Introduction
Leptomeningeal metastasis (LM), defined as the
invasion of metastatic tumour cells into the leptomeninges and
cerebrospinal fluid (CSF) of the subarachnoid space, is a
clinically devastating condition that occurs in the terminal stage
of cancer (1). Approximately 10%
of patients with metastatic cancer are diagnosed with LM during the
course of disease (2). The longer
survival achieved with more effective systemic cancer treatment and
the improvement in neuroimaging technologies has led to an increase
in the incidence of LM (3,4). Furthermore, several therapeutic
advances, such as small molecular weight targeted therapies and
immunotherapies, have led to prolonged survival of patients with LM
(4–6). Predicting the therapeutic efficacy of
active treatment in patients with LM has become increasingly
necessary.
Ventriculo-lumbar perfusion (VLP) chemotherapy is a
recently introduced treatment approach that alleviates CSF flow
disturbances and can aid in prolonging the survival of patients
with LM (7–10). Robust prognostic predictors for
this population are needed to design personalised therapeutic
strategies, and previous studies have reported several potential
CSF biomarkers (11,12). However, CSF prognostic biomarkers
are yet to become standardised in patients with LM, particularly
those treated with VLP chemotherapy.
Neurofilament light chain (NfL) is a cytoskeletal
protein that preserves the stability of neurons; NfL levels in the
CSF reflect neuroaxonal damage from numerous neurological
disorders, which results in neurological disability (13). A reliable quantification of the
extent of baseline neuroaxonal damage is reported to be helpful for
determining the prognosis in a wide spectrum of neurological
disorders (13). However, the
prognostic value of CSF NfL levels in LM has yet to be elucidated.
This study aimed to evaluate the prognostic performance of CSF NfL
in predicting survival in patients with LM treated with VLP
chemotherapy.
Patients and methods
Patients
From 2014 to 2021, patients with LM who i) were
included in a clinical trial for investigating the optimal
perfusion rate of VLP chemotherapy at the National Cancer Center in
Korea (10,14), and ii) had available stored CSF
samples obtained from the lumbar subarachnoid space immediately
before initiation of VLP chemotherapy were enrolled. Patients with
known central nervous system (CNS) disorders, including
neurodegenerative and neurovascular diseases, which could result in
elevated CSF NfL levels due to neuro-axonal damage, were excluded
(13). The diagnosis of LM was
made via either the cytological confirmation of malignant cells in
the CSF or the presence of magnetic resonance imaging (MRI)
features compatible with LM (1).
In 42 participants with LM, VLP chemotherapy was administered with
a perfusion rate of 15 ml/h, and a daily dose of methotrexate 24 mg
(14). All participants completed
the induction of VLP chemotherapy as previously described (14).
Methods
NfL levels were analyzed in the reserved CSF samples
stored at −80°C. CSF NfL levels were measured using a single
molecular array assay (Simoa). CSF NfL concentrations were measured
in duplicate by an independent investigator who was blinded to the
clinical information. The mean inter-assay and intra-assay
coefficients of variation were 7.4 and 5.3%, respectively.
The primary outcome was overall survival, which was
defined as the time from the initiation of VLP chemotherapy to
death. The previously known prognostic factors for LM, including
Karnofsky performance status (KPS), CSF protein levels, presence of
increased intracranial pressure (ICP), prior or concurrent
radiotherapy, and prior systemic chemotherapy involving more than
three different regimens, were evaluated at the beginning of VLP
chemotherapy (1). NfL levels are
influenced by neurodegenerative neuro-axonal injury and CSF
turn-over reduction due to the aging process; therefore, we
performed a sub-analysis with the patients classified according to
age group (15).
The Institutional Review Board Committee at the
National Cancer Center approved the current study (approval no.
NCC2021-0162), and written informed consent was obtained from all
participants.
Statistical analyses
The prognostic cut-off level was calculated using
the method proposed by Contal and O'Quigley. After the CSF NfL was
sorted, it was dichotomized at each threshold and the Q statistic
was calculated based on the log-rank test statistics. The final
cut-off was defined as the value that maximizes the Q statistics,
meaning the maximal difference between the two groups (16). Kaplan-Meier survival curves of
patients divided into groups according to CSF NfL cut-off levels
are presented and were assessed using the log rank test. The Cox
proportional hazards model was used to evaluate the prognostic
performance of variables associated with survival, such as CSF NfL,
protein levels, KPS, ICP, presence of prior or concurrent
radiotherapy, and prior systemic chemotherapy over three different
regimens, which were previously described prognostic factors for LM
(1). The variables with P<0.2
in univariable analysis were pre-selected, and the final
multivariable model was determined using backward selection method
with an elimination criterion of P>0.05. The reported P-values
are two-sided and statistical significance was defined at
P<0.05. All statistical analyses were performed using SAS
version 9.4 (SAS Institute Inc.) and R project software (version
4.1.1).
Results
Demographics
The characteristics of the 42 patients with LM are
summarised in Table I. There were
27 women and 15 men, and the median age at CSF sampling was 55
years. The median KPS at the initiation of VLP chemotherapy was 70.
The most commonly observed primary tumour type was lung cancer
(n=29, 69%), including 1 small cell lung cancer, followed by breast
cancer (n=7, 17%) and ovarian cancer (n=4, 10%).
 | Table I.Patient demographics (n=42). |
Table I.
Patient demographics (n=42).
| Demographic
information | Value |
|---|
| Median age at
sampling, years (IQR) | 55 (46–62) |
| Female/male (%) | 27 (64.3)/15
(35.7) |
| Median overall
survival, days (95% CI) | 174 (104–237) |
| Median survival after
initiation of VLP, days (95% CI) | 151 (84–201) |
| Median KPS at the
initiation of VLP (IQR) | 70 (60–70) |
| Median ICP at the
initiation of VLP (IQR) | 200 (150–250) |
| Median CSF protein
levels at the initiation of VLP, mg/dl (IQR) | 40 (25–66) |
| Median CSF NfL levels
at the initiation of VLP, ng/ml (IQR) | 8.15
(2.11-14.30) |
| Primary cancer |
|
| Lung
(NSCLC + SCLC) | 29 (28+1) |
|
Breast | 7 |
|
Ovarian | 4 |
|
Melanoma | 1 |
|
Unknown | 1 |
Prognostic performance of CSF NfL
levels in patients with LM
At the time of the analysis, 40 (95%) of the 42
patients with LM treated with VLP chemotherapy died; 12 patients
died from CNS-related causes (17), 10 died from systemic as well as CNS
cancer progression, and the remaining 18 died from undetermined
causes. The median overall survival after diagnosis was 174 days
[95% confidence interval (CI): 104–237 days], and the median
overall survival after initiation of VLP chemotherapy was 151 days
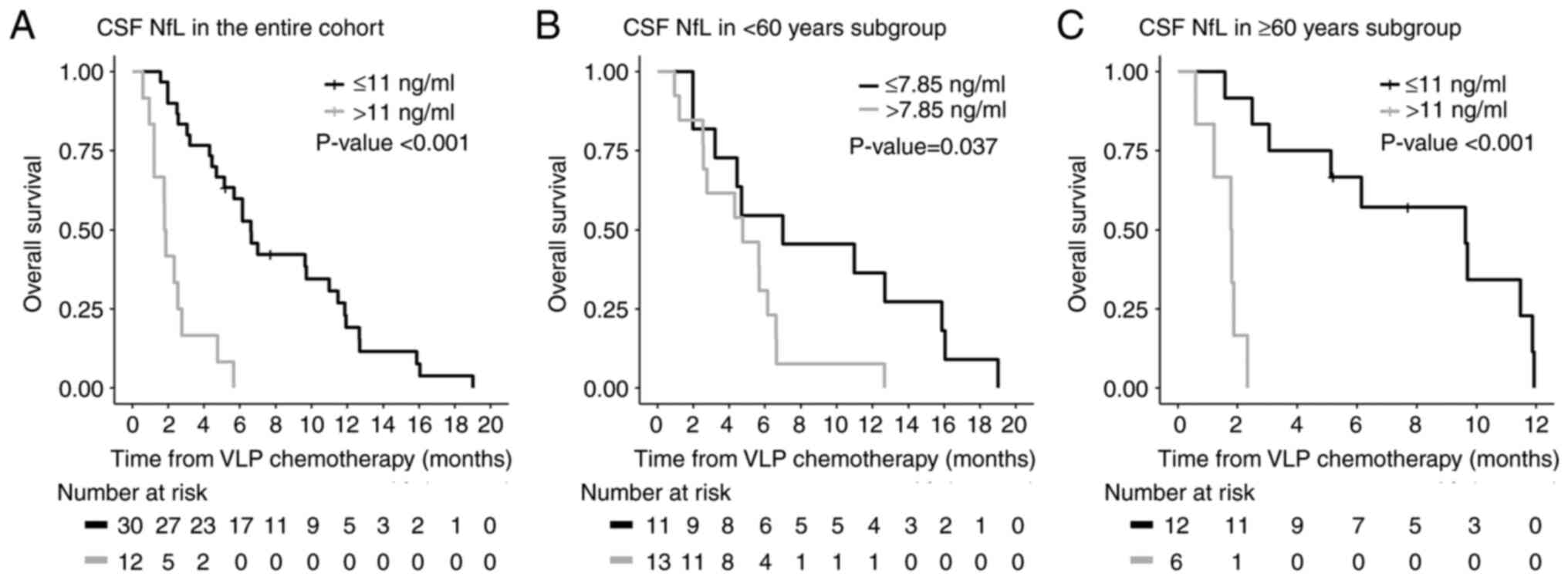
(95% CI: 84–201 days). The median CSF NfL level was 8.15 ng/ml
(interquartile range, 2.11-14.30). The estimated overall prognostic
cut-off value was 11 ng/ml, and 12 (30%) of 40 patients had CSF NfL
levels higher than this cut-off value. The median overall survival
after initiation of VLP chemotherapy was longer in LM patients with
low CSF NfL levels at the start of VLP chemotherapy than in those
with high CSF NfL levels [201 (95% CI: 143–334) vs. 56 (95% CI:
29–84) days, P<0.001; Fig.
1A].
In the univariable analysis, CSF NfL >11 ng/ml,
KPS <70, and a primary cancer type other than non-small cell
lung cancer showed a significant association with unfavourable
survival after initiation of VLP chemotherapy (Table IIA). The ICP at the initiation of
VLP chemotherapy, CSF protein level >50 mg/dl, presence of prior
or concurrent radiation therapy, and systemic chemotherapy
involving over three different regimens did not show significant
associations. In the multivariable analysis, CSF NfL >11 ng/ml
and KPS <70 continued to be significant variables.
 | Table II.Multivariable analysis of the entire
cohort and the different age groups [<60 years (n=24), ≥60 years
(n=18)]. |
Table II.
Multivariable analysis of the entire
cohort and the different age groups [<60 years (n=24), ≥60 years
(n=18)].
| A, Entire cohort (n=
42) |
|---|
|
|---|
|
| Univariable
analysis | Multivariable
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| KPS |
|
|
|
|
| ≥70 | 1 |
| 1 |
|
|
<70 | 2.60 (1.32-5.13) | 0.006 | 2.22 (1.09-4.50) | 0.028 |
| ICP |
|
|
|
|
|
<200 | 1 |
|
|
|
| ≥200 | 1.07 (0.56-2.03) | 0.837 |
|
|
| CSF protein,
mg/dl |
|
|
|
|
| ≤50 | 1 |
|
|
|
|
>50 | 1.06 (0.55-2.03) | 0.865 |
|
|
| Prior/concurrent
RTx |
|
|
|
|
| No | 1 |
|
|
|
| Yes | 0.75 (0.39-1.45) | 0.391 |
|
|
| NSCLC |
|
|
|
|
| No | 1 |
|
|
|
| Yes | 0.48 (0.24-0.93) | 0.031 |
|
|
| Number of
chemotherapy regimen |
|
|
|
|
| ≤3 | 1 |
|
|
|
|
>3 | 1.11 (0.58-2.14) | 0.750 |
|
|
| NfL, ng/ml |
|
|
|
|
| ≤11 | 1 |
| 1 |
|
|
>11 | 7.26
(3.10-17.02) | <0.001 | 6.63
(2.76-15.94) | <0.001 |
|
| B, <60 years
(n=24) |
|
|
| Univariable
analysis | Multivariable
analysis |
|
|
|
|
| Variable | HR (95%
CI) | P-value | HR (95%
CI) | P-value |
|
| KPS |
|
|
|
|
|
≥70 | 1 |
| 1 |
|
|
<70 | 2.54
(1.04-6.20) | 0.041 | 2.97
(1.14-7.78) | 0.027 |
| ICP |
|
|
|
|
|
<200 | 1 |
|
|
|
|
≥200 | 1.48
(0.64-3.40) | 0.360 |
|
|
| CSF protein,
mg/dl |
|
|
|
|
|
≤50 | 1 |
|
|
|
|
>50 | 0.98
(0.38-2.52) | 0.962 |
|
|
| Prior/concurrent
RTx |
|
|
|
|
| No | 1 |
| 1 |
|
|
Yes | 0.57
(0.25-1.33) | 0.195 | 0.31
(0.12-0.80) | 0.015 |
| NSCLC |
|
|
|
|
| No | 1 |
|
|
|
|
Yes | 0.51
(0.21-1.23) | 0.133 |
|
|
| Number of
chemotherapy regimen |
|
|
|
|
| ≤3 | 1 |
|
|
|
|
>3 | 1.28
(0.55-2.97) | 0.570 |
|
|
| NfL, ng/ml |
|
|
|
|
|
≤7.85 | 1 |
| 1 |
|
|
>7.85 | 2.68
(1.02-7.01) | 0.045 | 3.73
(1.32-10.57) | 0.013 |
|
| C, ≥60 years
(n=18) |
|
|
| Univariable
analysis | Multivariable
analysis |
|
|
|
|
|
Variable | HR (95%
CI) | P-value | HR (95%
CI) | P-value |
|
| KPS |
|
|
|
|
|
≥70 | 1 |
|
|
|
|
<70 | 3.28
(1.06-10.18) | 0.039 |
|
|
| ICP |
|
|
|
|
|
<200 | 1 |
|
|
|
|
≥200 | 0.68
(0.24-1.96) | 0.473 |
|
|
| CSF protein,
mg/dl |
|
|
|
|
|
≤50 | 1 |
|
|
|
|
>50 | 0.90
(0.33-2.50) | 0.841 |
|
|
| Prior/concurrent
RTx |
|
|
|
|
| No | 1 |
|
|
|
|
Yes | 0.87
(0.27-2.78) | 0.820 |
|
|
| NSCLC |
|
|
|
|
| No | 1 |
|
|
|
|
Yes | 0.33
(0.11-1.03) | 0.055 |
|
|
| Number of
chemotherapy regimen |
|
|
|
|
| ≤3 | 1 |
|
|
|
|
>3 | 1.53
(0.40-5.83) | 0.532 |
|
|
| NfL, ng/ml |
|
|
|
|
|
≤11 | 1 |
| 1 |
|
|
>11 | 24.44
(2.80-213.72) | 0.004 | 24.44
(2.80-213.72) | 0.004 |
We subsequently calculated specific cut-off values
according to patient age. In the <60- and ≥60-year subgroups,
the prognostic cut-off values were estimated as 7.85 and 11 ng/ml,
respectively. Using these cut-off values, the median overall
survival after initiation of VLP chemotherapy was longer in LM
patients with low CSF NfL levels than in those with high CSF NfL
levels in both age groups [213 (95% CI: 60–483) vs. 145 (95% CI:
77–187) days, P=0.037 in the <60 group; 293 (95% CI: 76–361) vs.
54.5 (95% CI: 18–71) days, P<0.001 in the ≥60 group; Fig. 1B and C]. In the multivariable
analysis, CSF NfL levels continued to show a significant
association with survival in both age groups (Table IIB and C).
Discussion
The median overall survival after commencement of
VLP chemotherapy in LM patients with low CSF NfL levels was longer
than that in those with high CSF NfL levels. In the multivariable
analysis with diverse potential prognostic factors and sub-analysis
of different age groups, statistical significance was maintained.
CSF NfL levels at the initiation of VLP chemotherapy may be a
potential prognostic factor for predicting overall survival in
patients with LM, and it would be helpful to design individualized
therapeutic strategies.
In previous studies, the most consistent prognostic
factor in LM has been performance status at diagnosis (1). In line with the previous results,
clinical performance status represented by the KPS score was a
significant prognostic factor in patients with LM from the
multivariable analysis of the current study. Prior and/or
concurrent radiotherapy was a significant prognostic factor in an
age group under 60 years but not significant for those over 60
years, and there was a discrepancy in whether radiotherapy was
associated with overall survival in previous studies (1). To the best of our knowledge, this
study is the first to demonstrate the prognostic significance of
CSF NfL as a quantitative and objective marker in LM.
NfL is a biomarker of acute and chronic neuro-axonal
damage, and baseline CSF NfL levels are associated with
neurological deterioration and/or survival in various neurological
diseases including dementia, amyotrophic lateral sclerosis, and
multiple sclerosis (13). Similar
to the context of previous reports, in this study, we found that
baseline CSF NfL levels are associated with overall survival after
treatment in LM. A caveat is that NfL levels are influenced by the
aging process; therefore, we needed the cut-off value estimated per
individual age groups (15). Given
the small sample size in the present study, we could only divide
patients into two different age groups; the sub-analysis showed
that CSF NfL levels were significant prognostic factors in both age
groups. Further large studies including sufficient number of
patients from all age groups are required to establish universal
standardized age-dependent prognostic cut-off values.
VLP is a recently introduced development in the
treatment of LM that can help overcome disruption of CSF flow,
found in up to 50% of patients (8–10,18).
In order to increase drug delivery but reduce drug toxicity, the
VLP method was used, and several previous clinical trials showed
the efficacy of VLP chemotherapy, using a small dosage of
chemo-agent and slow perfusion rate to improve survival of LM
patients (10,14). However, no robust data on the
prognostic factors in patients with LM treated with VLP
chemotherapy are available. The prognostic value of CSF CYFRA 21-1
has been reported in previous studies (11,12),
and herein we observed the prognostic value of CSF NfL in LM.
Combining these biomarkers would be helpful in building an
integrated prognostic system that aids in making personalised
therapeutic decisions. Unfortunately, we could not simultaneously
estimate the levels of CSF CYFRA 21-1 because of the shortage of
CSF samples in the current study. Additional studies are required,
including simultaneous measurement of such biomarkers and their
combination with clinical prognostic factors, to establish a more
reliable system for predicting the prognosis of LM.
One limitation of this study is that the
retrospective design and single referral centre setting may carry
the risk of unintentional selection bias. Additionally, we could
not analyse the patients with CNS-related causes of death
separately because of the small sample size (17). Finally, to demonstrate the
universal usefulness of CSF NfL as a prognostic biomarker, external
validation in larger LM patient cohorts with individual specific
primary tumour types and other therapeutic strategies beyond VLP
chemotherapy, including conventional intrathecal chemotherapy or
targeted/immunotherapies, is needed.
In conclusion, CSF NfL could be a putative
prognostic biomarker in patients with LM undergoing VLP
chemotherapy. On the basis of current results, we could move one
step forward to predict the therapeutic outcome of LM, which would
facilitate the selection of candidates who might benefit most from
active treatment.
Acknowledgements
Not applicable.
Funding
This study was supported by National Cancer Center in Korea
(grant no. 2011510).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JWH conceived the study. JWH and EYP confirm the
authenticity of all the raw data. JWH, YK, KHK, SHK, EYP, JHY, HY,
HSG and HJK interpreted and/or analysed the data. JWH, EYP and HJK
wrote the manuscript. JWH, YK, KHK, SHK, EYP, JHY, HY, HSG and HJK
revised the manuscript. JWH, HSG and HJK supervised the study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Review Board Committee at the
National Cancer Center approved the current study (approval no.
NCC2021-0162), and written informed consent was obtained from all
participants.
Patient consent for publication
Consent for publication was covered by the written
informed consent.
Competing interests
Kim Y, Kim KH, Park EY, Youn JH, Yoo H, Gwak HS
report no conflict of interest. Hyun JW has received a grant from
the National Cancer Center and the National Research Foundation of
Korea. Kim SH has lectured, consulted, and received honoraria from
Bayer Schering Pharma, Biogen, Genzyme, Merck Serono, and UCB and
received a grant from the National Research Foundation of Korea.
Kim HJ received a grant from the National Research Foundation of
Korea and research support from Aprilbio and Eisai; received
consultancy/speaker fees from Alexion, Aprilbio, Biogen, Celltrion,
Daewoong, Eisai, GC Pharma, HanAll BioPharma, MDimune, Merck
Serono, Novartis, Roche, Sanofi Genzyme, Teva-Handok, UCB, and
Viela Bio; and is a co-editor for the Multiple Sclerosis Journal
and an associated editor for the Journal of Clinical Neurology.
References
|
1
|
Le Rhun E, Weller M, Brandsma D, Van den
Bent M, de Azambuja E, Henriksson R, Boulanger T, Peters S, Watts
C, Wick W, et al: EANO-ESMO clinical practice guidelines for
diagnosis, treatment and follow-up of patients with leptomeningeal
metastasis from solid tumours. Ann Oncol. 28 (Suppl_4):iv84–iv99.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chamberlain M, Junck L, Brandsma D,
Soffietti R, Rudà R, Raizer J, Boogerd W, Taillibert S, Groves MD,
Le Rhun E, et al: Leptomeningeal metastases: A RANO proposal for
response criteria. Neuro Oncol. 19:484–492. 2017.PubMed/NCBI
|
|
3
|
Freilich RJ, Krol G and DeAngelis LM:
Neuroimaging and cerebrospinal fluid cytology in the diagnosis of
leptomeningeal metastasis. Ann Neurol. 38:51–57. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Groves MD: New strategies in the
management of leptomeningeal metastases. Arch Neurol. 67:305–312.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yi HG, Kim HJ, Kim YJ, Han SW, Oh DY, Lee
SH, Kim DW, Im SA, Kim TY, Kim CS, et al: Epidermal growth factor
receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for
leptomeningeal metastasis from non-small cell lung cancer patients
with sensitive EGFR mutation or other predictive factors of good
response for EGFR TKI. Lung Cancer. 65:80–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brastianos PK, Lee EQ, Cohen JV, Tolaney
SM, Lin NU, Wang N, Chukwueke U, White MD, Nayyar N, Kim A, et al:
Single-arm, open-label phase 2 trial of pembrolizumab in patients
with leptomeningeal carcinomatosis. Nat Med. 26:1280–1284. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gwak HS and Lee SH, Park WS, Shin SH, Yoo
H and Lee SH: Recent advancements of treatment for leptomeningeal
carcinomatosis. J Korean Neurosurg Soc. 58:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakagawa H, Fujita T, Kubo S, Izumoto S,
Nakajima Y, Tsuruzono K, Tokiyoshi K and Hayakawa T:
Ventriculolumbar perfusion chemotherapy with methotrexate and
cytosine arabinoside for meningeal carcinomatosis: A pilot study in
13 patients. Surg Neurol. 45:256–264. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gwak HS, Lim HS, Shin SH, Yoo H, Han JY,
Kim HT, Yun T, Lee JS and Lee SH: Ventriculolumbar perfusion
chemotherapy for the treatment of leptomeningeal carcinomatosis: A
phase I study with pharmacokinetic data. Am J Clin Oncol.
36:491–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gwak HS, Joo J, Shin SH, Yoo H, Han JY,
Kim HT, Yun T, Ro J, Lee JS and Lee SH: Ventriculolumbar perfusion
chemotherapy with methotrexate for treating leptomeningeal
carcinomatosis: A phase II study. Oncologist. 19:1044–1045. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gauthier H, Guilhaume MN, Bidard FC,
Pierga JY, Girre V, Cottu PH, Laurence V, Livartowski A, Mignot L
and Diéras V: Survival of breast cancer patients with meningeal
carcinomatosis. Ann Oncol. 21:2183–2187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hyun JW, Park JH, Kang BG, Park EY, Park
B, Joo J, Kim JK, Kim SH, Jeong JH, Lee HW, et al: Diagnostic and
prognostic values of cerebrospinal fluid CYFRA 21-1 in patients
with leptomeningeal carcinomatosis. Oncotarget. 8:53326–53335.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khalil M, Teunissen CE, Otto M, Piehl F,
Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas
F, et al: Neurofilaments as biomarkers in neurological disorders.
Nat Rev Neurol. 14:577–589. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi YH, Gwak HS, Joo J, Kwon JW, Shin SH,
Yoo H, Lee JH and Youn JH: Efficacy of slow rate ventriculolumbar
perfusion chemotherapy for leptomeningeal carcinomatosis: Interim
result of a phase II study. Brain Tumor Res Treat. 7:85–91. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khalil M, Pirpamer L, Hofer E, Voortman
MM, Barro C, Leppert D, Benkert P, Ropele S, Enzinger C, Fazekas F,
et al: Serum neurofilament light levels in normal aging and their
association with morphologic brain changes. Nat Commun. 11:8122020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Contal C and O'Quigley J: An application
of change point methods in studying the effect of age on survival
in breast cancer. Comp Stat Data Anal. 30:253–270. 1999. View Article : Google Scholar
|
|
17
|
Ko Y, Gwak HS, Park EY, Joo J, Lee YJ, Lee
SH, Kwon JW, Shin SH and Yoo H: Association of MRI findings with
clinical characteristics and prognosis in patients with
leptomeningeal carcinomatosis from non-small cell lung cancer. J
Neurooncol. 143:553–562. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chamberlain MC: Radioisotope CSF flow
studies in leptomeningeal metastases. J Neurooncol. 38:135–140.
1998. View Article : Google Scholar : PubMed/NCBI
|