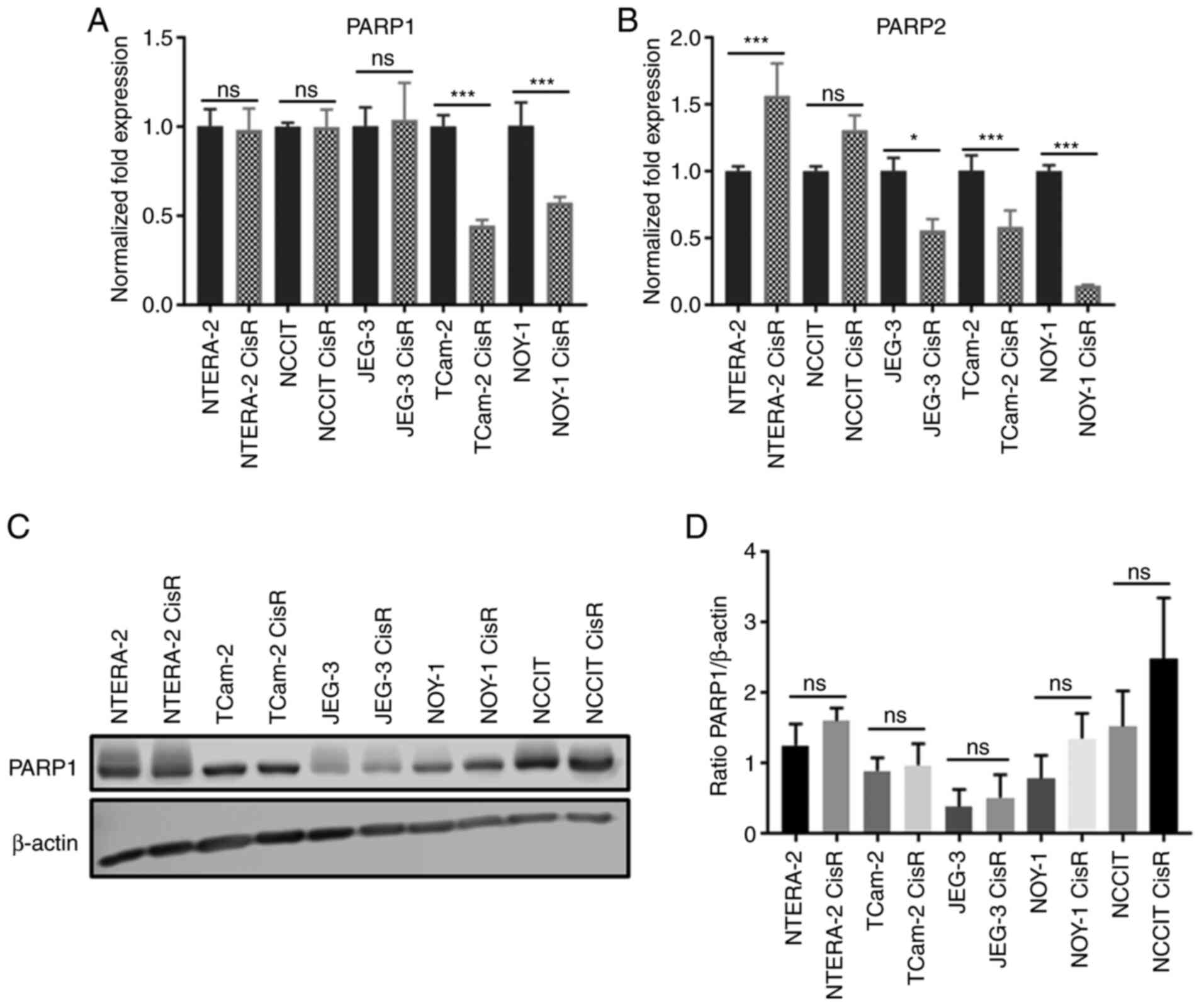
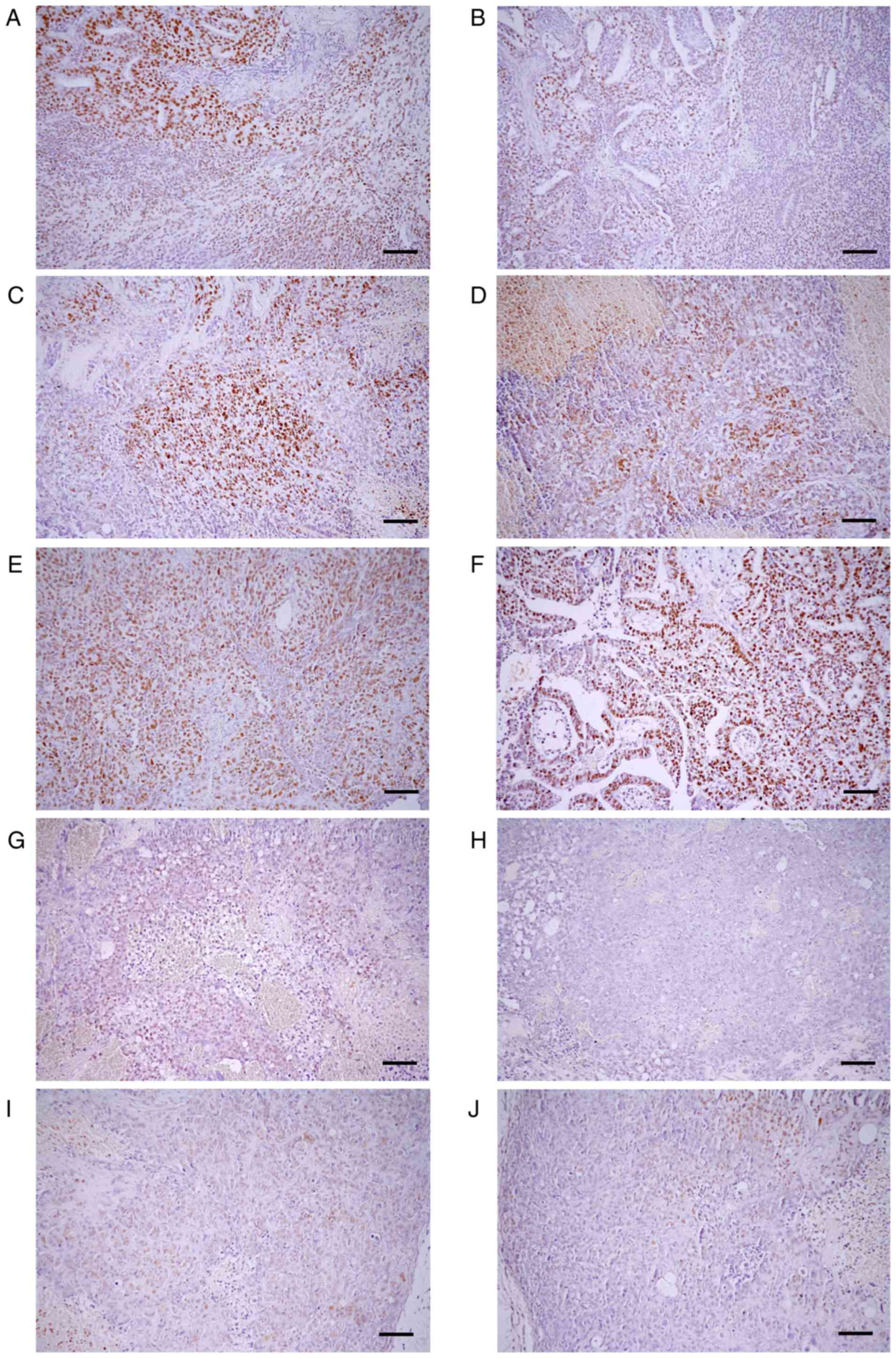
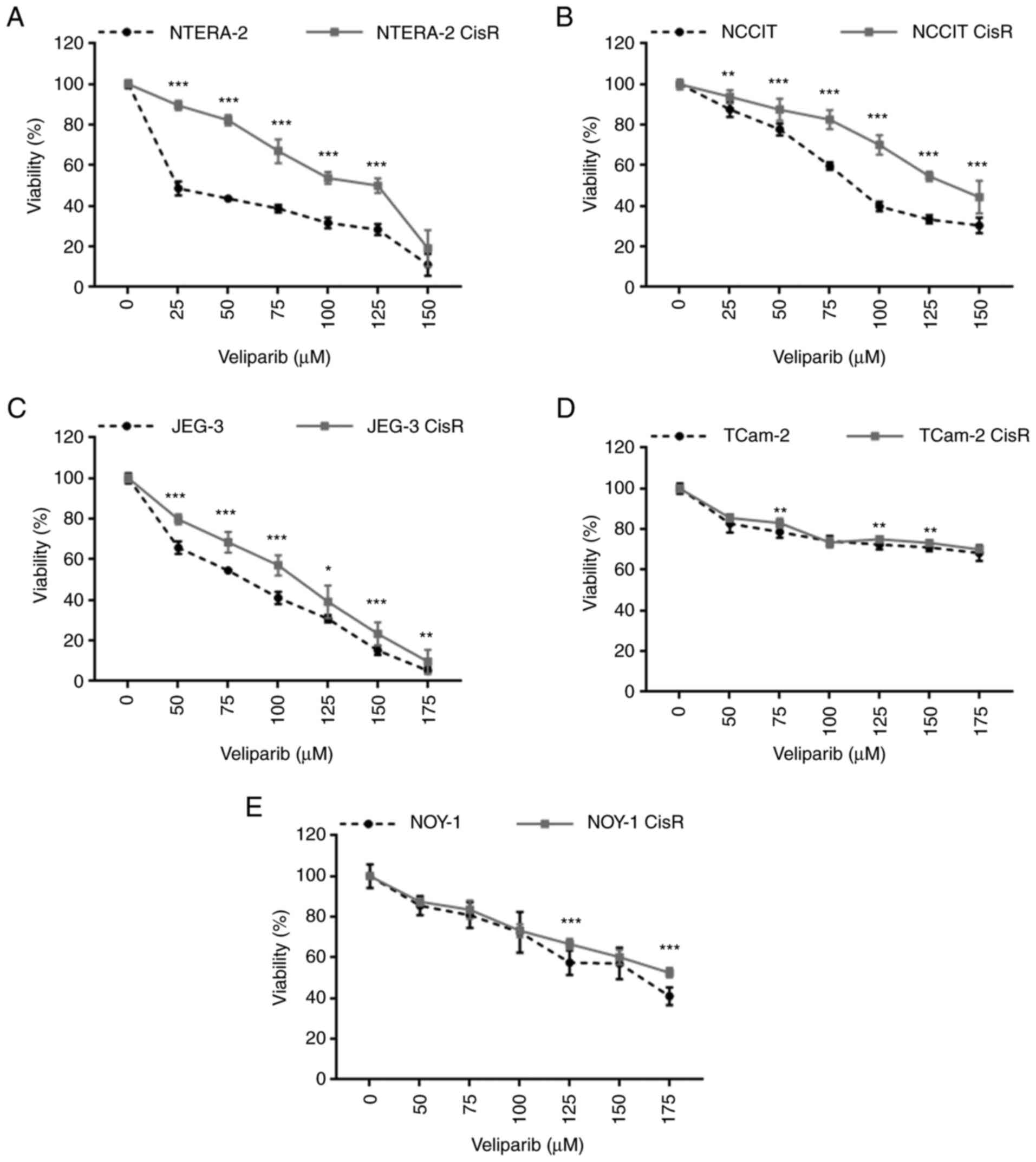
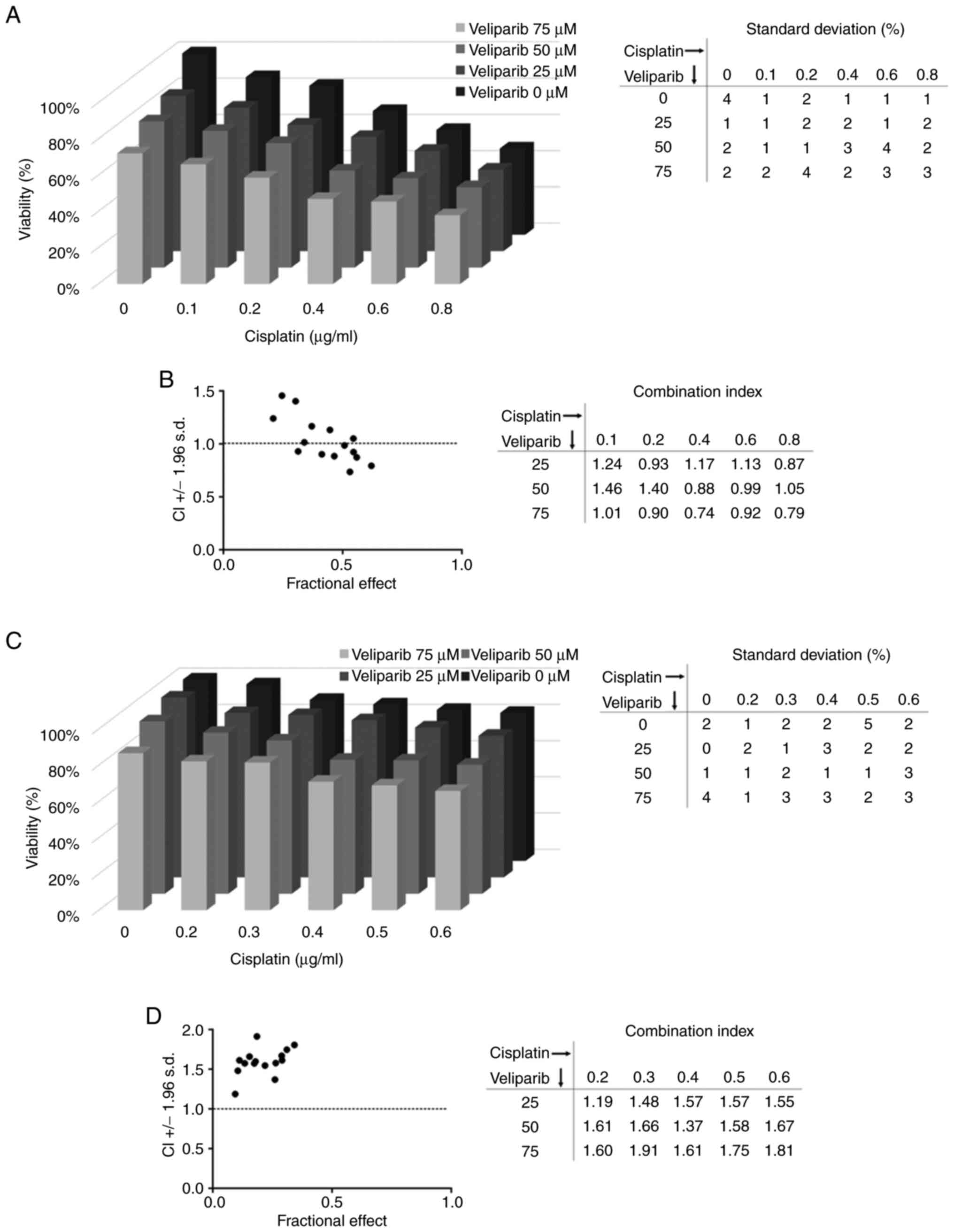
|
1
|
Oosterhuis JW and Looijenga LHJ: Human
germ cell tumours from a developmental perspective. Nat Rev Cancer.
19:522–537. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cai Q, Chen Y, Zhang D, Pan J, Xie Z, Xu
C, Li S, Zhang X, Gao Y, Hou J, et al: Estimates of over-time
trends in incidence and mortality of testicular cancer from 1990 to
2030. Transl Androl Urol. 9:182–195. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alsdorf W, Seidel C, Bokemeyer C and Oing
C: Current pharmacotherapy for testicular germ cell cancer. Expert
Opin Pharmacother. 20:837–850. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feldman DR, Patil S, Trinos MJ, Carousso
M, Ginsberg MS, Sheinfeld J, Bajorin DF, Bosl GJ and Motzer RJ:
Progression-free and overall survival in patients with
relapsed/refractory germ cell tumors treated with single-agent
chemotherapy: Endpoints for clinical trial design. Cancer.
118:981–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmidtova S, Kalavska K and Kucerova L:
Molecular mechanisms of cisplatin chemoresistance and its
circumventing in testicular germ cell tumors. Curr Oncol Rep.
20:882018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lobo J, Jeronimo C and Henrique R:
Cisplatin resistance in testicular germ cell tumors: Current
challenges from various perspectives. Cancers (Basel). 12:16012020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh R, Fazal Z, Freemantle SJ and
Spinella MJ: Mechanisms of cisplatin sensitivity and resistance in
testicular germ cell tumors. Cancer Drug Resist. 2:580–594.
2019.PubMed/NCBI
|
|
9
|
Al-Obaidy KI, Chovanec M and Cheng L:
Molecular characteristics of testicular germ cell tumors:
Pathogenesis and mechanisms of therapy resistance. Expert Rev
Anticancer Ther. 20:75–79. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mego M, Cierna Z, Svetlovska D, Macak D,
Machalekova K, Miskovska V, Chovanec M, Usakova V, Obertova J,
Babal P and Mardiak J: PARP expression in germ cell tumours. J Clin
Pathol. 66:607–612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Beek L, McClay É, Patel S, Schimpl M,
Spagnolo L and Maia de Oliveira T: PARP power: A structural
perspective on PARP1, PARP2, and PARP3 in DNA damage repair and
nucleosome remodelling. Int J Mol Sci. 22:51122021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hottiger MO, Boothby M, Koch-Nolte F,
Lüscher B, Martin NM, Plummer R, Wang ZQ and Ziegler M: Progress in
the function and regulation of ADP-ribosylation. Sci Signal.
4:mr52011.PubMed/NCBI
|
|
13
|
Zhu G and Lippard SJ: Photoaffinity
labeling reveals nuclear proteins that uniquely recognize
cisplatin-DNA interstrand cross-links. Biochemistry. 48:4916–4925.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fong PC, Boss DS, Yap TA, Tutt A, Wu P,
Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, et
al: Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA
mutation carriers. N Engl J Med. 361:123–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fong PC, Yap TA, Boss DS, Carden CP,
Mergui-Roelvink M, Gourley C, De Greve J, Lubinski J, Shanley S,
Messiou C, et al: Poly(ADP)-ribose polymerase inhibition: Frequent
durable responses in BRCA carrier ovarian cancer correlating with
platinum-free interval. J Clin Oncol. 28:2512–2519. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dockery LE, Gunderson CC and Moore KN:
Rucaparib: The past, present, and future of a newly approved PARP
inhibitor for ovarian cancer. Onco Targets Ther. 10:3029–3037.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coleman RL, Fleming GF, Brady MF, Swisher
EM, Steffensen KD, Friedlander M, Okamoto A, Moore KN, Efrat
Ben-Baruch N, Werner TL, et al: Veliparib with first-line
chemotherapy and as maintenance therapy in ovarian cancer. N Engl J
Med. 381:2403–2415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Farmer H, McCabe N, Lord CJ, Tutt AN,
Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I,
Knights C, et al: Targeting the DNA repair defect in BRCA mutant
cells as a therapeutic strategy. Nature. 434:917–921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bryant HE, Schultz N, Thomas HD, Parker
KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ and Helleday T:
Specific killing of BRCA2-deficient tumours with inhibitors of
poly(ADP-ribose) polymerase. Nature. 434:913–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rose M, Burgess JT, O'Byrne K, Richard DJ
and Bolderson E: PARP inhibitors: Clinical relevance, mechanisms of
action and tumor resistance. Front Cell Dev Biol. 8:5646012020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakogawa K, Aoki Y, Misumi K, Hamai Y, Emi
M, Hihara J, Shi L, Kono K, Horikoshi Y, Sun J, et al: Involvement
of homologous recombination in the synergism between cisplatin and
poly (ADP-ribose) polymerase inhibition. Cancer Sci. 104:1593–1599.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng H, Zhang Z, Borczuk A, Powell CA,
Balajee AS, Lieberman HB and Halmos B: PARP inhibition selectively
increases sensitivity to cisplatin in ERCC1-low non-small cell lung
cancer cells. Carcinogenesis. 34:739–749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rottenberg S, Jaspers JE, Kersbergen A,
van der Burg E, Nygren AO, Zander SA, Derksen PW, de Bruin M,
Zevenhoven J, Lau A, et al: High sensitivity of BRCA1-deficient
mammary tumors to the PARP inhibitor AZD2281 alone and in
combination with platinum drugs. Proc Natl Acad Sci USa.
105:17079–17084. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Evers B, Drost R, Schut E, de Bruin M, van
der Burg E, Derksen PW, Holstege H, Liu X, van Drunen E, Beverloo
HB, et al: Selective inhibition of BRCA2-deficient mammary tumor
cell growth by AZD2281 and cisplatin. Clin Cancer Res.
14:3916–3925. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Plummer R, Jones C, Middleton M, Wilson R,
Evans J, Olsen A, Curtin N, Boddy A, McHugh P, Newell D, et al:
Phase I study of the poly(ADP-ribose) polymerase inhibitor,
AG014699, in combination with temozolomide in patients with
advanced solid tumors. Clin Cancer Res. 14:7917–7923. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Plummer R, Lorigan P, Steven N, Scott L,
Middleton MR, Wilson RH, Mulligan E, Curtin N, Wang D, Dewji R, et
al: A phase II study of the potent PARP inhibitor, rucaparib
(PF-01367338, AG014699), with temozolomide in patients with
metastatic melanoma demonstrating evidence of chemopotentiation.
Cancer Chemother Pharmacol. 71:1191–1199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bell-McGuinn KM, Brady WE, Schilder RJ,
Fracasso PM, Moore KN, Walker JL, Duska LR, Mathews CA, Chen A,
Shepherd SP, et al: A phase I study of continuous veliparib in
combination with IV carboplatin/paclitaxel or IV/IP
paclitaxel/cisplatin and bevacizumab in newly diagnosed patients
with previously untreated epithelial ovarian, fallopian tube, or
primary peritoneal cancer: An NRG oncology/gynecologic oncology
group study. J Clin Oncol. 33 (Suppl 15):S55072015. View Article : Google Scholar
|
|
28
|
Landrum LM, Brady WE, Armstrong DK, Moore
KN, DiSilvestro PA, O'Malley DM, Tenney ME, Rose PG and Fracasso
PM: A phase I trial of pegylated liposomal doxorubicin (PLD),
carboplatin, bevacizumab and veliparib in recurrent,
platinum-sensitive ovarian, primary peritoneal, and fallopian tube
cancer: An NRG oncology/gynecologic oncology group study. Gynecol
Oncol. 140:204–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishio S, Takekuma M, Takeuchi S, Kawano
K, Tsuda N, Tasaki K, Takahashi N, Abe M, Tanaka A, Nagasawa T, et
al: Phase 1 study of veliparib with carboplatin and weekly
paclitaxel in Japanese patients with newly diagnosed ovarian
cancer. Cancer Sci. 108:2213–2220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gray HJ, Bell-McGuinn K, Fleming GF,
Cristea M, Xiong H, Sullivan D, Luo Y, McKee MD, Munasinghe W and
Martin LP: Phase I combination study of the PARP inhibitor
veliparib plus carboplatin and gemcitabine in patients with
advanced ovarian cancer and other solid malignancies. Gynecol
Oncol. 148:507–514. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wilson RH, Evans TJ, Middleton MR, Molife
LR, Spicer J, Dieras V, Roxburgh P, Giordano H, Jaw-Tsai S, Goble S
and Plummer R: A phase I study of intravenous and oral rucaparib in
combination with chemotherapy in patients with advanced solid
tumours. Br J Cancer. 116:884–892. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mego M, Svetlovska D, Reckova M, Angelis
D, Kalavska K, Obertova J, Palacka P, Rejlekova K, Sycova-Mila Z,
Chovanec M and Mardiak J: Gemcitabine, carboplatin and veliparib in
multiple relapsed/refractory germ cell tumours: The GCT-SK-004
phase II trial. Invest New Drugs. 39:1664–1670. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schmidtova S, Kalavska K, Gercakova K,
Cierna Z, Miklikova S, Smolkova B, Buocikova V, Miskovska V,
Durinikova E, Burikova M, et al: Disulfiram overcomes cisplatin
resistance in human embryonal carcinoma cells. Cancers (Basel).
11:12242019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schmidtova S, Dorssers LCJ, Kalavska K,
Gillis AJM, Oosterhuis JW, Stoop H, Miklikova S, Kozovska Z,
Burikova M, Gercakova K, et al: Napabucasin overcomes cisplatin
resistance in ovarian germ cell tumor-derived cell line by
inhibiting cancer stemness. Cancer Cell Int. 20:3642020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmidtova S, Kalavska K, Liskova V, Plava
J, Miklikova S, Kucerova L, Matuskova M, Rojikova L, Cierna Z,
Rogozea A, et al: Targeting of deregulated Wnt/β-catenin signaling
by PRI-724 and LGK974 inhibitors in germ cell tumor cell lines. Int
J Mol Sci. 22:42632021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pfaffl MW, Horgan GW and Dempfle L:
Relative expression software tool (REST) for group-wise comparison
and statistical analysis of relative expression results in
real-time PCR. Nucleic Acids Res. 30:e362002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stehlik P, Paulikova H and Hunakova L:
Synthetic isothiocyanate indole-3-ethyl isothiocyanate (homoITC)
enhances sensitivity of human ovarian carcinoma cell lines A2780
and A2780/CP to cisplatin. Neoplasma. 57:473–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Klauschen F, von Winterfeld M, Stenzinger
A, Sinn BV, Budczies J, Kamphues C, Bahra M, Wittschieber D,
Weichert W, Striefler J, et al: High nuclear
poly-(ADP-ribose)-polymerase expression is prognostic of improved
survival in pancreatic cancer. Histopathology. 61:409–416. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou J, Gelot C, Pantelidou C, Li A, Yücel
H, Davis RE, Färkkilä A, Kochupurakkal B, Syed A, Shapiro GI, et
al: A first-in-class polymerase theta inhibitor selectively targets
homologous-recombination-deficient tumors. Nat Cancer. 2:598–610.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zatreanu D, Robinson HMR, Alkhatib O,
Boursier M, Finch H, Geo L, Grande D, Grinkevich V, Heald RA,
Langdon S, et al: Poltheta inhibitors elicit BRCA-gene synthetic
lethality and target PARP inhibitor resistance. Nat Commun.
12:36362021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ray Chaudhuri A and Nussenzweig A: The
multifaceted roles of PARP1 in DNA repair and chromatin
remodelling. Nat Rev Mol Cell Biol. 18:610–621. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang M, Wu W, Wu W, Rosidi B, Zhang L,
Wang H and Iliakis G: PARP-1 and Ku compete for repair of DNA
double strand breaks by distinct NHEJ pathways. Nucleic Acids Res.
34:6170–6182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Langelier MF, Riccio AA and Pascal JM:
PARP-2 and PARP-3 are selectively activated by 5′ phosphorylated
DNA breaks through an allosteric regulatory mechanism shared with
PARP-1. Nucleic Acids Res. 42:7762–7775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ménissier de Murcia J, Ricoul M, Tartier
L, Niedergang C, Huber A, Dantzer F, Schreiber V, Amé JC, Dierich
A, LeMeur M, et al: Functional interaction between PARP-1 and
PARP-2 in chromosome stability and embryonic development in mouse.
EMBO J. 22:2255–2263. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cavallo F, Graziani G, Antinozzi C,
Feldman DR, Houldsworth J, Bosl GJ, Chaganti RS, Moynahan ME, Jasin
M and Barchi M: Reduced proficiency in homologous recombination
underlies the high sensitivity of embryonal carcinoma testicular
germ cell tumors to cisplatin and poly (adp-ribose) polymerase
inhibition. PLoS One. 7:e515632012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ogino H, Nakayama R, Sakamoto H, Yoshida
T, Sugimura T and Masutani M: Analysis of poly(ADP-ribose)
polymerase-1 (PARP1) gene alteration in human germ cell tumor cell
lines. Cancer Genet Cytogenet. 197:8–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Murai J, Huang SY, Das BB, Renaud A, Zhang
Y, Doroshow JH, Ji J, Takeda S and Pommier Y: Trapping of PARP1 and
PARP2 by CLinical PARP inhibitors. Cancer Res. 72:5588–5599. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dedes KJ, Wilkerson PM, Wetterskog D,
Weigelt B, Ashworth A and Reis-Filho JS: Synthetic lethality of
PARP inhibition in cancers lacking BRCA1 and BRCA2 mutations. Cell
Cycle. 10:1192–1199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Golan T, Hammel P, Reni M, Van Cutsem E,
Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, et
al: Maintenance olaparib for germline BRCA-mutated metastatic
pancreatic cancer. N Engl J Med. 381:317–327. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Robson ME, Tung N, Conte P, Im SA, Senkus
E, Xu B, Masuda N, Delaloge S, Li W, Armstrong A, et al: OlympiAD
final overall survival and tolerability results: Olaparib versus
chemotherapy treatment of physician's choice in patients with a
germline BRCA mutation and HER2-negative metastatic breast cancer.
Ann Oncol. 30:558–566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
de Bono J, Mateo J, Fizazi K, Saad F,
Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, et al:
Olaparib for metastatic castration-resistant prostate cancer. N
Engl J Med. 382:2091–2102. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Abida W, Campbell D, Patnaik A, Shapiro
JD, Sautois B, Vogelzang NJ, Voog EG, Bryce AH, McDermott R, Ricci
F, et al: Non-BRCA DNA damage repair gene alterations and response
to the PARP inhibitor rucaparib in metastatic castration-resistant
prostate cancer: Analysis from the phase II TRITON2 study. Clin
Cancer Res. 26:2487–2496. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
George RR, Thomas R, Davice A and Mathew
MS: Veliparib for the treatment of solid malignancies. J Oncol
Pharm Pract. 28:924–934. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rodler ET, Kurland BF, Griffin M, Gralow
JR, Porter P, Yeh RF, Gadi VK, Guenthoer J, Beumer JH, Korde L, et
al: Phase I study of veliparib (ABT-888) combined with cisplatin
and vinorelbine in advanced triple-negative breast cancer and/or
BRCA mutation-associated breast cancer. Clin Cancer Res.
22:2855–2864. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Diéras V, Han HS, Kaufman B, Wildiers H,
Friedlander M, Ayoub JP, Puhalla SL, Bondarenko I, Campone M,
Jakobsen EH, et al: Veliparib with carboplatin and paclitaxel in
BRCA-mutated advanced breast cancer (BROCADE3): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.
21:1269–1282. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Byers LA, Bentsion D, Gans S, Penkov K,
Son C, Sibille A, Owonikoko TK, Groen HJM, Gay CM, Fujimoto J, et
al: Veliparib in combination with carboplatin and etoposide in
patients with treatment-Naïve extensive-stage small cell lung
cancer: A phase 2 randomized study. Clin Cancer Res. 27:3884–3895.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Clarke JM, Patel JD, Robert F, Kio EA,
Thara E, Ross Camidge D, Dunbar M, Nuthalapati S, Dinh MH and Bach
BA: Veliparib and nivolumab in combination with platinum doublet
chemotherapy in patients with metastatic or advanced non-small cell
lung cancer: A phase 1 dose escalation study. Lung Cancer.
161:180–188. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lobo J, Constancio V, Guimaraes-Teixeira
C, Leite-Silva P, Miranda-Gonçalves V, Sequeira JP, Pistoni L,
Guimarães R, Cantante M, Braga I, et al: Promoter methylation of
DNA homologous recombination genes is predictive of the
responsiveness to PARP inhibitor treatment in testicular germ cell
tumors. Mol Oncol. 15:846–865. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Caggiano C, Cavallo F, Giannattasio T,
Cappelletti G, Rossi P, Grimaldi P, Feldman DR, Jasin M and Barchi
M: Testicular germ cell tumors acquire cisplatin resistance by
rebalancing the usage of DNA repair pathways. Cancers (Basel).
13:7872021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
De Giorgi U, Schepisi G, Gurioli G, Pisano
C, Basso U, Lolli C, Petracci E, Casadei C, Cecere SC, Attademo L,
et al: Olaparib as salvage treatment for advanced germ cell tumors
after chemotherapy failure: Results of the open-label, single-arm,
IGG-02 phase II trial. J Clin Oncol. 38 (Suppl 15):S50582020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shen Y, Rehman FL, Feng Y, Boshuizen J,
Bajrami I, Elliott R, Wang B, Lord CJ, Post LE and Ashworth A: BMN
673, a novel and highly potent PARP1/2 inhibitor for the treatment
of human cancers with DNA repair deficiency. Clin Cancer Resh.
19:5003–5015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jiang Y, Meng XY, Deng NN, Meng C, Li LH,
He ZK, Wang XY, Song ZY and Cui RJ: Effect and safety of
therapeutic regimens for patients with germline BRCA
mutation-associated breast cancer: A network meta-analysis. Front
Oncol. 11:7187612021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kim DS, Camacho CV and Kraus WL: Alternate
therapeutic pathways for PARP inhibitors and potential mechanisms
of resistance. Exp Mol Med. 53:42–51. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dias MP, Moser SC, Ganesan S and Jonkers
J: Understanding and overcoming resistance to PARP inhibitors in
cancer therapy. Nat Rev Clin Oncol. 18:773–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Edwards SL, Brough R, Lord CJ, Natrajan R,
Vatcheva R, Levine DA, Boyd J, Reis-Filho JS and Ashworth A:
Resistance to therapy caused by intragenic deletion in BRCA2.
Nature. 451:1111–1115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ter Brugge P, Kristel P, van der Burg E,
Boon U, de Maaker M, Lips E, Mulder L, de Ruiter J, Moutinho C,
Gevensleben H, et al: Mechanisms of therapy resistance in
patient-derived xenograft models of BRCA1-deficient breast cancer.
J Natl Cancer Inst. 108:2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Taglialatela A, Alvarez S, Leuzzi G,
Sannino V, Ranjha L, Huang JW, Madubata C, Anand R, Levy B, Rabadan
R, et al: Restoration of replication fork stability in BRCA1- and
BRCA2-deficient cells by inactivation of SNF2-family fork
remodelers. Mol Cell. 68:414–430.e8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rondinelli B, Gogola E, Yücel H, Duarte
AA, van de Ven M, van der Sluijs R, Konstantinopoulos PA, Jonkers
J, Ceccaldi R, Rottenberg S and D'Andrea AD: EZH2 promotes
degradation of stalled replication forks by recruiting MUS81
through histone H3 trimethylation. Nat Cell Biol. 19:1371–1378.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jaspers JE, Sol W, Kersbergen A, Schlicker
A, Guyader C, Xu G, Wessels L, Borst P, Jonkers J and Rottenberg S:
BRCA2-deficient sarcomatoid mammary tumors exhibit multidrug
resistance. Cancer Res. 75:732–741. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gogola E, Duarte AA, de Ruiter JR, Wiegant
WW, Schmid JA, de Bruijn R, James DI, Guerrero Llobet S, Vis DJ,
Annunziato S, et al: Selective loss of PARG restores PARylation and
counteracts PARP inhibitor-mediated synthetic lethality. Cancer
Cell. 35:950–952. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bajrami I, Frankum JR, Konde A, Miller RE,
Rehman FL, Brough R, Campbell J, Sims D, Rafiq R, Hooper S, et al:
Genome-wide profiling of genetic synthetic lethality identifies
CDK12 as a novel determinant of PARP1/2 inhibitor sensitivity.
Cancer Res. 74:287–297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jiang J, Yang ES, Jiang G, Nowsheen S,
Wang H, Wang T, Wang Y, Billheimer D, Chakravarthy AB, Brown M, et
al: p53-dependent BRCA1 nuclear export controls cellular
susceptibility to DNA damage. Cancer Res. 71:5546–5557. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yang ES, Nowsheen S, Rahman MA, Cook RS
and Xia F: Targeting BRCA1 localization to augment breast tumor
sensitivity to poly(ADP-Ribose) polymerase inhibition. Cancer Res.
72:5547–5555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
LoRusso P, Pilat MJP, Santa-Maria CA,
Connolly, Roesch EE, Afghahi A, Han HS, Nanda R, Wulf GM, Assad H,
et al: Trial in progress: A phase II open-label, randomized study
of PARP inhibition (olaparib) either alone or in combination with
anti-PD-L1 therapy (atezolizumab) in homologous DNA repair (HDR)
deficient, locally advanced or metastatic non-HER2-positive breast
cancer. J Clin Oncol. 38 (Suppl 15):TPS11022020. View Article : Google Scholar
|
|
78
|
Moore KN, Chambers SK, Hamilton EP, Chen
LM, Oza AM, Ghamande SA, Konecny GE, Plaxe SC, Spitz DL, Geenen
JJJ, et al: Adavosertib with chemotherapy in patients with primary
platinum-resistant ovarian, fallopian tube, or peritoneal cancer:
An open-label, four-arm, phase II study. Clin Cancer Res. 28:36–44.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tutt A, Stephens C, Frewer P, Pierce A,
Rhee J, Edgington S, Ottesen L, Ah-See ML, Hollingsworth SJ and
Dean E: VIOLETTE: A randomized phase II study to assess the DNA
damage response inhibitors AZD6738 or AZD1775 in combination with
olaparib (Ola) versus Ola monotherapy in patients (pts) with
metastatic, triple-negative breast cancer (TNBC). J Clin Oncol. 37
(Suppl 15):TPS11122019. View Article : Google Scholar
|
|
80
|
Choi YE, Battelli C, Watson J, Liu J,
Curtis J, Morse AN, Matulonis UA, Chowdhury D and Konstantinopoulos
PA: Sublethal concentrations of 17-AAG suppress homologous
recombination DNA repair and enhance sensitivity to carboplatin and
olaparib in HR proficient ovarian cancer cells. Oncotarget.
5:2678–2687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kim H, George E, Ragland R, Rafail S,
Zhang R, Krepler C, Morgan M, Herlyn M, Brown E and Simpkins F:
Targeting the ATR/CHK1 axis with PARP inhibition results in tumor
regression in BRCA-mutant ovarian cancer models. Clin Cancer Res.
23:3097–3108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Murai J, Feng Y, Yu GK, Ru Y, Tang SW,
Shen Y and Pommier Y: Resistance to PARP inhibitors by SLFN11
inactivation can be overcome by ATR inhibition. Oncotarget.
7:76534–76550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Muvarak NE, Chowdhury K, Xia L, Robert C,
Choi EY, Cai Y, Bellani M, Zou Y, Singh ZN, Duong VH, et al:
Enhancing the cytotoxic effects of PARP inhibitors with DNA
demethylating agents-a potential therapy for cancer. Cancer Cell.
30:637–650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Pulliam N, Fang F, Ozes AR, Tang J,
Adewuyi A, Keer H, Lyons J, Baylin SB, Matei D, Nakshatri H, et al:
An effective epigenetic-PARP inhibitor combination therapy for
breast and ovarian cancers independent of BRCA mutations. Clin
Cancer Res. 24:3163–3175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jiao S, Xia W, Yamaguchi H, Wei Y, Chen
MK, Hsu JM, Hsu JL, Yu WH, Du Y, Lee HH, et al: PARP inhibitor
upregulates PD-L1 expression and enhances cancer-associated
immunosuppression. Clin Cancer Res. 23:3711–3720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mehta AK, Cheney EM, Hartl CA, Pantelidou
C, Oliwa M, Castrillon JA, Lin JR, Hurst KE, de Oliveira Taveira M,
Johnson NT, et al: Targeting immunosuppressive macrophages
overcomes PARP inhibitor resistance in BRCA1-associated
triple-negative breast cancer. Nat Cancer. 2:66–82. 2021.
View Article : Google Scholar : PubMed/NCBI
|