Introduction
Lung cancer is a malignant tumor with the highest
morbidity and mortality rates, and the fastest growth rate
globally, with non-small cell lung cancer (NSCLC) accounting for
80–85% of cases (1,2). In the early stages of lung cancer,
there are no visible signs. Even when there are symptoms, the
non-specificity of the clinical presentation affects the speed and
accuracy of diagnosis. Nearly 80% of the initial diagnoses are made
at the advanced stage (3),
excluding the possibility of surgical therapy.
The typical treatment approach for patients with
advanced NSCLC who are negative for driving gene mutations is
platinum-based combination chemotherapy. The traditional treatment
scheme is inefficient and has a high rate of adverse responses
(4), decreasing the quality of
life and treatment compliance of patients. For advanced NSCLC
positive for driver gene mutations, the first-line treatment is
targeted therapy, which can effectively prolong the survival of
patients and improve their quality of life (5,6).
However, at the moment, the positive rate of driving gene mutation
in patients with advanced NSCLC is >50% (7,8), and
so approximately one-half of the patients are ineligible for
targeted treatment.
NSCLC was formerly considered to be a
non-immune-related malignancy. Nonetheless, recent 5-year
investigations have revealed that negative immune regulation
centered on aberrant immunological checkpoints plays an important
role in the formation and progression of NSCLC (9–11).
With the development of immunology and precision medicine, novel
immunotherapeutic agents targeting programmed death protein 1
(PD-1) and its ligands (PD-L1 and PD-L2) have become important
measures and trends in the treatment of advanced NSCLC.
Sintilimab (trade name, Daboshu) is a humanized IgG4
monoclonal antibody targeting PD-1 developed by Innovent Biologics
and Eli Lilly and Company (12,13).
On December 24, 2018, the China Food and Drug Administration
formally authorized sintilimab to treat recurrent or refractory
classical Hodgkin lymphoma following at least second-line system
chemotherapy (12). According to a
recent study on sintilimab, the drug is a broad-spectrum medication
with strong affinity, long-lasting stability and an enhanced target
occupancy rate (14). Sintilimab
is now being tested in various stage I, II and III clinical studies
in China to treat a range of solid malignancies, including NSCLC
and esophageal cancer. To the best of our knowledge, in the
domestic and foreign literature to date, there are no meta-analyses
on the use of sintilimab for advanced NSCLC. Meta-analyses of
PD-1/PD-L1 receptor inhibitors for advanced NSCLC treatment have
only included a small number of sintilimab-related studies
(15,16). A meta-analysis was performed in in
the present study to assess the safety and effectiveness of
sintilimab in the treatment of advanced NSCLC, in order to serve as
a model for the therapeutic use of sintilimab in advanced
NSCLC.
Materials and methods
Inclusion and exclusion criteria
Inclusion criteria
The inclusion criteria were as follows: i) Study
type: Randomized controlled trial (RCT), with the language limited
to Chinese or English; ii) Subjects: Patients with NSCLC diagnosed
by cytology or pathology; iii) Intervention measures: The trial
group received sintilimab alone, or sintilimab combined with
chemotherapy, and the control group received general chemotherapy;
iv) Evaluation indicators: Overall survival (OS) time,
progression-free survival (PFS) time, overall effective rate,
objective remission rate, overall adverse drug reaction (ADR)
incidence and grade 3–5 ADR incidence (17).
Exclusion criteria
The exclusion criteria were as follows: i)
Literature that does not meet the diagnostic criteria; ii)
literature on animal experiments; iii) non-RCT studies; iv)
repeatedly published literature; and v) incomplete outcome
indicators.
Document retrieval
Chinese and English databases, including PubMed
(https://pubmed.ncbi.nlm.nih.gov/), The
Cochrane Library (https://www.cochranelibrary.com/), Embase (www.embase.com/), Chinese Biomedical Literature
(http://www.sinomed.ac.cn/index.jsp),
China National Knowledge Infrastructure (https://www.cnki.net/), VIP Chinese Science and
Technology Journal (http://www.cqvip.com/) and Wanfang Medical (https://www.wanfangdata.com.cn/) databases, were
searched. The search time limit was from the establishment of each
database until October 2021. The language of publication was
limited to Chinese and English. The key words used for the search
in both languages were ‘sintilimab’ and ‘non-small cell lung
cancer’, with ‘NSCLC’ also used in the English key word search.
Literature screening and data
extraction
Two researchers conducted the literature screening
and data extraction independently, and the included information was
cross-checked. Differences were resolved by discussion with a third
party. The contents of the data extracted mainly included the
following: i) General data, such as the study title, author,
literature source and publication year; ii) intervention measures
and trial implementation in the test group and control group; iii)
relevant elements of research type and bias risk assessment; and
iv) outcome index and result.
Document quality evaluation
The selected literature was evaluated according to
the evaluation criteria of the Cochrane bias risk assessment tool
(18). The evaluation items
included whether the random allocation method was correct, whether
there was allocation concealment, whether the blind method was
implemented, whether the result data were complete, whether there
was selective reporting of the research results and whether there
were other sources of bias.
Statistical analysis
RevMan5.3 software (The Cochrane Collaboration) was
used for the statistical data analysis. The evaluation indexes OS
and PFS used the risk ratio [hazard ratio (HR)] for the statistical
quantity of effect analysis; objective remission rate, overall
effective rate, overall ADR incidence and drug grade 3–5 ADR
incidence were the binary variables. The results used relative risk
(RR) as the statistical quantity of effect analysis. The difference
was considered statistically significant when the confidence
interval (CI) was limited to 95%, with P<0.05. The χ2
test was used as the heterogeneity test. When P>0.1 and
I2<50%, this indicated that there was no
heterogeneity among the research results, and the fixed effects
model was used for the analysis. When P≤0.1 and I2≥50%,
this indicated that there was statistical heterogeneity among the
research results, and a random effects model was used for the
analysis.
Results
Basic information of included
studies
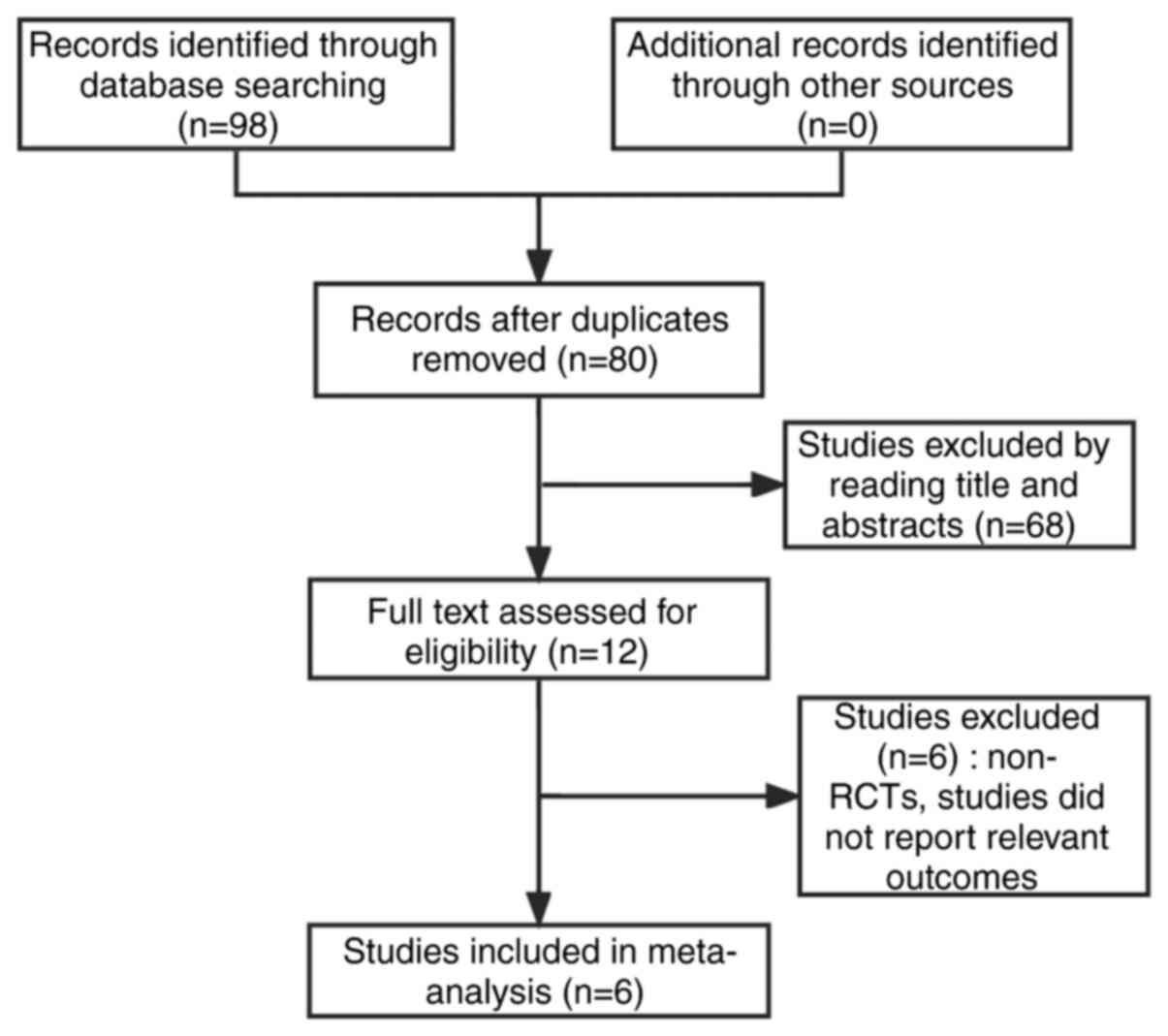
According to the established literature retrieval
strategy, 98 articles were obtained. Following application of the
exclusion criteria, 6 articles, including 1,162 patients, were
finally included in the meta-analysis. The screening process is
shown in Fig. 1, and the essential
information from the 6 studies are shown in Table I.
 | Table I.Baseline characteristics included in
the randomized controlled trials. |
Table I.
Baseline characteristics included in
the randomized controlled trials.
|
|
|
|
|
| Intervention
measures |
|
|---|
|
|
|
|
|
|
|
|
|---|
| First author/s,
year | Research type | Location | No. of patients | Diagnosis | Test group | Control group | Outcome index | (Refs.) |
|---|
| Yang et al,
2020 | Polycentric, random,
double-blind, phase III | China | 397 | Non-squamous
non-small cell carcinoma, stage IIIB-IV, EGFR and ALK
mutation-negative | Sintilimab (200 mg)
combined with pemetrexed and platinum chemotherapy | Placebo combined with
pemetrexed and platinum chemotherapy | PFS, OS, objective
remission rate, ADR incidence rate, incidence of grade 3–5
ADRs | (19) |
| Zhou et al,
2021 | Polycentric, random,
double-blind, phase III | China | 357 | Lung squamous cell
carcinoma, stage IIIB/IIIC or IV, EGFR, ALK mutation-negative | Sintilimab (200 mg)
combined with gemcitabine/platinum chemotherapy | Placebo combined with
gemcitabine and platinum chemotherapy | PFS, OS, objective
remission rate, ADR incidence rate, incidence of grade 3–5
ADRs | (20) |
| Liang and Wei,
2021 | Single-center,
random | China | 120 | Advanced non-small
cell lung cancer | Sintilimab (200 mg)
combined with gemcitabine chemotherapy | Gemcitabine
chemotherapy | Overall effective
rate, objective remission rate | (21) |
| He et al,
2021 | Single-center,
random | China | 70 | Non-small cell lung
cancer stage IV, EGFR, ALK mutation-negative | Sintilimab (200 mg)
combined with albumin paclitaxel chemotherapy | Albumin-bound
chemotherapy | Overall effective
rate, objective remission rate, ADR incidence rate | (22) |
| Hu et al,
2021 | Single-center,
random | China | 160 | Lung squamous cell
carcinoma, NSCLC stage IV | Sintilimab (200 mg)
combined with gemcitabine chemotherapy | Gemcitabine
chemotherapy | OS, overall
effective rate, objective remission rate, ADR incidence rate,
incidence of grade 3–5 ADRs | (23) |
| Chen et al,
2021 | Polycentric,
Random | China | 58 | Non-small cell lung
cancer phase IIIB and IV | Sintilimab (200 mg)
combined with anlotinib chemotherapy | Anlotinib
chemotherapy | PFS, overall
effective rate, objective remission rate, ADR incidence rate | (24) |
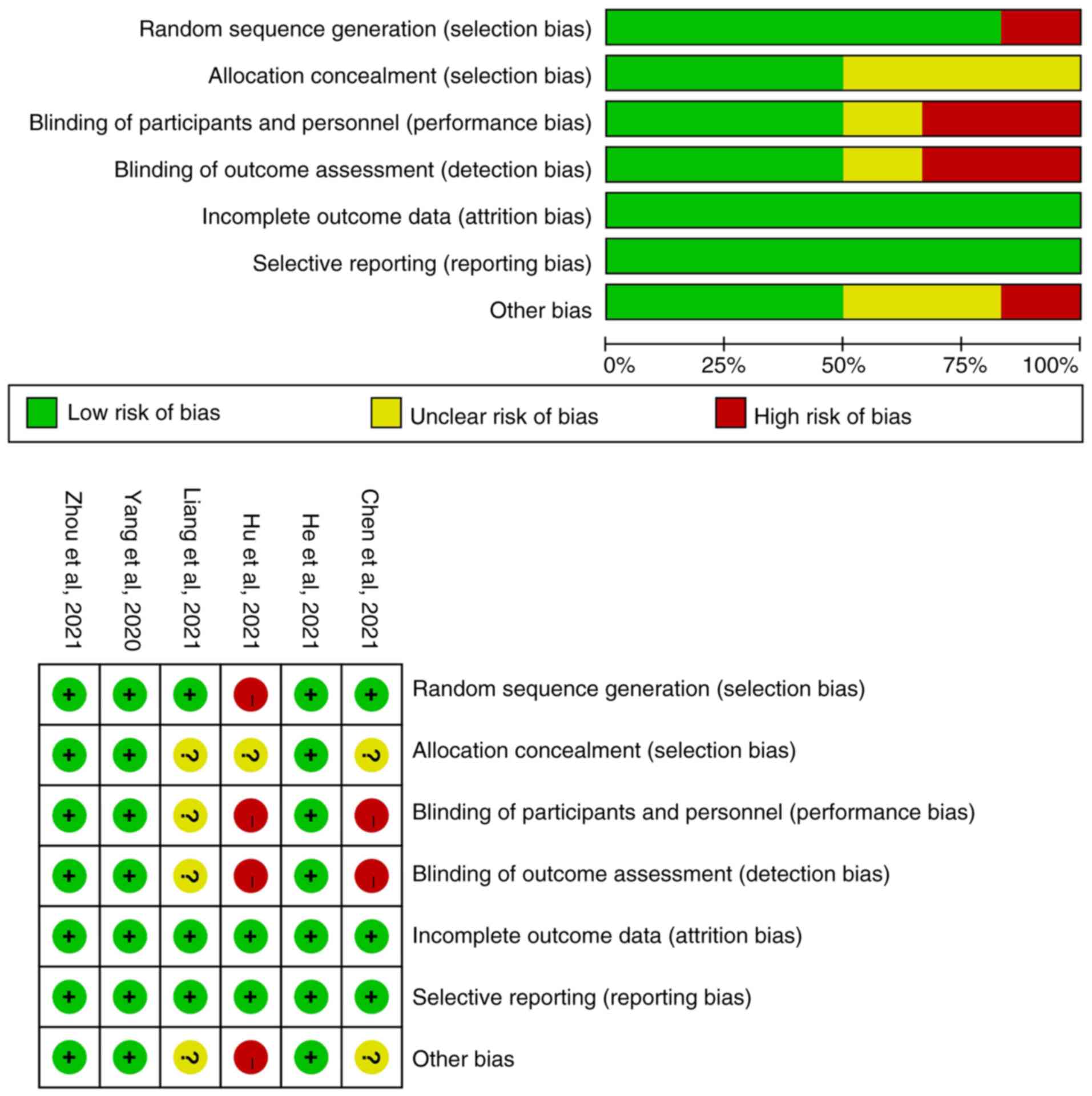
Document quality evaluation
The Cochrane bias risk assessment tool of RevMan5.3
software was used to evaluate the quality of the 6 RCT studies. It
was determined that 3 studies were of good quality and that 3 had a
high/unclear risk of bias. The specific literature quality is shown
in Fig. 2.
Meta-analysis results
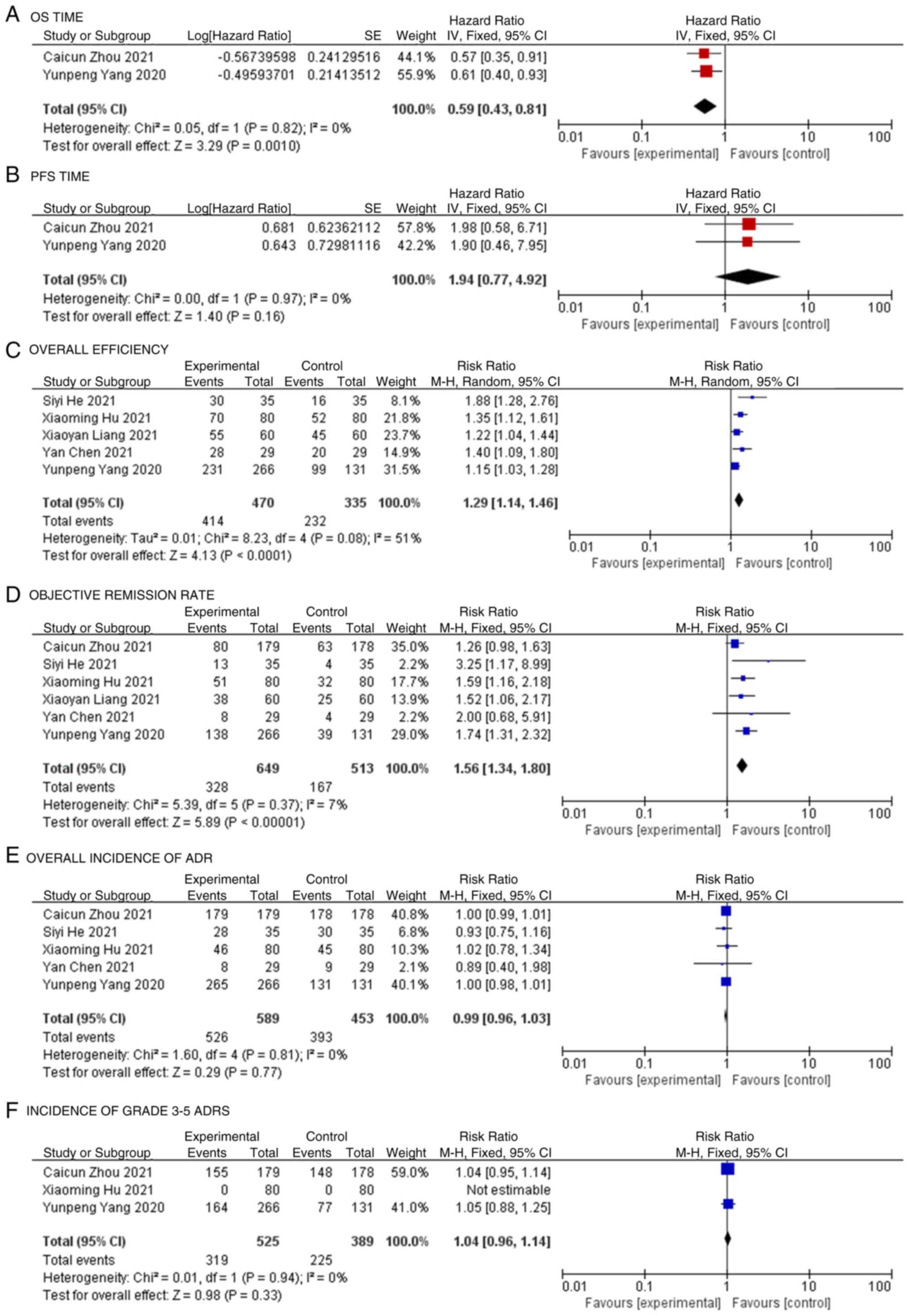
OS time
Among the 6 included studies, only 2 reported the HR
value and 95% CI for OS, and these 2 were incorporated into the
meta-analysis (19,20). The research results showed no
significant difference in heterogeneity (P=0.82; I2=0%;
Fig. 3A), so the fixed effects
model was used for the statistical analysis. The meta-analysis
showed that the test group exhibited significantly higher OS times
compared with the control group in the patients with NSCLC (HR,
0.59; 95% CI, 0.43-0.81; P=0.0010; Fig. 3A).
PFS time
Only 2 studies reported the HR value and 95% CI for
PFS among the 6 articles, and these 2 papers were incorporated into
the meta-analysis (19,20). There was no significant
heterogeneity between the results of each study (P=0.97;
I2=0%), so the fixed effects model was used for the
statistical analysis. The meta-analysis showed that the test group
exhibited higher PFS times compared with the control group in the
patients with NSCLC; however, the difference was not statistically
significant (HR, 1.94; 95% CI, 0.77-4.92; P=0.16; Fig. 3B).
Overall efficiency
Among the 6 included studies, 5 reported the overall
effective rate, and these 5 studies were combined for the
meta-analysis (19,21–23).
The heterogeneity difference among the research results was
statistically significant (P=0.08; I2=51%), so the
random effects model was used for the statistical analysis. The
meta-analysis showed that the overall effective rate of the test
group was significantly higher than that of the control group (RR,
1.29; 95% CI, 1.14-1.46; P<0.0001; Fig. 3C).
Objective remission rate
The objective remission rate was reported in all 6
studies, and these 6 studies were combined for the meta-analysis
(19–24). There was no significant
heterogeneity among the research results (P=0.37;
I2=7%), so the fixed effects model was used for the
statistical analysis. The meta-analysis showed that the objective
remission rate of the test group was significantly higher than that
of the control group (RR, 1.56; 95% CI, 1.34-1.80; P<0.00001;
Fig. 3D).
Overall incidence of ADR
Among the 6 studies, 5 reported the overall
incidence of ADRs, and these 5 were included in the meta-analysis
(19,20,22–24).
The results showed no significant difference in heterogeneity
(P=0.81; I2=0%), so the fixed effects model was used for
the statistical analysis. The meta-analysis showed no significant
difference in the overall incidence of ADRs between the test group
and the control group (RR, 0.99; 95% CI, 0.96-1.03; P=0.77;
Fig. 3E).
Incidence of grade 3–5 ADRs
Among the 6 studies, 3 reported the incidence of
grade 3–5 ADRs. In the 3 studies included in the meta-analysis
(19,20,23),
there was no statistically significant difference in heterogeneity
among the results (P=0.94; I2=0%), so the fixed effects
model was used for the statistical analysis. The meta-analysis
showed no statistically significant difference in grade 3–5 ADR
incidence between the experimental and control groups (RR, 1.04;
95% CI, 0.96-1.14; P=0.33; Fig.
3F).
Discussion
Lung cancer is the leading cause of death from
malignant tumors in China, and NSCLC accounts for >80% of cases
(1). Although targeted and
traditional therapy has brought significant survival benefits to
patients with advanced NSCLC, disease progression and drug
resistance have become critical issues limiting existing treatment
application and the development of new treatments. The discovery
and clinical application of immune checkpoint inhibitors has
provided significant progress in the treatment of a variety of
solid and non-solid malignant tumors. The PD-1/PD-L1 pathway, as
the final rate-limiting step of the antitumor immune response, can
effectively block the inhibitory regulation of immune checkpoints
and strengthen the antitumor immune response, which brings new hope
for the treatment of patients with advanced NSCLC (25). The National Comprehensive Cancer
Network guidelines (26) and the
Chinese Society of Clinical Oncology (27) recommend immunotherapy combined with
chemotherapy as the first-line treatment for PD-L1-positive NSCLC.
Nivolumab and pembrolizumab, approved for marketing in Japan and
the United States, respectively, in 2015, are the earliest PD-1
inhibitors with significant clinical efficacy (28). However, there are also ADRs such as
immune-associated pneumonia, enteritis and rash. As the first
domestic PD-1 inhibitor on the market, sintilimab is able to
effectively block the PD-1/PD-L1 pathway. Compared with nivolumab
and pembrolizumab, sintilimab has different binding epitopes and a
greater binding affinity for PD-1 (14). The receptor occupancy rate is
>95%, and the effect is lasting and stable; sintilimab has a
similar antitumor effect and better safety in advanced NSCLC
(29). In June 2021, sintilimab
was approved by the National Medical Products Administration for
the first-line treatment of advanced squamous NSCLC in combination
with chemotherapy. Further clinical trials of sintilimab will
change the treatment pattern of lung cancer. At present, clinical
trials have been performed on tumor types including, but not
limited to, second-line squamous NSCLC (NCT03150875; ORIENT-3),
first-line squamous NSCLC (NCT03629925; ORIENT-12), first-line
non-squamous NSCLC (NCT03607539; ORIENT-11), and locally advanced
epidermal growth factor receptor (EGFR)-mutated or metastatic
non-squamous NSCLC (NCT03802240; ORIENT-31) previously treated with
EGFR-tyrosine kinase inhibitor (30).
According to the results of the present study,
sintilimab combined with chemotherapy can significantly improve the
overall disease efficiency and objective remission rate of patients
with NSCLC, simultaneously prolonging the PFS and OS times of
patients. Sintilimab is superior to ordinary chemotherapy in the
treatment of NSCLC. OS time is the time from the beginning of
randomization to death (for any reason). Improving the OS time of
patients with advanced tumors is the main goal of clinical
treatment, and it is also the most important gold standard to
evaluate the efficacy of certain antineoplastic drugs. PFS time is
the time between the randomization of the patient and the
progression of tumor (any aspect) or death (for any reason). To a
certain extent, PFS can reflect the quality of life of patients
with advanced tumors, and it is also a highly valued indicator in
clinical treatment. However, since only 2 studies (19,20)
extracted the data for PFS and OS time in the present
meta-analysis, the long-term efficacy of sintilimab combined with
chemotherapy in the treatment of NSCLC was not determined.
Regarding safety, the overall incidence of ADRs to sintilimab
combined with chemotherapy was similar to that of conventional
chemotherapy, with no statistically significant difference. The
common adverse reactions included anemia, neutropenia, leukopenia,
thrombocytopenia, nausea, vomiting, diarrhea, rash, liver function
damage and renal function damage. The incidence of grade 3–5
adverse reactions of sintilimab combined with chemotherapy was
slightly higher than that of conventional chemotherapy, but the
results of the meta-analysis showed that there was no statistically
significant difference, and the types of grade 3 and above adverse
reactions of the two groups were similar, mainly including
leucopenia, thrombocytopenia, neutropenia, immune-associated
pneumonia, hyponatremia and rash (19,20),
which is consistent with that reported in the literature (31). In addition, there have been
reported adverse reactions associated with immunotherapy during the
treatment of sintilimab (19,20,22).
PD-1 inhibitors activate immune cells to kill tumor cells, and this
non-specific activation of the immune system may lead to immune
damage to other organs and tissues. Most immunotherapy-related
adverse reactions occurred within 1–6 months after the initiation
of treatment (32), with varying
periods, and their clinical manifestations were mostly
non-specific, atypical and reversible. Among the literature
included in the present meta-analysis, 3 studies (19,20,22)
reported the occurrence of adverse reactions associated with
immunotherapy. The typical adverse reactions associated with
immunotherapy included myelosuppression, hypothyroidism,
hyperthyroidism, fever, immune-associated pneumonia, diarrhea, skin
reaction, immune-related hepatitis and glomerulonephritis.
Nevertheless, the overall incidence of drug withdrawal and
mortality caused by sintilimab combined with chemotherapy due to
ADRs was low, indicating that it is well tolerated and the safety
is controllable.
The present study aimed to evaluate the efficacy and
safety of sintilimab in the treatment of advanced NSCLC by
comprehensively searching the relevant literature, and by combining
and analyzing the results according to the statistical methods of
evidence-based medicine, so as to provide the evidence-based basis
for the clinical treatment of advanced NSCLC with sintilimab.
However, this study still has certain limitations: i) Some of the
evaluation indicators in the literature with survival as the
endpoint was not included as the data could not be converted to an
HR value for the meta-analysis; ii) in the included studies, there
is specific heterogeneity in the study design, follow-up time and
outcome index data, and the quality of some of the included
literature was only average; iii) sintilimab has a short time to
market, there are few published associated clinical randomized
controlled studies, and the number of included cases is also
limited.
Compared with ordinary chemotherapy, the efficacy
and safety of sintilimab combined with chemotherapy in the
treatment of advanced NSCLC is worth affirming, with a particular
clinical application value and being worthy of clinical promotion.
However, limited by the number and quality of included studies, the
aforementioned conclusions still need to be verified by further
large-scale and high-quality studies.
Acknowledgements
The authors are grateful to Professor Baochang He
(Fujian Medical University School of Public Health, Fuzhou, China)
for providing guidance and suggestions on writing this
manuscript.
Funding
This research was supported by the Startup Fund for scientific
research, Fujian Medical University (grant no. 2019QH1260) and the
Natural Science Foundation of Fujian Province (grant no.
2018J01187).
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Author contributions
JX, XW, JW, FH and LX contributed to the conception
and design of the study. JX and LX wrote the manuscript. JX and FH
searched the literature, extracted data from the collected
literature and analyzed the data. LX revised the manuscript. All
authors read and approved the final version of the manuscript. JX,
XW, JW, FH and LX confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lan L, Zhao F, Cai Y, Wu RX and Meng Q:
Analysis of epidemiological characteristics of malignant tumor
mortality among Chinese residents in 2015. Zhonghua Liu Xing Bing
Xue Za Zhi. 39:32–34. 2018.(In Chinese). PubMed/NCBI
|
|
2
|
Chen WQ: Cancer statistics: Updated cancer
burden in China. Chin J Cancer Res. 27:12015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhi XY, Zou XN, Hu M, Jiang Y, Jia MM and
Yang GH: Increased lung cancer mortality rates in the Chinese
population from 1973–1975 to 2004–2005: An adverse health effect
from exposure to smoking. Cancer. 121:3107–3112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen G, Zheng F, Ren D, Du F, Dong Q, Wang
Z, Zhao F, Ahmad R and Zhao J: Anlotinib: A novel multitargeting
tyrosine kinase inhibitor in clinical development. J Hematol Oncol.
11:1202018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hida T, Nokihara H, Kondo M, Kim YH, Azuma
K, Seto T, Takiguchi Y, Nishio M, Yoshioka H, Imamura F, et al:
Alectinib versus crizotinib in patients with ALK-positive
non-small-cell lung cancer (J-ALEX): An open-label, randomised
phase 3 trial. Lancet. 390:29–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung
cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong J, Yang X, Kong H, Yang MX and Xie
WP: Analysis of EGFR and ALK driving genes in 2394 patients with
lung adenocarcinoma. J Nanjing Med Univ (Natural Science).
40:675–680. 2020.(In Chinese).
|
|
8
|
Qiao XL, Ai D, Liang H, Mu D and Guo Q:
Analysis of gene expression and clinical characteristics of
molecular targeted therapy for non-small cell lung cancer in
Shandong. Zhongguo Fei Ai Za Zhi. 20:14–20. 2017.(In Chinese).
PubMed/NCBI
|
|
9
|
Dermani FK, Samadi P, Rahmani G, Kohlan AK
and Najafi R: PD-1/PD-L1 immune checkpoint: Potential target for
cancer therapy. J Cell Physiol. 234:1313–1325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leprieur EG, Dumenil C, Julie C, Giraud V,
Dumoulin J, Labrune S and Chinet T: Immunotherapy revolutionises
non-small-cell lung cancer therapy: Results, perspectives and new
challenges. Eur J Cancer. 78:16–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sheng Z, Zhu X, Sun Y and Zhang Y: The
efficacy of anti-PD-1/PD-L1 therapy and its comparison with
EGFR-TKIs for advanced non-small-cell lung cancer. Oncotarget.
8:57826–5735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Innovent Biologics: China's NMPA approves
Innovent's antipd-l antibody Tyvyt® (sintilimab
injection) for Hodgkins lymphoma [media release]. Innovent
Biologics (Suzhou) Co., Ltd.; Suzhou: 2018, http://innoventbio.com/en/#/news/123December
26–2018
|
|
13
|
Innovent Biologics, .
Tyvyt®(sintilimab): Chinese prescribing information.
Innovent Biologics (Suzhou) Co., Ltd.; Suzhou: 2019
|
|
14
|
Wang J, Fei K, Jing H, Wu Z, Wu W, Zhou S,
Ni H, Chen B, Xiong Y, Liu Y, et al: Durable blockade of PD-1
signaling links preclinical efficacy of sintilimab to its clinical
benefit. MAbs. 11:1443–1451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Qian Y, Li J, Cui C, Chen L, Qu S
and Lu S: Indirect comparison of sintilimab and other PD-L1
inhibitors for first-line treatment of non-squamous non-small-cell
lung cancer. Future Oncol. 18:1896–1905. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye Z, Yang W, Xuan B, Li X, He J, Si H and
Ma W: Efficacy and safety evaluation of sintilimab for cancer
treatment: A systematic review and meta-analysis of randomized
controlled trials. Front Pharmacol. 13:8951872022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Cancer Institute, . Common
Terminology Criteria for Adverse Events (CTCAE) v4.0. NIH;
Bethesda, MD: 2009
|
|
18
|
Cumpston MS, McKenzie JE, Welch VA and
Brennan SE: Strengthening systematic reviews in public health:
Guidance in the cochrane handbook for systematic reviews of
interventions. (2nd edition). J Public Health (Oxf).
28:fdac0362022. View Article : Google Scholar
|
|
19
|
Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang
S, Chen G, Mei X, Yang Z, Ma R, et al: Efficacy and safety of
sintilimab plus pemetrexed and platinum as first-line treatment for
locally advanced or metastatic nonsquamous NSCLC: A randomized,
double-blind, phase 3 study (Oncology pRogram by InnovENT
anti-PD-1-11). J Thorac Oncol. 15:1636–1646. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen
G, Zhang L, Huang D, Cang S, Yang Z, et al: Sintilimab plus
platinum and gemcitabine as first-line treatment for advanced or
metastatic squamous NSCLC: Results from a randomized, double-blind,
phase 3 trial (ORIENT-12). J Thorac Oncol. 16:1501–1511. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang X and Wei Z: Effect of sintilimab
combined with chemotherapy on tumor markers and immune function of
advanced non-small-cell lung cancer. Pak J Med Sci. 37:1063–1068.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He SY, Zhang M, Xu Z, Lu XL, Chen ZP and
Zuo Y: Efficacy of sintilimab combined with chemotherapy in the
treatment of non-small cell lung cancer and its effect on immune
function. Chin Pract Med. 16:12–15. 2021.(In Chinese).
|
|
23
|
Hu XM, Li GY and Wang L: Clinical study of
gemcitabine combined with Sintilimab in the treatment of stage IV
lung squamous cell carcinoma. Chin J Clin Oncol Rehabilitation.
28:302–305. 2021.(In Chinese).
|
|
24
|
Chen Y, Liu W, Liang YH, Chen HF and Dong
ZH: Observation on the efficacy of Sintilimab combined with
amlotinib in the treatment of advanced non-small cell lung cancer.
J Guangdong Medical University. 39:615–618. 2021.
|
|
25
|
Ai C, Ji SS, Tang L, Wang L, Yan X and
Zhou MZ: Progress in antitumor clinical research of PD-1/PD-L1
inhibitors. Clin Med J. 19:62021.
|
|
26
|
National Comprehensive Cancer Network
(NCCN), . NCCN Clinical Practice Guidelines in Oncology: Non-small
cell lung cancer (version 1). NCCN; Plymouth Meeting, PA: 2021,
https://www.nccn.org/professionals/physician_gls/
|
|
27
|
Guidelines Working Committee of the
Chinese Society of Clinical Oncology, Chinese Society of Clinical
Oncology (CSCO), . Chinese Society of Clinical Oncology (CSCO):
Guidelines for the diagnosis and treatment of non-small cell lung
cancer 2020. People's Health Publishing House; Beijing, China: pp.
42021
|
|
28
|
Cai JX, Siying Chen SY, Jianping Qin JP,
Yue Zhang Y, Ruifeng Qi RF and Ye YM: Analysis of the efficacy and
safety of Sintilimab in the treatment of patients with advanced
colorectal cancer. Chin J Pharmacovigilance. 19:164–168. 2022.(In
Chinese).
|
|
29
|
Zhang L, Mai WQ, Jiang WY and Geng Q:
Sintilimab: A promising antitumor PD-1 antibody. Front Oncol.
10:5945582020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu D, Li YY, Song YQ and Li YJ: Clinical
research progress of PD-1 inhibitor Sintilimab. Chinese J Hospital
Pharmacy. 40:120–123. 2020.
|
|
31
|
Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan
X, Shen C, Duma N, Aguilera JV, Chintakuntlawar A, et al:
Treatment-related adverse events of PD-1 and PD-L1 inhibitors in
clinical trials: A systematic review and meta-analysis. JAMA Oncol.
5:1008–1019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eigentler TK, Hassel JC, Berking C, Aberle
J, Bachmann O, Grünwald V, Kähler KC, Loquai C, Reinmuth N, Steins
M, et al: Diagnosis, monitoring and management of immune-related
adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat
Rev. 45:7–18. 2016. View Article : Google Scholar : PubMed/NCBI
|