Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-related deaths worldwide, accounting for
approximately 80–90% of primary liver cancer (1). Despite the progression in treatment
strategies, the prognosis of HCC remains poor (2). Therefore, elucidation of the
molecular mechanism of HCC progression may contribute to better
prognosis.
L-type amino acid transporter 3 (LAT3) is a system
L-amino acid transporter that transports neutral amino acids such
as leucine, isoleucine, valine, phenylalanine, and methionine
(3). System L-amino acid
transporters transport large branched and aromatic neutral amino
acids in almost all cell types independent of Na+
(4). These transporters provide
cancer cells with the essential amino acids required for protein
synthesis and growth stimulation (5). The liver is the central organ for
amino acid metabolism, and the importance of amino acid metabolism
in HCC has been reported in recent years (6). Regarding cancer progression, LAT3 is
highly expressed in primary and recurrent prostate cancer, and
upregulated LAT3 levels result in increased intracellular leucine
levels and subsequent cell proliferation in prostate cancer cells
(7,8). However, the role of LAT3 in HCC
remains unclear.
Multiple kinase signaling pathways including the
PI3K/AKT pathway play central roles in the development of various
cancers (9). Furthermore, it was
reported that activation of PI3K/AKT signaling can affect the
proliferation, invasion, and apoptosis of HCC cells (10). AKT is a serine/threonine protein
kinase that plays a pivotal role in regulating diverse biological
functions, including protein synthesis, cell metabolism, cell
survival, and cell cycle progression, following its phosphorylation
(11). Phosphorylated-AKT (p-AKT)
was reported to stabilize LAT3 protein levels and subsequent cell
proliferation in prostate cancer cell lines (12). Therefore, we hypothesized that high
expression of LAT3 is associated with poor prognosis in HCC and
that p-AKT activation is the mechanism of its malignant
potential.
In the current study, we investigated the
relationship between LAT3 expression and the clinicopathological
factors of HCC including prognosis by immunohistochemical analysis,
and investigated its mechanism with respect to p-AKT
expression.
Materials and methods
Patients
One hundred thirty-five patients with histologically
proved HCC who underwent hepatectomy at Tokushima University
Hospital between January 2008 and December 2017 were enrolled in
this study. In this patient cohort, median and range of age was 68
(33–88) years old. We included patients who: (a) had no history of
treatment prior to surgery; and (b) had no extrahepatic metastasis.
Pathological and morphological parameters and Japanese
Tumor-Node-Metastasis stage were determined in accordance with the
Liver Cancer Study Group of Japan (13). This study was approved by the
institutional review board of our institute (no. 4144).
Immunohistochemical analysis and
evaluation
Immunohistochemical analysis was performed in
accordance with the protocol used in our department, which has been
previously reported by Ishikawa et al (14). Briefly, sections were
deparaffinized with xylene, followed by rehydration in a graded
ethanol series. The sections were treated with 3% hydrogen peroxide
in methanol for 10 min to quench the endogenous peroxidase
activity. Antigen retrieval was performed by boiling in 10 mM
citrate buffer (pH 6) using a microwave. After incubation with 1%
bovine serum albumin to block nonspecific antibody binding, the
sections were incubated with primary antibodies against rabbit
monoclonal LAT3 antibody (1:100 dilution, NBP1-87332; Novus, CO,
USA) and rabbit polyclonal phospho-AKT antibody (1:100 dilution,
ab81283; Abcam, Tokyo, Japan) for 60 min at room temperature. After
washing with phosphate-buffered saline, the sections were subjected
to the Dako REAL EnVision/HRP detection system (Dako Corporation,
Tokyo, Japan) for 60 min at room temperature. The peroxidase
reaction was developed with 3,3′-diaminobenzidine as the chromogen.
The sections were counterstained with 10% Mayer's hematoxylin,
dehydrated in a graded series of ethanol, treated with xylene, and
mounted in a synthetic resin. LAT3 expression scores were assessed
based on the extent of obvious staining in the cell membrane or
cytoplasm, as follows: 0, <5% of the tumor area stained; 1,
5–10% stained; 2, 11–25% stained; 3, 26–50% stained; and 4, ≥51%
stained. Tumors in which the stained tumor cells were scored >1
were considered to show positive expression (15). Regarding p-AKT staining, when
>10% of the tumor cells were stained in the cytoplasm and/or
nucleus, the samples were considered positive (16). Microscopic observation was
performed using an Olympus BX43 (Tokyo, Japan) with UPlanFL N
objective lenses (4×/0.13, 10×/0.30, 20×/0.5 and 40×/0.75; Olympus)
at room temperature, and then images were acquired using camera
(DP27, Olympus) and cellSens Standard software (version
1.17.16030.0, Olympus). Representative images of positive and
negative expression of LAT3 and p-AKT are shown in Fig. 1. Fig.
1A showed LAT3 expression in tumor tissue and adjacent normal
tissue from the same patient, and LAT3 was highly expressed in
tumor tissue compared with adjacent normal tissue.
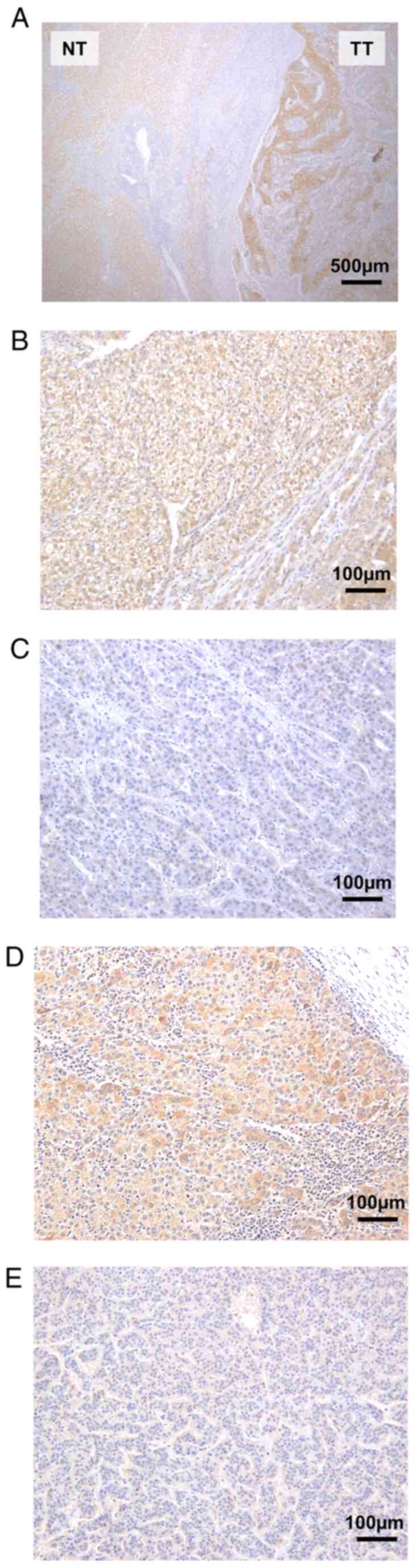 | Figure 1.Representative images of
immunohistochemical analysis. (A) LAT3 expression in TT and
adjacent NT (magnification, ×40; scale bar, 500 µm). (B) LAT3-high.
(C) LAT3-low (magnification, ×200; scale bar, 100 µm). (D) p-Akt
positive. (E) p-Akt negative (magnification, ×200; scale bar, 100
µm). LAT3, L-type amino acid transporter 3; NT, normal tissue;
p-AKT, phosphorylated AKT; TT, tumor tissue. |
Statistical analysis
The unpaired Mann-Whitney U test or the chi-squared
test was used to compare clinicopathological factors between
LAT3-high and -low expression groups. Cancer-specific survival and
disease-free survival curves were obtained using the Kaplan-Meier
method, and differences were compared using the log-rank test in
univariate analysis. Multivariate analysis was conducted using the
Cox proportional hazard regression model. For all statistical
analyses, P<0.05 was considered significant. All
statistical analyses were performed using JMP 8.0.1 statistical
software (SAS Campus Drive, Cary, NC, USA).
Results
Clinicopathological factors
On the basis of the immunohistochemical analysis,
135 patients were divided into LAT3-high (n=46) and LAT3-low (n=89)
groups. Table I summarizes the
clinicopathological factors of the patients in both groups. The
LAT3-high group showed significantly higher levels of protein
induced by vitamin K absence-II (PIVKA-II) (P<0.05). There were
no significant differences in terms of age, sex, viral infection
status, and other tumor factors between the two groups.
 | Table I.Patient characteristics in the
LAT3-high and LAT3-low expression groups. |
Table I.
Patient characteristics in the
LAT3-high and LAT3-low expression groups.
| Variable | High (n=46) | Low (n=89) | P-value |
|---|
| Age, years | 66.6±10.5 | 68.4±9.9 | 0.515 |
| Sex
(male/female) | 30/16 | 66/23 | 0.281 |
| HBV (−/+) | 30/16 | 70/19 | 0.096 |
| HCV (−/+) | 28/18 | 58/31 | 0.623 |
| Child-Pugh
(5/>6) | 36/10 | 75/14 | 0.392 |
| Growth pattern
(Eg/Ig) | 42/4 | 75/14 | 0.240 |
| Tumor size (<2/≥2
cm) | 13/33 | 19/70 | 0.375 |
| Tumor number
(single/multi) | 37/9 | 75/14 | 0.578 |
| Differentiation
(poorly/others) | 2/44 | 9/80 | 0.331 |
| Portal vein invasion
(−/+) | 33/13 | 75/14 | 0.090 |
| Stage
(I/II/III/IV) | 10/16/17/3 | 15/48/23/3 | 0.194 |
| AFP (<100/>100
ng/ml) | 31/15 | 69/20 | 0.208 |
| PIVKA-II
(<400/>400 mAU/ml) | 25/21 | 67/22 | 0.014 |
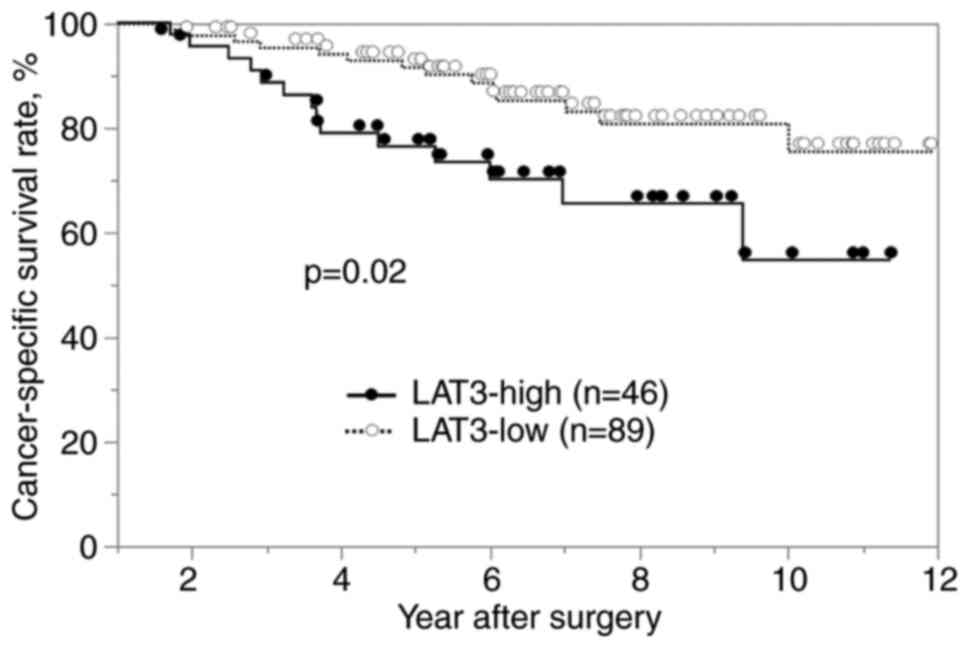
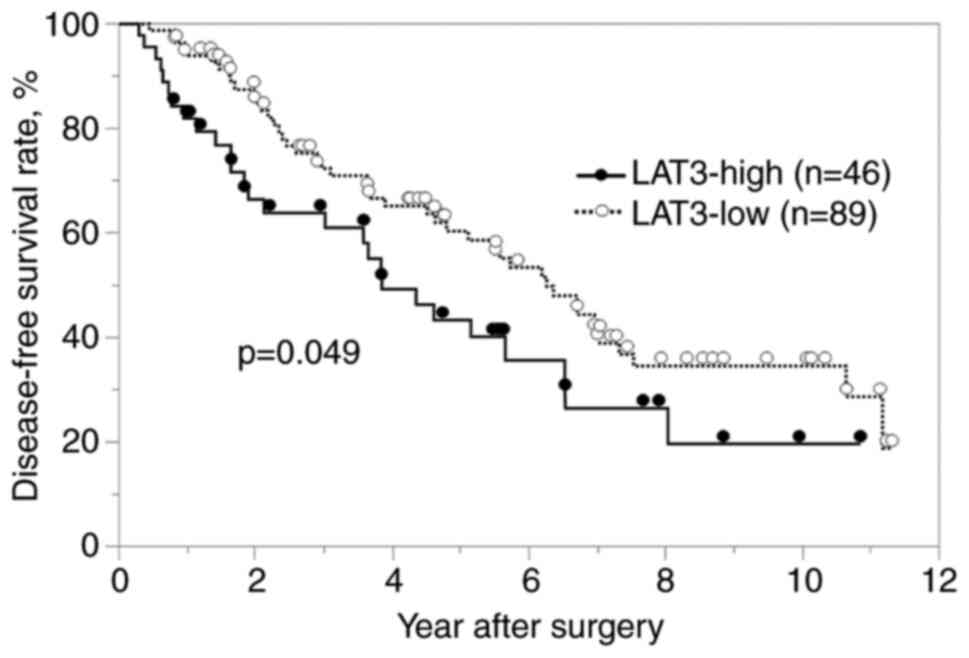
Cancer-specific and disease-free
survival
The cancer-specific and disease-free survival rates
were significantly worse in the LAT3-high group than in the
LAT3-low group (P<0.05; Figs. 2
and 3). In the univariate analysis
of cancer-specific survival, multiple tumors, portal vein invasion,
PIVKA-II level (>400 mAU/ml) and high LAT3 expression were
defined as prognostic factors. In the multivariate analysis,
multiple tumors and high LAT3 expression were determined to be
independent prognostic factors (Table
II). In the univariate analysis of disease-free survival,
multiple tumors, high PIVKA-II level (>400 mAU/ml) and high LAT3
expression were defined as prognostic factors. In the multivariate
analysis, multiple tumors and high PIVKA-II level (>400 mAU/ml)
were determined to be independent prognostic factors (Table III), but high LAT3 expression was
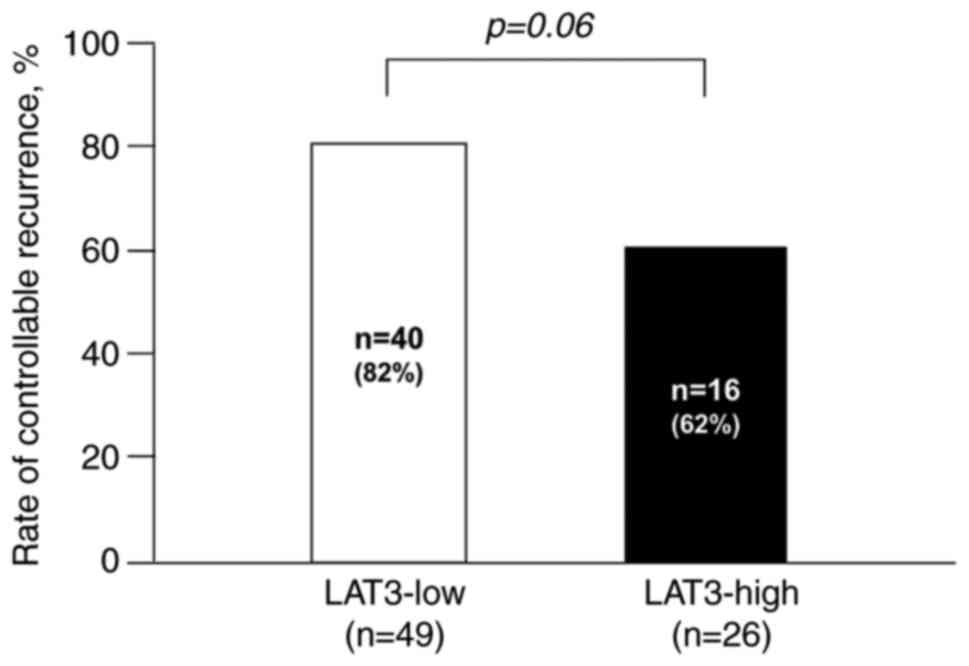
not. Regarding recurrence, the rate of controllable recurrence with
local therapy tended to be higher in the LAT3-high group than in
the LAT3-low group (P=0.06) (Fig.
4).
 | Table II.Multivariate analysis of
cancer-specific survival. |
Table II.
Multivariate analysis of
cancer-specific survival.
| Variable | 3-year survival rate,
% | Univariate
P-value | Hazard ratio (95%
CI) | Multivariate
P-value |
|---|
| Age (<70/≥70
years) | 90.1/87.4 | 0.372 | 1.880
(0.835-4.231) | 0.127 |
| Sex
(male/female) | 90.2/86.3 | 0.945 | 0.919
(0.381-2.216) | 0.850 |
| Child-Pugh
(5/>6) | 88.5/91.3 | 0.961 | 0.841
(0.297-2.381) | 0.744 |
| Growth pattern
(Eg/Ig) | 90.1/80.8 | 0.201 | 1.549
(0.545-4.398) | 0.411 |
| Tumor size (<2/≥2
cm) | 89.7/87.6 | 0.351 | 1.537
(0.516-4.577) | 0.440 |
| Tumor number
(single/multi) | 93.5/66.0 | <0.001 | 4.169
(1.686-10.310) | 0.002 |
| Differentiation
(poorly/others) | 80.8/89.8 | 0.941 | 1.359
(0.302-6.113) | 0.689 |
| Portal vein
invasion (−/+) | 92.2/76.6 | 0.025 | 2.295
(0.871-6.044) | 0.093 |
| AFP
(<100/>100 mIU) | 91.5/81.9 | 0.539 | 0.539
(0.192-1.510) | 0.240 |
| PIVKA-II
(<400/>400 mAU) | 93.1/80.7 | 0.040 | 1.233
(0.490-3.103) | 0.656 |
| LAT3
(low/high) | 94.2/78.8 | 0.020 | 2.558
(1.105-5.922) | 0.028 |
 | Table III.Multivariate analysis of disease-free
survival. |
Table III.
Multivariate analysis of disease-free
survival.
| Variable | 3-year survival
rate, % | Univariate
P-value | Hazard ratio (95%
CI) | Multivariate
P-value |
|---|
| Age (<70/≥70
years) | 67.2/73.0 | 0.915 | 0.986
(0.596-1.631) | 0.955 |
| Sex
(male/female) | 67.3/76.1 | 0.142 | 0.679
(0.387-1.193) | 0.178 |
| Child-Pugh
(5/>6) | 71.7/57.4 | 0.330 | 1.343
(0.692-2.608) | 0.384 |
| Growth pattern
(Eg/Ig) | 70.2/62.5 | 0.945 | 0.779
(0.330-1.838) | 0.568 |
| Tumor size
(<2/≥2 cm) | 68.5/69.0 | 0.101 | 0.570
(0.320-1.015) | 0.056 |
| Tumor number
(single/multi) | 73.4/48.2 | 0.021 | 2.372
(1.192-4.720) | 0.014 |
| Differentiation
(poorly/others) | 63.5/70.1 | 0.357 | 0.747
(0.225-2.481) | 0.634 |
| Portal vein
invasion (−/+) | 69.1/73.5 | 0.906 | 0.954
(0.446-2.039) | 0.903 |
| AFP
(<100/>100 mIU) | 68.4/73.9 | 0.172 | 0.555
(0.274-1.124) | 0.102 |
| PIVKA-II
(<400/>400 mAU) | 77.7/52.1 | 0.024 | 2.340
(1.259-4.349) | 0.007 |
| LAT3
(low/high) | 72.7/64.2 | 0.049 | 1.471
(0.883-2.453) | 0.139 |
p-Akt expression
The LAT3-high group showed a significantly higher
rate of p-AKT positivity compared with the LAT3-low group
(P<0.01; Fig. 5).
Discussion
The present study evaluated the clinical
significance of LAT3 expression in HCC. The LAT family of proteins
consists of four Na+-independent neutral amino acid transporters.
The members of this family are divided into two sub-families:
namely, SLC7 (LAT1 and LAT2) and SLC43 (LAT3 and LAT4) (3). In cancer progression, amino acid
uptake is essential for cell proliferation. LAT1 is the most
commonly known transporter regarding cancer and transports large
neutral amino acids, such as leucine, isoleucine, valine,
phenylalanine, tyrosine, tryptophan, methionine, and histidine.
However, LAT3 transports a narrow range of neutral amino acids,
including leucine, isoleucine, valine, phenylalanine, and
methionine (3). In particular,
leucine plays pivotal roles in cancer progression, not only in
protein synthesis but as a signaling factor (4). Compared with LAT1, LAT3 shows a more
restricted expression pattern in some types of cancer, although
reports are increasing about prostate cancer and leukemia. Rii
et al (17) reported that
high LAT3 expression was related to poor prognosis after
prostatectomy, and knockdown of SLC43A1, which encodes LAT3,
suppressed the proliferation of prostate cancer cells and arrested
the cell cycle. Xu et al (18) showed that cellular proliferation
and the mTOR pathway, which activates AKT, were significantly
reduced when LAT3 was blocked. There is also a report of LAT3
expression in HepG2 HCC cells (19); however, there is no report
regarding LAT3 expression in clinical HCC samples. To our
knowledge, this is the first report about LAT3 correlated with
prognosis in HCC.
LAT3 is an amino acid transporter that uptakes
neutral amino acids, most of which are essential amino acids. Among
these amino acids, leucine has been reported to promote
myofibroblast differentiation via the AKT signaling pathway
(20). Because the AKT signaling
pathway is correlated with cell survival, cell cycle progression,
and apoptosis inhibition (11),
AKT signaling was investigated in this study. Zhang et al
(12) reported that LAT3 protein
levels were increased when AKT was phosphorylated, and that AKT and
LAT3 were co-localized on the plasma membrane in prostate cancer
cell lines. They confirmed that activated PI3K/AKT signaling
regulates leucine transport through LAT3 in prostate cancer. In
this study, the LAT3-high expression group showed a significantly
higher rate of p-AKT positivity. Therefore, LAT3 expression might
accelerate the malignant potential of HCC via AKT signaling. In
this study, the LAT3-high group showed significantly higher
PIVKA-II levels. PIVKA-II is commonly known as a prognostic factor
in HCC (21). In fact, the
LAT3-high group showed a worse cancer-specific survival rate
compared with the LAT-3-low group. Although there was no
significant difference in the disease-free survival rate between
the two groups, more aggressive recurrence tended to occur in the
LAT3-high group. This may have promoted the worse cancer-specific
survival in the LAT3-high group. Thus, high LAT3 expression seemed
to be related to the malignant potential of HCC.
In terms of LAT inhibitors, JPH203 has already been
reported to inhibit LAT1, and clinical trials are ongoing in
various cancers, including biliary and breast cancer (22). Regarding LAT3, ESK246 from
Pittosporum has been reported to inhibit LAT-mediated
leucine transport in prostate cancer cells compared with leucine
analog (23). This inhibitor has
potential as a new anti-HCC agent.
This study has several limitations. First, we only
investigated LAT3 and p-AKT expression in resected specimens by
immunohistochemistry. In this study sample, number of specimens
which was available for RT-PCR was small. We plan to perform an
in vivo study using an HCC cell line with an inhibitor such
as ESK246. Second, this was a single-center study and the number of
cases was relatively small. Thus, prospective studies in larger
patient populations are necessary in the future.
In conclusion, LAT3 expression was associated with
the poor prognosis of HCC and higher expression of p-AKT.
Acknowledgements
The authors would like to thank Ms. Yoshie Hara
(Department of Surgery, Tokushima University, Tokushima, Tokushima,
Japan) for her assistance with the immunohistochemistry studies.
The authors would also like to thank Dr H. Nikki March for editing
a draft of this manuscript.
Funding
The present study was supported by the Japan Agency for Medical
Research and Development (AMED) (grant no. JP19fk0210048 and
JP20fk0210048) and Grant-in-Aid for Scientific Research (grant no.
20K08957).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BS and SY designed the experiments, acquired,
analyzed and interpreted the data, and drafted and revised the
manuscript. TI, YS, CT and HT acquired and analyzed the data, and
revised the manuscript. YM and MS designed the experiments and
revised the manuscript. All authors have read and approved the
final manuscript. BS and SY confirm the authenticity of raw
data.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Tokushima University Hospital (Tokushima, Japan; no.
4144). The requirement for informed consent was waived, and an
information disclosure statement was uploaded onto the homepage of
our hospital website for opt-out.
Patient consent for publication
An information disclosure statement that the text,
data and images are published, and they will be freely available on
the internet and may be seen by the general public was uploaded
onto the homepage of the hospital website for opt-out.
Competing interests
MS declares receiving an unrestricted research grant
from Bayer Yakuhin, Co. Ltd., Japan. All other authors declare that
they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
LAT3
|
L-type amino acid transporter 3
|
|
HCC
|
hepatocellular carcinoma
|
|
p-AKT
|
phosphorylated AKT
|
|
PIVKA-II
|
protein induced by vitamin K
absence-II
|
|
AFP
|
α-fetoprotein
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lan T, Yuan K, Yan X, Xu L, Liao H, Hao X,
Wang J, Liu H, Chen X, Xie K, et al: LncRNA SNHG10 facilitates
hepatocarcinogenesis and metastasis by modulating its homolog
SCARNA13 via a positive feedback loop. Cancer Res. 79:3220–3234.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Q and Holst J: L-type amino acid
transport and cancer: Targeting the mTORC1 pathway to inhibit
neoplasia. Am J Cancer Res. 5:1281–1294. 2015.PubMed/NCBI
|
|
4
|
Kanai Y: Amino acid transporter LAT1
(SLC7A5) as a molecular target for cancer diagnosis and
therapeutics. Pharmacol Ther. 230:1079642022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaira K, Takahashi T, Murakami H, Shukuya
T, Kenmotsu H, Naito T, Oriuchi N, Kanai Y, Endo M, Kondo H, et al:
Relationship between LAT1 expression and response to platinum-based
chemotherapy in non-small cell lung cancer patients with
postoperative recurrence. Anticancer Res. 31:3775–3782.
2011.PubMed/NCBI
|
|
6
|
Zhao Y, Zhang J, Wang S, Jiang Q and Xu K:
Identification and validation of a nine-gene amino acid
metabolism-related risk signature in HCC. Front Cell Dev Biol.
9:7317902021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Q, Bailey CG, Ng C, Tiffen J, Thoeng
A, Minhas V, Lehman ML, Hendy SC, Buchanan G, Nelson CC, et al:
Androgen receptor and nutrient signaling pathways coordinate the
demand for increased amino acid transport during prostate cancer
progression. Cancer Res. 71:7525–7536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Q, Tiffen J, Bailey CG, Lehman ML,
Ritchie W, Fazli L, Metierre C, Feng YJ, Li E, Gleave M, et al:
Targeting amino acid transport in metastatic castration-resistant
prostate cancer: effects on cell cycle, cell growth, and tumor
development. J Natl Cancer Inst. 105:1463–1473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu P, Liu T and Hu Y: PI3K inhibitors for
cancer therapy: What has been achieved so far? Curr Med Chem.
16:916–930. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin E, Choi CM, Kim HR, Jang SJ and Park
YS: Immunohistochemical characterization of the mTOR pathway in
stage-I non-small-cell lung carcinoma. Lung Cancer. 89:13–18. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang BK, Moran AM, Bailey CG, Rasko JE,
Holst J and Wang Q: EGF-activated PI3K/Akt signalling coordinates
leucine uptake by regulating LAT3 expression in prostate cancer.
Cell Commun Signal. 17:832019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liver Cancer Study group of Japan, . The
general rules for the clinical and pathological study of primary
liver cancer. 3rd English edition. Tokyo: Kanehara & Co., Ltd;
2010
|
|
14
|
Ishikawa D, Shimada M, Utsunomiya T,
Morine Y, Imura S, Ikemoto T, Arakawa Y, Kanamoto M, Iwahashi S,
Saito Y, et al: Effect of Twist and Bmi1 on intraductal papillary
mucinous neoplasm of the pancreas. J Gastroenterol Hepatol.
29:2032–2037. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Namikawa M, Kakizaki S, Kaira K, Tojima H,
Yamazaki Y, Horiguchi N, Sato K, Oriuchi N, Tominaga H, Sunose Y,
et al: Expression of amino acid transporters (LAT1, ASCT2 and xCT)
as clinical significance in hepatocellular carcinoma. Hepatol Res.
45:1014–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmitz KJ, Wohlschlaeger J, Lang H,
Sotiropoulos GC, Malago M, Steveling K, Reis H, Cicinnati VR,
Schmid KW and Baba HA: Activation of the ERK and AKT signalling
pathway predicts poor prognosis in hepatocellular carcinoma and ERK
activation in cancer tissue is associated with hepatitis C virus
infection. J Hepatol. 48:83–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rii J, Sakamoto S, Sugiura M, Kanesaka M,
Fujimoto A, Yamada Y, Maimaiti M, Ando K, Wakai K, Xu M, et al:
Functional analysis of LAT3 in prostate cancer: Its downstream
target and relationship with androgen receptor. Cancer Sci.
112:3871–3883. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu SM, Tang K, Meng L and Tang Y:
Suppression of amino acid transporter LAT3 expression on
proliferation of K562 cells. J Huazhong Univ Sci Technolog Med Sci.
33:632–635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ritchie JW and Taylor PM: Tryptophan and
iodothyronine transport interactions in HepG2 human hepatoma cells.
Amino Acids. 38:1361–1367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang S, Chen X, Huang Z, Chen D, Yu B,
Chen H, Luo J, He J, Zheng P and Yu J: Leucine promotes
differentiation of porcine myoblasts through the protein kinase B
(Akt)/Forkhead box O1 signalling pathway. Br J Nutr. 119:727–733.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nanashima A, Morino S, Yamaguchi H, Tanaka
K, Shibasaki S, Tsuji T, Hidaka S, Sawai T, Yasutake T and Nakagoe
T: Modified CLIP using PIVKA-II for evaluating prognosis after
hepatectomy for hepatocellular carcinoma. Eur J Surg Oncol.
29:735–742. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okano N, Naruge D, Kawai K, Kobayashi T,
Nagashima F, Endou H and Furuse J: First-in-human phase I study of
JPH203, an L-type amino acid transporter 1 inhibitor, in patients
with advanced solid tumors. Invest New Drugs. 38:1495–1506. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Q, Grkovic T, Font J, Bonham S,
Pouwer RH, Bailey CG, Moran AM, Ryan RM, Rasko JE, Jormakka M, et
al: Monoterpene glycoside ESK246 from Pittosporum targets
LAT3 amino acid transport and prostate cancer cell growth. ACS Chem
Biol. 9:1369–1376. 2014. View Article : Google Scholar : PubMed/NCBI
|