Introduction
Laryngeal carcinoma is a common type of airway
cancer, and squamous cell carcinoma is the pathological type most
often observed in the respiratory system (1). Although laryngeal tumors represent
only 2–5% of all carcinomas, they are of significant interest as
the larynx plays a vital role in swallowing and speaking.
Additionally, laryngeal tumors can also severely affect a patient's
quality of life. Currently, conventional management of LSCC
consists of radiotherapy (RT), chemoradiotherapy (CRT), and
surgery.
The incidence and mortality rates of laryngeal
cancer are 1.86/100,000 and 1.01/100,000, respectively, ranking
21st most common cause of cancer-associated death among all cancer
types in China (2).
The value of postoperative adjuvant therapy for
locally advanced cancer with lymph node-negative status (N0)
remains unclear (3). Furthermore,
it is yet to be determined what the best adjuvant treatment is for
locally advanced N0 cancer in patients who have undergone primary
surgical treatment (4–7). While RT is used to preserve laryngeal
function, it may also lead to adverse effects following laryngeal
surgery, such as xerostomia, fibrosis, laryngeal edema, tissue
necrosis, and dysphagia, and eventually reduce a patient's quality
of life. Therefore, the value of adjuvant therapies should be
balanced against any possible complications, and thus patients
should be fully informed before making a decision (7).
The National Comprehensive Cancer Network guiding
principle (version 02.2020) recommends PORT for patients with pT4N0
tumors, positive or close margins, and destructive pathological
features. In contrast, for patients with pT3 tumors, adjuvant
management is elective (8).
Some retrospective studies reported that laryngeal
preservation with CRT, rather than removing the larynx entirely,
for locally advanced cancer resulted in reduced survival rates
among laryngeal carcinoma (LC) patients (9–11).
Whether PORT is beneficial in T3-4a N0M0 laryngeal
cancer patients remains contested. Given the doubt surrounding
suitable adjuvant treatments for T3N0 and T4aN0 LSCC, this study
aimed to determine the oncological results of T3-4a N0M0 glottic
LSCC treated with the operating method and to determine whether
PORT is related to increased survival rates in surgically managed
T3-4aN0M0 LSCC patients with negative margins.
Materials and methods
Patients and preoperative
procedures
This study was approved by the Ethics Committee of
the Eye, Ear, Nose, and Throat Hospital at Fudan University
(approval no. 2021039). The study was conducted following the
ethical standards of The Committee on Human Experimentation of the
Eye, Ear, Nose and Throat Hospital at Fudan University as well as
that described in the Declaration of Helsinki of 1975 as revised in
1983 (12). All patients provided
informed written consent to participate in this study. A total of
369 patients diagnosed with T3-4aN0M0 glottis LSCC between January
2005 and December 2010 were included. Overall, 357 (96.7%) patients
were men and 12 (3.3%) patients were women. The mean age was
60.8±10.9 years (range, 30–85 years; median age, 60 years). The
sex, smoking status, age, alcohol consumption, clinical and tumor,
node, and metastasis (TNM) stage are shown in Table I. This study also recorded
post-surgery information on PORT, surgical margins, and recurrence.
When LSCC was diagnosed, the different treatment options were
discussed with these patients and their families to assist them in
determining the optimal treatment plan for them. Cancer staging was
based on the TNM grouping criteria for LSCC staging designated by
the Union for International Cancer Control (UICC) (13).
 | Table I.Clinical characteristics of the
patients. |
Table I.
Clinical characteristics of the
patients.
| Factor | Value | % | 5 years CSS, % |
P-valuec |
|---|
| Age, years |
|
|
|
|
|
Mean | 60.8±10.9 | - | - |
|
|
Range | 30-85 | - | - |
|
| Age, n |
|
|
|
|
|
<60 | 180 | 48.8 | 71.1 | 0.042a |
|
≥60 | 189 | 51.2 | 64.0 |
|
| Sex, n |
|
|
|
|
|
Male | 357 | 97.3 | 66.6 | 0.060 |
|
Female | 12 | 2.7 | 91.7 |
|
| Smoking status,
n |
|
|
|
|
|
Smoking | 255 | 69.1 | 68.2 | 0.430 |
| No
smoking | 114 | 30.9 | 65.8 |
|
| Drinking status,
n |
|
|
|
|
|
Drinking | 162 | 43.9 | 66.5 | 0.961 |
| No
Drinking | 207 | 56.1 | 68.2 |
|
| Margin situation,
n |
|
|
|
|
|
Negative | 315 | 85.4 | 67.7 | 0.920 |
|
Positive with rescue
therapy | 54 | 14.6 | 66.1 |
|
| PORT with negative
margins, n |
|
|
|
|
|
Yes | 57 | 18.1 | 62.5 | 0.074 |
| No | 258 | 81.9 | 69.5 |
|
| Clinical stage,
n |
|
|
|
|
| III
(T3) | 282 | 76.4 | 73.6 |
<0.001b |
| IV
(T4a) | 87 | 23.6 | 47.4 |
|
Operational procedures
The T3-4aN0M0 LSCC patients underwent either partial
or total laryngectomy based on the assessment of malignancy, and
the operations were performed as described previously (14). We defined margins <5 mm as close
margins, which is still considered as negative margins, but PORT
was routinely recommended in these cases (15). All patients were confirmed to have
LSCC based on their postoperative pathological reports. The
inclusion criteria were: i) Patients who received primary surgery,
including total laryngectomy and partial laryngectomy, in the Eye,
Ear, Nose and Throat Hospital; ii) patients diagnosed with LSCC
pathologically; and iii) patients who had resectable LSCC and had
an operation with the purpose of curing the cancer. The exclusion
criteria were: i) Patients treated with only primary RT or CRT and
ii) patients treated with neoadjuvant chemotherapy.
Postoperative procedures
The postoperative procedures were performed as
described previously (14). When
the postoperative margins were positive, we recommend that the
patient undergo rescue therapy, including postoperative
supplemental RT and CRT. According to the physician's opinion (for
these patients, we routinely recommend postoperative radiotherapy)
and the patient's conditions, certain patients with negative
surgical margins also underwent PORT. A total of 54 patients with
positive margins received adjuvant chemotherapy in addition to
PORT. Complementary RT or CRT was generally started within 4–6
weeks after surgery. RT and chemotherapy were performed as
described previously (16). The
curative irradiation dose was 60–74 Gy (1.8–2.0 Gy/fraction) from
weeks 6–7. Radiation fields covered both the primary tumor location
and the involved lymph nodes. Platinum-based concurrent CRT was
performed based on one of three regimens: i) cisplatin (DDP)
regimen-DDP (45–50 mg/m2/day) on days 1–3; ii) DDP
combined with 5-fluorouracil (5-FU) (PF regimen)-DDP (40
mg/m2/day) on days 1–3 and 5-FU (750–800
mg/m2/day) on days 1–4; or iii) carboplatin (CBP)-based
regimen-CBP, calcium folinate, and tegafur (200, 300, or 1,000
mg/m2/day, respectively) on days 1–3 for 1–2 cycles.
Follow-up
Follow-up time was documented from the day of
operation until the day of the last contact or death. The follow-up
methods primarily included outpatient regular follow-up, telephone
consultation, and email consultation. The standard of local control
is based on the results of electronic laryngoscopy and CT/MRI
examination, with no new recurrence of the larynx and cervical
lymph nodes.
Statistical analyses
The demographics and clinical features are described
as frequencies and proportions. Statistical analysis was performed
using SPSS version 19.0 (IBM Corp.). Follow-up time was documented
from the day of operation until the day of the latest encounter or
death. Life table examination was conducted to determine the DFS,
CSS, and OS rates after 5 and 10 years. Kaplan-Meier analysis was
used to assess the CSS, DFS, and OS rates among the different
groups, and a log-rank test was used to compare survival. The 95%
confidence interval (CI) and hazard ratio associated with the
prognostic agents were also assessed. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical characteristics and
demographics
This study included 369 T3-4aN0M0 glottic LSCC
patients who underwent laryngectomy. The mean ± SD follow-up time
was 95.3±39.8 months (range, 6.6-139.4 months), and the median
period of follow-up was 89 months. The average hospitalization
phase was 25.89±8.7 days, with a range of 11–98 days. The mean ± SD
nasogastric feeding tube removal period was 13.54±11.18 days,
ranging from 4–192 days. The clinical demographics and
characteristics are described in Table
I. Of the patients, 255 (69.1%) were smokers, and 162 (43.9%)
consumed alcohol. A total of 282 (76.4%) patients had stage III
tumor, and 87 (23.6%) had a stage IV tumor. Among them, 315 (85.4%)
patients had negative surgical margins. Of the patients with
negative margins, 57 (18.1%) underwent PORT.
Surgery that involved total laryngectomy (261,
70.7%), partial laryngectomy (108, 29.3%), including vertical
partial laryngectomy (VPL) (64, 59.3%), cricohyoidoepiglottopexy
(CHEP) (41, 38.0%), cricohyoidopexy (CHP) (2, 1.9%), CO2
laser surgery (1, 0.9%), and Turker (1, 0.9%), as well as neck
lymph node dissection are documented in Table II.
 | Table II.Summary of the surgical
treatments. |
Table II.
Summary of the surgical
treatments.
| Treatment | n | % |
|---|
| Total
laryngectomy | 261 | 70.7 |
| Partial
laryngectomy | 108 | 29.3 |
|
Vertical partial
laryngectomy | 64 | 59.3 |
|
Cricohyoidoepiglottopexy | 41 | 38.0 |
|
Cricohyoidopexy | 2 |
1.9 |
|
Turker | 1 |
0.9 |
| Neck
dissection |
|
|
|
Unilateral radical neck
dissection | 19 |
5.1 |
|
Unilateral modified neck
dissection | 4 |
1.1 |
|
Unilateral selective neck
dissection | 10 |
2.7 |
| One
side radical neck dissection, one side selective neck
dissection | 1 |
0.3 |
| Bilateral neck
dissection |
|
|
|
Bilateral modified neck
dissection | 1 |
0.3 |
|
Bilateral selective neck
dissection | 1 |
0.3 |
DFS and CSS results
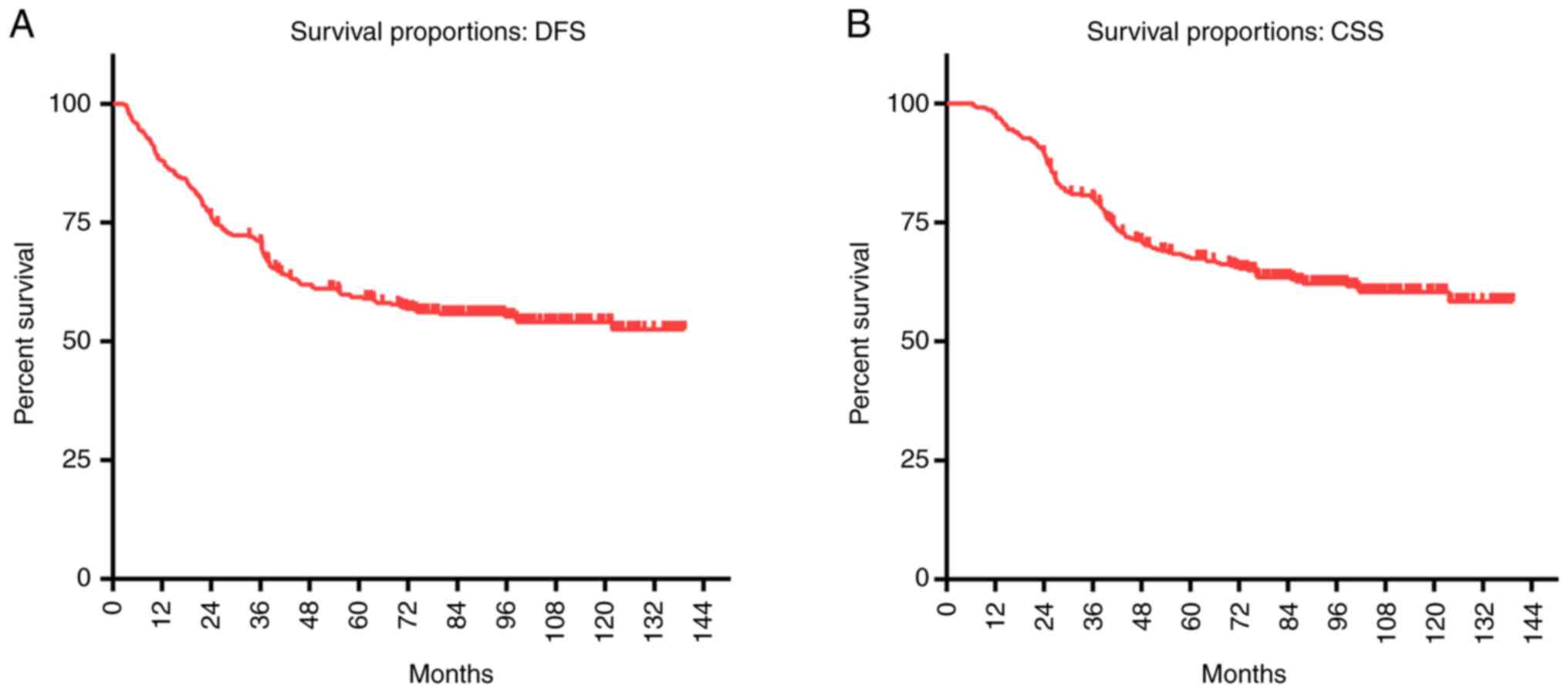
The 5-year DFS of the 369 T3-4aN0M0 glottic LSCC
patients was 59.3% (Table III,
Fig. 1). The 5-year CSS was 67.5%,
and the OS was 66.7% (Table III,
Fig. 1).
 | Table III.OS, DFS and CSS rates of T3-4aN0M0
glottic LSCC patients distributed among different groups. |
Table III.
OS, DFS and CSS rates of T3-4aN0M0
glottic LSCC patients distributed among different groups.
|
| 5 years | 10 years |
|---|
|
|
|
|
|---|
| Factor | OS % | CSS % | DFS % | OS % | CSS % | DFS % |
|---|
| T3,4aN0M0 glottic
LSCC with laryngectomy | 66.7 | 67.5 | 59.3 | 58 | 60.3 | 54 |
| Operation
method |
|
|
|
|
|
|
| Total
laryngectomy | 61.7 | 62.5 | 56.2 | 51.7 | 54.7 | 50 |
| Partial
laryngectomy | 78.5 | 79.3 | 65.4 | 72.9 | 73.6 | 63.4 |
| Clinical stage |
|
|
|
|
|
|
| Stage
III | 72.9 | 73.6 | 63.4 | 65.2 | 67 | 59.7 |
| Stage
IV | 46.3 | 47.4 | 44.5 | 34.8 | 38.3 | 36.2 |
| Margin status |
|
|
|
|
|
|
|
Negative | 67.1 | 67.7 | 59 | 57.6 | 60.1 | 53.5 |
|
Positive with rescue
therapy | 64.6 | 66.1 | 61 | 60.7 | 62.1 | 57.1 |
| PORT with negative
margins |
|
|
|
|
|
|
| No | 68.7 | 69.5 | 63.8 | 61.6 | 64.7 | 60.6 |
|
Yes | 62.5 | 62.5 | 59.1 | 49.4 | 49.4 | 41.9 |
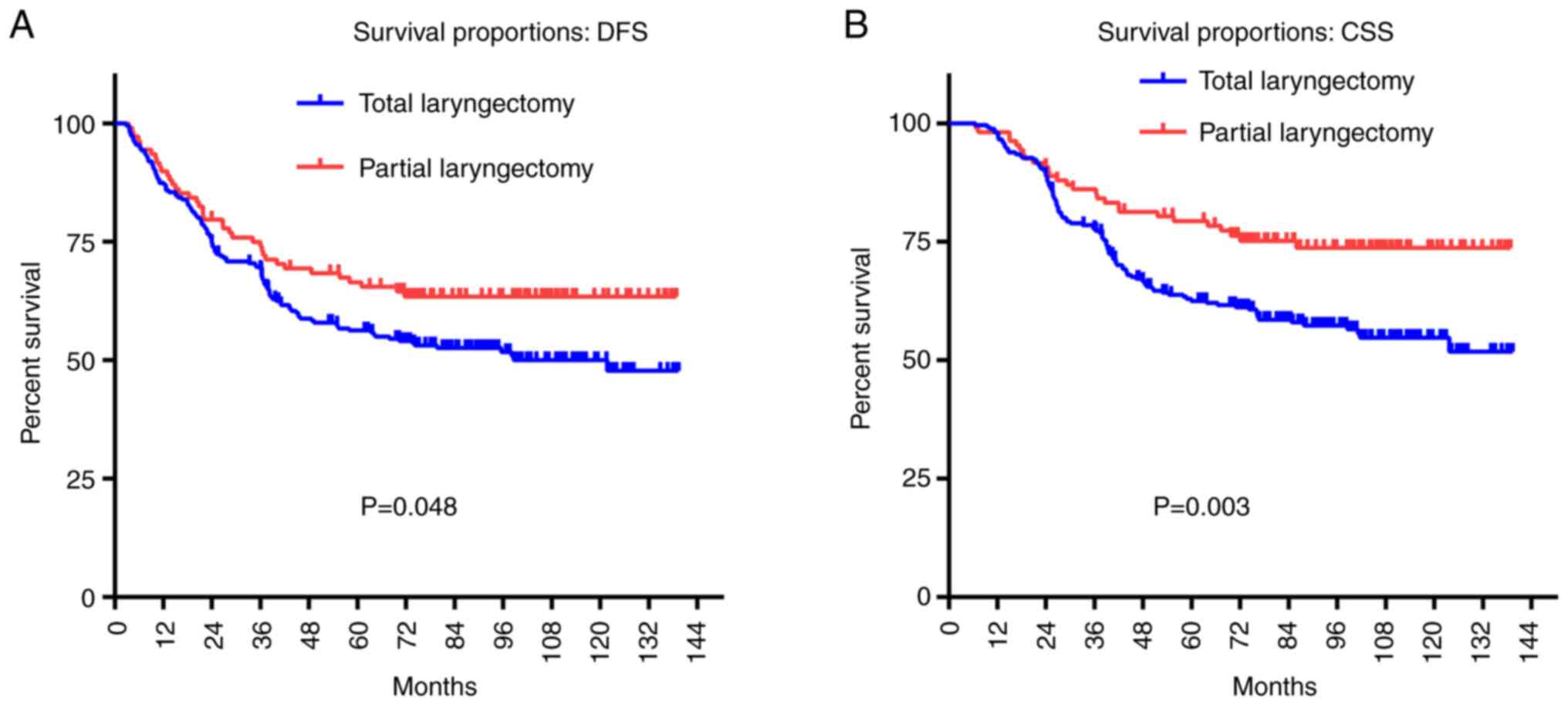
For T3-4aN0M0 glottic patients who underwent total
laryngectomy, the 5-year DFS rate was 56.2%, and the 5-year CSS
rate was 62.5%. Conversely, the 5-year DFS and CSS rates for
patients who underwent partial laryngectomy were 65.4% (P=0.048)
and 79.3% (P=0.003), respectively, which were both higher than that
in the patients who underwent total laryngectomy (Fig. 2, Tables III and IV).
 | Table IV.Multivariate Cox proportional hazards
regression analysis for DFS and CSS in patients with T3-4aN0M0
glottic LSCC. |
Table IV.
Multivariate Cox proportional hazards
regression analysis for DFS and CSS in patients with T3-4aN0M0
glottic LSCC.
|
Characteristics | DFS, HR (95%
CI) | P-value | CSS, HR (95%
CI) | P-value |
|---|
| Operation
method |
|
|
|
|
| Total
laryngectomy | 1.00 | 0.048a | 1.000 | 0.003a |
| Partial
laryngectomy | 0.70
(0.51-1.00) |
| 0.53
(0.40-0.83) |
|
| Clinical stage |
|
|
|
|
| Stage
III | 1.000 |
<0.001c | 1.000 |
<0.001b |
| Stage
IV | 2.15
(1.74-3.80) |
| 2.77
(2.48-5.92) |
|
| Margin status |
|
|
|
|
|
Negative | 1.00 | 0.732 | 1.00 | 0.920 |
|
Positive with rescue
therapy | 0.93
(0.60-1.43) |
| 1.03
(0.64-1.65) |
|
| Postoperative
radiotherapy with negative margins |
|
|
|
|
| No | 1.00 | 0.057 | 1.00 | 0.074 |
|
Yes | 1.64
(0.98-3.40) |
| 1.64
(0.94-3.54) |
|
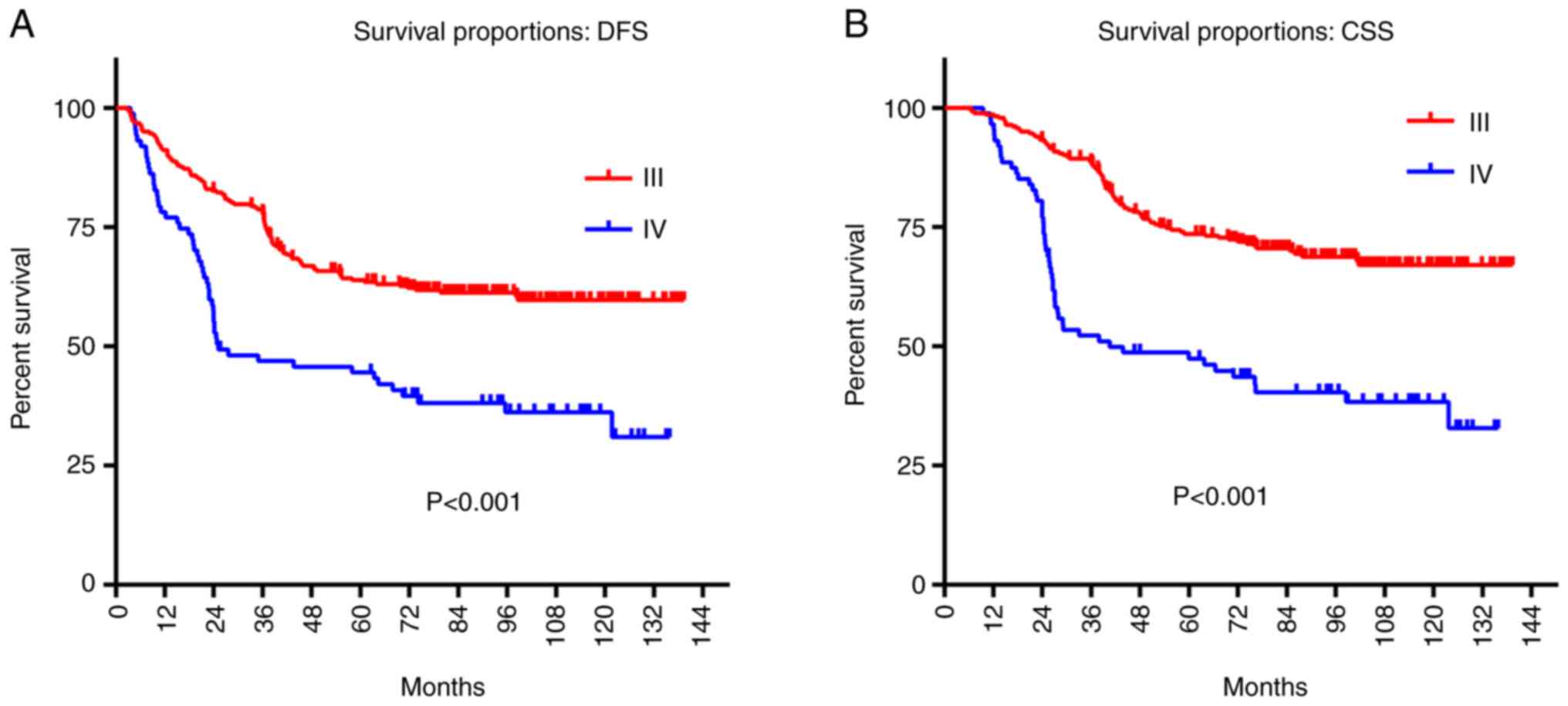
To assess the relationship between DFS, CSS, and
T3-4aN0M0 glottic LSCC tumor stage, log-rank tests were used. The
5-year DFS and CSS rates of patients with stage III tumors were
higher than those of patients with stage IV tumors (DFS, 63.4 vs.
44.5%, P<0.01; CSS, 73.6 vs. 47.4%, P<0.01; Fig. 3, Tables III and IV).
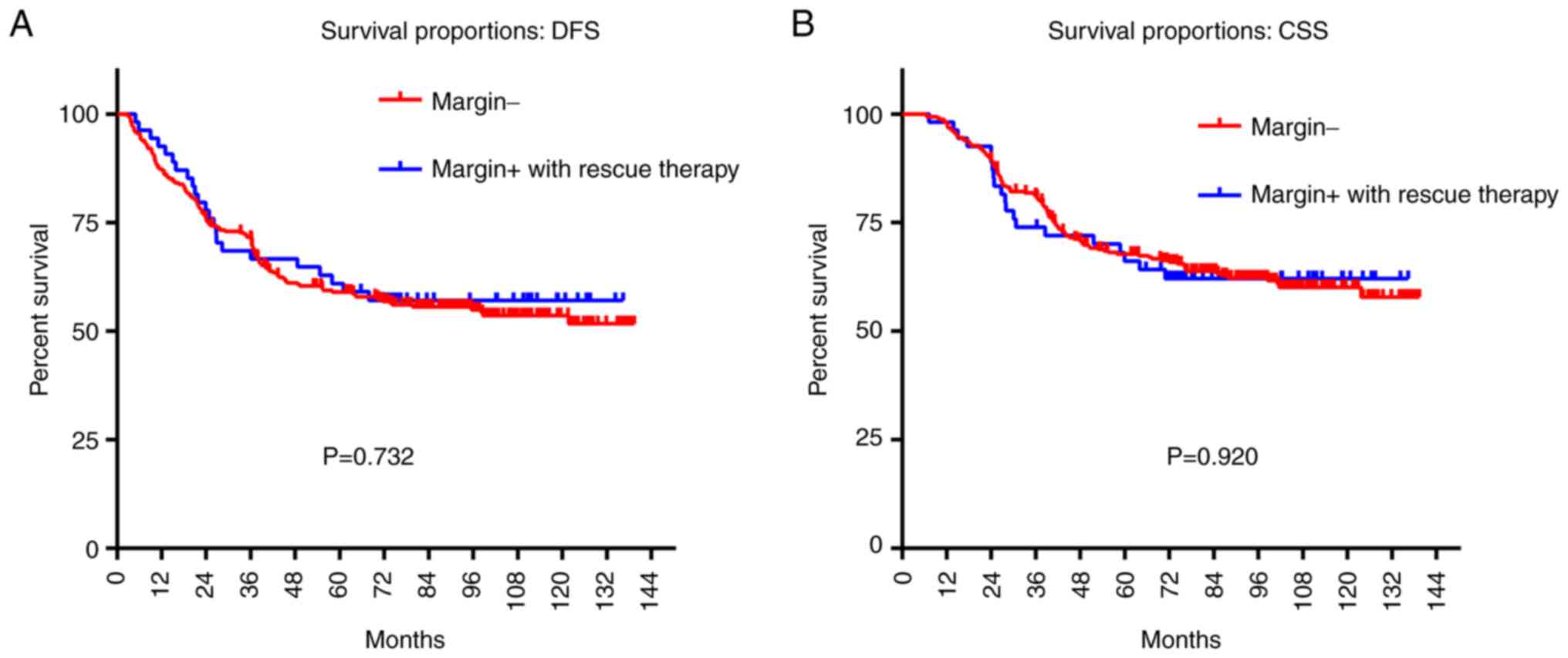
This study also investigated the relationship
between the DFS, CSS, and surgical margin status of the T3-4a N0M0
glottic LSCC. The LSCC patients with positive surgical margins or
close margins underwent PORT, and certain patients were also
treated with chemotherapy. No significant difference was detected
in the 5-year CSS and DFS rates between T3-4a N0M0 glottic patients
with negative margins and those with positive margins following
rescue therapy (DFS, 59.0 vs. 61.0%, P=0.732; CSS, 67.7 vs. 66.1%,
P=0.920; Fig. 4).
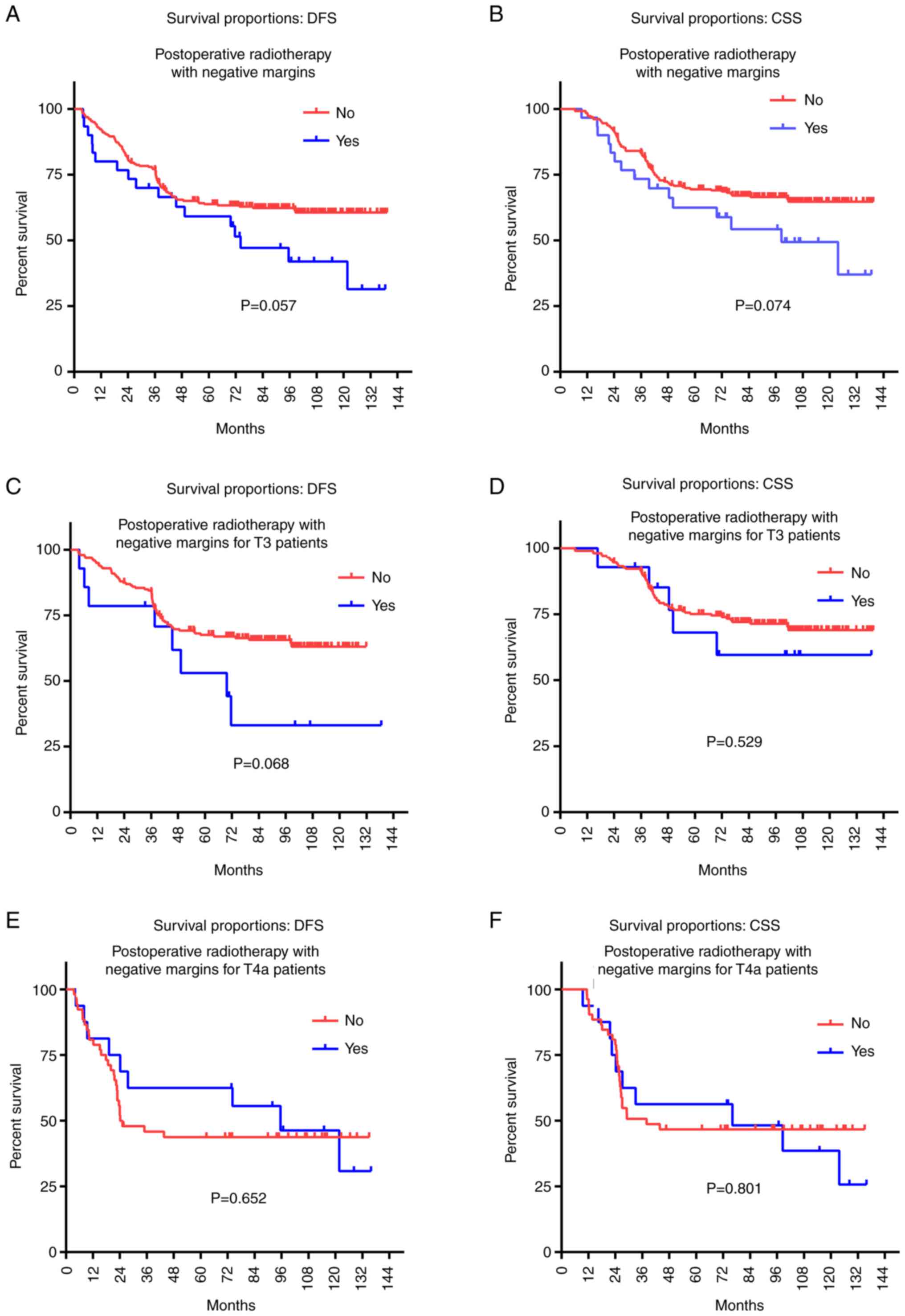
Finally, the relationships between the CSS, DFS, and
PORT status of T3-4aN0M0 glottic patients with negative margins
were assessed. No significant difference was detected in the CSS
and DFS rates between the patients with negative margins who
underwent PORT compared with those who did not (57 cases vs. 258
cases; DFS, 59.1 vs. 63.8%, P=0.057; CSS, 62.5 vs. 69.5%, P=0.074;
Tables III and IV, Fig.
5). However, T3-4aN0M0 glottic patients with negative margins
who did not receive PORT tended to have a better survival rate than
those who received PORT. This trend was especially evident in the
10-year survival rate (DFS, 41.9 vs. 60.6%; CSS, 49.4 vs. 64.7%;
Tables III and IV). To avoid the clinical staging from
interfering with this result, we studied the effect of the PORT
status of LSCC with negative margins in T3 and T4a patients
separately. Similar results were obtained (for T3 LSCC patients:
DFS, P=0.068, CSS, P=0.529; for T4a LSCC patients: DFS, P=0.652,
CSS, P=0.801; Tables III and
IV, Fig. 5).
Discussion
LSCC is a unique carcinoma, the survival rates of
which have worsened in the last 2 decades. Surprisingly,
retrospective studies have reported reduced OS rates in patients
with advanced laryngeal tumors even after the widespread adoption
of RT-based laryngeal preservation methods (10,11,17–19).
The guideline recommendations for the optimal management of locally
advanced LC are focused on approaches that maximize laryngeal
function without compromising the oncological results (20).
For LSCC patients treated with surgery, the ideal
adjuvant treatment remains uncertain, especially for locally
advanced lymph node-negative disease (21). To help answer this unresolved
question, we assessed the survival outcomes of the current surgical
approaches for T3-4a N0M0 glottic LSCC in China.
The results showed the oncological outcomes of 369
surgically treated patients, with a 66.7% 5-year OS rate, a 59.3%
5-year DFS rate, and a 67.5% CSS rate. The survival rate of LSCC
patients with partial laryngectomy was significantly higher than
that of those with total laryngectomy for the following reasons: i)
The vast majority of the patients who underwent partial
laryngectomy had earlier stage tumors, with a higher proportion of
T3 patients (97.2 vs. 67.8%, representing partial laryngectomy and
total laryngectomy, respectively); ii) patients with total
laryngectomy tended to be older and have more cardiopulmonary and
other systemic comorbidities. Therefore, for patients with
T3-4aN0M0 glottic LSCC, surgical laryngectomy techniques, including
total laryngectomy, VPL, CHP, and CHEP, can achieve acceptable
functional and oncological outcomes. Our findings are in agreement
with previous reports that showed 5-year DFS and OS rates of 60–79
and 48–71%, respectively (4,14,22),
following open laryngectomy.
Previous studies suggested that radical radiotherapy
achieved higher laryngeal preservation rates with the same survival
rate as surgery. However, the 5-year survival rate is only 53%
(23), which is lower than the
survival rate observed at our center. It is thus suggested that the
treatment strategy for laryngeal cancer should be based on
increasing the survival rate.
The results of the present study showed that
patients with stage IV T3-4a N0M0 glottic LSCC had poorer CSS and
DFS rates than stage III patients. No significant difference was
detected in the CSS and DFS rates between patients whose margins
were negative compared with those who were positive following PORT
or CRT. Nguyen-Tan et al (24) discovered a strong relationship
between T-stage LSCC and survival rates, with 5-year OS rates for
pT4 and pT3 of 38 and 54%, respectively. In this study, the 5 -year
DFS and CSS rates of patients with T3-4a N0M0 glottic LSCC stage
III were better than those of stage IV patients. The prospective
predictive role of radical operation was emphasized by Hinerman
et al (25), who found a
5-year locoregional control of 56 and 89% for patients with
positive and negative surgical margins, respectively. In this
study, PORT or CRT was given to LSCC patients with positive
margins. No significant difference was observed in the 5-year CSS
and DFS rates between patients with T3-4a N0M0 glottic LSCC whose
margins were negative and those whose margins were positive
following rescue therapy. Hence, both PORT and CRT are useful
strategies for treating patients with T3-4a glottis LSCC with
positive margins.
In the present study, no significant difference was
observed in the CSS and DFS rates between the T3-4a glottis LSCC
patients with negative margins who underwent PORT and those who did
not. These results were consistent with that described by Graboyes
et al (3), who presented no
significant difference in the CSS and OS rates of cT3N0 glottic
LSCC among certain management groups (definitive RT, total
laryngectomy, and total laryngectomy with adjuvant RT). Kim et
al (6) conducted a study
involving 60 T3-4 LSCC patients to assess the influence of PORT on
the 5-year DFS, OS, and CSS rates. No significant difference was
detected in the rates between patients who underwent primary
surgery only and those who had adjuvant RT.
Although PORT is a broadly used method for treatment
of patients with intermediate-advanced stage LC, delayed toxicity
may later severely affect a patient's swallowing and speech
function due to the regular use of RT and platinum-based
chemotherapy (26) resulting in
dysphagia and speech disorders. Notably, aspiration after RT for
head and neck tumors is often unnoticeable because of an
ineffective cough reflex in those patients, leading to death from
aspiration pneumonia (27,28). A ~20% decline in the 5-year
survival rates of locally advanced glottic tumor patients was
detected from 1977 to 2003. There was an increase in the number of
CRT procedures for all-stage glottic tumors between 2003 to 2006,
which seemed to be consistent with the timing of the publication of
the RTOG 91–11 and VA laryngeal studies (29,30).
Nevertheless, the use of CRT for T4 cancers has been significantly
reduced since 2006, possibly consistent with reports suggesting
that the decrease in survival rates in laryngeal tumor patients
might be driven by advanced malignancies increasingly being cured
without surgery (11,31).
Researchers have questioned the role of PORT for T3
N0-1 LSCC patients cured following the operation (3,32),
primarily as RT may severely influence the functional results after
laryngeal operation, eventually reducing the quality of life
(7).
During the present study, T3-4aN0M0 glottic patients
with negative margins who did not receive PORT tended to have a
better survival rate than those who received PORT. This trend was
especially evident in the 10-year survival rate (DFS: 41.9 vs.
60.6%; CSS: 49.4 vs. 64.7%). Lin et al (33) described that 70.5% of advanced
tumor patients had regular operating management, and 29.5% received
concurrent chemoradiotherapy (CCRT). Nevertheless, 22.4% of the
CCRT patients withdrew from the treatment due to side effects, such
as xerostomia, dysphagia, neutropenic fever, percutaneous
endoscopic gastrostomy tube placement, and mucositis, during the
course of RT (34).
This study showed that T3-4aN0 patients did not
benefit from PORT, which is consistent with previously published
research and single-institution research that also reported no
survival advantage for these patients. In single-institution
studies (6,35,36),
whether T4aN0 LSCC patients benefited from the addition of PORT has
sometimes been questioned. These statistics may assist
decision-making regarding whether to undergo PORT for T3-4aN0M0
glottic patients with negative margins.
In this study, most patients with negative margins
did not receive RT, primarily because of the physician's experience
and the patient's willingness greatly influenced the regimen during
the early years of treatment for LC. For patients with T3-4aN0M0
tumors that did not penetrate the perichondrium of thyroid
cartilage or cricoid cartilage, certain surgeons do not recommend
PORT after radical surgery (such as total laryngectomy). In
addition, a considerable number of patients refused RT because of
economic concerns and/or concerns regarding the side effects.
Although this is inconsistent with the latest clinical guidelines,
it is closer to real-world studies, and our findings also precisely
show that the RT group did exhibit survival benefits after radical
surgery, which may provide some guidance for future treatments.
However, this research was limited by its
retrospective nature and intrinsic biases. Because the duration of
this study was relatively long and different surgeons and RT
physicians may have differences in surgical methods and RT
standards, the mode of postoperative adjuvant therapy was not a
unified standard, although this is more in line with real-world
settings. In this study, the majority of the previous patients were
not evaluated for performance status; thus this data was not shown
in the present study. The PRT and chemotherapeutic regimens of some
patients in the dataset of this study were not homogeneous and/or
incomplete, and there were also some deficiencies. Future,
prospective cohort studies are required to obtain more convincing
clinical data.
In conclusion, tumor classification is a prognostic
factor for T3-4aN0M0 glottic LSCC patients. Partial laryngectomy is
still recommended for select patients without compromising
survival. PORT and CRT are valid rescue therapies for T3-4aN0M0
glottic LSCC patients with positive surgical margins. However, PORT
did not increase survival in surgically managed pT3-4aN0M0 LSCC
patients with negative margins, and worse outcomes were observed in
the 10-year survival rate with the use of PORT.
Acknowledgements
The authors would like to thank Dr Ji Li from the
Department of Radiation Oncology, Eye, Ear, Nose and Throat
Hospital, Fudan University, for his work on radiation therapy in
patients.
Funding
This work was sponsored by the Major Clinical Research Project
of Shanghai Shen-kang Hospital Clinical Development Center (grant
no. SHDC2020CR6011); the Science and Technology Innovation Project
of Shanghai Shen-kang Hospital Clinical Development Center (grant
no. SHDC12015114); the National Natural Science Foundation of China
(grant nos. 82003178, 81772878, 30801283, and 30972691); Shanghai
Sailing Program (grant no. 19YF1405900), the Shanghai Municipal Key
Clinical Specialty (grant no. shslczdzk00801); the Science and
Technology Committee of Shanghai (grant no. 20MC1920200); the
Science and Technology Commission of Shanghai Municipality (grant
no. 20Y11902200); the Shanghai Anti-Cancer Development Foundation
(grant no. H6001-008); and the Training Program of the Excellent
Doctors of Fudan University (grant no. QT00140).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, YH, YY, XZ, LZ, HG, CX and LT were involved in
the conception and design of the study, in the analysis of the
data, and in the interpretation of data. JZ, YH, CX and TL drafted
the manuscript and revised it for important intellectual content.
All authors have read and approved the final manuscript. CX and TL
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Eye, Ear, Nose, and Throat Hospital at Fudan University
(approval no. 2021039). The study was conducted in accordance with
the ethical standards of the committee on human experimentation of
the Eye, Ear, Nose and Throat Hospital at Fudan University and that
described in the Declaration of Helsinki of 1975 as revised in
1983. All patients provided informed written consent to participate
in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Steuer CE, El-Deiry M, Parks JR, Higgins
KA and Saba NF: An update on larynx cancer. CA Cancer J Clin.
67:31–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Y, Zhao Q, Ding G, Zhu Y, Li W and
Chen W: Incidence and mortality of laryngeal cancer in China,
2008–2012. Chin J Cancer Res. 30:299–306. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Graboyes EM, Zhan KY, Garrett-Mayer E,
Lentsch EJ, Sharma AK and Day TA: Effect of postoperative
radiotherapy on survival for surgically managed pT3N0 and pT4aN0
laryngeal cancer: Analysis of the national cancer data base.
Cancer. 123:2248–2257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sessions DG, Lenox J, Spector GJ, Newland
D, Simpson J, Haughey BH and Chao KSC: Management of T3N0M0 glottic
carcinoma: Therapeutic outcomes. Laryngoscope. 112:1281–1288. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spector GJ, Sessions DG, Lenox J, Newland
D, Simpson J and Haughey BH: Management of stage IV glottic
carcinoma: Therapeutic outcomes. Laryngoscope. 114:1438–1446. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SH, Lee YS, Kwon M, Kim JW, Jong-Lyel
R, Choi SH, Kim SY, Lee SW and Nam SY: Adjuvant role of radiation
therapy for locally advanced laryngeal cancer without pathological
lymph node metastasis. Acta Otolaryngol. 136:703–710. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laccourreye O, Hans S, Borzog-Grayeli A,
Maulard-Durdux C, Brasnu D and Housset M: Complications of
postoperative radiation therapy after partial laryngectomy in
supraglottic cancer: A long-term evaluation. Otolaryngol Head Neck
Surg. 122:752–757. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pfister DG, Spencer S, Adelstein D, Adkins
D, Anzai Y, Brizel DM, Bruce JY, Busse PM, Caudell JJ, Cmelak AJ,
et al: Head and neck cancers, version 2.2020, NCCN clinical
practice guidelines in oncology. J Natl Compr Cancer Netw.
18:873–898. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen AY and Halpern M: Factors predictive
of survival in advanced laryngeal cancer. Arch Otolaryngol Head
Neck Surg. 133:1270–1276. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grover S, Swisher-McClure S, Mitra N, Li
J, Cohen RB, Ahn PH, Lukens JN, Chalian AA, Weinstein GS, O'Malley
BW Jr and Lin A: Total laryngectomy versus larynx preservation for
T4a larynx cancer: Patterns of care and survival outcomes. Int J
Radiat Oncol Biol Phys. 92:594–601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoffman HT, Porter K, Karnell LH, Cooper
JS, Weber RS, Langer CJ, Ang KK, Gay G, Stewart A and Robinson RA:
Laryngeal cancer in the United States: Changes in demographics,
patterns of care, and survival. Laryngoscope. 116:1–13. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Human D: Declaration of Helsinki. Lancet.
357:2362001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Zhou L, Tao L, Zhang M, Wu H, Chen
X, Li X, Li C and Gong H: Oncologic outcomes of surgical treatment
for T3 glottic laryngeal squamous cell carcinoma. Head Neck.
40:1734–1742. 2018.PubMed/NCBI
|
|
15
|
Holsinger FC, Tomeh C, Moore MW, Yan W,
Chen C and Laccourreye O: Supracricoid partial laryngectomy with
cricohyoidoepiglottopexy: Surgical technique illustrated in the
anatomy laboratory. Head Neck. 37:906–908. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu X, Heng Y, Zhou L, Tao L and Zhang M:
A prognostic nomogram for predicting risk of recurrence in
laryngeal squamous cell carcinoma patients after tumor resection to
assist decision making for postoperative adjuvant treatment. J Surg
Oncol. 120:698–706. 2019.PubMed/NCBI
|
|
17
|
Dziegielewski PT, O'Connell DA, Klein M,
Fung C, Singh P, Mlynarek MA, Fung D, Harris JR and Seikaly H:
Primary total laryngectomy versus organ preservation for T3/T4a
laryngeal cancer: A population-based analysis of survival. J
Otolaryngol Head Neck Surg. 41 (Suppl 1):S56–S64. 2012.PubMed/NCBI
|
|
18
|
Timmermans AJ, de Gooijer CJ,
Hamming-Vrieze O, Hilgers FJ and van den Brekel MW: T3-T4 laryngeal
cancer in The Netherlands cancer institute; 10-year results of the
consistent application of an organ-preserving/-sacrificing
protocol. Head Neck. 37:1495–1503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lechien JR, Maniaci A, Hans S, Iannella G,
Fakhry N, Mayo-Yáñez M, Ayad T, Mannelli G and Chiesa-Estomba CM:
Epidemiological, clinical and oncological outcomes of young
patients with laryngeal cancer: A systematic review. Eur Arch
Otorhinolaryngol. 2:10.1007/s00405–022-07466-9. 2022.
|
|
20
|
Forastiere AA, Ismaila N, Lewin JS, Nathan
CA, Adelstein DJ, Eisbruch A, Fass G, Fisher SG, Laurie SA, Le QT,
et al: Use of larynx-preservation strategies in the treatment of
laryngeal cancer: American society of clinical oncology clinical
practice guideline update. J Clin Oncol. 36:1143–1169. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hinni ML, Salassa JR, Grant DG, Pearson
BW, Hayden RE, Martin A, Christiansen H, Haughey BH, Nussenbaum B
and Steiner W: Transoral laser microsurgery for advanced laryngeal
cancer. Arch Otolaryngol Head Neck Surg. 133:1198–1204. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mannelli G, Lazio MS, Luparello P and
Gallo O: Conservative treatment for advanced T3-T4 laryngeal
cancer: Meta-analysis of key oncological outcomes. Eur Arch
Otorhinolaryngol. 275:27–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ko HC, Harari PM, Chen S, Wieland AM, Yu
M, Baschnagel AM, Kimple RJ and Witek ME: Survival outcomes for
patients with T3N0M0 squamous cell carcinoma of the glottic larynx.
JAMA Otolaryngol Head Neck Surg. 143:1126–1133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nguyen-Tan PF, Le QT, Quivey JM, Singer M,
Terris DJ, Goffinet DR and Fu KK: Treatment results and prognostic
factors of advanced T3-4 laryngeal carcinoma: The University of
California, San Francisco (UCSF) and Stanford University hospital
(SUH) experience. Int J Radiat Oncol Biol Phys. 50:1172–1180. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hinerman RW, Morris CG, Amdur RJ, Lansford
CD, Werning JW, Villaret DB and Mendenhall WM: Surgery and
postoperative radiotherapy for squamous cell carcinoma of the
larynx and pharynx. Am J Clin Oncol. 29:613–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Machtay M, Moughan J, Trotti A, Garden AS,
Weber RS, Cooper JS, Forastiere A and Ang KK: Factors associated
with severe late toxicity after concurrent chemoradiation for
locally advanced head and neck cancer: An RTOG analysis. J Clin
Oncol. 26:3582–3589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nguyen NP, Moltz CC, Frank C, Millar C,
Smith HJ, Dutta S, Nguyen PD, Nguyen LM, Lemanski C, Ludin A, et
al: Effectiveness of the cough reflex in patients with aspiration
following radiation for head and neck cancer. Lung. 185:243–248.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nguyen NP, Smith HJ, Dutta S, Alfieri A,
North D, Nguyen PD, Lee H, Martinez T, Lemanski C, Ludin A, et al:
Aspiration occurence during chemoradiation for head and neck
cancer. Anticancer Res. 27:1669–1672. 2007.PubMed/NCBI
|
|
29
|
Foote RL, Foote RT, Brown PD, Garces YI,
Okuno SH and Strome SE: Organ preservation for advanced laryngeal
carcinoma. Head Neck. 28:689–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pfister DG, Laurie SA, Weinstein GS,
Mendenhall WM, Adelstein DJ, Ang KK, Clayman GL, Fisher SG,
Forastiere AA, Harrison LB, et al: American Society of Clinical
Oncology clinical practice guideline for the use of
larynx-preservation strategies in the treatment of laryngeal
cancer. J Clin Oncol. 24:3693–3704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cosetti M, Yu GP and Schantz SP: Five-year
survival rates and time trends of laryngeal cancer in the US
population. Arch Otolaryngol Head Neck Surg. 134:370–379. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sanguineti G, Vidiri A and Pellini R: Is
postoperative radiotherapy routinely indicated after total
laryngectomy for pT3N0-1 supraglottic carcinoma? Oral Oncol.
107:1048252020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin CC, Fedewa SA, Prickett KK, Higgins KA
and Chen AY: Comparative effectiveness of surgical and nonsurgical
therapy for advanced laryngeal cancer. Cancer. 122:2845–2856. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee M, Buchanan MA, Riffat F and Palme CE:
Complications after CO2 laser surgery for early glottic cancer: An
institutional experience. Head Neck. 38 (Suppl 1):E987–E990. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Richard JM, Sancho-Garnier H, Pessey JJ,
Luboinski B, Lefebvre JL, Dehesdin D, Stromboni-Luboinski M and
Hill C: Randomized trial of induction chemotherapy in larynx
carcinoma. Oral Oncol. 34:224–228. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Patel SA, Qureshi MM, Dyer MA, Jalisi S,
Grillone G and Truong MT: Comparing surgical and nonsurgical
larynx-preserving treatments with total laryngectomy for locally
advanced laryngeal cancer. Cancer. 125:3367–3377. 2019. View Article : Google Scholar : PubMed/NCBI
|