Introduction
Complement Clq/TNF-related protein 6 (CTRP6) is one
of the CTRP family members homologous to adiponectin discovered in
recent years (1–3). All CTRP members are secreted
proteins, which are widely expressed in various tissues and cell
types (1). Previous studies have
found that CTRP6 plays a role in fat metabolism, glucose
metabolism, cardiometabolism, inflammatory response and
autoimmunity (2,3); however, research on the role of CTRP6
in cancer is an emerging area. Previous studies have revealed that
the overexpression of CTRP6 is associated with a poor prognosis in
lung adenocarcinoma (1,3), and CTRP6 is also able to serve as a
marker for the diagnosis and prognosis of renal clear cell
carcinoma (4). By contrast, a
previous study also revealed a role of CTRP6 in the inhibition of
cancer cell metastasis in ovarian cancer (5). From this, it can be argued that CTRP6
has both oncogenic and antitumor effects, which may be related to
the cancer type. However, little is known about the role of CTRP6
in digestive tumors. It was not until recently that the
relationship between CTRP6 and gastric, liver and colon cancer was
gradually revealed (6–8). The present study reviews the
pathophysiological role of CTRP6 in the development of
tumorigenesis in the digestive system and explores the possible
mechanisms.
CTRP6: General characteristics
CTRP6 is found in serum and is widely expressed in
the human uterus, skin, placenta, lung, fat and other tissues
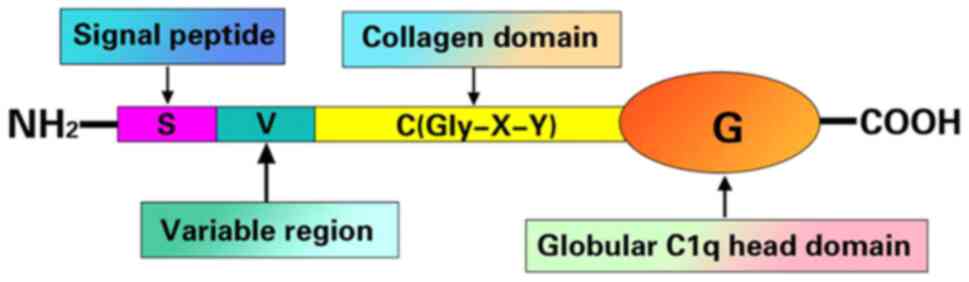
(1). CTRP6 contains an
amino-terminal signal peptide, a short variable domain, a collagen
domain and a carboxyl-terminal spherical domain homologous to the
complement protein, C1q, where the spherical domain is important
for the stimulation of p42/44MAPK phosphorylation pathways
(9,10) (Fig.
1). In humans, CTRP6 induces IL-10 mRNA and protein expression
in monocytes, and when blocking phosphorylation by cotreating cells
with selective p42/44MAPK inhibitors, CTRP6-mediated IL-10
expression is abolished (11).
Another study found that the globular domain of human CTRP6 shares
up to 33% amino acid identity with adiponectin, suggesting that the
actions of CTRP6 may share some similarities to the physiological
effects of adiponectin; for example, they both participate in the
regulation of a number of physiological and pathophysiological
processes such as glucose and lipid metabolism, and inflammation
(12). Fig. 1 shows the schematic structure of
the CTRP family (13).
A number of the proteins in the CTRP family are
involved in tumor regulation. A recent study suggested that CTRP1
may promote human glioblastoma progression and predict a poor
prognosis (14). CTRP3 reduces
glucose levels by reducing hepatic gluconeogenesis and induces
hepatic Akt activation, which subsequently stimulates the
proliferation of chondrogenic cells (15,16).
CTRP4 can act as a regulator of tumor-promoting inflammation
(17), while CTRP8 is involved in
brain cancer formation (18).
The regulatory role of CTRP6 in tumor biology is
associated with multiple mechanisms and has different roles
depending on the tumor type. CTRP6 inhibits the proliferation and
migration of epithelial ovarian cancer cells by blocking the
IL-8/VEGF pathway (5). CTRP6 can
also inhibit the progression of oral squamous cell cancer cells by
disrupting the lamin-laminin receptor axis (19). CTRP6 also has a potential role in
promoting tumor growth, invasion and metastasis, and can serve as a
novel cancer diagnostic and prognostic biomarker for clear cell
renal cell carcinoma (4).
The study of CTRP6 in digestive tumors is currently
focused on, liver, colon and gastric cancer. Previous studies have
shown that CTRP6 is highly expressed in liver and colon cancer
tissues compared with non-cancerous tissues, and may be used as an
early marker for the diagnosis of these diseases (8,20).
CTRP6 is overexpressed in gastric cancer and is involved in the
division and migration of gastric cancer cells (7). The regulatory mechanisms of CTRP6
will be detailed in the following sections.
Regulation of inflammation
CTRP6, a novel metabolic immunomodulator that binds
to multiple endogenous ligands, is an intermediate link in obesity
with adipose tissue inflammation and insulin resistance (21,22).
CTRP6 serves a role in regulating the secretion of inflammatory
factors and may have a proinflammatory or inhibitory inflammatory
effect depending on the site of action. In a previous study, the
knockdown of CTRP6 resulted in a significant reduction in the
expression of TNF-α, IL-1 and IL-6 in high glucose-induced
glomerular mesilial cells (23).
Overexpression of CTRP6 can activate PI3K/Akt signaling by
inhibiting the Ras homologue family A/Rho associated kinase/PTEN
pathway and improve the inflammatory damage caused by cerebral
ischemia/reperfusion (24).
Activation of the PI3K/Akt pathway is one of the
common molecular mechanisms in human tumor development. PI3K/Akt
signaling negatively regulates processes such as cell growth and
proliferation, glucose metabolism and cell migration, and is
considered to play a key regulatory role in tumor invasiveness
(25). One of the specific
mechanisms by which the PI3K/Akt pathway promotes tumorigenesis is
through the dysregulation of inflammatory mediators and immunity.
It has been shown that rosmarinic acid subsequently prevents lung
tumor invasion by reducing the production of inflammatory factors,
such as IL-6, IL-8, TNF-α and cyclooxygenase-2, by inhibiting Akt
phosphorylation (26). We
speculate that this mechanism of action of the PI3K/Akt pathway is
equally applicable during the pathogenesis of digestive tumors. A
recent study has shown that the chronic inflammatory status due to
obesity is a risk factor for the development of colorectal cancer,
and that the PI3K/Akt pathway is one of the important pathways to
mediate this process (27). The
PI3K/Akt pathway also mediates the aggressive role of
cancer-associated fibroblasts in gastric cancer, while IL-8
enhances expression of PI3K/Akt pathway expression and increases
chemoresistance to gastric cancer (28,29).
In a recent study on hepatoma, royal jelly increased IL-2 and TNF-α
levels in serum by inhibiting PI3K expression and phosphorylating
Akt, thereby preventing and controlling hepatocarcinogenesis in
mice (30). From the
aforementioned studies, we can speculate that CTRP6 may promote
tumorigenesis in the digestive system by releasing inflammatory
factors and activating the PI3K/Akt pathway. This speculation is
supported by the study by Wan et al (6), where it was shown that the inhibition
of CTRP6 blocked Akt signaling and in turn prevented the survival
and migration of hepatocellular carcinoma (HCC).
It has recently been shown that overexpression of
CTRP6 enhances the proliferation, migration and invasion of lung
adenocarcinoma cells by regulating the MAPK signaling pathway
(3). The MAPK/NF-κB pathway is one
of the common intersection pathways of various cellular signaling
pathways, such as inflammation and stress, and is involved in
cellular activity, including carcinogenesis (31). Activation of MAPK/NF-κB signaling
enhances the secretion of IL-1β and IL-18, and leads to the
development of renal inflammation (32). In a study by Eyre et al
(33), it was found that the
globular domain of CTRP6 could stimulate the phosphorylation of
MAPK/ERK1/2, and when human serum were treated with selective
MAPK/ERK1/2 inhibitors, CTRP6-mediated IL-10 expression was
eliminated (24). In an additional
study, it was revealed that digestive tract tumors are regulated by
inflammatory factor secretion by the MAPK signaling pathway. IL-1β,
IL-6 and TNF-α, the inflammatory factors produced by inhibiting the
MAPK pathway, could effectively delay the progression of colorectal
cancer (34). In gastric cancer,
IL-6 promotes tumor growth and metastasis, and resveratrol can
prevent this by blocking Raf/MAPK signaling (35). Piperine in turn inhibits
IL-1β-induced IL-6 expression by inhibiting the MAPK and STAT3
pathways in gastric cancer cells (36). Additional studies have also
demonstrated the oncogenic role of inflammatory factors such as
IL-1β and IL-6 in gastric, colorectal and liver cancer (37–40).
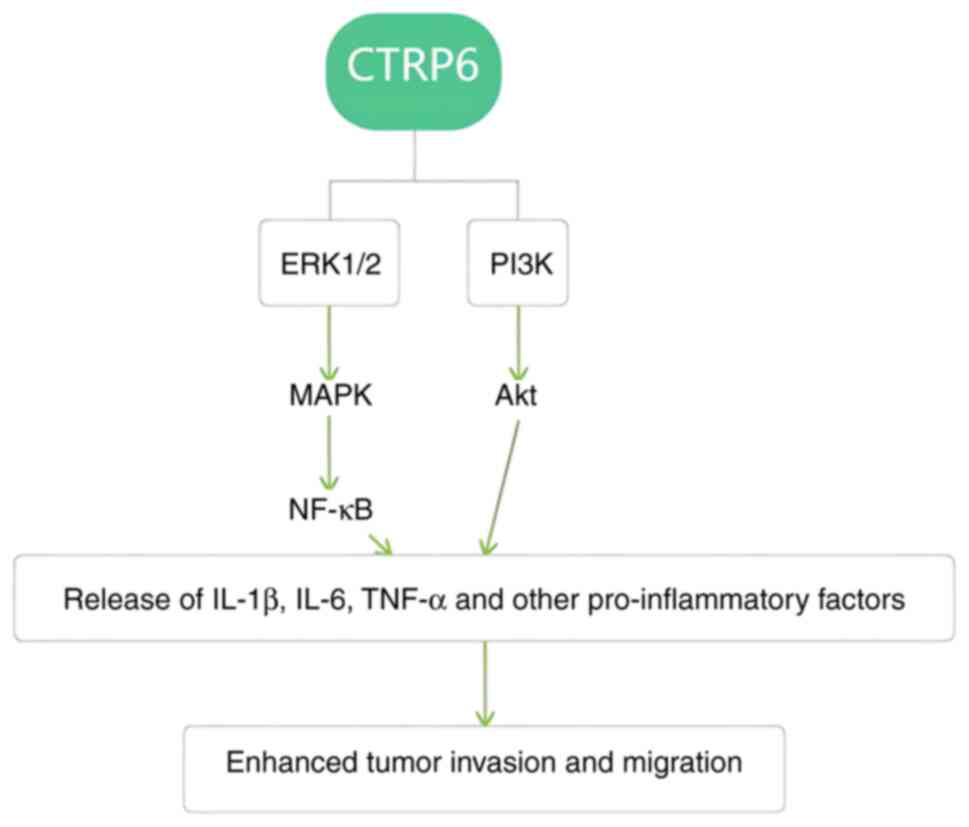
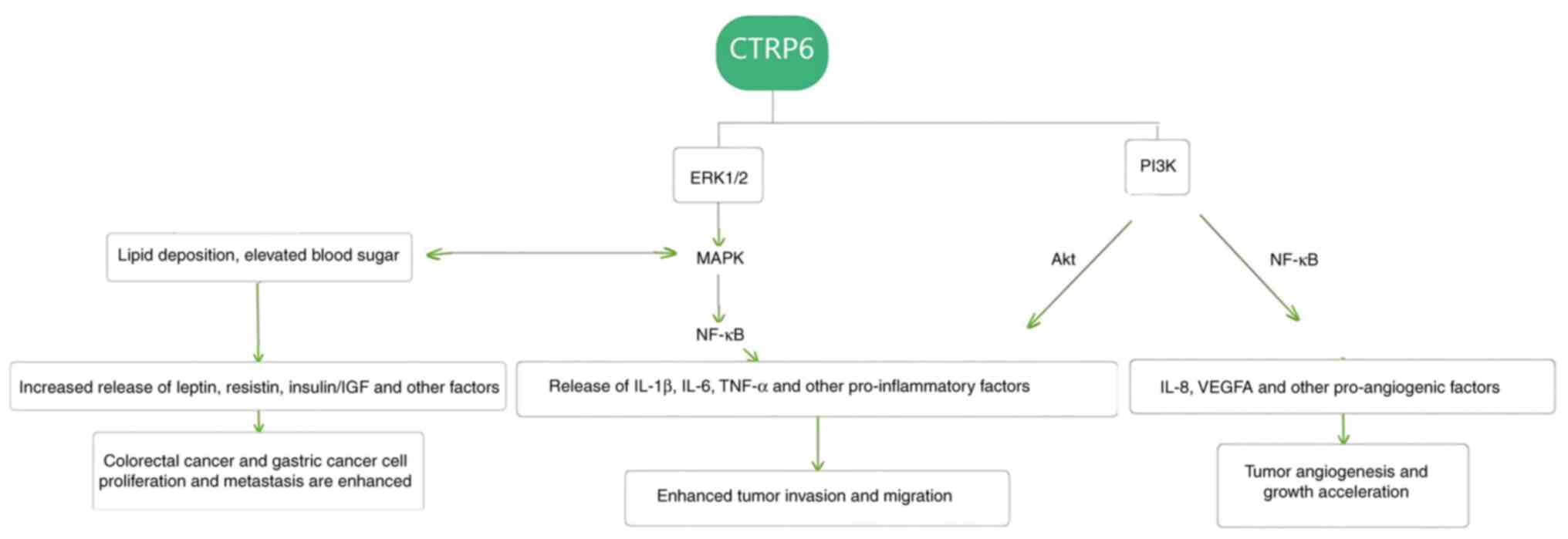
We hypothesize that CTRP6 activation of the MAPK/ERK1/2/NF-κB
pathway promotes the secretion of inflammatory factors such as
IL-1β, IL-6 and TNF-α, and in turn accelerates tumor
progression.
In conclusion, it can be speculated that one role of
CTRP6 in digestive system tumors is to regulate tumor development
through the activation of the Akt pathway and the regulation of
inflammatory factors in the MAPK pathway (Fig. 2).
Regulation of glycolipid metabolism
Disordered glucose and lipid metabolism are the two
main processes that increase tumor risk and severity. Abnormal
lipid metabolism leads to disturbances in adipokine secretion,
which is strongly associated with tumorigenesis and tumor
progression (41). Obesity due to
abnormal lipid metabolism is an independent risk factor for
tumorigenesis and tumor development in a variety of liver,
pancreatic, ovarian and colorectal cancer types (26,40,42).
The role of CTRP6 in glycolipid metabolism has been extensively
studied (43). Animal experiments
showed that CTRP6 could affect pig adipogenesis by activating
Akt/PKA/MAPK signaling, while knockdown of CTRP6 reduced muscle and
subcutaneous fat deposition through alternative signaling pathways
(43,44). Cellular experiments showed that
knockdown of CTRP6 inhibited adipogenesis by inhibiting the
expression of adipogenesis-related genes and the MAPK/ERK1/2
signaling pathway (45). The
aforementioned studies revealed the role of CTRP6 in promoting fat
deposition. Clinical experiments related to glucose metabolism have
demonstrated that CTRP6 may be associated with insulin resistance
and type 2 diabetes (46).
MAPK is a key molecule in the regulation of
bioenergy metabolism and is expressed in various metabolically
related organs (47,48). In adipose metabolism, one of the
mechanisms by which obesity becomes a risk factor for rectal cancer
is due to the metabolic disturbance of adipokines (48). Obesity increases the expression of
leptin, estrogen, resistin, macrophage migration inhibitor factor,
monocyte chemoattractant protein 1 and insulin/insulin-like growth
factor, and reduces the expression of adiponectin, which promotes
obesity-related tumors (e.g., breast, pancreatic, ovarian and
colorectal cancer) proliferation, invasion and metastasis (41). Obesity-induced gastric cancer
stimulates the binding of chemotactic protein and stromal
cell-derived factor 1 to CXC chemokine receptor 4 (CXCR4) and CXCR7
to regulate cancer cell motility and angiogenic regeneration, a
process mediated by the p38 MAPK pathway (49). The MAPK pathway remains important
in the glucose metabolism of tumors. In a previous study, the
knockdown of glucose-regulated protein 94 inhibited the ability of
cancer cell proliferation and metastasis in colorectal cancer cells
by inhibiting the expression of the MAPK pathway, including
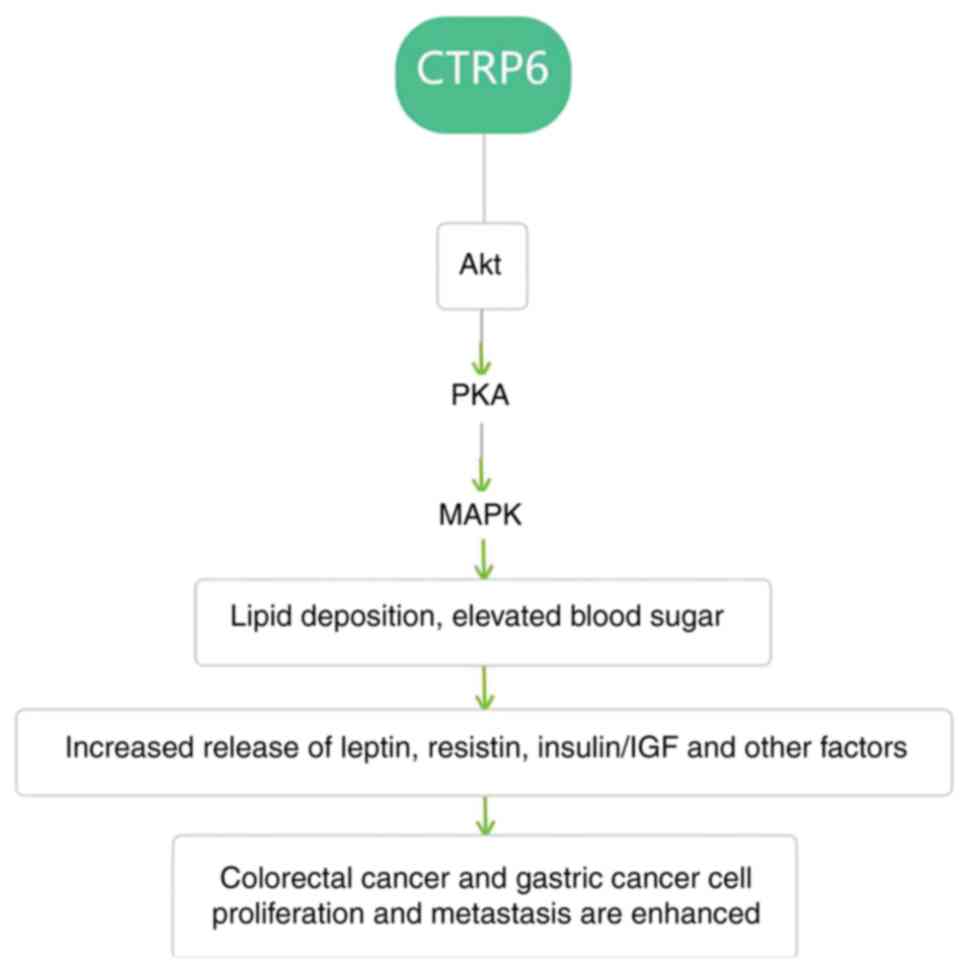
ERK/p-ERK, JNK/p-JNK and p38/p-p38 signaling (50). It can be speculated from the
aforementioned studies that the effect of CTRP6 on colorectal and
gastric cancer is at least partly due to the regulation of
glycolipid metabolism through the MAPK-PKA pathway (Fig. 3), but direct evidence of CTRP6
tumor promotion through MAPK pathway activation is currently
lacking. The study by Lei et al (22) examined the effect of CTRP6 on cell
metabolism by activating/blocking the pathway between colorectal
cancer and gastric cancer cells. It was demonstrated that CTRP6
induces inflammatory factors by regulating glycan and lipid
metabolism (22), which is in
agreement with the aforementioned speculation.
Angiogenesis
Tumor angiogenesis is a key factor in tumor growth,
progression and metastasis, and inhibiting tumor angiogenesis can
be used as an effective means to treat tumors (51). PI3K/Akt signaling is one of the
classical pathways leading to increased vessel number and vascular
permeability, achieving the purpose of revascularization by
enabling the transformation of the vascular smooth muscle cell
(VSMC) phenotype (52). In the
CTRP family, various factors such as CTRP1, CTRP3 and CTRP5 can
regulate inflammatory factors and glycolipid metabolism by
activating pathways such as the PI3K/Akt/endothelial NO synthase
and p38/MAPK/NF-κB pathways, and in turn regulate vascularization
due to chronic inflammation (53).
A recent study found that CTRP9 is correlated with Akt and
AMP-activated protein kinase (AMPK) pathway activation by promoting
endothelial cell function and ischemia-induced revascularization
(54). We consider that the
aforementioned development process is equally suitable for the role
of CTRP6 in digestive tract tumors.
Digestive tumors such as those of gastric, liver and
colorectal cancer are all regulated by the Akt signaling pathway
(55–57). A recent study confirmed that the
CDK5 regulatory subunit-associated protein 3 gene improves patient
prognosis by inhibiting tumor angiogenesis through the
downregulation of gastric neuroendocrine cancer
Akt/hypoxia-inducible factor-1α/VEGFA signaling (58). In colorectal cancer, highly dry
human colorectal cancer cells promote angiogenesis through the
activation of the angiogenic cytokines, IL-8 and VEGFA, produced by
the EGFR/Akt/NF-κB pathway, and lactoferrin suppresses colon cancer
angiogenesis by regulating the PI3K/ERK1/2/Akt pathway (59,60).
Moreover, Wang et al (61)
found that IL-6 activates STAT3 to stimulate angiogenesis in
gastric cancer and, as discussed previously, CTRP6 promotes
pro-inflammatory factors such as IL-6 through the PI3K/Akt and MAPK
pathways (27,28,34,35).
Thus, in gastric cancer, CTRP6 most likely serves a role in
promoting gastric cancer angiogenesis by activating the
Akt/IL-6/STAT3 or MAPK/IL-6/STAT3 pathway. It has previously been
demonstrated that CTRP6 promotes hepatoma angiogenesis and
subsequently reduces HepG2 cell necrosis by regulating Akt
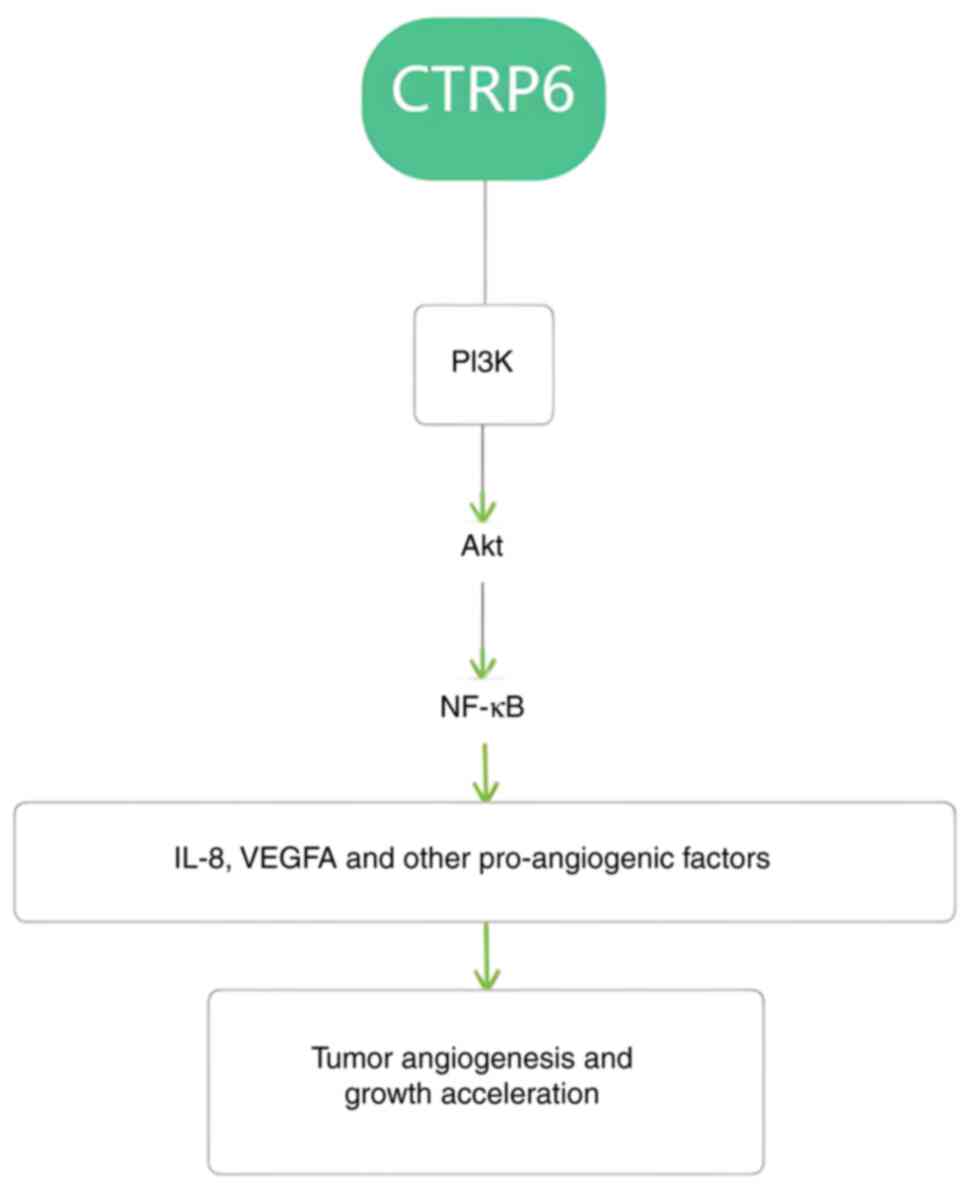
signaling in HCC cells (8). It is
reasonable to speculate that CTRP6 regulates colorectal and liver
cancer, and other tumors, by the activation of proangiogenic
factors such as PI3K/Ak/NF-κB (Fig.
4). However, there is no direct basis for CTRP6 to activate the
Akt pathway to promote vascular effects in other digestive tumors
outside HCC.
AMPK is a major regulator of glycolipid metabolism
and protein synthesis, and also functions in regulating
angiogenesis (62,63). Activation of AMPK-related pathways
can serve as a prognostic marker for colon tumors and can also play
a role in regulating angiogenesis in gastric cancer and HCC
(64–66). It was recently demonstrated that
dual-loaded liposomes containing apigenin and 5-fluorouracil
inhibited tumor angiogenesis by inhibiting AMPK phosphorylation in
colorectal cancer (67). CTRP6 can
activate the AMPK pathway in various tissues and thus plays
different roles in mechanisms such as the promotion of cellular
differentiation and antifibrosis (68,69).
In colon cancer, CTRP6 was shown to be highly expressed, and its
expression level was not correlated with patient age, sex or
pathological type, among others (4). However, this evidence is insufficient
to deduce whether the role of CTRP6 in colon cancer is related to
tumor angiogenesis associated with AMPK pathway activation. By
contrast, it has been suggested that adiponectin inhibits tumor
angiogenesis by regulating the AMPK pathway in colon cancer
(70). Due to the proposed
structural similarity of CTRP6 and adiponectin, we can even
speculate that CTRP6 may have an effect on inhibiting tumor
angiogenesis (12). More research
should be conducted to confirm this speculation.
Alternative views
Iwata et al (71) observed that the overexpression of
CTRP6 had an inhibitory effect on tumor stromal fibrosis in gastric
cancer, the development of which is considered to promote cancer
progression and confer chemoresistant properties in malignant
tissues. This suggests that CTRP6 may also function as an inhibitor
of gastric cancer progression. However, after the addition of
recombinant CTRP6 protein, the study did not record changes in the
proliferation rate and invasiveness of the gastric cancer cells
(71). Therefore, without
additional research, we still tend to consider that CTRP6 plays a
major tumor-promoting role in gastric cancer.
Murayama et al (72) found that the recombinant human
CTRP6 protein increased the expression of the anti-inflammatory
factor IL-10 in mice and inhibited CTRP6-mediated IL-10 expression
after pretreatment with the selective ERK1/2 inhibitor U0126 and
then resolved the symptoms of arthritis. It is thus inferred that
CTRP6 may play an anti-inflammatory effect in the induction of
IL-10 expression through the ERK1/2 pathway. An additional study
also found that CTRP6 overexpression decreased the expression of
inflammatory factors IL-1β, IL-6 and TNF-α, and increased the
expression of anti-inflammatory factor IL-10 (73). This seems to contradict the
speculation that CTRP6 promotes inflammatory factor release through
the activation of the Akt and MAPK pathways. However, the current
evidence is insufficient to prove which mechanism is dominant or
coexisting in digestive tumors.
A previous study also found that the secretion
levels of IL-8 in ovarian cancer were opposite to those of CTRP6
and were dose-dependent, so it was hypothesized that CTRP6 may be
involved in inhibiting the proliferation and metastasis of ovarian
cancer cells by inhibiting IL-8 and vascular endothelial growth
factors (5). CTRP6 inhibits
platelet-derived growth factor-BB-induced VSMC proliferation and
migration, at least in part by the inhibition of PI3K/Akt/mTOR
signaling, and thus may be a potential target for the treatment of
atherosclerosis (74). The
aforementioned studies contradict the observation that CTRP6
promotes tumor angiogenesis in HCC. We speculate that this
contradiction may result from a different regulatory role of CTRP6
in blood vessels and in different tissues, as the structurally
similar adiponectin showed similar properties in recent studies
(75,76).
Additionally, in a recent study, Zhang et al
(77) proposed that microRNA
(miR)-148a inhibited HCC cell growth by targeting death receptors
and downregulating epithelial-to-mesenchymal transition and
PI3K/Akt signaling pathways. Moreover, methylsulfonylmethane
inhibits iron metabolism and modulates p38/p53/ERK signaling and
miR expression targeted to inhibit the proliferation of embryonic
cancer stem cells (78). These
studies suggest that miRs are likely to be involved in the
regulation of CTRPs in gastrointestinal tumors, miRs may be
involved in the regulation of CTRPs on gastrointestinal tumors by
activating the PI3K/Akt pathway. More research needs to be
conducted to confirm this conclusion.
Conclusion
CTRP6 plays a role in promoting tumorigenesis and
development in digestive system tumors through multiple mechanisms.
In gastric cancer tissues and cells, the overexpression of CTRP6
affects the proliferation, migration, invasion and apoptosis of
tumor cells through the release of pro-inflammatory factors
(8). In colon cancer, CTRP6
expression is significantly higher than in non-cancerous tissues
and may influence the development of colon cancer by regulating
glycolipid metabolism and inflammatory response, and thus may serve
as a marker for the early screening of colon cancer (4,33,37).
In HCC, CTRP6 is highly expressed and promotes the survival and
migration of HCC cells through mechanisms such as the promotion of
tumor angiogenesis (6,7). The induction of the aforementioned
mechanism is mainly realized by the activation of the Akt and MAPK
pathways, as summarized in Table I
and Fig. 5. CTRP6 may also
regulate the aforementioned tumors via activation of the AMPK
pathway (67,68) but there is insufficient evidence to
prove this.
 | Table I.Review of the underlying mechanisms
of C1qTNF-related protein 6 in digestive tract tumors. |
Table I.
Review of the underlying mechanisms
of C1qTNF-related protein 6 in digestive tract tumors.
| Cancer type | Related signaling
pathways | Proinflammatory
function | Glycolipid
metabolism | Angiogenesis | (Refs.) |
|---|
| Colorectal | PI3K/Akt,
MAPK/ERK1/2/NF-κB, AMPK | Activation of the
PI3K/Akt and MAPK/ERK1/2/NF-κB pathways releases inflammatory
factors such as IL-1β, IL-6 and TNF-α | Activation of
Akt/PKA/MAPK to release lepokines such as leptin, estrogen and
resistin | Activation of the
PI3K/Ak/NF-κB pro-angiogenic factor, IL-8 and VEGFA | (26,33,37–39,47,49,55,58,59) |
| Gastric | PI3K/Akt, Raf/MAPK,
Akt/IL-6/STAT3 or MAPK/IL-6/STAT3/AMPK | PI3K/Akt mediates
fibroblast invasion in gastric cancer and is enhanced by IL-8.
Mediation of the MAPK pathway to promote IL-6 and IL-1β
release | Activation of
Akt/PKA/MAPK to release chemokines such as SDF-1 | Activation of
Akt/IL-6/STAT3 or MAPK/IL-6/STAT3 promotes angiogenesis | (27,28,34–36,46,48,54) |
| Liver | PI3K/Akt/NF-κB,
MAPK/ERK1/2/NF-κB, AMPK | Activation of the
MAPK pathway promotes IL-1β, IL- 6 and TNF-α release | - | Activation of the
PI3K/Ak/NF-κB pro-angiogenic factor, IL-8 and VEGFA | (6,29,39,56,61) |
The biological function of CTRP6 is complex and has
received attention as a regulator of metabolism in previous
studies, although there are less current studies analyzing CTRP6 in
cancer. Continuing research into CTRP6 will deepen our
understanding of its biological function and help increase the
understanding of its role in tumor regulation. Current findings
suggest that CTRP6 and its downstream pathways may become drug
targets for tumor therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MK wrote the manuscript, and AZ, XZ and ZP reviewed
and revised the manuscript. All authors approved the final version
of this manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang CL, Wu LL and Li L: Research
progress of complement-C1q/tumor necrosis factor-related protein 3.
Sheng Li Xue Bao. 69:666–676. 2017.PubMed/NCBI
|
|
2
|
Schäffler A and Buechler C: CTRP family:
Linking immunity to metabolism. Trends Endocrinol Metab.
23:194–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han M, Wang B, Zhu M and Zhang Y: C1QTNF6
as a novel biomarker regulates cellular behaviors in A549 cells and
exacerbates the outcome of lung adenocarcinoma patients. In Vitro
Cell Dev Biol Anim. 55:614–621. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin W, Chen X, Chen T, Liu J, Ye Y, Chen
L, Qiu X, Chia-Hsien Cheng J, Zhang L, Wu J and Qiu S: C1QTNF6 as a
novel diagnostic and prognostic biomarker for clear cell renal cell
carcinoma. DNA Cell Biol. 39:1000–1011. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Liu Z, Duan L, Ma B and Sun Z: C1q
tumor necrosis factor-related protein 6 (CTRP6) inhibits the
proliferation and migration of ovarian cancer cells. Xi Bao Yu Fen
Zi Mian Yi Xue Za Zhi. 31:1664–1668. 2015.(In Chinese). PubMed/NCBI
|
|
6
|
Wan X, Zheng C and Dong L: Inhibition of
CTRP6 prevented survival and migration in hepatocellular carcinoma
through inactivating the AKT signaling pathway. J Cell Biochem.
120:17059–17066. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu HX, Cui L, Meng XY, Wang ZJ, Cui YX, Yu
YP, Wang D and Jiang XJ: C1QTNF6 is overexpressed in gastric
carcinoma and contributes to the proliferation and migration of
gastric carcinoma cells. Int J Mol Med. 43:621–629. 2019.PubMed/NCBI
|
|
8
|
Takeuchi T, Adachi Y and Nagayama T:
Expression of a secretory protein C1qTNF6, a C1qTNF family member,
in hepatocellular carcinoma. Anal Cell Pathol (Amst). 34:113–121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kishore U, Gaboriaud C, Waters P, Shrive
AK, Greenhough TJ, Reid KB, Sim RB and Arlaud GJ: C1q and tumor
necrosis factor superfamily: Modularity and versatility. Trends
Immunol. 25:551–561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wong GW, Krawczyk SA, Kitidis-Mitrokostas
C, Revett T, Gimeno R and Lodish HF: Molecular, biochemical and
functional characterizations of C1q/TNF family members:
Adipose-tissue-selective expression patterns, regulation by
PPAR-gamma agonist, cysteine-mediated oligomerizations,
combinatorial associations and metabolic functions. Biochem J.
416:161–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim MJ, Lee W, Park EJ and Park SY:
C1qTNF-related protein-6 increases the expression of interleukin-10
in macrophages. Mol Cells. 30:59–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong GW, Wang J, Hug C, Tsao TS and Lodish
HF: A family of Acrp30/adiponectin structural and functional
paralogs. Proc Natl Acad Sci USA. 101:10302–10307. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kong M, Gao Y, Guo X, Xie Y and Yu Y: Role
of the CTRP family in tumor development and progression. Oncol
Lett. 22:7232021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen L and Su G: Identification of CTRP1
as a prognostic biomarker and oncogene in human glioblastoma.
Biomed Res Int. 2019:25824162019.PubMed/NCBI
|
|
15
|
Maeda T, Jikko A, Abe M, Yokohama-Tamaki
T, Akiyama H, Furukawa S, Takigawa M and Wakisaka S: Cartducin, a
paralog of Acrp30/adiponectin, is induced during chondrogenic
differentiation and promotes proliferation of chondrogenic
precursors and chondrocytes. J Cell Physiol. 206:537–544. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akiyama H, Furukawa S, Wakisaka S and
Maeda T: CTRP3/cartducin promotes proliferation and migration of
endothelial cells. Mol Cell Biochem. 304:243–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao J, Gu G, Lu L and Wang X: Research
progress of C1q tumor necrosis factor-related protein 4.
International Journal of Cardiovascular Diseases 99–101; 2016
|
|
18
|
Thanasupawat T, Glogowska A, Burg M, Wong
GW, Hoang-Vu C, Hombach-Klonisch S and Klonisch T: RXFP1 is
targeted by complement C1q tumor necrosis factor-related factor 8
in brain cancer. Front Endocrinol (Lausanne). 6:1272015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hano K, Hatano K, Saigo C, Kito Y, Shibata
T and Takeuchi T: An adiponectin paralog protein, CTRP6 decreased
the proliferation and invasion activity of oral squamous cell
carcinoma cells: Possible interaction with laminin receptor
pathway. Mol Biol Rep. 46:4967–4973. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gou J, Kang S, Qu H and Cui Y: Expression
of complement C1q/tumor necrosis factor-related protein 6 in colon
cancer. Journal of Gastroenterology and Hepatology. 28:313–316.
2019.
|
|
21
|
Kirketerp-Møller N, Bayarri-Olmos R,
Krogfelt KA and Garred P: C1q/TNF-Related protein 6 is a pattern
recognition molecule that recruits collectin-11 from the complement
system to ligands. J Immunol. 204:1598–1606. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei X, Seldin MM, Little HC, Choy N,
Klonisch T and Wong GW: C1q/TNF-related protein 6 (CTRP6) links
obesity to adipose tissue inflammation and insulin resistance. J
Biol Chem. 292:14836–14850. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu E, Yin C, Yi X and Liu Y: Knockdown of
CTRP6 inhibits high glucose-induced oxidative stress, inflammation
and extracellular matrix accumulation in mesangial cells through
regulating the Akt/NF-kB pathway. Clin Exp Pharmacol Physiol.
47:1203–1211. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Sun J, Gu L and Gao X: Protective
effect of CTRP6 on cerebral ischemia/reperfusion injury by
attenuating inflammation, oxidative stress and apoptosis in PC12
cells. Mol Med Rep. 22:344–352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pintha K, Chaiwangyen W, Yodkeeree S,
Suttajit M and Tantipaiboonwong P: Suppressive effects of
rosmarinic acid rich fraction from perilla on oxidative stress,
inflammation and metastasis ability in A549 cells exposed to PM via
C-Jun, P-65-Nf-kb and Akt signaling pathways. Biomolecules.
11:10902021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bou Malhab LJ and Abdel-Rahman WM: Obesity
and inflammation: Colorectal cancer engines. Curr Mol Pharmacol.
15:620–646. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu X, Yao L, Qu J, Liu L, Lu N, Wang J
and Zhang J: Cancer-associated fibroblast infiltration in gastric
cancer: The discrepancy in subtypes pathways and immunosuppression.
J Transl Med. 19:3252021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhai J, Shen J, Xie G, Wu J, He M, Gao L,
Zhang Y, Yao X and Shen L: Cancer-associated fibroblasts-derived
IL-8 mediates resistance to cisplatin in human gastric cancer.
Cancer Lett. 454:37–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chi X, Liu Z, Wei W, Hu X, Wang Y, Wang H
and Xu B: Selenium-rich royal jelly inhibits hepatocellular
carcinoma through PI3K/AKT and VEGF pathways in H22 tumor-bearing
mice. Food Funct. 12:9111–9127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y
and Li Y: Inflammation and tumor progression: signaling pathways
and targeted intervention. Signal Transduct Target Ther. 6:2632021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, Chi H, Zhu W, Yang G, Song J, Mo L,
Zhang Y, Deng Y, Xu F, Yang J, et al: Cadmium induces renal
inflammation by activating the NLRP3 inflammasome through
ROS/MAPK/NF-kB pathway in vitro and in vivo. Arch Toxicol.
95:3497–3513. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eyre S, Hinks A, Bowes J, Flynn E, Martin
P, Wilson AG, Morgan AW, Emery P, Steer S, Hocking LJ, et al:
Overlapping genetic susceptibility variants between three
autoimmune disorders: Rheumatoid arthritis, type 1 diabetes and
coeliac disease. Arthritis Res Ther. 12:R1752010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ray AL, Berggren KL, Restrepo Cruz S, Gan
GN and Beswick EJ: Inhibition of MK2 suppresses IL-1β, IL-6, and
TNF-α-dependent colorectal cancer growth. Int J Cancer.
142:1702–1711. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang T, Zhang J, Zhou J, Zhu M, Wang L and
Yan L: Resveratrol inhibits Interleukin-6 induced invasion of human
gastric cancer cells. Biomed Pharmacother. 99:766–773. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xia Y, Khoi PN, Yoon HJ, Lian S, Joo YE,
Chay KO, Kim KK and Jung YD: Piperine inhibits IL-1β-induced IL-6
expression by suppressing p38 MAPK and STAT3 activation in gastric
cancer cells. Mol Cell Biochem. 398:147–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shokrzadeh M, Mohammadpour A, Hoseini V,
Abediankenari S, Ghassemi-Barghi N and Tabari YS: Serum cytokine OF
IL-2, IL-10 and IL-12 levels in patients with stomach
adenocarcinoma. Arq Gastroenterol. 55:385–389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mohamed EA, Bassiouny K, Alshambky AA and
Khalil H: Anticancer properties of N,N-dibenzylasparagine as an
Asparagine (Asp) analog, using colon cancer caco-2 cell line. Asian
Pac J Cancer Prev. 23:2531–2540. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Neurath MF: IL-23 in inflammatory bowel
diseases and colon cancer. Cytokine Growth Factor Rev. 45:1–8.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kern L, Mittenbühler MJ, Vesting AJ,
Ostermann AL, Wunderlich CM and Wunderlich FT: Obesity-Induced TNFα
and IL-6 signaling: The missing link between obesity and
inflammation-driven liver and colorectal cancers. Cancers (Basel).
11:242018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pu X and Chen D: Targeting adipokines in
obesity-related tumors. Front Oncol. 11:6859232021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sadeghi A, Fadaei R, Moradi N, Fouani FZ,
Roozbehkia M, Zandieh Z, Ansaripour S, Vatannejad A and
Doustimotlagh AH: Circulating levels of C1q/TNF-α-related protein 6
(CTRP6) in polycystic ovary syndrome. IUBMB Life. 72:1449–1459.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu W, Xu K, Li M, Zhang J and Wang Y:
MicroRNA-29b/29c targeting CTRP6 influences porcine adipogenesis
via the AKT/PKA/MAPK Signalling pathway. Adipocyte. 10:264–274.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu W, Ji M, Xu K, Zhang D, Yin Y, Huang X,
Peng Y and Zhang J: Knockdown of CTRP6 reduces the deposition of
intramuscular and subcutaneous fat in pigs via different signaling
pathways. Biochim Biophys Acta Mol Cell Biol Lipids.
1865:1587292020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu WJ, Mo DL, Zhao CZ, Zhao C, Chen YS,
Pang WJ and Yang GS: Knockdown of CTRP6 inhibits adipogenesis via
lipogenic marker genes and Erk1/2 signalling pathway. Cell Biol
Int. 39:554–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang M, Tang X, Li L, Liu D, Liu H, Zheng
H, Deng W, Zhao X and Yang G: C1q/TNF-related protein-6 is
associated with insulin resistance and the development of diabetes
in Chinese population. Acta Diabetol. 55:1221–1229. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen HY, Yang MD, Chou YC, Ma YS, Peng SF,
Liao CL, Chen PY, Hsia TC, Lien JC and Chen CH: Ouabain Suppresses
Cell Migration and Invasion in Human Gastric Cancer AGS Cells
Through the Inhibition of MMP Signaling Pathways. Anticancer Res.
41:4365–4375. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kasprzak A: Insulin-Like Growth Factor 1
(IGF-1) Signaling in Glucose Metabolism in Colorectal Cancer. Int J
Mol Sci. 22:64342021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kruszyna Ł, Murawa D, Jagodziński PP,
Oszkinis G and Krasiński Z: The expression and prognostic
significance of VEGF and CXCR4 in gastric cancer: Correlation with
Angiogenesis, Lymphangiogenesis and Progression. Curr Issues Mol
Biol. 44:3075–3088. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Batzorig U, Wei PL, Wang W, Huang CY and
Chang YJ: Glucose-Regulated Protein 94 Mediates the Proliferation
and Metastasis through the Regulation of ETV1 and MAPK Pathway in
Colorectal Cancer. Int J Med Sci. 18:2251–2261. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wiśniewska W, Kopka M, Siemiątkowska K,
Fudalej MM, Sobiborowicz A and Badowska-Kozakiewicz AM: The
complexity of tumour angiogenesis based on recently described
molecules. Contemp Oncol (Pozn). 25:33–44. 2021.PubMed/NCBI
|
|
52
|
Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah
G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I,
Nagy JA, et al: Pathological angiogenesis is induced by sustained
Akt signaling and inhibited by rapamycin. Cancer Cell. 10:159–170.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Si Y, Fan W and Sun L: A review of the
relationship between CTRP family and coronary artery disease. Curr
Atheroscler Rep. 22:222020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yamaguchi S, Shibata R, Ohashi K, Enomoto
T, Ogawa H, Otaka N, Hiramatsu-Ito M, Masutomi T, Kawanishi H,
Murohara T and Ouchi N: C1q/TNF-Related protein 9 promotes
revascularization in response to ischemia via an eNOS-Dependent
manner. Front Pharmacol. 11:13132020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
He L, Wang W, Shi H, Jiang C, Yao H, Zhang
Y, Qian W and Lin R: THBS4/integrin α2 axis mediates BM-MSCs to
promote angiogenesis in gastric cancer associated with chronic
Helicobacter pylori infection. Aging (Albany NY). 13:19375–19396.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Auyeung KK and Ko JK: Angiogenesis and
oxidative stress in metastatic tumor progression: Pathogenesis and
novel therapeutic approach of colon cancer. Curr Pharm Des.
23:3952–3961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhao Z, Gao J, Li C, Xu X, Hu Y and Huang
S: Reactive oxygen species induce endothelial differentiation of
liver cancer stem-like sphere cells through the activation of
Akt/IKK signaling pathway. Oxid Med Cell Longev. 2020:16216872020.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lin JX, Weng XF, Xie XS, Lian NZ, Qiu SL,
Wang JB, Lu J, Chen QY, Cao LL, Lin M, et al: CDK5RAP3 inhibits
angiogenesis in gastric neuroendocrine carcinoma by modulating
AKT/HIF-1α/VEGFA signaling. Cancer Cell Int. 19:2822019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chung SY, Chao TC and Su Y: The
stemness-high human colorectal cancer cells promote angiogenesis by
producing higher amounts of angiogenic cytokines via activation of
the Egfr/Akt/Nf-kB pathway. Int J Mol Sci. 22:13552021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li HY, Li M, Luo CC, Wang JQ and Zheng N:
Lactoferrin exerts antitumor effects by inhibiting angiogenesis in
a HT29 human colon tumor model. J Agric Food Chem. 65:10464–10472.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang L, Yi T, Kortylewski M, Pardoll DM,
Zeng D and Yu H: IL-17 can promote tumor growth through an
IL-6-Stat3 signaling pathway. J Exp Med. 206:1457–1464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Carling D: AMPK. Curr Biol. 14:R2202004.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Huang H, Wang L, Qian F, Chen X, Zhu H,
Yang M, Zhang C, Chu M, Wang X and Huang X: Liraglutide via
activation of AMP-Activated protein kinase-hypoxia inducible
factor-1α-Heme Oxygenase-1 signaling promotes wound healing by
preventing endothelial dysfunction in diabetic mice. Front Physiol.
12:6602632021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zulato E, Bergamo F, De Paoli A, Griguolo
G, Esposito G, De Salvo GL, Mescoli C, Rugge M, Nardin M, Di Grazia
L, et al: Prognostic significance of AMPK activation in advanced
stage colorectal cancer treated with chemotherapy plus bevacizumab.
Br J Cancer. 111:25–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dai W, Wang Y, Yang T, Wang J, Wu W and Gu
J: Downregulation of exosomal CLEC3B in hepatocellular carcinoma
promotes metastasis and angiogenesis via AMPK and VEGF signals.
Cell Commun Signal. 17:1132019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang Y, Wang B, Guerram M, Sun L, Shi W,
Tian C, Zhu X, Jiang Z and Zhang L: Deoxypodophyllotoxin suppresses
tumor vasculature in HUVECs by promoting cytoskeleton remodeling
through LKB1-AMPK dependent Rho A activatio. Oncotarget.
6:29497–29512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sen K, Banerjee S and Mandal M: Dual drug
loaded liposome bearing apigenin and 5-Fluorouracil for synergistic
therapeutic efficacy in colorectal cancer. Colloids Surf B
Biointerfaces. 180:9–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Qu LH, Hong X, Zhang Y, Cong X, Xiang RL,
Mei M, Su JZ, Wu LL and Yu GY: C1q/tumor necrosis factor-related
protein-6 attenuates TNF-α-induced apoptosis in salivary acinar
cells via AMPK/SIRT1-modulated miR-34a-5p expression. J Cell
Physiol. 236:5785–5800. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xie YH, Xiao Y, Huang Q, Hu XF, Gong ZC
and Du J: Role of the CTRP6/AMPK pathway in kidney fibrosis through
the promotion of fatty acid oxidation. Eur J Pharmacol.
892:1737552021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Moon HS, Liu X, Nagel JM, Chamberland JP,
Diakopoulos KN, Brinkoetter MT, Hatziapostolou M, Wu Y, Robson SC,
Iliopoulos D and Mantzoros CS: Salutary effects of adiponectin on
colon cancer: In vivo and in vitro studies in mice. Gut.
62:561–570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Iwata Y, Yasufuku I, Saigo C, Kito Y,
Takeuchi T and Yoshida K: Anti-fibrotic properties of an
adiponectin paralog protein, C1q/TNF-related protein 6 (CTRP6), in
diffuse gastric adenocarcinoma. J Cancer. 12:1161–1168. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Murayama MA, Kakuta S, Inoue A, Umeda N,
Yonezawa T, Maruhashi T, Tateishi K, Ishigame H, Yabe R, Ikeda S,
et al: CTRP6 is an endogenous complement regulator that can
effectively treat induced arthritis. Nat Commun. 6:84832015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lei H, Wu D, Wang JY, Li L, Zhang CL, Feng
H, Fu FY and Wu LL: C1q/tumor necrosis factor-related protein-6
attenuates post-infarct cardiac fibrosis by targeting RhoA/MRTF-A
pathway and inhibiting myofibroblast differentiation. Basic Res
Cardiol. 110:352015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dong X, Hu H, Fang Z, Cui J and Liu F:
CTRP6 inhibits PDGF-BB-induced vascular smooth muscle cell
proliferation and migration. Biomed Pharmacother. 103:844–850.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li R, Du J, Yao Y, Yao G and Wang X:
Adiponectin inhibits high glucose-induced angiogenesis via
inhibiting autophagy in RF/6A cells. J Cell Physiol.
234:20566–20576. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shah D, Sandhu K, Das P and Bhandari V:
Adiponectin ameliorates hyperoxia-induced lung endothelial
dysfunction and promotes angiogenesis in neonatal mice. Pediatr
Res. 91:545–555. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang N, Zhou J, Zhou Y and Guan F:
MicroRNA-148a inhibits hepatocellular carcinoma cell growth via
epithelial-to-mesenchymal transition and PI3K/AKT signaling
pathways by targeting death receptor-5. Appl Biochem Biotechnol.
194:2731–2746. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sp N, Kang DY, Jo ES, Lee JM and Jang KJ:
Iron metabolism as a potential mechanism for inducing
TRAIL-Mediated extrinsic apoptosis using methylsulfonylmethane in
embryonic cancer stem cells. Cells. 10:28472021. View Article : Google Scholar : PubMed/NCBI
|