Introduction
Follicular dendritic cell sarcoma (FDCS) is a rare
tumor type. The majority of FDCSs are located in lymph nodes, while
the majority of extra-lymph node lesions are found in the liver,
spleen, nasopharynx and soft tissues of the neck, which are rich in
lymphoid tissue (1). Inflammatory
pseudotumor-like follicular dendritic cell sarcoma (IPT-like FDCS)
is a rare type of low-grade malignancy that was defined by Cheuk
et al (2) in 2001. IPT-like
FDCS is much rarer than classical FDCS and is a specific subtype of
FDCS that occurs mainly in the liver and spleen. The clinical
manifestations of splenic IPT-like FDCS are nonspecific; most
patients do not have any obvious symptoms and splenic tumors are
typically discovered unintentionally during physical examination. A
small number of patients may present with upper abdominal
discomfort, abdominal pain, fever and/or weight loss. The present
study reported the pathological and imaging data of a case of
IPT-like FDCS admitted to our hospital and reviewed the literature
published since Cheuk et al (2) defined IPT-like FDCS in 2001, to gain
a deeper understanding of this rare tumor type and to assist
clinicians in developing a treatment plan.
Case report
A 29-year-old female patient presented at the First
People's Hospital of Zunyi (Guizhou, China) in July 2021, where a
large splenic mass was incidentally detected during an abdominal
ultrasound examination for their company-required annual physical
examination. Physical examination revealed mild tenderness to
palpation in the left upper quadrant. The levels of the tumor
markers α-fetoprotein, carcinoembryonic antigen, carbohydrate
antigen 125 (CA125) and CA199 were all normal. The patient denied
having any remarkable medical personal or family history. Abdominal
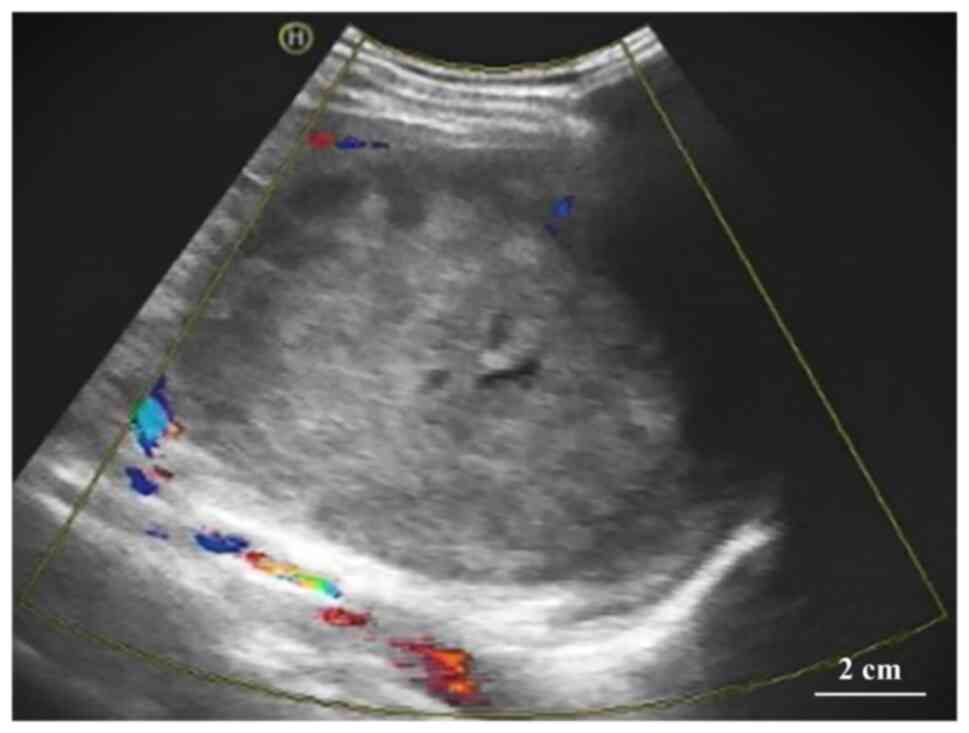
sonography revealed a heterogeneous echogenic mass measuring
~12.8×11.4×12.1 cm with hazy borders and poorly defined surrounding
tissue. There was a fluid hypoechoic region within the mass, as
well as dotted and streaked blood hyposignals in and around the
mass (Fig. 1).
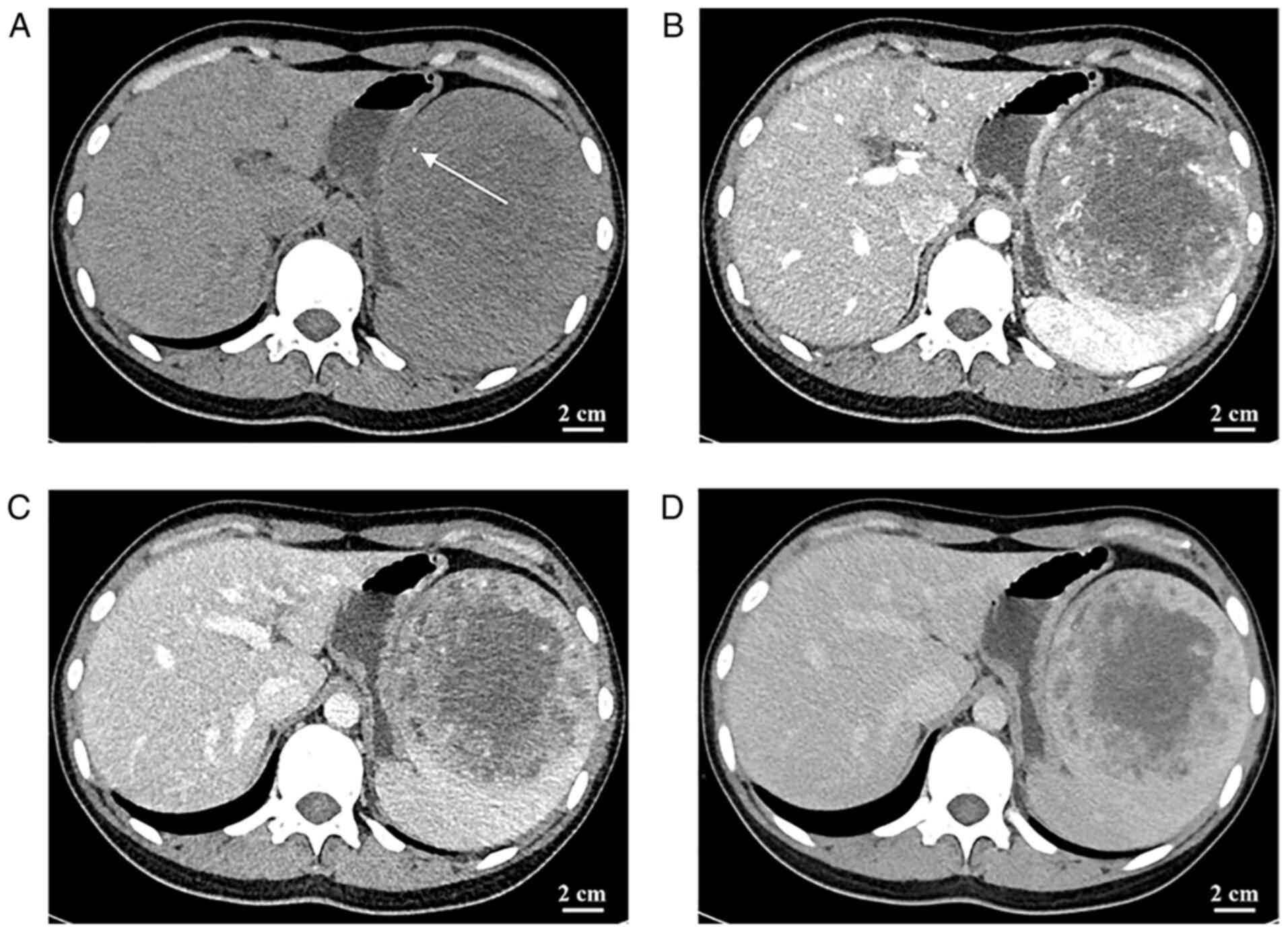
The CT of the abdomen revealed a mass of
heterogeneous density with patchy, slightly hyperdense and poorly
defined borders. In addition, punctate calcification was observed
in the tumor. The parenchymal portion of the tumor had progressive
enhancement, whereas the central liquefied necrotic region
exhibited no discernible enhancement. The boundary between the
tumor and the surrounding tissue was visible following enhancement
(Fig. 2). Abdominal CT displayed a
slightly hypodense, ill-defined mass. In addition, small patchy
hemorrhage as well as calcification were seen within the mass
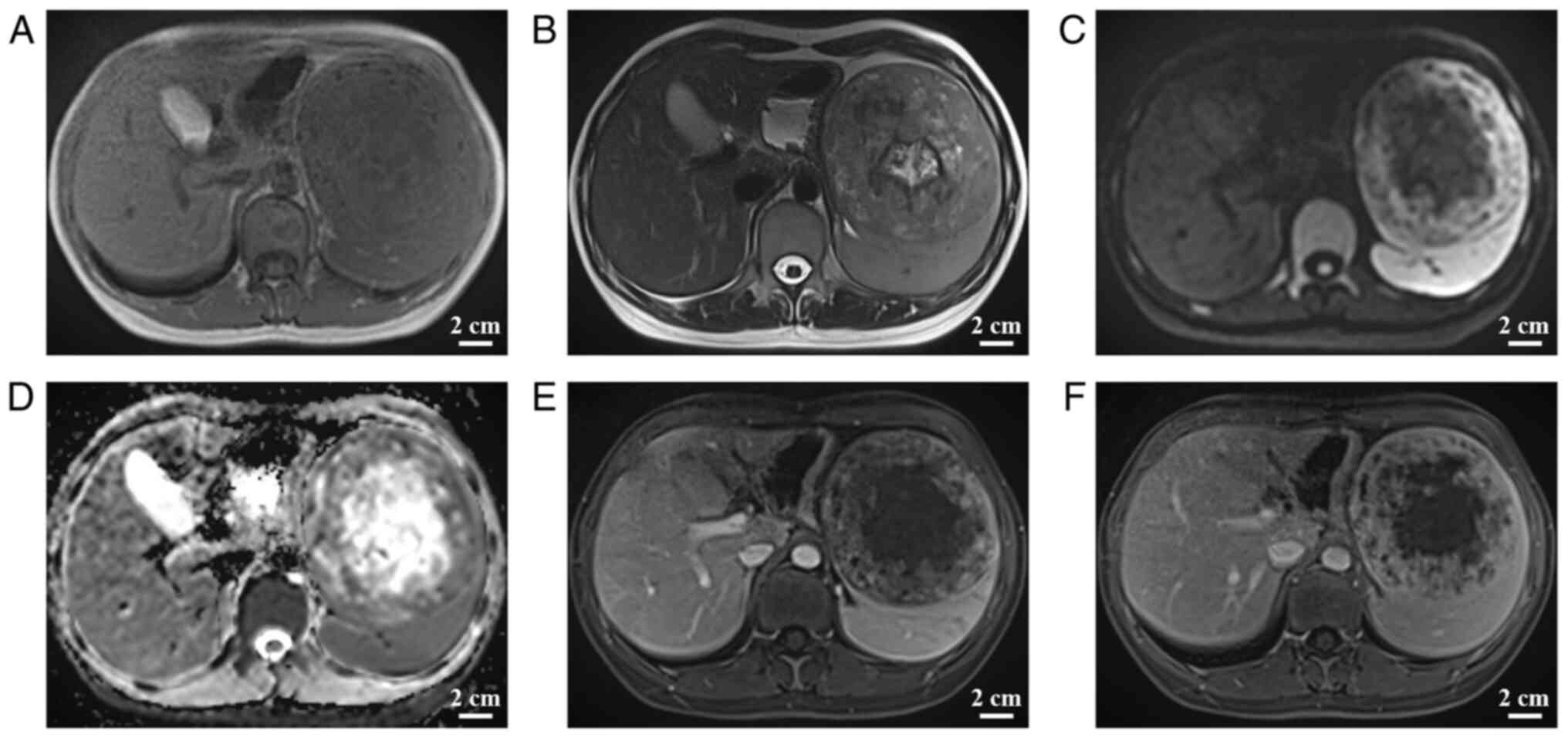
(Fig. 2). Abdominal magnetic
resonance imaging (MRI), including conventional MRI and enhanced
abdominal MRI, indicated a mixed-signal mass in the spleen,
measuring ~10.6×10.6×10.1 cm, with well-defined borders. The tumor
margin had an envelope-like structure and the parenchyma exhibited
progressive enhancement with no enhancement in the central necrotic
area. The parenchymal part of the neoplasm had a high signal
(b=1,000 sec/mm2) on diffusion-weighted imaging (DWI)
and a low signal on the apparent diffusion coefficient map,
suggesting that the spread of the parenchymal part of the neoplasm
was restricted; by contrast, the central necrotic area was not
restricted (Fig. 3). The
radiologist initially diagnosed a vascular tumor originating from
the spleen, such as hemangioma, based on this patient's clinical
presentation and the imaging features. However, it was not possible
to exclude other benign or malignant neoplastic lesions.
After a multidisciplinary team discussion, the
clinicians decided that surgical resection was the most appropriate
treatment strategy. This patient subsequently underwent an open
splenectomy with the surgical incision located under the left
subcostal area. Macroscopically, the mass was round in shape,
measuring ~11.0×9.5×9.6 cm, with solid gray-white tissue on the cut
surface of the mass, clearly demarcated from the surrounding
tissues, and a necrotic area visible in the center of the lesion
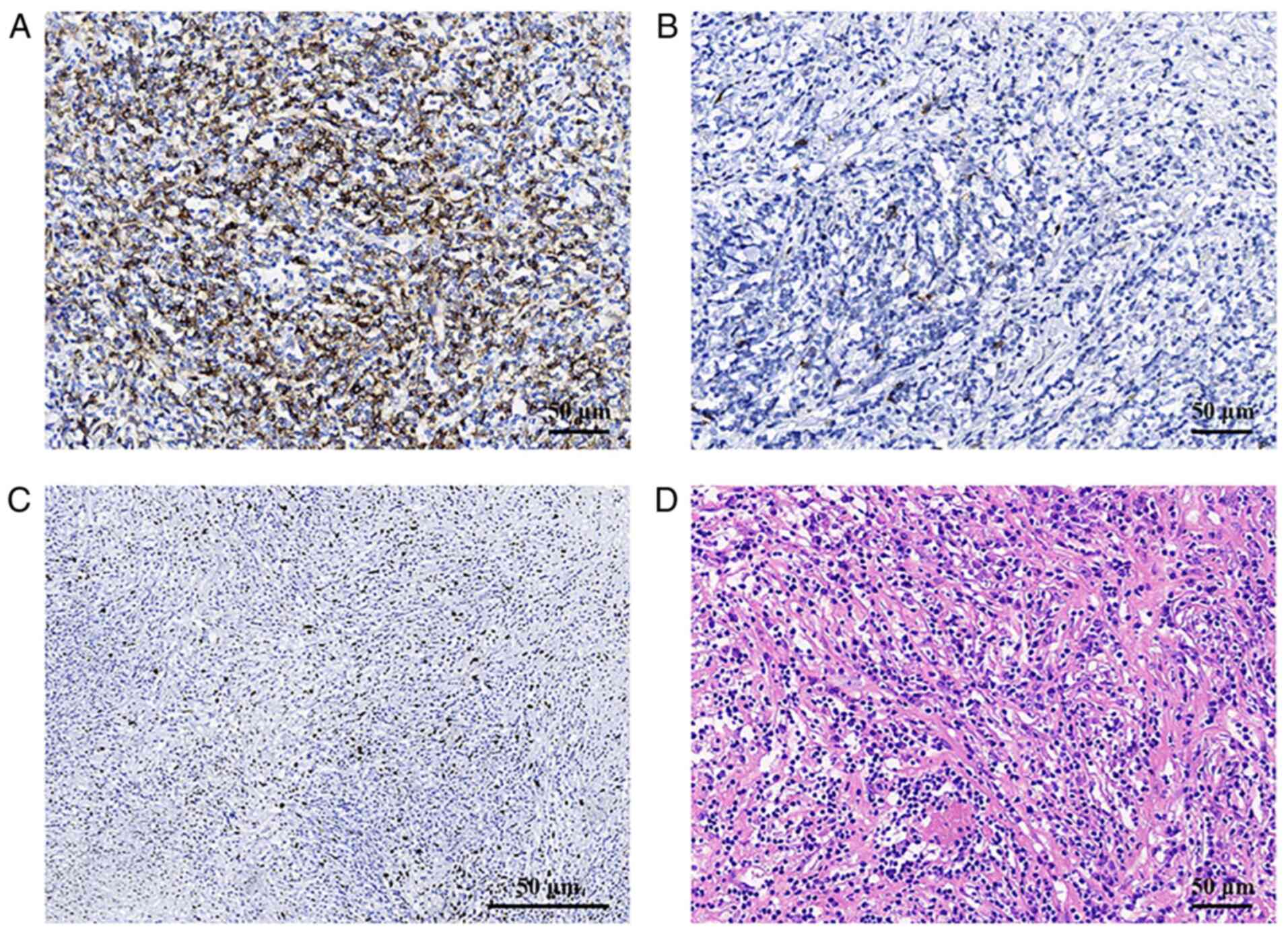
within the mass. Histologically, tumor cells were scattered or
arranged in faint bundles in a prominent lymphoplasmacytic
infiltrate (Fig. 4). The tumor
cells were shuttle-shaped with indistinct borders and abundant red
cytoplasm. The nuclei were elongated and vesicular, with small but
distinct nucleoli. Histopathological examination diagnosed splenic
inflammatory pseudotumor-like follicular dendritic cell sarcoma
(IPT-like FDCS). Immunohistochemical staining (3) using antibodies from Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd. revealed positivity for CD21
(cat. no. ZA-0525; prediluted by the manufacturer), CD23 (cat. no.
ZA-0516; prediluted by the manufacturer), CD35 (cat. no. ZA-0638;
prediluted by the manufacturer) and a high Ki67 (cat. no. ZM-0378;
prediluted by the manufacturer) proliferation index (~10%);
furthermore, Epstein-Barr virus (EBV)-encoded RNA (EBER) was
detected by in situ hybridization (cat. no. ZM-0105;
dilution, 1:20) (4) (Fig. 4). The patient received
postoperative anti-infective treatment; the patient's vital signs
were stable and the patient recovered well, and the patient was
discharged from the hospital 10 days after surgery. The patient was
followed up for 12 months and is now in a healthy condition, with
good treatment results, and no recurrence or metastasis was
detected during an abdominal MRI as well as an abdominal ultrasound
examination in the last month.
Discussion
FDCS was first described by Monda et al
(5) in 1986. FDCS is considered a
rare, low-grade malignancy that originates from follicular
dendritic cells in the follicle-generating centers of lymph nodes
or the lymphoid tissue outside of lymph nodes. IPT-like FDCS is a
new type defined by Cheuk et al (2) in 2001 and is closely related to EBV
infection and certain histological features of inflammatory
pseudotumor (IPT) (6). In a
literature search performed as part of the present study, Google
Scholar (https://scholar.google.com) and
PubMed (https://pubmed.ncbi.nlm.nih.gov) were used as
databases and all English-language literature was searched from
2001, the year when Cheuk et al (2) officially named the tumor IPT-like
FDCS, to the present, using the search terms ‘IPT-like FDCS’ or
‘inflammatory pseudotumor-like follicular dendritic cell sarcoma’.
Each report was carefully red, excluding duplicates and those that
studies that were not on IPT-like FDCS. Finally, 106 cases with a
definite diagnosis of IPT-FDCS published in the English language
were retrieved and Table I details
the epidemiologically relevant characteristics of these cases
(further information provided in Table SI). The following features of the
disease were identified by summarizing previous studies: i) This
disease has a wide age range of distribution of 19–88 years
(2,7), but patients are predominantly
middle-aged and elderly, with a mean age of 54.59 years; ii) female
patients were more common (~62.26%); iii) the most common organ
affected was the spleen (62 cases), followed by the liver (26
cases), and other sites included the colon (8 cases), both the
liver and spleen concomitantly (5 cases), pancreas and mesentery (2
cases each) and lung (1 case); and iv) the vast majority of
IPT-like FDCS cases were associated with EBV infection and only
three cases reported previously in the literature were negative for
EBV-encoded RNA by in situ hybridization. EBV infection
starts in the oropharynx, and subsequently, the virus enters the
circulation of humans and binds to the CD21 receptor on B
lymphocytes (8); therefore,
positive expression of CD21 may be detected by immunohistochemistry
to examine EBV infection. Of note, Takeuchi et al (9) reported an increase in the number of
EBV-infected cells in IgG4-associated lymphadenopathy, suggesting
that IgG4-associated disease may be associated with EBV. In
addition, Choe et al (10)
reported that a large number of IgG4-positive plasma cells were
found in six EBV-positive patients with IPT-like FDCS, suggesting
that EBV has a key role in IPT-like FDCS. EBV is associated not
only with IPT-like FDCS but also with Burkitt's lymphoma,
nasopharyngeal carcinoma and Hodgkin's disease (HD). In addition to
the features mentioned above, another important finding of the
present literature search was that, compared to developed
countries, developing countries have higher rates of EBV infection
and consequently a higher incidence of EBV-related diseases
(11), including IPT-like FDCS. By
analyzing the available relevant literature, it was indicated that
IPT-like FDCS is more common in East Asia, accounting for 81.13% of
worldwide cases, which may be due to the food culture (e.g.,
Chinese-style salted fish) and level of economic development in
East Asian countries (12)
(Table I).
 | Table I.Epidemiologic features of inflammatory
pseudotumor-like follicular dendritic cell sarcoma. |
Table I.
Epidemiologic features of inflammatory
pseudotumor-like follicular dendritic cell sarcoma.
|
|
| Sex, n |
|
|---|
|
|
|
|
|
|---|
| Region | Cases, n | Female | Male | Location |
|---|
| East Asia | 86 | 52 | 34 | Spleen (n=49), liver
(n=24), colon (n=6), liver and spleen (n=4), peri-pancreas (n=1),
lung (n=1), ileum and mesentery (n=1) |
| America | 13 | 10 | 3 | Spleen (n=10),
pancreas and spleen (n=1), colon (n=1), liver and spleen (n=1) |
| Europe | 6 | 3 | 3 | Spleen (n=3), liver
(n=2), ileum and mesentery (n=1) |
| South Asia | 1 | 1 | 0 | Colon (n=1) |
The imaging presentation was summarized based on
previous reports in the literature (7,13).
In most cases of IPT-like FDCS, the lesion appears on CT as a
round, hypodense mass with well-defined borders, frequently with
hemorrhage, necrosis and calcification. The MRI features of
IPT-like FDCS were a well-defined soft tissue mass with fibrous
envelope-like structures. Contrast enhancement on both CT and MRI
indicated progressive enhancement of the parenchyma, demonstrating
that the parenchyma of the tumor is rich in capillary blood supply,
and the border becomes clearer in the arterial phase. On DWI, the
parenchymal part of the tumor is diffusion-limited, while the
liquefied necrotic area is not limited, suggesting that the solid
part of the tumor has a higher tumor cell density. In the present
study, the MRI signal of the solid part of the tumor was not
homogeneous and multiple small patches of the hypersignal were
visible on T2-weighted images Since the MR signal intensity varies
with the composition of the parenchymal part (14), it was considered that this may be
due to microhemorrhagic foci in the parenchymal part or related to
the number of inflammatory cells. The central necrotic area does
not exhibit a distinct hypointense signal on T1-weighted images but
an isosignal, which was speculated to be due to the necrotic area
of this tumor not being liquefied necrosis but coagulative
necrosis, and a ring of granulation or fibrous tissue may be seen
at the edge of this necrotic area. This ring of granulation/fibrous
tissue exhibits a typical magnetic resonance signal pattern. The
tumor was of a large size that it was rarely encountered in the
previous literature, to an extent that adjacent tissues and organs
are compressed, which may lead to the appearance of corresponding
clinical symptoms. In addition, the tumor of the present case did
not exhibit any aggressive biological behavior. This further
supports that the IPT-like FDCS is relatively inert and/or has a
slow growth rate (10).
IPT-like FDCS requires to be distinguished from
hemangioma of the spleen, lymphoma of the spleen and metastases
occurring on the spleen on imaging presentation. Hemangioma of the
spleen is the most common benign tumor type occurring in the
spleen. It is usually accompanied by multiple foci of punctate
calcification. Due to the abundant blood sinuses and slow blood
flow within the hemangioma, it is characterized by a significant
hypersignal on T2-weighted images. Splenic lymphoma is the most
common malignancy that occurs in the spleen. Splenic lymphomas may
be divided into HD and non-Hodgkin's lymphoma. In patients with HD,
the spleen is frequently the first or even the only organ involved.
Splenic lymphoma has a variety of imaging presentations. When HD
appears as an isolated large mass, it is difficult to distinguish
it from IPT-like FDCS on CT in terms of the mass itself, but
lymphoma is frequently associated with enlarged lymph nodes around
the spleen or elsewhere, which is different from IPT-like FDCS. The
spleen is a rare site of tumor metastasis, but splenic metastasis
is the second most common malignancy of the spleen. These
metastases usually present as multiple foci in the spleen and a
single lesion is less common. Melanoma is one of the most common
sources of splenic metastases (15). Metastases from melanoma have a
unique imaging presentation, such as a hypersignal on T1-weighted
images and a hyposignal on T2-weighted images, which is not
difficult to distinguish from IPT-like FDCS. The diagnosis of
splenic metastases is not difficult when the patient has a history
of primary tumor or other organ metastases; otherwise, a
pathological biopsy is required to make a definitive diagnosis.
IPT-like FDCS is a low-grade malignant tumor with a
relatively inert biological behavior that has been reported in the
previous literature to have lower metastasis and recurrence rates
than classic FDCS, which exhibits more aggressive and higher
mortality rates (2). In an earlier
report in the literature, three out of nine patients with IPT-like
FDCS experienced recurrence within three years, i.e., a recurrence
rate of ~33% at three years (2).
Chan et al (16) determined
that indicators of poor prognosis were tumors with a diameter of
>6 cm in with coagulative necrosis, tumor cells with significant
heterogeneity and nuclear schistosomes >5/10 high-power fields.
However, it remains elusive whether these indicators are also
applicable to IPT-like FDCS occurring in the spleen. For the
treatment of this tumor type, radical surgical resection is still
considered the treatment of choice due to the controversial effects
of radiotherapy and chemotherapy on IPT-like FDCS, and chemotherapy
combined with radiotherapy may be considered for patients with
recurrence or those who are not able to tolerate surgery, but the
use of adjuvant radiotherapy and chemotherapy is still
controversial (17). Adjuvant
radiotherapy is thought to have a role in prolonging patient
survival, but adjuvant chemotherapy has demonstrated inconsistent
results, with no significant change in local recurrence rates or
metastasis rates compared to previously reported rates. Therefore,
it is essential that surgical resection of the tumor is complete,
so it may be suggested that surgeons carefully examine the
surgically resected specimens of this tumor type to ensure that the
tumor is completely removed to improve patient survival.
Compared with previous relevant studies, the present
case report suggests for the first time that the prevalence of
IPT-like FDCS is significantly higher in East Asia than in other
regions, which may be related to the increased prevalence of
EBV-related diseases due to diet and lifestyle habits (18–20);
in addition, the clinical characteristics, treatment and follow-up
results of all previous case reports were reviewed; and finally, a
younger age of the cases was observed. However, the clinical
diagnostic methods of all previously reported cases were not
compared and no gross anatomical images of the tumor after surgical
resection were provided, which are limitations of the present
study. The possibility of IPT-like FDCS should be considered when a
single round-like well-defined mass-like lesion is present in the
spleen of a female patient, particularly with the progressive
enhancement of the parenchyma, although the disease is sporadic.
Pathological immunohistology is ultimately required to confirm the
diagnosis, i.e., by detection of immunological markers such as
CD21, CD23 and CD35, as well as by in situ hybridization
with EBV probes (21–23).
In summary, the present study reported a sporadic
case of splenic IPT-like FDCS including its imaging features, and,
more importantly, the literature review indicated for the first
time, to the best of our knowledge, that the disease is more
prevalent in the Asian population. The present case has unique
features that we hope will help physicians further understand the
diagnosis and treatment of this disease.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JRZ and LJ designed the study and wrote the
manuscript. LH, JW and YQ performed all of the experiments. JRZ
performed the literature review. LJ and XJM were involved in the
acquisition of the data and confirm the authenticity of all the raw
data. JRZ revised the manuscript and interpreted the data. All
authors agreed to the journal to which the article was submitted
and agreed to take responsibility for all aspects of the work. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen T and Gopal P: Follicular dendritic
cell sarcoma. Arch Pathol Lab Med. 141:596–599. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheuk W, Chan JK, Shek TW, Chang JH, Tsou
MH, Yuen NW, Ng WF, Chan AC and Prat J: Inflammatory
pseudotumor-like follicular dendritic cell tumor: A distinctive
low-grade malignant intra-abdominal neoplasm with consistent
Epstein-Barr virus association. Am J Surg Pathol. 25:721–731. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khorooshi R and Owens T: Detection and
cellular localization of phospho-STAT2 in the central nervous
system by immunohistochemical staining. Methods Mol Biol.
967:179–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Niedobitek G and Herbst H: In situ
detection of Epstein-Barr virus and phenotype determination of
EBV-infected cells. Methods Mol Biol. 326:115–137. 2006.PubMed/NCBI
|
|
5
|
Monda L, Warnke R and Rosai J: A primary
lymph node malignancy with features suggestive of dendritic
reticulum cell differentiation. A report of 4 cases. Am J Pathol.
122:562–572. 1986.PubMed/NCBI
|
|
6
|
Nguyen BD, Roarke MC and Yang M:
Synchronous hepatic and splenic inflammatory pseudotumour-like
follicular dendritic cell sarcomas. Liver Int. 35:19172015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu L, Ge R and Gao S: Imaging features and
radiologic-pathologic correlations of inflammatory pseudotumor-like
follicular dendritic cell sarcoma. BMC Med Imaging. 21:522021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Kenyon WJ, Li Q, Müllberg J and
Hutt-Fletcher LM: Epstein-Barr virus uses different complexes of
glycoproteins gH and gL to infect B lymphocytes and epithelial
cells. J Virol. 72:5552–5558. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takeuchi M, Sato Y, Yasui H, Ozawa H, Ohno
K, Takata K, Gion Y, Orita Y, Tachibana T, Itoh T, et al:
Epstein-Barr Virus-infected Cells in IgG4-related Lymphadenopathy
with comparison with extranodal IgG4-related disease. Am J Surg
Pathol. 38:946–955. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choe JY, Go H, Jeon YK, Yun JY, Kim YA,
Kim HJ, Huh J, Lee H, Shin DH and Kim JE: Inflammatory
pseudotumor-like follicular dendritic cell sarcoma of the spleen: A
report of six cases with increased IgG4-positive plasma cells.
Pathol Int. 63:245–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balfour HH Jr and Verghese P: Primary
Epstein-Barr virus infection: Impact of age at acquisition,
coinfection, and viral load. J Infect Dis. 207:1787–1789. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang ET, Ye W, Zeng YX and Adami HO: The
evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 30:1035–1047. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barat M, Hoeffel C, Aissaoui M, Dohan A,
Oudjit A, Dautry R, Paisant A, Malgras B, Cottereau AS and Soyer P:
Focal splenic lesions: Imaging spectrum of diseases on CT, MRI and
PET/CT. Diagn Interv Imaging. 102:501–513. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oz Puyan F, Bilgi S, Unlu E, Yalcin O,
Altaner S, Demir M and Cakir B: Inflammatory pseudotumor of the
spleen with EBV positivity: Report of a case. Eur J Haematol.
72:285–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schön CA, Görg C, Ramaswamy A and Barth
PJ: Splenic metastases in a large unselected autopsy series. Pathol
Res Pract. 202:351–356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan JK, Fletcher CD, Nayler SJ and Cooper
K: Follicular dendritic cell sarcoma. Clinicopathologic analysis of
17 cases suggesting a malignant potential higher than currently
recognized. Cancer. 79:294–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Youens KE and Waugh MS: Extranodal
follicular dendritic cell sarcoma. Arch Pathol Lab Med.
132:1683–1687. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim RH, Chang MS, Kim HJ, Song KS, Kim YS,
Choi BY and Kim WH: Medical history and lifestyle factors
contributing to Epstein-Barr virus-associated gastric carcinoma and
conventional gastric carcinoma in Korea. Anticancer Res.
30:2469–2475. 2010.PubMed/NCBI
|
|
19
|
Camargo MC, Koriyama C, Matsuo K, Kim WH,
Herrera-Goepfert R, Liao LM; Eurgast-EPIC Group, ; Yu J,
Carrasquilla G, Sung JJ, et al: Case-case comparison of smoking and
alcohol risk associations with Epstein-Barr virus-positive gastric
cancer. Int J Cancer. 134:948–953. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan G and Hashim MJ: Global burden of
deaths from Epstein-Barr virus attributable malignancies 1990–2010.
Infect Agent Cancer. 9:382014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Granados R, Aramburu JA, Rodríguez JM and
Nieto MA: Cytopathology of a primary follicular dendritic cell
sarcoma of the liver of the inflammatory pseudotumor-like type.
Diagn Cytopathol. 36:42–46. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kiryu S, Takeuchi K, Shibahara J, Uozaki
H, Fukayama M, Tanaka H, Maeda E, Akahane M and Ohtomo K:
Epstein-Barr virus-positive inflammatory pseudotumour and
inflammatory pseudotumour-like follicular dendritic cell tumour. Br
J Radiol. 82:e67–e71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vardas K, Manganas D, Papadimitriou G,
Kalatzis V, Kyriakopoulos G, Chantziara M, Exarhos D and
Drakopoulos S: Splenic inflammatory pseudotumor-like follicular
dendritic cell tumor. Case Rep Oncol. 7:410–416. 2014. View Article : Google Scholar : PubMed/NCBI
|