Introduction
Pancreatic cancer (PC) is a malignant tumor of the
digestive system that is challenging to diagnose and treat,
exhibits a high degree of malignancy and is associated with poor
prognosis (1). According to the
literature, the 5-year survival rate of PC is <8% (2), and >90% of PC cases are pancreatic
ductal adenocarcinoma (3). As the
onset of PC is often missed, patients are frequently diagnosed in
the first instance with metastatic or advanced cancer, which limits
the clinical treatment options. Lymph node metastasis, as the
primary pathway and an early event in PC, is an important factor
that influences the clinical stage, treatment and prognosis of
patients with PC (4). Clinical
studies have shown that the incidence of lymphatic invasion by
cancer cells is 3–5-fold higher than that of vascular invasion
(5). Although the lymph node
metastasis of PC is important clinically, the molecular mechanism
that induces PC cells to separate from the primary focus, invade
lymphatic vessels and metastasize to regional lymph nodes is
unclear. Therefore, it is of great value to explore the molecular
mechanism of lymph node metastasis of PC to improve the clinical
diagnosis, treatment and prognosis of patients with PC.
Lymphatic system vessels are the primary channels
through which cancer cells spread from a local tumor to lymph nodes
and then the lymphatic circulation to distant sites (6). Lymphangiogenesis is considered to be
the most critical and rate-limiting step in the development of
lymph node metastasis by malignant tumor cells (7). Due to the lack of specific molecular
markers for lymphatic endothelial cells, the study of lymph node
metastasis has been overshadowed by the study of vascular system
invasion. However, since specific markers for lymphatic endothelial
cells have been discovered, these now provide a basis for further
study of the mechanism of lymph node metastasis. VEGF-C has been
shown to be an important growth factor in the lymphangiogenesis of
solid malignant tumors, which promotes lymphangiogenesis and
microlymphangiogenesis by binding to VEGFR-3. The VEGF-C and
VEGFR-3 axis has been shown serve a central role in the initiation
of lymphangiogenesis (8). A study
by Ochi et al (9) showed
that the expression levels of VEGF-C and VEGFR-3 were significantly
correlated with each other in patients with pancreatic carcinoma.
The study also demonstrated that VEGFR-3 combined with VEGF-C to
stimulate the formation of lymphatic vessels in pancreatic
carcinoma, which induced the formation of new lymphatic capillaries
and increased the risk of lymph node metastasis. The aforementioned
studies indicate that VEGF-C/VEGFR-3 in PC tissues is an important
pathway that induces the formation of lymphatic vessels and lymph
node metastases. However, the regulatory mechanism regulating the
VEGF-C/VEGFR-3 pathway in PC has not been determined previously, to
the best of our knowledge. Therefore, the present study
investigated the potential regulatory role of BRAF-activated
non-protein coding RNA (BANCR) and hypoxia-inducible factor
(HIF)-1α in the VEGF-C/VEGFR-3 pathway in PC by knocking down their
expression.
Studies have shown that lncRNAs are abnormally
expressed in several types of human malignant tumors and
participate in the proliferation, invasion and metastasis of tumor
cells (10). Previous studies by
Jiang et al (11) and Shen
et al (12) have
demonstrated the important role of the long non-coding RNA (lncRNA)
BANCR in the tumor lymph node metastasis of breast and colorectal
cancer, respectively. BANCR was first reported by Flockhart et
al (13), who identified it in
melanoma cells in 2012. It is a ~693-bp lncRNA that is present on
chromosome 9 and specifically activated by a mutation in the
BRAFV600E gene; its expression is upregulated in
melanoma cells and it plays an important role in the promotion of
lymph node metastasis. With increased interest in BANCR, subsequent
studies found that in addition to melanoma, BANCR also serves a key
role in other types of tumors (14). Studies have detected the
upregulated expression of BANCR in gastric cancer (15), colorectal cancer (12), hepatocellular carcinoma (16), esophageal squamous cell carcinoma
(17), osteosarcoma (18) and thyroid cancer (19,20),
and shown BANCR to be significantly associated with a poor
prognosis, tumor cell proliferation, invasion and local lymph node
metastasis. Notably, a study by Wu et al (4) demonstrated that the expression of
BANCR was upregulated in PC tissues and cell lines, namely PANC-1
and SW1990, and found that BANCR upregulation was closely
associated with lymph node metastasis in patients with PC. In
addition, the study demonstrated that interfering with the
expression of BANCR effectively inhibited the proliferation and
invasion of PC cells.
The present study aimed to verify the important role
of BANCR in PC proliferation, invasion and lymph node metastasis.
The expression levels of BANCR in human PC cells were compared with
those in normal human pancreatic cells, and the role and molecular
mechanisms of BANCR in the lymphangiogenesis of PC were determined
for the first time, to the best of our knowledge.
A hypoxic microenvironment is common for the
occurrence and development of malignant tumors. In particular, a
hypoxic microenvironment is important in the induction of highly
invasive PC, and is established due to a poor blood supply to the
rapidly proliferating cells (21);
it is also a key factor underlying the high degree of malignancy
and poor curative outcomes (22).
A previous study on hypoxia focused on tumor angiogenesis and drug
resistance (23); however, little
is known regarding the mechanism of tumor lymphangiogenesis and
lymph node metastasis. The activation of HIF is the key molecular
characteristic of tumor cell changes occurring in a hypoxic
microenvironment, which is closely associated with the occurrence,
development, invasion and metastasis of tumors (24). HIF has three subtypes: HIF-1, −2
and −3, of which HIF-1 is the most important subtype in hypoxic
tumor cells and is widely expressed in a variety of human tumors
(25). HIF-1 is composed of HIF-1α
and HIF-1β, and the former is the key transcription factor in the
hypoxic response, due to its important role in the regulation of
hypoxic gene expression and in the signal transduction network
(26). Previous studies have shown
that the upregulated expression of HIF-1α in PC is associated with
tumorigenesis and progression (27,28).
Liu et al (29) confirmed
the high expression of HIF-1α in PC and its association with lymph
node metastasis and TNM staging. In the present study, the
expression levels of HIF-1α in PANC-1 and SW1990 PC cell lines were
assessed, and the effect of knocking down the expression HIF-1α was
evaluated. Furthermore, the ability of BANCR to regulate the
expression of HIF-1α was investigated, and the effect of HIF-1α on
the transcription and translation of VEGF-C in PC cells was
explored. The role of BANCR in the regulation of HIF-1α was thereby
revealed, and the potential value of the
BANCR/HIF-1α/VEGF-C/VEGFR-3 pathway in the lymphangiogenesis and
lymph node metastasis in PC was determined.
Materials and methods
Cell lines and culture conditions
The PC cell lines PANC-1 and SW1990, immortalized
human pancreatic ductal epithelial cells (HPDCs) and human
lymphatic tube endothelial cells (HDLECs) were purchased from
Guangzhou Genio Biotech Co., Ltd. The SW1990 and HPDC cells were
cultured in DMEM (MilliporeSigma), the PANC-1 cells were cultured
in RPMI-1640 (HyClone; Cytiva) and the HDLECs were cultured in
Endothelial Cell Medium (ScienCell Research Laboratories, Inc.),
each supplemented with 10% FBS (Thermo Fisher Scientific, Inc.) and
1% antibiotics (penicillin-streptomycin; Thermo Fisher Scientific,
Inc.). Culture was performed under normoxic or hypoxic conditions
in a humidified incubator at 37°C. The normoxic conditions were 20%
O2, 5% CO2 and 75% N2, and the
hypoxic conditions were 1% O2, 5% CO2 and 94%
N2. Cells in the logarithmic growth stage were selected
for subsequent experiments.
Cell transfection and grouping
PC cell lines in the logarithmic growth stage were
divided into two groups after digestion. Lipofectamine™
3000 (Thermo Fisher Scientific, Inc.) was used to transfect the PC
cell lines with a small interfering (si)RNA BANCR knockdown plasmid
(TIANpure Mini Plasmid Kit II; Tiangen Biotech Co., Ltd.) and
negative control plasmid (si-NC) to establish the si-BANCR and
si-NC groups, respectively. In brief, PC cells at 80% confluence
were plated in a 12-well plate. A total of 100 µl Opti-MEM was used
to dilute the Lipofectamine™ 3000 (4 µl) and
vector/pcDNA (6 µl; 100 ng/µl siRNA/plasmid), incubated at room
temperature for 5 min, mixed gently and then incubated for a
further 20 min at 37°C. Next, the cell medium was replaced with
Opti-MEM (700 µl/well; Thermo Fisher Scientific, Inc.), and the
aforementioned transfection mixture was added to each well. After 8
h at 37°C, the medium was replaced with standard supplemented
medium, and after transfection for 48 h, the transfected cells were
used for subsequent experiments. The si-BANCR sequence was
5′-GGUGTGGCGUCTUGCUUTT-3′. The si-NC sequence was
5′-GGCCGGUTTCCUUTTCUGCG-3′. In a subsequent experiment, the PC cell
lines were transfected with the si-HIF-1α sequence
5′-CTGATGACCACACAACTTGA-3′ to establish a HIF-1α knockdown group
using the aforementioned transfection protocol. Transfection
success was evaluated by the detection of green fluorescent protein
and reverse transcription-quantitative PCR (RT-qPCR) as shown in
Figs. S1 and S2.
Lymphangiogenesis experiments
PC cells stably transfected with si-BANCR or si-NC
were digested and HDLECs were added to establish a mixed cell
suspension. The mixed cell suspension (PC cells:HDLEC cells, 1:1;
7.5×103 cells/well) was seeded on a Matrigel basement
membrane (BD Biosciences) coating in 96-well plates at 100 µl/well,
with 3 wells per condition, and the cells were cultured under the
aforementioned normoxic or hypoxic conditions. The formation of
microlymphatic vessels was observed after culturing for 12 h.
Detection of MLVD
The lymphatic vessel distribution was observed using
a low magnification inverted fluorescence microscope (×100) and
assessed in a double-blinded manner by two pathologists.
Subsequently, 3 hot-spot areas (areas that appear to have a high
MLVD density) were selected and the positive structure of each area
was observed using a high-power field of view (×400). Finally, 5
regions were randomly selected for counting the number of vessels,
and the maximum value was selected as the MLVD of each hot-spot
area for statistical analysis.
RT-qPCR
A TRIzol® RNA extraction kit (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to extract total RNAs from
the cells according to the manufacturer's instruction. The RNA was
reverse transcribed into cDNA using PrimeScript RT Master Mix
(Takara Biotechnology Co., Ltd.) at 37°C for 15 min. The relative
expression levels of BANCR, HIF-1α, VEGF-C and VEGFR-3 in each
group were detected. RT-qPCR was performed in strict accordance
with the instructions of the SYBR® Primx Ex
Taq™ (TIi RNaseH Plus) (cat. no. RR420A; Takara Bio,
Inc.), with GAPDH as the internal reference gene in a reaction
system of 20 µl. The thermocycling conditions were as follows:
Pre-denaturation at 95°C for 5 min; followed by 38 cycles of
denaturation at 95°C for 30 sec, annealing at 65°C for 30 sec and
extension at 72°C for 30 sec; and a final extension step of 72°C
for 8 min. Primers were designed based on the following human gene
sequences in NCBI GeneBank: BANCR (NC_000009.12), HIF-1α
(NC_000014.9), VEGF-C (NC_000004.12), VEGFR-3 (NC_000005.10) and
GAPDH (NC_000012.12). The primers were synthesized by
Bio-Engineering Co., Ltd., and their sequences are provided in
Table I. Quantitative analysis of
relative gene expression data used the 2−ΔΔCq method,
and GAPDH was used as the internal reference control (30).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer name | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| GAPDH |
TCAAGAAGGTGGTGAAGCAGG |
TCAAAGGTGGAGGAGTGGGT |
| BANCR |
GAGCCTTGCCAGTTCCATTT |
TGCAGAGGTGAGATTCAGGT |
| HIF-1α |
ACTTCTGGATGCTGGTGATTTG |
GCTTCGCTGTGTGTTTTGTTCT |
| VEGF-C |
CTACAGATGTGGGGGTTGCT |
GATTGGCAAAACTGATTGTGAC |
| VEGFR-3 |
CTCTGACCTAGTGGAGATCCTG |
CTTCGGTGATATGTAGAGCTGTG |
Western blotting
The transfected cells were examined by western
blotting. Total protein was extracted under different treatment
conditions using RIPA buffer (cat. no. R0010; Beijing Solarbio
Science & Technology Co., Ltd.) and protein concentration was
quantified using the BCA method (Thermo Fisher Scientific, Inc.). A
total of 20 µg protein/lane was loaded for electrophoresis.
SDS-PAGE on a 10% gel was performed at a constant voltage of 100 V
for 40 min. After electrophoresis, the electroporation apparatus
was used to transfer the resolved proteins to a PVDF membrane using
a constant current of 250 mA for 2 h. The membranes were
subsequently blocked at room temperature for 1 h with 5% skimmed
milk/TBS-0.1% and Tween-20 (TBST) solution. Primary antibodies
against HIF-1α (cat. no. ab2185; 1:500; Abcam), VEGF-C (cat. no.
22601-1-AP; 1:1,000; ProteinTech Group, Inc.) and VEGFR-3 (cat. no.
Ab27278; 1:1,000; Abcam) were used. A GAPDH antibody (cat. no.
ab9485; 1:3,000; Abcam) was also used to detect GAPDH as an
internal reference. The membrane was incubated overnight with the
primary antibodies at 4°C. After washing the film with TBS-0.1% and
Tween-20 (TBST) three times, the film was incubated with the
secondary antibody (Alexa Fluor® 568; cat. no. ab175473;
1:5,000; Abcam) at room temperature for 1 h. TBST was used to wash
the films again three times, after which the signals were developed
and visualized using an ECL reagent (Thermo Fisher Scientific,
Inc.). A CanoScan Lide 120 scanner (Canon, Inc.) was used to scan
the film for densitometric analysis. Densitometric analysis was
performed using ImageJ 1.48 (National Institutes of Health).
Statistical analysis
SPSS version 19.0 (IBM Corp) was used for
statistical analysis. The relative expression levels of BANCR,
HIF-1α, VEGF-C and VEGFR-3 in PC cells and MLVD values are
expressed as the mean ± standard deviation. The relative expression
levels of BANCR in HPDCs and the PANC-1 and SW1900 cell lines were
analyzed by one-way ANOVA followed by Tukey's multiple comparison
test. Differences between two groups were compared using an
independent samples Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
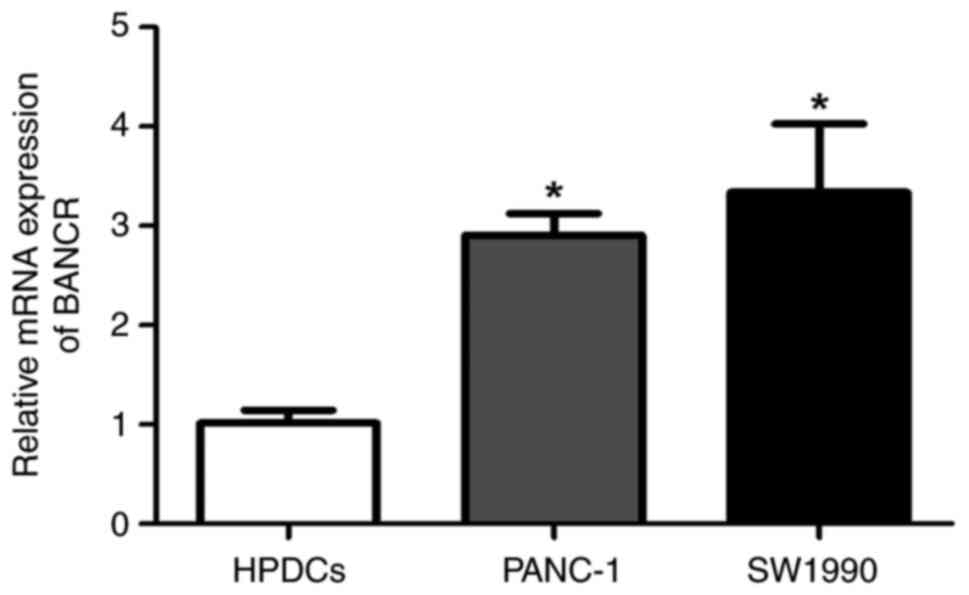
BANCR expression is upregulated in
PANC-1 and SW1990 cells
RT-qPCR was used to detect the relative expression
of BANCR in the HPDCs and the PANC-1 and SW1990 PC cell lines. The
results showed that the expression of BANCR in the PANC-1 and
SW1990 cells was significantly upregulated compared with that in
the HPDCs (P<0.05). No significant difference in the expression
of BANCR was detected between the PANC-1 and SW1990 cells (Fig. 1).
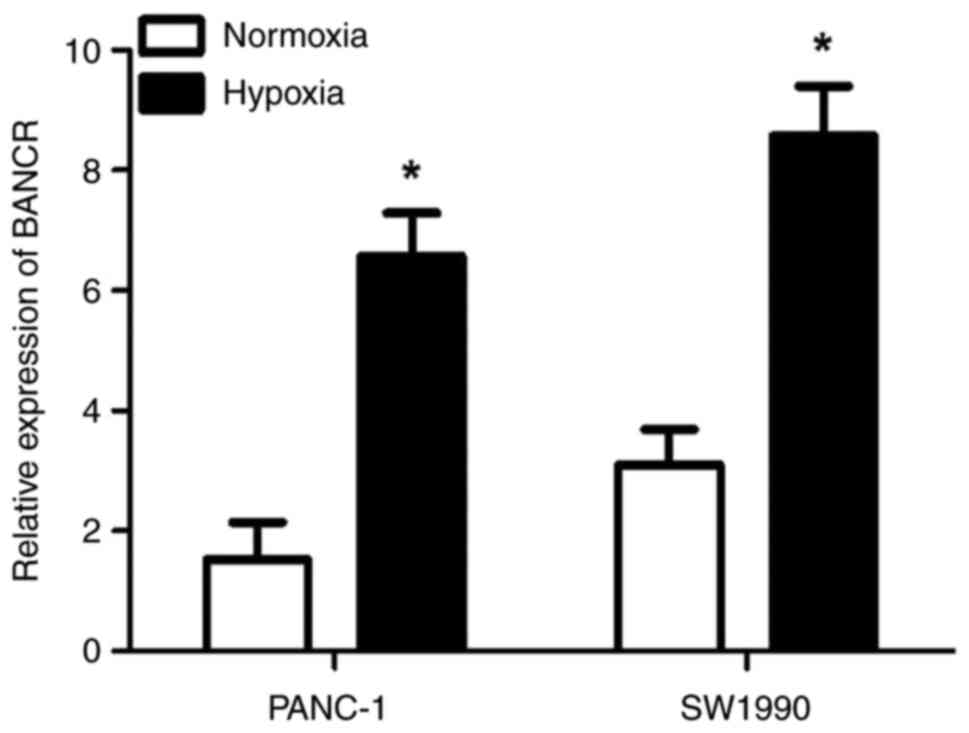
BANCR expression is upregulated in
PANC-1 and SW1990 cells under hypoxic conditions
RT-qPCR was used to detect the expression of BANCR
in PANC-1 and SW1990 cells under normoxic and hypoxic conditions.
The results showed that the expression of BANCR was significantly
higher under hypoxic conditions compared with normoxic conditions
(P<0.05; Fig. 2).
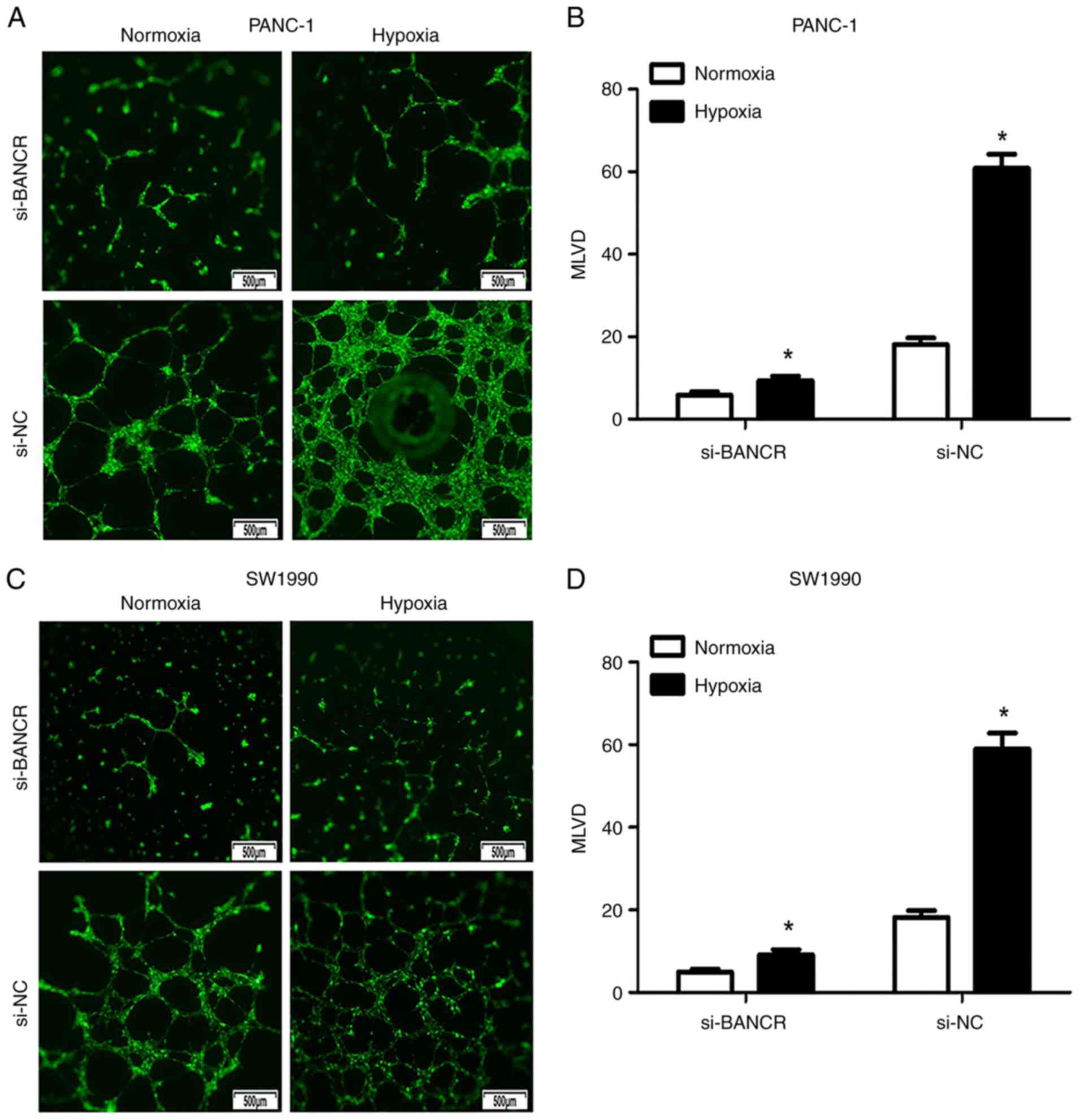
BANCR increases the MLVD of PC cells
under hypoxic conditions
BANCR expression was knocked down in PANC-1 and
SW1990 cells, after which the cells were co-cultured with HDLECs
under normoxic and hypoxic conditions. In the PANC-1 cell line, the
MLVD values in the si-NC group and the si-BANCR group increased
significantly under hypoxic conditions compared with those in cells
grown under normoxic conditions (Fig.
3A and B). In addition, the degree of MLVD was reduced
significantly following the knockdown of BANCR expression in PANC-1
cells (si-BANCR: hypoxia vs. normoxia: 9.133±3.925 vs. 5.8±3.189,
respectively, P<0.05; si-NC: hypoxia vs. normoxia: 61.6±12.53
vs. 18.13±6.128, respectively, P<0.05). Similar results were
observed in the SW1990 cells (si-BANCR: hypoxia vs. normoxia:
9.2±4.411 vs. 4.933±2.604, respectively, P<0.05; si-NC: hypoxia
vs. normoxia: 59.0±14.89 vs. 18.2±6.45, respectively, P<0.05;
Fig. 3C and D).
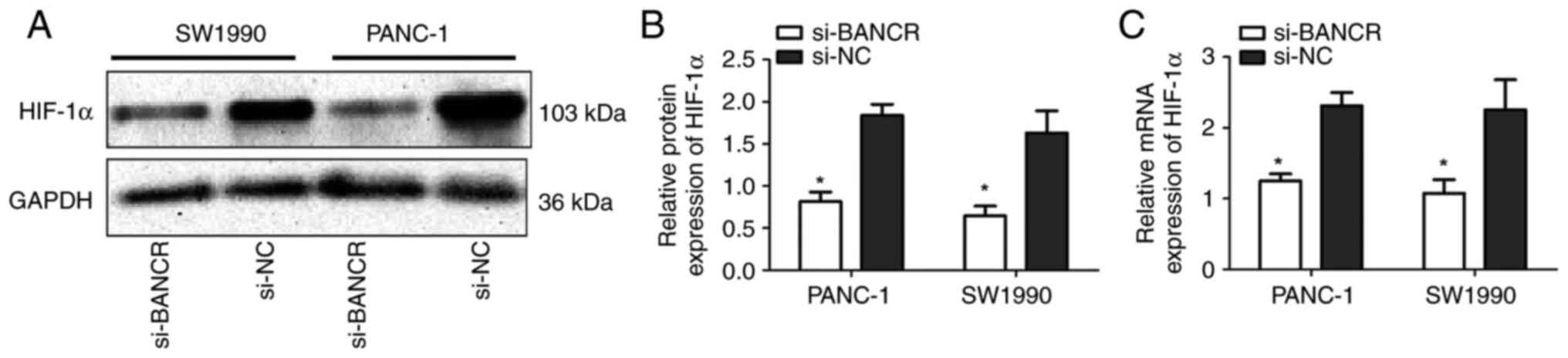
HIF-1 expression is upregulated by
BANCR at the transcriptional level
SW1990 and PANC-1 cells transfected with si-BANCR or
si-NC were cultured under hypoxic conditions. Western blotting was
used to detect the relative protein expression levels of HIF-1α in
each group, and the results are shown in Fig. 4A and B. The relative protein
expression of HIF-1α in the si-BANCR group was significantly lower
compared with that in the si-NC group in each cell line (SW1990:
0.646±0.200 vs. 1.630±0.453, respectively, P<0.05; PANC-1:
0.815±0.195 vs. 1.838±0.228, respectively, P<0.05). RT-qPCR was
used to detect the relative mRNA expression levels of HIF-1α in the
si-BANCR and si-NC groups and the results were consistent with the
protein results (SW1990: 1.076±0.430 vs. 2.253±0.950, P<0.05;
PANC-1: 1.248±0.228 vs. 2.309±0.426, P<0.05; Fig. 4C).
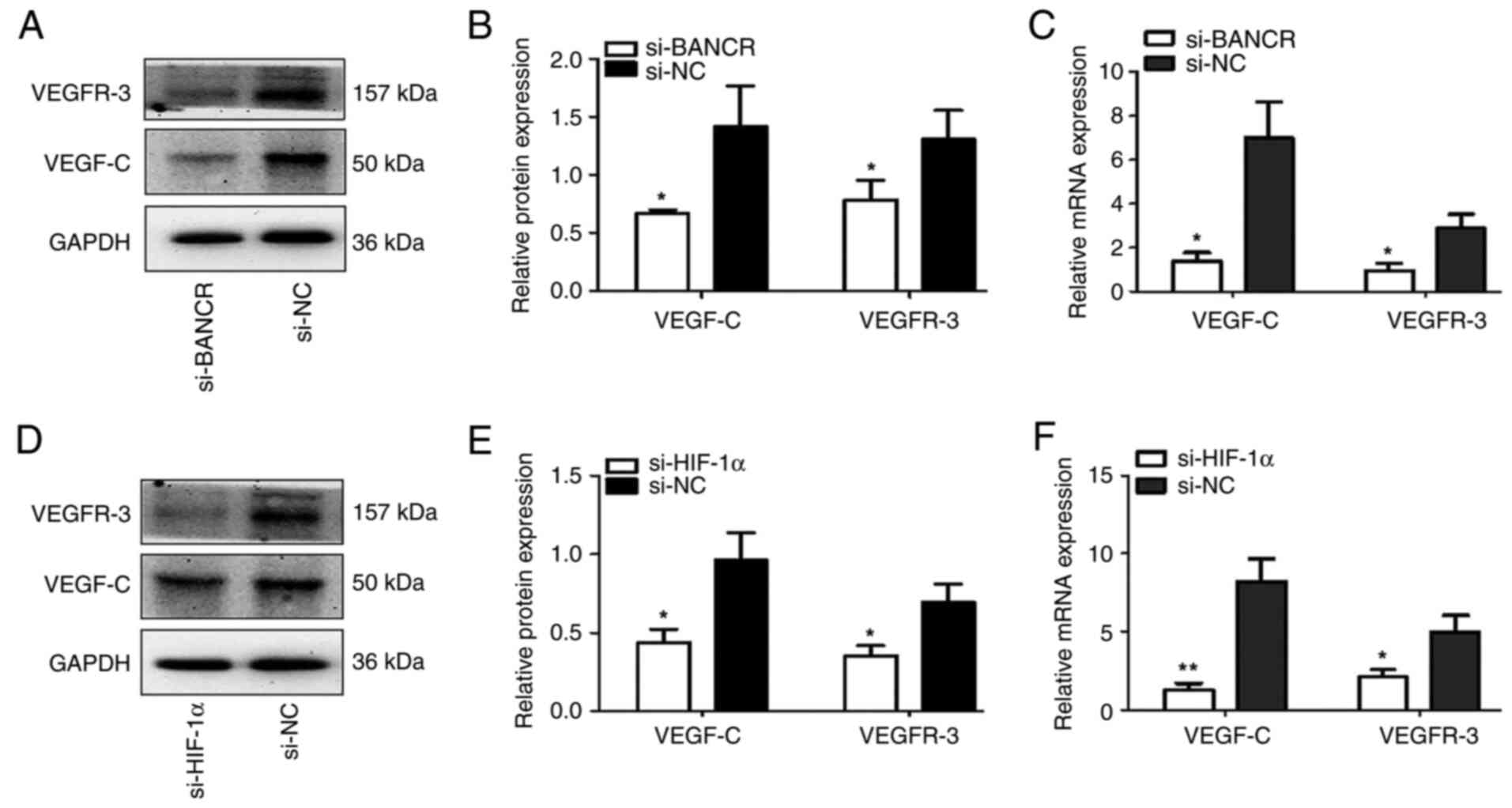
BANCR and HIF-1α upregulate the
expression of VEGF-C and VEGFR-3
RT-qPCR and western blotting were used to detect the
relative protein and mRNA expression levels of VEGF-C and VEGFR-3
in SW1990 cells transfected with si-BANCR, si-HIF-1α or si-NC under
hypoxic conditions. The relative protein and mRNA expression levels
of VEGF-C and VEGFR-3 the si-BANCR group were significantly lower
compared with those in the si-NC group (P<0.05; Fig. 5A-C). The relative protein and mRNA
expression levels of VEGF-C and VEGFR-3 in the si-HIF-1α group were
also decreased significantly compared with those in the si-NC group
(P<0.05; Fig. 5D-F). These
results indicate that BANCR may upregulate VEGF-C and VEGFR-3 by
regulating HIF-1α expression.
Discussion
PC exhibits a high degree of malignancy and is
associated with a poor prognosis. The majority of patients with PC
are first diagnosed with middle- to late-stage cancer, and this
limits the clinical curative effects of treatments. Despite
advancements in surgery, radiotherapy and chemotherapy, the
prognosis of patients with PC has not substantially improved in the
past 20 years (1). Lymph node
metastasis is the primary pathway and an early event in PC
metastasis, which affects the clinical stage, treatment and
prognosis of patients with PC (4).
Previous studies on the lymph node metastasis of PC have focused on
clinical high-risk factors and their correlations; however, less
research has been performed to determine the molecular mechanisms
underlying lymph node metastasis. In the present study, the
molecular mechanism underlying lymph node metastasis in PC was
preliminarily explored at the cellular level. PANC-1 and SW1990
cell lines were chosen for use in the study as they are the most
widely used representative PC cell lines, which are easy to
cultivate and use in lymphangiogenesis experiments and also exhibit
high BANCR expression levels. The present study demonstrated that
under hypoxic conditions, BANCR promoted the lymphangiogenesis of
PC cells by a mechanism that may be associated with increased
activity of an HIF-1α/VEGF-C/VEGFR-3 axis.
Lymphangiogenesis is the formation of new lymphatic
vessels in tissues. Liu et al (31) detected the presence of what they
termed microlymphatics, new lymphatic vessels, or a lymphoid
labyrinth in the tissues of patients with gastric or colorectal
cancer, and this was significantly positively associated with lymph
node metastasis in nude mice xenografts. In PC, Sipos et al
(32) detected microlymphatics in
PC tissues and an increased number of lymphatic vessels around
malignant tumor tissues. Additionally, a significant association
between the number of lymphatic vessels and lymph node metastasis
was observed. The aforementioned studies together indicated that
lymphangiogenesis is a key step in tumor lymph node metastasis. In
the present study, it was shown that lymphangiogenesis in PC cells
increased significantly under hypoxic conditions, and this was
decreased by knocking down the expression of BANCR. These results
reveal that a hypoxic microenvironment and upregulation of BANCR
expression are key factors in lymphangiogenesis of PC.
A study by Keklikoglou et al (33) revealed that the expression of
VEGF-C was upregulated in PC and positively associated with MLVD,
the Dukes stage and lymph node metastasis. VEGFR-3 was the first
marker of the tyrosine-protein kinase family to be discovered and
is the specific receptor for VEGF-C. Yang et al (34) demonstrated that downregulation of
VEGFR-3 inhibits lymphangiogenesis, which further inhibits the
lymphatic metastasis of bladder cancer. In addition, a review
conducted by Winder and Lenz (35)
described data indicating that VEGFR-3 combined with VEGF-C induces
the formation of new lymphatic capillaries and increases the risk
of lymph node metastasis in colon cancer. Furthermore, Ochi et
al (9) detected a correlation
between VEGF-C and VEGFR-3 expression levels in PC by analyzing the
clinical and pathological data from patients; the authors concluded
that VEGFR-3 combined with VEGF-C induced the formation of new
lymphatic capillaries and increased the risk of lymph node
metastasis in PC. These findings indicate that the VEGF-C/VEGFR-3
pathway is important in the formation of microlymphatic vessels and
lymph node metastasis in PC. However, the molecular mechanisms
regulated by the VEGF-C/VEGFR-3 pathway in PC remain to be
determined.
A meta-analysis showed that BANCR is upregulated in
a variety of solid malignancies and is closely associated with a
poor overall survival rate, lymph node metastasis and distant
metastasis (36). In the present
study, the expression of BANCR in SW1990 and PANC-1 cells was
detected. The results showed that BANCR was upregulated in PC
cells, and the upregulated expression of BANCR was significantly
associated with lymphangiogenesis. Furthermore, knocking down the
expression of BANCR significantly downregulated the expression of
HIF-1α, VEGF-C and VEGFR-3 at the transcriptional and translational
levels. Based on the aforementioned results, it may be assumed that
BANCR is upregulated in PC and can promote tumor lymphangiogenesis
via the HIF-1 α/VEGF-C/VEGFR-3 pathway, which may lead to tumor
lymph node metastasis.
In the present study, the effects of hypoxia on the
expression of BANCR in PC cells were also investigated. The results
showed that the expression of BANCR in PC cells was significantly
increased under hypoxic conditions. Therefore, it is suggested that
hypoxia and BANCR are closely associated with the occurrence and
development of PC. Microlymphangiogenesis was also assessed, and
the results showed that the MLVD of PC cells increased
significantly under hypoxic conditions, and the MLVD in the
negative control cells was higher than that in the cells in which
BANCR was knocked down. The aforementioned study by Sipos et
al (32) detected
microlymphatics, new lymphatics or a lymphoid labyrinth in the
tissues of patients with PC. Another study of tissue samples from
patients with PC, conducted by Cheng et al (37), obtained similar results, with the
observation of microlymphatics, new lymphatic vessels and/or a
lymphoid labyrinth in PC tissues. The aforementioned results
indicate that BANCR may promote the formation of PC microlymphatics
under hypoxic conditions.
Nakajima et al (38) reported a significant association
between the elevated expression of HIF-1α and VEGF-C mRNA and lymph
node metastasis in patients non-small cell lung cancer. In
addition, Schoppmann et al (39) provided evidence that HIF-1α is
involved in the regulation of VEGF-C expression and
lymphangiogenesis in breast cancer, and a study by Katsuta et
al (40) in esophageal cancer
presented a similar result. However, the role of HIF-1α in PC is
not fully understood. The activation of HIF-1α is the most notable
molecular tumor cell alteration that occurs under hypoxic
conditions, and its abnormal expression is associated with a poor
prognosis in numerous types of tumors (41). A recent study by Liu et al
(29) reported that the increased
expression of HIF-1α in the tumor tissues of patients with PC is
associated with tumor lymph node metastasis, late-stage tumors and
a poorer predicted prognosis at first diagnosis, and revealed the
high expression of HIF-1α in PC tissues and its relationship with
TNM stage and lymph node metastasis. The results of the present
study showed an association between the expression levels of BANCR
and HIF-1α in PC cells. Knocking down the expression of BANCR
induced a significant reduction in the expression of HIF-1α in PC
cells at the transcriptional and translational levels,
demonstrating the positive regulation effect of BANCR on HIF-1α in
PC cells.
An increase in lymphangiogenesis and the
infiltration of lymphatic vessels into solid malignant tumor
tissues are necessary conditions for local lymph node metastasis,
and MLVD is an important quantitative index of these processes.
VEGF-C has been shown to be an important growth factor in the
lymphangiogenesis of solid malignant tumors, which can promote
lymphangiogenesis and microlymphangiogenesis via its combination
with VEGFR-3. In the present study, the expression levels of VEGF-C
and VEGFR-3 were significantly reduced by knocking down the
expression of BANCR. Furthermore, VEGF-C and VEGFR-3 expression
levels were also significantly decreased by knocking down the
expression of HIF-1α. The present study revealed that the
expression of BANCR was significantly increased in PC cells under
hypoxic conditions, and higher levels of BANCR were associated with
higher expression of components of the HIF-1α/VEGF-C/VEGFR-3 axis
at the transcriptional and translational levels.
In conclusion, the expression of BANCR in PC cells
was significantly increased under hypoxic conditions and the
upregulation of BANCR promoted lymphangiogenesis and upregulated
the expression of all components of the HIF-1α/VEGF-C/VEGFR-3
pathway, which plays an important role in the process of PC lymph
node metastasis. These findings suggest that BANCR may be a useful
biomarker and potential novel target for the diagnosis, treatment
and prognostic prediction of PC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Science Technology
Innovation Special Fund of Tongzhou area (grant nos. KJ2021CX008-22
and KJ2021CX008-24).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SLH, WH, YJ and HS conceived the study. HWS, JHM, JY
and YCD designed the study. SLH, WH and HS analyzed the data. JY
and YCD wrote the manuscript. SLH and WH edited the paper. All
authors have read and approved the final manuscript. SLH and WH
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mizrahi JD, Surana R, Valle JW and Shroff
RT: Pancreatic cancer. Lancet. 395:2008–2020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwarz RE and Smith DD: Extent of lymph
node retrieval and pancreatic cancer survival: Information from a
large US population database. Ann Surg Oncol. 13:1189–1200. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chari ST, Sharma A and Maitra A: Early
detection of sporadic pancreatic ductal adenocarcinoma: Problems,
promise, and prospects. Ann Intern Med. 172:558–559. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu X, Xia T, Cao M, Zhang P, Shi G, Chen
L, Zhang J, Yin J, Wu P, Cai B, et al: LncRNA BANCR promotes
pancreatic cancer tumorigenesis via modulating
MiR-195-5p/Wnt/β-catenin signaling pathway. Technol Cancer Res
Treat. 18:15330338198879622019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ran S, Volk L, Hall K and Flister MJ:
Lymphangiogenesis and lymphatic metastasis in breast cancer.
Pathophysiology. 17:229–251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sundar SS and Ganesan TS: Role of
lymphangiogenesis in cancer. J Clin Oncol. 25:4298–4307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stacker SA, Williams SP, Karnezis T,
Shayan R, Fox SB and Achen MG: Lymphangiogenesis and lymphatic
vessel remodelling in cancer. Nat Rev Cancer. 14:159–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi JU, Chung SW, Al-Hilal TA, Alam F,
Park J, Mahmud F, Jeong JH, Kim SY and Byun Y: A heparin conjugate,
LHbisD4, inhibits lymphangiogenesis and attenuates lymph node
metastasis by blocking VEGF-C signaling pathway. Biomaterials.
139:56–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ochi N, Matsuo Y, Sawai H, Yasuda A,
Takahashi H, Sato M, Funahashi H, Okada Y and Manabe T: Vascular
endothelial growth factor-C secreted by pancreatic cancer cell line
promotes lymphatic endothelial cell migration in an in vitro model
of tumor lymphangiogenesis. Pancreas. 34:444–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kung JT, Colognori D and Lee JT: Long
noncoding RNAs: Past, present, and future. Genetics. 193:651–669.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang J, Shi SH, Li XJ, Sun L, Ge QD, Li C
and Zhang W: Long non-coding RNA BRAF-regulated lncRNA 1 promotes
lymph node invasion, metastasis and proliferation, and predicts
poor prognosis in breast cancer. Oncol Lett. 15:9543–9552.
2018.PubMed/NCBI
|
|
12
|
Shen X, Bai Y, Luo B and Zhou X:
Upregulation of lncRNA BANCR associated with the lymph node
metastasis and poor prognosis in colorectal cancer. Biol Res.
50:322017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Flockhart RJ, Webster DE, Qu K,
Mascarenhas N, Kovalski J, Kretz M and Khavari PA: BRAFV600E
remodels the melanocyte transcriptome and induces BANCR to regulate
melanoma cell migration. Genome Res. 22:1006–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang S, Liu Z, Guo Q, Chen C, Ke X and Xu
G: High BANCR expression is associated with worse prognosis in
human malignant carcinomas: An updated systematic review and
meta-analysis. BMC Cancer. 20:8702020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan YH, Ye MH, Wu L, Wu MJ, Lu SG and Zhu
XG: BRAF-activated lncRNA predicts gastrointestinal cancer patient
prognosis: A meta-analysis. Oncotarget. 8:6295–6303. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou T and Gao Y: Increased expression of
LncRNA BANCR and its prognostic significance in human
hepatocellular carcinoma. World J Surg Oncol. 14:82016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu ZH, Yang TX, Xu ZP, Lv J, Cao XF, et
al: Long non-coding RNA BANCR expression in esophageal squamous
cell carcinoma and its effects on cell growth and invasion. Prog
Mod Biomed. 16:4622–4627. 2016.(In Chinese).
|
|
18
|
Lou KX, Li ZH, Wang P, Liu Z, Chen Y, Wang
XL and Cui HX: Long non-coding RNA BANCR indicates poor prognosis
for breast cancer and promotes cell proliferation and invasion. Eur
Rev Med Pharmacol Sci. 22:1358–1365. 2018.PubMed/NCBI
|
|
19
|
Zheng H, Xu J, Hao S, Liu X, Ning J, Song
X, Jiang L and Liu Z: Expression of BANCR promotes papillary
thyroid cancer by targeting thyroid stimulating hormone receptor.
Oncol Lett. 16:2009–2015. 2018.PubMed/NCBI
|
|
20
|
Stojanović S, Šelemetjev S, Đorić I,
Rončević J, Janković Miljuš J, Živaljević V and Išić Denčić T:
Elevated BANCR expression levels have different effects on
papillary thyroid carcinoma progression depending on the presence
of the BRAFV600E mutation. Eur J Surg Oncol. 46:1835–1842. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koong AC, Mehta VK, Le QT, Fisher GA,
Terris DJ, Brown JM, Bastidas AJ and Vierra M: Pancreatic tumors
show high levels of hypoxia. Int J Radiat Oncol Biol Phys.
48:919–922. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dauer P, Nomura A, Saluja A and Banerjee
S: Microenvironment in determining chemo-resistance in pancreatic
cancer: Neighborhood matters. Pancreatology. 17:7–12. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang G, Hu M, Ren H, Wang J, Cheng X, Li
R, Yuan B, Balan Y, Bai Z and Huang H: VHH212 nanobody targeting
the hypoxia-inducible factor 1α suppresses angiogenesis and
potentiates gemcitabine therapy in pancreatic cancer in vivo.
Cancer Biol Med. 18:772–787. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang QK, Wang XX, Wang Y and Ni N:
Integrative analysis reveals the landscape of hypoxia-inducible
factor (HIF) family genes in pan-cancer. J Oncol. 2020:88731042020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harris AL: Hypoxia-a key regulatory factor
in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ye LY, Zhang Q, Bai XL, Pankaj P, Hu QD
and Liang TB: Hypoxia-inducible factor 1α expression and its
clinical significance in pancreatic cancer: A meta-analysis.
Pancreatology. 14:391–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin X, Dai L, Ma Y, Wang J and Liu Z:
Implications of HIF-1α in the tumorigenesis and progression of
pancreatic cancer. Cancer Cell Int. 20:2732020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu M, Zhong J, Zeng Z, Huang K, Ye Z,
Deng S, Chen H, Xu F, Li Q and Zhao G: Hypoxia-induced feedback of
HIF-1α and lncRNA-CF129 contributes to pancreatic cancer
progression through stabilization of p53 protein. Theranostics.
9:4795–4810. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu HD, Zhao LH, Zhang YF, Li YL, Li XD,
Yang SC, Li YM and Nie CS: Morphologic features of lymphatic in
periphery region of gastric carcinoma and colon carcinoma. Ai
Zheng. 24:699–703. 2005.PubMed/NCBI
|
|
32
|
Sipos B, Kojima M, Tiemann K, Klapper W,
Kruse ML, Kalthoff H, Schniewind B, Tepel J, Weich H, Kerjaschki D
and Klöppel G: Lymphatic spread of ductal pancreatic adenocarcinoma
is independent of lymphangiogenesis. J Pathol. 207:301–312. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Keklikoglou I, Hosaka K, Bender C, Bott A,
Koerner C, Mitra D, Will R, Woerner A, Muenstermann E, Wilhelm H,
et al: MicroRNA-206 functions as a pleiotropic modulator of cell
proliferation, invasion and lymphangiogenesis in pancreatic
adenocarcinoma by targeting ANXA2 and KRAS genes. Oncogene.
34:4867–4878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang H, Kim C, Kim MJ, Schwendener RA,
Alitalo K, Heston W, Kim I, Kim WJ and Koh GY: Soluble vascular
endothelial growth factor receptor-3 suppresses lymphangiogenesis
and lymphatic metastasis in bladder cancer. Mol Cancer. 10:362011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Winder T and Lenz HJ: Vascular endothelial
growth factor and epidermal growth factor signaling pathways as
therapeutic targets for colorectal cancer. Gastroenterology.
138:2163–2176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang G and Cai J: Evaluation of
prognostic value of lncRNA BANCR in tumor patients: A systematic
review and meta-analysis. J BUON. 24:2553–2559. 2019.PubMed/NCBI
|
|
37
|
Cheng P, Jin G, Hu X, Shi M, Zhang Y, Liu
R, Zhou Y, Shao C, Zheng J and Zhu M: Analysis of tumor-induced
lymphangiogenesis and lymphatic vessel invasion of pancreatic
carcinoma in the peripheral nerve plexus. Cancer Sci.
103:1756–1763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakajima T, Anayama T, Koike T, Shingyoji
M, Castle L, Kimura H, Yoshino I and Yasufuku K: Endobronchial
ultrasound doppler image features correlate with mRNA expression of
HIF1-α and VEGF-C in patients with non-small-cell lung cancer. J
Thorac Oncol. 7:1661–1667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schoppmann SF, Fenzl A, Schindl M,
Bachleitner-Hofmann T, Nagy K, Gnant M, Horvat R, Jakesz R and
Birner P: Hypoxia inducible factor-1alpha correlates with VEGF-C
expression and lymphangiogenesis in breast cancer. Breast Cancer
Res Treat. 99:135–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Katsuta M, Miyashita M, Makino H, Nomura
T, Shinji S, Yamashita K, Tajiri T, Kudo M, Ishiwata T and Naito Z:
Correlation of hypoxia inducible factor-1alpha with lymphatic
metastasis via vascular endothelial growth factor-C in human
esophageal cancer. Exp Mol Pathol. 78:123–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jun JC, Rathore A, Younas H, Gilkes D and
Polotsky VY: Hypoxia-inducible factors and cancer. Curr Sleep Med
Rep. 3:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|