According to global cancer data statistics, lung
cancer was the leading cause of cancer-related death in 2020
(1). Approximately 30% of
cancer-related deaths in China are related to lung cancer, which
remains the most common cancer type (2). Clinical statistics show that
non-small cell lung cancer (NSCLC) accounts for ~85% of lung cancer
cases, and lung adenocarcinoma is the most common type of NSCLC
(1). Recently, with the
introduction of molecular-targeted drugs and immune checkpoint
inhibitors, the survival outcomes of patients with advanced lung
cancer have improved greatly, but the 5-year overall survival rate
of patients with lung adenocarcinoma remains less than 20%
(3–5).
According to statistics, the incidence of epidermal
growth factor receptor (EGFR) mutations in Caucasians is ~20%
(6), whereas the rate is 44–50%
among Asian nonsmoking NSCLC patients (7,8). The
higher frequency of EGFR mutations appears to be beneficial for
Asian lung adenocarcinoma patients. EGFR tyrosine kinase inhibitors
(TKIs) are currently the first-line treatment for lung
adenocarcinoma patients with EGFR-sensitive mutations (4,9–14);
unfortunately, most patients develop acquired drug resistance after
10–14 months of EGFR-TKI treatment (14,15).
The mechanisms of acquisition of drug resistance to first- and
second-generation EGFR-TKIs are complex, and the most important
mechanism of acquired drug resistance is the secondary T790M
mutation, accounting for 50–60% of all cases (16). The third-generation EGFR inhibitor
Osimertinib is the most common drug used for the treatment of
patients with this mutation (17).
However, acquired drug resistance still emerges against
third-generation drugs typically 8–10 months after receiving
Osimertinib (17,18). The C797s mutation is the primary
mechanism of acquired drug resistance (19). Research on fourth-generation
EGFR-TKI drugs for the treatment of tumors with a C797s mutation is
currently at various experimental stages, although no drug has been
approved for clinical use.
Due to the relatively high frequency of EGFR
mutations in patients with lung adenocarcinoma in China, an
increasing number of patients develop EGFR-TKI resistance, and
subsequent treatment options are critical. This article reviews the
mechanisms of drug resistance and treatment progress after EGFR-TKI
resistance.
The mechanisms of acquired drug resistance against
first- and second-generation EGFR-TKIs are complex and can be
divided into three categories: Changes in EGFR, activation of
alternative bypass or downstream pathways, and changes in the
phenotype (Fig. 1).
A secondary T790M mutation is the most important
mechanism of acquired drug resistance against first-generation
EGFR-TKIs. The crystal structure of the ATP binding pocket is
altered due to this mutation, inhibiting the binding of TKIs and
ATP. Thus, downstream signal transduction cannot be inhibited by
TKIs, and these drugs do not subsequently restrict tumor growth
(20,21). The mechanism of action of the
first-generation EGFR-TKI differs from that of the
second-generation EGFR-TKI; the second-generation EGFR-TKI
irreversibly binds to the ErbB receptor, resulting in a more potent
effect than the first-generation drugs (22), but the mechanism of drug resistance
is similar (23–25). In the ARCHER1050 study, dacomitinib
had overall survival (OS) benefits relative to gefitinib in the
Chinese population (median overall survival (mOS) duration was 32.5
months vs. 24.9 months, P=0.0097) (26). The LUX-Lung7 trial compared the
efficacy of afatinib and gefitinib for the treatment of NSCLC
patients with EGFR mutations. The results showed that the
progression-free survival (PFS) duration of the afatinib group was
longer than that of the gefitinib group (11.0 vs. 10.9 months;
P=0.017) (11). These results
suggest that compared with first-generation EGFR-TKIs, the effects
of second-generation EGFR-TKIs are longer in the context of
T790M.
Osimertinib is the most widely used third-generation
EGFR-TKI and it can effectively and selectively inhibit tumors with
EGFR-sensitive and T790M drug-resistant mutations (27), exhibiting a significant effect in
NSCLC patients with brain metastases (28,29).
Almonertinib (30,31) and furmonertinib (32,33)
have also been approved in China, and several other
third-generation EGFR-TKI inhibitors are in different stages of
research and development (Table
I). Lazertinib achieved a 57% overall response rate (ORR) in
the T790M (+) population in a phase 2 clinical trial (34). The drug exhibited a potent
beneficial effect on brain lesions, and the intracranial disease
control rate in the entire population was 90.6% (35). In January 2021, the Korean Food and
Drug Administration (MFDS) approved the listing of lazertinib for
the treatment of patients with locally advanced or metastatic NSCLC
positive for EGFR T790M mutations who previously received EGFR-TKI
treatment (36). The
third-generation EGFR-TKIs olmutinib (37–39)
and nazartinib (40) are also
approved in South Korea.
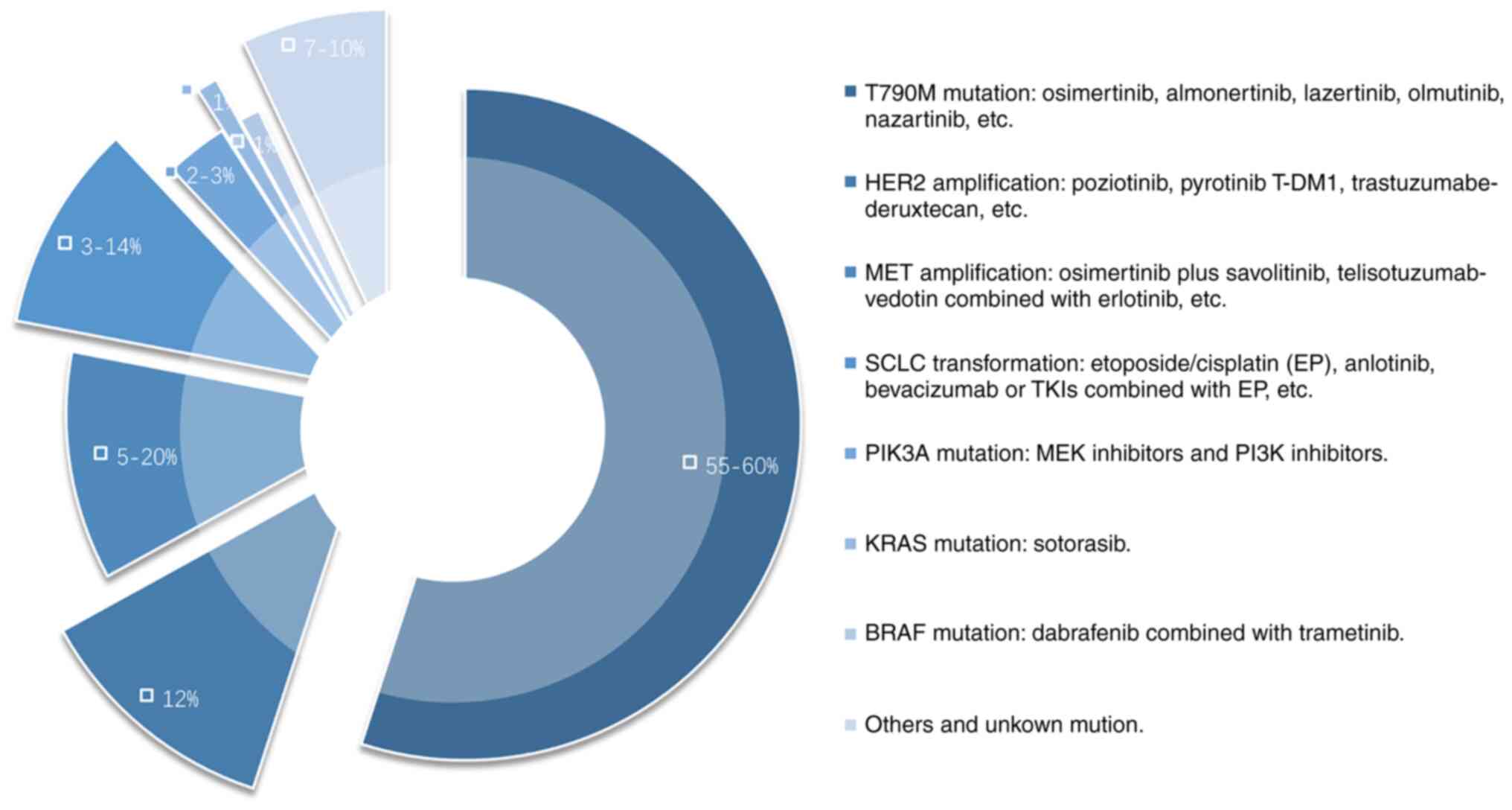
Activation of HER2, also known as ERBB2, triggers
functional abnormalities in several downstream signaling pathways,
such as the mitogen-activated protein kinase (MAPK), inosine
phosphate 3-kinase (PI3K)/protein kinase B (AKT), protein kinase C
(PKC), and signal transducer and transcriptional activator (STAT)
pathways, resulting in uncontrolled cell proliferation (48,49).
HER2 overexpression occurs in ~12% of NSCLC patients who are
resistant to first- and second-generation EGFR-TKIs and usually do
not co-exist with the T790M secondary mutation (50). Standard treatment for managing this
drug resistance mechanism is currently not available, and there is
insufficient evidence to show that existing anti-HER2 therapies are
effective. The selective HER2 tyrosine kinase inhibitors poziotinib
(51) and pyrotinib (52,53),
and the antibody conjugate drugs T-DM1 (54) and trastuzumabe-deruxtecan (55) are potential treatment options.
MET is a proto-oncogene and one of the key driver
genes in several types of cancer (56). The MET gene encodes c-Met [a
hepatocyte growth factor (HGF) receptor], which is responsible for
regulating important processes, such as cell differentiation,
proliferation, migration, and apoptosis (57). Hepatocyte growth factor (HGF) binds
to c-Met to phosphorylate tyrosine kinase residues in the catalytic
domain; activates the downstream pathways modulated by PI3K, MAPK,
and STAT3 signaling, and promotes cell transformation, cell
invasion, cell proliferation, and cell cycle progression (57,58).
MET amplification accounts for 2–4% of untreated NSCLC cases
(59) and for 5–20% of patients
with acquired drug resistance against first- and second-generation
EGFR-TKIs (58,60,61).
Lai et al (62) showed that
an increased copy number of the MET gene is not equal to MET
amplification; only MET amplification is a determinant of EGFR-TKI
resistance in NSCLC patients.
PIK3A can induce the phosphorylation and subsequent
activation of the downstream AKT signal transduction pathway and it
plays a central role in regulating tumor cell growth, reproduction,
migration, and apoptosis (71).
The role of PIK3CA mutations in NSCLC remains contested. Some
researchers consider PIK3CA mutations to be an independent risk
factor for NSCLC patient survival (72), and the survival time of patients
with EGFR and PIK3CA mutations treated with EGFR-TKIs was shown to
be shorter than that of people with only EGFR mutations (73). However, it has also been shown that
PIK3CA mutations have no significant effect on NSCLC patient
survival times (74). PIK3CA
mutations are a mechanism of acquired EGFR-TKI resistance in
patients with EGFR-mutated lung cancer (75). The frequency of PIK3CA mutations
after EGFR-TKI resistance is 2–3% (76). Preclinical studies have found that
double targeting of MEK and PI3K can effectively control the
proliferation of EGFR-TKI drug-resistant NSCLC cell lines (77). Alpelisib (a PI3K inhibitor) has
been approved by the Food and Drug Administration (FDA) for the
treatment of breast cancer (78),
but it has not been applied for NSCLC after the development of
resistance to TKIs.
KRAS mutations activate downstream pathways, such as
the MAPK and PI3K pathways, driving the occurrence and development
of tumors (79). The proportion of
KRAS mutations after the development of EGFR-TKI resistance is ~1%
(76). Tanaka et al
(80) suggested that the mechanism
underlying KRASG12C-acquired drug resistance to KRAS-TKI is related
to the activation of the RAS-MAPK signals and the production of
KRASY96D resistance genes. The FDA approved the KRASG12C inhibitor
sotorasib in May 2021 to treat NSCLC patients with KRASG12C
mutations after at least one previous systematic treatment
(81).
BRAF mutations increase the activity of RAF kinase,
activates downstream MEK, and regulates cell growth, proliferation,
differentiation, migration, and apoptosis (82). BRAFV600E is the most common BRAF
mutation, accounting for 36% of all BRAF mutations (83). BRAF mutations account for only 1%
of patients with acquired drug resistance to TKIs (84). Dabrafenib combined with trametinib
has been approved by the FDA for the treatment of metastatic NSCLC
with BRAFV600E mutations (85).
The AXL-mediated Gas6/Axl signaling pathway is
associated with tumor cell growth, metastasis, invasion, EMT,
angiogenesis, drug resistance, immune regulation, and stem cell
maintenance (86,87). In 2012, a study found that Axl
expression was upregulated in patients with acquired drug
resistance to EGFR-TKIs, and EGFR-TKI sensitivity was restored
after blocking Axl (88). Thus,
Axl is a promising therapeutic target for patients with acquired
drug resistance. Small molecule inhibitors, monoclonal antibodies,
and antibody-drug conjugates targeting Axl are currently under
development (89). DS-1205 (an AXL
inhibitor) combined with gefitinib (90) and BGB324 (an AXL inhibitor)
combined with erlotinib (91) were
evaluated, and preliminary results were promising.
PTEN negatively regulates the PTEN/PI3K/Akt
signaling pathway and regulates cell growth, apoptosis, and
migration (92). Studies have
shown that patients with EGFR mutations with PTEN deletions have
significantly shorter PFS durations than those without PTEN
deletions (6 vs. 18 months) (93).
According to Xun et al (92), the deletion of PTEN in lung cancer
promotes the carcinogenic function of STMN1 (overexpression of
which is related to tumor growth, metastasis, and poor survival)
through the PI3K/AKT pathway. Other reported drug resistance
mechanisms include loss of neurofibromin 1 activity (94), amplification of the CT10 homologous
oncogene of v-crk avian sarcoma virus (95), a multistep mechanism involved in
the insulin-like growth factor 1 receptor (IGF1R) pathway (96), and the fibroblast growth factor
(FGF) 2/FGF receptor 1 (FGFR1) autocrine growth pathway (97). As these drug resistance mutations
are rare, no drugs targeting them have been approved.
Compound mutations indicate the presence of more
than one EGFR mutation, either common or uncommon, within the same
tumor. Attili et al (98)
found high heterogeneity in the incidence of compound mutations
(4–26% of total EGFR mutant cases), with the variance possibly due
to the different testing methods adopted, and the specific
mutations considered. In various combinations, compound EGFR
mutations containing either exon 21 p. L858R or exon 19 deletions
were common (99). The response
rate of those tumors with compound mutations to EGFR-TKIs compared
with those with single mutations is contested. Rossi et al
(100) found a longer mOS in the
compound mutation group than in the single rare mutation group
(33.6 vs. 12 months; P=0.473), whereas Jiang et al (101) concluded that patients in the
single mutation group exhibited a longer mOS than those in the
co-mutation group (ORR: 64.6% vs. 27.4%, P<0.001). More
prospective randomized clinical trials (RCTs) are required to
reconcile these differences.
A co-mutation is defined as the coexistence of an
EGFR mutation along with one or more other gene mutations. The
co-mutation incidence rate was 66.0% in the retrospective study of
Jiang (101). Co-mutations,
including TP53 (102,103), HER family genes (104), KRAS, MET, and ROS1, are typically
considered to be associated with poor prognosis (105,106).
Among the patients who did not maintain a response
to EGFR-TKI treatment, 3–14% had tumors that showed morphological
transformation to SCLC (61,107). Although the tumors that
transformed into SCLC had persistent EGFR activation,
immunohistochemical analysis showed that EGFR expression decreased
sharply (108).
EGFR-TKI-resistant lung adenocarcinoma and SCLC share a common
clonal origin. Significant inactivation of Rb and TP53 (a common
mutation of classical SCLC) was found in patients with SCLC after
the development of drug resistance (108–110). In addition, PIK3CA (111) mutations and TERT amplification
(112) were also observed. The
specific mechanisms involved in this transition and TKI resistance
have not been determined. In addition to the above mutations, other
studies have suggested that the transformation may be related to
EMT (113,114). A retrospective analysis of this
mechanism of drug resistance showed that the etoposide/cisplatin
regimen is currently the most effective treatment (115). In this retrospective study,
patients treated with anlotinib also achieved an ORR of 66.7% and
an mPFS duration of 6.2 months. Another small-sample study reported
longer PFS durations were obtained with bevacizumab or other TKIs
combined with chemotherapy (116).
In recent years, several cases of transformation of
EGFR-mutated NSCLC to SCC have been reported (117–121), and some reports indicate the
association between the T790M mutation and SCC transformation
(122,123). As this morphological
transformation is rare, the mechanism is unclear, although it has
been shown that it may be related to changes in the PI3K/AKT/mTOR
pathway during EGFR-TKI therapy (124). For patients with drug-resistant
lung SCC, the prognosis is usually poor, and the mOS is only ~3.5
months (120). It is difficult to
choose follow-up treatments due to the low incidence; Liao et
al (121) reported the case
of a patient who received almonertinib for 6 months after detection
of the SCC phenotype. At the time of writing the study, the patient
was continuing almonertinib monotherapy and the disease was
stable.
EMT is a process in which epithelial cells lose
polarity and adhesion to gain increased migratory ability, and in
the process exhibit a mesenchymal phenotype characterized by
decreased E-cadherin and increased vimentin expression as well as
stem cell-like features (125,126). EMT is considered one of the
possible mechanisms of acquired drug resistance to EGFR-TKIs
(127). Increased expression of
Aurora kinase A (AURKA) can induce EMT and contribute to the
occurrence of acquired EGFR-TKI resistance (128). Nilsson et al (129) found that activation of the YAP
and FOXM1 axes serves as a driver of EMT-related EGFR-TKI
resistance. It has also been confirmed that reversing EMT can
restore sensitivity to EGFR-TKI drugs (116). The AURKA inhibitor alisertib can
restore the sensitivity of drug-resistant cells to EGFR-TKIs and
partially reverse the EMT process (130). It has also been found that
Bruton's tyrosine kinase (BTK) mediates dryness and EMT
characteristics, and the BTK inhibitor acalabrutinib can enhance
the effect of gefitinib and Osimertinib in TKI-resistant NSCLC
cells (131).
Regarding the aforementioned drug resistance
mechanisms, although researchers have performed extensive
treatment-related research, no drugs specifically developed for
acquired drug resistance mechanisms have been approved and marketed
given the low incidence of these causative mutations. For the
first- and second-generation TKI drug-resistant T790M-negative
population, platinum-containing dual-drug chemotherapy is currently
recommended, but its benefits are limited (132). Other treatment options are being
explored and are summarized below.
The relationships between EGFR mutations, EGFR-TKIs,
and immunotherapy efficacy are contested. A meta-analysis of large
RCTs showed that patients with EGFR mutations showed no significant
benefit from immunotherapy (133,134). In people with PD-L1 expression
levels <50%, the use of EGFR-TKI inhibitors resulted in a better
PFS rate and ORR (135). However,
studies have also shown that for patients with EGFR mutations, the
proportion of patients with PD-L1 expression levels ≥50% increased
after EGFR-TKI treatment, and the mPFS resulting from subsequent
treatment with PD-1 antibodies was longer than that of patients
with low PD-L1 expression (7.1 vs. 1.7 months; P=0.0033) (136). These results indicate that
EGFR-TKI drugs appear to have a positive effect on the tumor
microenvironment (TME).
According to the IMpower150 study, a subgroup
analysis of patients following EGFR-TKI failure showed that OS
benefits were obtained after addition of atrizumab; this is the
only study that has confirmed OS benefits from immunotherapy after
the development of EGFR-TKI resistance (137). Another ongoing phase II study
also showed that the addition of atezolizumab to the bevacizumab
regimen improved the disease control rate (DCR) and PFS outcome
(138). In the single-arm II
phase study by Lam et al (139), a 9.4-month PFS duration was
obtained using a quadruple combination of atezolizumab,
bevacizumab, carboplatin, and pemetrexed. A total of 42.5% of the
patients were resistant to first- and second-generation EGFR-TKIs.
The incidence of treatment-related adverse events was 37.5%
(15/40), which is within the range of controllable adverse events.
Thus, this combination appears to be a feasible treatment.
CT18 is the first prospective immunotherapy study in
patients with lung adenocarcinoma with EGFR mutations. The results
showed good clinical benefits (ORR=50%, mPFS=7 months, OS=23.5
months) in T790M-negative patients with acquired drug resistance
after treatment with toripalimab combined with carboplatin and
pemetrexed (140). A phase III
RCT (TREASURE) is underway, evaluating toripalimab plus
chemotherapy as second-line treatment in patients with
EGFR-mutant-advanced NSCLC who were previously treated with
EGFR-TKIs; patients with failed first-line EGFR-TKIs and those who
did not harbor T790M mutation were enrolled. (141). ORIENT-31 was a randomized,
double-blind, multicenter, phase III RCT that evaluated the
efficacy and safety of the combination of sintilimab and
bevacizumab for treating EGFR-mutated SCLC after EGFR-TKI
treatment. The study found that patients in the quadruple drug
group had prolonged mPFS (6.9 vs. 4.3 months) and median duration
of efficacy (8.3 vs. 7.0 months) outcomes compared to those in the
chemotherapy group (142).
Pemetrexed is an anti-folic acid drug that can
interfere with folic acid metabolism, resulting in aberrant DNA
synthesis in tumor cells (144).
A cancer registration cohort analysis from Taiwan showed that
pemetrexed may be suitable as a first choice for chemotherapy in
patients undergoing chemotherapy after progression with EGFR-TKI
treatment (145). A 2018
meta-analysis showed that second-line drugs combined pemetrexed
chemotherapy resulted in a longer PFS and OS duration than therapy
with pemetrexed (146). In a
phase II study, researchers compared cisplatin plus pemetrexed
against pemetrexed alone in patients with drug resistance and found
no significant difference in PFS and OS outcomes between the two
groups. The efficacy of pemetrexed in NSCLC patients with disease
progression after first-line EGFR-TKI treatment was not improved by
adding cisplatin (147).
Preclinical studies have shown that vascular
endothelial growth factor (VEGF) and EGFR share a common downstream
signaling pathway and acquired EGFR resistance is associated with
increased VEGF levels (148).
In vivo and in vitro studies have confirmed that
anlotinib (a small molecular multitarget tyrosine kinase inhibitor)
can overcome acquired resistance to EGFR-TKIs through modulation of
the FGFR1 signaling pathway (149,150). Phase II clinical trials have
shown that the use of bevacizumab combined with afatinib resulted
in an ORR of 22% and a PFS of 7.1 months in T790M-negative patients
who developed drug resistance (151). A patient with EGFRL858R and
KRASG12D mutations administered a combination of bevacizumab,
camrelizumab, and pemetrexed after developing EGFR-TKI resistance
achieved a benefit lasting ~17 months (152). A retrospective analysis in China
revealed that the longer the duration of the previous EGFR-TKI
treatment had been, the longer the PFS duration was when patients
received follow-up immunotherapy combined with chemotherapy and
antiangiogenic drugs (153). Due
to the limitations of the above studies, additional prospective
studies are needed to confirm the efficacy of antiangiogenic drugs
combined with targeted therapy or immunotherapy in the future.
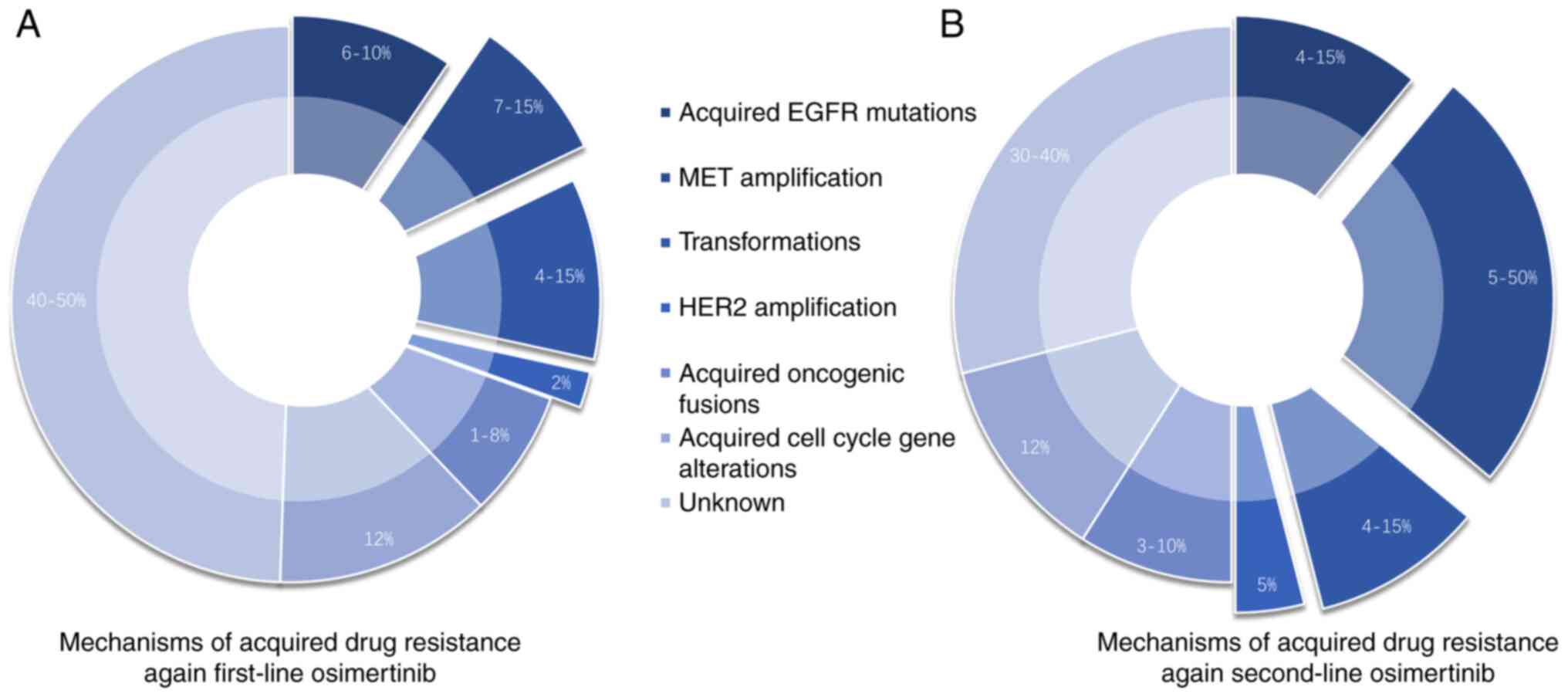
At present, Osimertinib is the only third-generation
EGFR-TKI preparation that is widely used and that has been studied
relatively extensively. Drug resistance mechanisms associated with
first-line use of Osimertinib are similar to those associated with
second-line therapy, but the proportion of patients developing
resistance differs (Fig. 2). At
present, the reported mechanisms of drug resistance can be divided
into EGFR-dependent and EGFR-independent mechanisms (154,155). The mechanisms of EGFR-dependent
drug resistance include EGFR mutations, amplification, deletion,
and ligand overexpression as well as tertiary EGFR mutations,
whereas EGFR-independent resistance mechanisms include activation
of abnormal accessory pathways, activation of downstream pathways,
and histological/phenotypic transformation (156).
The Aura3 study revealed that 49% of the patients
had T790M loss, and 14% had EGFRC797S mutations, the most common
mutations acquired after the development of Osimertinib resistance
(154). EGFRC797S mutations
include cis (98%) and trans mutations (2%). T790M and
C797S mutations that occur simultaneously in the same allele are
referred to as cis mutations, and mutations that occur in
different alleles are referred to as trans mutations
(157,158).
Recent studies have shown that a third-generation
TKI combined with a first-generation EGFR-TKI can change the
expression profile of drug resistance genes in lung adenocarcinoma
patients with EGFR activation mutations, and T790M and
trans-C797S triple mutations (158). Brigatinib combined with cetuximab
is an effective treatment strategy for these lung adenocarcinoma
patients with EGFR activation mutations and T790M and
cis-C797S triple mutations (158,159). Chang et al (160) reported the case of a patient with
lung adenocarcinoma with triple mutations (L858R, T790M, and
cis-G796s/cis-C797s). After treatment failure with
brigatinib combined with cetuximab, the patient responded to the
combination of brigatinib, Osimertinib, and bevacizumab. Other
reported treatments include Osimertinib combined with anlotinib
(161), chemotherapy combined
with antiangiogenic agents (162), and apatinib combined with
afatinib (163). At present,
fourth-generation EGFR-TKIs targeting drug-resistant T790M
mutations are under development, although no drug has been
approved. Fourth-generation EGFR-TKIs in clinical trials and
currently undergoing research and development and are described in
subsequent sections.
In a study where next-generation sequencing (NGS)
analysis was performed on 93 samples obtained after the development
of Osimertinib resistance, EGFRG796/C797, L792, and L718/G719
mutations were found in 24.7, 10.8, and 9.7% of cases, respectively
(164). G724 mutations were also
reported in some studies (165,166). At present, a drug that targets
the aforementioned mutated genes is not available. In vitro
studies have confirmed that L792 is still sensitive to gefitinib
(167) and that tumors with the
L718Q mutations remain sensitive to icotinib (168). In a patient with the
EGFRG724S/19del mutation after second-line Osimertinib resistance,
PFS was achieved after using afatinib for 3.8 months (169).
EGFR-independent mechanisms of drug resistance
primarily include activation of abnormal accessory and downstream
pathways and histological/phenotypic transformation. Most of the
mechanisms of Osimertinib drug resistance are the same as those of
first- and second-generation TKIs.
Abnormal FGFR expression can lead to the activation
of the FGFR cancer-related signaling pathway effectors (PI3K/AKT,
STAT, and MAPK) and affect cell proliferation, survival,
metabolism, and migration as well as the cell cycle (178). In vitro studies found that
hypoxia can lead to acquired resistance to EGFR-TKIs by increasing
the expression of FGFR1 (179).
The combined use of EGFR-TKIs and FGFR1 inhibitors (BGJ398) may
represent a potential therapeutic strategy for the management of
NSCLC (179,180). Upregulation of IGF1R is one of
the mechanisms of drug resistance to EGFR-TKIs, including
Osimertinib (181–183). In cells resistant to Osimertinib
that exhibit low levels of AXL expression, short-term IGF-1R
inhibition combined with Osimertinib can eradicate tumors and
prevent regrowth (184).
Histological transformation from lung
adenocarcinoma to SCLC, SCC and EMT was also observed in patients
with Osimertinib resistance (177,185). Platinum-containing dual-drug
chemotherapy is still recommended for these patients.
Drug resistance to third-generation targeted drugs
(Osimertinib) is a dilemma faced by several lung cancer patients
who receive targeted therapy. To date, the FDA has not approved
targeted therapy for progression after treatment with Osimertinib.
Thus, the research and development of fourth-generation EGFR-TKI
drugs have become a focus recently, and several drugs have shown
good results in clinical trials.
EAI045 is the first fourth-generation EGFR-TKI
drug. EAI045 combined with cetuximab significantly reduced the
tumor size in mice carrying L858R/T790M/C797S mutations, but no
obvious effect was observed with single-agent use (186). To improve the activity of EAI045
and the ability to use the drug as a single agent, To et al
(187) modified EAI045 and
obtained a new allosteric inhibitor, JBJ-04-12502, which exhibited
higher efficacy, lower toxicity, and efficacy against EGFR
mutations compared with the parent compound. JBJ-04-12502 inhibits
the triple-drug resistance mechanism of patients with
L858R/T790M/C797S mutations, the double mutation of EGFR-T70M, and
the L858R drug resistance mutation. The therapeutic effect of
JBJ-04-12502 in combination with Osimertinib is more potent,
although it is still in the research and development stage
(188). CH7233163 is a
fourth-generation EGFR-TKI inhibitor developed by Roche Chugai
Pharmaceuticals for patients with a Del19 mutation. After the
application of CH7233163 in Del19/L858R/T790M, L858R/T790M mutant,
and Del19 mice, a substantial reduction in tumor volume was
observed (189). The prospect of
CH7233163 appears to be more promising than that of
JBJ-04-12502.
Both BLU-945 and BLU-701 are fourth-generation
EGFR-TKI inhibitors developed by Blueprint Medicines. Both can
resist EGFR activation mutations (del19, 21L858R) as well as T790M
and C797S drug resistance mutation activity (190,191). BLU-945 combined with Osimertinib
or gefitinib provided a more significant tumor elimination effect
in n NSCLC mouse model (192).
BLU-701 also exhibited intracranial antitumor activity, and both
BLU-701 alone and in combination with BLU-945 showed strong
antitumor activity (193).
TQB3804 is a fourth-generation oral EGFR-targeted
drug developed by the Zhengda Tianqing Pharmaceutical Group. It not
only solves Osimertinib resistance caused by d746750
(19del)/T790M/C797S and L858R/T790M/C797S, but is also effective
against the d746-750/T790M and L858R/T790M double mutations
associated with resistance to first- and second-generation TKIs
(194). Correlative clinical
trials (NCT04128085 and NCT04180150) are currently underway
(195).
BBT-176 is an innovative EGFR-TKI developed by
Bridge Biotherapeutics in Korea. BBT-176 showed strong anticancer
activity in xenotransplantation animal models carrying triple
mutations Del19/T790M/C797S and L858R/T790M/C797S (196). Moreover, BBT-176 in combination
with the anti-EGFR antibody cetuximab showed significantly enhanced
activity (197).
The EGFR and MET bispecific antibody amivantamab is
also classified as a fourth-generation EGFR-TKI. This drug is
effective against the EGFR exon 20 insertion mutation (primary drug
resistance mutation) (198),
C797S mutation, and MET amplification after the acquisition of
Osimertinib resistance. Amivantamab combined with Lazertinib
effectively overcomes Osimertinib resistance. In 45 patients with
Osimertinib resistance, the disease control rate reached 60% with a
median follow-up period of 4 months (199). All the above drugs, except for
amivantamab, which has been approved for the treatment of the NSCLC
EGFR exon 20 insertion mutation, remain in different stages of
research and development or clinical trials. Thus, it will be
several years before these drugs are available for clinical
use.
U3-1402 is an antibody-drug conjugate (ADC)
developed by Daiichi Co., Ltd., which consists of patritumab acting
on HER3 antibodies and the cytotoxic drug DX-8951 (Exitecan, a
topoisomerase inhibitor). U3-1402 is effective against different
drug resistance mechanisms against EGFR-TKIs, as demonstrated in
phase I trial results published in 2019 (200). Another phase I study included 57
patients with EGFR-TKI resistance. The DCR was 68% in 44 patients
who had previously received Osimertinib and platinum-containing
chemotherapy. The mPFS reached 8.2 months (201). ADC drugs have considerable
potential for solving the problem of resistance to Osimertinib.
The COMPEL study was a randomized, double-blind
phase III clinical study that evaluated the efficacy and safety of
chemotherapy plus Osimertinib or chemotherapy plus placebo in
advanced NSCLC patients with progressive EGFR mutations after
first-line treatment with Osimertinib. The study is currently
underway and will be published in September 2024 (204).
The ORIENT-31 study included patients who were
T790M-negative after first- and second-generation EGFR-TKI
treatment and patients who received third-generation EGFR-TKI
treatment. The results showed that the PFS duration was
significantly prolonged in patients treated with sintilimab
combined with bevacizumab and chemotherapy compared with that of
patients treated with chemotherapy alone (142). This study was the first to
confirm that PD-1 inhibitors combined with antivascular drugs and
chemotherapy significantly improved PFS outcomes in EGFR-mutant
non-squamous NSCLC patients with progression after EGFR-TKI
treatment, providing options for the follow-up treatment of
drug-resistant patients after targeted treatment.
In a single-arm phase II study of patients
administered a quadruple combination of atezolizumab, bevacizumab,
carboplatin, and pemetrexed, the PFS duration was 9.4 months; 57.5%
of these patients had been treated with Osimertinib (139). The IMpower150 study is currently
the only randomized prospective phase III clinical trial that
demonstrated OS benefits in NSCLC patients in an EGFR-sensitive
mutation subgroup (137), showing
that the addition of atezolizumab to the standard therapy of
bevacizumab and chemotherapy represents a novel treatment
option.
The 21st century is the era of targeted cancer
treatment, and several promising options for lung adenocarcinoma
patients are available. Although novel treatments provide survival
benefits to varying degrees, the problem of drug resistance
inevitably leads to disease progression. It has been demonstrated
that tumors become increasingly molecularly heterogeneous following
targeted therapy (5,207). There is a large body of
literature implicating intratumoral heterogeneity as a major driver
of drug resistance (208,209). NGS and single-cell RNA sequencing
(scRNA-seq) are used to study the genetic and molecular
characteristics of tumor development at various stages (210), revealing the heterogeneity of
tumor cells and monitoring the progress of tumor development.
In recent years, modified T-cell therapy,
particularly those that use chimeric antigen receptor (CAR)-T
cells, has attracted growing interest in various solid tumors with
the clinical success of chimeric antigen receptor CAR T-cell
therapy in hematological malignancies (212,213). The CAR T strategy aims to isolate
T cells from the peripheral blood of patients or other donors and
genetically engineer T cells with CAR structures to equip them with
the capability of recognizing specific antigens on the tumor cell
surface. After infusion back into patients, these ‘super’ T cells
recognize and eliminate the cancer cells that express specific
target antigens (214). The major
difference between CAR T cells and tumor-specific T cells is that
the former cells are not limited by the major histocompatibility
complex (215,216). It is critical to identify
targeted tumor-associated antigens (TAAs). Ideal TAAs are highly
and selectively expressed in solid tumors, but weakly expressed or
absent in normal tissues (217).
The lung adenocarcinoma-associated TAAs currently
being investigated in clinical trials on CAR-T cells include
mesothelin (MSLN), mucin 1 (MUC1), carcinoembryonic antigen (CEA)
EGFR, PD-L1, prostate stem cell antigen (PSCA), disialoganglioside
GD2 (GD2), and c-Met (218–222).
For EGFR-mutated LUAD, EGFR is definitely an
optimum TAA. A phase I clinical trial of EGFR-targeting CAR T-cell
therapy to treat patients with EGFR-positive relapsed/refractory
NSCLC achieved initial success (NCT01869166). The results showed
that none of the patients exhibited significant toxic side effects
after anti-EGFR CAR-T-cell therapy, 2 patients achieved partial
remission, and 5 patients had stable disease for 2–8 months
(223). This result provides
preliminary evidence that EGFR-targeting CAR T therapy is safe and
feasible in certain cases of relapsed/refractory NSCLC. Currently,
there are two ongoing phase I clinical trials in patients with lung
cancer on C-X-C chemokine receptor type 5 modified EGFR-targeted
CAR-T cells (NCT05060796 and NCT04153799).
Although CAR-T-cell therapies have achieved great
success in hematological malignancies, the study of lung cancer is
still in the early exploration stage. Numerous clinical trials have
progressed slowly and have achieved very limited efficacy, and
several challenges and hurdles remain, such as on-target/off-tumor
toxicity, CAR-T cell trafficking and infiltration into the tumor,
TME heterogeneity, immune suppression, and cytokine release
syndrome (224–226). Recently, Vasic et al
(227) found that allogeneic
double-negative CAR-T cells inhibit tumor growth with no off-tumor
toxicity in either a lung cancer xenograft model or B-cell acute
lymphoblastic leukemia (B-ALL) was observed. Therefore,
double-negative CAR-T cells may serve as a patient-accessible form
of CAR-T cell therapy.
Due to China's large population and relatively high
EGFR mutation rate, the identification of the best treatment after
the development of EGFR-TKI resistance has become an urgent
problem. EGFR-TKIs have been continuously developed and are
currently in their fourth generation of iteration, and this process
is accompanied by the continuous optimization of pharmacological
mechanisms, the emergence of novel drug resistance mechanisms, and
the development of solutions to these new drug resistance
mechanisms. Although it may take considerably more research to
conquer cancer, significant levels of drug research and development
remain ongoing.
Not applicable.
The present study was supported by the Wu Jieping Medical
Foundation of China (grant no. 320.6750.2021-22-8).
Not applicable.
RS conceived the article, performed the literature
search and drafted the manuscript. ZH and YZ contributed their
knowledge on this topic and were involved in planning the structure
of this review. BJ made critical modifications to the content
within the manuscript. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao M, Li H, Sun D and Chen W: Cancer
burden of major cancers in China: A need for sustainable actions.
Cancer Commun (Lond). 40:205–210. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Camidge DR, Doebele RC and Kerr KM:
Comparing and contrasting predictive biomarkers for immunotherapy
and targeted therapy of NSCLC. Nat Rev Clin Oncol. 16:341–355.
2019. View Article : Google Scholar
|
|
4
|
Lee CK, Davies L, Wu YL, Mitsudomi T,
Inoue A, Rosell R, Zhou C, Nakagawa K, Thongprasert S, Fukuoka M,
et al: Gefitinib or erlotinib vs chemotherapy for EGFR
mutation-positive lung cancer: Individual patient data
meta-analysis of overall survival. J Natl Cancer Inst. 109:2017.
View Article : Google Scholar
|
|
5
|
Rotow J and Bivona TG: Understanding and
targeting resistance mechanisms in NSCLC. Nat Rev Cancer.
17:637–658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kobayashi Y and Mitsudomi T: Not all
epidermal growth factor receptor mutations in lung cancer are
created equal: Perspectives for individualized treatment strategy.
Cancer Sci. 107:1179–1186. 2016. View Article : Google Scholar
|
|
7
|
Shi Y, Au JS, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
prospective, molecular epidemiology study of EGFR mutations in
Asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar
|
|
8
|
Shi Y, Li J, Zhang S, Wang M, Yang S, Li
N, Wu G, Liu W, Liao G, Cai K, et al: Molecular Epidemiology of
EGFR mutations in asian patients with advanced non-small-cell lung
cancer of adenocarcinoma histology-Mainland China Subset analysis
of the PIONEER study. PLoS One. 10:e01435152015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong
Z, Lu S, Cheng Y, Han B, Chen L, et al: First-line erlotinib versus
gemcitabine/cisplatin in patients with advanced EGFR
mutation-positive non-small-cell lung cancer: Analyses from the
phase III, randomized, open-label, ENSURE study. Ann Oncol.
26:1883–1889. 2015. View Article : Google Scholar
|
|
11
|
Schuler M, Paz-Ares L, Sequist LV, Hirsh
V, Lee KH, Wu YL, Lu S, Zhou C, Feng J, Ellis SH, et al: First-line
afatinib for advanced EGFRm+ NSCLC: Analysis of long-term
responders in the LUX-Lung 3, 6, and 7 trials. Lung Cancer.
133:10–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa
K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, et al: Dacomitinib
versus gefitinib as first-line treatment for patients with
EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A
randomised, open-label, phase 3 trial. Lancet Oncol. 18:1454–1466.
2017. View Article : Google Scholar
|
|
13
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-Mutated advanced NSCLC. N Engl J Med. 382. pp.
41–50. 2020, View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi YK, Wang L, Han BH, Li W, Yu P, Liu
YP, Ding CM, Song X, Ma ZY, Ren XL, et al: First-line icotinib
versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for
patients with advanced EGFR mutation-positive lung adenocarcinoma
(CONVINCE): A phase 3, open-label, randomized study. Ann Oncol.
28:2443–2450. 2017. View Article : Google Scholar
|
|
15
|
Rebuzzi SE, Alfieri R, La Monica S, Minari
R, Petronini PG and Tiseo M: Combination of EGFR-TKIs and
chemotherapy in advanced EGFR mutated NSCLC: Review of the
literature and future perspectives. Crit Rev Oncol Hematol.
146:1028202020. View Article : Google Scholar
|
|
16
|
Huang L and Fu L: Mechanisms of resistance
to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B. 5:390–401.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-Positive lung
cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thress KS, Paweletz CP, Felip E, Cho BC,
Stetson D, Dougherty B, Lai Z, Markovets A, Vivancos A, Kuang Y, et
al: Acquired EGFR C797S mutation mediates resistance to AZD9291 in
non-small cell lung cancer harboring EGFR T790M. Nat Med.
21:560–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang S, Tsui ST, Liu C, Song Y and Liu D:
EGFR C797S mutation mediates resistance to third-generation
inhibitors in T790M-positive non-small cell lung cancer. J Hematol
Oncol. 9:592016. View Article : Google Scholar
|
|
20
|
Lim SM, Syn NL, Cho BC and Soo RA:
Acquired resistance to EGFR targeted therapy in non-small cell lung
cancer: Mechanisms and therapeutic strategies. Cancer Treat Rev.
65:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagano T, Tachihara M and Nishimura Y:
Mechanism of resistance to epidermal growth factor
receptor-tyrosine kinase inhibitors and a potential treatment
strategy. Cells. 7:2122018. View Article : Google Scholar
|
|
22
|
Park K, Tan EH, O'Byrne K, Zhang L, Boyer
M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, et al: Afatinib versus
gefitinib as first-line treatment of patients with EGFR
mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase
2B, open-label, randomised controlled trial. Lancet Oncol.
17:577–589. 2016. View Article : Google Scholar
|
|
23
|
Jänne PA, Ou SI, Kim DW, Oxnard GR,
Martins R, Kris MG, Dunphy F, Nishio M, O'Connell J, Paweletz C, et
al: Dacomitinib as first-line treatment in patients with clinically
or molecularly selected advanced non-small-cell lung cancer: A
multicentre, open-label, phase 2 trial. Lancet Oncol. 15:1433–1441.
2014. View Article : Google Scholar
|
|
24
|
Wu SG, Liu YN, Tsai MF, Chang YL, Yu CJ,
Yang PC, Yang JC, Wen YF and Shih JY: The mechanism of acquired
resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib
in lung adenocarcinoma patients. Oncotarget. 7:12404–12413. 2016.
View Article : Google Scholar
|
|
25
|
Cabanero M, Sangha R, Sheffield BS, Sukhai
M, Pakkal M, Kamel-Reid S, Karsan A, Ionescu D, Juergens RA, Butts
C and Tsao MS: Management of EGFR-mutated non-small-cell lung
cancer: Practical implications from a clinical and pathology
perspective. Curr Oncol. 24:111–119. 2017. View Article : Google Scholar
|
|
26
|
Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa
K, Niho S, Chawla A, Rosell R, Corral J, Migliorino MR, et al:
Updated overall survival in a randomized study comparing
dacomitinib with gefitinib as first-line treatment in patients with
advanced non-small-cell lung cancer and EGFR-Activating mutations.
Drugs. 81:257–266. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-Mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu YL, Ahn MJ, Garassino MC, Han JY,
Katakami N, Kim HR, Hodge R, Kaur P, Brown AP, Ghiorghiu D, et al:
CNS efficacy of osimertinib in patients with T790M-Positive
advanced non-small-cell lung cancer: Data from a Randomized phase
III Trial (AURA3). J Clin Oncol. 36:2702–2709. 2018. View Article : Google Scholar
|
|
29
|
Reungwetwattana T, Nakagawa K, Cho BC,
Cobo M, Cho EK, Bertolini A, Bohnet S, Zhou C, Lee KH, Nogami N, et
al: CNS response to osimertinib versus standard epidermal growth
factor receptor tyrosine kinase inhibitors in patients with
untreated EGFR-Mutated advanced non-small-cell lung cancer. J Clin
Oncol. Aug 28–2018.(Epub ahead of print). View Article : Google Scholar
|
|
30
|
Lu S, Wang Q, Zhang G, Dong X, Yang CT,
Song Y, Chang GC, Lu Y, Pan H, Chiu CH, et al: Efficacy of
aumolertinib (HS-10296) in patients with advanced EGFR T790M+
NSCLC: Updated post-national medical products administration
approval results from the APOLLO registrational trial. J Thorac
Oncol. 17:411–422. 2022. View Article : Google Scholar
|
|
31
|
Lu S, Wang Q, Zhang G, Dong X, Yang C,
Song Y, Chang GC, LU Y, Pan H, Chiu CH, et al: 1208P Final results
of APOLLO study: Overall survival (OS) of aumolertinib in patients
with pretreated EGFR T790M-positive locally advanced or metastatic
non-small cell lung cancer (NSCLC). Ann Oncol. 32:S9622021.
View Article : Google Scholar
|
|
32
|
Shi Y, Hu X, Zhang S, Lv D, Wu L, Yu Q,
Zhang Y, Liu L, Wang X, Cheng Y, et al: Efficacy, safety, and
genetic analysis of furmonertinib (AST2818) in patients with EGFR
T790M mutated non-small-cell lung cancer: A phase 2b, multicentre,
single-arm, open-label study. Lancet Respir Med. 9:829–839. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deeks ED: Furmonertinib: First approval.
Drugs. 81:1775–1780. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ahn MJ, Han JY, Kim SW, Ki Hyeong Lee5,
Kim DW, Lee YG, Cho EK, Lee GW, Lee JS, Kim JH, et al: Lazertinib,
a 3rd generation EGFR-TKI, in patients with EGFR-TKI resistant
NSCLC: Updated results of phase I/II Study. Abstract #9037. May
31-June 4. 2019.
|
|
35
|
Kim SW, Ahn MJ, Han JY, Lee KH, Cho EK,
Lee YG, Kim DW, Kim JH, Lee JS, Lee GW, et al: Intracranial
anti-tumor activity of lazertinib in patients with advanced NSCLC
who progressed after prior EGFR TKI therapy: Data from a phase I/II
study. Am Soc Clin Oncol. 38:95712020. View Article : Google Scholar
|
|
36
|
Dhillon S: Lazertinib: First approval.
Drugs. 81:1107–1113. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim ES: Olmutinib: First global approval.
Drugs. 76:1153–1157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim DW, Lee DH, Han JY, Lee J, Cho BC,
Kang JH, Lee KH, Cho EK, Kim JS, Min YJ, et al: Safety,
tolerability, and anti-tumor activity of olmutinib in non-small
cell lung cancer with T790M mutation: A single arm, open label,
phase 1/2 trial. Lung Cancer. 135:66–72. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park K, Jӓnne PA, Kim DW, Han JY, Wu MF,
Lee JS, Kang JH, Lee DH, Cho BC, Yu CJ, et al: Olmutinib in
T790M-positive non-small cell lung cancer after failure of
first-line epidermal growth factor receptor-tyrosine kinase
inhibitor therapy: A global, phase 2 study. Cancer. 127:1407–1416.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tan DS, Leighl NB, Riely GJ, Yang JC,
Sequist LV, Wolf J, Seto T, Felip E, Aix SP, Jonnaert M, et al:
Safety and efficacy of nazartinib (EGF816) in adults with
EGFR-mutant non-small-cell lung carcinoma: A multicentre,
open-label, phase 1 study. Lancet Respir Med. 8:561–572. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Costa DB, Schumer ST, Tenen DG and
Kobayashi S: Differential responses to erlotinib in epidermal
growth factor receptor (EGFR)-mutated lung cancers with acquired
resistance to gefitinib carrying the L747S or T790M secondary
mutations. J Clin Oncol. 26:1182–1184; author reply 1184–1186.
2008. View Article : Google Scholar
|
|
42
|
Balak MN, Gong Y, Riely GJ, Somwar R, Li
AR, Zakowski MF, Chiang A, Yang G, Ouerfelli O, Kris MG, et al:
Novel D761Y and common secondary T790M mutations in epidermal
growth factor receptor-mutant lung adenocarcinomas with acquired
resistance to kinase inhibitors. Clin Cancer Res. 12:6494–6501.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bean J, Riely GJ, Balak M, Marks JL,
Ladanyi M, Miller VA and Pao W: Acquired resistance to epidermal
growth factor receptor kinase inhibitors associated with a novel
T854A mutation in a patient with EGFR-mutant lung adenocarcinoma.
Clin Cancer Res. 14:7519–7525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Grolleau E, Haddad V, Boissière L,
Falchero L and Arpin D: Clinical efficacy of osimertinib in a
patient presenting a double EGFR L747S and G719C mutation. J Thorac
Oncol. 14:e151–e153. 2019. View Article : Google Scholar
|
|
45
|
Chiba M, Togashi Y, Bannno E, Kobayashi Y,
Nakamura Y, Hayashi H, Terashima M, De Velasco MA, Sakai K, Fujita
Y, et al: Efficacy of irreversible EGFR-TKIs for the uncommon
secondary resistant EGFR mutations L747S, D761Y, and T854A. BMC
Cancer. 17:2812017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu Y, Tang J, Li X, Qin T and Wei Y:
Durable response to osimertinib in a Chinese patient with
metastatic lung adenocarcinoma harboring a rare EGFR L858R/D761Y
compound mutation. Onco Targets Ther. 13:10447–10451. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang L, Yang X, Ming Z, Shi J, Lv X, Li
W, Yuan B, Chen Y, Liu B, Qin K, et al: Molecular characteristics
of the uncommon EGFR Exon 21 T854A Mutation and response to
osimertinib in patients with non-small cell lung cancer. Clin Lung
Cancer. 23:311–319. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Patil T, Mushtaq R, Marsh S, Azelby C,
Pujara M, Davies KD, Aisner DL, Purcell WT, Schenk EL, Pacheco JM,
et al: Clinicopathologic characteristics, treatment outcomes, and
acquired resistance patterns of atypical EGFR mutations and HER2
alterations in stage IV non-small-cell lung cancer. Clin Lung
Cancer. 21:e191–e204. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Riudavets M, Sullivan I, Abdayem P and
Planchard D: Targeting HER2 in non-small-cell lung cancer (NSCLC):
A glimpse of hope? An updated review on therapeutic strategies in
NSCLC harbouring HER2 alterations. ESMO Open. 6:1002602021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Takezawa K, Pirazzoli V, Arcila ME, Nebhan
CA, Song X, de Stanchina E, Ohashi K, Janjigian YY, Spitzler PJ,
Melnick MA, et al: HER2 amplification: A potential mechanism of
acquired resistance to EGFR inhibition in EGFR-mutant lung cancers
that lack the second-site EGFRT790M mutation. Cancer Discov.
2:922–933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Elamin YY, Robichaux JP, Carter BW, Altan
M, Gibbons DL, Fossella FV, Lam VK, Patel AB, Negrao MV, Le X, et
al: Poziotinib for patients With HER2 Exon 20 mutant non-small-cell
lung cancer: Results from a phase II Trial. J Clin Oncol.
40:702–709. 2022. View Article : Google Scholar
|
|
52
|
Song Z, Lv D, Chen SQ, Huang J, Li Y, Ying
S, Wu X, Hua F, Wang W, Xu C, et al: Pyrotinib in patients with
HER2-Amplified advanced non-small cell lung cancer: A prospective,
multicenter, single-arm trial. Clin Cancer Res. 28:461–467. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhou C, Li X, Wang Q, Gao G, Zhang Y, Chen
J, Shu Y, Hu Y, Fan Y, Fang J, et al: Pyrotinib in HER2-Mutant
advanced lung adenocarcinoma after platinum-based chemotherapy: A
multicenter, open-label, single-arm, phase II Study. J Clin Oncol.
38:2753–2761. 2020. View Article : Google Scholar
|
|
54
|
Li BT, Shen R, Buonocore D, Olah ZT, Ni A,
Ginsberg MS, Ulaner GA, Offin M, Feldman D, Hembrough T, et al:
Ado-Trastuzumab emtansine for patients with HER2-Mutant lung
cancers: Results from a phase II basket trial. J Clin Oncol.
36:2532–2537. 2018. View Article : Google Scholar
|
|
55
|
Li BT, Smit EF, Goto Y, Nakagawa K,
Udagawa H, Mazières J, Nagasaka M, Bazhenova L, Saltos AN, Felip E,
et al: Trastuzumab deruxtecan in HER2-Mutant non-small-cell lung
cancer. N Engl J Med. 386:241–251. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Koch JP, Aebersold DM, Zimmer Y and Medová
M: MET targeting: Time for a rematch. Oncogene. 39:2845–2862. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pasquini G and Giaccone G: C-MET
inhibitors for advanced non-small cell lung cancer. Expert Opin
Investig Drugs. 27:363–375. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Engelman JA, Zejnullahu K, Mitsudomi T,
Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen
J, et al: MET amplification leads to gefitinib resistance in lung
cancer by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bean J, Brennan C, Shih JY, Riely G, Viale
A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, et al: MET
amplification occurs with or without T790M mutations in EGFR mutant
lung tumors with acquired resistance to gefitinib or erlotinib.
Proc Natl Acad Sci USA. 104:20932–20937. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumor specimens at the time of acquired resistance to
EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lai GG, Lim TH, Lim J, Liew PJ, Kwang XL,
Nahar R, Aung ZW, Takano A, Lee YY, Lau DP, et al: Clonal MET
amplification as a determinant of tyrosine kinase inhibitor
resistance in epidermal growth factor receptor-mutant
non-small-cell lung cancer. J Clin Oncol. 37:876–884. 2019.
View Article : Google Scholar
|
|
63
|
Dulak AM, Gubish CT, Stabile LP, Henry C
and Siegfried JM: HGF-independent potentiation of EGFR action by
c-Met. Oncogene. 30:3625–3635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dong Y, Xu J, Sun B, Wang J and Wang Z:
MET-Targeted therapies and clinical outcomes: A systematic
literature review. Mol Diagn Ther. 26:203–227. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu YL, Zhang L, Kim DW, Liu X, Lee DH,
Yang JC, Ahn MJ, Vansteenkiste JF, Su WC, Felip E, et al: Phase
Ib/II study of capmatinib (INC280) plus gefitinib after failure of
epidermal growth factor receptor (EGFR) inhibitor therapy in
patients with EGFR-Mutated, MET factor-dysregulated non-small-cell
lung cancer. J Clin Oncol. 36:3101–3109. 2018. View Article : Google Scholar
|
|
66
|
Wu YL, Cheng Y, Zhou J, Lu S, Zhang Y,
Zhao J, Kim DW, Soo RA, Kim SW, Pan H, et al: Tepotinib plus
gefitinib in patients with EGFR-mutant non-small-cell lung cancer
with MET overexpression or MET amplification and acquired
resistance to previous EGFR inhibitor (INSIGHT study): An
open-label, phase 1b/2, multicentre, randomised trial. Lancet
Respir Med. 8:1132–1143. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sequist LV, Han JY, Ahn MJ, Cho BC, Yu H,
Kim SW, Yang JC, Lee JS, Su WC, Kowalski D, et al: Osimertinib plus
savolitinib in patients with EGFR mutation-positive, MET-amplified,
non-small-cell lung cancer after progression on EGFR tyrosine
kinase inhibitors: Interim results from a multicentre, open-label,
phase 1b study. Lancet Oncol. 21:373–386. 2020. View Article : Google Scholar
|
|
68
|
Camidge D, Barlesi F, Goldman J,
Morgensztern D, Heist R, Vokes E, Spira A, Angevin E, Su W, Hong D,
Strickler J, Motwani M, Sun Z, et al: MA14. 03 EGFR M+ Subgroup of
Phase 1b study of telisotuzumab vedotin (Teliso-V) plus erlotinib
in c-Met+ non-small cell lung cancer. J Thor Oncol. 14:S305–S306.
2019. View Article : Google Scholar
|
|
69
|
McCoach CE, Yu A, Gandara DR, Riess JW,
Vang DP, Li T, Lara PN, Gubens M, Lara F, Mack PC, et al: Phase
I/II study of capmatinib plus erlotinib in patients with
MET-positive non-small-cell lung cancer. JCO Precis Oncol.
1:PO.20.00279. 2021.
|
|
70
|
Camidge DR, Moran T, Demedts I, Grosch H,
Mileham K, Molina J, Juan-Vidal O, Bepler G, Goldman JW, Park K, et
al: A Randomized, open-label phase II study evaluating emibetuzumab
plus erlotinib and emibetuzumab monotherapy in MET
immunohistochemistry positive NSCLC patients with acquired
resistance to erlotinib. Clin Lung Cancer. 23:300–310. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Alqahtani A, Ayesh HSK and Halawani H:
PIK3CA gene mutations in solid malignancies: Association with
clinicopathological parameters and prognosis. Cancers (Basel).
12:932019. View Article : Google Scholar
|
|
72
|
Wang Y, Wang Y, Li J, Li J and Che G:
Clinical significance of PIK3CA gene in non-small-cell lung cancer:
A systematic review and meta-analysis. Biomed Res Int.
2020:36082412020.
|
|
73
|
Qiu X, Wang Y, Liu F, Peng L, Fang C, Qian
X, Zhang X, Wang Q, Xiao Z, Chen R, et al: Survival and prognosis
analyses of concurrent PIK3CA mutations in EGFR mutant non-small
cell lung cancer treated with EGFR tyrosine kinase inhibitors. Am J
Cancer Res. 11:3189–3200. 2021.PubMed/NCBI
|
|
74
|
Song Z, Yu X and Zhang Y: Mutation and
prognostic analyses of PIK3CA in patients with completely resected
lung adenocarcinoma. Cancer Med. 5:2694–2700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Westover D, Zugazagoitia J, Cho BC, Lovly
CM and Paz-Ares L: Mechanisms of acquired resistance to first- and
second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 29
(Suppl 1):i10–i19. 2018. View Article : Google Scholar
|
|
77
|
Qu GP, Shi M, Wang D, Wu JH, Wang P, Gong
ML and Zhang ZJ: Dual targeting of MEK and PI3K effectively
controls the proliferation of human EGFR-TKI resistant non-small
cell lung carcinoma cell lines with different genetic backgrounds.
BMC Pulm Med. 21:2082021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Markham AJD: Alpelisib: First global
approval. Drugs. 79:1249–1253. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Skoulidis F, Li BT, Dy GK, Price TJ,
Falchook GS, Wolf J, Italiano A, Schuler M, Borghaei H, Barlesi F,
et al: Sotorasib for lung cancers with KRAS p.G12C Mutation. N Engl
J Med. 384:2371–2381. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tanaka N, Lin JJ, Li C, Ryan MB, Zhang J,
Kiedrowski LA, Michel AG, Syed MU, Fella KA, Sakhi M, et al:
Clinical acquired resistance to KRASG12C inhibition
through a Novel KRAS Switch-II pocket mutation and polyclonal
alterations converging on RAS-MAPK Reactivation. Cancer Discov.
11:1913–1922. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang SS; Nagasaka MJLCT and Therapy, :
Spotlight on Sotorasib (AMG 510) for KRASG12C positive
non-small cell lung cancer. Lung Cancer (Auckl). 12:115–122.
2021.PubMed/NCBI
|
|
82
|
Pratilas CA, Hanrahan AJ, Halilovic E,
Persaud Y, Soh J, Chitale D, Shigematsu H, Yamamoto H, Sawai A,
Janakiraman M, et al: Genetic predictors of MEK dependence in
non-small cell lung cancer. Cancer Res. 68:9375–9383. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ai X, Li Y, Chen R, Gu D and Mao Y: P59.
07 mutation profile of BRAF in Chinese non-small cell lung cancer
patients. J Thorac Oncol. 16:S11492021. View Article : Google Scholar
|
|
84
|
Ohashi K, Sequist LV, Arcila ME, Moran T,
Chmielecki J, Lin YL, Pan Y, Wang L, de Stanchina E, Shien K, et
al: Lung cancers with acquired resistance to EGFR inhibitors
occasionally harbor BRAF gene mutations but lack mutations in KRAS,
NRAS, or MEK1. Proc Natl Acad Sci USA. 109:E2127–E2133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Odogwu L, Mathieu L, Blumenthal G, Larkins
E, Goldberg KB, Griffin N, Bijwaard K, Lee EY, Philip R, Jiang X,
et al: FDA approval summary: Dabrafenib and trametinib for the
treatment of metastatic non-small cell lung cancers harboring BRAF
V600E mutations. Oncologist. 23:740–745. 2018. View Article : Google Scholar
|
|
86
|
Zhu C, Wei Y and Wei X: AXL receptor
tyrosine kinase as a promising anti-cancer approach: Functions,
molecular mechanisms and clinical applications. Mol Cancer.
18:1532019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Goyette MA and Côté JF: AXL receptor
tyrosine kinase as a promising therapeutic target directing
multiple aspects of cancer progression and metastasis. Cancers
(Basel). 14:4662022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhang Z, Lee JC, Lin L, Olivas V, Au V,
LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK, et al:
Activation of the AXL kinase causes resistance to EGFR-targeted
therapy in lung cancer. Nat Genet. 44:852–860. 2012. View Article : Google Scholar
|
|
89
|
Sang YB, Kim JH, Kim CG, Hong MH, Kim HR,
Cho BC and Lim SM: The Development of AXL inhibitors in lung
cancer: Recent progress and challenges. Front Oncol. 12:8112472022.
View Article : Google Scholar
|
|
90
|
Nishio M, Okamoto I, Murakami H,
Horinouchi H, Toyozawa R, Takeda M, Uno M, Crawford N, Jimbo T,
Ishigami M, et al: 570P A first-in-human phase I study of the AXL
inhibitor DS-1205c in combination with gefitinib in subjects with
EGFR-mutant NSCLC. Ann Oncol. 31:S4882020. View Article : Google Scholar
|
|
91
|
Byers LA, Gold KA and Peguero JA: Ph I/II
study of oral selective AXL inhibitor bemcentinib (BGB324) in
combination with erlotinib in patients with advanced EGFRm NSCLC:
End of trial update. Wolters Kluwer Health; 2021, View Article : Google Scholar
|
|
92
|
Xun G, Hu W and Li B: PTEN loss promotes
oncogenic function of STMN1 via PI3K/AKT pathway in lung cancer.
Sci Rep. 11:143182021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ferrara MG, Martini M, D'Argento E,
Forcella C, Vita E, Di Noia V, Sperduti I, Bilotta M, Ribelli M,
Damiano P, et al: PTEN loss as a predictor of tumor heterogeneity
and poor prognosis in patients with EGFR-mutant advanced
non-small-cell lung cancer receiving tyrosine kinase inhibitors.
Clin Lung Cancer. 22:351–360. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Redig AJ, Capelletti M, Dahlberg SE, Sholl
LM, Mach S, Fontes C, Shi Y, Chalasani P and Jänne PA: Clinical and
molecular characteristics of NF1-mutant lung cancer. Clin Cancer
Res. 22:3148–3156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Cheung HW, Du J, Boehm JS, He F, Weir BA,
Wang X, Butaney M, Sequist LV, Luo B, Engelman JA, et al:
Amplification of CRKL induces transformation and epidermal growth
factor receptor inhibitor resistance in human non-small cell lung
cancers. Cancer Discov. 1:608–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cortot AB, Repellin CE, Shimamura T,
Capelletti M, Zejnullahu K, Ercan D, Christensen JG, Wong KK, Gray
NS and Jänne PA: Resistance to irreversible EGF receptor tyrosine
kinase inhibitors through a multistep mechanism involving the IGF1R
pathway. Cancer Res. 73:834–843. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ware KE, Hinz TK, Kleczko E, Singleton KR,
Marek LA, Helfrich BA, Cummings CT, Graham DK, Astling D, Tan AC
and Heasley LE: A mechanism of resistance to gefitinib mediated by
cellular reprogramming and the acquisition of an FGF2-FGFR1
autocrine growth loop. Oncogenesis. 2:e392013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Attili I, Passaro A, Pisapia P, Malapelle
U and de Marinis F: Uncommon EGFR compound mutations in non-small
cell lung cancer (NSCLC): A systematic review of available
evidence. Curr Oncol. 29:255–266. 2022. View Article : Google Scholar
|
|
99
|
Hayashi T, Kohsaka S, Takamochi K, Hara K,
Kishikawa S, Sano K, Takahashi F, Suehara Y, Saito T, Takahashi K,
et al: Clinicopathological characteristics of lung adenocarcinoma
with compound EGFR mutations. Hum Pathol. 103:42–51. 2020.
View Article : Google Scholar
|
|
100
|
Rossi S, Damiano P, Toschi L, Finocchiaro
G, Giordano L, Marinello A, Bria E, D'Argento E and Santoro A:
Uncommon single and compound EGFR mutations: Clinical outcomes of a
heterogeneous subgroup of NSCLC. Curr Probl Cancer. 46:1007872022.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Jiang D, Fu Y, Zhou X, Li Y, Cui Y, Hong
L, Jin H, Shi K, Huang F, Zhang X, et al: The prognosis of EGFR
complex mutation or co-mutation with tyrosine kinase inhibitor
treatment in non-small cell lung cancer. Am Soc Clin Oncol.
40:e210862022. View Article : Google Scholar
|
|
102
|
Wang R, Pan S and Song X: Research
Advances of EGFR-TP53 Co-mutation in advanced non-small cell lung
cancer. Zhongguo Fei Ai Za Zhi. 25:174–182. 2022.(In Chinese).
|
|
103
|
Wang F, Zhao N, Gao G, Deng HB, Wang ZH,
Deng LL, Yang Y and Lu C: Prognostic value of TP53 co-mutation
status combined with EGFR mutation in patients with lung
adenocarcinoma. J Cancer Res Clin Oncol. 146:2851–2859. 2020.
View Article : Google Scholar
|
|
104
|
Cheng Y, Ma L, Liu Y, Zhu J, Xin Y, Liu X,
Wang Y, Zhang T, Yang C, Wang S, et al: Comprehensive
characterization and clinical impact of concomitant genomic
alterations in EGFR-mutant NSCLCs treated with EGFR kinase
inhibitors. Lung Cancer. 145:63–70. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang Y, Li S, Lyu Z, Cai J, Zheng N, Li
Y, Xu T and Zeng H: The co-mutation of EGFR and tumor-related genes
leads to a worse prognosis and a higher level of tumor mutational
burden in Chinese non-small cell lung cancer patients. J Thorac
Dis. 14:185–193. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Li HS, Liu CM and Wang Y: Limited role of
KRAS mutation in guiding immunotherapy in advanced non-small-cell
lung cancer. Future Oncol. 18:2433–2443. 2022. View Article : Google Scholar
|
|
107
|
Marcoux N, Gettinger SN, O'Kane G, Arbour
KC, Neal JW, Husain H, Evans TL, Brahmer JR, Muzikansky A, Bonomi
PD, et al: EGFR-Mutant adenocarcinomas that transform to small-cell
lung cancer and other neuroendocrine carcinomas: Clinical outcomes.
J Clin Oncol. 37:278–285. 2019. View Article : Google Scholar
|
|
108
|
Niederst MJ, Sequist LV, Poirier JT,
Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN,
Moran T, et al: RB loss in resistant EGFR mutant lung
adenocarcinomas that transform to small-cell lung cancer. Nat
Commun. 6:63772015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lee JK, Lee J, Kim S, Kim S, Youk J, Park
S, An Y, Keam B, Kim DW, Heo DS, et al: Clonal history and genetic
predictors of transformation into small-cell carcinomas from lung
adenocarcinomas. J Clin Oncol. 35:3065–3074. 2017. View Article : Google Scholar
|
|
110
|
Offin M, Chan JM, Tenet M, Rizvi HA, Shen
R, Riely GJ, Rekhtman N, Daneshbod Y, Quintanal-Villalonga A,
Penson A, et al: Concurrent RB1 and TP53 alterations define a
subset of EGFR-Mutant lung cancers at risk for histologic
transformation and inferior clinical outcomes. J Thorac Oncol.
14:1784–1793. 2019. View Article : Google Scholar
|
|
111
|
Mambetsariev I, Arvanitis L, Fricke J,
Pharaon R, Baroz AR, Afkhami M, Koczywas M, Massarelli E and Salgia
R: Small cell lung cancer transformation following treatment in
EGFR-Mutated non-small cell lung cancer. J Clin Med. 11:14292022.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Mc Leer A, Foll M, Brevet M, Antoine M,
Novello S, Mondet J, Cadranel J, Girard N, Giaj Levra M, Demontrond
P, et al: Detection of acquired TERT amplification in addition to
predisposing p53 and Rb pathways alterations in EGFR-mutant lung
adenocarcinomas transformed into small-cell lung cancers. Lung
Cancer. 167:98–106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Song KA, Niederst MJ, Lochmann TL, Hata
AN, Kitai H, Ham J, Floros KV, Hicks MA, Hu H, Mulvey HE, et al:
Epithelial-to-Mesenchymal transition antagonizes response to
targeted therapies in lung cancer by suppressing BIM. Clin Cancer
Res. 24:197–208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shaurova T, Zhang L, Goodrich DW and
Hershberger PA: Understanding lineage plasticity as a path to
targeted therapy failure in EGFR-Mutant non-small cell lung cancer.
Front Genet. 11:2812020. View Article : Google Scholar
|
|
115
|
Wang W, Xu C, Chen H, Jia J, Wang L, Feng
H, Wang H, Song Z, Yang N and Zhang Y: Genomic alterations and
clinical outcomes in patients with lung adenocarcinoma with
transformation to small cell lung cancer after treatment with EGFR
tyrosine kinase inhibitors: A multicenter retrospective study. Lung
Cancer. 155:20–27. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang C, Zhang S, Yao Y, Huang J, Peng K,
Gao Q, Chen H, Xu C, Zhang X, Wu Y, Yang J, et al: MA12. 08
Chemotherapy plus EGFR TKIs or bevacizumab versus chemotherapy
alone in SCLC-transformed EGFR-mutant lung adenocarcinoma. J Thor
Oncol. 16:S178–S179. 2021. View Article : Google Scholar
|
|
117
|
Kuiper JL, Ronden MI, Becker A, Heideman
DA, van Hengel P, Ylstra B, Thunnissen E and Smit EF:
Transformation to a squamous cell carcinoma phenotype of an
EGFR-mutated NSCLC patient after treatment with an EGFR-tyrosine
kinase inhibitor. J Clin Pathol. 68:320–321. 2015. View Article : Google Scholar
|
|
118
|
Levin PA, Mayer M, Hoskin S, Sailors J,
Oliver DH and Gerber DE: Histologic transformation from
adenocarcinoma to squamous cell carcinoma as a mechanism of
resistance to EGFR inhibition. J Thorac Oncol. 10:e86–e88. 2015.
View Article : Google Scholar
|
|
119
|
Longo L, Mengoli MC, Bertolini F, Bettelli
S, Manfredini S and Rossi G: Synchronous occurrence of
squamous-cell carcinoma ‘transformation’ and EGFR exon 20 S768I
mutation as a novel mechanism of resistance in EGFR-mutated lung
adenocarcinoma. Lung Cancer. 103:24–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Roca E, Pozzari M, Vermi W, Tovazzi V,
Baggi A, Amoroso V, Nonnis D, Intagliata S and Berruti A: Outcome
of EGFR-mutated adenocarcinoma NSCLC patients with changed
phenotype to squamous cell carcinoma after tyrosine kinase
inhibitors: A pooled analysis with an additional case. Lung Cancer.
127:12–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Liao J, Li Y, Liu C, Long Q and Wang J:
Case report: EGFR-Positive early-stage lung adenocarcinoma
transforming to squamous cell carcinoma after TKI treatment. Front
Oncol. 11:6968812021. View Article : Google Scholar
|
|
122
|
Jukna A, Montanari G, Mengoli MC, Cavazza
A, Covi M, Barbieri F, Bertolini F and Rossi G: Squamous Cell
Carcinoma ‘Transformation’ concurrent with secondary T790M mutation
in resistant EGFR-Mutated Adenocarcinomas. J Thorac Oncol.
11:e49–e51. 2016. View Article : Google Scholar
|
|
123
|
Bugano DDG, Kalhor N, Zhang J, Neskey M
and William WN Jr: Squamous-cell transformation in a patient with
lung adenocarcinoma receiving erlotinib: Co-occurrence with T790M
mutation. Cancer Treat Comm. 4:34–36. 2015. View Article : Google Scholar
|
|
124
|
Park S, Shim JH, Lee B, Cho I, Park WY,
Kim Y, Lee SH, Choi Y, Han J, Ahn JS, et al: Paired genomic
analysis of squamous cell carcinoma transformed from EGFR-mutated
lung adenocarcinoma. Lung Cancer. 134:7–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Weng CH, Chen LY, Lin YC, Shih JY, Lin YC,
Tseng RY, Chiu AC, Yeh YH, Liu C, Lin YT, et al:
Epithelial-mesenchymal transition (EMT) beyond EGFR mutations per
se is a common mechanism for acquired resistance to EGFR TKI.
Oncogene. 38:455–468. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Brabletz S, Schuhwerk H, Brabletz T and
Stemmler MP: Dynamic EMT: A multi-tool for tumor progression. EMBO
J. 40:e1086472021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhu X, Chen L, Liu L and Niu X:
EMT-Mediated Acquired EGFR-TKI resistance in NSCLC: Mechanisms and
strategies. Front Oncol. 9:10442019. View Article : Google Scholar
|
|
128
|
Miralaei N, Majd A, Ghaedi K, Peymani M
and Safaei M: Integrated pan-cancer of AURKA expression and drug
sensitivity analysis reveals increased expression of AURKA is
responsible for drug resistance. Cancer Med. 10:6428–6441. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Nilsson MB, Sun H, Robichaux J, Pfeifer M,
McDermott U, Travers J, Diao L, Xi Y, Tong P, Shen L, et al: A
YAP/FOXM1 axis mediates EMT-associated EGFR inhibitor resistance
and increased expression of spindle assembly checkpoint components.
Sci Transl Med. 12:eaaz45892020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wang CY, Lee MH, Kao YR, Hsiao SH, Hong SY
and Wu CW: Alisertib inhibits migration and invasion of EGFR-TKI
resistant cells by partially reversing the epithelial-mesenchymal
transition. Biochim Biophys Acta Mol Cell Res. 1868:1190162021.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Yeh CT, Chen TT, Satriyo PB, Wang CH, Wu
ATH, Chao TY, Lee KY, Hsiao M, Wang LS and Kuo KT: Bruton's
tyrosine kinase (BTK) mediates resistance to EGFR inhibition in
non-small-cell lung carcinoma. Oncogenesis. 10:562021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Liao BC, Griesing S and Yang JC:
Second-line treatment of EGFR T790M-negative non-small cell lung
cancer patients. Ther Adv Med Oncol. Nov 25–2019.(Epub ahead of
print). View Article : Google Scholar
|
|
133
|
Lee CK, Man J, Lord S, Links M, Gebski V,
Mok T and Yang JC: Checkpoint Inhibitors in Metastatic EGFR-Mutated
non-small cell lung cancer-A meta-analysis. J Thorac Oncol.
12:403–407. 2017. View Article : Google Scholar
|
|
134
|
Lee CK, Man J, Lord S, Cooper W, Links M,
Gebski V, Herbst RS, Gralla RJ, Mok T and Yang JC: Clinical and
molecular characteristics associated with survival among patients
treated with checkpoint inhibitors for advanced non-small cell lung
carcinoma: A systematic review and meta-analysis. JAMA Oncol.
4:210–216. 2018. View Article : Google Scholar
|
|
135
|
Yang CY, Liao WY, Ho CC, Chen KY, Tsai TH,
Hsu CL, Su KY, Chang YL, Wu CT, Hsu CC, et al: Association between
programmed death-ligand 1 expression, immune microenvironments, and
clinical outcomes in epidermal growth factor receptor mutant lung
adenocarcinoma patients treated with tyrosine kinase inhibitors.
Eur J Cancer. 124:110–122. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Isomoto K, Haratani K, Hayashi H, Shimizu
S, Tomida S, Niwa T, Yokoyama T, Fukuda Y, Chiba Y, Kato R, et al:
Impact of EGFR-TKI treatment on the tumor immune microenvironment
in EGFR mutation-positive non-small cell lung cancer. Clin Cancer
Res. 26:2037–2046. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Reck M, Mok TS, Nishio M, Jotte RM,
Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu
D, Moro-Sibilot D, et al: Atezolizumab plus bevacizumab and
chemotherapy in non-small-cell lung cancer (IMpower150): Key
subgroup analyses of patients with EGFR mutations or baseline liver
metastases in a randomised, open-label phase 3 trial. Lancet Respir
Med. 7:387–401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wu SG, Ho CC, Yang JC, Lia BC, Yang CY,
Lin YT, Yu CJ, Liao WY and Shih JY: 12P A phase II study of
atezolizumab in combination with bevacizumab, carboplatin or
cisplatin, and pemetrexed for EGFR-mutant metastatic NSCLC patients
after failure of EGFR TKIs. Ann Oncol. 33:S33–S34. 2022. View Article : Google Scholar
|
|
139
|
Lam TC, Tsang KC, Choi HC, Lee VH, Lam KO,
Chiang CL, So TH, Chan WW, Nyaw SF, Lim F, et al: Combination
atezolizumab, bevacizumab, pemetrexed and carboplatin for
metastatic EGFR mutated NSCLC after TKI failure. Lung Cancer.
159:18–26. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ren S, Zhang J, Zhao Y, Zhou J, Fan Y, Shu
Y, Liu X, Zhang H, He J, Gao G, et al: A multi-center phase II
study of toripalimab with chemotherapy in patients with EGFR mutant
advanced NSCLC patients resistant to EGFR TKIs: Efficacy and
biomarker analysis. Am Soc Clin Oncol. 6:3552020.
|
|
141
|
Jiang T, Wang P, Zhang J, Zhao Y, Zhou J,
Fan Y, Shu Y, Liu X, Zhang H, He J, et al: Toripalimab plus
chemotherapy as second-line treatment in previously EGFR-TKI
treated patients with EGFR-mutant-advanced NSCLC: A multicenter
phase-II trial. Signal Transduct Target Ther. 6:3552021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Lu S, Wu L, Jian H, Cheng Y, Wang Q, Fang
J, Wang Z, Hu Y, Sun M, Han L, et al: VP9-2021: ORIENT-31: Phase
III study of sintilimab with or without IBI305 plus chemotherapy in
patients with EGFR mutated nonsquamous NSCLC who progressed after
EGFR-TKI therapy. Ann Oncol. 33:112–113. 2022. View Article : Google Scholar
|
|
143
|
Hayashi H, Sugawara S, Fukuda Y, Fujimoto
D, Miura S, Ota K, Ozawa Y, Hara S, Tanizaki J, Azuma K, et al: A
randomized phase II study comparing nivolumab with
carboplatin-pemetrexed for EGFR-mutated NSCLC with resistance to
EGFR tyrosine kinase inhibitors (WJOG8515L). Clin Cancer Res.
28:893–902. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
de Rouw N, Piet B, Derijks HJ, van den
Heuvel MM and Ter Heine R: Mechanisms, management and prevention of
pemetrexed-related toxicity. Drug Saf. 44:1271–1281. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liang SK, Keng LT, Chang CH, Wen YF, Lee
MR, Yang CY, Wang JY, Ko JC, Shih JY and Yu CJ: Treatment options
of first-line tyrosine kinase inhibitors and subsequent systemic
chemotherapy agents for advanced EGFR mutant lung adenocarcinoma
patients: Implications from Taiwan cancer registry cohort. Front
Oncol. 10:5903562021. View Article : Google Scholar
|
|
146
|
Li Z, Guo H, Lu Y, Hu J, Luo H and Gu W:
Chemotherapy with or without pemetrexed as second-line regimens for
advanced non-small-cell lung cancer patients who have progressed
after first-line EGFR TKIs: A systematic review and meta-analysis.
Onco Targets Ther. 11:3697–3703. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Yoo KH, Lee SJ, Cho J, Lee KH, Park KU,
Kim KH, Cho EK, Choi YH, Kim HR, Kim HG, et al: A randomized,
open-label, Phase II study comparing pemetrexed plus cisplatin
followed by maintenance pemetrexed versus pemetrexed alone in
patients with epidermal growth factor receptor (EGFR)-mutant
non-small cell lung cancer after failure of first-line EGFR
tyrosine kinase inhibitor: KCSG-LU12-13. Cancer Res Treat.
51:718–726. 2019. View Article : Google Scholar
|
|
148
|
Le X, Nilsson M, Goldman J, Reck M,
Nakagawa K, Kato T, Ares LP, Frimodt-Moller B, Wolff K,
Visseren-Grul C, et al: Dual EGFR-VEGF Pathway inhibition: A
promising strategy for patients with EGFR-Mutant NSCLC. J Thorac
Oncol. 16:205–215. 2021. View Article : Google Scholar
|
|
149
|
Lian Z, Du W, Zhang Y, Fu Y, Liu T, Wang
A, Cai T, Zhu J, Zeng Y, Liu Z and Huang JA: Anlotinib can overcome
acquired resistance to EGFR-TKIs via FGFR1 signaling in non-small
cell lung cancer without harboring EGFR T790M mutation. Thorac
Cancer. 11:1934–1943. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Zhang C, Cao H, Cui Y, Jin S, Gao W, Huang
C and Guo R: Concurrent use of anlotinib overcomes acquired
resistance to EGFR-TKI in patients with advanced EGFR-mutant
non-small cell lung cancer. Thorac Cancer. 12:2574–2584. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Hata A, Katakami N, Kaji R, Yokoyama T,
Kaneda T, Tamiya M, Inoue T, Kimura H, Yano Y, Tamura D, et al:
Afatinib plus bevacizumab combination after acquired resistance to
EGFR tyrosine kinase inhibitors in EGFR-mutant non-small cell lung
cancer: Multicenter, single-arm, phase 2 trial (ABC Study). Cancer.
124:3830–3838. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Yang R, Wang D, Li X, Mao K, Wang J, Li P,
Shi X, Zhang S and Wang Y: An advanced non-small cell lung cancer
patient with EGFR and KRAS mutations, and PD-L1 positive, benefited
from immunotherapy: A case report. Ann Transl Med. 10:3812022.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Bai M, Wang W, Gao X, Wu L, Jin P, Wu H,
Yu J and Meng X: Efficacy of immune checkpoint inhibitors in
patients with EGFR Mutated NSCLC and potential risk factors
associated with prognosis: A single institution experience. Front
Immunol. 13:8324192022. View Article : Google Scholar
|
|
154
|
Mu Y, Hao X, Xing P, Hu X, Wang Y, Li T,
Zhang J, Xu Z and Li J: Acquired resistance to osimertinib in
patients with non-small-cell lung cancer: Mechanisms and clinical
outcomes. J Cancer Res Clin Oncol. 146:2427–2433. 2020. View Article : Google Scholar
|
|
155
|
Leonetti A, Sharma S, Minari R, Perego P,
Giovannetti E and Tiseo M: Resistance mechanisms to osimertinib in
EGFR-mutated non-small cell lung cancer. Br J Cancer. 121:725–737.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
He J, Huang Z, Han L, Gong Y and Xie C:
Mechanisms and management of 3rd-generation EGFR-TKI resistance in
advanced non-small cell lung cancer (Review). Int J Oncol.
59:902021. View Article : Google Scholar
|
|
157
|
Papadimitrakopoulou V, Wu YL, Han JY, Ahn
MJ, Ramalingam SS, John T, Okamoto I, Yang JC, Bulusu K, Laus G, et
al: Analysis of resistance mechanisms to osimertinib in patients
with EGFR T790M advanced NSCLC from the AURA3 study. Annal Oncol.
29:viii7412018. View Article : Google Scholar
|
|
158
|
Piotrowska Z, Nagy R, Fairclough S, Lanman
R, Marcoux N, Gettinger S, Owonikoko T, Ramalingam S and Sequist L:
Characterizing the genomic landscape of EGFR C797S in lung cancer
using ctDNA next-generation sequencing. J Thorac Oncol.
12:S17672017. View Article : Google Scholar
|
|
159
|
Wang X, Zhou L, Yin JC, Wu X, Shao YW and
Gao B: Lung adenocarcinoma harboring EGFR 19del/C797S/T790M triple
mutations responds to brigatinib and Anti-EGFR antibody combination
therapy. J Thorac Oncol. 14:e85–e88. 2019. View Article : Google Scholar
|
|
160
|
Chang Y, Liu S, Jiang Y, Hua L and Wen L:
Effective treatment of pulmonary adenocarcinoma harboring triple
EGFR mutations of L858R, T790M, cis-G796s/cis-C797s by osimertinib,
brigatinib, and bevacizumab combination therapy: A case report.
Respir Med Case Rep. 36:1015822022.PubMed/NCBI
|
|
161
|
Zhou R, Song L, Zhang W, Shao L and Li X
and Li X: Combination of osimertinib and anlotinib may overcome the
resistance mediated by in cis EGFR T790M-C797S in NSCLC: A case
report. Onco Targets Ther. 14:2847–2851. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Yang Y, Xu H, Ma L, Yang L, Yang G, Zhang
S, Ai X, Zhang S and Wang Y: Possibility of brigatinib-based
therapy, or chemotherapy plus anti-angiogenic treatment after
resistance of osimertinib harboring EGFR T790M-cis-C797S mutations
in lung adenocarcinoma patients. Cancer Med. 10:8328–8337. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhao Y, Chen Y, Huang H, Li X, Shao L and
Ding H: Significant benefits of afatinib and apatinib in a
refractory advanced NSCLC patient resistant to osimertinib: A case
report. OncoTargets Ther. 14:3063–3067. 2021. View Article : Google Scholar
|
|
164
|
Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu
X, Bao H, Tong X, Wang X, Shao YW, et al: Investigating novel
resistance mechanisms to third-generation EGFR tyrosine kinase
inhibitor osimertinib in non-small cell lung cancer patients. Clin
Cancer Res. 24:3097–3107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Zhang Y, He B, Zhou D, Li M and Hu C:
Newly emergent acquired EGFR exon 18 G724S mutation after
resistance of a T790M specific EGFR inhibitor osimertinib in
non-small-cell lung cancer: A case report. OncoTargets Ther.
12:51–56. 2018. View Article : Google Scholar
|
|
166
|
Schoenfeld AJ, Chan JM, Kubota D, Sato H,
Rizvi H, Daneshbod Y, Chang JC, Paik PK, Offin M, Arcila ME, et al:
Tumor analyses reveal squamous transformation and off-target
alterations as early resistance mechanisms to first-line
osimertinib in EGFR-Mutant lung cancer. Clin Cancer Res.
26:2654–2663. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Fairclough SR, Kiedrowski LA, Lin JJ,
Zelichov O, Tarcic G, Stinchcombe TE, Odegaard JI, Lanman RB, Shaw
AT and Nagy RJ: Identification of osimertinib-resistant EGFR L792
mutations by cfDNA sequencing: oncogenic activity assessment and
prevalence in large cfDNA cohort. Exp Hematol Oncol. 8:242019.
View Article : Google Scholar
|
|
168
|
Ma L, Chen R, Wang F, Ma LL, Yuan MM, Chen
RR and Liu J: EGFR L718Q mutation occurs without T790M mutation in
a lung adenocarcinoma patient with acquired resistance to
osimertinib. Ann Transl Med. 7:2072019. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Fang W, Huang Y, Gan J, Zheng Q and Zhang
L: Emergence of EGFR G724S after progression on osimertinib
responded to afatinib monotherapy. J Thorac Oncol. 15:e36–e37.
2020. View Article : Google Scholar
|
|
170
|
Zhang Y, Yang Q, Zeng X, Wang M, Dong S,
Yang B, Tu X, Wei T, Xie W, Zhang C, et al: MET amplification
attenuates lung tumor response to immunotherapy by inhibiting
STING. Cancer Discov. 11:2726–2737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Syed YY: Amivantamab: First approval.
Drugs. 81:1349–1353. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Amivantamab OK'd for EGFR-Mutant NSCLC, .
Cancer Discov. 11:16042021. View Article : Google Scholar
|
|
173
|
Neijssen J, Cardoso RM, Chevalier KM,
Wiegman L, Valerius T, Anderson GM, Moores SL, Schuurman J, Parren
PW, Strohl WR and Chiu ML: Discovery of amivantamab (JNJ-61186372),
a bispecific antibody targeting EGFR and MET. J Biol Chem.
296:1006412021. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Planchard D, Loriot Y, André F, Gobert A,
Auger N, Lacroix L and Soria JC: EGFR-independent mechanisms of
acquired resistance to AZD9291 in EGFR T790M-positive NSCLC
patients. Ann Oncol. 26:2073–2078. 2015. View Article : Google Scholar
|
|
175
|
Ramalingam S, Cheng Y, Zhou C, Ohe Y,
Imamura F, Cho BC, Lin M, Majem M, Shah R, Rukazenkov Y, et al:
Mechanisms of acquired resistance to first-line osimertinib:
preliminary data from the phase III FLAURA study. OncologyPro.
29:viii7402018.
|
|
176
|
Oxnard GR, Hu Y, Mileham KF, Husain H,
Costa DB, Tracy P, Feeney N, Sholl LM, Dahlberg SE, Redig AJ, et
al: Assessment of resistance mechanisms and clinical implications
in patients With EGFR T790M-Positive lung cancer and acquired
resistance to osimertinib. JAMA Oncol. 4:1527–1534. 2018.
View Article : Google Scholar
|
|
177
|
Qu F, Zhou Y and Yu WJA-CD: A review of
research progress on mechanisms and overcoming strategies of
acquired osimertinib resistance. Anticancer Drugs. 33:e76–e83.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Beenken A and Mohammadi M: The FGF family:
Biology, pathophysiology and therapy. Nat Rev Drug Discov.
8:235–253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Lu Y, Liu Y, Oeck S, Zhang GJ, Schramm A
and Glazer PM: Hypoxia induces resistance to EGFR inhibitors in
lung cancer cells via upregulation of FGFR1 and the MAPK pathway.
Cancer Res. 80:4655–4667. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Quintanal-Villalonga A, Molina-Pinelo S,
Cirauqui C, Ojeda-Márquez L, Marrugal Á, Suarez R, Conde E,
Ponce-Aix S, Enguita AB, Carnero A, et al: FGFR1 Cooperates with
EGFR in lung cancer oncogenesis, and their combined inhibition
shows improved efficacy. J Thorac Oncol. 14:641–655. 2019.
View Article : Google Scholar
|
|
181
|
Hayakawa D, Takahashi F, Mitsuishi Y,
Tajima K, Hidayat M, Winardi W, Ihara H, Kanamori K, Matsumoto N,
Asao T, et al: Activation of insulin-like growth factor-1 receptor
confers acquired resistance to osimertinib in non-small cell lung
cancer with EGFR T790M mutation. Thorac Cancer. 11:140–149. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Zhao Y, Wang H and He C: Drug resistance
of targeted therapy for advanced non-small cell lung cancer
harbored EGFR mutation: From mechanism analysis to clinical
strategy. J Cancer Res Clin Oncol. 147:3653–3664. 2021. View Article : Google Scholar
|
|
183
|
Makimoto G, Ninomiya K, Kubo T, Sunami R,
Kato Y, Ichihara E, Ohashi K, Rai K, Hotta K, Tabata M, et al: A
novel osimertinib-resistant human lung adenocarcinoma cell line
harbouring mutant EGFR and activated IGF1R. Jpn J Clin Oncol.
51:956–965. 2021. View Article : Google Scholar
|
|
184
|
Wang R, Yamada T, Kita K, Taniguchi H,
Arai S, Fukuda K, Terashima M, Ishimura A, Nishiyama A, Tanimoto A,
et al: Transient IGF-1R inhibition combined with osimertinib
eradicates AXL-low expressing EGFR mutated lung cancer. Nat Commun.
11:46072020. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Lin CC, Shih JY, Yu CJ, Ho CC, Liao WY,
Lee JH, Tsai TH, Su KY, Hsieh MS, Chang YL, et al: Outcomes in
patients with non-small-cell lung cancer and acquired Thr790Met
mutation treated with osimertinib: A genomic study. Lancet Respir
Med. 6:107–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Jia Y, Yun CH, Park E, Ercan D, Manuia M,
Juarez J, Xu C, Rhee K, Chen T, Zhang H, et al: Overcoming
EGFR(T790M) and EGFR(C797S) resistance with mutant-selective
allosteric inhibitors. Nature. 534:129–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
To C, Jang J, Chen T, Park E, Mushajiang
M, De Clercq DJH, Xu M, Wang S, Cameron MD, Heppner DE, et al:
Single and dual targeting of mutant EGFR with an allosteric
inhibitor. Cancer Discov. 9:926–943. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Tripathi SK and Biswal BK: Allosteric
mutant-selective fourth-generation EGFR inhibitors as an efficient
combination therapeutic in the treatment of non-small cell lung
carcinoma. Drug Discov Today. 26:1466–1472. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Kashima K, Kawauchi H, Tanimura H,
Tachibana Y, Chiba T, Torizawa T and Sakamoto H: CH7233163
overcomes osimertinib-resistant EGFR-Del19/T790M/C797S Mutation.
Mol Cancer Ther. 19:2288–2297. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Schalm S, Dineen T, Lim S, Park CW, Hsieh
J, Woessner R, Zhang Z, Wilson K, Eno M, Wilson D, et al: 1296P
BLU-945, a highly potent and selective 4th generation EGFR TKI for
the treatment of EGFR T790M/C797S resistant NSCLC. Ann Oncol.
31:S8392020. View Article : Google Scholar
|
|
191
|
Conti C, Campbell J, Woessner R, Guo J,
Timsit Y, Iliou M, Wardwell S, Davis A, Chicklas S, Hsieh J, et al:
BLU-701 is a highly potent, brain-penetrant and WT-sparing
next-generation EGFR TKI for the treatment of sensitizing (ex19del,
L858R) and C797S resistance mutations in metastatic NSCLC. Cancer
Res. 81 (Suppl 13):12622021. View Article : Google Scholar
|
|
192
|
Lim SM, Park CW, Zhang Z, Woessner R,
Dineen T, Stevison F, Hsieh J, Eno M, Wilson D, Campbell J, et al:
BLU-945, a fourth-generation, potent and highly selective epidermal
growth factor receptor tyrosine kinase inhibitor with intracranial
activity, demonstrates robust in vivo anti-tumor activity in models
of osimertinib-resistant non-small cell lung cancer. Cancer Res. 81
(Suppl 13):14672021. View Article : Google Scholar
|
|
193
|
Tavera L, Zhang Z, Wardwell S, Job E,
McGinn K, Chen M, Iliou M, Albayya F, Campbell J, Eno M, et al:
BLU-701 tumour suppression and intracranial activity as a single
agent and in combination with BLU-945 in models of non-small cell
lung cancer (NSCLC) driven by EGFR mutations. Mol Cell Biol.
165:S372022.
|
|
194
|
Liu X, Zhang X, Yang L, Chen S, Tian X,
Dong T, Ding CZ, Hu L, Wu L, Zhao L, Mao J, et al: Preclinical
evaluation of TQB3804, a potent EGFR C797S inhibitor. Cancer Res.
79 (Suppl 13):13202019. View Article : Google Scholar
|
|
195
|
Huang J and Wang H: Targeted therapy and
mechanism of drug resistance in non-small cell lung cancer with
epidermal growth factor receptor gene mutation. Zhongguo Fei Ai Za
Zhi. 25:183–192. 2022.(In Chinese).
|
|
196
|
Lim S, Kim DW, Jung JE, Lee G, Ryou JH,
Kang SU, Lee YH, Shin HJ, Yum SY and Yim Ε: A Phase 1/2, open-label
study of BBT-176, a triple mutation targeting EGFR TKI, in patients
with NSCLC who progressed after prior EGFR TKI therapy. Ann Oncol.
32:S949–S1039. 2021. View Article : Google Scholar
|
|
197
|
Lim S, Kim D and Jung J: A phase I/II,
open-label study of BBT-176, a triple mutation targeting EGFR TKI,
in patients with NSCLC who progressed after prior EGFR TKI therapy.
Ann Oncol. 32:S1035(Suppl 5):2021.
|
|
198
|
Park K, Haura EB, Leighl NB, Mitchell P,
Shu CA, Girard N, Viteri S, Han JY, Kim SW, Lee CK, et al:
Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung
cancer progressing on platinum chemotherapy: Initial results from
the CHRYSALIS phase I study. J Clin Oncol. 39:3391–3402. 2021.
View Article : Google Scholar
|
|
199
|
Cho B, Lee K, Cho E, Kim DW, Lee JS, Han
JY, Kim SW, Spira A, Haura EB, Sabari JK, et al: 1258O Amivantamab
(JNJ-61186372), an EGFR-MET bispecific antibody, in combination
with lazertinib, a 3rd-generation tyrosine kinase inhibitor (TKI),
in advanced EGFR NSCLC. Ann Oncol. 31:S813(Suppl 4):2020.
View Article : Google Scholar
|
|
200
|
Yu H, Johnson M, Steuer C, Vigliotti M,
Chen S, Kamai Y, Yu C and Jänne P: Preliminary phase 1 results of
U3-1402-A novel HER3-targeted antibody-drug conjugate-in EGFR
TKI-resistant, EGFR-mutant NSCLC. Mol Cell Biol. 14:S336–S337.
2019.
|
|
201
|
Jänne PA, Baik C, Su WC, Johnson ML,
Hayashi H, Nishio M, Kim DW, Koczywas M, Gold KA, Steuer CE, et al:
Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR
inhibitor-resistant, EGFR-mutated non-small cell lung cancer.
Cancer Discov. 12:74–89. 2022. View Article : Google Scholar
|
|
202
|
Soo RA, Han JY, Dafni U, Cho BC, Yeo CM,
Nadal E, Carcereny E, de Castro J, Sala MA, Bernabé R, et al: A
randomised phase II study of osimertinib and bevacizumab versus
osimertinib alone as second-line targeted treatment in advanced
NSCLC with confirmed EGFR and acquired T790M mutations: The
European Thoracic Oncology Platform (ETOP 10–16) BOOSTER trial. Ann
Oncol. 33:181–192. 2022. View Article : Google Scholar
|
|
203
|
Cui Q, Hu Y, Cui Q, Wu D, Mao Y, Ma D and
Liu H: Osimertinib rechallenge with bevacizumab vs. chemotherapy
plus bevacizumab in EGFR-Mutant NSCLC patients with osimertinib
resistance. Front Pharmacol. 12:7467072022. View Article : Google Scholar
|
|
204
|
Sequist L, Peled N, Tufman A, Servidio L,
Li J, Taylor R and Zhao J: COMPEL: Chemotherapy with/without
osimertinib in patients with EGFRm advanced NSCLC and progression
on first-line osimertinib. J Thor Oncol. 16:S11012021. View Article : Google Scholar
|
|
205
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 phase 3
Randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.
View Article : Google Scholar
|
|
206
|
Tamiya M, Kunimasa K, Nishino K, Matsumoto
S, Kawachi H, Kuno K, Inoue T, Kuhara H, Imamura F, Goto K and
Kumagai T: Successful treatment of an osimertinib-resistant lung
adenocarcinoma with an exon 18 EGFR mutation (G719S) with afatinib
plus bevacizumab. Invest New Drugs. 39:232–236. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Blakely CM, Watkins TBK, Wu W, Gini B,
Chabon JJ, McCoach CE, McGranahan N, Wilson GA, Birkbak NJ, Olivas
VR, et al: Evolution and clinical impact of co-occurring genetic
alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet.
49:1693–1704. 2017. View Article : Google Scholar
|
|
208
|
Dagogo-Jack I and Shaw AT: Tumour
heterogeneity and resistance to cancer therapies. Nat Rev Clin
Oncol. 15:81–94. 2018. View Article : Google Scholar
|
|
209
|
Assaraf YG, Brozovic A, Gonçalves AC,
Jurkovicova D, Linē A, Machuqueiro M, Saponara S, Sarmento-Ribeiro
AB, Xavier CPR and Vasconcelos MH: The multi-factorial nature of
clinical multidrug resistance in cancer. Drug Resist Updat.
46:1006452019. View Article : Google Scholar
|
|
210
|
Zhang Y, Wang D, Peng M, Tang L, Ouyang J,
Xiong F, Guo C, Tang Y, Zhou Y, Liao Q, et al: Single-cell RNA
sequencing in cancer research. J Exp Clin Cancer Res. 40:812021.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Maynard A, McCoach CE, Rotow JK, Harris L,
Haderk F, Kerr DL, Yu EA, Schenk EL, Tan W, Zee A, et al:
Therapy-Induced evolution of human lung cancer revealed by
single-cell RNA sequencing. Cell. 182:1232–1251.e22. 2020.
View Article : Google Scholar
|
|
212
|
Kim DW and Cho JY: Recent advances in
allogeneic CAR-T cells. Biomolecules. 10:2632020. View Article : Google Scholar
|
|
213
|
Patel AJ, Richter A, Drayson MT and
Middleton GW: The role of B lymphocytes in the immuno-biology of
non-small-cell lung cancer. Cancer Immunol Immunother. 69:325–342.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Hung LVM, Ngo HT and Van Pham P: Clinical
trials with cytokine-induced killer cells and CAR-T cell
transplantation for non-small cell lung cancer treatment. Adv Exp
Med Biol. 1292:113–130. 2020. View Article : Google Scholar
|
|
215
|
Johnson LA and June CH: Driving
gene-engineered T cell immunotherapy of cancer. Cell Res. 27:38–58.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Xu J, Zhang Q, Tian K, Wang H, Yin H and
Zheng J: Current status and future prospects of the strategy of
combining CAR-T with PD-1 blockade for antitumor therapy (Review).
Mol Med Rep. 17:2083–2088. 2018.PubMed/NCBI
|
|
217
|
Kandra P, Nandigama R, Eul B, Huber M,
Kobold S, Seeger W, Grimminger F and Savai R: Utility and drawbacks
of chimeric antigen receptor T Cell (CAR-T) therapy in lung cancer.
Front Immunol. 13:9035622022. View Article : Google Scholar
|
|
218
|
Xu C, Ju D and Zhang X: Chimeric antigen
receptor T-cell therapy: Challenges and opportunities in lung
cancer. Antib Ther. 5:73–83. 2022.PubMed/NCBI
|
|
219
|
Yang P, Qiao Y, Meng M and Zhou Q:
Cancer/Testis antigens as biomarker and target for the diagnosis,
prognosis, and therapy of lung cancer. Front Oncol. 12:8641592022.
View Article : Google Scholar
|
|
220
|
Yeku O, Li X and Brentjens RJ: Adoptive
T-Cell therapy for solid tumors. Am Soc Clin Oncol Educ Book.
37:193–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Reppel L, Tsahouridis O, Akulian J, Davis
IJ, Lee H, Fucà G, Weiss J, Dotti G, Pecot CV and Savoldo B:
Targeting disialoganglioside GD2 with chimeric antigen
receptor-redirected T cells in lung cancer. J Immunother Cancer.
10:e0038972022. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Min J, Long C, Zhang L, Duan J, Fan H, Chu
F and Li Z: c-Met specific CAR-T cells as a targeted therapy for
non-small cell lung cancer cell A549. Bioengineered. 13:9216–9232.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Feng K, Guo Y, Dai H, Wang Y, Li X, Jia H
and Han W: Chimeric antigen receptor-modified T cells for the
immunotherapy of patients with EGFR-expressing advanced
relapsed/refractory non-small cell lung cancer. Sci China Life Sci.
59:468–479. 2016. View Article : Google Scholar
|
|
224
|
Xiao BF, Zhang JT, Zhu YG, Cui XR, Lu ZM,
Yu BT and Wu N: Chimeric antigen receptor T-Cell therapy in lung
cancer: Potential and challenges. Front Immunol. 12:7827752021.
View Article : Google Scholar
|
|
225
|
Qu J, Mei Q, Chen L and Zhou J: Chimeric
antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer
(NSCLC): Current status and future Aperspectives. Cancer Immunol
Immunother. 70:619–631. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Chen L, Chen F, Li J, Pu Y, Yang C, Wang
Y, Lei Y and Huang Y: CAR-T cell therapy for lung cancer: Potential
and perspective. Thorac Cancer. 13:889–899. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Vasic D, Lee JB, Leung Y, Khatri I, Na Y,
Abate-Daga D and Zhang L: Allogeneic double-negative CAR-T cells
inhibit tumor growth without off-tumor toxicities. Sci Immunol.
7:eabl36422022. View Article : Google Scholar
|