collagen I
coated plates were washed with 1 ml of isotonic, pH 7.4
phosphate-buffered saline (PBS) twice and incubated with PBS for 10
min at 37°C (preincubation) to remove the culture medium. Then, to
evaluate the effects of FAP, FAP (Proemend) and Tween 80 on the
intracellular accumulation of EPI, cells were incubated for 10 min
at 37°C with PBS containing a mixture of EPI 20 µg/ml and FAP (0,
1.5, 15 or 150 µg/ml), a mixture of EPI 20 µg/ml and FAP (Proemend)
(0, 1.5, 15 or 150 µg/ml), or a mixture of EPI 20 µg/ml and Tween
80 at a concentration of 0, 7.88, or 78.8 µg/ml, respectively.
Solutions of FAP (Proemend) (1.5, 15 or 150 µg/ml) contain 0.788,
7.88, or 78.8 µg/ml of Tween 80, respectively. After 10-min
incubation of cells with a medium containing the above compound(s),
the culture medium was discarded, cells were washed 3 times with
1-ml PBS and were dissolved with PBS containing 0.1% Triton-X. The
concentrations of EPI and protein in cell lysates were determined
fluorometrically at 458 nm for excitation and 538 nm for emission
and photometrically at 562 nm using the TaKaRa BCA protein assay
kit (Takara Bio, Siga, Japan), respectively.
Effect of cell washing on cytotoxicity
of combined use of FAP and EPI (evaluated by viability)
The effect of cell washing on the cytotoxicity of
FAP (Proemend) alone at different concentrations (0, 1.5, 7.5, 15,
30, 45, 75, 150 µg/ml) was evaluated. HUEhT-1 cells were incubated
with a culture medium containing FAP at different concentrations
for 30 min, the medium was discarded, and then cells were incubated
with a fresh culture medium alone for 24 h (cytotoxicity of FAP
(Proemend), without washing). For comparison, HUEhT-1 cells were
incubated with a culture medium containing FAP (Proemend) at
different concentrations (0, 1.5, 7.5, 15, 30, 45, 75, 150 µg/ml)
for 30 min, the medium was discarded, the cell surface was washed
with culture medium (100 µl each), and then incubated for 24 h with
fresh culture medium (cytotoxicity of FAP (Proemend), with
washing). Separately, the effect of cell washing on the
cytotoxicity of a combination of FAP (Proemend) at different
concentrations (0, 1.5, 7.5, 15, 30, 45, 75, 150 µg/ml) and EPI 1.0
µg/ml was evaluated. After HUEhT-1 cells were incubated with FAP
(Proemend) at different concentrations (0, 1.5, 7.5, 15, 30, 45,
75, 150 µg/ml) for 30 min, the culture medium was discarded. Then,
cells were cultured with fresh medium containing EPI 1.0 µg/ml (a
non-cytotoxic concentration) for 24 h (cytotoxicity of combined
use, without washing). For comparison, the cell surface was washed
with a culture medium (100 µl each) after 30-min incubation of
cells with FAP (Proemend) at different concentrations (0, 1.5, 7.5,
15, 30, 45, 75, 150 µg/ml) and then incubated for 24 h with a
culture medium containing EPI 1.0 µg/ml (cytotoxicity of combined
use, with washing). Cell viability was estimated after 24
h-incubation by WST-1 assay.
Analysis of EPI in cells
The concentrations of EPI accumulated in HUEhT-1
cells after incubation were determined in the same manner as
reported previously by high performance liquid chromatography
(HPLC) (10). Briefly, the HPLC
column used was a YMC-Triart C18 column (YMC Inc., Kyoto, Japan).
The mobile phase was a mixture of 1% acetic acid and acetonitrile
in a ratio of 7:3 (v/v%), and the flow rate was set at 1.0 ml/min.
EPI was detected fluorometrically at an excitation wavelength of
470 nm and an emission wavelength of 585 nm, respectively.
Statistical analysis
The data were presented as the mean ± SE
(experiments of cell culture were repeated with four independent
repetitions), and statistical analysis was performed by one-way
ANOVA, followed by the Tukey-Kramer method for multiple
comparisons. The level of significance was set at P<0.05.
Results
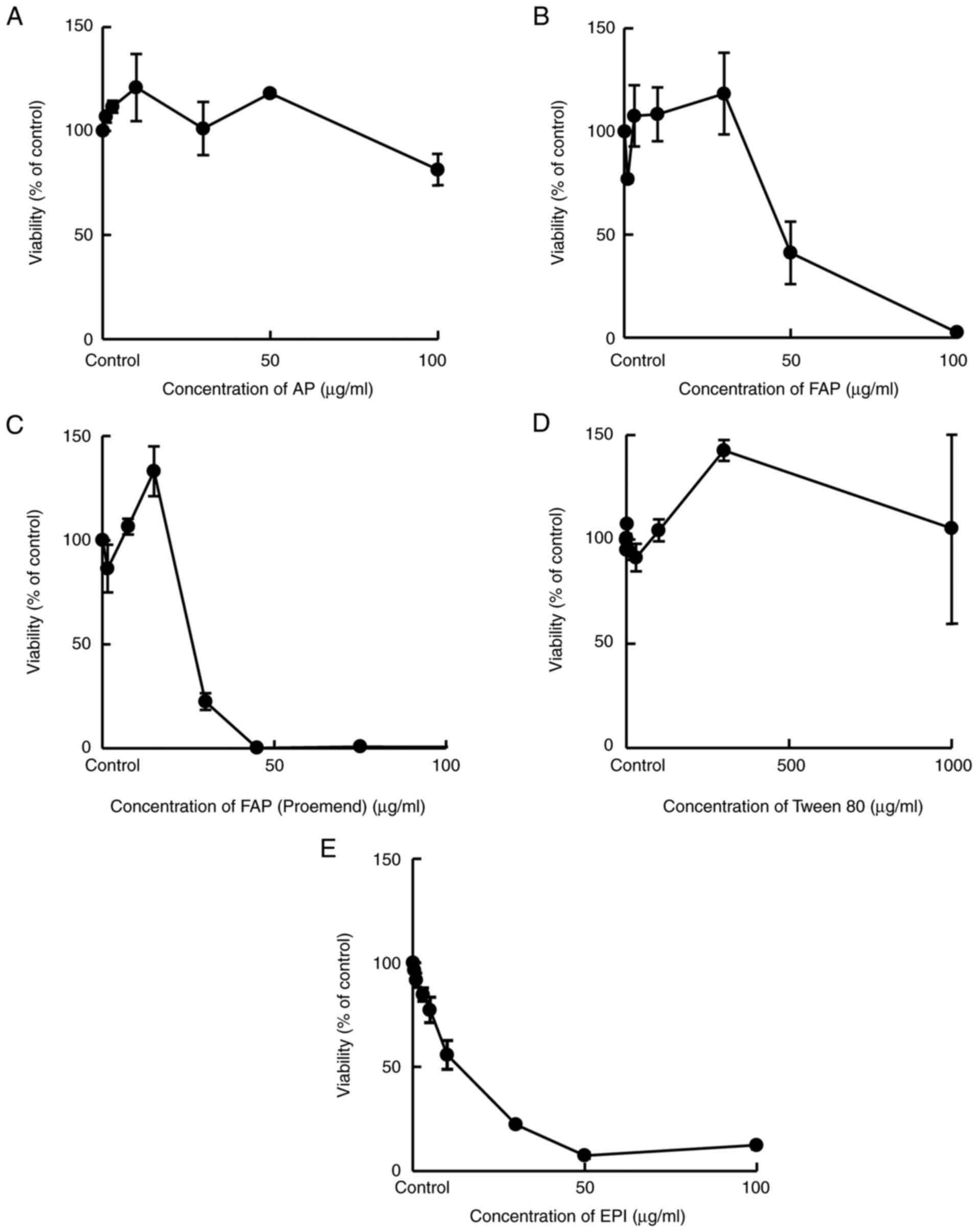
Cytotoxicity of AP, FAP, FAP
(Proemend), Tween 80 and EPI alone on HUEhT-1 cells
(viability)
HUEhT-1 cells were incubated for 24 h with a medium
containing AP, FAP, FAP (Proemend), Tween 80, or EPI at different
concentrations and the viability of treated cells was evaluated by
WST-1 assay. AP and Tween 80 alone exerted no cytotoxicity in a
concentration range from 0 to 50 µg/ml and from 0 to 1,000 µg/ml,
respectively. In contrast, FAP, FAP (Proemend) and EPI exhibited
cytotoxicity in a concentration-dependent manner (decrease in
viability) at more than 30, 15 and 1.0 µg/ml, respectively,
indicating the potency of cytotoxicity was in the following order:
EPI > FAP (Proemend) > FAP (Fig.
1).
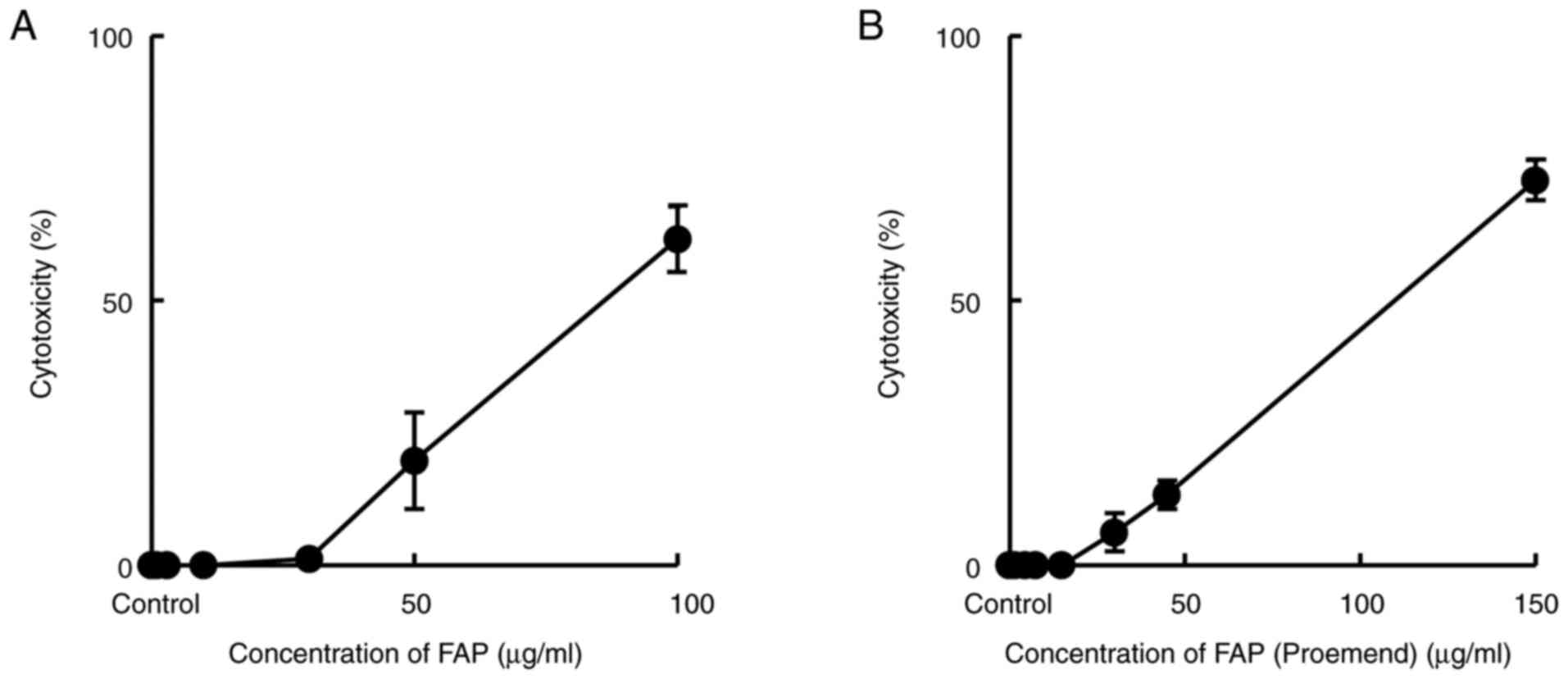
Cytotoxicity of FAP and FAP (Proemend)
each on HUEhT-1 cells (LDH leakage)
HUEhT-1 cells were incubated for 24 h with either
FAP or FAP (Proemend) at different concentrations, and their
cytotoxic potencies were evaluated by measuring LDH leakage from
cells. The leakage of LDH was induced at more than 30 and 15 µg/ml
of FAP and FAP (Proemend), respectively. No significant difference
was observed in the potency of cytotoxicity between FAP and FAP
(Proemend) when evaluated at a concentration of 50 µg/ml of FAP
(Fig. 2).
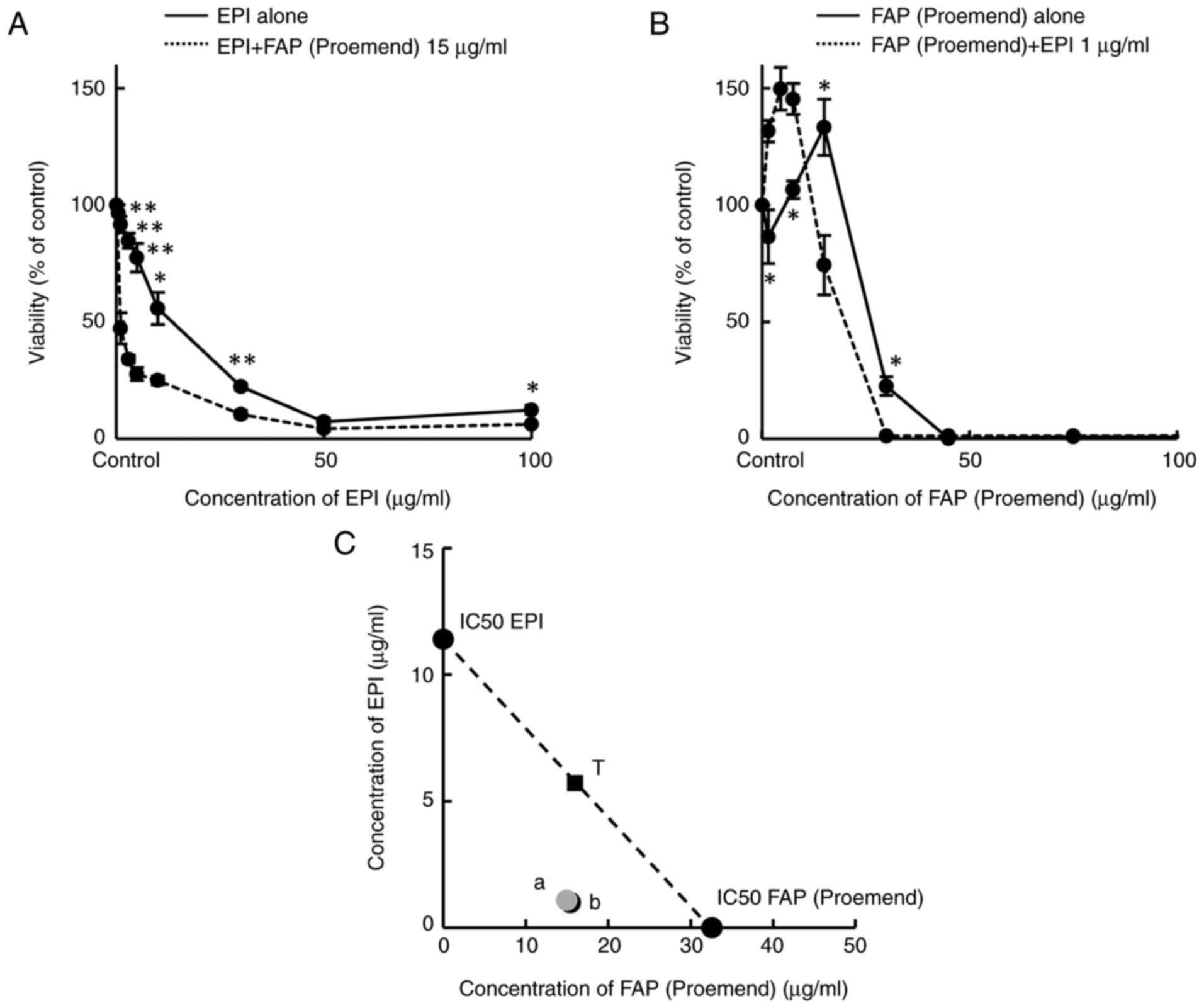
Cytotoxicity of combined use of FAP
(Proemend) and EPI
In HUEhT-1 cells, the co-existence of FAP (Proemend)
at a concentration of 15 µg/ml significantly increased the
cytotoxicity of EPI (Fig. 3A),
although FAP (Proemend) alone at a concentration of 15 µg/ml showed
no cytotoxicity (Fig. 1C).
Similarly, the co-existence of a non-toxic concentration of EPI (1
µg/ml) significantly increased the cytotoxicity of FAP (Proemend)
(Fig. 3B). These results indicate
the synergistic cytotoxicity of FAP (Proemend) and EPI. Using these
concentration-viability curves, values of IC50 were estimated for
isobolographic analysis (Fig. 3C).
Estimated IC50 values were 11.4 µg/ml for EPI alone, 0.96 µg/ml for
a combination of EPI and FAP (Proemend), 32.6 µg/ml for FAP
(Proemend) alone, and 15.4 µg/ml for a combination of FAP
(Proemend) and EPI. These results indicate that the cytotoxicity of
EPI can be greatly increased by the presence of FAP (Proemend) even
at a non-toxic concentration, although the increase in the
cytotoxicity of FAP (Proemend) is small even in the co-presence of
a non-toxic concentration of EPI.
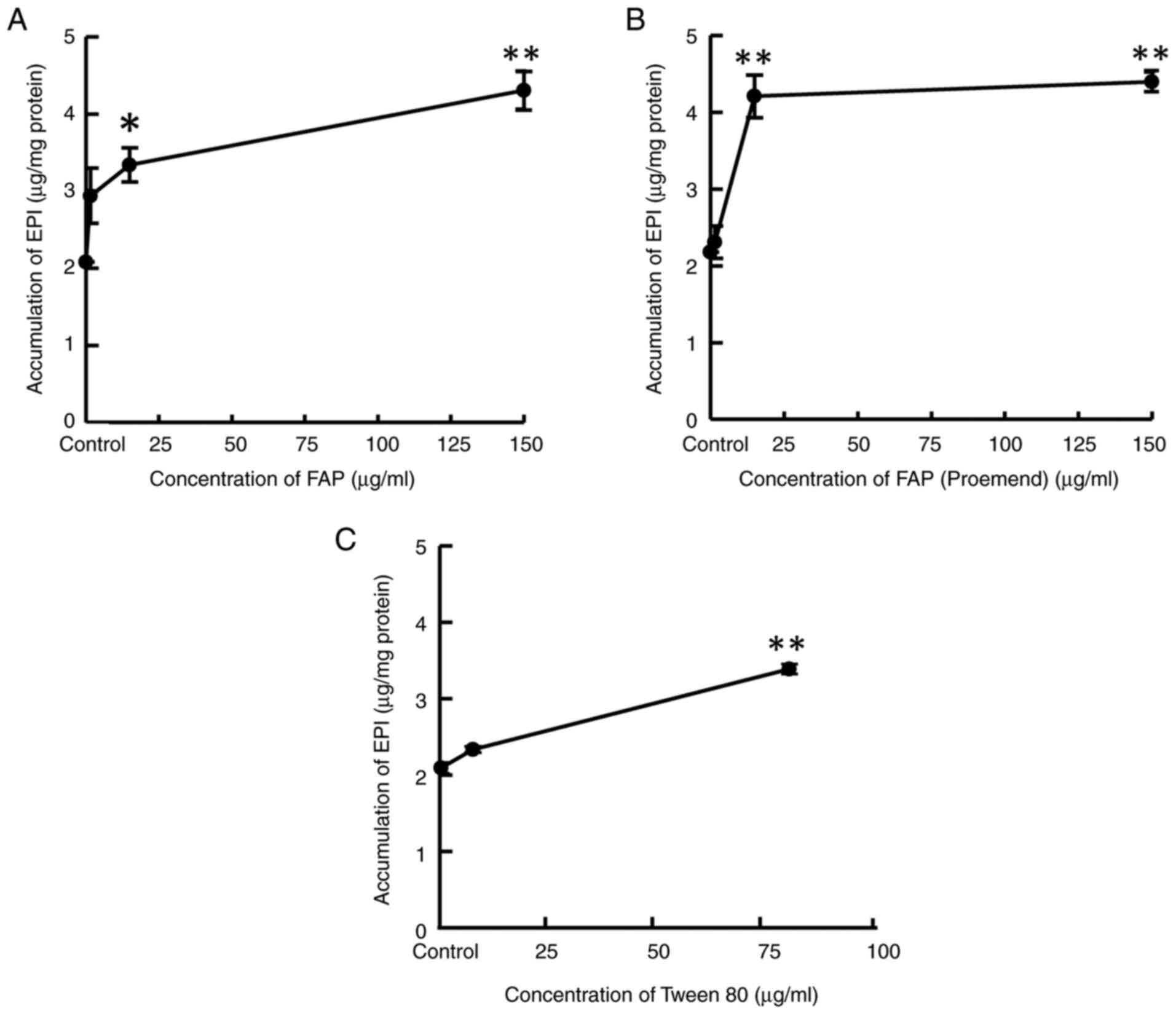
Effect of FAP, FAP (Proemend) and
Tween 80 on the intracellular accumulation of EPI
HUEhT-1 cells were incubated for 10 min with a
culture medium containing EPI 20 µg/ml and either FAP, FAP
(Proemend) or Tween 80 at different concentrations to evaluate the
effect of each compound on the intracellular accumulation of EPI.
FAP and FAP (Proemend) at concentrations of more than 15 µg/ml and
Tween 80 at a concentration of 78.8 µg/ml increased the
intracellular EPI concentration significantly (Fig. 4A-C).
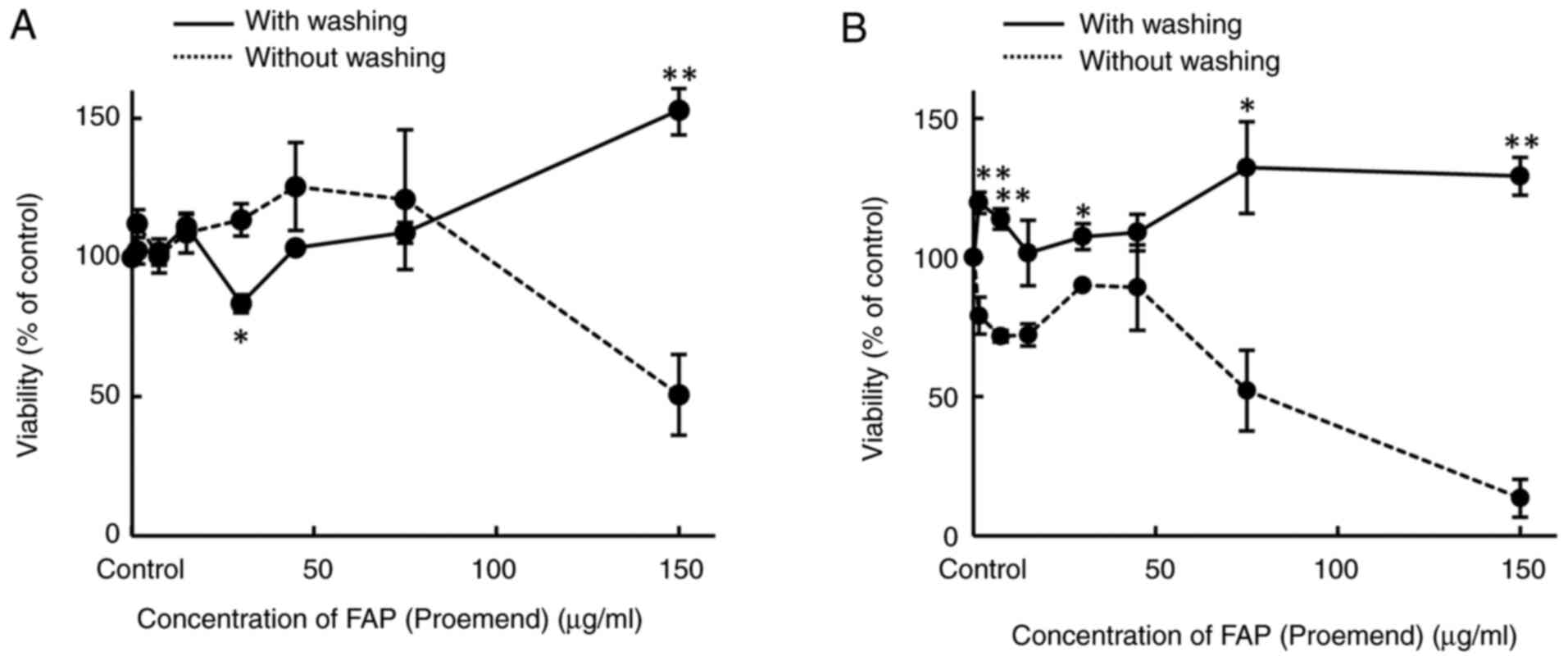
Effect of cell surface washing on
cytotoxicity of combined use of EPI and FAP (Proemend)
(viability)
The effect of cell surface washing on the
cytotoxicity of FAP (Proemend) alone or a combination of FAP
(Proemend) and EPI 1 µg/ml was examined in HUEhT-1 cells. The
cytotoxicity of FAP (Proemend) observed at a concentration of 150
µg/ml was eliminated by washing the cell surface with a fresh
culture medium after 30-min incubation of HUEhT-1 cells with FAP
(Proemend) (Fig. 5A). The
viability of washed cells that were incubated with 150 µg/ml FAP
(Proemend) rather increased by about 1.5-fold of control (100%)
(Fig. 5A). In addition, the
washing of the cell surface after incubation of cells with FAP
(Proemend) at different concentrations eliminated completely the
subsequently evoked synergic cytotoxicity of FAP (Proemend) and EPI
(Fig. 5B).
Discussion
FEC and EC regimens in the chemotherapy for breast
cancer patients contain EPI, an anthracycline drug with a high
emetic risk, and the use of oral AP, intravenous FAP,
dexamethasone, or olanzapine, an atypical antipsychotic is
recommended (15). The combined
use of intravenous infusion of FAP and EPI that is frequently used
for outpatients, however, can cause infusion-site adverse events
compared with the combination of oral AP and EPI or intravenous FAP
and cisplatin (1,15). We also observed the induction of
infusion-site adverse events in breast cancer chemotherapy with the
FEC regimen with intravenous FAP and studied the mechanism of
adverse events based on the viewpoint of the perivascular tissue
distribution of EPI by comparing three different treatments groups
using rats (10). In the FAP-S
group, FAP and EPI were infused into the jugular vein using the
same IV tube. In the FAP-D group, FAP and EPI were infused into
different jugular veins using two IV tubes (right and left jugular
vein), respectively, and in the AP group, AP was administered
orally, and EPI was infused into the jugular vein using an IV tube.
The concentrations of EPI in plasma and perivascular tissue were
compared among the FAP-S, FAP-D, and AP groups at 30 min and 24 h
after the 5-min constant-rate infusion of EPI. There was no
significant difference in the plasma EPI concentrations among the
three groups. However, concentrations of EPI in perivascular
tissues of infusion-site at 30 min and 24 h after EPI infusion were
scattered greatly as follows: FAP-S group, the mean concentration
was 2.30 µg/g at 30 min and 3.86 µg/g at 24 h; FAP-D group, 0.96
and 0.76 µg/g; AP group, 0.66 and 0.28 µg/g, respectively. The
magnitude of histological damage at infusion-site adverse events
was in the following order: EPI-infusion-site of the FAP-S group
>> EPI-infusion-site of the FAP-D group >>
EPI-infusion-site of the AP group and FAP-infusion-site of the
FAP-D group (no damage). These results suggested that EPI has more
potent cytotoxicity than FAP, and the co-existence of FAP at a
higher concentration increased perivascular tissue concentrations
of EPI infused thereafter and caused severe infusion-site adverse
events, indicating the synergic cytotoxicity between FAP and EPI.
Based on these results, we previously suggested that the infusion
of FAP and EPI from different peripheral veins (right and left) can
reduce the infusion-site adverse events greatly (10).
In the present study, the possible synergistic
cytotoxicity of FAP and EPI and avoiding method of infusion-side
adverse events were further studied employing HUEhT-1 cells. As
shown in Fig. 1, the incubation of
cells with FAP, FAP (Proemend) and EPI alone showed cytotoxicity of
HUEhT-1 cells in a concentration-dependent manner, and the potency
of cytotoxicity was in the following order: EPI > FAP (Proemend)
> FAP when the cytotoxicity was evaluated by viability with
WST-1 assay. These results suggested that Tween 80 contained in
Proemend IV Infusion can increase the cytotoxicity of FAP, although
Tween 80 alone showed no cytotoxicity in a concentration range from
0 to 1.0 mg/ml (Fig. 1). In
contrast, the greater cytotoxicity of FAP (Proemend) compared to
FAP was not clearly detected when evaluated by the LDH leakage
assay, although FAP (Proemend) induced LDH leakage at a lower
concentration compared to FAP (Fig.
2). It was reported that the assay with the neutral red and the
MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium
bromide] was the most sensitive in detecting cytotoxic events
compared to the LDH leakage and the protein assays when the
cytotoxicity of hepatoma cell lines following exposure to cadmium
chloride was detected (16). It
was also reported that the WST-1 reagent presents several
advantages compared to the two other tetrazolium salt-based cell
proliferation reagents, MTT and XTT
[2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide],
including water-solubility, rapidity, greater stability and
sensitivity (17,18). Thus, in the present study, the
WST-1 assay was mainly used to evaluate the viability, or
cytotoxicity, of each test compound. In these studies, however, a
low concentration of FAP and/or FAP (Proemend) appeared to increase
cell viability as shown in Figs.
1C and 3B. Similar phenomena
were also observed in cells that were incubated with FAP (Proemend)
150 µg/ml for 30-min and then the cell surface was washed (Fig. 5A and B). In addition, large
variability in cell viability was observed at a concentration of
Tween 80 1 mg/ml in the medium (Fig.
1D). As shown in Fig. 4C,
Tween 80 can increase the EPI distribution into HUEhT-1 cells and
would cause cytotoxicity more or less. It may be considered that a
slight cytotoxic effect rather stimulates the viability of cells.
Further study is necessary to clarify the mechanism of such cell
stimulation.
The effect of the combined use of FAP and EPI on
cytotoxicity was examined, in which the presence of a nontoxic
concentration of FAP (15 µg/ml) and EPI (1.0 µg/ml) significantly
increased the cytotoxicity of EPI and FAP (Proemend), respectively
(Fig. 3A and B). The synergic
cytotoxicity of EPI and FAP (Proemend) was clearly detected by
isobolographic analysis, in which the co-presence of nontoxic
concentration of FAP (Proemend) greatly increased the cytotoxicity
of EPI as evaluated by IC50 values (Fig. 3C), and the co-presence of either
FAP or FAP (Proemend) was found to significantly increase the
intracellular accumulation of EPI, in which Tween 80 alone also
increases the EPI cell distribution depending on the concentration
(Fig. 4). To avoid such synergic
cytotoxicity of FAP (Proemend) and EPI, the effect of cell-surface
washing after application of FAP (Proemend) on the synergic
cytotoxicity was examined (Fig.
5). The washing of cell surface with culture medium after
incubation with FAP (Proemend) eliminated the cytotoxicity caused
by FAP (Proemend) alone and synergic cytotoxicity of FAP (Proemend)
and EPI almost completely (Fig.
5).
These findings obtained in in-vitro HUEhT-1
cell studies imply the efficacy of washing the infusion in avoiding
the infusion-site adverse events in chemotherapy using FEC or EC
regimen and Proemend IV Infusion. Detailed preclinical animal
studies are necessary to examine the efficacy of infusion-site
washing in avoiding infusion-site adverse events in chemotherapy
with EPI and intravenous FAP, including the effect of the timing of
infusion-site washing and the quantity of saline for infusion-site
washing. Regarding the timing of infusion-site washing, it may be
considered as follows: FAP meglumine, a negatively charged
phosphoryl prodrug for AP (19),
and Tween 80 (polysorbate 80) with HLB 15.0 are both water-soluble
compounds, and the adsorption on the cell surface or on the
vascular endothelial cells would not be strong, because the surface
layer of endothelial cells is covered with a negatively charged,
brush-like glycocalyx (20). In
contrast, the adsorption to the cell surface, or charged
interaction, and the intracellular accumulation of weakly basic
drugs, AP with a pKa value of 9.7 (19) and EPI with a pKa value of around
8.5 (21), are considered to be
strong, because many weakly basic lipophilic drugs with a pKa value
of more than 6.5 bind to acidic phospholipids, especially
phosphatidylserine, in the cellular membrane (22,23).
In addition, the cytotoxicity of EPI was greatly increased in the
presence of FAP (Proemend) even at a nontoxic concentration,
compared to the combination of FAP (Proemend) and nontoxic
concentration of EPI (Fig. 3C).
Taken together, in in-vivo preclinical studies, it will be
important to administer Proemend at first and wash the vascular
infusion-site via IV tube with an efficient amount of saline
immediately after the IV infusion of Proemend, and thereafter
administer EPI by infusion to avoid infusion-site adverse events in
pharmacotherapy with EPI and Proemend. Regarding the washing of IV
tube, the flushing of IV tube post administration of medications or
between medications in IV administrations of multiple agents is
recommended to prevent medicine loss (or flushing of residual
medication from the line), to improve cannula patency (prevention
of loss of function of peripheral intravenous catheters), or to
prevent incompatibility issues between medication (24–27).
However, to eliminate the adsorbed FAP and Tween 80 on the
infusion-site vascular tissue almost completely, a greater volume
of saline than the volume of IV tube flushing, for example, the
same volume used for Proemend infusion (100–150 ml), will be
necessary. Taking these considerations into account, detailed
preclinical animal studies are needed to clarify the interaction
mechanism between FAP and EPI and evaluate the efficacy of
infusion-site washing in avoiding infusion-site adverse events.
In conclusion, the washing of the cell surface with
culture medium after incubation with FAP (Proemend) was found to
greatly decrease the synergic cytotoxicity of FAP, EPI and Tween 80
in HUEhT-1 cells. Based on our previous (10) and present studies, we would like to
suggest that washing the infusion site after the application of
Proemend with saline through an IV tube, or infusion of Proemend
and EPI from different peripheral veins (right and left) may avoid
or reduce the infusion-site adverse events.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MY, KOd, KOm, YM and TM designed the study, and KOm
approved the study. MY and KOd performed cellular experiments using
HUEhT-1 cells, and TI, MM, ST, KOm, NM and TN assisted in the
biological assay. MY and KOd analyzed the data. MY, KOd, YM and TM
confirm the authenticity of all the raw data. MY and TM wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AP
|
aprepitant
|
|
EPI
|
epirubicin
|
|
FAP
|
fosaprepitant
|
References
|
1
|
Pritchett W and Kinsley K: Benefits and
Risks of fosaprepitant in patients receiving emetogenic regimens.
Clin J Oncol Nurs. 20:555–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sato Y, Kondo M, Inagaki A, Komatsu H,
Okada C, Naruse K, Sahashi T, Kuroda J, Ogura H, Uegaki S, et al:
Highly frequent and enhanced injection site reaction induced by
peripheral venous injection of fosaprepitant in
anthracycline-treated patients. J Cancer. 5:390–397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujii T, Nishimura N, Urayama KY, Kanai H,
Ishimaru H, Kawano J, Takahashi O, Yamauchi H and Yamauchi T:
Differential impact of fosaprepitant on infusion site adverse
events between cisplatin- and anthracycline-based chemotherapy
regimens. Anticancer Res. 35:379–383. 2015.PubMed/NCBI
|
|
4
|
Hegerova LT, Leal AD, Grendahl DC, Seisler
DK, Sorgatz KM, Anderson KJ, Hilger CR and Loprinzi CL: An analysis
of fosaprepitant-induced venous toxicity in patients receiving
highly emetogenic chemotherapy. Support Care Cancer. 23:55–59.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boccia R, Geller RB, Clendeninn N and
Ottoboni T: Hypersensitivity and infusion-site adverse events with
intravenous fosaprepitant after anthracycline-containing
chemotherapy: A retrospective study. Future Oncol. 15:297–303.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lundberg JD, Crawford BS, Phillips G,
Berger MJ and Wesolowski R: Incidence of infusion-site reactions
associated with peripheral intravenous administration of
fosaprepitant. Support Care Cancer. 22:1461–1466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chau E, Lundberg J, Phillips G, Berger M
and Wesolowski R: Updated report on incidence of infusion-site
reactions associated with peripheral intravenous administration of
fosaprepitant. J Oncol Pharm Pract. 25:1053–1057. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ottoboni T, Keller MR, Cravets M,
Clendeninn N and Quart B: Bioequivalence of HTX-019 (aprepitant IV)
and fosaprepitant in healthy subjects: A phase I, open-label,
randomized, two-way crossover evaluation. Drug Des Devel Ther.
12:429–435. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ottoboni T, Lauw M, Keller MR, Cravets M,
Manhard K, Clendeninn N and Quart B: Safety of HTX-019 (intravenous
aprepitant) and fosaprepitant in healthy subjects. Future Oncol.
14:2849–2859. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamasaki M, Kimura R, Mayahara S, Maeda Y,
Takahashi M, Nishida T, Oda K and Murakami T: Study on the
infusion-site adverse events and vascular distribution of
epirubicin in chemotherapy with epirubicin and fosaprepitant. Mol
Clin Oncol. 11:43–49. 2019.PubMed/NCBI
|
|
11
|
Oda K, Umakoshi T, Mori N, Kasai R and
Murakami T: Biopharmaceutical properties of tubeimoside-1: A
cytotoxic amphipathic cyclic bisdesmoside. Int J Clin Pharmacol
Pharmacother. 2:1262017. View Article : Google Scholar
|
|
12
|
Varela-Castillo O, Cordero P,
Gutiérrez-Iglesias G, Palma I, Rubio-Gayosso I, Meaney E,
Ramirez-Sanchez I, Villarreal F, Ceballos G and Nájera N:
Characterization of the cytotoxic effects of the combination of
cisplatin and flavanol (−)-epicatechin on human lung cancer cell
line A549. An isobolographic approach. Exp Oncol. 40:19–23. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chuang SH and Reddy DS: Isobolographic
analysis of antiseizure activity of the GABA type A
receptor-modulating synthetic neurosteroids brexanolone and
ganaxolone with tiagabine and midazolam. J Pharmacol Exp Ther.
372:285–298. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teno N, Iguchi Y, Oda K, Yamashita Y,
Masuda A, Fujimori K, Une M and Gohda K: Discovery of orally active
and nonsteroidal farnesoid X receptor (FXR) antagonist with
propensity for accumulation and responsiveness in ileum. ACS Med
Chem Lett. 12:420–425. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Olver IN: Prevention of
chemotherapy-induced nausea and vomiting: Focus on fosaprepitant.
Ther Clin Risk Manag. 4:501–506. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fotakis G and Timbrell JA: In vitro
cytotoxicity assays: Comparison of LDH, neutral red, MTT and
protein assay in hepatoma cell lines following exposure to cadmium
chloride. Toxicol Lett. 160:171–17. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eccardt AM, Bell TP, Mattathil L, Prasad
R, Kelly SC and Fisher JS: Trans-plasma membrane electron transport
and ascorbate efflux by skeletal muscle. Antioxidants (Basel).
6:892017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scarcello E, Lambremont A, Vanbever R,
Jacques PJ and Lison D: Mind your assays: Misleading cytotoxicity
with the WST-1 assay in the presence of manganese. PLoS One.
15:e02316342020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Zou M, Piao H, Liu Y, Tang B, Gao
Y, Ma N and Cheng G: Characterization and pharmacokinetic study of
aprepitant solid dispersions with soluplus®. Molecules.
20:11345–11356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cosgun ZC, Fels B and Kusche-Vihrog K:
Nanomechanics of the endothelial glycocalyx: From structure to
function. Am J Pathol. 190:732–741. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurbanoglu S, Palabiyik BB, Gumustas M,
Şanlı S and Uslu B; Ozkan Ankara University SA, : Development and
validation of a stability-indicating RP-LC method for the
determination of anticancer drug epirubicin in pharmaceuticals. J
Liq Chromatogr Relat Technol. 37:1583–1596. 2014. View Article : Google Scholar
|
|
22
|
Yata N, Toyoda T, Murakami T, Nishiura A
and Higashi Y: Phosphatidylserine as a determinant for the tissue
distribution of weakly basic drugs in rats. Pharm Res. 7:1019–1025.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murakami T and Yumoto R: Role of
phosphatidylserine binding in tissue distribution of
amine-containing basic compounds. Expert Opin Drug Metab Toxicol.
7:353–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wotton K, Gassner LA and Ingham E:
Flushing an i.v. line: A simple but potentially costly procedure
for both patient and health unit. Contemp Nurse. 17:264–273. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flint A, McIntosh D and Davies MW:
Continuous infusion versus intermittent flushing to prevent loss of
function of peripheral intravenous catheters used for drug
administration in newborn infants. Cochrane Database Syst Rev.
CD0045932005.PubMed/NCBI
|
|
26
|
Perez A, Feuz I, Brotschi B and Bernet V:
Intermittent flushing improves cannula patency compared to
continuous infusion for peripherally inserted venous catheters in
newborns: Results from a prospective observational study. J Perinat
Med. 40:311–314. 2012.PubMed/NCBI
|
|
27
|
Kovacevich DS, Corrigan M, Ross VM,
McKeever L, Hall AM and Braunschweig C: American society for
parenteral and enteral nutrition guidelines for the selection and
care of central venous access devices for adult home parenteral
nutrition administration. JPEN J Parenter Enteral Nutr. 43:15–31.
2019. View Article : Google Scholar : PubMed/NCBI
|