Introduction
Colorectal cancer (CRC) remains a life-threatening
disease worldwide (1), with an
incidence rate that increases annually by ~2% and a death rate that
increases annually by ~1.3% among those <50 years of age
(2). In recent years, a group of
prognostic markers obtained from patients' routine blood tests,
such as the neutrophil to lymphocyte ratio (NLR) (3) and the lymphocyte to monocyte ratio
(LMR) (4), have been established.
These indicators are non-invasive and easily accessible in
practice; however, their prognostic efficacies have not been
conventionally compared with traditional markers.
Body mass index (BMI), which is calculated as weight
in kilograms divided by height in meters squared
(kg/m2), is a traditional prognostic marker for numerous
malignancies, including breast (5), ovarian (6), esophageal (7), lung (8) and gastric (9) cancer, as well as CRC (10–12).
However, there are still unresolved problems regarding the
prognostic value of BMI in CRC. On the one hand, the BMI criteria
to group patients have been inconsistent in previous studies; for
example, Guercio et al (13) divided patients into 5 subgroups
[<21 (underweight), 21–24.9 (normal), 25–29.9, 30–34.9 and 35
kg/m2], as did Chiu et al (11); however, in the latter study,
underweight (<18.50 kg/m2) and normal weight
(18.50-24.99 kg/m2) patients were not in line with those
in the former study. Furthermore, Song et al found that 20.2
kg/m2 was the best discrimination point for survival
(12). In addition, the final
conclusion for the role of BMI in CRC is conflicting, with some
studies indicating that patients with an underweight BMI (<18.50
kg/m2) would have poor survival (10–12),
but other studies failing to reproduce these results (13) or even yielding opposite results
(14). Based on this background,
it is reasonable to improve the prognostic efficacy of BMI with a
combination of other markers. In fact, other studies have tried to
combine BMI with other markers, including the NLR (15,16);
however, these studies did not routinely report the prognostic
efficacy of the new indicators or statistically compare them when
it was generated.
The carcinoembryonic antigen (CEA) level is another
long-term established tumor marker that is recommended by the
American Society of Clinical Oncology for CRC (17) and has been incorporated into the
Tumor-Node-Metastasis (TNM) system (the so-called C-stage) to
provide additional prognostic information (18). However, apart from the fact that
only 21–36% of patients are positive for CEA at diagnosis (19), limitations such as a low
sensitivity of a single CEA value for prognosis cannot be ignored
(20), and its efficacy could be
largely attenuated in conditions such as type II diabetes (21) or smoking (22). Notably, in previous studies, the
serum concentration of CEA was closely correlated with BMI; for
example, an increase or decrease in BMI in patients with cancer was
correlated with a fluctuation in systemic inflammatory factors such
as interleukin-6 (IL-6) (23,24),
which can promote the secretion of CEA in CRC cells (25,26).
Based on these factors, it is reasonable to explore the prognostic
value of the CEA to BMI ratio (CBR) in CRC, as the significance of
CEA levels could be balanced to some extent by BMI in these
patients; however, to the best of our knowledge, associated reports
are still absent.
The present study aimed to explore the prognostic
value of the CBR, as well as its prognostic efficacy when compared
with individual CEA, BMI and other inflammatory prognostic
indicators in CRC.
Materials and methods
Patients
Between January 2012 and October 2021, patients with
CRC who underwent radical resection of primary lesions at Hainan
Hospital of Chinese People's Liberation Army (PLA) General Hospital
(Sanya, China) were retrospectively included in the present study.
Patients meeting any one of the following criteria were excluded:
i) Patients with an absence of preoperative laboratory test results
or abnormal aminotransferase or serum creatinine levels, since such
abnormalities could cause an altered metabolism or excretion of CEA
(27,28); ii) patients with distant lesions;
iii) patients who were missing any TNM information in their
postoperative pathological reports; iv) patients with multiple or
recurrent malignancies or in situ lesions; v) and patients
with a follow-up time of <36 months. In addition, patients who
received neoadjuvant chemotherapy were also excluded, since such
therapy could cause problems in being able to accurately confirm
the pT/pN stages in post-operative pathological findings, in
particular for those who reached a tumor regression grading of 2 or
3 (29). A binary system was
applied for patients with cancer with or without mucinous elements,
tumor deposits (TDs) and risk factors (with perineural or
lymphovascular invasion). Other clinicopathological parameters were
recorded as described in previous studies (30,31).
The study was performed in line with the principles stated in the
Declaration of Helsinki and was approved by the Ethics Committee of
Hainan Hospital of Chinese PLA General Hospital (approval no.
301HLFYLS15). All patients or their authorized relatives provided
written informed consent.
Determination of the CBR, NLR, LMR,
platelet to lymphocyte ratio (PLR) and prognostic nutritional index
(PNI), and their correlations
Laboratory tests were performed as described in our
previous studies (30,31). The CBR was calculated as the
concentration of serum CEA (reference, 0–5 µg/ml) divided by the
BMI. In addition, other inflammatory prognostic indicators,
including NLR, LMR, PLR and PNI, were also determined according to
previous studies (3,4,32,33).
The correlation of CBR with NLR, LMR, PLR and PNI was also
determined.
Definitions of disease-free survival
(DFS) and overall survival (OS)
The follow-up was conducted as previously described
(31). Briefly, patients were
interviewed every 3–6 months for the first 2 years and then every
6–12 months after for those who survived for >2 years. DFS time
was defined as the time from the date of surgery until the date of
the first recurrence or metastasis at any location, or death from
any reason. OS time was defined from the same initial point to the
date of death from any cause. The latest follow-up point was
December 2021.
Statistical analysis
Statistical analyses were conducted using SPSS 20.0
(IBM Corp.) and MedCalc v19.0.7 (MedCalc Software bvba). The
optimal discriminator point of the CBR was calculated by receiver
operating characteristic (ROC) curve analysis, and then the area
under the curve (AUC) was compared using the methods described by
DeLong et al (34). The
differences in the clinicopathological parameters between the CBR
subgroups were estimated by χ2 test. The association of
CBR with other inflammatory prognostic indicators was determined by
Pearson's correlation analysis. Survival differences for the low or
high CBR subgroups were determined by Kaplan-Meier analysis
followed by log-rank tests. A Cox proportional hazards model was
applied to select the risk factors for survival (forward likelihood
ratio model). Based on the results of the multivariable analysis,
nomograms were established using R (version 4.1.1; http://www.r-project.org) with the survival and RMS
package, and the C-index was used to determine the prediction
efficacy. P<0.05 (two-sided) was considered to indicate a
statistically significant difference.
Results
Demographic features and the
prognostic efficacy of the CBR
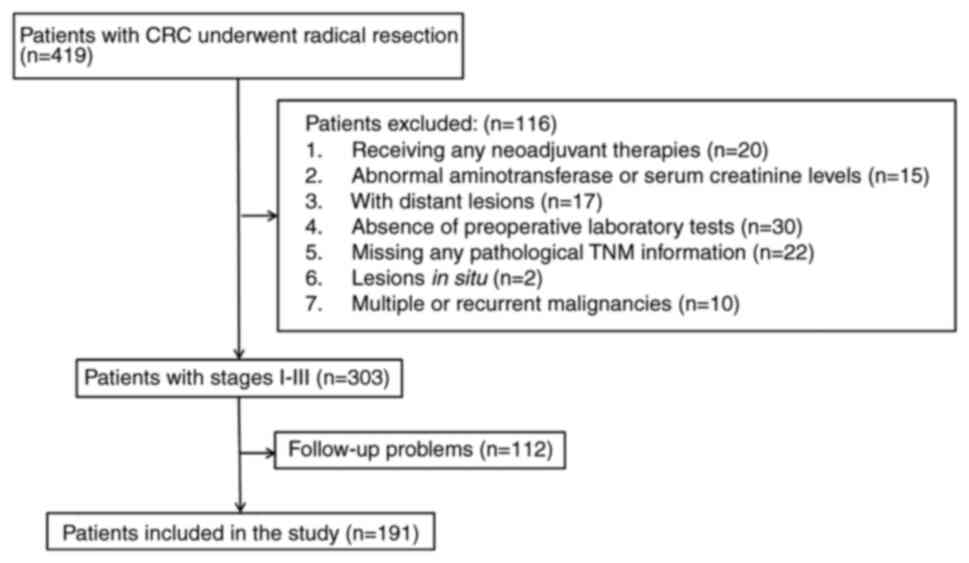
As shown in Fig. 1,
a total of 191 patients (68 females and 123 males) were finally
included in the study. The mean age of the patients was 59.3 years
(range, 24–85 years), with a mean follow-up period of 51.1 months
(range, 1–111 months). By ROC analysis, taking 0.28 as the optimal
cutoff point (according to the Youden index), 29.84% (57/191) of
the patients were assigned to the high CBR group (≥0.28), and
70.16% (134/191) were assigned to the low CBR group (<0.28). The
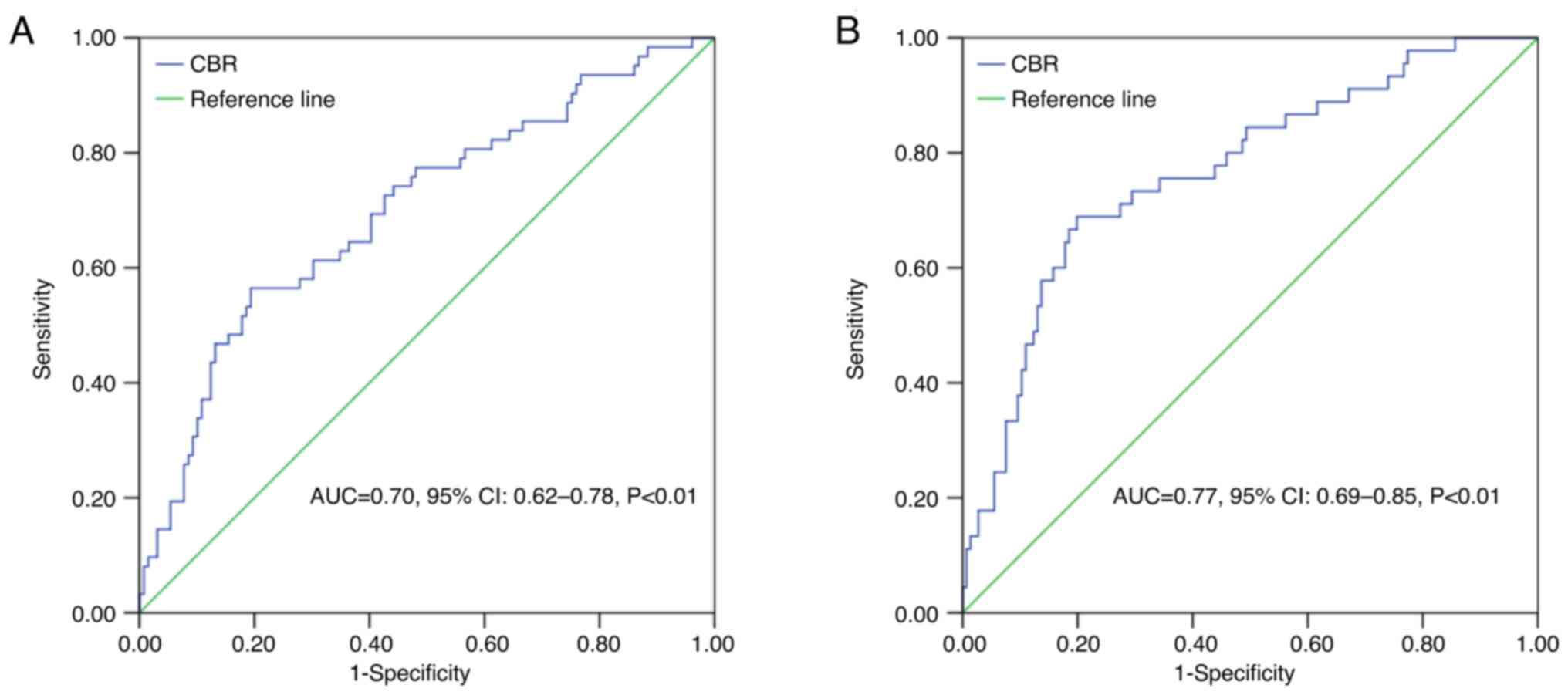
CBR presented a sensitivity of 56.50 and 68.90%, and a specificity
of 80.60 and 80.10% for DFS and OS, respectively (both P<0.01)
(Fig. 2). Next, a further
comparison of the prognostic efficacy of the CBR in predicting OS
with individual CEA (Z=2.35, P=0.02), BMI (Z=2.01, P=0.04), NLR
(Z=2.90, P<0.01), LMR (Z=2.42, P=0.02) and PLR (Z=2.49, P=0.01)
values indicated significant differences, with the exception of PNI
(Z=1.16, P=0.25).
Correlation of CBR with NLR, LMR, PLR
and PNI
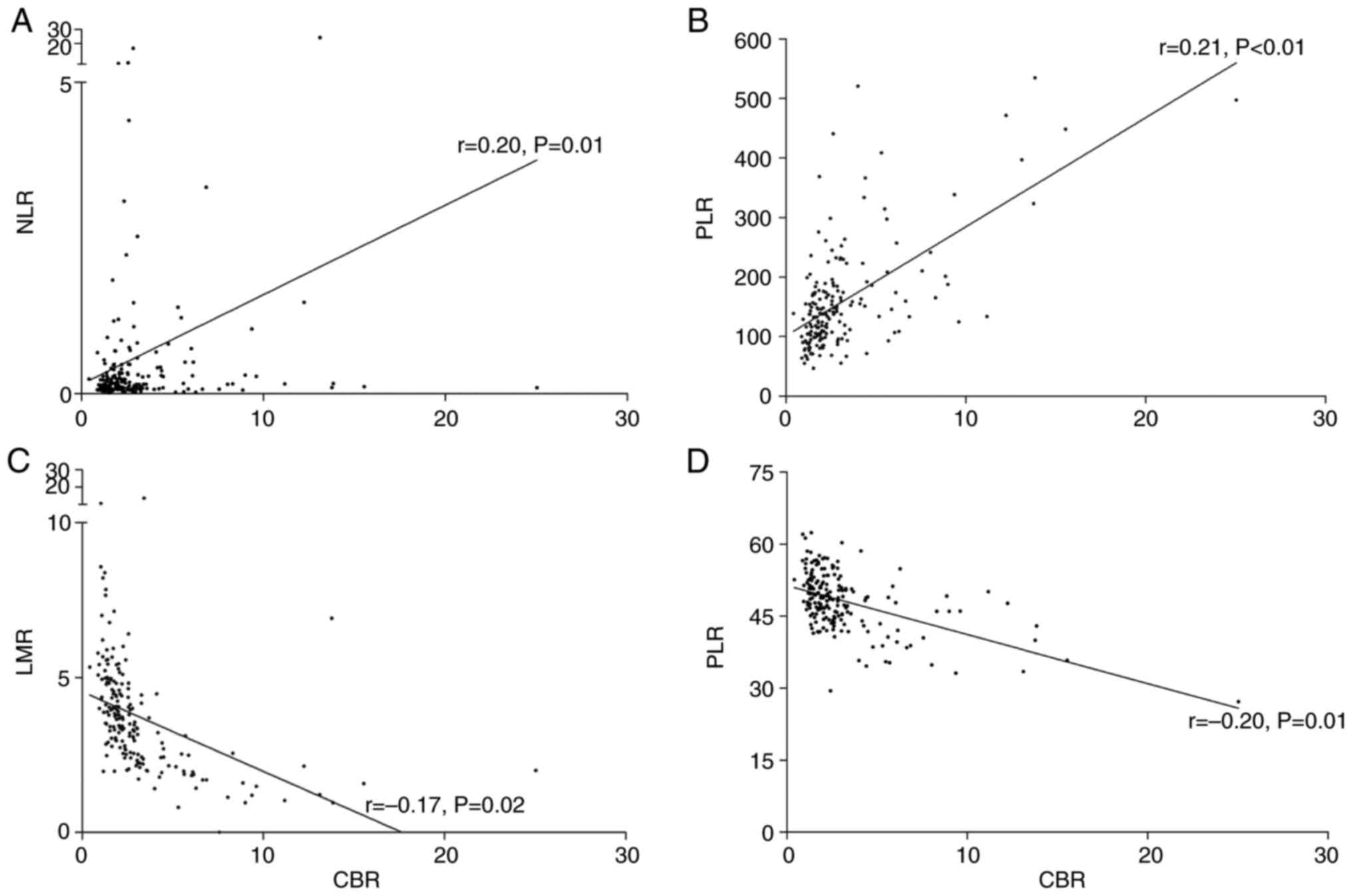
According to Pearson's correlation analysis, the CBR
displayed a significant positive association with NLR (r=0.20,
P=0.01) and PLR (r=0.21, P<0.01), whereas a negative association
was exhibited with LMR (r=−0.17, P=0.02) and PNI (r=−0.20, P=0.01)
(Fig. 3).
Clinicopathological parameter
differences between the high- and low-CBR subgroups
According to the χ2 test, patients who
underwent a laparotomy (P=0.03), or those with advanced T stages
(T3+T4) (P<0.01), the presence of TD(s)
(P<0.01) and advanced TNM stages (stage II or III) (P<0.01)
were more commonly found in the high CBR group. No significant
differences were found for the other clinicopathological parameters
between the high and low CBR subgroups (Table I).
 | Table I.Differences in clinicopathological
parameters among CBR subgroups. |
Table I.
Differences in clinicopathological
parameters among CBR subgroups.
|
|
| CBR |
|---|
|
|
|
|
|---|
| Characteristic | Total patients,
n | Low, n | High, n | P-value |
|---|
| Age, years |
|
|
| 0.35 |
|
<60 | 91 | 67 | 24 |
|
|
≥60 | 100 | 67 | 33 |
|
| Sex |
|
|
| 0.74 |
|
Female | 68 | 49 | 19 |
|
|
Male | 123 | 85 | 38 |
|
| Type of
resection |
|
|
| 0.03a |
|
Laparotomy | 29 | 15 | 14 |
|
|
Laparoscopy | 162 | 119 | 43 |
|
| Tumor location |
|
|
| 0.25 |
|
Right | 42 | 26 | 16 |
|
|
Left | 149 | 108 | 41 |
|
| Histological
grade |
|
|
| 0.38 |
| Well +
moderate | 162 | 116 | 46 |
|
|
Poor | 29 | 18 | 11 |
|
| Mucinous
element |
|
|
| 0.16 |
|
Present | 37 | 22 | 15 |
|
|
Absent | 154 | 112 | 42 |
|
| T stages |
|
|
|
<0.01a |
|
T1 +
T2 | 51 | 44 | 7 |
|
|
T3 +
T4 | 140 | 90 | 50 |
|
| N stages |
|
|
| 0.11 |
|
N0 | 115 | 86 | 29 |
|
|
N1 +
N2 | 76 | 48 | 28 |
|
| Tumor deposits |
|
|
|
<0.01a |
|
Present | 171 | 126 | 45 |
|
|
Absent | 20 | 8 | 12 |
|
| TNM stages |
|
|
|
<0.01a |
| I | 40 | 38 | 2 |
|
| II | 75 | 48 | 27 |
|
|
III | 76 | 48 | 28 |
|
| Risk factors |
|
|
| 0.09 |
|
Present | 24 | 13 | 11 |
|
|
Absent | 167 | 121 | 46 |
|
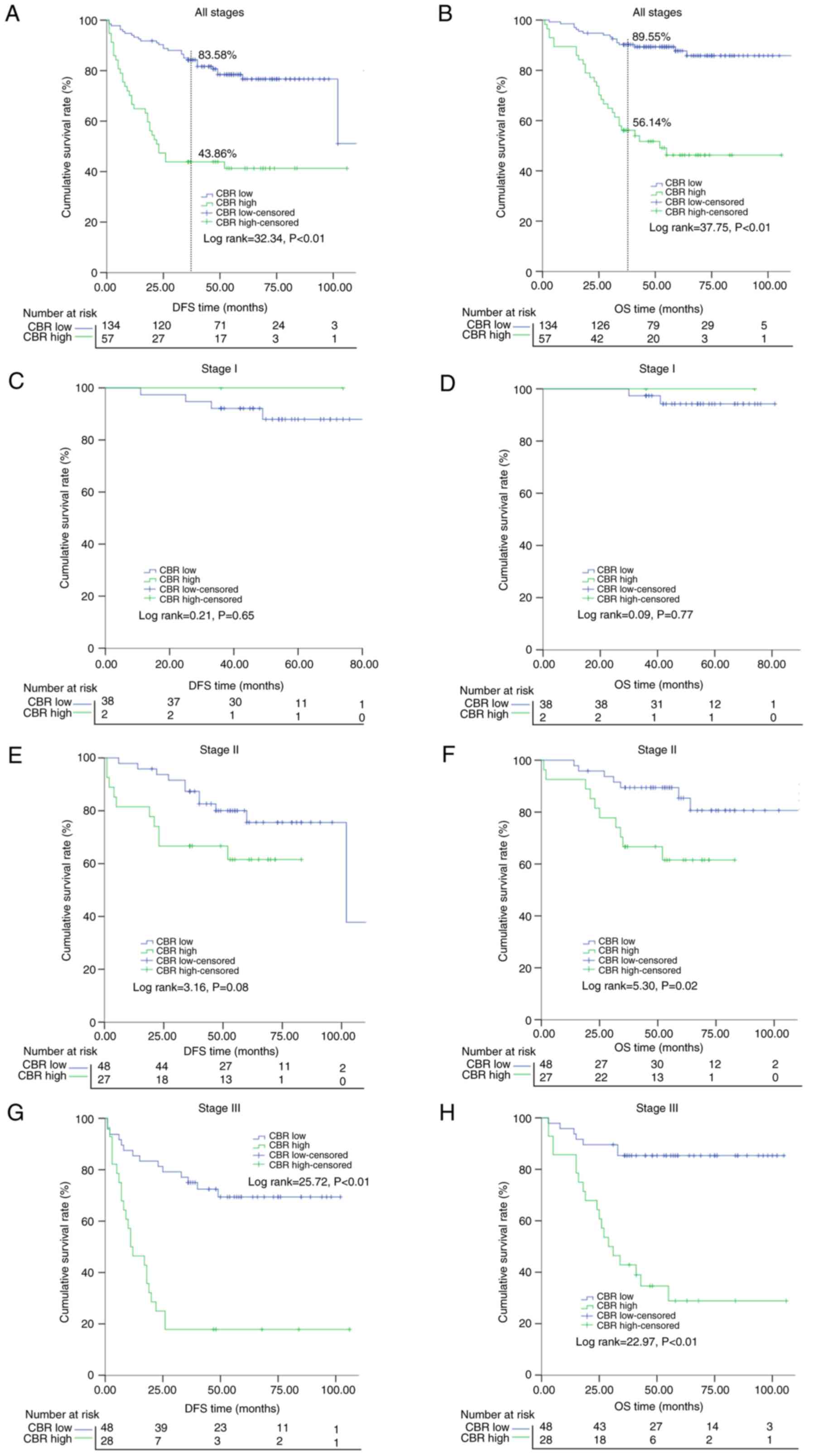
Survival differences between the high
and low CBR subgroups in terms of DFS and OS
After the 3-year follow-up period, significant
differences in DFS (43.86 vs. 83.58%; P<0.01) and OS (56.14 vs.
89.55%; P<0.01) rates were found between the high and low CBR
subgroups, respectively (Fig. 4A and
B). Subsequently, the survival differences of the low and high
CBR subgroups were further compared in different stages, and it was
indicated that patients with a low CBR would have a superior DFS
and OS in stage II (with a tendency but non-significant result for
DFS) and stage III patients but not in stage I cases (Fig. 4).
Univariate and multivariate analyses
of the prognostic factors for DFS and OS
Univariate tests indicated that type of resection,
T, N and TNM stages, TDs, risk factors, pre-operative NLR, LMR,
PLR, PNI and CBR were all factors that could affect DFS (Table II), and that these, in addition to
histological grade and mucinous elements, were factors that could
affect OS (Table III). When
these significant factors (only those P<0.05) were included in
the multivariate tests for DFS and OS, the results indicated that
the TDs, PNI and CBR were independent prognostic factors for both
DFS (CBR: HR, 3.48; 95% CI, 2.04-5.91; P<0.01) and OS (CBR: HR,
3.71; 95% CI, 1.95-7.08; P<0.01). Additionally, N stage was an
independent prognostic factor for DFS, and histological grade,
mucinous element and risk factors were independent prognostic
factor for OS (Tables II and
III).
 | Table II.Univariate and multivariate analyses
of risk factors for disease-free survival. |
Table II.
Univariate and multivariate analyses
of risk factors for disease-free survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Characteristic | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Age, years |
|
|
|
|
|
|
|
<60 | 1.00 |
|
|
|
|
|
|
≥60 | 0.86 | 1.05 | 0.63-1.72 |
|
|
|
| Sex |
|
|
|
|
|
|
|
Female | 1.00 |
|
|
|
|
|
|
Male | 0.27 | 1.36 | 0.79-2.35 |
|
|
|
| Type of
resection |
|
|
|
|
|
|
|
Laparotomy | 1.00 |
|
|
|
|
|
|
Laparoscopy |
<0.01a | 0.39 | 0.22-0.68 |
|
|
|
| Tumor location |
|
|
|
|
|
|
|
Right | 1.00 |
|
|
|
|
|
|
Left | 0.84 | 0.94 | 0.52-1.71 |
|
|
|
| Histological
grade |
|
|
|
|
|
|
| Well +
moderate | 1.00 |
|
|
|
|
|
|
Poor | 0.08 | 1.74 | 0.94-3.22 |
|
|
|
| Mucinous
element |
|
|
|
|
|
|
|
Present | 1.00 |
|
|
|
|
|
|
Absent | 0.06 | 0.58 | 0.33-1.02 |
|
|
|
| T stages |
|
|
|
|
|
|
|
T1 +
T2 | 1.00 |
|
|
|
|
|
|
T3 +
T4 |
<0.01a | 3.96 | 1.71-9.20 |
|
|
|
| N stages |
|
|
|
|
|
|
|
N0 | 1.00 |
|
| 1.00 |
|
|
|
N1 +
N2 |
<0.01a | 2.85 | 1.71-4.74 |
<0.01a | 2.30 | 1.30-4.08 |
| Tumor deposits |
|
|
|
|
|
|
|
Present | 1.00 |
|
| 1.00 |
|
|
|
Absent |
<0.01a | 0.13 | 0.07-0.24 |
<0.01a | 0.34 | 0.17-0.67 |
| TNM stages |
|
|
|
|
|
|
| I | 1.00 |
|
|
|
|
|
| II | 0.05a | 2.98 | 1.02-8.69 |
|
|
|
|
III |
<0.01a | 6.45 | 2.30-18.14 |
|
|
|
| Risk factors |
|
|
|
|
|
|
|
Present | 1.00 |
|
|
|
|
|
|
Absent | 0.01a | 0.43 | 0.23-0.79 |
|
|
|
| Preoperative
measures |
|
|
|
|
|
|
|
NLR |
<0.01a | 1.08 | 1.03-1.14 |
|
|
|
|
LMR | 0.02a | 0.82 | 0.69-0.97 |
|
|
|
|
PLR | 0.02 | 1.00 | 1.00-1.01 |
|
|
|
|
PNI |
<0.01a | 0.93 | 0.90-0.97 |
<0.01a | 0.93 | 0.90-0.97 |
| CBR |
|
|
|
|
|
|
|
Low | 1.00 |
|
| 1.00 |
|
|
|
High |
<0.01a | 3.86 | 2.33-6.37 |
<0.01a | 3.48 | 2.04-5.91 |
 | Table III.Univariate and multivariate tests of
risk factors for overall survival. |
Table III.
Univariate and multivariate tests of
risk factors for overall survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Characteristic | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Age, years |
|
|
|
|
|
|
|
<60 | 1.00 |
|
|
|
|
|
|
≥60 | 0.13 | 1.60 | 0.88-2.93 |
|
|
|
| Sex |
|
|
|
|
|
|
|
Female | 1.00 |
|
|
|
|
|
|
Male | 0.20 | 1.54 | 0.80-2.99 |
|
|
|
| Type of
resection |
|
|
|
|
|
|
|
Laparotomy | 1.00 |
|
|
|
|
|
|
Laparoscopy | 0.03a | 0.47 | 0.24-0.93 |
|
|
|
| Tumor location |
|
|
|
|
|
|
|
Right | 1.00 |
|
|
|
|
|
|
Left | 0.96 | 0.98 | 0.47-1.98 |
|
|
|
| Histological
grade |
|
|
|
|
|
|
| Well +
moderate | 1.00 |
|
| 1.00 |
|
|
|
Poor | 0.01a | 2.32 | 1.20-4.50 | 0.01a | 2.54 | 1.26-5.11 |
| Mucinous
element |
|
|
|
|
|
|
|
Present | 1.00 |
|
| 1.00 |
|
|
|
Absent | 0.04a | 0.50 | 0.26-0.95 | 0.05a | 0.49 | 0.24-0.98 |
| T stages |
|
|
|
|
|
|
|
T1 +
T2 | 1.00 |
|
|
|
|
|
|
T3 +
T4 | 0.01a | 4.12 | 1.48-11.51 |
|
|
|
| N stages |
|
|
|
|
|
|
|
N0 | 1.00 |
|
|
|
|
|
|
N1 +
N2 |
<0.01a | 2.38 | 1.32-4.30 |
|
|
|
| Tumor deposits |
|
|
|
|
|
|
|
Present | 1.00 |
|
| 1.00 |
|
|
|
Absent |
<0.01a | 0.12 | 0.06-0.23 |
<0.01a | 0.24 | 0.12-0.47 |
| TNM stages |
|
|
|
|
|
|
| I | 1.00 |
|
|
|
|
|
| II | 0.03a | 4.87 | 1.12-21.07 |
|
|
|
|
III |
<0.01a | 8.23 | 1.95-34.70 |
|
|
|
| Risk factors |
|
|
|
|
|
|
|
Present | 1.00 |
|
| 1.00 |
|
|
|
Absent |
<0.01a | 0.34 | 0.17-0.67 | 0.01a | 0.37 | 0.18-0.78 |
| Preoperative
measures |
|
|
|
|
|
|
|
NLR | 0.01a | 1.09 | 1.03-1.16 |
|
|
|
|
LMR | 0.04a | 0.82 | 0.67-0.99 |
|
|
|
|
PLR | 0.04a | 1.00 | 1.00-1.01 |
|
|
|
|
PNI |
<0.01a | 0.91 | 0.87-0.95 |
<0.01a | 0.89 | 0.85-0.94 |
| CBR |
|
|
|
|
|
|
|
Low | 1.00 |
|
| 1.00 |
|
|
|
High |
<0.01a | 5.50 | 2.98-10.15 |
<0.01a | 3.71 | 1.95-7.08 |
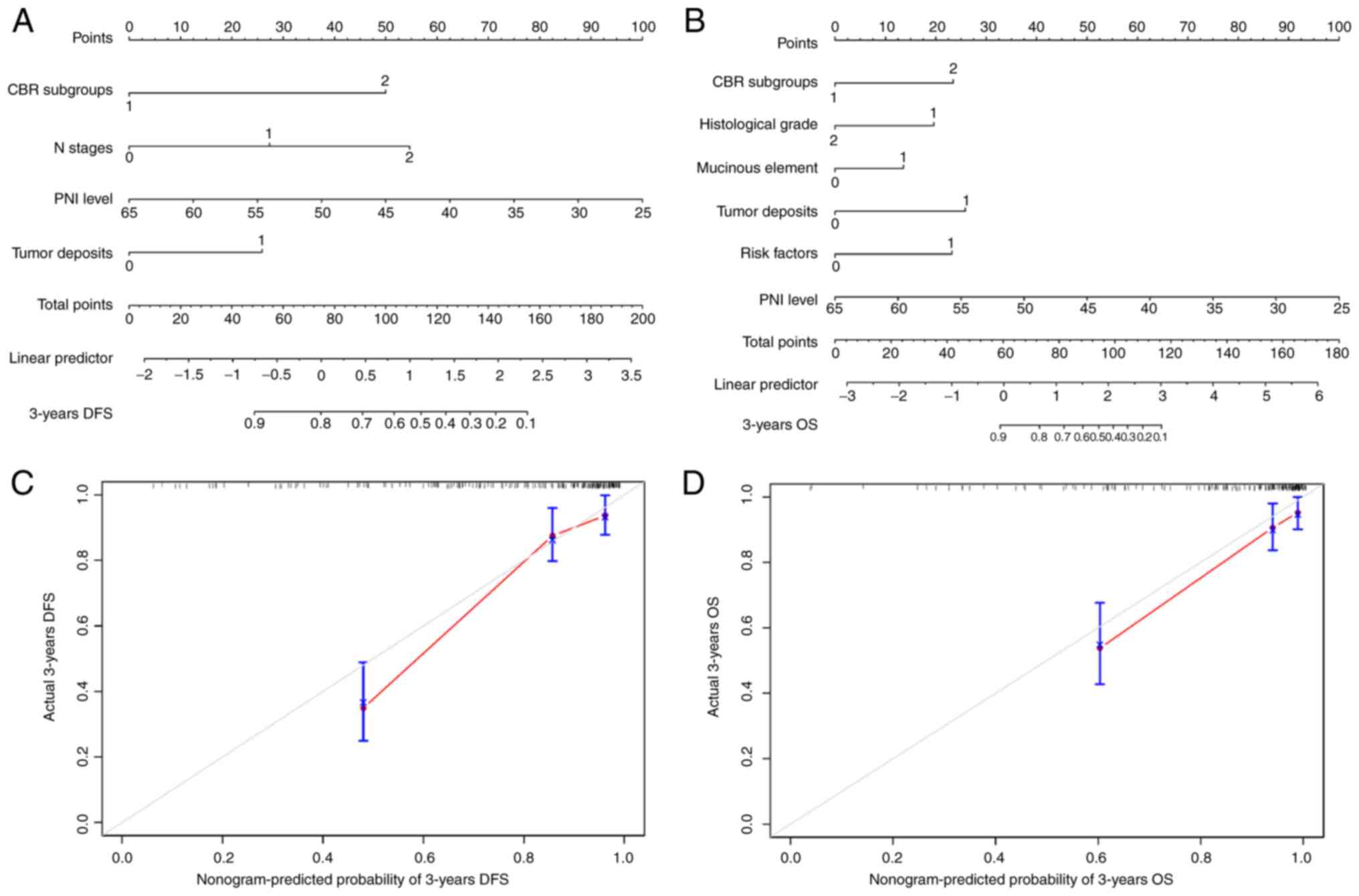
Development of nomograms for
predicting CRC prognosis
To predict DFS and OS rates for the patients,
nomograms were constructed based on the multivariate analyses in
Tables II and III. Each factor received a
corresponding total point according to the nomograms. As shown in
Fig. 5, the C-indexes for the
prediction of 3-year DFS and OS were 0.81 (95% CI, 0.72-0.91) and
0.84 (95% CI, 0.71-0.96), respectively (Fig. 5A and B), which indicated that the
model provided good discrimination. In addition, the calibration
curves displayed a good consistency between the predicted results
and the actual results (Fig. 5C and
D).
Discussion
The present study found that the CBR was significant
in the prognosis of CRC, and that the prognostic efficacy of the
CBR was superior to individual CEA, BMI, NLR, PLR and LMR values,
with a relatively high sensitivity and specificity. Patients with a
high CBR had a worse outcome than patients with a low CBR. Taking
into consideration that the CBR was less likely to be influenced by
common acute complications such as infection, bleeding and
obstruction in CRC, it should be considered a robust prognostic
indicator in practice. To the best of our knowledge, the present
study is the first report concerning the value of the CBR in
CRC.
Both CEA levels and BMI are traditional prognostic
markers in CRC, but have individual limitations. In previous
studies on CEA levels, the positive rate was relatively low
(19), and although patients with
a normal range of CEA also had prognostic significance based on
reduced cutoff points (35,36),
the sensitivity was relatively low (46.00%) (37) with a limited AUC in predicting
survival that ranged from 0.636 (cutoff, 11 µg/ml) (38) and 0.645 (cutoff, 5 µg/ml) (39) to 0.740 (cutoff, 12.5 µg/ml)
(40). A recent study indicated
that the CEA to maximum tumor diameter ratio has prognostic value;
however, the AUC of the new marker in predicting 3-year OS was
reported to be only 0.704 (41).
For BMI, its application in prognosis was largely blocked by
inconsistent criteria and conflicting results, as aforementioned
(10–14). Similarly, some reports indicated
that combining BMI with other markers, such as lymphocyte counts,
could improve the prognostic efficacy for patients with head and
neck cancer who underwent radiation therapy (16). Recently, Xie et al (42) conducted a study that included 2,471
patients with CRC and found that the neutrophil-BMI ratio was a
useful prognostic marker. In the present study, CBR presented with
a relatively high sensitivity and specificity for OS. Furthermore,
the prognostic efficacy was markedly stronger than that of
individual CEA and BMI values, and even greater than NLR, LMR and
PLR values.
Taking into consideration the individual role of CEA
levels and BMI in CRC prognosis, these results could be explained
from the following perspectives. First, for patients falling in a
certain BMI range, a relatively high CEA (equal to a high CBR)
would correlate with poor survival, which has been well established
by previous clinical studies (35,43).
Molecularly, it is well known that up to 90% of CRC cells can
release CEA (44,45), and CEA can trigger CRC progression
by inducing epithelial-mesenchymal transition, increasing cancer
cell invasiveness and inhibiting apoptotic signaling (46). In addition, CEA can also cause
radioresistance in CRC cells in the presence of M2 macrophages
(47). Based on these facts, a
high CBR weighted by a high CEA was likely to be associated with
poor outcomes in the patients. Second, for those with a similar
concentration of CEA, a lower BMI (also indicating a high CBR)
would lead to worse outcomes, which was also in line with previous
observations (10–12). However, it is also notable that
certain studies indicated that a high BMI, particularly for class I
(BMI, 30–35 kg/m2) and II (BMI, ≥35 kg/m2)
obesity, was correlated with poor survival in CRC (48,49).
In fact, previous studies found an increasing incidence of newly
diagnosed liver disorder in obese patients (50), and a relationship between BMI and
serum liver enzyme activity (51);
subsequently, the metabolism or excretion of CEA was altered and
had a tendency to be higher in patients with a high BMI (27). However, studies concerning the CEA
level in individuals with a high BMI, particularly in those with
class I or class II obesity, are rare, and it is still largely
unknown whether the CBR is also applicable in such a scenario (only
5 patients had a BMI of >30 in the present study).
Cancer-related inflammation has a profound effect in
regulating cancer development and is regarded as a hallmark of it
(52). High CBR associated with
poor survival could be also understood from the perspective of
inflammation. Previous studies indicated that CEA in patients with
CRC could induce the secretion of IL-6, and that the levels of CEA
and IL-6 were positively correlated (16,53).
It was noted that IL-6 could induce fat loss in cancer cachexia via
different approaches, such as promoting white adipose tissue
lipolysis (54) and decreasing
muscle mass (55), which could
result in a decreased BMI in these patients (56). Based on these facts, it was
plausible that a high CBR indicated a high level of inflammation
and a high tendency for reduced BMI, which could present as
cachexia and poor survival. The present study also found that a
high CBR was associated with advanced T stage
(T3+T4), and the presence of TDs. CBD was
positively associated with NLR or PLR and negatively associated
with LMR or PNI on correlation analysis; in fact, these parameters
were also previously reported to be useful prognostic indicators in
CRC. For example, Lino-Silva et al (57) included 392 patients of all stages
and found that stage I–III patients who presented with TDs
displayed similar mortality rates to stage IV patients.
Furthermore, Moon et al (58) published a systematic review that
included 90,455 stage III patients and found that those who
presented with TDs had a worse 5-year DFS rate. In addition to TDs,
the association of a low LMR with poor survival was also recorded;
for example, Naszai et al (59) conducted a meta-analysis that
included 32,788 patients and found that a pre-treatment high NLR
was a significant predictor of poor OS. In line with this, Tan
et al (4) also conducted a
meta-analysis that concluded that a low LMR was a significant
predictor of poor OS. All this evidence supports the fact that
patients with CRC and a high CBR have poor survival rates.
In recent years, a small subgroup of patients with
CRC featuring mismatch repair deficiency (dMMR) were demonstrated
to exhibit better outcomes than those with mismatch
repair-proficient tumors (pMMR) in certain stage cases, such as
stage II (60,61), and they also had a significantly
good response when receiving immunotherapies in metastatic cases
when compared to pMMR cases (62).
Notably, a lower BMI was found to be more common in patients with
dMMR than in those with pMMR (63); additionally, CEA levels were
significantly higher in the pMMR subtype (but only in stage III
patients) (64). These results
suggested that for patients in certain stages, the CBR would be
higher than for those in other stages. However, in the present
study, no such differences were found either for BMI (n=112,
P=0.64; data not shown) or for CEA levels (n=47, P=0.92; data not
shown). Taking into consideration the complex prognostic role of
MMR status in CRC, additional studies with larger samples are still
needed.
The present study also had some limitations. First,
it was conducted with a relatively small sample size; in
particular, the patients with a follow-up time of <36 months
were excluded since these cases may lack definite OS information
and cause problems in calculating the specific survival rate, which
may also result in a biased finding. Second, both CEA levels and
BMI could present extreme values, and the prognostic value of the
CBR was not validated in these cases. Third, definite information
for those stage II patients with high risks or the stage III
patients on adjuvant therapy was not sufficient, and it was well
established that adjuvant chemotherapy could improve the DFS in
patients with radical surgery (65,66).
However, it was notable that a number of other factors could
influence the efficacy of these therapies in the real world,
including the delay, early discontinuation and dose reduction of
the treatment. This is a complex problem that requires additional
elegant studies. Further studies are also needed to further
validate the present results.
Overall, the present results indicated that,
compared with individual CEA, BMI, NLR and LMR values, the CBR was
a more robust prognostic factor in CRC, and patients with a
relatively high CBR presented with inferior survival rates.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BY was responsible for the conception of the work.
JX, MD and JL obtained the data and contributed to the data
analysis. TX, QY and BY checked and analyzed the data. BY wrote the
manuscript. JX and BY confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was performed in line with the principles
stated in the Declaration of Helsinki and was approved by the
Ethics Committee of Hainan Hospital of Chinese People's Liberation
Army General Hospital (approval no. 301HLFYLS15). The patients or
their authorized relatives provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Sauer AG, Fedewa SA,
Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal A:
Colorectal cancer statistics, 2020. CA Cancer J Clin. 70:145–164.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song Y, Yang Y, Gao P, Chen X, Yu D, Xu Y,
Zhao J and Wang Z: The preoperative neutrophil to lymphocyte ratio
is a superior indicator of prognosis compared with other
inflammatory biomarkers in resectable colorectal cancer. BMC
Cancer. 17:7442017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan D, Fu Y, Tong W and Li F: Prognostic
significance of lymphocyte to monocyte ratio in colorectal cancer:
A meta-analysis. Int J Surg. 55:128–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takada K, Kashiwagi S, Asano Y, Goto W,
Ishihara S, Morisaki T, Shibutani M, Tanaka H, Hirakawa K and Ohira
M: Clinical verification of body mass index and tumor immune
response in patients with breast cancer receiving preoperative
chemotherapy. BMC Cancer. 21:11292021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zamorano AS, Hagemann AR, Morrison L, Lee
JA, Liao LM, Brinton LA, Park Y and Toriola AT: Pre-diagnosis body
mass index, physical activity and ovarian cancer mortality. Gynecol
Oncol. 155:105–111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang P, Li Y, Sun H, Liu S, Zhang R, Liu X
and Zhu Z: Predictive value of body mass index for short-term
outcomes of patients with esophageal cancer after esophagectomy: A
meta-analysis. Ann Surg Oncol. 26:2090–2103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shukla S, Babcock Z, Pizzi L and Brunetti
L: Impact of body mass index on survival and serious adverse events
in advanced non-small cell lung cancer treated with bevacizumab: A
meta-analysis of randomized clinical trials. Curr Med Res Opin.
37:811–817. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye Z, Wei S, Zeng Y, Wang Y, Lin Z, Chen
S, Xie Y, Zheng Q and Chen L: Prognostic value of preoperative body
mass index for diabetic patients with non-metastasis gastric
cancer: A single center experience. BMC Surg. 21:3202021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee S, Lee DH, Lee JH, Shin SJ, Lee HS,
Park EJ, Baik SH, Lee KY and Kang J: Association of body mass index
with survival in Asian patients with colorectal cancer. Cancer Res
Treat. 54:860–872. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiu CC, Ho CH, Hung CM, Chao CM, Lai CC,
Chen CM, Liao KM, Wang JJ, Wu YC, Shi HY, et al: Correlation of
body mass index with oncologic outcomes in colorectal cancer
patients: A large population-based study. Cancers. 13:35922021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song N, Huang D, Jang D, Kim MJ, Jeong SY,
Shin A and Park JW: Optimal body mass index cut-off point for
predicting colorectal cancer survival in an asian population: A
national health information database analysis. Cancers (Basel).
12:8302020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guercio BJ, Zhang S, Venook AP, Ou FS,
Niedzwiecki D, Lenz HJ, Innocenti F, Mullen BC, O'Neil BH, Shaw JE,
et al: Body mass index and weight loss in metastatic colorectal
cancer in CALGB (Alliance)/SWOG 80405. JNCI Cancer Spectr.
4:pkaa0242020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwak HD, Ju JK, Kang DW, Baek SJ, Kwak JM,
Kim J and Kim SH: Outcomes according to body mass index following
laparoscopic surgery in patients with colorectal cancer. J Minim
Access Surg. 14:134–139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dragomir R, Dragomir AS, Negru A, Săftescu
S, Popovici D, Schenker M, Lupușoru R and Negru Ș: Role of
combining neutrophil-to-lymphocyte ratio and pretreatment body mass
index in predicting progression-free survival in patients with
non-small cell lung cancer treated with nivolumab. Exp Ther Med.
21:5262021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu YY, Chang KP, Lin CY, Pai PC, Wang HM,
Hsu CL, Liao CT, Yen TC, Fang TJ, Huang SF, et al: Prognostic
significance of combined pretreatment lymphocyte counts and body
mass index in patients with head and neck cancer treated with
radiation therapy. Cancer Med. 7:2808–2815. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Locker GY, Hamilton S, Harris J, Jessup
JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF and Bast RC Jr:
ASCO 2006 update of recommendations for the use of tumor markers in
gastrointestinal cancer. J Clin Onol. 24:5313–5327. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thirunavukarasu P, Sukumar S, Sathaiah M,
Mahan M, Pragatheeshwar KD, Pingpank JF, Zeh H III, Bartels CJ, Lee
KKW and Bartlett DL: C-stage in colon cancer: implications of
carcinoembryonic antigen biomarker in staging, prognosis, and
management. J Natl Cancer Inst. 103:689–697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carriquiry LA and Piñeyro A: Should
carcinoembryonic antigen be used in the management of patients with
colorectal cancer? Dis Colon Rectum. 42:921–929. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ailawadhi S, Sunga A, Rajput A, Yang GY,
Smith J and Fakih M: Chemotherapy-induced carcinoembryonic antigen
surge in patients with metastatic colorectal cancer. Oncology.
70:49–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang CS, Chen CY, Huang LK, Wang WS and
Yang SH: Postoperative serum carcinoembryonic antigen levels cannot
predict survival in colorectal cancer patients with type II
diabetes. J Chin Med Assoc. 83:911–917. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang CS, Chen CY, Huang LK, Wang WS and
Yang SH: Prognostic value of postoperative serum carcinoembryonic
antigen levels in colorectal cancer patients who smoke. PLoS One.
15:e02336872020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kacel EL, Kirsch JL, Sannes TS, Patidar S,
Postupack R, Jensen S, Wong S, Garey S, Dodd S, Ulfig CM, et al:
Interleukin-6 and body mass index, tobacco use, and sleep in
gynecologic cancers. Health Psychol. 38:866–877. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khaodhiar L, Ling PR, Blackburn GL and
Bistrian BR: Serum levels of interleukin-6 and C-reactive protein
correlate with body mass index across the broad range of obesity.
JPEN J Parenter Enteral Nutr. 28:410–415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ullmann CD, Schlom J and Greiner JW:
Interleukin-6 increases carcinoembryonic antigen and
histocompatibility leukocyte antigen expression on the surface of
human colorectal carcinoma cells. J Immunother (1991). 12:231–241.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Belluco C, Nitti D, Frantz M, Toppan P,
Basso D, Plebani M, Lise M and Jessup JM: Interleukin-6 blood level
is associated with circulating carcinoembryonic antigen and
prognosis in patients with colorectal cancer. Ann Surg Oncol.
7:133–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bullen AW, Losowsky MS, Carter S, Patel S
and Neville AM: Diagnostic usefulness of plasma carcinoembryonic
antigen levels in acute and chronic liver disease.
Gastroenterology. 73:673–678. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang CS, Huang LK, Chen CY, Wang WS and
Yang SH: Prognostic value of postoperative serum carcinoembryonic
antigen levels in colorectal cancer patients with chronic kidney
disease. Am J Surg. 221:162–167. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
MacGregor TP, Maughan TS and Sharma RA:
Pathological grading of regression following neoadjuvant
chemoradiation therapy: The clinical need is now. J Clin Pathol.
65:867–871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu R, You YJ, Li F and Yan B:
Postoperative fasting blood glucose predicts prognosis in stage
I–III colorectal cancer patients undergoing resection.
Gastroenterol Res Prac. 2020:24824092020.PubMed/NCBI
|
|
31
|
Zhang YC, Liu Y, Qiu XM and Yan B:
Concurrent comparison of the prognostic values of tumor budding,
tumor stroma ratio, tumor infiltrating pattern and
lymphocyte-to-monocyte ratio in colorectal cancer patients. Technol
Cancer Res Treat. 20:153303382110458262021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, Zhao R, Cui Y, Zhou Y and Wu X: The
dynamic change of neutrophil to lymphocyte ratio can predict
clinical outcome in stage I–III colon cancer. Sci Rep. 8:94532018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie H, Huang S, Yuan G, Tang S and Gan J:
Prognostic significance of preoperative fibrinogen-to-prealbumin
ratio in patients with stage I–III colorectal cancer undergoing
surgical resection: A retrospective cohort study. Biomed Res Int.
2021:39053532021.PubMed/NCBI
|
|
34
|
DeLong ER, DeLong DM and Clarke-Pearson
DL: Comparing the areas under two or more correlated receiver
operating characteristic curves: A nonparametric approach.
Biometrics. 44:837–845. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Beom SH, Shin SJ, Kim CG, Kim JH, Hur H,
Min BS, Lee KY, Kim NK and Ahn JB: Clinical significance of
preoperative serum carcinoembryonic antigen within the normal range
in colorectal cancer patients undergoing curative resection. Ann
Surg Oncol. 27:2774–2783. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cai Z, Xiao J, He X, Ke J, Zou Y, Chen Y,
Wu X, Li X, Wang L, Wang J, et al: Accessing new prognostic
significance of preoperative carcinoembryonic antigen in colorectal
cancer receiving tumor resection: More than positive and negative.
Cancer Biomark. 19:161–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Z, Zhang Y, Niu Y, Li K, Liu X, Chen H
and Gao C: A systematic review and meta-analysis of diagnostic and
prognostic serum biomarkers of colorectal cancer. PLoS One.
9:e1039102014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tumay V and Guner OS: The utility and
prognostic value of CA 19-9 and CEA serum markers in the long-term
follow up of patients with colorectal cancer. A single-center
experience over 13 years. Ann Ital Chir. 91:494–503.
2020.PubMed/NCBI
|
|
39
|
Björkman K, Mustonen H, Kaprio T, Kekki H,
Pettersson K, Haglund C and Böckelman C: CA125: A superior
prognostic biomarker for colorectal cancer compared to CEA, CA19-9
or CA242. Tumour Biol. 43:57–70. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Iacuzzo C, Germani P, Troian M, Mis TC,
Giudici F, Osenda E, Bortul M and de Manzini N: Serum
carcinoembryonic antigen pre-operative level in colorectal cancer:
Revisiting risk stratification. ANZ J Surg. 91:E367–E374. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Xiong Z, Xie M, Huang Q, Jin L, Yin
S, Chen S, Lan P and Lian L: Prognostic value of the ratio of
carcinoembryonic antigen concentration to maximum tumor diameter in
patients with stage II colorectal cancer. J Gastrointest Oncol.
12:1470–1481. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xie W, Huang X, Wei C, Mo X, Ru H, Zhang
L, Ge L, Tang W and Liu J: Preoperative neutrophil-BMI ratio as a
promising new marker for predicting tumor outcomes in colorectal
cancer. Technol Cancer Res Treat. Feb 28–2022.(Epub ahead of
print). View Article : Google Scholar
|
|
43
|
Baqar AR, Wilkins S, Staples M, Lee CH,
Oliva K and McMurrick P: The role of preoperative CEA in the
management of colorectal cancer: A cohort study from two cancer
centres. Int J Surg. 64:10–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gold P and Freedman SO: Demonstration of
tumor-specific antigens in huamn colonic carcinoma by immunological
tolerance and absorption techniques. J Exp Med. 121:439–462. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Goldstein MJ and Mitchell EP:
Carcinoembryonic antigen in the staging and follow-up of patients
with colorectal cancer. Cancer Invest. 23:338–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bajenova O, Gorbunova A, Evsyukov I, Rayko
M, Gapon S, Bozhokina E, Shishkin A and O'Brien SJ: The genome-wide
analysis of carcinoembryonic antigen signaling by colorectal cancer
cells using RNA sequencing. PLoS One. 11:e01612562016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang EY, Chang JC, Chen HH, Hsu CY, Hsu
HC and Wu KL: Carcinoembryonic antigen as a marker of
radioresistance in colorectal cancer: A potential role of
macrophages. BMC Cancer. 18:3212018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee J, Meyerhardt JA, Giovannucci E and
Jeon JY: Association between body mass index and prognosis of
colorectal cancer: A meta-analysis of prospective cohort studies.
PLoS One. 10:e01207062015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Daniel CR, Shu X, Ye Y, Gu J, Raju GS,
Kopetz S and Wu X: Severe obesity prior to diagnosis limits
survival in colorectal cancer patients evaluated at a large cancer
centre. Br J Cancer. 114:103–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Meier CR, Krähenbühl S, Schlienger RG and
Jick H: Association between body mass index and liver disorders: An
epidemiological study. J Hepatol. 37:741–747. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Salvaggio A, Periti M, Miano L, Tavanelli
M and Marzorati D: Body mass index and liver enzyme activity in
serum. Clin Chem. 37:720–723. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jessup JM, Laguinge L, Lin S, Samara R,
Aufman K, Battle P, Frantz M, Edmiston KH and Thomas P:
Carcinoembryonic antigen induction of IL-10 and IL-6 inhibits
hepatic ischemic/reperfusion injury to colorectal carcinoma cells.
Int J Cancer. 111:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Han J, Meng Q, Shen L and Wu G:
Interleukin-6 induces fat loss in cancer cachexia by promoting
white adipose tissue lipolysis and browning. Lipids Health Dis.
17:142018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Carson JA and Baltgalvis KA: Interleukin 6
as a key regulator of muscle mass during cachexia. Exerc Sport Sci
Rev. 38:168–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Webster JM, Kempen LJAP, Hardy RS and
Langen RCJ: Inflammation and skeletal muscle wasting during
cachexia. Front Physiol. 11:5976752020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lino-Silva LS, Anchondo-Nunez P,
Chit-Huerta A, Aguilar-Romero E, Morales-Soto J, Salazar-Garcia JA,
Guzman-Lopez CJ, Maldonado-Martinez HA, Meneses-Garcia A and
Salcedo-Hernández RA: Stage I–III colon cancer patients with tumor
deposits behave similarly to stage IV patients. Cross-section
analysis of 392 patients. J Surg Oncol. 120:300–307.
2019.PubMed/NCBI
|
|
58
|
Moon JY, Lee MR and Ha GW: Prognostic
value of tumor deposits for long-term oncologic outcomes in
patients with stage III colorectal cancer: A systematic review and
meta-analysis. Int J Colorectal Dis. 37:141–151. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Naszai M, Kurjan A and Maughan TS: The
prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio
(NLR) in colorectal cancer: A systematic review and meta-analysis.
Cancer Med. 10:5983–5997. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gkekas I, Novotny J, Fabian P, Nemecek R,
Palmqvist R, Strigard K, Pecen L, Svoboda T, Gurlich R and
Gunnarsson U: Deficient mismatch repair as a prognostic marker in
stage II colon cancer patients. Eur J Surg Oncol. 45:1854–1861.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang B, Li F, Zhou X, Ma Y and Fu W: Is
microsatellite instability-high really a favorable prognostic
factor for advanced colorectal cancer? A meta-analysis. World J
Surg Oncol. 17:1692019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Flaherty DC, Jalas JR, Sim MS,
Stojadinovic A, Protic M, Lee DJ and Bilchik AJ: The negative
impact of body mass index on the tumor microenvironment in colon
cancer: Results of a prospective trial. Ann Surg Oncol.
25:1374–1380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Søreide K, Søreide JA and Kørner H:
Prognostic role of carcinoembryonic antigen is influenced by
microsatellite instability genotype and stage in locally advanced
colorectal cancers. World J Surg. 35:888–894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
André T, Meyerhardt J, Iveson T, Sobrero
A, Yoshino T, Souglakos I, Grothey A, Niedzwiecki D, Saunders M,
Labianca R, et al: Effect of duration of adjuvant chemotherapy for
patients with stage III colon cancer (IDEA collaboration): final
results from a prospective, pooled analysis of six randomised,
phase 3 trials. Lancet Oncol. 21:1620–1629. 2020. View Article : Google Scholar : PubMed/NCBI
|