Introduction
Standard postoperative radiotherapy with concomitant
and adjuvant temozolomide (TMZ) has been shown to be beneficial for
glioblastoma treatment, according to a randomized phase III trial
by The European Organization for Research and Treatment of Cancer
and The National Cancer Institute of Canada Clinical Trials Group
in 2019 (1). Subsequently,
radiotherapy with concomitant TMZ followed by adjuvant TMZ
chemotherapy became the global standard treatment for malignant
gliomas, particularly glioblastomas. Although these treatments
improve the survival rate of patients with glioblastoma, the
prognosis is unsatisfactory, as the 5-year survival rate remains
<5% (2). Therefore, novel
therapies are needed.
Glutamate is one of the main excitatory
neurotransmitters in the central nervous system. Dysregulation of
glutamate signaling is extremely important in seizure induction by
initiating and synchronizing glutamatergic transmission (3,4).
Epileptic seizures are a common symptom of primary brain tumors.
Approximately 25–60% of patients with high-grade gliomas (World
Health Organization grades 3 and 4) suffer from seizures
(tumor-associated epilepsy) and ~15–25% of patients with high-grade
gliomas develop drug-resistant epilepsy (5). Furthermore, glioma cells express
α-amino-3- hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)
receptors, which are stimulated by glutamate in autocrine and
paracrine responses and subsequently tumor growth and invasion,
possibly through the activation of oncogenic signaling cascades and
cytoskeletal remodeling (6–11).
The pathophysiology of malignant gliomas and
tumor-associated epilepsy is not fully understood; however, AMPA
receptor antagonists are promising candidates that may mediate both
anticonvulsive and antitumoral effects on glioma cells.
Furthermore, blocking AMPA receptors was reported to increase the
effectiveness of conventional cytotoxic chemotherapy in certain
cancer cell lines, including lung carcinoma, astrocytoma,
neuroblastoma and rhabdomyosarcoma/medulloblastoma cells. (6,12).
Talampanel is an orally administered non-competitive antagonist of
AMPA receptor with good permeability across the blood-brain barrier
and good tolerability in clinical trials for epilepsy and other
neurologic disorders (9,13). A phase II trial of talampanel in
combination with radiotherapy and TMZ demonstrated a survival
advantage for patients with newly diagnosed glioblastoma compared
to patients not treated with talampanel (14,15);
however, talampanel failed to demonstrate significant antitumor
activity as a single agent in patients with recurrent malignant
glioma (9). Another
non-competitive AMPA receptor antagonist, perampanel, has excellent
brain penetration, a long half-life and a good drug concentration
profile compared to talampanel (4,15–18).
Perampanel is effective against drug-resistant epilepsy in patients
with glioma (5,15,17,19).
Recently, perampanel treatment was reported to be effective for
uncontrollable epilepsy with gliomas, and magnetic resonance
imaging was reported to demonstrate inhibition of tumor growth
and/or reduction of peritumoral brain edema (17). Perampanel has antiproliferative
effects on glioblastoma cell lines by reducing cell metabolism
owing to a decrease in glucose uptake (15). Recently, the antitumoral effects of
perampanel were reported to be mediated by the induction of
apoptosis and a synergistic effect with TMZ in glioblastoma cell
lines (20). The present study
used much higher concentrations of perampanel than the usual
maintenance blood concentration of perampanel when used as an
anticonvulsant; the results suggested that perampanel may act as an
anticonvulsant and may also have additional antitumoral effects on
malignant gliomas. However, the detailed mechanisms of the
antitumoral action remains unknown.
The poor prognosis for patients with glioblastoma
may be partly due to the migratory and invasive behavior of tumor
cells that exhibit widespread infiltration into the adjacent brain
parenchyma (21). Recently,
overexpression of SERPINE1, a clade E member of the serine protease
inhibitor superfamily that is the main regulator of the plasminogen
activator system, has been proposed as a factor for tumor migration
and invasion in numerous types of cancer, including glioma, which
results in poor patient prognosis (21). Furthermore, SERPINE1 has been
reported to serve roles in CSF dissemination, angiogenesis,
apoptosis, drug resistance, etc (21,22).
Preliminary experiments (data not shown) suggested that
SERPINE1 expression was involved in perampanel
susceptibility; perampanel, may also be associated with invasion
and migration.
The present study evaluated the relationship between
perampanel and SERPINE1, and assessed the mechanisms of their
antitumoral activities.
Materials and methods
Cell lines, culture conditions and
materials
Human malignant glioma cell lines A-172 (cat. no.
JCRB0228; lot no. 021999), AM-38 (cat. no. IFO50492; lot no.
12082003), T98G (cat. no. IFO50303; lot no. 1007), U-251MG (cat.
no. IFO50288; lot no. 12132002) and YH-13 (cat. no. IFO50493; lot
no. 1164) were purchased from Health Science Research Resources
Bank. The human glioblastoma U-138MG cell line (cat. no. HTB-16;
lot no. 1104428) was purchased from the American Type Culture
Collection.
Cells were cultured in Dulbecco's modified Eagle's
minimum essential medium (DMEM) (Nissui Pharmaceutical, Co., Ltd.)
supplemented with 5% fetal calf serum (Thermo Fisher Scientific,
Inc.) using plastic culture flasks (Corning, Inc.) in a 37°C
humidified incubator with an atmosphere containing 5%
CO2.
Perampanel was gifted by Eisai Co., Ltd. and TMZ was
purchased from Tokyo Chemical Industry Co., Ltd.
Cell culture viability
experiments
The inhibitory effect of perampanel on the viability
of malignant glioma cells was evaluated by counting the number of
cells following exposure to the drug. Briefly, cells were plated at
1×104 cells/well in 24-well, flat-bottomed plates (Iwaki
Cell Biology) and incubated with DMEM (Nissui Pharmaceutical) with
5% fetal calf serum (Thermo Fisher Scientific) at 37°C for 24 h.
The cells were subsequently washed twice with medium and further
incubated for 72 h with fresh medium (control) or medium containing
0.01, 0.1, 1 or 10 µM perampanel. Subsequently, cells were detached
by trypsinization and counted using a Coulter Counter Z1 (Beckman
Coulter, Inc.). The experiment was repeated at each concentration
in at least three independent systems and cell numbers were
quantified at least eight times in total.
Subsequent experiments used 1.0 µM perampanel, based
the blood concentrations previously reported as being achieved
using oral administration of 4 mg or 8 mg perampanel as an
antiepileptic drug of 0.84 µM or 1.48 µM, respectively (17).
Furthermore, as a low concentration of perampanel
demonstrated significant anti-viability effects on the T98G and
U-251MG cell lines, which are widely used in brain tumor
experiments, these cell lines were used in the subsequent
experiments (cell cycle distribution analysis and assessment of
apoptosis). T98G expresses MGMT and U-251MG does not express MGMT
(23).
Cell cycle distribution analysis
Perampanel-induced changes in the cell cycle
distribution were assessed using flow cytometry. T98G and U-251MG
cells were seeded in 6-well plates (Iwaki Cell Biology) at
1×106 cells/plate and incubated at 37°C for 24 h. The
culture medium was replaced with fresh medium (control) or medium
with 1.0 µM perampanel and incubated at 37°C for 0, 6, 24 and 48 h.
Then the cells were harvested using trypsin-EDTA solution and fixed
in ice-cold 70% ethanol for 2 h. The fixed cells were treated with
0.5% RNase A (Roche Diagnostics GmbH) for 30 min and stained with
1.0 µg/ml propidium iodide (PI) solution (Miltenyi Biotech, Inc.)
for 30 min at room temperature. Fluorescence was measured using a
BD fluorescence activated cell sorter (FACS)-Calibur Flow Cytometer
(BD Biosciences) at a wavelength of 610 nm. The histograms were
analyzed using FlowJo software (version 10.5.3. BioLegend, Inc.).
The experiments were repeated at least four times.
Assessment of apoptosis
Apoptosis induced by perampanel in T98G and U-251MG
malignant glioma cells was assessed using flow cytometry and
western blotting.
Flow cytometry
Apoptosis was assessed by flow cytometry, with
Annexin V/PI double staining. Cells were seeded in 6-well plates
(Iwaki Cell Biology) at 1×106 cells/well and incubated
at 37°C for 24 h. The culture medium was then replenished with
fresh medium (control) or with medium containing perampanel (0.01,
0.1, 1 or 10 µM) at 37°C for 48 h. The cells were then washed with
phosphate-buffered saline (PBS) and collected using trypsin-EDTA
solution. Following centrifugation (two times at 15,300 × g for 5
min at 4°C) and washing in PBS, the solution was agitated with 100
µl of binding buffer (Wako Pure Chemical Industries, Ltd.), into
which 5 µl of Annexin V-Alexa Fluor 488 conjugate (Thermo Fisher
Scientific, Inc.) and 10 µl of PI solution (Miltenyi Biotech, Inc.)
were added and incubated at room temperature for 10 min. An
additional binding buffer was added to give a total sample volume
of 500 µl. Fluorescence was measured using a BD FACS-Calibur Flow
Cytometer (BD Biosciences). The apoptotic cells were analyzed using
FlowJo software (version 10.5.3. BioLegend, Inc). The experiments
were repeated at least four times to confirm reproducibility.
Western blotting
Western blotting was performed after treatment with
1.0 µM perampanel at 37°C for 0, 4, 8 or 24 h. Proteins were
isolated from cells lysed in RIPA buffer (Wako Pure Chemical
Industries, Ltd.) supplemented with protease inhibitor complex mix
(cOmplete, Mini, EDTA-free. Roche Diagnostics GmbH). The protein
concentrations were determined using a Pierce BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.). A total of 50 µg of protein per
lane was separated by 12% SDS-PAGE (Tefco Co., Ltd.) and
transferred onto nitrocellulose membranes (Cytiva) for 60 min at 15
V using Bio-Rad Trans-Blot transfer system (Bio-Rad Laboratories,
Inc.). The membranes were blocked using 1% skimmed milk dissolved
in washing buffer (PBS +0.2% Tween-20) for 60 min at room
temperature, then incubated with primary antibodies at 4°C
overnight as follows: Anti-caspase-3 mouse monoclonal antibody
(mAb; 1:500; cat. no. sc-7272. Santa Cruz biotechnology, Inc.) and
anti-β-actin mouse mAb (1:200; cat. no. sc-47778; Santa Cruz
biotechnology, Inc.), which was utilized as a loading control. The
membranes were incubated for 60 min at room temperature with the
HRP conjugated goat anti-rabbit IgG secondary antibody (1:100). The
band patterns were analyzed using an ImageQuant LAS-4000 (Cytiva)
after treatment with ECL Prime Western Blotting Detection Reagent
(Cytiva). The experiments were repeated three times.
Enhanced effects of TMZ by
perampanel
Whether a combination of TMZ and perampanel could
produce an additive antitumor effect compared with TMZ-only
treatment in malignant glioma cells was assessed. Glioma cells,
A-172, AM-38, T98G, U-138MG, U-251MG and YH-13, were plated at
1×104 cells/well in 24-well, flat-bottomed plates (Iwaki
Cell Biology) and incubated with DMEM (Nissui Pharmaceutical) with
5% fetal calf serum (Thermo Fisher Scientific) at 37°C for 24 h.
Subsequently, the cells were incubated with medium containing
perampanel (0.01, 0.1, 1, 10, 100 µM) with or without 10 µM TMZ.
The TMZ concentration was chosen to represent a clinically relevant
concentration of TMZ (24). After
exposure to the various concentrations of perampanel with or
without TMZ for 72 h, cells were detached by trypsinization and
counted using a Coulter Counter Z1 (Beckman Coulter, Inc.). The
experiments were repeated at least four times at each concentration
of perampanel.
Enhancement of perampanel by inhibitor
of SERPINE1
Overexpression of SERPINE1 is an important factor
for tumor migration and invasion in numerous types of cancer and is
considered to indicate poor prognosis (21,22).
In a preliminary experiment (data not shown), western blotting
demonstrated that the SERPINE1 protein expression level was high in
U-138MG and A-172 cells that were resistant to perampanel.
Furthermore, RNA-sequencing was performed by the Bioengineering
Lab. Co., Ltd. which demonstrated that the SERPINE1 mRNA
expression level in U-138MG cells was ~100 times higher compared
with that in T98G cells, which were highly sensitive to perampanel
(data not shown). Both T98G and U-138MG cells expressed MGMT
(23).
The combination of perampanel and tiplaxtinin, a
selective and orally efficacious inhibitor of SERPINE1, to produce
an additive antitumor effect compared with perampanel-only
treatment was evaluated in malignant glioma cells. U-138MG cells
were plated at 1×104 cells/well in 24-well,
flat-bottomed plates (Iwaki Cell Biology) and incubated with medium
for 24 h, as for the aforementioned cell viability studies, and
then incubated at 37°C in medium with or without 1.0 µM perampanel
and with or without 5.0 µM tiplaxtinin (MilliporeSigma). After
incubation for 72 h, the cells were detached by trypsinization and
counted using a Coulter Counter Z1 (Beckman Coulter, Inc.). The
experiments were repeated at least nine times for each
condition.
Statistical analysis
Unpaired Student's t-test was used to compare the
data between two groups, and one-way ANOVA followed by the
Tukey-Kramer post hoc test was used for multiple comparisons, using
the SPSS (version 21.0; IBM Corp.). Data are presented as the mean
± SEM and were considered significantly different at P<0.05.
Results
Our previous study demonstrated, by reverse
transcription PCR and western blotting, that
O6-methylguanine-DNA methyltransferase (MGMT), a key
factor of alkylating agents, was expressed in T98G, U-138MG and
YH-13 cells (23). Consistent with
an earlier study (25), the
present study also confirmed in our laboratory that T98G (M237I)
and U-251MG (R273H) both had a point mutation in the p53
gene (data not shown).
Antitumoral effects and cell
sensitivity to perampanel in human malignant glioma cell lines
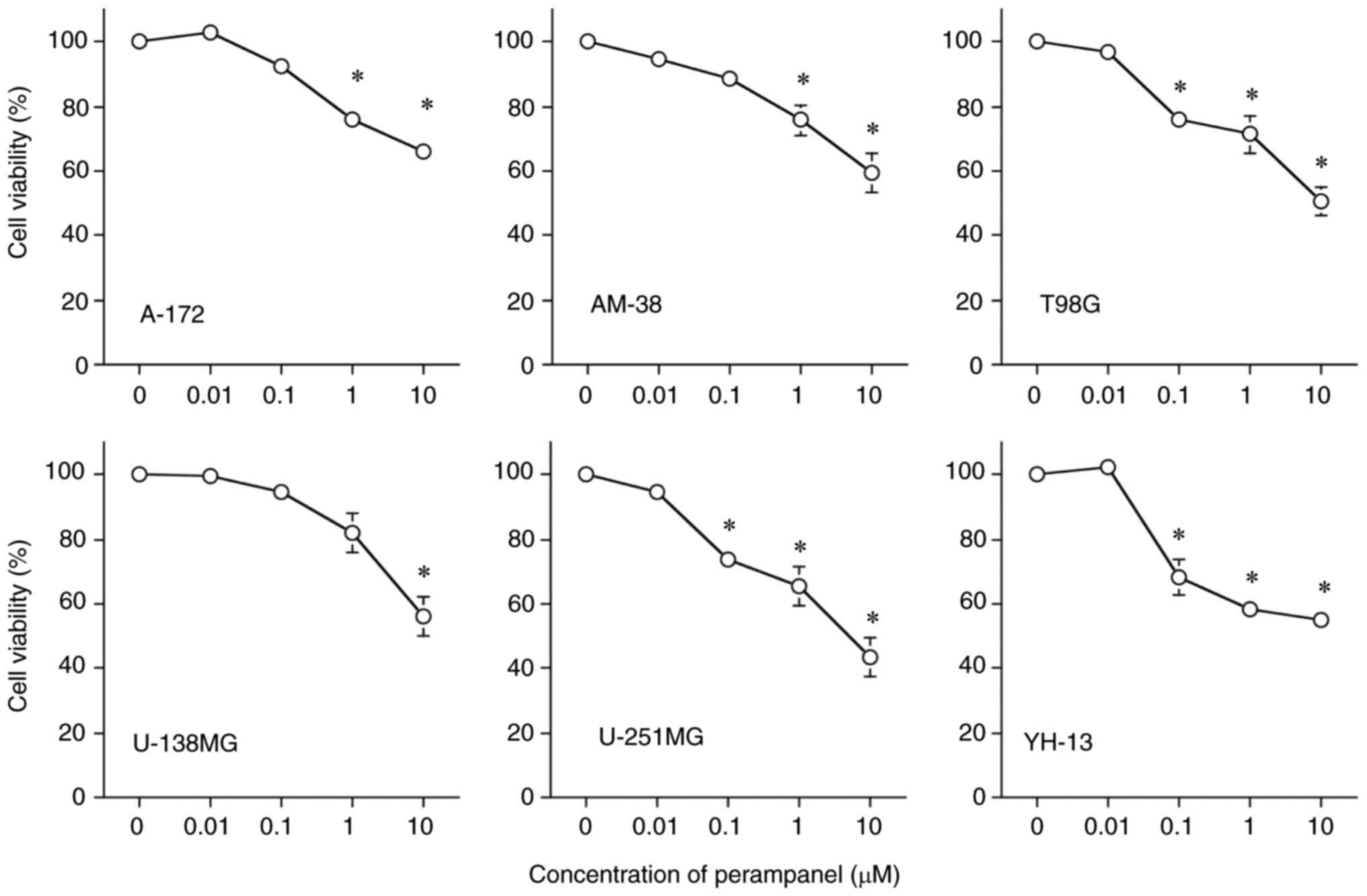
Fig. 1 demonstrates
the inhibitory effects of perampanel on cell viability in a
dose-dependent manner in all tumor cell lines, compared with the
respective untreated control. However, the sensitivity of the cell
lines to perampanel treatment varied, U-251MG was the most
sensitive (lowest IC50 for perampanel among the 6 cell
lines) and U-138MG was the least sensitive to perampanel. The
IC50 of perampanel for U-251MG was <10 µM. The
IC50 of perampanel for T98G was slightly higher than 10
µM, while the IC50 for the other four cell lines, A-172,
AM-38, U-138MG and YH-13, was higher than 10 µM. Cell viability was
significantly inhibited in five cell lines, excluding U-138MG,
compared to the control when treated with perampanel at a
concentration of 1.0 µM, which is the blood concentration achieved
by oral administration as an antiepileptic drug (17).
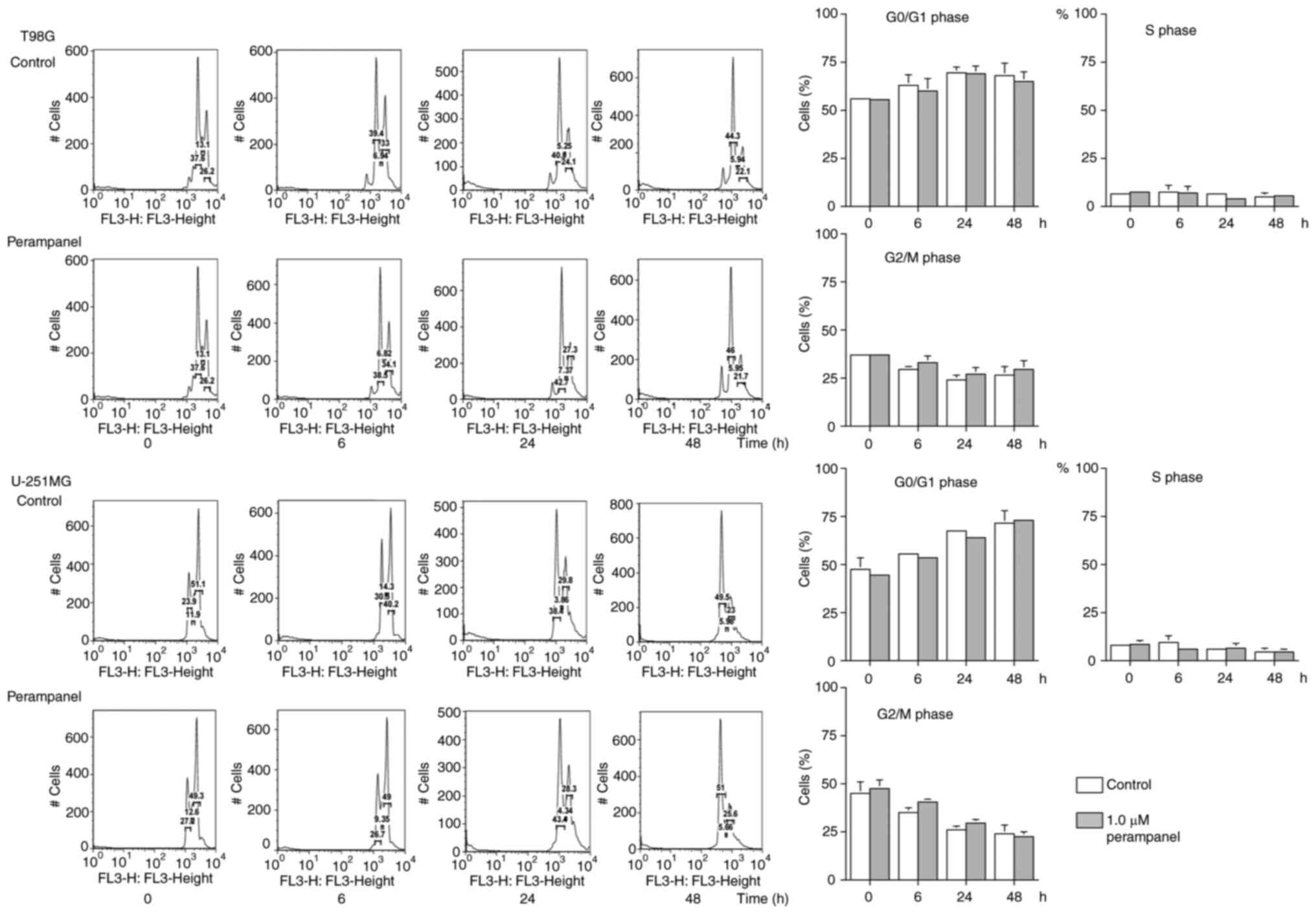
Cell cycle distribution analysis
DNA histograms and the proportions of T98G or
U-251MG cells in each cell cycle phase are presented in Fig. 2. Consistent results were obtained
from at least four repeats; however, statistically significant
differences were not observed between the control and perampanel
groups. No significant increase in the populations of G0/G1, S or
G2/M phases were observed in the cells following 1.0 µM perampanel
treatment compared with the untreated control at 6, 24 and 48 h,
which indicated that the antitumor effect of 1.0 µM of perampanel
may not be due to the accumulation of cells at specific cell cycle
phases.
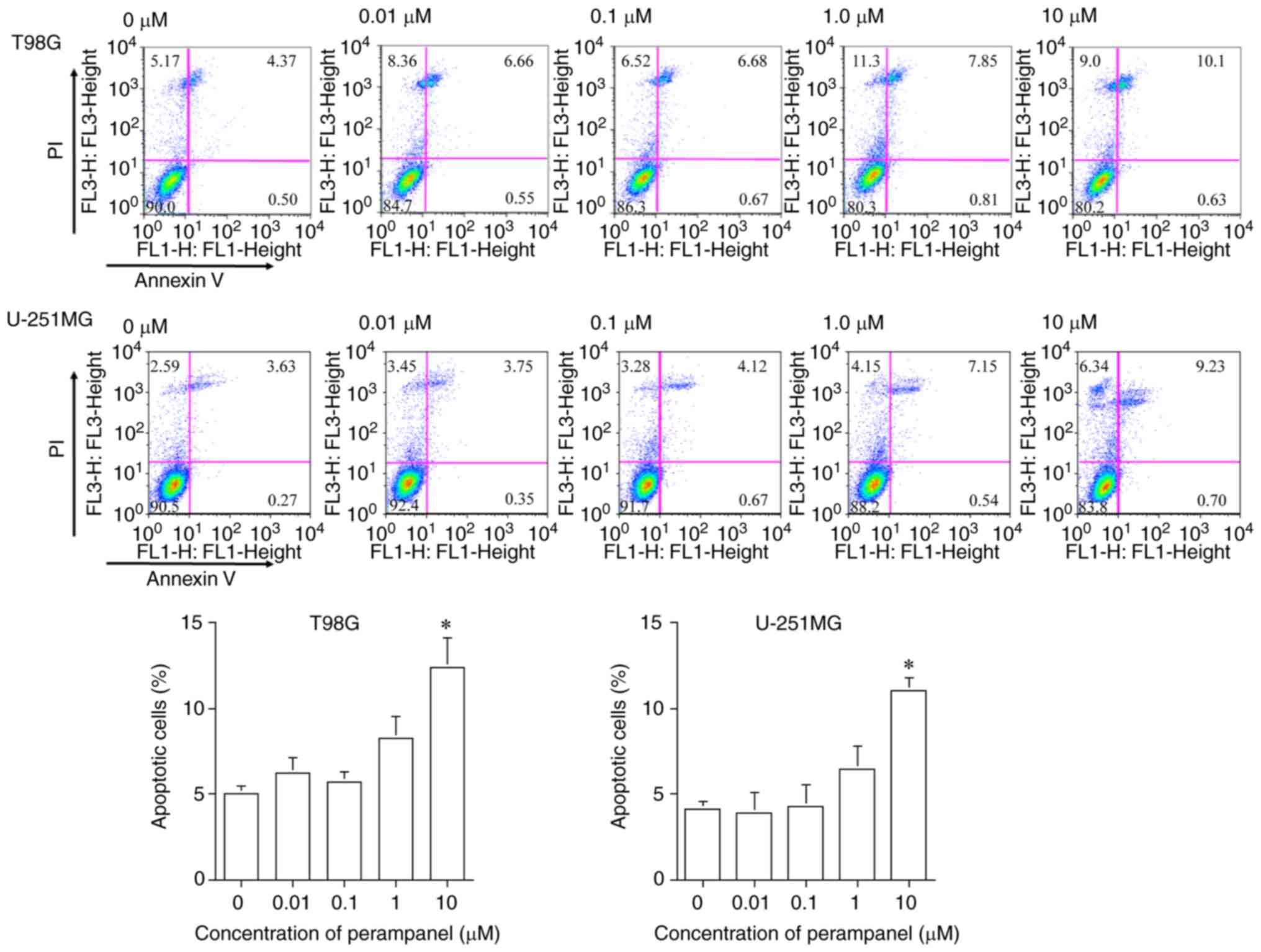
Detection of apoptosis using flow
cytometry and protein expression
The results presented in Fig. 3 demonstrate that 10 µM perampanel
treatment induced significant apoptosis after 48 h, compared with
the untreated control, in both T98G and U-251MG cells. The
proportion of apoptotic cells (Annexin V-positive: early-stage
apoptosis; Annexin V/PI-positive: late-stage apoptosis) following
10 µM perampanel treatment (T98G, 12.41±1.66%; U-251MG,
10.99±0.78%) was significantly higher compared with the proportion
without treatment in both T98G and U-251MG cells (T98G, 5.03±0.44%;
U-251MG, 4.16±0.41%). These findings did not contradict previously
reported results (20).
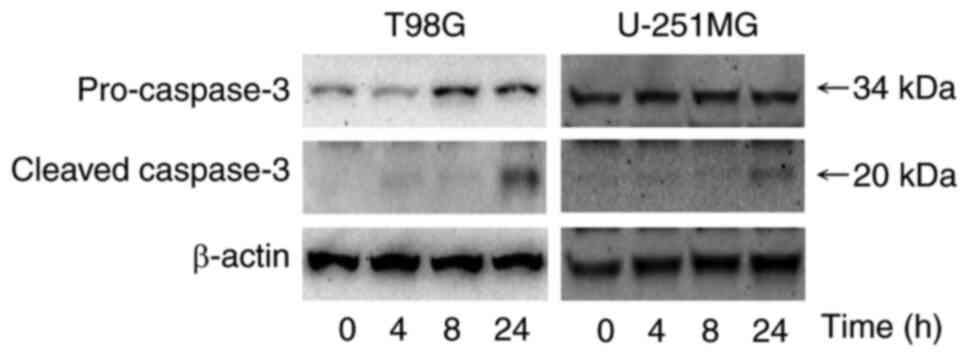
Western blotting was used to evaluate the protein
expression levels of caspase-3, an effector caspase related to
apoptosis. Fig. 4 demonstrated
that the protein expression level of cleaved caspase-3 was markedly
greater after treatment with 1.0 µM perampanel for 24 h compared
with the control in T98G and U-251MG cells.
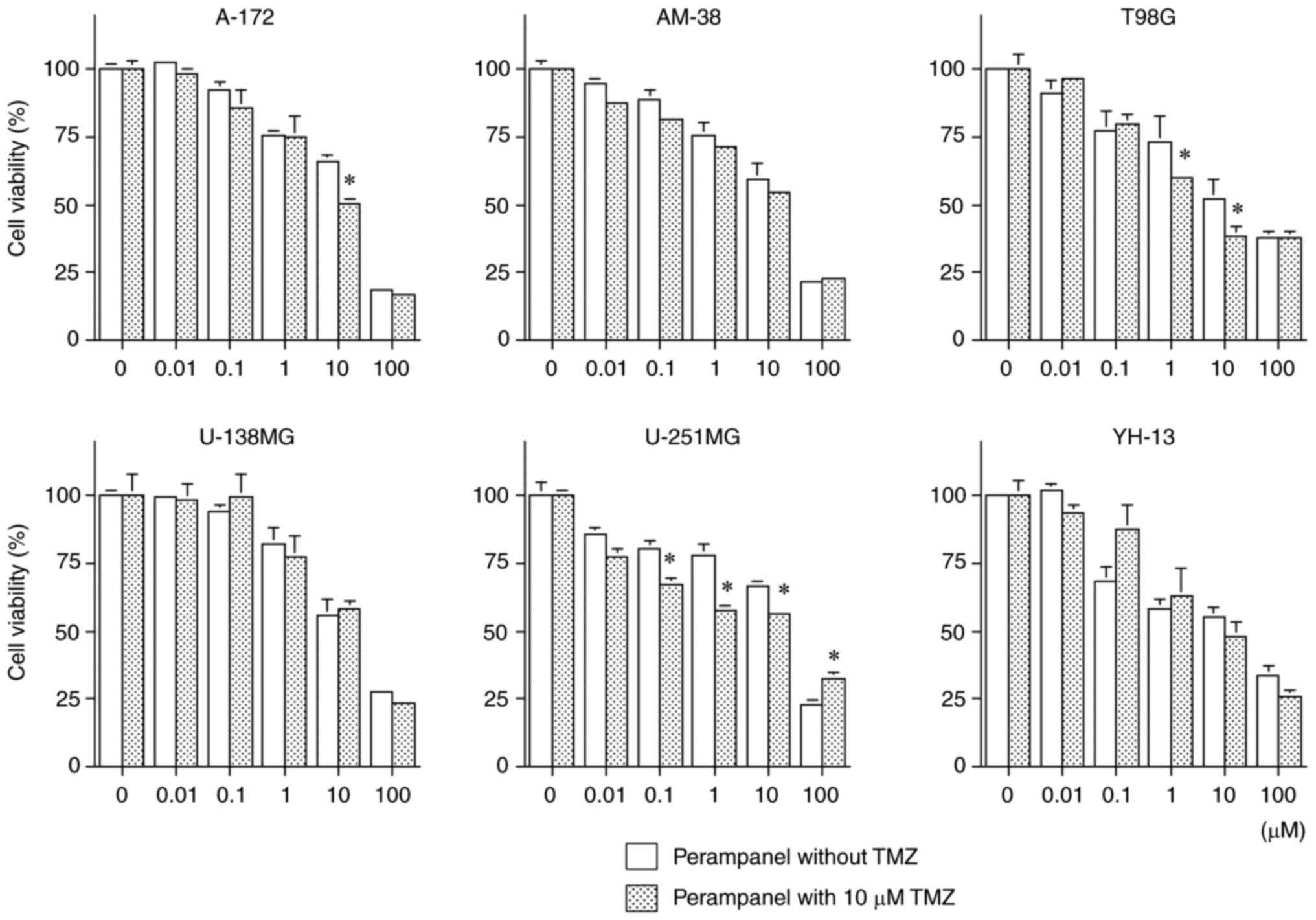
Antitumoral effects of combined
perampanel and TMZ
Fig. 5 presents the
results of combined treatment with perampanel and TMZ, which
demonstrated further significant inhibition of cell viability
compared with the same concentration of perampanel without TMZ
co-treatment, in the A-172, T98G and U-251MG cell lines at ≥10 µM
perampanel (a clinically relevant concentration). Significant
inhibitory effects on cell viability were observed in the T98G and
U-251MG cell lines co-treated with 1.0 µM perampanel with TMZ
compared with 1.0 µM perampanel alone, which is achieved clinically
by oral administration. Conversely, combined treatment with
perampanel and TMZ demonstrated no further cell viability
inhibitory effect in the AM-38, U-138MG and YH-13 cell lines.
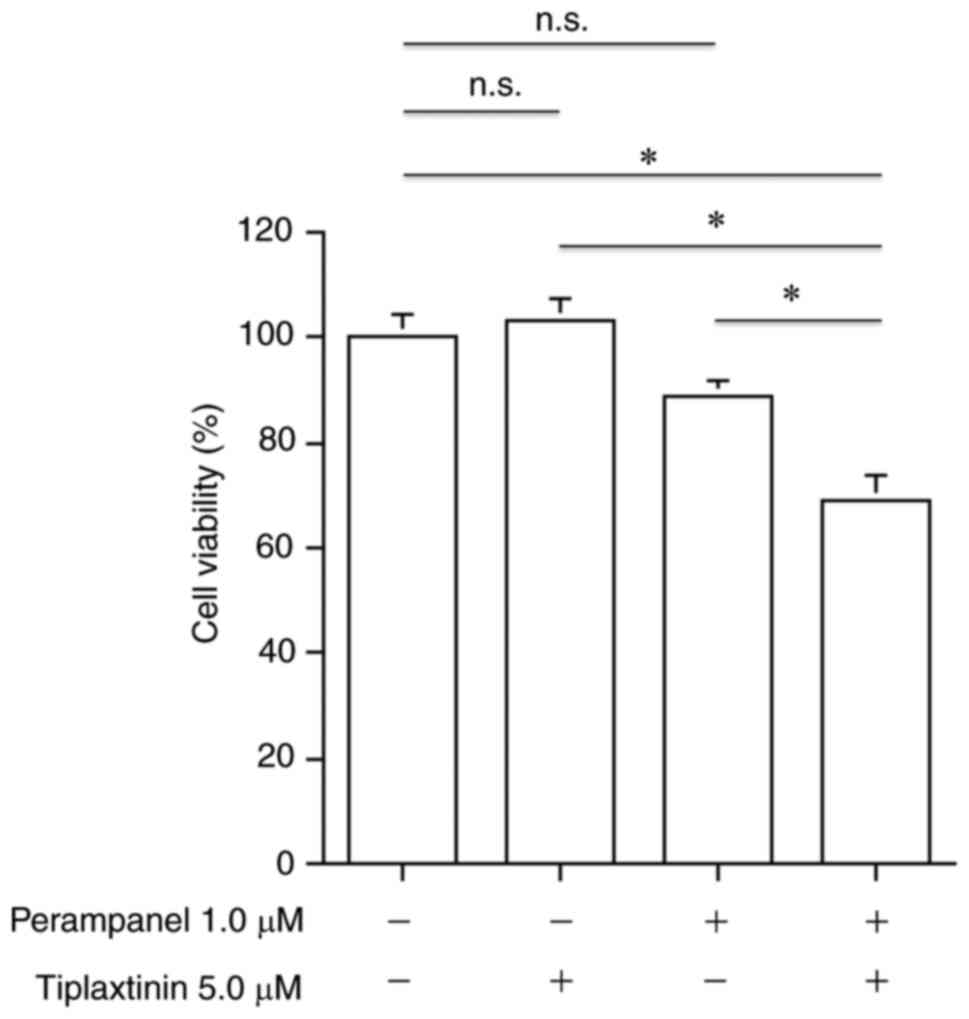
Antitumoral effects of combined
perampanel and tiplaxtinin
Cell viability was further inhibited following
combined treatment for 72 h with 1.0 µM perampanel and 5.0 µM
tiplaxtinin, a SERPINE1 inhibitor, compared with no treatment or
either treatment alone (Fig. 6).
However, no effect on viability was observed with only 1.0 µM
perampanel or 5.0 µM tiplaxtinin alone compared with the untreated
control in the perampanel-resistant U-138MG cell line.
Discussion
The present study demonstrated that the AMPA
receptor non-competitive antagonist perampanel, an antiepileptic
drug, had a dose-dependent inhibitory effect on cell viability in
the six human malignant glioma cell lines examined. The clinical
blood concentration levels of perampanel administered as an
antiepileptic drug were reported to be 0.84 µM at 4 mg dosage and
1.48 µM at 8 mg dosage (17). In
the present study, 5 of 6 cell liens exhibited significantly
reduced cell viability with 1.0 µM perampanel treatment; the
IC50 of perampanel was ≤10 µM in the U-251MG cell line.
Therefore, perampanel may have an antitumor effect on malignant
glioma in certain cases, even if only given at antiepileptic drug
dosages.0
In the present study, the antitumoral effects of
perampanel in malignant glioma were further evaluated by assessing
the effects on the cell cycle and the induction of apoptosis. No
significant change was observed in the proportions of cells in the
G0/G1, S and G2/M phases under 1.0 µM perampanel treatment in T98G
and U-251MG cells, despite observing the inhibitory effect of
perampanel on cell viability in this study. The protein expression
levels of the apoptosis-related protein caspase-3 demonstrated the
induction of apoptosis in both glioma cell lines under 10 µM
perampanel treatment. Conversely, Lange et al (15) reported that the antitumor effect of
perampanel was not due to cell cycle-related viability inhibition
or apoptosis induction in malignant glioma cell lines established
from patients. In their study, caspase activation analysis
indicated no induction of apoptosis even using 30 and 100 µM
perampanel. Therefore, it was concluded that the ultimate antitumor
effect of perampanel was due to a decrease in cell metabolism
(15). Salmaggi et al
(20) reported that cell
proliferation was suppressed by perampanel owing to induction of
apoptosis in four types of malignant glioma cell lines (U87, U138,
A172 and SW1783) and also indicated negative results for the cell
cycle-related effects of perampanel, but reported that perampanel
induced apoptosis. These differences in apoptosis findings may have
been due to different cell lines and measurement methods (20). Furthermore, both studies used
perampanel at higher concentrations than in the present study, so a
different mechanism of action from the present antitumor effect may
have been involved. These results indicated that the antitumor
effect of perampanel may vary between cell lines, but the
differences in susceptibility and mechanism of action need further
investigation. Assessment of the expression of cleaved caspase-8
(initiator caspase in the extrinsic apoptotic pathway), caspase-9
(initiator caspase in the intrinsic mitochondrial pathway) and PARP
are required to further elucidate this mechanism of action;
however, in the present study, only the protein expression levels
of the effector caspase, caspase-3/cleaved caspase-3, was assessed
using western blotting. Furthermore, it is also important to
evaluate the anti-tumor effects of perampanel on glioma cells in
the presence of caspase inhibitors such as Z-VAD-FMK.
The antitumor effect of perampanel combined with
TMZ, a standard chemotherapeutic drug for malignant glioma, was
also assessed. Certain cell lines demonstrated significantly
increased inhibition of cell viability when treated with ≤10 µM
perampanel and 10 µM TMZ (a clinically relevant concentration)
compared with perampanel alone; therefore, perampanel given at
antiepileptic drug dosages in addition to TMZ may have further
antitumoral effects in some patients. The mechanism of the combined
antitumor effect of TMZ and perampanel remains unknown, but further
effects were demonstrated in two (T98G and U-251MG) of the three
cell lines in which perampanel demonstrated a good antitumor
effect. A previous study, using different doses and regimens from
the present study, reported synergistic antitumoral effects of a
combination of perampanel and TMZ in three cell lines (U87, U138
and A172) (20). A172 cells were
reported to be the most sensitive, and this cell line also
demonstrated statistically significant effects from the
co-treatment with 10 µM perampanel and 10 µM TMZ in the present
study. MGMT is expressed in 45–75% of malignant gliomas (26). MGMT expression is strongly involved
in the sensitivity of cells to TMZ; T98G cells express MGMT and are
resistant to TMZ (23). However,
in the present study, the co-treatment of perampanel with TMZ
enhanced the antitumor effect against T98G cells. Further research
is needed, but the results of the present study indicated that
perampanel may exert antiepileptic as well as antitumor effect,
which would be beneficial clinically.
Preliminary RNA-sequencing experiments demonstrated
~100-fold higher mRNA expression levels of SERPINE1 in
U-138MG, with low sensitivity to perampanel, compared with T98G
which demonstrated high sensitivity. SERPINE1 expression was
reported to be correlated with worse prognosis in patients with
malignant glioma and knockdown of SERPINE1 in malignant
glioma cells was reported to have suppressed tumor growth and
invasion (21). Therefore, in the
present study, how the sensitivity of perampanel changed in
perampanel-resistant cells as SERPINE1 was suppressed by
tiplaxtinin, a selective inhibitor of SERPINE1, was evaluated.
Significantly decreased cell viability was observed when cells were
treated with a combination of perampanel and tiplaxtinin compared
with perampanel or tiplaxtinin alone in the U-138MG cell line.
Therefore, the antitumoral effect of perampanel may not occur in
malignant gliomas with high protein expression levels of SERPINE1.
The present study did not assess how the action of SERPNE1 was
involved in susceptibility to the perampanel, but increased
understanding of this may lead to improved treatment of malignant
glioma.
In the present study the antitumoral effect of
perampanel on glioblastoma cell lines was assessed. The results did
not examine the relationship between malignant glioma and related
epilepsy, which is also an important therapeutic challenge.
Therefore, the study of malignant glioma and associated epilepsy is
crucial for malignant glioma patients and requires further study in
the future.
The present study demonstrated marked differences in
susceptibility to perampanel among glioma cells, but the results
suggested that perampanel, an anticonvulsant, exhibited antitumoral
effects, such as cell viability inhibition and apoptosis induction,
which were greater when used in combination with TMZ in some
malignant glioma cells. The results also suggested that
SERPINE1 expression may be involved in perampanel
susceptibility. These results may lead to a new therapeutic
strategy for malignant glioma.
Acknowledgements
Certain parts of the present study were incorporated
within a Japanese-language thesis submitted for JT's PhD at Nihon
University School of Medicine (Tokyo, Japan).
Funding
This work was supported in part by Grants-in-Aid for Scientific
Research from The Japan Society for the Promotion of Science (grant
no. 19K09491).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JT and ES developed the experimental design,
performed experiments and data analysis, and drafted the
manuscript. YH, CY, SY, KS, KT, KK and SKA were involved in the
conception and design of the study, performed experiments, analyzed
the data and drafted the manuscript. HH and YK supervised the study
including the development of the experimental design and proofread
the manuscript. AY contributed to the experimental design, data
analysis and writing of the manuscript. JT, ES and AY confirm the
authenticity of all raw data. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent to participate
Not applicable.
Competing interests
AY, the corresponding author, has received research
funds for other research projects from Medtronic Japan Co., Ltd.
and Eisai Co., Ltd. Note that Eisai Co., Ltd. provided the
antiepileptic agent perampanel used in the present study. AY has
also, in accordance with the rules, reported any competing
interests (including a small amount of research funds for other
research projects) to his main academic society, The Japan
Neurosurgical Society.
References
|
1
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Preusser M, de Ribaupierre S, Wöhrer A,
Erridge SC, Hegi M, Weller M and Stupp R: Current concepts and
management of glioblastoma. Ann Neurol. 70:9–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi J, Stradmann-Bellinghausen B, Yakubov
E, Savaskan NE and Régnier-Vigouroux A: Glioblastoma cells induce
differential glutamatergic gene expressions in human
tumor-associated microglia/macrophages and monocyte-derived
macrophages. Cancer Biol Ther. 16:1205–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanada T, Hashizume Y, Tokuhara N,
Takenaka O, Kohmura N, Ogasawara A, Hatakeyama S, Ohgoh M, Ueno M
and Nishizawa Y: Perampanel: A novel, orally active, noncompetitive
AMPA-receptor antagonist that reduces seizure activity in rodent
models of epilepsy. Epilepsia. 52:1331–1440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vecht C, Duran-Peña A, Houillier C, Durand
T, Capelle L and Huberfeld G: Seizure response to perampanel in
drug-resistant epilepsy with gliomas: Early observations. J
Neurooncol. 133:603–607. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Groot JF, Piao Y, Lu L, Fuller GN and
Yung WK: Knockdown of GluR1 expression by RNA interference inhibits
glioma proliferation. J Neurooncol. 88:121–133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishiuchi S, Tsuzuki K, Yoshida Y, Yamada
N, Hagimura N, Okado H, Miwa A, Kurihara H, Nakazato Y, Tamura M,
et al: Blockage of Ca(2+)-permeable AMPA receptors suppresses
migration and induces apoptosis in human glioblastoma cells. Nat
Med. 8:971–978. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishiuchi S, Yoshida Y, Sugawara K, Aihara
M, Ohtani T, Watanabe T, Saito N, Tsuzuki K, Okado H, Miwa A, et
al: Ca2+-permeable AMPA receptors regulate growth of human
glioblastoma via Akt activation. J Neurosci. 27:7987–8001. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iwamoto FM, Kreisl TN, Kim L, Duic JP,
Butman JA, Albert PS and Fine HA: Phase 2 trial of talampanel, a
glutamate receptor inhibitor, for adults with recurrent malignant
gliomas. Cancer. 116:1776–1782. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takano T, Lin JH, Arcuino G, Gao Q, Yang J
and Nedergaard M: Glutamate release promotes growth of malignant
gliomas. Nat Med. 7:1010–1015. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wirsching HG and Weller M: Does neuronal
activity promote glioma progression? Trends Cancer. 6:1–3. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rzeski W, Ikonomidou C and Turski L:
Glutamate antagonists limit tumor growth. Biochem Pharmacol.
64:1195–1200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Howes JF and Bell C: Talampanel.
Neurotherapeutics. 4:126–129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grossman SA, Ye X, Piantadosi S, Desideri
S, Nabors LB, Rosenfeld M, Fisher J and NABTT CNS Consortium:
Survival of patients with newly diagnosed glioblastoma treated with
radiation and temozolomide in research studies in the United
States. Clin Cancer Res. 16:2443–2449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lange F, Weßlau K, Porath K, Hörnschemeyer
J, Bergner C, Krause BJ, Mullins CS, Linnebacher M, Köhling R and
Kirschstein T: AMPA receptor antagonist perampanel affects
glioblastoma cell growth and glutamate release in vitro. PLoS One.
14:e02116442019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hibi S, Ueno K, Nagato S, Kawano K, Ito K,
Norimine Y, Takenaka O, Hanada T and Yonaga M: Discovery of
2-(2-oxo-1-phenyl-5-pyridin-2-yl-1,2-dihydropyridin-3-yl)benzonitrile
(perampanel): A novel, noncompetitive α-amino-3-hydroxy-5-
methyl-4-isoxazolepropanoic acid (AMPA) receptor antagonist. J Med
Chem. 55:10584–10600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Izumoto S, Miyauchi M, Tasaki T, Okuda T,
Nakagawa N, Nakano N, Kato A and Fujita M: Seizures and tumor
progression in glioma patients with uncontrollable epilepsy treated
with perampanel. Anticancer Res. 38:4361–4366. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patsalos PN: The clinical pharmacology
profile of the new antiepileptic drug perampanel: A novel
noncompetitive AMPA receptor antagonist. Epilepsia. 56:12–27. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rösche J, Piek J, Hildebrandt G, Grossmann
A, Kirschstein T and Benecke R: Perampanel in the treatment of a
patient with glioblastoma multiforme without IDH1 mutation and
without MGMT promotor methylation. Fortschr Neurol Psychiatr.
83:286–289. 2015.(In German). PubMed/NCBI
|
|
20
|
Salmaggi A, Corno C, Maschio M, Donzelli
S, D'Urso A, Perego P and Ciusani E: Synergistic effect of
perampanel and temozolomide in human glioma cell lines. J Pers Med.
11:3902021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seker F, Cingoz A, Sur-Erdem İ, Erguder N,
Erkent A, Uyulur F, Esai Selvan M, Gümüş ZH, Gönen M, Bayraktar H,
et al: Identification of SERPINE1 as a regulator of glioblastoma
cell dispersal with transcriptome profiling. Cancers (Basel).
11:16512019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pavón MA, Arroyo-Solera I, Céspedes MV,
Casanova I, León X and Mangues R: uPA/uPAR and SERPINE1 in head and
neck cancer: Role in tumor resistance, metastasis, prognosis and
therapy. Oncotarget. 7:57351–57366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshino A, Ogino A, Yachi K, Ohta T,
Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N, Sano E,
et al: Gene expression profiling predicts response to temozolomide
in malignant gliomas. Int J Oncol. 36:1367–1377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ostermann S, Csajka C, Buclin T, Leyvraz
S, Lejeune F, Decosterd LA and Stupp R: Plasma and cerebrospinal
fluid population pharmacokinetics of temozolomide in malignant
glioma patients. Clin Cancer Res. 10:3728–3736. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wischhusen J, Naumann U, Ohgaki H,
Rastinejad F and Weller M: CP-31398, a novel p53-stabilizing agent,
induces p53-dependent and p53-independent glioma cell death.
Oncogene. 22:8233–8245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bello MJ, Alonso ME, Amiñoso C, Anselmo
NP, Arjona D, Gonzalez-Gomez P, Lopez-Marin I, de Campos JM,
Gutierrez M, Isla A, et al: Hypermethylation of the DNA repair gene
MGMT: Association with TP53 G:C to A:T transitions in a series of
469 nervous system tumors. Mutat Res. 554:23–32. 2004. View Article : Google Scholar : PubMed/NCBI
|