Introduction
Malignant tumors threaten human life worldwide.
Treating malignant tumors includes surgery, chemotherapy, targeted
therapy, immunotherapy and, importantly, radiation therapy. In
recent years, the effect of radiation therapy has been demonstrated
repeatedly. Radiotherapy is mainly applied to local solid tumors,
such as head and neck tumors, skin cancer, lymphoma, lung cancer,
esophageal cancer, etc. It may also be used as an adjuvant
treatment, combined with chemotherapy and surgery, for the
treatment of breast cancer, cervical cancer, gastrointestinal
tumors, etc. For cancers of the blood, radiotherapy has little
effect. However, its further clinical application has been
substantially restricted as it damages normal tissues. Radiation
therapy uses an external radiation beam, which sends radiation into
the body tissue and causes damage (1). When deep tumors are irradiated,
healthy pre-tumor normal tissue receives a higher dose of ionizing
radiation than tumor tissue, resulting in extra damage. In
addition, for certain superficial tumors, if the radiation passes
directly through the surface of the tumor, normal tissue behind the
tumor may also be exposed to radiation, although the tumor is
directly irradiated. In either case, the normal tissues,
particularly those tissues or organs sensitive to radiation, are
severely damaged. Thus, the dose-limiting toxicity of normal
tissues is one of the significant obstacles to the development of
tumor radiotherapy (2).
Current directions of clinical radiotherapy
technology developments are to improve the ability of radiotherapy
machines and change the local mode of radiotherapy to maximize the
accuracy of irradiation of tumor tissue, avoiding damage to normal
tissue. It is exciting that FLASH radiotherapy (FLASH-RT), as an
ultra-high dose rate (UHDR) radiotherapy, has been proposed as a
new method in recent years. The FLASH effect was first reported in
1959. However, due to technical limitations, it was relatively
challenging to translate the results into clinical practice and
further research on this new radiotherapy was severely limited
(3,4). However, due to the improvement of
radiotherapy equipment technology, in-depth progression of
radiation source research and advances in radiobiology research,
the great potential of FLASH-RT has been recognized again as a tool
for clinical radiotherapy. FLASH-RT is a new non-invasive external
irradiation radiotherapy technology that is able to extend
patients' treatment window and significantly change the
radiotherapy and tumor treatment pattern by delivering ultra-high
dose rays at an UHDR (5). Compared
with conventional radiotherapy (Con-RT), FLASH-RT delivers doses
higher than 8 Gy in a short time (<1 sec) and the dose rate may
exceed 50 Gy/sec (1). In several
studies, radiation toxicity to normal surrounding healthy tissues
was significantly reduced and tumor growth was inhibited, with a
degree of tumor control similar to that of traditional dose rate
irradiation. While certain researchers remain skeptical about the
effectiveness of FLASH-RT in cancer patients, it is widely thought
that FLASH irradiation holds substantial promise for the future and
is perhaps the most significant recent discovery in the history of
radiation therapy (5). In the
present review, the research progress of FLASH-RT is summarized and
its various special influences, including the research status of
FLASH-RT and its influencing factors, are outlined. At the same
time, the mechanism of FLASH-RT is also summarized, which provides
insight for future cancer treatment.
Effect of FLASH
Normal tissue responses to
FLASH-RT
UHDR irradiation damages normal tissues to a lesser
extent than Con-RT. In 1959, the FLASH effect was observed for the
first time. Dewey and Boag (6)
found that when bacteria were subjected to large-pulse electron
radiation, radiosensitivity was reduced as compared to ordinary
dose rate irradiation. In 1967, Town (7) obtained the corresponding rays in the
form of pulse waves and improved the radiation dose rate by
shortening the time. These results were consistent with Dewey and
Boag (6). They also indicated that
damage to normal tissue decreased with increasing and constant
total doses. This reduction was confirmed in the 1970s by Field and
Bewley (8), as well as Hornsey and
Alper (4), using mouse intestines.
In 1969, Berry et al (9)
demonstrated that normal mammalian cells exposed to UHDR
irradiation had more robust viability than those exposed to
conventional dose rate irradiation. In 1971, Hornsey and Bewley
(10) reported that a high dose
rate electron beam (60 Gy/min and above) caused tissue hypoxia,
reducing the radiosensitivity of tissues. The research on the FLASH
effect in the 1960s and 1970s was not successfully translated into
clinical applications and research stagnated again.
In 2014, Favaudon et al (11) evaluated the effects of FLASH UHDR
or conventional dose rate irradiation on lung tissue by local
irradiation of mice. The results indicated that all mice irradiated
at the conventional dose rate of 17 Gy developed severe pneumonia
and fibrosis. This was the opposite of the result in
FLASH-irradiated mice; no pneumonia or fibrosis was observed in
these mice after the same dose of FLASH. When the dose was
increased to 30 Gy, FLASH began to induce pneumonia and fibrosis.
The authors found that 17-Gy FLASH irradiation prevented the
activation of TGF-β and acute apoptosis of bronchi and blood
vessels.
In 2017, Montay-Gruel et al (12) performed several studies on the
effects of FLASH-RT on brain tissues and found that spatial memory
was significantly impaired after irradiation with a total dose of
10 Gy at a conventional dose rate of 0.1 Gy/sec. However, the
spatial memory was significantly protected when the average dose
rate of radiation was >100 Gy/sec. Even 2 months after the mice
received radiotherapy, the animals' ability to recognize new
objects was significantly better in the FLASH-RT group than in the
conventional dose rate group. In addition, the discrimination of
newborn neurons in the mouse hippocampus indicated that the
protective effect of FLASH-RT on nerve regeneration depended on the
protective effect of neural stem cells. Further studies were
performed in 2018 and 2019; these concluded that FLASH-RT had a
more substantial protective effect on normal brain tissue than
conventional dose rate radiotherapy (13,14).
Alaghband et al (15) indicated that, compared with
conventional dose rate (0.077 Gy/sec, 6 MeV electron) irradiation,
irradiation of the whole brain of mice with an UHDR
(4.4×106 Gy/sec; 6 MeV beam) had an evident radiation
protection FLASH effect. As the animals passed further cognitive
tests, the authors observed no significant difference in the brains
of the FLASH-irradiated mice compared to the control group, while
the brains of the mice exposed to conventional dose rates were
significantly damaged. Mice irradiated at conventional dose rates
has considerably lower levels of immature and mature neurons after
4 months. Regarding pituitary function, the authors found a
two-fold decrease in plasma growth hormone in mice exposed to
conventional dose rates but not in mice exposed to FLASH. These
findings illustrate the benefits of FLASH irradiation over
conventional dose rate irradiation. In large mammals, a phase I
single-dose escalation trial (25–41 Gy) studied six cats with
locally advanced T2/T3N0M0 nasal plane squamous cell carcinoma with
hair loss and fibrinoid necrosis as acute and late endpoints and
observed a ‘protective effect’ (damage to normal tissue is less
than that of Con-RT) of FLASH-RT (5). Further histological analysis revealed
no acute toxicity in three cats, moderate/mild transient mucositis
in three cats and depilation in all cats. The 16-month
progression-free survival in the experimental group was 84%. This
finding confirmed the potential benefits of FLASH-RT and provided a
basis for further evaluation of FLASH-RT effects in humans.
Numerous studies have examined the effects of FLASH in normal
tissues, which are summarized in Table
I.
 | Table I.Studies examining the effects of
different modes of FLASH irradiation on normal tissue. |
Table I.
Studies examining the effects of
different modes of FLASH irradiation on normal tissue.
| Author, year | System | Dose, Gy | Dose rate,
Gy/sec | Assay | (Refs.) |
|---|
| Hornsey and Bewley,
1971 | Mouse
intestine | 11.9 | 17-83 | LD50/5 | (10) |
| Field and Bewley,
1974 | Mouse foot
skin | 24 | 56-83 | Early and late
reactions | (8) |
| Hendry et
al, 1982 | Mouse tail
skin | 50 | 17-170 | Necrosis ND50 | (95) |
| Favaudon et
al, 2014 | Mouse lung | 15-17 | 40-60 | Lung fibrosis | (11) |
| Montay-Gruel et
al, 2017 | Mouse brain | 10 |
100-106 | Memory tests | (12) |
| Vozenin et
al, 2019 | Mouse
intestine | 14.7 | 70-210 | LD50/5
(survival) | (56) |
| Montay-Gruel et
al, 2018 | Mouse brain | 10 | 37 | Neurocognitive
tests | (13) |
| Simmons et
al, 2019 | Mouse brain | 30 | 200/300 | Neurocognitive
tests | (71) |
| Montay-Gruel et
al, 2019 | Mouse brain | 10 | >100 | Neurocognitive
tests | (14) |
| Abel et al,
2019 | Mouse lung | 15/17.5/20 | 40 | Survival,
dermatitis, breathing function | (96) |
| Girdhani et
al, 2019 | Mouse lung | 15/17.5/20 | 40 | Lung fibrosis, skin
dermatitis | (35) |
| Vozenin et
al, 2019 | Mini-pig skin | 22-34 | 300 | Skin
toxicity/injury | (5) |
| Montay-Gruel et
al, 2019 | Zebrafish
embryo | 8 | >100 | Morphology | (14) |
| Alaghband et
al, 2020 | Mouse brain | 8 |
4.4×106 | Neurocognitive
tests | (15) |
| Fouillade et
al, 2020 | Mouse lung | 17 | 40-60 | Cellular
proliferation, inflammation | (76) |
| Levy et al,
2020 | Mouse abdomen | 12-16 | 216 | Crypt cells, stool
production, survival, regeneration | (57) |
| Diffenderfer et
al, 2020 | Mouse abdomen | 15 | 78 | Intestinal crypt
cell proliferation | (38) |
| Diffenderfer et
al, 2020 | Mouse
intestine | 18 | 78 | Fibrosis | (38) |
| Cao et al,
2021 | Mouse mammary
gland | 20 | 300 | Oxygen depletion
test | (60) |
| Liew et al,
2021 | Mouse skin | 30 | 125 | Survival | (32) |
| Cunningham et
al, 2021 | Mouse skin | 15,35 | 57,115 | Plasma and skin
levels of TGF-β1 and skin | (97) |
| Velalopoulou et
al, 2021 | Mouse skin, muscle,
bone | 30,45 | 69-124 | Survival,
histology, pathology | (98) |
| Montay-Gruel et
al, 2021 | Mouse brain | 10-30 |
1.8×106 | Survival,
neurocognitive tests | (99) |
Tumor control effect
Numerous studies indicated that FLASH reduces
irradiation damage in normal tissues; however, its therapeutic
effect on tumor tissues remained to be determined. Favaudon et
al (11) observed no
difference in anti-tumor efficiency when an orthotopic mouse lung
cancer model was exposed to FLASH-RT or Con-RT. They also indicated
that only 20% of the mice irradiated at the conventional dose rate
of 15 Gy were tumor-free at weeks 8–9 as opposed to 70% of the mice
treated with 28 Gy FLASH-RT. These findings suggest that FLASH
irradiation may enhance tumor inhibition. Numerous studies
suggested that flash-RT and Con-RT have almost the same therapeutic
effect on tumors when used at equal doses (5,11,16).
For instance, the first pre-clinical study of nasal plane
spontaneous squamous cell carcinoma in felines indicated no
significant difference in the efficacy of FLASH compared with
Con-RT (5).
The results of FLASH irradiation have also been
validated in humans; a recent study by Bourhis et al
(17) reported the first patient
receiving FLASH-RT. A 75-year-old patient presented with a
multiresistant CD30+ T-cell cutaneous lymphoma
disseminated throughout the whole skin surface. Prior to this
treatment, Localized skin RT has been previously used over 110
times for various ulcerative and/or painful cutaneous lesions
progressing despite systemic treatments. However, due to the
emergence of a new skin tumor (3.5 cm in diameter), FLASH-RT was
administered for a total dose of 15 Gy. The purpose of FLASH
irradiation is to minimize the toxicity of surrounding normal
tissues. After 3 weeks of treatment, the toxicity of normal tissues
decreased significantly and the tumor was well controlled. This was
the world's first clinical report of FLASH applied to humans,
providing a stimulus for further basic research and clinical
applications of FLASH. Subsequently, further studies on the
clinical application of FLASH radiotherapy were published. Van de
Water et al (16) conducted
a clinical study on four patients with head-and-neck cancer who
received four treatment plans (the clinical treatment plan, a
‘standard’ spot-reduced plan, an ‘arc’ spot-reduced plan and an
‘arc-shoot-through’ spot-reduced plan). They indicated that FLASH
dose rates were not achieved for conventional planning and clinical
spot-scanning machines. As such, increased spot-wise beam
intensities, spot-reduced planning, hypofractionation and
arc-shoot-through plans were required to achieve FLASH-compatible
dose rates. In addition, Wei et al (18) performed a dosimetric clinical study
on two patients with lung cancer and observed that the
single-energy Bragg peak plans achieve superior dosimetry
performances in organ-at-risk sparing (OARs) to transmission plans
with comparable dose rate performances for lung cancer FLASH
therapy. Beam angle optimization may further improve the OAR
dosimetry parameters with similar 3D FLASH dose rate coverage.
Factors affecting FLASH irradiation
Dose effect
Earlier FLASH studies used monopulse and nanosecond
X-ray irradiation, and the instantaneous dose rate was up to
7×108 Gy/sec (9). The
dose rate mentioned in FLASH-RT studies is the average dose rate of
the whole irradiation process. Smyth et al (19) found that, compared with a cathode
ray tube, synchrotron broad-beam radiation therapy with a high dose
rate (37–41 Gy/sec) and an equivalent dose did not provide normal
tissue protection. These results suggest that the protective effect
of FLASH-RT on normal tissues may not be universal and the dose
rate required to induce the FLASH effect may not be universal.
Montay-Gruel et al (12)
studied whole brains irradiated with 10 Gy of 4.5 and 6 MeV
electrons at dose rates from 0.1 to 500 Gy/sec. When the dose rate
was ≥30 Gy/sec, the neuroprotective FLASH effect was significant;
when the dose rate was ≥100 Gy/sec, the maximum FLASH effect was
induced; however, when the dose rate was <30 Gy/sec, the
neuroprotective effect disappeared. Another study indicated that
FLASH-X-ray whole-brain irradiation of 37 Gy/sec has a more
significant memory protection effect than Con-RT (13). Contrary to these results,
Venkatesulu et al (20)
suggested that FLASH-RT at 3 5Gy/sec gave higher toxicity than
Con-RT at 0.1 Gy/sec.
The total radiation dose used in pre-clinical
FLASH-RT studies is not uniform. Bourhis et al (21) suggested using low-segmented FLASH
delivery because low-segmented FLASH has the same efficacy as
Con-RT in controlling orthotopic glioma in mice. By contrast,
Vozenin et al (5) used
FLASH-RT to treat cats with locally advanced nasal squamous cell
carcinoma and found that a single dose of up to 41 Gy failed to
reach the maximum tolerated dose; no dose-limiting toxicity was
observed under a single dose of 25–41 Gy. Meanwhile, normal tissue
had good tolerance.
Although there has been much research on FLASH-RT,
the optimal dose rate for FLASH-RT remains undefined. Zhou et
al (22) found that
FLASH-RT-induced transient hypoxia protected normal tissue better
than Con-RT; they analyzed the order of magnitude of the minimum
dose rate required by ultra-short radiation pulse FLASH-RT through
dimensionality. The results indicated that the lower limit of the
dose rate of FLASH-RT may be very close to the minimum dose rate in
pre-clinical trials (>40 Gy/sec). In addition, if FLASH-RT is
used in the clinical treatment of deep tumors while delivering an
UHDR for deep tissues, normal tissues along the radiation beam
trajectory may receive a low dose rate (lower than the minimum dose
rate to induce the FLASH effect) and the damage of normal tissues
along the radiation path also requires to be considered.
The linear quadratic formula (L-Q model) (or α/β
equation) was proposed by Kellerer and has been used in
radiobiology research and clinical radiotherapy, where it had a
profound influence on theoretical research and clinical application
of radiobiology (23). According
to the linear quadratic model, cells are killed if two strands of a
DNA or two arms of a chromosome are damaged simultaneously
(24). There are two mechanisms of
damage in this event: i) Cell death caused by radiation hitting two
strands of DNA at once and ii) the single-target multiple-hit
effect, with cell death caused by radiation hitting two strands of
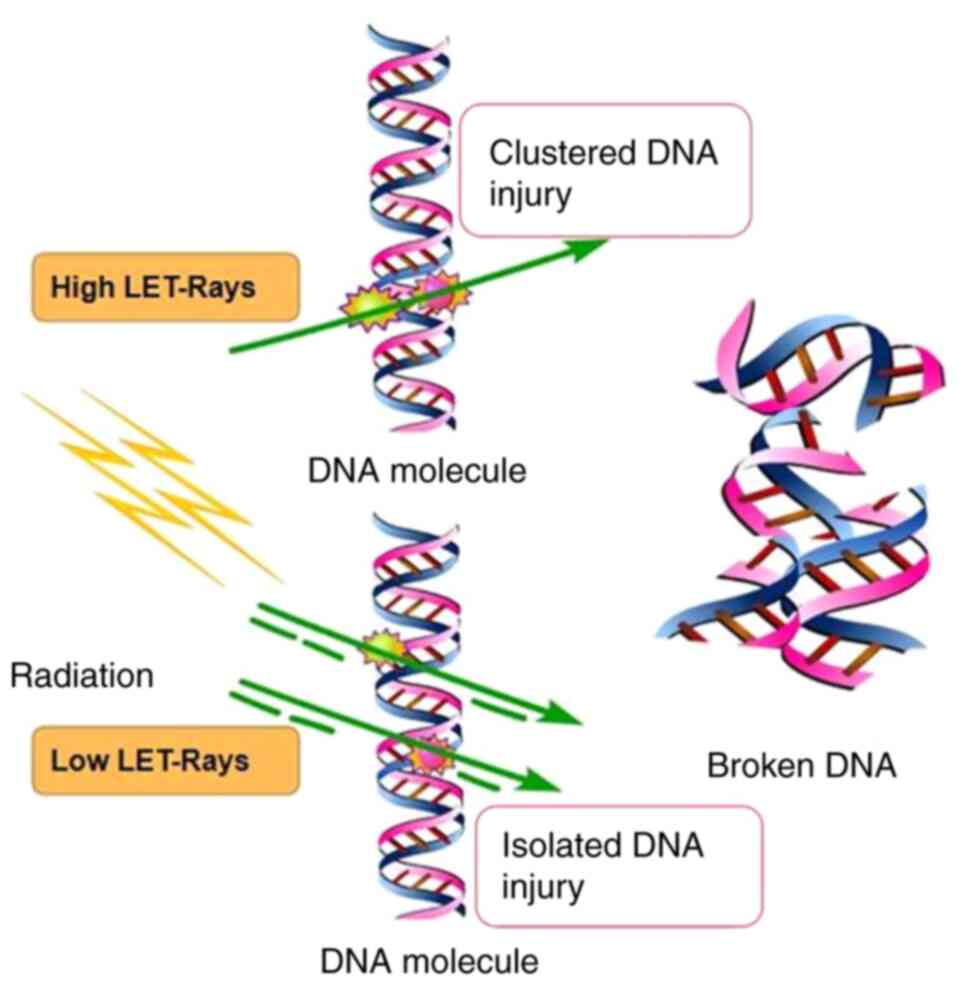
DNA separately (Fig. 1). Rays with
energy particles that accomplish this effect alone are called
high-let rays, while particles that require multiple particles to
cause DNA damage are called low-let rays. However, certain authors
were skeptical about the L-Q model. Steel et al (25) indicated that the L-Q model was not
able to predict the cell survival rate when the cell population
received a fractional irradiation dose, suggesting that factors
other than the local dose affected the survival rate after
irradiation. Hadrons are being used for clinical cancer treatments,
predominantly with proton and carbon-ion beams but with increasing
interest in the application of other ions. As particles slow down
in the body, they become more densely ionized, eventually losing
all energy and stopping moving. One result of this is that as the
particles decelerate and the LET of these beams increases,
resulting in an increase in relative bioavailability (RBE),
depending on the energy and particle type (26,27).
In radiobiology, the increase in RBE is driven by the production of
more complex or clustered DNA damage that cells find increasingly
difficult to repair. With the increase of LET (28,29),
the dose savings of the graded treatment decreased and the low dose
rate savings also decreased.
Therefore, it is necessary to consider the dose.
Venkatesulu et al (20)
observed that FLASH-RT (35 Gy/sec) was more likely to induce acute
gastrointestinal syndrome than Con-RT (0.1 Gy/sec) for the same
single 16 Gy abdominal irradiation. Tissues with low α/β values
(such as the spinal cord, lung, kidney, liver, bone and the
vascular system) are sensitive to the fractional dose and the late
response of tissues is aggravated when the fractional dose is
increased in Con-RT. However, a single high dose of FLASH-RT
significantly protected normal brain and lung tissues (10,21).
Tissues with high α/β values (such as small intestine and skin)
were more sensitive to the total treatment time and premature
tissue response was aggravated when the total treatment time was
shortened. This finding suggests that the efficacy of FLASH-RT is
associated with tissue specificity and has considerable complexity.
Rothwell et al (30)
analyzed the effects of different doses and dose rates on FLASH.
The authors indicated that within a specific range (<1,000
Gy/sec), a higher dose rate was associated with a greater ∆OER,
which was consistent with previous studies (22,31).
The authors also found that, even at these high dose rates, no
significant change in ∆OER was observed without a sufficiently high
dose. This finding reaffirms the importance of the dose and dose
rate for the FLASH effect.
Based on these results, further studies analyzed the
influencing factors of FLASH irradiation and established models to
predict the effects of FLASH irradiation under various influencing
factors. To facilitate the radiobiological investigation of FLASH
phenomena and the assessment of clinical applicability, Liew et
al (32) presented an
extension of the mechanistic radiobiological model ‘UNified and
VERSatile Bio Response Engine’ (UNIVERSE), which reproduces the
dose-, dose rate and oxygen tension-dependent influence on cell
killing. For these systems, the findings suggest that the extent of
the cell/tissue sparing effect, if present, strongly depends on
beam quality used for reference conventional irradiation. For
instance, the dose rate effects are associated with survival and
survival was observed from doses and dose rates of ~8 Gy and ~40
Gy/sec, respectively. Although this model may predict the response
to FLASH radiotherapy, it is difficult to estimate the dose and
dose rate accurately. In addition, other models may help us
understand and predict the FLASH effect. These include the model
developed by Rothwell et al (30) using parameters involved in oxygen
consumption, including those related to dose transfer,
radiochemical oxygen consumption dynamics and tissue inherent
biological characteristics. Although the FLASH study indicates the
need for low initial oxygen concentrations and high doses, this
study provides a quantitative tool to determine more precise values
for different situations. After establishing a clinically relevant
parameter space for flash radiotherapy, the model may be used to
answer complex questions surrounding the mechanism of action.
Influence of radiation sources
The FLASH effect is thought to exist in
electron-wire radiotherapy, which has been preliminarily verified
in small animal models (33,34);
relevant human experiments are in progress (17). In addition to electron wires, FLASH
effects have also been observed for X-irradiation (13). Due to its physical properties,
proton beam radiotherapy has a protective effect on normal tissue
and the proton FLASH effect may add additional protection to normal
tissue. Girdhani et al (35) demonstrated for the first time that
proton FLASH-RT reduced normal tissue toxicity in a mouse model.
Beyreuther et al (36)
irradiated zebrafish embryos with a conventional dose rate proton
beam of 5 Gy/min and a proton FLASH beam of 100 Gy/sec and observed
no difference in toxicity. However, Buonanno et al (37) indicated that proton FLASH
irradiation in vitro did not increase the survival rate of
normal human lung fibroblasts. The reason for these findings may be
that the pulse rate of radiation affected the FLASH effect. This
finding suggests that the role of protons in FLASH mode requires to
be further studied. Several studies discussed the design,
implementation and in vivo verification of a new proton
FLASH-RT system (38), clarifying
the importance and clinical significance of FLASH-RT research in
proton plasma radiotherapy (39).
UHDR proton beam therapy is already under consideration (40).
The correlation between the FLASH proton and the
FLASH effect has been confirmed in numerous studies. Several
studies examined the effect of proton transport in FLASH mode
(36–39,41).
The proton relative bioavailability values using the point scanning
system are almost identical to those using the passive scattering
system. In a recent review of the data from these studies,
Colangelo and Azzam (39) provided
corresponding evidence for the FLASH effect in their study. These
studies were performed at aerobic levels; therefore, the results
were limited. Certain experiments under very low oxygen tension
established the relationship between the FLASH effect and proton
FLASH in vitro (42–44).
Buonanno et al (37)
conducted experiments using normal non-fibrocytes and compared
conventional dose rate and proton FLASH irradiation. The expression
of TGF-β in pre-senescent cells decreased with increasing dose
rate. These findings provide evidence for the long-term effects of
proton-derived FLASH. A colony-forming cell assay indicated that
the cell survival index at a constant dose rate had an exponential
relationship with the increased dose. The ability of proton-derived
FLASH-RT to reduce long-term radiotoxicity in normal tissue
compared with conventional dose rate radiotherapy may be due to
differences in the types or amounts of DNA damage. These findings
provide another aspect of the proton-derived FLASH effect. Proton
beam therapy (PBT) may be the ideal solution for treating certain
deep tumors. Due to its high energy and dose rate, FLASH
irradiation has been applied in clinical practice (16). The PBT beam has also been applied
to other devices for testing innovations in equipment and devices
(38,45). However, the limitations of PBT
hamper its further popularization. Proton scattering is necessary
for patients with large tumors, resulting in the loss of scattered
particles and reducing the total delivery dose. Furthermore, the
total duration increases to provide UHDR at each point; therefore,
the total dose rates decrease and may not be sufficient to cause
FLASH effects (21,46).
Although the majority of current radiation
treatments are performed using X-rays, preclinical studies
examining results of exposure to X-rays are rare. Montay-Gruel
et al (47) have summarized
the different methods that may be used to generate X-rays, their
beam properties and their effects. Schüler et al (33) provided a comprehensive review of
the numerous results generated from electron FLASH experiments.
They suggest the following set to be at a minimum in terms of dose
parameter: The dose and dose parameters should be defined to a dose
specification point (DSP) at the center of the irradiated volume of
interest. If a highly irregular volume is considered, a
representative DSP in this volume should be defined and used. The
reporting of the dose parameters should be accompanied by the
coordinates of the DSP as well as dose profile measurements along
the lateral and axial directions, centered on the DSP.
Weber et al (48) discussed the technical challenges in
beam delivery and provided a promising solution using 3D
range-modulators in order to apply UHDRs compatible with FLASH with
carbon ions. Next, they also discussed the possible outcome of
C-ion therapy at UHDR on the level of the radiobiological and
radiochemical effects. Although a large number of studies have
assessed the preclinical FLASH status of different radiation types,
there is still no consensus on the normalization and
standardization of radiation types, which still requires to be
confirmed by further studies.
FLASH-RT biological mechanism
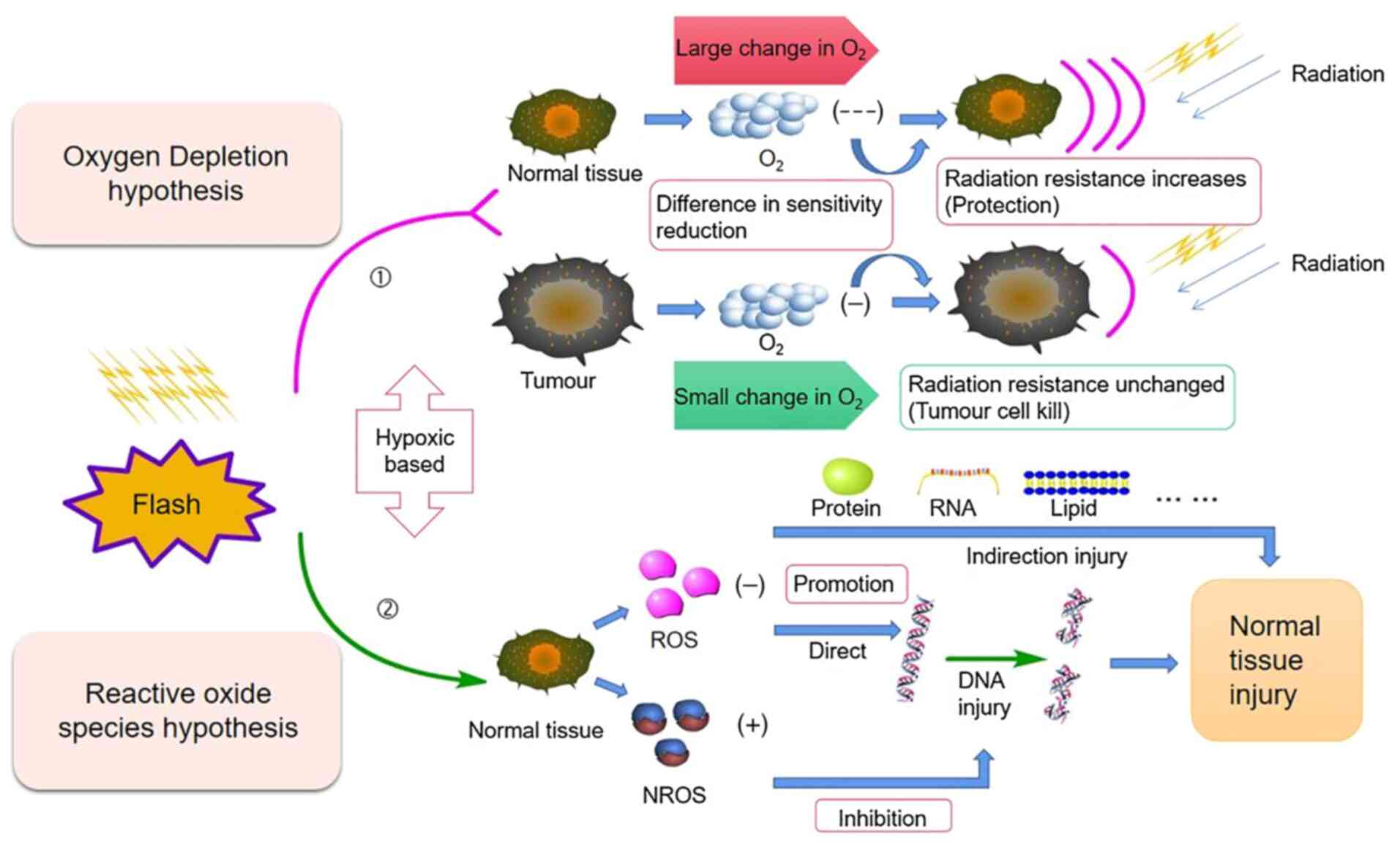
Oxygen consumption hypothesis
Traditional radiotherapy is based on the classical
‘4R’ theories of radiobiology (repair, reoxygenation,
redistribution of cell cycle and repopulation) (49). Regarding the biological mechanism
of FLASH-RT, early studies focused on the possibility of oxygen
metabolism in the environment surrounding cells (50). FLASH-RT irradiation results in
radiochemical oxygen depletion, which leads to an acute period of
hypoxia in the irradiated tissue and transient radiation
resistance. Oxygen is a critical factor affecting the FLASH effect
and a physical parameter that evaluates the FLASH effect. There is
evidence that numerous normal tissues are able to maintain a small
number of cell populations for continuous renewal/regeneration at
low physiological oxygenation levels (51). High doses rapidly deplete oxygen at
high dose rates, allowing it to diffuse to maintain adequate
oxygenation levels, and normal tissue responds as hypoxic tissue.
As a result, UHDRs deplete oxygen, mimicking hypoxia and increasing
tissue resistance to radiation. In the case of hypoxic
(radiation-resistant) tumors surrounded by oxidized normal tissue
(radiosensitivity), UHDRs increase the radiation resistance of
normal tissue with minimal effects on already hypoxic tumor tissue
(52). In common ionizing
radiation reactions, water in cells breaks down as a result of
radiation and produces reactive oxygen species (ROS), which
indirectly damage DNA, including the common process of hydroxyl
radicals attacking DNA (53). In
low-linear-energy transfer radiation, DNA damage caused by ROS may
account for up to 70% and the remainder is caused by direct
interactions between DNA and radiation (31,54,55).
According to the oxygen fixation hypothesis, if oxygen radicals
cause this indirect DNA damage, the damage is fixed by the presence
of molecular oxygen by forming more damaging peroxy radicals
(56).
After FLASH-RT, radiochemical depletion of oxygen
occurs in tumor tissue, leading to radiation resistance of the
tumor. Petersson et al (42) provided a reliable quantitative
model for understanding the biological effects of FLASH-RT that was
compatible with experimental observations of FLASH effects. The
model suggests that oxygen levels may be depleted at moderate
oxygen concentrations (but not at high or very low concentrations);
oxygen levels may be depleted sufficiently to affect
radiosensitivity. Under physiologically relevant oxygen
concentrations (relative partial pressure 1.6–20%), Adrian et
al (43) reported that the
FLASH effect depended on the oxygen concentration in vitro
and the survival rate of hypoxic prostate cancer cells (1.6%) was
significantly improved by FLASH irradiation. However, numerous
studies suggested that FLASH-RT was able to maintain an anti-tumor
response similar to Con-RT (21,57);
in certain cases, FLASH-RT may have generated anti-tumor responses
better than those to Con-RT (34,58).
Several studies on oxygen in FLASH-RT indicated that oxygen and
hypoxic environments may have a critical role in the FLASH effect.
There are numerous descriptions of the traditional mechanics of the
FLASH effect (Table II). For
instance, Petersson et al (42) used a model of oxygen dynamics
during irradiation to develop a time-dependent model of the oxygen
enhancement ratio in mammalian cells, which includes oxygen
depletion. Then the characteristics of the model were discussed in
terms of dose and dose rate dependence of the oxygen enhancement
ratio. Eventually, they determined that only under moderate oxygen
tension, oxygen levels were able to be depleted in quantities
sufficient to affect radiation sensitivity. Boscolo et al
(59) studied the yield as a
function of time and LET for all radioactive substances simulated
for different ionic radiation and different oxygenation levels at
different energies within 1 µsec after radiation passage. Under
oxygenated conditions, high production of two highly toxic species,
O2•− and HO2•, was predicted,
particularly at low LET radiation. All of these studies have
further promoted the mechanistic research progress on FLASH-RT.
 | Table II.Studies exploring the mechanisms of
FLASH radiotherapy. |
Table II.
Studies exploring the mechanisms of
FLASH radiotherapy.
| Author, year | Dose rate,
Gy/sec | Dose, Gy | Type of ray | Core theory | System | (Refs.) |
|---|
| Petersson et
al, 2020 | 0-100 | 0-30 | Electrons | Oxygen
consumption | In vitro and
in vivo | (42) |
| Labarbe et
al, 2020 |
10−3−107 | 10 | Electrons or
photons | ROS | In
vitro | (78) |
| Boscolo et
al, 2020 | - | - | Ion and proton | Oxygen
consumption | In
vitro | (59) |
| Liew et al,
2021 |
0.01-104 | 2-32 | Electrons and
x-rays | ‘UNIVERSE’ | In vitro and
in vivo | (32) |
| Cao et al,
2021 | 0-300 | 0-30 | Electrons | Oxygen
consumption | In vitro and
in vivo | (60) |
| Boscolo et
al, 2021 | 109 | 0-150 | Electrons | Oxygen
consumption | In
vitro | (64) |
| Jansen et
al, 2021 | 0-340 | 10 | X-rays, protons and
carbon ions | Oxygen
consumption | In
vitro | (63) |
| Rothwell et
al, 2021 | 40–150+ | 2-50 | - | Oxygen
consumption | - | (30) |
| Tinganelli et
al, 2022 | 0-70 | 0-7.5 | Ions | Oxygen
consumption | In
vitro | (61) |
Although the oxygen consumption mechanism of FLASH
irradiation is widely known, this mechanism is being challenged due
to progress in-depth research. FLASH irradiation is unlikely to
consume oxygen to radiation-related levels of hypoxia in large
tissues. At the same dose, FLASH irradiation led to less oxygen
consumption than conventional in vitro irradiation, which
may be related to the protective effect of FLASH. However, the
difference in oxygen consumption between FLASH and conventional
exposure cannot be quantified in vivo because measurements
of oxygen consumption under conventional exposure are hampered by
oxygen supplementation in the blood (60). Tinganelli et al (61) studied the effect of FLASH
irradiation on CHO-K1 cells in oxygenation content ranging from 0
to 21%. The authors determined that the FLASH protection effect was
oxygenation-dependent and the protective effect was more
significant in the presence of lower oxygen content. This finding
affirms the opinion of researchers who were skeptical about the
mechanism of oxygen consumption (43,62).
These studies (60–62) are significant to the research on
the mechanisms of FLASH. Studies reported a slight reduction in
oxygenation after FLASH irradiation, claiming a negligible effect
on radiosensitivity (32,63,64).
Cao et al (60) used a
fluorescence quenching method and a water-soluble molecular probe
to measure oxygen in vivo and in vitro, and
quantified the change of oxygen per unit dose by irradiation with a
10 MeV electron beam. In in vitro experiments, the oxygen
consumption g values of conventional irradiation ranged from 0.19
to 0.21 mm Hg/Gy (0.34 to 0.37 µM/Gy) and that of UHDR irradiation
ranged from 0.16 to 0.17 mm Hg/Gy (0.28 to 0.30 µM/Gy). In
vivo, the oxygen content decreased by 2.3±0.3 mm Hg in normal
tissues and 1.0±0.2 mm Hg in tumor tissues after a single dose of
20 Gy FLASH irradiation, while no reduction in oxygen was observed
with a single fraction of 20 Gy applied in the conventional mode.
Therefore, it is indicated that FLASH irradiation induces less
oxygen consumption than conventional irradiation at the same dose,
which may be related to the retention effect of FLASH. However, the
difference in oxygen consumption between FLASH and conventional
irradiation cannot be quantified in vivo, as measurements of
oxygen consumption under conventional irradiation is replenished by
oxygen supplementation in the blood. Jansen et al (63) experimentally investigates whether
oxygen depletion occurs during FLASH irradiation by measuring the
oxygen concentration in vitro during irradiation of water by
photons, protons and carbon ions. They observed that oxygen
consumption in water was only related to radiation dose, dose rate
and linear energy transfer, with a higher dose rate associated with
lower oxygen consumption. They also found no clinically relevant
oxygen consumption limits. Eventually, they concluded that the
FLASH did consume oxygen, but not a sufficient amount to use up all
of it. For high dose rates, less oxygen was consumed than for the
standard radiotherapy dose rate and loss of oxygen of any analyzed
radiation type was found when using FLASH for 10-Gy dose delivery.
These studies also challenge the traditional hypothesis of oxygen
consumption.
ROS-mediated cell damage
Other biochemical mechanisms are thought to be
responsible for the FLASH effect on ROS and free radicals. A study
indicated that the electronic irradiation of zebrafish embryos with
Con-RT and FLASH-RT only had a minor effect on their morphology 5
days after fertilization due to the low production of ROS,
suggesting that the radiation resistance to FLASH of normal tissues
was significantly associated with reduced ROS levels (14). Abolfath et al (65) performed molecular dynamics
simulations to study the generation of reactive species around DNA
at various dose rates and oxidation levels. Under normoxic
conditions at high dose rates, individual ROS aggregate to form
resonant or metastable molecular states linked by hydrogen bonds.
The resulting clusters have low diffusivity and are known as
non-reactive oxygen species (NROS), which, unlike ROS, have a
limited biological damage potential. At low dose rates and low
oxygen tension, the production of NROS is reduced, resulting in a
higher proportion of free ROS. Montay-Gruel et al (14) indicated that oxygen consumption of
UHDR promotes the protection of normal tissues by inhibiting ROS
production. The authors irradiated water with 4% oxygen (to
simulate physiological oxygen tension) with FLASH-RT or Con-RT.
After FLASH irradiation, the concentration of
H2O2 in an aqueous solution was significantly
decreased. Spitz et al (31) indicated that the FLASH effect was
related to the instantaneous generation of free radicals and
inherent differences in redox and free radical chemistry between
normal and tumor tissue. The study suggested that the content of
unstable iron in normal tissue cells was less than that in tumor
tissue cells; therefore, the further reaction of ROS was more
easily restricted, reducing cell damage (31). Tumor tissue cells contain a large
amount of unstable iron ions and internal reactions are more likely
to occur. This phenomenon results in a similar reduction in
radiation sensitivity in FLASH-RT with less reduction in tumor cell
sensitivity and a greater reduction in normal cell sensitivity. The
difference in sensitivity of tumor cells and normal cells to
FLASH-RT resulted in different tissue responses. Both Con-RT and
Flash-RT mechanisms are related to oxygen and their related
mechanisms will be discussed below (Fig. 2).
Favaudon et al (66) proposed three hypotheses and
compared them with the current results. The radiation-induced
transient oxygen depletion (TOD) hypothesis suggests that normal
tissue preservation at UHDRs is the result of transient hypoxic
radiation protection due to oxygen depletion. Although in
vivo data (14) suggested that
local oxygen tension had a strong effect on the final results, the
isoefficiency of tumor cell killing under normoxic and hypoxic
conditions was less supportive. In addition, both direct
measurement of oxygen consumption during FLASH irradiation by
optical methods in vitro and in vivo and observations
of the FLASH effect in aerated cultured cells with DNA damage and
survival as endpoints support the TOD hypothesis, and free radical
self-destruction appears to be a more plausible explanation
(66).
Immune and inflammatory
hypothesis
Another explanation is that chromatin remodeling is
mediated by poly (adenosine diphosphate ribose) polymerase and
inflammatory/anti-inflammatory cell signaling may depend on the
duration of treatment. As certain circulating blood cells are
irradiated, they protect the immune system better than under
traditional dose irradiation. The chromosomal aberrations of
circulating blood lymphocytes depend strongly on the amount and
duration of irradiation. In FLASH exposure, time reduction allows
more circulating immune cells to survive. In this case, the
efficacy of fractionated irradiation is lost (67). It has been reported that the TGF-β
signaling pathway is lower in FLASH-irradiated mice than in mice
subjected to Con-RT (11). One
study reported that the key to radiation resistance of
tumor-infiltrating T cells is TGF-β (68), while other studies indicated that
TGF-β signaling inhibits the immune system and promotes cancer
progression, concluding that inhibitors of the TGF-β pathway may
enhance the treatment of malignant tumors (69). Rama et al (58) reported that FLASH proton radiation
improved lung tumor control, possibly due to the recruitment of
CD3+ T lymphocytes into the tumor. In several studies,
FLASH-RT and Con-RT were compared in immunocompromised animals;
however, no differences in tumor response were observed. Girdhani
et al (35) found that
proton FLASH-RT had lower toxicity to normal tissue in pre-clinical
mouse models than Con-RT. Subsequent genome-wide microarray
analysis suggested that extensive activation and maturation of the
immune system were inhibited in FLASH-RT mice (10,13,70).
FLASH may provide better immune responses due to reduced exposure
of circulating immune cells because of the short exposure time,
although segmental FLASH-RT may reduce this effect (38). A study on whole-brain irradiation
in C57BL/6J mice indicated lower levels of pro-inflammatory
cytokines in the hippocampus after FLASH than conventional dose
rate irradiation (71). A
significant increase in five of 10 cytokines (IL-6, IL-1β, TNF-α,
KC/GRO and IL-4) measured at conventional dose rates was reported
10 weeks after irradiation, whereas FLASH only produced increases
in three cytokines (IL-1β, TNF-α and KC/GRO).
There is evidence that radiation may lead to
pro-inflammatory immune stimulation and anti-inflammatory
immunosuppressive responses, and the potential of this
immunomodulatory response is the main theoretical basis for
numerous recent clinical trials (72). Durante et al (73) noted that the difference in the high
dose rate and total treatment time may reduce the proportion of
circulating blood cells irradiated, thereby protecting the immune
system, and predicted that this would be more effective than
subconventional dosing. They indicated that chromosomal aberrations
detected in circulating lymphocytes following radiation exposure
depend on exposure time and volume (73), but this has not been confirmed for
flash exposure. Rama et al (58) demonstrated that Flash-RT improves T
cell infiltration in irradiated tumors. It is speculated that
routine and flash irradiation may directly alter the expression
levels of immune factors and active immune cells, but indirectly
affect immune reactivity by affecting DNA damage or the
microenvironment. It has also been suggested that radiation
exposure leads to the expression of a range of other immune
factors, including interleukins, interferons, immune checkpoint
ligands and other cytokines. These may induce more than direct
radiation responses (74,75).
DNA damage, senescence and
fibrosis
There is also considerable evidence of differences
in DNA damage in normal tissues after Con-RT and FLASH irradiation.
Fouillade et al (76)
studied the phosphorylation of histone H2AX and recruitment of
cohesion protein 53BP1 at DNA damage sites in MRC5 and IMR90 normal
human fibroblasts and A549 human lung adenocarcinoma cells and
indicated that after FLASH (5×106 Gy/sec) and Con-RT (5
Gy) irradiation, no significant difference was observed in the
number of H2AX lesions between the three cell lines and the two
irradiation modes. However, it was noteworthy that flash-RT-induced
53BP1 lesions were significantly fewer than CONV lesions in the two
fibroblast cell lines. It has been observed that tumor cells are
frequently deficient in radiation-induced G1 arrest and that
pathologic DNA repair features and defects are present in
double-strand breaks reconnected at G0/G1 (77). Reduced production of the 53BP1
substrate DNA damage subset through free radical recombination and
defective repair of G1 tumor cells are also considered to be two
important processes in the differential response of normal and
tumor cells to FLASH-RT (78).
FLASH irradiation has been indicated to protect
tissues from radiation-induced fibrosis similar to that seen in
Con-RT. This has been demonstrated in a large number of animal
studies (5,11,76).
Fouillade et al (76)
analyzed the characteristics of radiation-induced lung senescence
in mice using immunofluorescence and transcriptome techniques and
observed that FLASH-RT was less efficient than Con-RT in inducing
p53BP1 lesions. The persistent focal accumulation of 53BP1 at
chromosomal damage sites was associated with DNA fragments and
chromatin-enhanced senescence. Furthermore, they demonstrated that
FLASH-RT was able to prevent the induction of senescent cells in
irradiated lungs. They also pioneered the use of single-cell RNA
sequencing in radiation biology, allowing the identification of
cell-type-specific transcriptional changes (79).
Pre-clinical application prospect of
FLASH
FLASH-RT is as lethal to tumors as conventional dose
rate radiotherapy and has low side effects on normal tissue.
Therefore, FLASH-RT will likely have a considerable clinical impact
in the future. This inference is supported by the researchers who
studied the protection of mouse brain tissue during FLASH-RT
(80), thereby suggesting that
FLASH-RT modifies a common initial event that may control the
development of both acute and delayed toxicity. To date, the FLASH
effect has been demonstrated in several in vivo models,
mostly in wild-type mice, and in several organ systems. These
organs comprise the so-called acute reaction organs, such as the
gut and hematopoietic system, (81,82),
and delayed reaction organs such as the brain, lung and skin
(76,83–87).
In 2019, researchers from Stanford University, the SLAC National
Accelerator Laboratory, and Indiana University solved the problem
of FLASH-RT device architecture (88). FLASH-RT has two primary clinical
purposes. First, the FLASH effect may increase the total dose for
treating radio-resistant tumors associated with poor outcomes
(5). Larger doses of radiation may
be delivered to the tumor without the severe toxicity to
surrounding normal tissue that Con-RT is expected to cause.
Furthermore, analysis of the distribution of initial DNA damage
after a high dose rate irradiation showed that the distribution of
DNA damage after high-dose rate irradiation shifted in the
direction of severe damage and the distribution range widened
(89,90). These findings suggest that
increasing the dose rate and changing the pulse frequency of
ultrafast electrons increases the complexity of DNA damage and
reduces its repairability (91). A
modified UHDR electron FLASH-RT (eFLASH-RT) linear accelerator was
proposed by Rahman et al (92). This device was the first functional
beam model commissioned in a clinical treatment planning system for
eFLASH-RT, enabling planning and evaluation with minimal deviation
from the Con-RT workflow. The device facilitates clinical
translation because eFLASH-RT and Con-RT plan quality were
comparable for humans with complex geometries and tissue
heterogeneity. The methods may be expanded to model other eFLASH
irradiators with different beam characteristics. The differences
between conventional dose rate radiotherapy and FLASH-RT are
significant in numerous aspects, such as in the dose rate. Compared
with Con-RT, the dose rate of FLASH-RT may be >40 Gy/sec and the
irradiation time may be <1 sec. At the same time, the
irradiation source, the equipment used and the corresponding
mechanism also exhibit certain differences. The most important
difference, however, is in the extent of damage to normal tissue
(Table III).
 | Table III.Comparison of Con-RT and
FLASH-RT. |
Table III.
Comparison of Con-RT and
FLASH-RT.
| Item | Con-RT | FLASH |
|---|
| Equipment | Proton and ion
accelerator, X-knife, γ-knife, | Proton
accelerators, linear accelerator |
| Cost | Proven equipment,
technology, research and clinical translation | Higher technology
cost, equipment cost, personnel cost, research cost, automation and
clinical efficiency |
| Dose rate,
Gy/sec | 0.002-0.017 | >40 |
| Ray | X- and γ-ray,
proton, heavy ion, electron | Proton, X-ray,
electron |
| Time, sec | ≥120 | <1 |
| Tumor control
effect | Efficient | Efficient |
| Damage degree of
normal tissue | High | Low |
| Factors | Tissue
radiosensitivity, dose | Dose, dose
segmentation, oxygen content, pulse |
| Mechanism | Oxygen depletion
hypothesis, ROS, immunoinflammatory hypothesis | Repair,
reoxygenation, redistribution, repopulation, oxygen depletion
hypothesis, ROS |
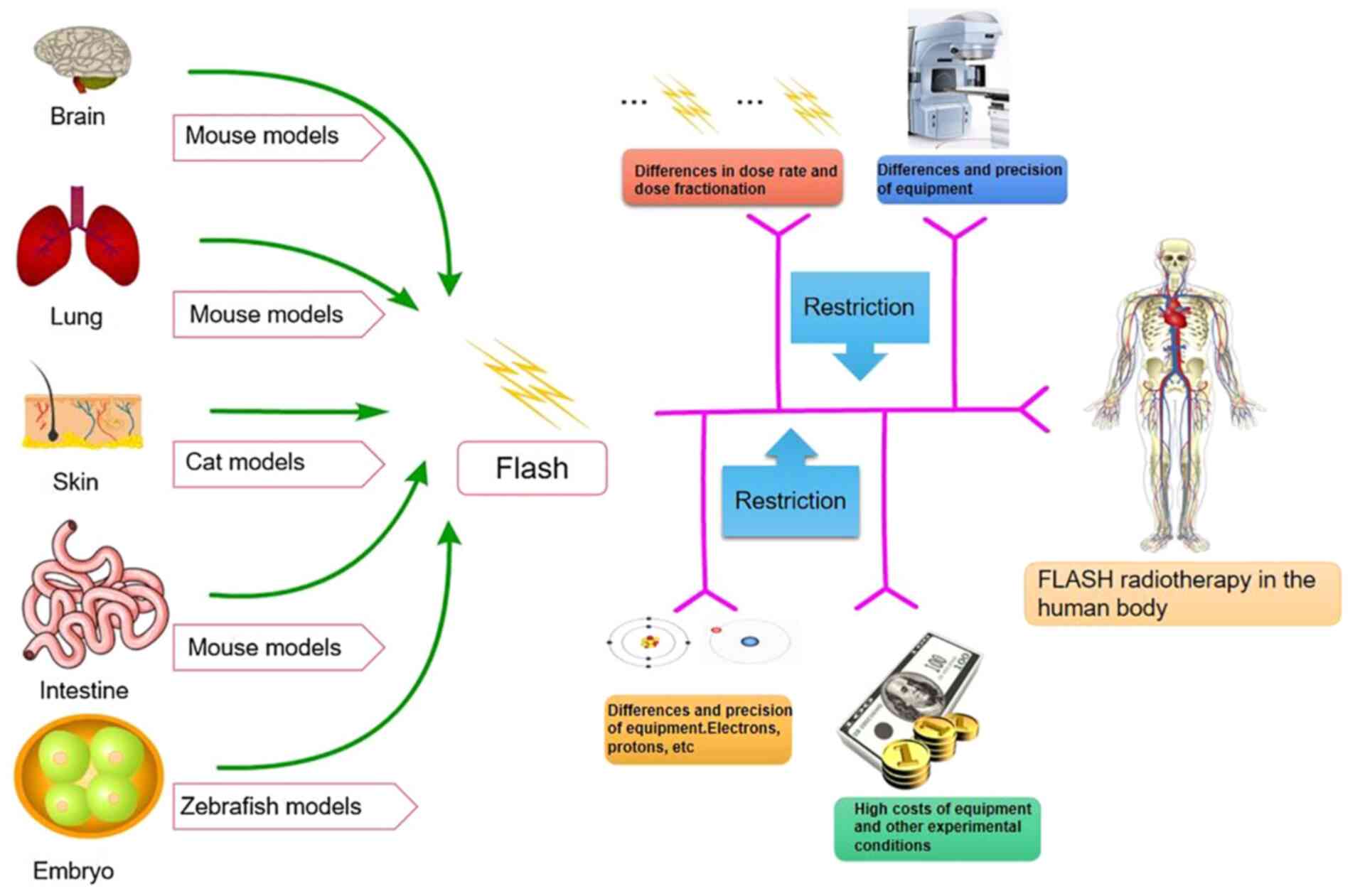
Although several FLASH-related animal studies have
been performed, the limitations of FLASH in human studies remain
evident (Fig. 3). First, FLASH-RT
systems are scarce and high-energy electron or proton FLASH-RT is
required for deep tumor radiotherapy. Safety is paramount when
patients are exposed to such high dose rates. Developing a
comprehensive dose monitoring system is necessary to ensure safety
(17,93). The issue of fractional treatment is
also an area to be further explored; the correct dose of
therapeutic irradiation must be determined in fractional treatment
and single fractional treatment within 1 sec (89). Furthermore, treatment equipment
limits FLASH-RT. The linear accelerator cannot generate the
irradiation dose at the required rate. In addition, pre-clinical
studies lack relevant information, such as radiobiology studies to
ensure that their findings may be replicated in different settings
and to assess potential long-term effects while further exploring
specific mechanisms (89).
Furthermore, financial factors limit FLASH-RT. It is essential to
reduce the cost of technology and make equipment more economical,
compact and compatible with existing facilities. Taylor et
al (94) warn that although
many studies have reported the efficacy of FLASH (95–99),
it is essential to conduct prospective clinical trials safely and
effectively for FLASH. In this paper, the status of FLASH quality
assurance and safety system is reviewed in the aspects of FLASH
standards, beam monitoring, calibration and machine quality
assurance, external peer review of programs and system dose. For
instance, in the clinical trial of FLASH-RT, challenges include no
charge to current standards, multiple datasets required for trial
submission when it appeared relevant, end-to-end testing required,
motion phantom when relevant, planning goals, special guidance on
beam arrangements by modality, detailed delivery log files, minimum
of a registry for all patients. Further questions that need to be
addressed are how well we can select, measure, optimize and
reproduce the fine structure of the UHDR dose delivery process;
whether it is possible to measure if the biological response to
FLASH treatment varies from patient to patient and from tumor to
tumor from day to day, in particular if medications or drugs vary
the critical biology of FLASH therapy; if FLASH treatment may be
delivered across realistic, deep and/or larger volumes, how easy it
will be to introduce FLASH therapy into the overall care matrix of
a patient; and whether FLASH irradiation causes any long-term
damage to tissues. These points all require to be addressed prior
to the clinical implementation of FLASH-RT. In addition, further
clinical trials of FLASH-RT have been proposed (100,101); for instance, it has been
suggested that organizations such as The American Association of
Physicists in Medicine, European Society Therapeutic Radiation
Oncology and European Federation of Organizations For Medical
Physics continue to collaborate on guidance and collect
time-structure information in clinical trials to ensure a balanced
and retrospective analysis; late effects occur at a later time and
clinical access is equitable. In conclusion, the clinical trials
and applications of FLASH-RT face numerous challenges.
Conclusion
FLASH-RT is a particular irradiation method that
has been in development for ~60 years. The modality has been
studied from bacteria to cells in vitro, from mice to small
animals, and eventually in patients. FLASH-RT began with UHDR
radiotherapy and the FLASH effect was discovered in the development
process of UHDR radiotherapy. Subsequently, scholars continued
in-depth research, hypothesized the possible radiobiological
mechanism of FLASH-RT and designed experiments to verify it. The
essence of FLASH-RT lies in its robust tumor control and specific
protective effect on normal tissues. The success of FLASH-RT in the
first patient substantially increased confidence in applications
for clinical patients. In the future, FLASH-RT may eventually have
a critical role in the treatment of various liver, pancreatic and
colon tumors, and the improvement and popularization of
radiotherapy equipment. FLASH-RT may even replace certain surgical
treatments, significantly reducing the pain and economic pressure,
improving survival rates and reducing the side effects of
radiotherapy. FLASH-RT has considerable potential for tumor
treatment.
Several countries are at the forefront of FLASH-RT
research. FLASH-RT may fundamentally overturn the current
theoretical system of radiotherapy in the future. There is much
speculation about the biological mechanisms that support the FLASH
effect. Radiation leads to radiochemical depletion of oxygen and
this is particularly evident at high dose rates. It may be safely
concluded that oxygen consumption is at least partly responsible
for the FLASH effect. Based on available data, the extent of its
contribution remains unclear and requires further investigation. In
addition to oxygen consumption, the FLASH effect is involved in
immune regulation; however, the evidence supporting this view is
scarce and preliminary. Similarly, any potential contribution of
FLASH-mediated immune effects requires to be explored. In addition
to mechanistic insight, the most important question remains the
clinical translational potential of FLASH-RT.
In conclusion, FLASH-RT is expected to serve as an
example of radiation therapy innovation that improves the
therapeutic index. Despite the complexity of its technology and the
uncertainty of its efficacy, future studies on the mechanism will
facilitate translation to clinical practice for the benefit of
patients. In the previous special issue (33,46–48),
relevant reviews frequently describe in detail a specific point
about FLASH radiotherapy, such as FLASH effects, mechanisms, or
potential and obstacles to clinical application. However, the
present review provided a unified and comprehensive overview of
FLASH in numerous aspects. It may also serve as an introduction to
those researchers who are not familiar with FLASH-RT. In
particular, for non-radiology researchers, such as surgical
researchers, it may provide new ideas for the interdisciplinary
treatment of malignant tumors.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Key
Technologies R&D Program (grant no. 2018YFC1106800).
Availability of data and materials
Not applicable.
Authors' contributions
YHL, ZW and YL conceived the study. YHL, TL, XPF
and JZ conducted the literature search. YHL, HC, XM and WWL wrote
and prepared the original draft of the manuscript. YHL, YL, ZW, TL,
XPF, HC, JL, XM, JPD, GMH, WWL, KFY and HW participated in the
writing and reviewing the article. YHL, JPD, GMH, KFY and HW edited
the article. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Con-RT
|
conventional radiotherapy
|
|
PBT
|
proton beam therapy
|
|
ROS
|
reactive oxygen species
|
|
LET
|
linear energy transfer
|
|
NROS
|
non-reactive oxygen species
|
|
PFS
|
progression-free survival
|
|
CRT
|
cathode ray tube
|
References
|
1
|
Kurup A, Pasternak J, Taylor R, Murgatroyd
L, Ettlinger O, Shields W, Nevay L, Gruber S, Pozimski J, Lau HT,
et al: Simulation of a radiobiology facility for the centre for the
clinical application of particles. Phys Med. 65:21–28. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Durante M, Bräuer-Krisch E and Hill M:
Faster and safer? FLASH ultra-high dose rate in radiotherapy. Br J
Radiol. 91:201706282018.PubMed/NCBI
|
|
3
|
Berry RJ: Effects of radiation dose-rate
from protracted, continuous irradiation to ultra-high dose-rates
from pulsed accelerators. Br Med Bull. 29:44–47. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hornsey S and Alper T: Unexpected
dose-rate effect in the killing of mice by radiation. Nature.
210:212–213. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vozenin MC, De Fornel P, Petersson K,
Favaudon V, Jaccard M, Germond JF, Petit B, Burki M, Ferrand G,
Patin D, et al: The Advantage of FLASH radiotherapy confirmed in
mini-pig and cat-cancer patients. Clin Cancer Res. 25:35–42. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dewey DL and Boag JW: Modification of the
oxygen effect when bacteria are given large pulses of radiation.
Nature. 183:1450–1451. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Town CD: Radiobiology. Effect of high dose
rates on survival of mammalian cells. Nature. 215:847–848. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Field SB and Bewley DK: Effects of
dose-rate on the radiation response of rat skin. Int J Radiat Biol
Relat Stud Phys Chem Med. 26:259–267. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berry RJ, Hall EJ, Forster DW, Storr TH
and Goodman MJ: Survival of mammalian cells exposed to × rays at
ultra-high dose-rates. Br J Radiol. 42:102–107. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hornsey S and Bewley DK: Hypoxia in mouse
intestine induced by electron irradiation at high dose-rates. Int J
Radiat Biol Relat Stud Phys Chem Med. 19:479–483. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Favaudon V, Caplier L, Monceau V,
Pouzoulet F, Sayarath M, Fouillade C, Poupon MF, Brito I, Hupé P,
Bourhis J, et al: Ultrahigh dose-rate FLASH irradiation increases
the differential response between normal and tumor tissue in mice.
Sci Transl Med. 6:245ra932014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Montay-Gruel P, Petersson K, Jaccard M,
Boivin G, Germond JF, Petit B, Doenlen R, Favaudon V, Bochud F,
Bailat C, et al: Irradiation in a flash: Unique sparing of memory
in mice after whole brain irradiation with dose rates above
100Gy/s. Radiother Oncol. 124:365–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Montay-Gruel P, Bouchet A, Jaccard M,
Patin D, Serduc R, Aim W, Petersson K, Petit B, Bailat C, Bourhis
J, et al: X-rays can trigger the FLASH effect: Ultra-high dose-rate
synchrotron light source prevents normal brain injury after whole
brain irradiation in mice. Radiother Oncol. 129:582–588. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Montay-Gruel P, Acharya MM, Petersson K,
Alikhani L, Yakkala C, Allen BD, Ollivier J, Petit B, Jorge PG,
Syage AR, et al: Long-term neurocognitive benefits of FLASH
radiotherapy driven by reduced reactive oxygen species. Proc Natl
Acad Sci USA. 116:10943–10951. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alaghband Y, Cheeks SN, Allen BD,
Montay-Gruel P, Doan NL, Petit B, Jorge PG, Giedzinski E, Acharya
MM, Vozenin MC and Limoli CL: Neuroprotection of radiosensitive
juvenile mice by ultra-high dose rate FLASH irradiation. Cancers
(Basel). 12:16712020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van de Water S, Safai S, Schippers JM,
Weber DC and Lomax AJ: Towards FLASH proton therapy: The impact of
treatment planning and machine characteristics on achievable dose
rates. Acta Oncol. 58:1463–1469. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bourhis J, Sozzi WJ, Jorge PG, Gaide O,
Bailat C, Duclos F, Patin D, Ozsahin M, Bochud F, Germond JF, et
al: Treatment of a first patient with FLASH-radiotherapy. Radiother
Oncol. 139:18–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei S, Lin H, Choi JI, Simone CB II and
Kang M: A novel proton pencil beam scanning FLASH RT delivery
method enables optimal OAR sparing and ultra-high dose rate
delivery: A comprehensive dosimetry study for lung tumors. Cancers
(Basel). 13:57902021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smyth LML, Donoghue JF, Ventura JA,
Livingstone J, Bailey T, Day LRJ, Crosbie JC and Rogers PAW:
Comparative toxicity of synchrotron and conventional radiation
therapy based on total and partial body irradiation in a murine
model. Sci Rep. 8:120442018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Venkatesulu BP, Sharma A, Pollard-Larkin
JM, Sadagopan R, Symons J, Neri S, Singh PK, Tailor R, Lin SH and
Krishnan S: Ultra high dose rate (35 Gy/sec) radiation does not
spare the normal tissue in cardiac and splenic models of
lymphopenia and gastrointestinal syndrome. Sci Rep. 9:171802019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bourhis J, Montay-Gruel P, Gonçalves Jorge
P, Bailat C, Petit B, Ollivier J, Jeanneret-Sozzi W, Ozsahin M,
Bochud F, Moeckli R, et al: Clinical translation of FLASH
radiotherapy: Why and how? Radiother Oncol. 139:11–17. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou S, Zheng D, Fan Q, Yan Y, Wang S, Lei
Y, Besemer A, Zhou C and Enke C: Minimum dose rate estimation for
pulsed FLASH radiotherapy: A dimensional analysis. Med Phys.
47:3243–3249. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fowler JF and Stern BE: Dose-rate effects:
Some theoretical and practical considerations. Br J Radiol.
33:389–395. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orton CG: A unified approach to
dose-effect relationships in radiotherapy. II: Inhomogeneous dose
distributions. Int J Radiat Oncol Biol Phys. 14:557–560. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steel H, Brüningk SC, Box C, Oelfke U and
Bartzsch SH: Quantification of differential response of tumour and
normal cells to microbeam radiation in the absence of FLASH
effects. Cancers (Basel). 13:32282021. View Article : Google Scholar
|
|
26
|
Schaub L, Harrabi SB and Debus J: Particle
therapy in the future of precision therapy. Br J Radiol.
93:202001832020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blakely EA: The 20th Gray lecture 2019:
Health and heavy ions. Br J Radiol. 93:202001722020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ando K and Kase Y: Biological
characteristics of carbon-ion therapy. Int J Radiat Biol.
85:715–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parodi K: The biological treatment
planning evolution of clinical fractionated radiotherapy using high
LET. Int J Radiat Biol. 94:752–755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rothwell BC, Kirkby NF, Merchant MJ,
Chadwick AL, Lowe M, Mackay RI, Hendry JH and Kirkby KJ:
Determining the parameter space for effective oxygen depletion for
FLASH radiation therapy. Phys Med Biol. 66:0550202021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spitz DR, Buettner GR, Petronek MS,
St-Aubin JJ, Flynn RT, Waldron TJ and Limoli CL: An integrated
physico-chemical approach for explaining the differential impact of
FLASH versus conventional dose rate irradiation on cancer and
normal tissue responses. Radiother Oncol. 139:23–27. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liew H, Mein S, Dokic I, Haberer T, Debus
J, Abdollahi A and Mairani A: Deciphering time-dependent DNA damage
complexity, repair, and oxygen tension: A mechanistic model for
FLASH-dose-rate radiation therapy. Int J Radiat Oncol Biol Phys.
110:574–586. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schüler E, Acharya M, Montay-Gruel P, Loo
BW Jr, Vozenin MC and Maxim PG: Ultra-high dose rate electron beams
and the FLASH effect: From preclinical evidence to a new
radiotherapy paradigm. Med Phys. 49:2082–2095. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Correction to lancet diabetes endocrinol.
2019.7:288–99. Lancet Diabetes Endocrinol. 7:e52019.PubMed/NCBI
|
|
35
|
Girdhani S, Abel E, Katsis A, Rodriquez A,
Senapati S, KuVillanueva A, Jackson IL, Eley J, Vujaskovic Z and
Parry R: Abstract LB-280: FLASH: A novel paradigm changing tumor
irradiation platform that enhances therapeutic ratio by reducing
normal tissue toxicity and activating immune pathways. Cancer Res.
79 (Suppl 13):LB–280. 2019. View Article : Google Scholar
|
|
36
|
Beyreuther E, Brand M, Hans S, Hideghéty
K, Karsch L, Leßmann E, Schürer M, Szabó ER and Pawelke J:
Feasibility of proton FLASH effect tested by zebrafish embryo
irradiation. Radiother Oncol. 139:46–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Buonanno M, Grilj V and Brenner DJ:
Biological effects in normal cells exposed to FLASH dose rate
protons. Radiother Oncol. 139:51–55. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Diffenderfer ES, Verginadis II, Kim MM,
Shoniyozov K, Velalopoulou A, Goia D, Putt M, Hagan S, Avery S, Teo
K, et al: Design, implementation, and in vivo validation of a novel
proton FLASH radiation therapy system. Int J Radiat Oncol Biol
Phys. 106:440–448. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Colangelo NW and Azzam EI: The importance
and clinical implications of FLASH ultra-high dose-rate studies for
proton and heavy ion radiotherapy. Radiat Res. 193:1–4. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
van Marlen P, Dahele M, Folkerts M, Abel
E, Slotman BJ and Verbakel WFAR: Bringing FLASH to the clinic:
Treatment planning considerations for ultrahigh dose-rate proton
beams. Int J Radiat Oncol Biol Phys. 106:621–629. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Diffenderfer ES, Sørensen BS, Mazal A and
Carlson DJ: The current status of preclinical proton FLASH
radiation and future directions. Med Phys. 49:2039–2054. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Petersson K, Adrian G, Butterworth K and
McMahon SJ: A quantitative analysis of the role of oxygen tension
in FLASH radiation therapy. Int J Radiat Oncol Biol Phys.
107:539–547. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Adrian G, Konradsson E, Lempart M, Bäck S,
Ceberg C and Petersson K: The FLASH effect depends on oxygen
concentration. Br J Radiol. 93:201907022020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim MM, Verginadis II, Goia D, Haertter A,
Shoniyozov K, Zou W, Maity A, Busch TM, Metz JM, Cengel KA, et al:
Comparison of FLASH proton entrance and the spread-out bragg peak
dose regions in the sparing of mouse intestinal crypts and in a
pancreatic tumor model. Cancers (Basel). 13:42442021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Patriarca A, Fouillade C, Auger M, Martin
F, Pouzoulet F, Nauraye C, Heinrich S, Favaudon V, Meyroneinc S,
Dendale R, et al: Experimental set-up for FLASH proton irradiation
of small animals using a clinical system. Int J Radiat Oncol Biol
Phys. 102:619–626. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nesteruk KP, Togno M, Grossmann M, Lomax
AJ, Weber DC, Schippers JM, Safai S, Meer D and Psoroulas S:
Commissioning of a clinical pencil beam scanning proton therapy
unit for ultra-high dose rates (FLASH). Med Phys. 48:4017–4026.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Montay-Gruel P, Corde S, Laissue JA and
Bazalova-Carter M: FLASH radiotherapy with photon beams. Med Phys.
49:2055–2067. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Weber UA, Scifoni E and Durante M: FLASH
radiotherapy with carbon ion beams. Med Phys. 49:1974–1992. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pajonk F, Vlashi E and McBride WH:
Radiation resistance of cancer stem cells: The 4 R's of
radiobiology revisited. Stem Cells. 28:639–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wilson JD, Hammond EM, Higgins GS and
Petersson K: Corrigendum: Ultra-high dose rate (FLASH)
radiotherapy: Silver bullet or fool's gold? Front Oncol.
10:2102020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mohyeldin A, Garzón-Muvdi T and
Quiñones-Hinojosa A: Oxygen in stem cell biology: A critical
component of the stem cell niche. Cell Stem Cell. 7:150–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wilson P, Jones B, Yokoi T, Hill M and
Vojnovic B: Revisiting the ultra-high dose rate effect:
Implications for charged particle radiotherapy using protons and
light ions. Br J Radiol. 85:e933–e939. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Morgan WF and Sowa MB: Effects of ionizing
radiation in nonirradiated cells. Proc Natl Acad Sci USA.
102:14127–14128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Santivasi WL and Xia F: Ionizing
radiation-induced DNA damage, response, and repair. Antioxid Redox
Signal. 21:251–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Grimes DR and Partridge M: A mechanistic
investigation of the oxygen fixation hypothesis and oxygen
enhancement ratio. Biomed Phys Eng Express. 1:0452092015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Vozenin MC, Hendry JH and Limoli CL:
Biological benefits of ultra-high dose rate FLASH radiotherapy:
Sleeping beauty awoken. Clin Oncol (R Coll Radiol). 31:407–415.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Levy K, Natarajan S, Wang J, Chow S,
Eggold JT, Loo P, Manjappa R, Lartey FM, Schüler E, Skinner L, et
al: FLASH irradiation enhances the therapeutic index of abdominal
radiotherapy for the treatment of ovarian cancer. bioRxiv.
2019.2012.2012.873414. 2020.
|
|
58
|
Rama N, Saha T, Shukla S, Goda C, Milewski
D, Mascia AE, Vatner RE, Sengupta D, Katsis A, Abel E, et al:
Improved tumor control through T-cell infiltration modulated by
ultra-high dose rate proton FLASH using a clinical pencil beam
scanning proton system. Int J Radiat Oncol Biol Phys. 105 (Suppl
1):S164–S165. 2019. View Article : Google Scholar
|
|
59
|
Boscolo D, Krämer M, Fuss MC, Durante M
and Scifoni E: Impact of target oxygenation on the chemical track
evolution of ion and electron radiation. Int J Mol Sci. 21:4242020.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cao X, Zhang R, Esipova TV, Allu SR,
Ashraf R, Rahman M, Gunn JR, Bruza P, Gladstone DJ, Williams BB, et
al: Quantification of oxygen depletion during FLASH irradiation in
vitro and in vivo. Int J Radiat Oncol Biol Phys. 111:240–248. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tinganelli W, Sokol O, Quartieri M,
Puspitasari A, Dokic I, Abdollahi A, Durante M, Haberer T, Debus J,
Boscolo D, et al: Ultra-high dose rate (FLASH) carbon ion
irradiation: Dosimetry and first cell experiments. Int J Radiat
Oncol Biol Phys. 112:1012–1022. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kranzer R, Poppinga D, Weidner J, Schüller
A, Hackel T, Looe HK and Poppe B: Ion collection efficiency of
ionization chambers in ultra-high dose-per-pulse electron beams.
Med Phys. 48:819–830. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jansen J, Knoll J, Beyreuther E, Pawelke
J, Skuza R, Hanley R, Brons S, Pagliari F and Seco J: Does FLASH
deplete oxygen? Experimental evaluation for photons, protons, and
carbon ions. Med Phys. 48:3982–3990. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Boscolo D, Scifoni E, Durante M, Krämer M
and Fuss MC: May oxygen depletion explain the FLASH effect? A
chemical track structure analysis. Radiother Oncol. 162:68–75.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Abolfath R, Grosshans D and Mohan R:
Oxygen depletion in FLASH ultra-high-dose-rate radiotherapy: A
molecular dynamics simulation. Med Phys. 47:6551–6561. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Favaudon V, Labarbe R and Limoli CL: Model
studies of the role of oxygen in the FLASH effect. Med Phys.
49:2068–2081. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fernet M, Ponette V, Deniaud-Alexandre E,
Ménissier-De Murcia J, De Murcia G, Giocanti N, Megnin-Chanet F and
Favaudon V: Poly(ADP-ribose) polymerase, a major determinant of
early cell response to ionizing radiation. Int J Radiat Biol.
76:1621–1629. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Arina A, Beckett M, Fernandez C, Zheng W,
Pitroda S, Chmura SJ, Luke JJ, Forde M, Hou Y, Burnette B, et al:
Tumor-reprogrammed resident T cells resist radiation to control
tumors. Nat Commun. 10:39592019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Holmgaard RB, Schaer DA, Li Y, Castaneda
SP, Murphy MY, Xu X, Inigo I, Dobkin J, Manro JR, Iversen PW, et
al: Targeting the TGFβ pathway with galunisertib, a TGFβRI small
molecule inhibitor, promotes anti-tumor immunity leading to
durable, complete responses, as monotherapy and in combination with
checkpoint blockade. J Immunother Cancer. 6:472018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zlobinskaya O, Siebenwirth C, Greubel C,
Hable V, Hertenberger R, Humble N, Reinhardt S, Michalski D, Röper
B, Multhoff G, et al: The effects of ultra-high dose rate proton
irradiation on growth delay in the treatment of human tumor
xenografts in nude mice. Radiat Res. 181:177–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Simmons DA, Lartey FM, Schüler E, Rafat M,
King G, Kim A, Ko R, Semaan S, Gonzalez S, Jenkins M, et al:
Reduced cognitive deficits after FLASH irradiation of whole mouse
brain are associated with less hippocampal dendritic spine loss and
neuroinflammation. Radiother Oncol. 139:4–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Friedl AA, Prise KM, Butterworth KT,
Montay-Gruel P and Favaudon V: Radiobiology of the FLASH effect.
Med Phys. 49:1993–2013. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Durante M, Yamada S, Ando K, Furusawa Y,
Kawata T, Majima H, Nakano T and Tsujii H: Measurements of the
equivalent whole-body dose during radiation therapy by cytogenetic
methods. Phys Med Biol. 44:1289–1298. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Prise KM and O'Sullivan JM:
Radiation-induced bystander signalling in cancer therapy. Nat Rev
Cancer. 9:351–360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dewan MZ, Galloway AE, Kawashima N,
Dewyngaert JK, Babb JS, Formenti SC and Demaria S: Fractionated but
not single-dose radiotherapy induces an immune-mediated abscopal
effect when combined with anti-CTLA-4 antibody. Clin Cancer Res.
15:5379–5388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Fouillade C, Curras-Alonso S, Giuranno L,
Quelennec E, Heinrich S, Bonnet-Boissinot S, Beddok A, Leboucher S,
Karakurt HU, Bohec M, et al: FLASH irradiation spares lung
progenitor cells and limits the incidence of radio-induced
senescence. Clin Cancer Res. 26:1497–1506. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Scully R, Panday A, Elango R and Willis
NA: DNA double-strand break repair-pathway choice in somatic
mammalian cells. Nat Rev Mol Cell Biol. 20:698–714. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Labarbe R, Hotoiu L, Barbier J and
Favaudon V: A physicochemical model of reaction kinetics supports
peroxyl radical recombination as the main determinant of the FLASH
effect. Radiother Oncol. 153:303–310. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kulkarni A, Anderson AG, Merullo DP and
Konopka G: Beyond bulk: A review of single cell transcriptomics
methodologies and applications. Curr Opin Biotechnol. 58:129–136.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lohse I, Lang S, Hrbacek J, Scheidegger S,
Bodis S, Macedo NS, Feng J, Lütolf UM and Zaugg K: Effect of high
dose per pulse flattening filter-free beams on cancer cell
survival. Radiother Oncol. 101:226–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Levy K, Natarajan S, Wang J, Chow S,
Eggold JT, Loo PE, Manjappa R, Melemenidis S, Lartey FM, Schüler E,
et al: Abdominal FLASH irradiation reduces radiation-induced
gastrointestinal toxicity for the treatment of ovarian cancer in
mice. Sci Rep. 10:216002020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chabi S, To THV, Leavitt R, Poglio S,
Jorge PG, Jaccard M, Petersson K, Petit B, Roméo PH, Pflumio F, et
al: Ultra-high-dose-rate FLASH and conventional-dose-rate
irradiation differentially affect human acute lymphoblastic
leukemia and normal hematopoiesis. Int J Radiat Oncol Biol Phys.
109:819–829. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Vozenin MC, Montay-Gruel P, Limoli C and
Germond JF: All irradiations that are ultra-high dose rate may not
be FLASH: The critical importance of beam parameter
characterization and in vivo validation of the FLASH effect. Radiat
Res. 194:571–572. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Jorge PG, Jaccard M, Petersson K, Gondré
M, Durán MT, Desorgher L, Germond JF, Liger P, Vozenin MC, Bourhis
J, et al: Dosimetric and preparation procedures for irradiating
biological models with pulsed electron beam at ultra-high
dose-rate. Radiother Oncol. 139:34–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ko RB, Soto LA, von Eyben R, Melemenidis
S, Rankin EB, Maxim PG, Graves EE and Loo BW: Evaluating the
reproducibility of mouse anatomy under rotation in a custom
immobilization device for conformal FLASH radiotherapy. Radiat Res.
194:600–606. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Soto LA, Casey KM, Wang J, Blaney A,
Manjappa R, Breitkreutz D, Skinner L, Dutt S, Ko RB, Bush K, et al:
FLASH irradiation results in reduced severe skin toxicity compared
to conventional-dose-rate irradiation. Radiat Res. 194:618–624.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Khan S, Bassenne M, Wang J, Manjappa R,
Melemenidis S, Breitkreutz DY, Maxim PG, Xing L, Loo BW Jr and
Pratx G: Multicellular spheroids as in vitro models of oxygen
depletion during FLASH irradiation. Int J Radiat Oncol Biol Phys.
110:833–844. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Maxim PG, Keall P and Cai J: FLASH
radiotherapy: Newsflash or flash in the pan? Med Phys.
46:4287–4290. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Prempree T, Michelsen A and Merz T: The
repair time of chromosome breaks induced by pulsed x-rays on
ultra-high dose-rate. Int J Radiat Biol Relat Stud Phys Chem Med.
15:571–574. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Evans SE, Grigoryan A and Szalai VA:
Oxidation of guanine in double-stranded DNA by [Ru(bpy)2dppz]Cl2 in
cationic reverse micelles. Inorg Chem. 46:8349–8361. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lapi A, Pratviel G and Meunier B: Guanine
oxidation in double-stranded DNA by MnTMPyP/KHSO(5): At least three
independent reaction pathways. Met Based Drugs. 8:47–56. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rahman M, Ashraf MR, Gladstone DJ, Bruza
P, Jarvis LA, Schaner PE, Cao X, Pogue BW, Hoopes PJ and Zhang R:
Treatment planning system for electron FLASH radiation therapy:
Open-source for clinical implementation. Int J Radiat Oncol Biol
Phys. 112:1023–1032. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lin B, Gao F, Yang Y, Wu D, Zhang Y, Feng
G, Dai T and Du X: FLASH radiotherapy: History and future. Front
Oncol. 11:6444002021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Taylor PA, Moran JM, Jaffray DA and
Buchsbaum JC: A roadmap to clinical trials for FLASH. Med Phys.
49:4099–4108. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hendry JH, Moore JV, Hodgson BW and Keene
JP: The constant low oxygen concentration in all the target cells
for mouse tail radionecrosis. Radiat Res. 92:172–181. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Abel E, Girdhani S, Jackson I, Eley J,
Katsis A, Marshall A, Rodriguez A, Senapati S, Bentzen SM,
Vujaskovic Z, et al: Characterization of radiation-induced lung
fibrosis and mode of cell death using single and multi-pulsed
proton flash irradiation. Int J Radiat Oncol Biol Phys. 105 (Suppl
1):E652–E653. 2019. View Article : Google Scholar
|
|
97
|
Cunningham S, McCauley S, Vairamani K,
Speth J, Girdhani S, Abel E, Sharma RA, Perentesis JP, Wells SI,
Mascia A and Sertorio M: FLASH proton pencil beam scanning
irradiation minimizes radiation-induced leg contracture and skin
toxicity in mice. Cancers (Basel). 13:10122021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Velalopoulou A, Karagounis IV, Cramer GM,
Kim MM, Skoufos G, Goia D, Hagan S, Verginadis II, Shoniyozov K,
Chiango J, et al: FLASH proton radiotherapy spares normal
epithelial and mesenchymal tissues while preserving sarcoma
response. Cancer Res. 81:4808–4821. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Montay-Gruel P, Acharya MM, Gonçalves
Jorge P, Petit B, Petridis IG, Fuchs P, Leavitt R, Petersson K,
Gondré M, Ollivier J, et al: Hypofractionated FLASH-RT as an
effective treatment against glioblastoma that reduces
neurocognitive side effects in mice. Clin Cancer Res. 27:775–784.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
AAPM, . PUBLICATIONS-REPORTS. https://www.aapm.org/pubs/reports/August
26–2021
|
|
101
|
ESTRO, . ESTRO Guidelines. https://www.estro.org/Science/GuidelinesAugust
26–2021
|