Introduction
Renal carcinoma (RC) is responsible for
approximately 90% of all kidney cancers in adults (1). RC is classified into several
histological cell types based on the genetic, histological and
clinical phenotypes; clear cells, granular cells, mixture cells and
undifferentiated cells (2–4). The cancer cells are resistant to
radiation, chemical and hormone therapies in RC patients and cannot
be treated without surgery (5,6).
Therefore, it is essential to identify more efficient
chemotherapeutic agents for RC treatment.
Dapagliflozin is a new type 2 diabetes drug that
decreases blood glucose levels by inhibition of sodium/glucose
cotransporter (SGLT2) in the kidney (7–9). It
has been reported that empagliflozin, a SGLT2 inhibitor, mediates
apoptosis through inhibition of sonic hedgehog signaling molecule
expression and migration by activating adenosine
monophosphate–activated protein kinase (AMPK) in cervical cancer
(10). Previous studies
demonstrate that dapagliflozin exerts anti-proliferative and
anti-tumor activity in human kidney and breast tumor cells through
cell cycle arrest and apoptosis, tumor growth inhibition or
AMPK/mTOR signaling pathways (11,12).
Moreover, dapagliflozin reduces tumor volume and activates
caspase-3, beclin-1 and JNK in solid Ehrlich carcinoma mice
(13). Nevertheless, the mechanism
underlying dapagliflozin-induced apoptosis has not been presented
in human RC.
The cellular Fas-associated death domain-like
interleukin-1-converting enzyme-inhibitory protein (cFLIP) is an
important apoptosis-regulatory protein associated with apoptosis
(14). cFLIP has
cFLIPL, cFLIPS and cFLIPR isoforms
(15). Each of these isoforms has
different effects on apoptotic pathways through different
mechanisms (16–19). Overexpression of cFLIP suppresses
death ligand-mediated cell death and confers resistance to
chemotherapeutic agents (20).
Constant cFLIP mRNA levels and cFLIP protein stability decrease the
sensitivity to anti-cancer drugs in the cFLIP-overexpressing
bladder and colorectal cancers (21–23).
Hence, modulation of cFLIP, an anti-apoptotic protein, plays a key
role in elucidating the mechanism of chemopreventive–mediated
apoptosis.
In the present investigation, we showed that
dapagliflozin mediated apoptosis in human RC Caki-1 cells by
caspase-dependent pathway via the downregulation of
cFLIPL and an increase in cFLIPS
instability.
Materials and methods
Cell culture and materials
Caki-1 cells were purchased from American Type
Culture Collection (HTB-46) and maintained in DMEM (LM 001–05;
WelGene) with 10% FBS (S001-07; WelGene) and 1% anti-biotic
anti-mycotic (AA) solution (LS 203-01; WelGene). HK-2 cells were
obtained from the Korean Cell Line Bank (22190) and maintained in
RPMI1640 medium (LM 011-01; WelGene) supplemented with 10% FBS and
1% AA solution. The cells were maintained at 37°C under 5%
CO2 condition. z-VAD-fmk (627610) was obtained from
Calbiochem. Dapagliflozin (SC-364481), N-acetylcysteine (NAC;
A7250) and cycloheximide (CHX; C1988) were obtained from
Sigma-Aldrich.
Cell viability assay
2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)–2H-tetrazolium-5-carboxanilide
(XTT) assays were analyzed using the Welcount Cell Viability Assay
Kit (TR055-01; WelGene). Caki-1 and HK-2 cells were seeded
(0.2×105 cells/well) into three 96-well plates
containing DMEM or RPMI1640 with 10% FBS. Cells were expose to 0,
20, 40, 60, 80 and 100 µM of dapagliflozin for 24 h and then
cultivated with XTT reagent for 2–3 h at room temperature. The
density was measured at 450 nm using a microplate reader (Thermo
LabSystems) at 450/690 nm.
FACS analysis
0.4×106 cells were suspended in 100 µl of
cold phosphate-buffered saline (PBS; 70011044; Thermo Fisher
Scientific) and 200 µl of 95% ethanol (1.00983.1011; Merck) was
mixed, and the sample was while vortexing. The cells were
cultivated for 3 h at 4°C, washed twice with cold PBS, and
resuspended in 250 µl of 1.12% sodium citrate buffer (pH 8.4) with
12.5 µg of RNase A (R4875; Sigma-Aldrich; Merck KGaA). The cells
were cultivated for 30 min at 37°C, mixed with 250 µl of 50 µg/ml
propidium iodide (PI; P4170; Sigma-Aldrich; Merck KGaA) for 20 min
at 37°C. Cells were analyzed by fluorescence-activated cell sorting
(FACS) using CytoFLEX (B53000; Beckman Coulter).
Western blotting
The total cell lysates were prepared by resuspending
0.45×106 cells in 20–50 µl of RIPA lysis buffer (50 mM
Tris buffer, 20 mM HEPES, 100 mM NaF; 120 mM NaCl, 0.5% Triton
X-100, 100 µM Na3VO4, pH 7.6) Total lysates
was quantified using a BCA kit (#23225; Thermo Fisher Scientific)
according to the manufacturer's protocols. The proteins (30–70 µg)
were isolated using 10 or 12% SDS-PAGE gels and electrotransferred
onto NC membranes (GE Healthcare). Target proteins were identified
using the respective antibodies and Immobilon Western
Chemiluminescent HRP Substrate Solution (WBKLS0100; Millipore) and
visualized by Davinch-Chemi (CAS-400SM; Davinch-K). Anti-PARP
antibody (1:1,000; #9542) and anti-Bax (1:1,000; #2772) were obtain
from Cell Signaling Technology. Anti-caspase-3 (1:3,000;
ADI-AAP-113) and anti-FLIP (1:700; ALX-804-961-0100) were purchased
from Enzo Life Sciences. Anti-Bcl-2 (1:700; sc-7832), anti-Bcl-xL
(1:1,000, sc-634), anti-Mcl-1 (1:1,000; sc-12756), anti-cIAP1
(1:1,000; sc-7943), anti-cIAP2 (1:1,000; sc-517317) and
anti-β-actin antibody (1:5,000; sc-47778) were supplied by Santa
Cruz Biotechnology, and anti-XIAP (1:10,000; 610717) antibody was
obtained from BD Biosciences.
Annexin V/PI double staining
Fluorescein isothiocyanate (FITC) Annexin V
apoptosis detection kit I (556547; BD Biosciences) was used to
determine cell death type. The cells were washed twice with cold
PBS, and then cell pellets resuspended in 1X binding buffer. This
suspended cells (100 µl) were stained with 5 µl of FITC-conjugated
Annexin V and then 5 µl PI. The cells were incubated for 15 min at
room temperature in the dark. After adding 400 µl of 1X binding
buffer to each tube, the cells were analyzed using CytoFLEX
(Beckman Coulter).
RNA isolation and RT-PCR
The levels of cFLIPL and
cFLIPS mRNA was confirmed using RT-PCR. Total RNA was
isolated using Easy-Blue reagent (17061; iNtRON Biotechnology).
cDNA was prepared using the M-MLV Reverse Transcriptase (18057018;
Thermo Fisher Scientific) according to the manufacturer's
protocols. GAPDH was used as an internal control. The primers used
to target genes of cFLIPL, cFLIPS and GAPDH:
for cFLIPL, 5′-CGGACTATAGAGTGCTGATGG-3′ (forward) and
5′-GATTATCAGGCAGATTCCTAG-3′ (reverse); cFLIPS,
5′-TAAGCTGTCTGTCGGGGACT-3′ (forward) and
5′-AGATCAGGACAATGGGCATAG-3′ (reverse); GAPDH,
5′-AGGTCGGAGTCAACGGATTTG-3′ (forward) and
5′-GTGATGGCATGGACTGTGGT-3′ (reverse). The amplified PCR products
were separated by electrophoresing on a 1.5% agarose gel with 0.1%
ETBR, and the DNA bands were detected by an ultraviolet light gel
doc (WGD30; DAIHAN).
Stable transfection
Caki-1 cells were seeded onto 6-well culture plates
(0.25×106 cells/well) and cultivated overnight at 37°C.
Cells were transfected with the pcDNA 3.1-cFLIPL, pcDNA
3.1-cFLIPS or control pcDNA 3.1 plasmid vectors using
LipofectAMINE2000® (11668-019; Thermo Fisher Scientific)
in Opti-MEM medium (31985-070; Thermo Fisher Scientific). After 48
h of transfection, the transfected cells were selected using
culture medium containing 800 µg/ml G418 (10131-035; Thermo Fisher
Scientific). The cells were then exposed to dapagliflozin for 24 h
and analyzed for cFLIPL and cFLIPS protein
expression using western blotting. After 2 or 3 weeks, to rule out
the possibility of clonal differences between the generated stable
cell lines, pooled Caki-1/pcDNA 3.1, Caki-1/cFLIPL and
Caki-1/cFLIPS clones were analyzed for cFLIPL
and cFLIPS protein expression using western
blotting.
Statistical analysis
The experiments were performed three independent
experiments. One-way ANOVA and post hoc comparisons (Scheffe) and
two-way ANOVA followed by post hoc test (Tukey's HSD) were used
when comparing the situations. Statistical Package for Social
Sciences 27.0 (IBM SPSS Inc.) was utilized for the data analysis.
The data were expressed as the mean ± SD, and P-values <0.05
were considered significant.
Results
Dapagliflozin decreases cell viability
and induces apoptosis in Caki-1 cells
The anti-cancer effect of dapagliflozin on RC Caki-1
cells, the cells was investigated by treating the cells with 0, 20,
40, 60, 80 and 100 µM of dapagliflozin. As shown in Fig. 1A, treatment of Caki-1 cells with
dapagliflozin showed a dose-dependent reduction in cell viability.
High concentrations (80 and 100 µM) dapagliflozin induced the
rounded cells of considerable number in Caki-1 cells under light
microscopy (Fig. 1B). We then
performed flow cytometry analysis of the dapagliflozin-treated
Caki-1 cells. Dapagliflozin treatment for 24 h significantly
increased the sub-G1 fraction in a dose-dependent manner (Fig. 1C). Exposure to dapagliflozin
increased the expression levels of cleavage form of PARP and
caspase-3 in Caki-1 cells (Fig.
1D). To determine cell death type induced by dapagliflozin, we
analyzed FITC-conjugated Annexin V/PI staining using flow
cytometry. Treatment with 100 µM of dapagliflozin increased Annexin
V/PI positive cells (Fig. 1E).
These observations supported that dapagliflozin induces apoptosis
in Caki-1 cells.
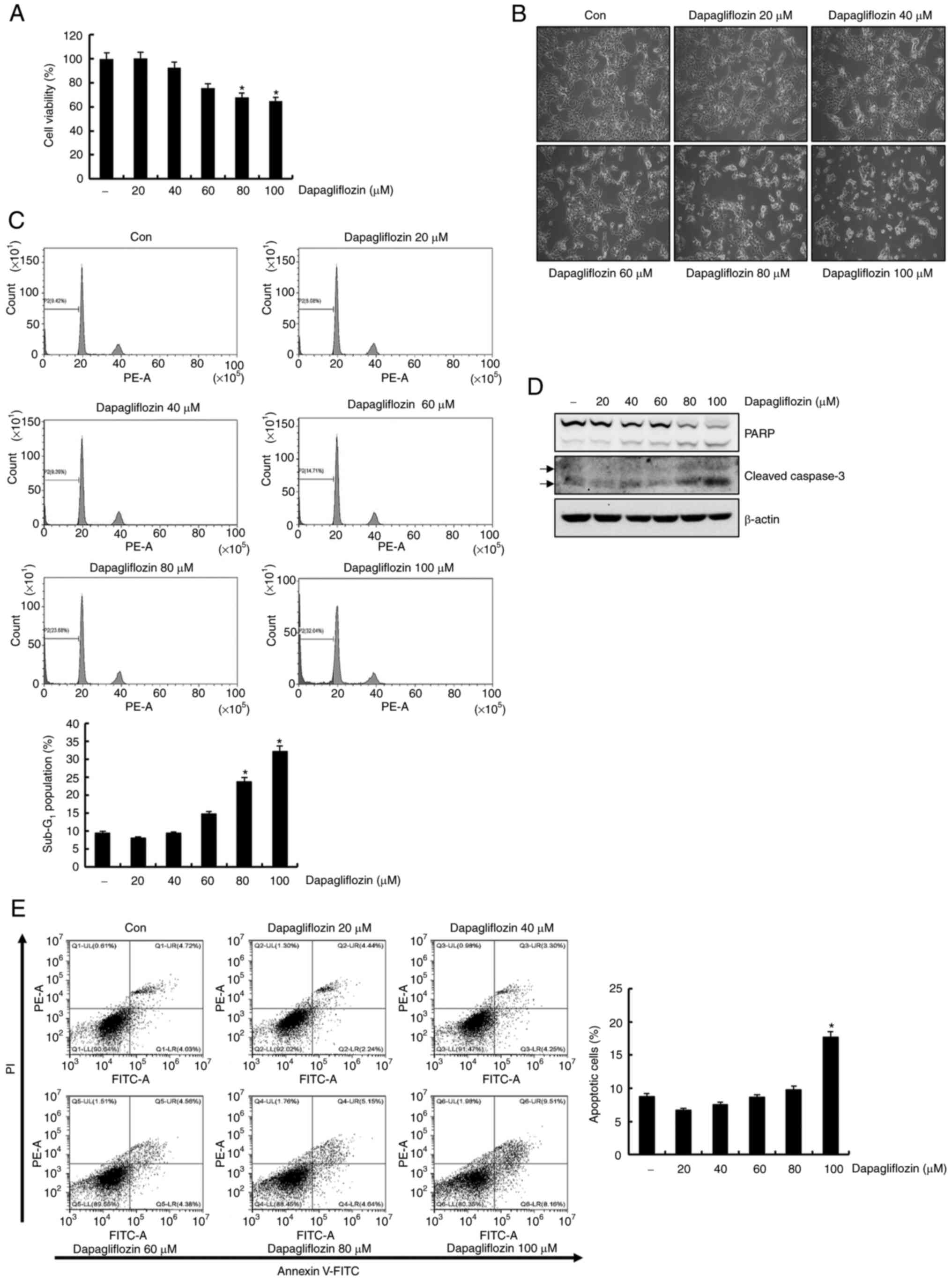 | Figure 1.Dapagliflozin induces apoptosis in
Caki-1 cells. (A) Cells were treated with dapagliflozin for 24 h.
Subsequently, cell viability was analyzed using a
2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
assay kit. (B) Caki-1 cells were treated with dapagliflozin for 24
h and morphological findings were observed under a light microscope
at a magnification of ×200. (C) Caki-1 cells were exposed to
dapagliflozin for 24 h. The Sub-G1 fraction was measured
via flow cytometry. The FACS data are indicated in the upper panel.
The percentage of the sub-G1 population is shown in the
lower panel. (D) Cells were treated with varying concentrations of
dapagliflozin for 24 h. PARP, cleaved caspase-3 and β-actin protein
expression was examined by western blotting. β-actin was used as
the protein loading control. Cleaved caspase-3 is indicated by
arrows. (E) Caki cells were treated with dapagliflozin for 24 h.
The type of cell death, which is apoptosis or necrosis, was
confirmed via flow cytometry after FITC-conjugated Annexin V/PI
staining. The percentage of cells in each quadrant (Q1-UL, necrotic
cells; Q1-UR, late apoptotic cells; Q1-LL, live cells; Q1-LR, early
apoptotic cells) is indicated (left panel). The percentage of
apoptotic cells is shown in the right panel. Data were acquired in
three independent experiments and presented as the mean ± SD (n=3).
*P<0.05 compared with non-treated cells. Con, dapagliflozin 0
µM; PARP, poly (ADP-ribose) polymerase. |
Dapagliflozin-induced apoptosis
reduces cFLIPL and cFLIPS expression
levels
The detailed molecular mechanism associated with
dapagliflozin-induced apoptosis was studied by treating Caki-1
cells with dapagliflozin and analyzing the expression levels of
apoptotic regulatory proteins using western blotting. As shown in
Fig. 2A, cFLIPL and
cFLIPS protein expressions markedly reduced in
dapagliflozin-treated Caki-1 cells. However, protein expression
levels of Bcl-2, Bcl-xL, Bax, Mcl-1, XIAP, cIAP1 and cIAP2 did not
change in response to dapagliflozin treatment. These results
demonstrated that dapagliflozin-induced apoptosis suppressed the
expression of cFLIPL and cFLIPS in Caki-1
cells. To determine whether the dapagliflozin-mediated
cFLIPL and cFLIPS reduction in protein
expression was regulated at the transcriptional level, we confirmed
RT-PCR. Exposure Caki-1 cells to dapagliflozin had no effect on
cFLIPL or cFLIPS expression at the
transcriptional level (Fig. 2B).
Therefore, dapagliflozin-mediated downregulation of
cFLIPL and cFLIPS expression levels is
modulated at the post-transcriptional level.
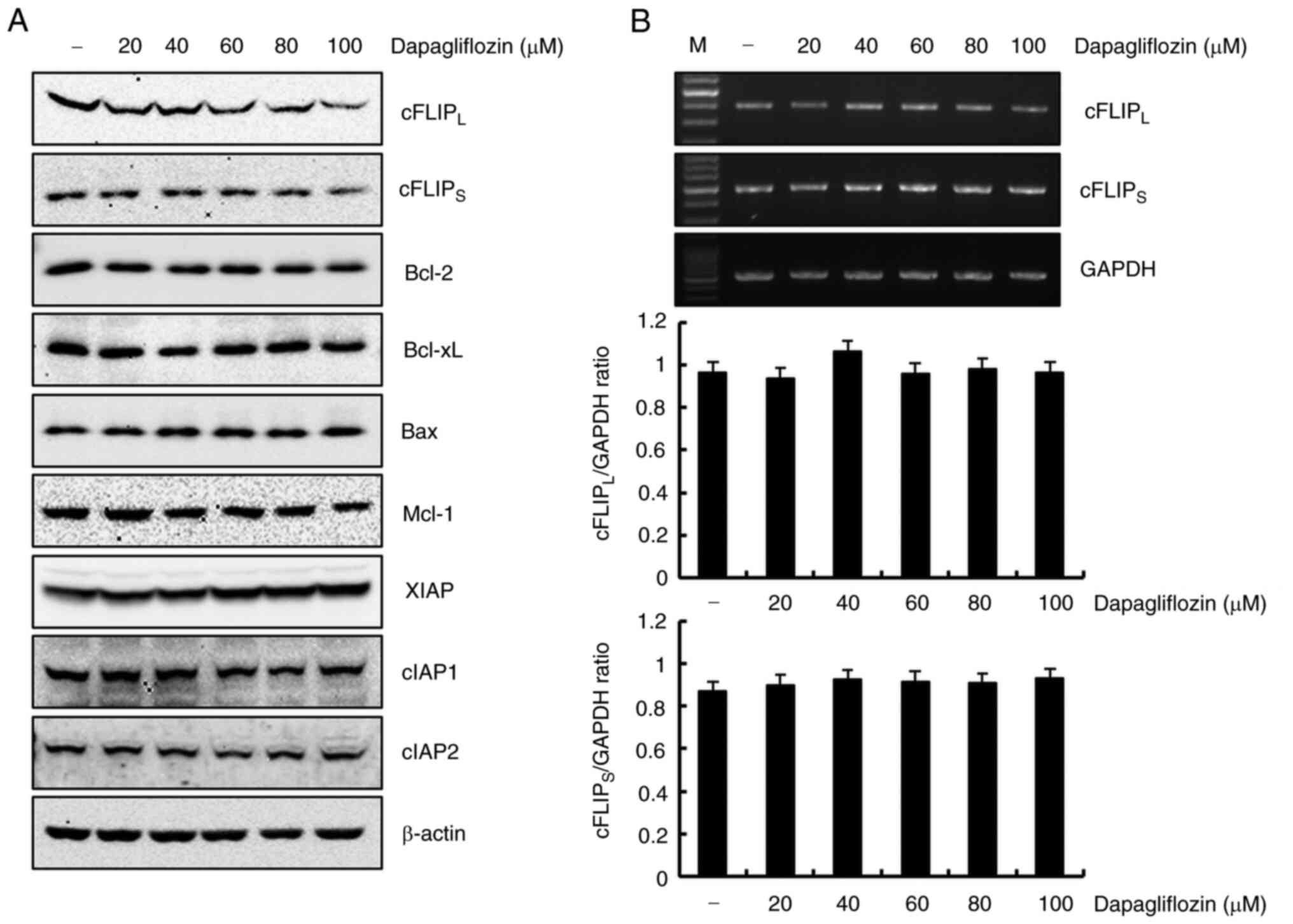 | Figure 2.Dapagliflozin inhibits
cFLIPL and cFLIPS protein expression in
Caki-1 cells. (A) Cells were treated with various concentrations of
dapagliflozin. At 24 h after treatment, protein expression levels
of cFLIPL, cFLIPS, Bcl-2, Bcl-xL, Bax, Mcl-1,
XIAP, cIAP1, cIAP2 and β-actin were analyzed using western
blotting. β-actin served as the protein loading control. (B) Caki-1
cells were treated with different concentrations dapagliflozin.
After 24 h, the levels of cFLIPL, cFLIPS and
GAPDH mRNA (upper panel) were determined using RT-PCR. GAPDH was
used as a loading control. The density of cFLIPL,
cFLIPS and GAPDH was analyzed using ImageJ software.
Data obtained from RT-PCR of cFLIPL, cFLIPS
and GAPDH were used to evaluate the effect of dapagliflozin on the
cFLIPL/GAPDH ratio (middle panel) and
cFLIPS/GAPDH ratio (lower panel). Data were acquired in
three independent experiments. PARP, poly (ADP-ribose) polymerase;
cFLIP, cellular Fas-associated death domain-like
interleukin-1-converting enzyme-inhibitory protein Mcl-1, myeloid
cell leukemia-1; XIAP, X-linked inhibitor of apoptosis protein;
cIAP, cellular inhibitor of apoptosis protein; RT-PCR, reverse
transcription PCR; M, marker of RT-PCR. |
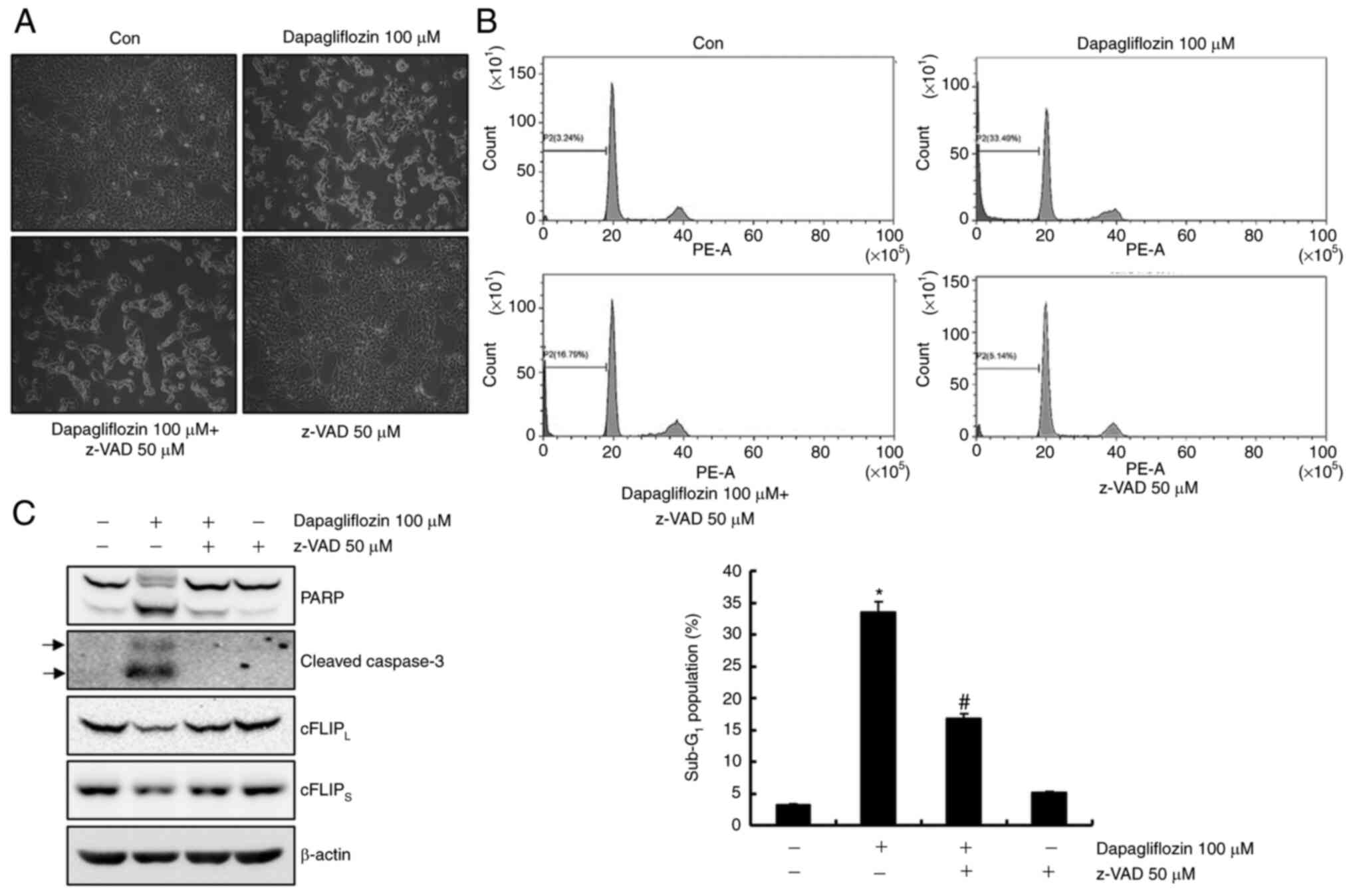
Dapagliflozin-mediated apoptosis is
inhibited via a caspase signaling pathway
To identify whether activation of the caspase
signaling pathway plays an important role in dapagliflozin-mediated
apoptosis, Caki-1 cells were pretreatment with a pan-caspase
inhibitor, z-VAD-fmk. As shown in Fig,
3A and B, pretreatment with z-VAD-fmk inhibited
dapagliflozin-mediated apoptosis. Moreover, pretreatment of Caki-1
cells with z-VAD-fmk prevented the cleavage forms of PARP and
caspase-3 and restored the cFLIPL and cFLIPS
expressions levels (Fig. 3C).
These findings suggest that dapagliflozin-induced apoptosis is
modulated by a caspase signaling pathway through cFLIPL
and cFLIPS downregulation in Caki-1 cells.
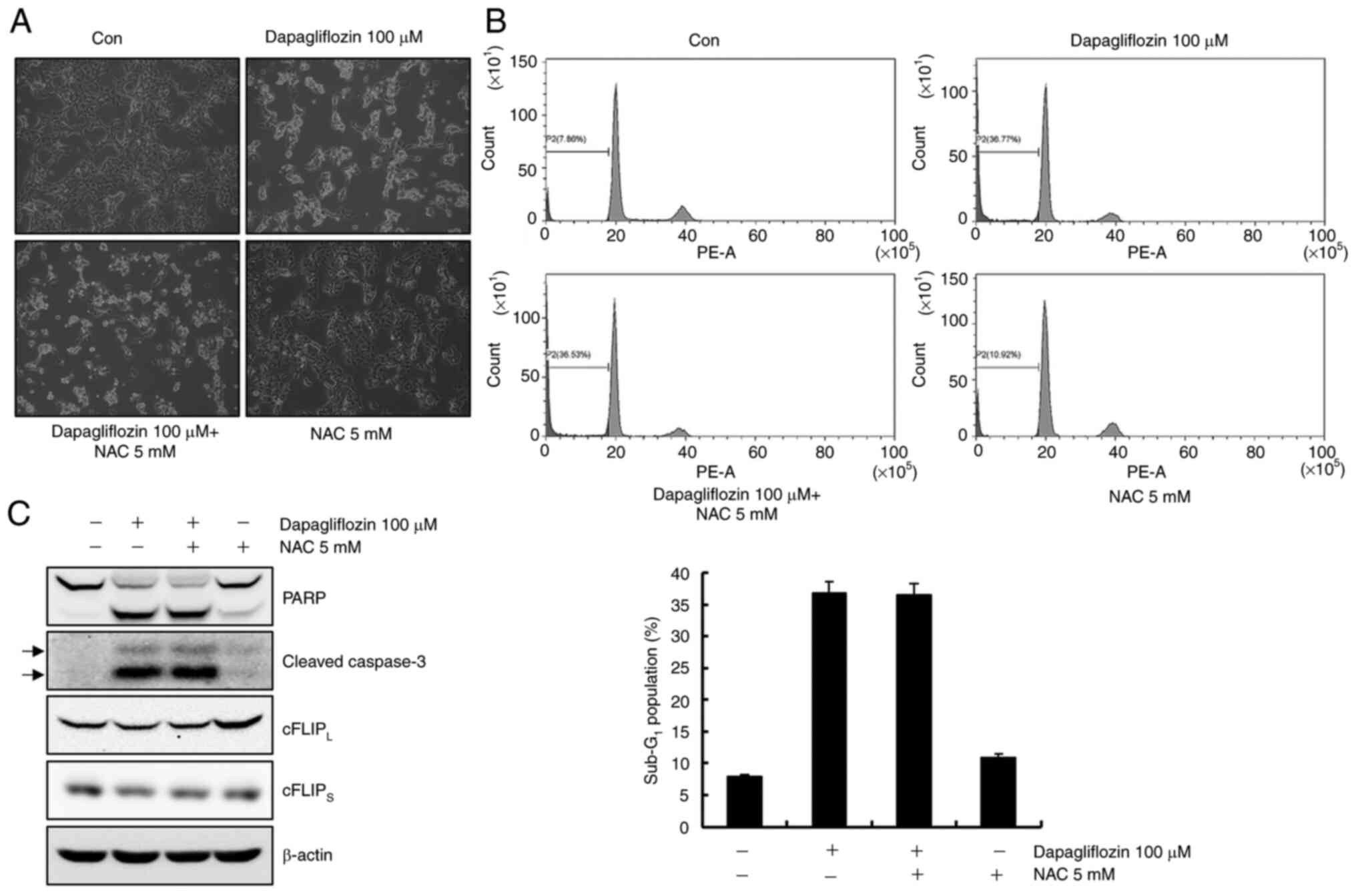
Dapagliflozin-mediated apoptosis is
not involved in reactive oxygen species (ROS)
ROS causes apoptosis by modulating the expression
levels of cFLIP (24). Caki-1
cells were pretreated with the ROS scavenger, NAC, for 1 h and then
cultivated with dapagliflozin for 24 h to investigate whether ROS
plays a key role in dapagliflozin-induced apoptosis. As shown in
Fig. 4A and B, pretreatment with
NAC did not prevent dapagliflozin-mediated apoptosis. Furthermore,
NAC failed to prevent PARP cleavage and caspase activation and did
not restore cFLIPL and cFLIPS expression
levels in dapagliflozin-treated cells (Fig. 4C). These data suggest that ROS
generation is not affected by dapagliflozin-mediated apoptosis.
Dapagliflozin-mediated apoptosis is
partially recovered through cFLIPL downregulation
To determine whether the downregulation of
cFLIPL and cFLIPS plays an important role in
dapagliflozin-induced apoptosis in Caki-1 cells, cFLIPL-
and cFLIPS-overexpressing cells were exposed to
dapagliflozin. As shown in Fig.
5A, treatment with dapagliflozin considerably caused apoptosis
in Caki-1/vector cells, whereas overexpression of cFLIPL
partially inhibited dapagliflozin-mediated apoptosis. In contrast,
the overexpression of cFLIPS did not prevent
dapagliflozin-induced apoptosis (Fig.
5B). The expression of cleavage forms of PARP and caspase-3
induced by dapagliflozin treatment was partially blocked by the
overexpression of cFLIPL (Fig. 5C). However, treatment with
dapagliflozin in Caki-1/cFLIPS cells did not affect the
cleavage forms of PARP and caspase-3 expression levels (Fig. 5D). These results reveal that the
downregulation of cFLIPL contributes to
dapagliflozin-mediated apoptosis. In addition, dapagliflozin may
mediate apoptosis in Caki-1/vector cells and even in
cFLIPS-overexpressed cells.
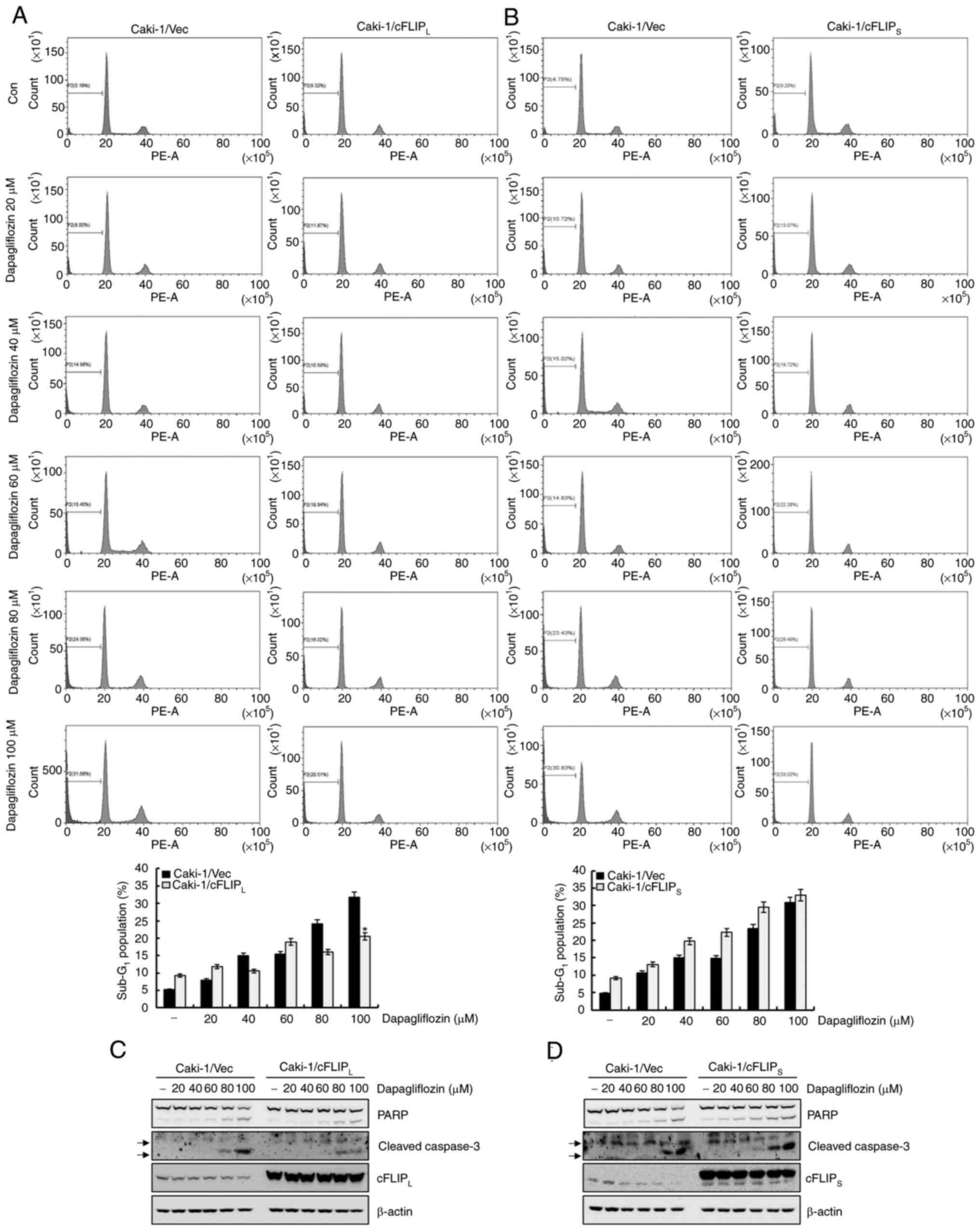 | Figure 5.Downregulation of cFLIPL
contributes to dapagliflozin-mediated apoptosis. (A and B)
Caki-1/Vec, Caki-1/cFLIPL and Caki-1/cFLIPS
cells were treated for 24 h with the indicated concentrations of
dapagliflozin. The apoptosis levels were determined based on the
sub-G1 fraction using flow cytometry. The FACS data are
indicated in the upper panels. The percentage of the
sub-G1 population is shown in the lower panels. (C)
Caki-1/vector and Caki-1/cFLIPL cells were treated with
the indicated concentrations of dapagliflozin. After 24 h, the
protein expression levels of PARP, cleaved caspase-3,
cFLIPL and β-actin were analyzed by western blotting.
(D) Caki-1/vector and Caki-1/cFLIPS cells were treated
with dapagliflozin for 24 h. Protein expression levels of PARP,
cleaved caspase-3, cFLIPS and β-actin were detected
using western blotting. β-actin was used as a loading control.
Cleaved caspase-3 is indicated by arrows. Data were acquired in
three independent experiments and are presented as the mean ± SD
(n=3). *P<0.05 compared with dapagliflozin-treated Caki-1/Vec
cells. Vec, vector; Con, dapagliflozin 0 µM; PARP, poly
(ADP-ribose) polymerase; cFLIP, cellular Fas-associated death
domain-like interleukin-1-converting enzyme-inhibitory protein. |
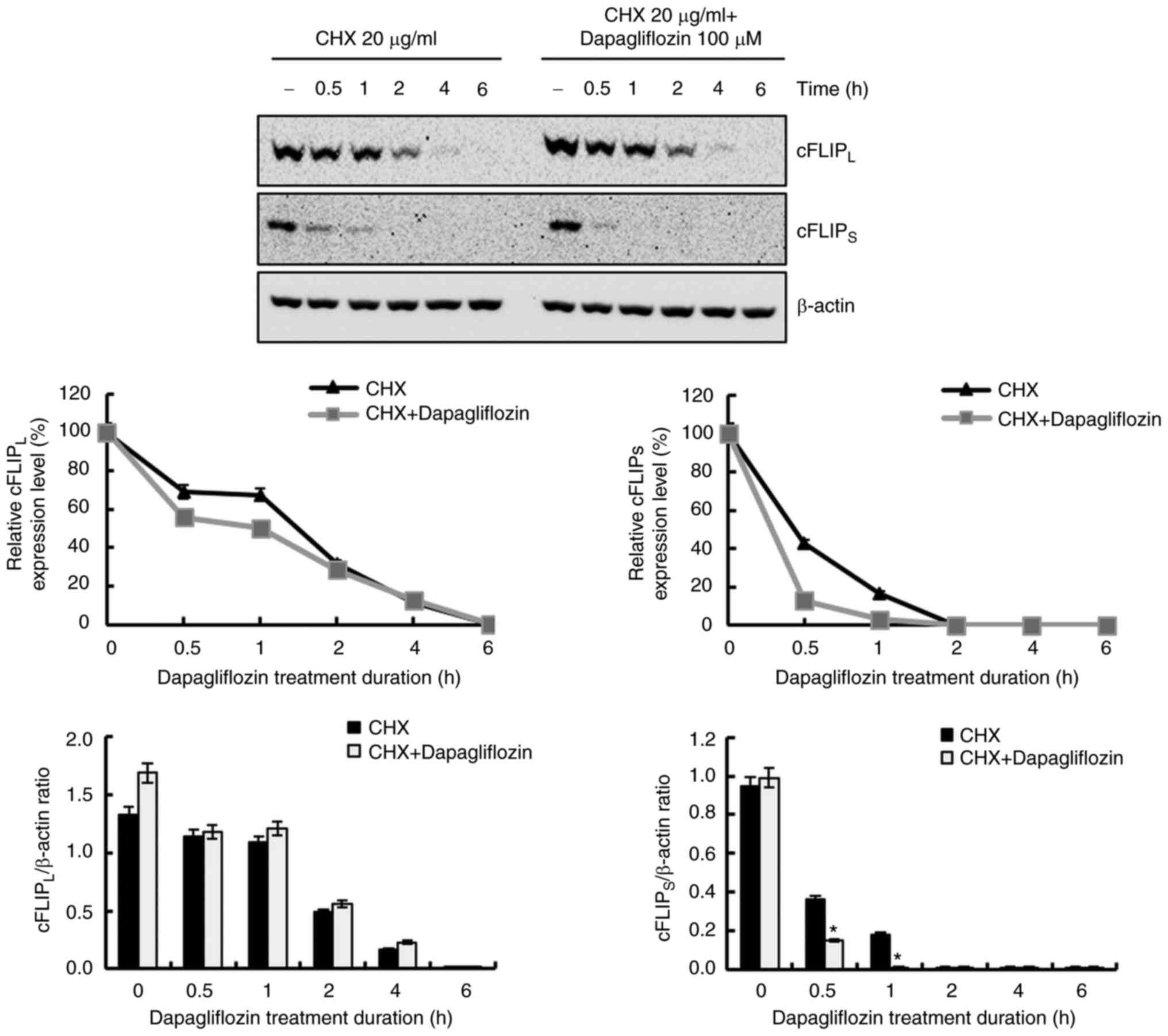
Dapagliflozin reduces the expression
level of cFLIPS ascribed by the increase in protein
instability
To further investigate the molecular mechanism
underlying the reduction of cFLIPL and cFLIPS
expression levels in dapagliflozin-treated cells, we studied
protein stability assays of cFLIPL and
cFLIPS. Treatment with dapagliflozin did not affect
cFLIPL or cFLIPS expression at the
transcriptional level (Fig. 2B).
After pretreating Caki-1 cells with CHX for 1 h, the cells were
treated with dapagliflozin for varying lengths of time; degradation
of the cFLIPS was promoted by dapagliflozin treatment,
but not by cFLIPL (Fig.
6). These findings indicate that the degradation of
cFLIPS protein is facilitated by dapagliflozin treatment
and that dapagliflozin treatment induces cFLIPS protein
instability.
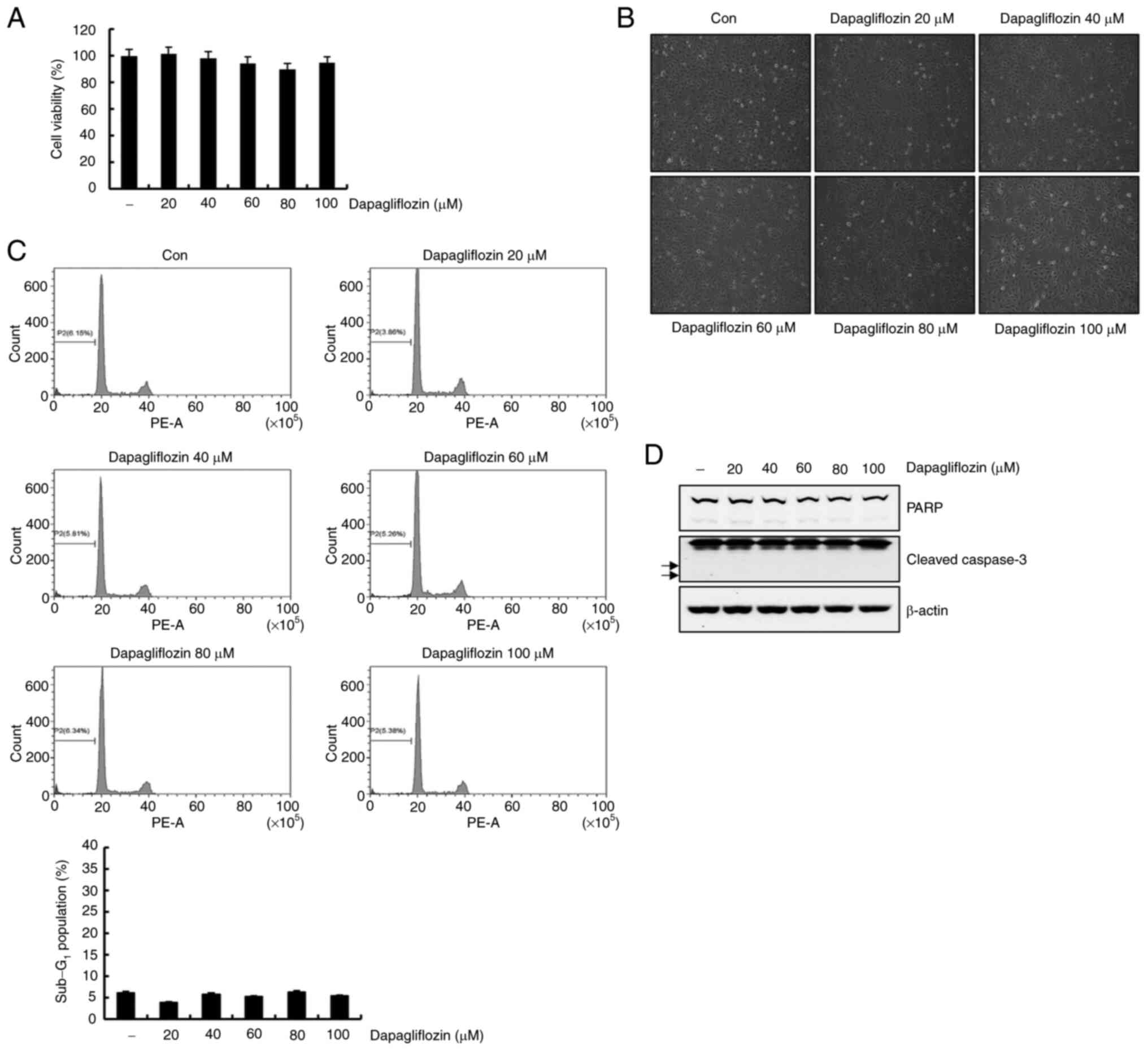
Dapagliflozin does not affect cell
death in normal human kidney HK-2 cells
We examined the effect of dapagliflozin on normal
human kidney HK-2 cells. Dapagliflozin had no effects on cell
viability and morphology (Fig. 7A and
B). Then, flow cytometry analysis of the dapagliflozin-treated
HK-2 cells was conducted. Sub-G1 population was not affected by
dapagliflozin treatment (Fig. 7C).
Additionally, protein bands of cleavage form of PARP and caspase-3
were not detected in response to dapagliflozin treatment (Fig. 7D). These data indicate that
dapagliflozin did not affect cell death in HK-2 cells.
Discussion
In this study, we demonstrated that dapagliflozin
exerts potential anti-tumor effects on human RC Caki-1 cells.
Dapagliflozin-mediated apoptosis is caused by caspase signaling
pathways in Caki-1 cells. Furthermore, the detailed molecular
mechanism in dapagliflozin-induced apoptosis is associated with
caspase-mediated degradation of cFLIPL and increase of
cFLIPS instability.
Previous studies have reported that dapagliflozin
exerts anti-proliferative and anti-tumor effect (11,12).
Consistent with previous studies, our study showed that
dapagliflozin treatment significantly increased the sub-G1 fraction
in a dose-dependent manner and increased the levels of cleavage
forms of PARP and caspase-3. Annexin V/PI positive cells were
detected. These findings reveal that dapagliflozin induces
apoptosis in Caki-1 cells.
Apoptosis refers to programmed cell death related
with caspase activation (25–27).
The caspase activation is determined by the regulation of anti-
and/or pro-apoptotic proteins (28) Dapagliflozin-mediated downregulation
of cFLIPL and cFLIPS was caused by their
increased degradation, whereas protein expression levels of Bcl-2,
Bcl-xL, Bax, Mcl-1, XIAP, cIAP1 and cIAP2 were no changed. These
data indicate that dapagliflozin-induced apoptosis decreases the
expression levels of cFLIPL and cFLIPS.
cFLIP is a regulator of the apoptotic signaling
pathway and is expressed in various cancer cell lines (15,29).
Previous studies have demonstrated that cFLIP expression levels are
modulated at the proteasome-mediated post-translational level
(30,31). In this study, dapagliflozin
treatment did not alter cFLIPL and cFLIPS
mRNA levels. The findings suggest that the dapagliflozin-mediated
reduction in the cFLIPL and cFLIPS expression
levels is modulated at the post-translational level.
Caspase activation regulates apoptosis-regulatory
proteins (32,33). Previous studies have shown that
dapagliflozin does not affect caspase activation in colon cancer
cell lines (34). Contrary to
these studies, the present study showed that pretreatment with
z-VAD-fmk inhibited sub-G1 cell accumulation, cleavage forms of
PARP and caspase-3, and restored the cFLIPL and
cFLIPS expression levels. These results suggested that
dapagliflozin-induced apoptosis might be modulated by the caspase
signaling pathway through the downregulation of cFLIPL
and cFLIPS in Caki-1 cells.
Previous investigations have shown that
overexpression of cFLIP modulates apoptosis in several cancer cell
lines (35–37). In the present study, overexpression
of cFLIPL partially inhibited dapagliflozin-induced
apoptosis, while the overexpression of cFLIPS failed to
inhibit dapagliflozin-induced apoptosis. These data indicate that
dapagliflozin-mediated apoptosis is blocked in
cFLIPL-overexpressing cells, implying that
dapagliflozin-induced apoptosis occurs by the downregulation of
cFLIPL.
It has been reported that the downregulation of
cFLIPS is occurred by the increase in protein
instability during endoplasmic reticulum (ER) stress-induced
apoptosis in human colon tumor cells (38). In the present study, reduction of
cFLIPS expression was ascribed by the increased protein
instability of cFLIPS in dapagliflozin-treated cells.
This demonstrated that dapagliflozin facilitated the degradation of
the cFLIPS, leading to increase instability of
cFLIPS.
ROS is an important apoptosis regulator in human
cancer cells (39,40). Previous studies reported that ROS
regulates cFLIP expression and increases apoptosis (41,42).
In the present study, pretreatment with of Caki-1 cells did not
inhibit dapagliflozin-mediated apoptosis. These data suggest that
dapagliflozin-mediated apoptosis is independent of ROS generation.
However, recent studies have shown that dapagliflozin decreases ROS
production (43–45). Consistent with the present study,
dapagliflozin appears to be a feasible option for reducing ROS
production.
Interestingly, dapagliflozin suppresses ER
stress-induced apoptosis in normal HK-2 cells (46). In contrast, dapagliflozin did not
affect cell death in HK-2 cells in this study.
Taken together, our data indicates that
dapagliflozin–induced apoptosis is modulated by caspase signaling
pathways through the downregulation of cFLIPL and an
increase in cFLIPS protein instability in Caki-1 cells.
In conclusion, dapagliflozin is a potential chemotherapeutic agent
against human renal cancer.
Acknowledgements
Not applicable.
Funding
This research was supported by Basic Science Research Program
through the National Research Foundation of Korea (NRF) funded by
the Ministry of Education (grant no. 2020R1I1A1A01068857).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JYK conceived and designed the experiments. JHJ
performed most of the experiments and analyzed the data. TJL, EGS
and IHS conducted data analyses for light microscopy, Annexin V/PI
staining and RT-PCR. JHJ drafted and wrote the manuscript. JYK
revised the manuscript accordingly. JHJ provided the funding. JHJ
and JYK confirmed the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Low G, Huang G, Fu W, Moloo Z and Girgis
S: Review of renal cell carcinoma and its common subtypes in
radiology. World J Radiol. 8:484–500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ray RP, Mahapatra RS, Khullar S, Pal DK
and Kundu AK: Clinical characteristics of renal cell carcinoma:
Five years review from a tertiary hospital in Eastern India. Indian
J Cancer. 53:114–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jonasch E, Gao J and Rathmell WK: Renal
cell carcinoma. BMJ. 349:g47972014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen HT and McGovern FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rabinovitch RA, Zelefsky MJ, Gaynor JJ and
Fuks Z: Patterns of failure following surgical resection of renal
cell carcinoma: Implications for adjuvant local and systemic
therapy. J Clin Oncol. 12:206–212. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Zhang H and Chen X: Drug
resistance and combating drug resistance in cancer. Cancer Drug
Resist. 2:141–160. 2019.PubMed/NCBI
|
|
7
|
Bakris GL, Fonseca VA, Sharma K and Wright
EM: Renal sodium-glucose transport: Role in diabetes mellitus and
potential clinical implications. Kidney Int. 75:1272–1277. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mosley JF II, Smith L, Everton E and
Fellner C: Sodium-glucose linked transporter 2 (SGLT2) inhibitors
in the management of type-2 diabetes: A drug class overview. P T.
40:451–462. 2015.PubMed/NCBI
|
|
9
|
Gnudi L, Coward RJM and Long DA: Diabetic
nephropathy: Perspective on novel molecular mechanisms. Trends
Endocrinol Metab. 27:820–830. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie Z, Wang F, Lin L, Duan S, Liu X, Li X,
Li T, Xue M, Cheng Y, Ren H and Zhu Y: An SGLT2 inhibitor modulates
SHH expression by activating AMPK to inhibit the migration and
induce the apoptosis of cervical carcinoma cells. Cancer Lett.
495:200–210. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuang H, Liao L, Chen H, Kang Q, Shu X and
Wang Y: Therapeutic effect of sodium glucose Co-transporter 2
inhibitor dapagliflozin on renal cell carcinoma. Med Sci Monit.
23:3737–3745. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou J, Zhu J, Yu SJ, Ma HL, Chen J, Ding
XF, Chen G, Liang Y and Zhang Q: Sodium-glucose co-transporter-2
(SGLT-2) inhibition reduces glucose uptake to induce breast cancer
cell growth arrest through AMPK/mTOR pathway. Biomed Pharmacother.
132:1108212020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kabel AM, Arab HH and Elmaaboud MA: Effect
of dapagliflozin and/or L-arginine on solid tumor model in mice:
The interaction between nitric oxide, transforming growth
factor-beta 1, autophagy, and apoptosis. Fundam Clin Pharmacol.
35:968–978. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shirley S and Micheau O: Targeting c-FLIP
in cancer. Cancer Lett. 332:141–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ivanisenko NV, Seyrek K, Hillert-Richter
LK, König C, Espe J, Bose K and Lavrik IN: Regulation of extrinsic
apoptotic signaling by c-FLIP: Towards targeting cancer networks.
Trends Cancer. 8:190–209. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He MX and He YW: A role for c-FLIP(L) in
the regulation of apoptosis, autophagy, and necroptosis in T
lymphocytes. Cell Death Differ. 20:188–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bagnoli M, Canevari S and Mezzanzanica D:
Cellular FLICE-inhibitory protein (c-FLIP) signalling: A key
regulator of receptor-mediated apoptosis in physiologic context and
in cancer. Int J Biochem Cell Biol. 42:210–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kataoka T: The caspase-8 modulator c-FLIP.
Crit Rev Immunol. 25:31–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smyth P, Sessler T, Scott CJ and Longley
DB: FLIP(L): The pseudo-caspase. FEBS J. 287:4246–4260. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poukkula M, Kaunisto A, Hietakangas V,
Denessiouk K, Katajamäki T, Johnson MS, Sistonen L and Eriksson JE:
Rapid turnover of c-FLIPshort is determined by its unique
C-terminal tail. J Biol Chem. 280:27345–27355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Korkolopoulou P, Goudopoulou A, Voutsinas
G, Thomas-Tsagli E, Kapralos P, Patsouris E and Saetta AA: c-FLIP
expression in bladder urothelial carcinomas: Its role in resistance
to Fas-mediated apoptosis and clinicopathologic correlations.
Urology. 63:1198–1204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ullenhag GJ, Mukherjee A, Watson NF,
Al-Attar AH, Scholefield JH and Durrant LG: Overexpression of FLIPL
is an independent marker of poor prognosis in colorectal cancer
patients. Clin Cancer Res. 13:5070–5075. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ryu BK, Lee MG, Chi SG, Kim YW and Park
JH: Increased expression of cFLIP(L) in colonic adenocarcinoma. J
Pathol. 194:15–19. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Azad N and Iyer AKV: Reactive oxygen
species and apoptosis. Systems biology of free radicals and
antioxidants. Laher I: Springer Berlin Heidelberg; Berlin,
Heidelberg: pp. 113–135. 2014, View Article : Google Scholar
|
|
25
|
Jan R and Chaudhry GE: Understanding
apoptosis and apoptotic pathways targeted cancer therapeutics. Adv
Pharm Bull. 9:205–218. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Plati J, Bucur O and Khosravi-Far R:
Apoptotic cell signaling in cancer progression and therapy. Integr
Biol (Camb). 3:279–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Plati J, Bucur O and Khosravi-Far R:
Dysregulation of apoptotic signaling in cancer: Molecular
mechanisms and therapeutic opportunities. J Cell Biochem.
104:1124–1149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Longley DB, Wilson TR, McEwan M, Allen WL,
McDermott U, Galligan L and Johnston PG: c-FLIP inhibits
chemotherapy–induced colorectal cancer cell death. Oncogene.
25:838–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Safa AR, Day TW and Wu CH: Cellular
FLICE-like inhibitory protein (C-FLIP): A novel target for cancer
therapy. Curr Cancer Drug Targets. 8:37–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oztürk S, Schleich K and Lavrik IN:
Cellular FLICE-like inhibitory proteins (c-FLIPs): Fine-tuners of
life and death decisions. Exp Cell Res. 318:1324–1331. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kiraz Y, Adan A, Yandim MK and Baran Y:
Major apoptotic mechanisms and genes involved in apoptosis. Tumour
Biol. 37:8471–8486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pfeffer CM and Singh ATK: Apoptosis: A
target for anticancer therapy. Int J Mol Sci. 19:4482018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saito T, Okada S, Yamada E, Shimoda Y,
Osaki A, Tagaya Y, Shibusawa R, Okada J and Yamada M: Effect of
dapagliflozin on colon cancer cell [Rapid Communication]. Endocr J.
62:1133–1137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu X, Yue P, Schönthal AH, Khuri FR and
Sun SY: Cellular FLICE-inhibitory protein down-regulation
contributes to celecoxib-induced apoptosis in human lung cancer
cells. Cancer Res. 66:11115–11119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen S, Liu X, Yue P, Schönthal AH, Khuri
FR and Sun SY: CCAAT/enhancer binding protein homologous
protein-dependent death receptor 5 induction and
ubiquitin/proteasome-mediated cellular FLICE-inhibitory protein
down-regulation contribute to enhancement of tumor necrosis
factor-related apoptosis-inducing ligand-induced apoptosis by
dimethyl-celecoxib in human non small-cell lung cancer cells. Mol
Pharmacol. 72:1269–1279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin Y, Liu X, Yue P, Benbrook DM, Berlin
KD, Khuri FR and Sun SY: Involvement of c-FLIP and survivin
down-regulation in flexible heteroarotinoid-induced apoptosis and
enhancement of TRAIL-initiated apoptosis in lung cancer cells. Mol
Cancer Ther. 7:3556–3565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mora-Molina R, Stöhr D, Rehm M and
López-Rivas A: cFLIP downregulation is an early event required for
endoplasmic reticulum stress-induced apoptosis in tumor cells. Cell
Death Dis. 13:1112022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yodkeeree S, Sung B, Limtrakul P and
Aggarwal BB: Zerumbone enhances TRAIL-induced apoptosis through the
induction of death receptors in human colon cancer cells: Evidence
for an essential role of reactive oxygen species. Cancer Res.
69:6581–6589. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sheikh BY, Sarker MMR, Kamarudin MNA and
Mohan G: Antiproliferative and apoptosis inducing effects of citral
via p53 and ROS-induced mitochondrial-mediated apoptosis in human
colorectal HCT116 and HT29 cell lines. Biomed Pharmacother.
96:834–846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kanayama A and Miyamoto Y: Apoptosis
triggered by phagocytosis-related oxidative stress through FLIPS
down-regulation and JNK activation. J Leukoc Biol. 82:1344–1352.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nitobe J, Yamaguchi S, Okuyama M, Nozaki
N, Sata M, Miyamoto T, Takeishi Y, Kubota I and Tomoike H: Reactive
oxygen species regulate FLICE inhibitory protein (FLIP) and
susceptibility to Fas-mediated apoptosis in cardiac myocytes.
Cardiovasc Res. 57:119–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Uthman L, Homayr A, Juni RP, Spin EL,
Kerindongo R, Boomsma M, Hollmann MW, Preckel B, Koolwijk P, van
Hinsbergh VWM, et al: Empagliflozin and dapagliflozin reduce ROS
generation and restore NO bioavailability in tumor necrosis factor
α-stimulated human coronary arterial endothelial cells. Cell
Physiol Biochem. 53:865–886. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu Y, Xu Q, Li H, Meng Z, Hao M, Ma X, Lin
W and Kuang H: Dapagliflozin reduces apoptosis of diabetic retina
and human retinal microvascular endothelial cells through
ERK1/2/cPLA2/AA/ROS pathway independent of hypoglycemic. Front
Pharmacol. 13:8278962022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Leng W, Wu M, Pan H, Lei X, Chen L, Wu Q,
Ouyang X and Liang Z: The SGLT2 inhibitor dapagliflozin attenuates
the activity of ROS-NLRP3 inflammasome axis in steatohepatitis with
diabetes mellitus. Ann Transl Med. 7:4292019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shibusawa R, Yamada E, Okada S, Nakajima
Y, Bastie CC, Maeshima A, Kaira K and Yamada M: Dapagliflozin
rescues endoplasmic reticulum stress-mediated cell death. Sci Rep.
9:98872019. View Article : Google Scholar : PubMed/NCBI
|