Introduction
Colorectal cancer (CRC) ranks as the third most
prevalent cancer, and ~1.4 million new cases of CRC are diagnosed
annually all over the world (1–3). As
early diagnosis for patients with CRC is still challenging, a
substantial number of patients with CRC are diagnosed at late
stages, which is generally associated with a poor prognosis
(4). Despite the application of
multiple modalities for CRC treatment, such as surgical resection
and radio-chemotherapy, the patient prognosis remains poor
(5,6). Therefore, the identification of key
molecules that are involved in the carcinogenesis and deterioration
of CRC is important for the early prevention and treatment of the
cancer.
C-X-C motif chemokine ligand 12 (CXCL12), also known
as stromal cell-derived factor-1, was initially identified as a key
cytokine involved in the metastasis of tumor cells (7,8).
Located on the long arm of chromosome 10, the CXCL12 gene
was first cloned from a bone marrow-derived stromal cell line, and
was then identified as a pre-B cell growth stimulating factor
(9,10). In humans, CXCL12 exists as six
different splice variants (CXCL12α to φ) (11), which share the first three exons
and are encoded by the same CXCL12 gene (11). The variants differ by the fourth
exon, which determines the splice variant length. All CXCL12
isoforms have the first 67 amino acids in common and then exhibit
different lengths, with CXCL12α to φ being 68, 72, 98, 119, 69 and
79 amino acids long, respectively (11). The amino-terminal domain of CXCL12
binds to the second extracellular loop of C-X-C chemokine receptor
type 4 (CXCR4) and activates the signaling pathways downstream
(9,10). The third intracellular loop of
CXCR4 is necessary for Gαi-dependent signaling, and intracellular
loops 2 and 3, as well as the CXCR4 C-terminus, are required for
chemotaxis (9,10). Typically, the binding of CXCL12 to
CXCR4 triggers multiple signal transduction pathways that control
the regulation of intracellular calcium flux, transcription,
chemotaxis and cell survival (12,13).
In addition, CXCL12 is constitutively expressed in tissues that are
vulnerable to metastasis, such as the lung, bone marrow and liver
tissues (13). Subsequent
preclinical studies showed that expression levels of CXCL12 in
certain human tumors were correlated with dedifferentiation and
malignant tumor behaviors (14,15).
For patients with CRC, accumulating studies have been performed to
evaluate the association between tumor expression levels of CXCL12
and survival outcomes (16,17).
However, results of these studies are not always consistent
(18–30). Therefore, in the present study, a
meta-analysis was performed to comprehensively investigate the
possible predictive role of tumor CXCL12 expression for the
prognosis of patients with CRC.
Materials and methods
The Preferred Reporting Items for Systematic Reviews
and Meta-Analyses (PRISMA) statement (31,32)
was followed in conceiving, conducting and reporting the study, and
the methodology of the meta-analysis was in accordance with the
recommendations of the Cochrane's Handbook (33) guidelines.
Literature retrieval
Studies were retrieved by searching the PubMed,
Embase and Web of Science electronic databases from the inception
of the databases until March 22, 2022. Combined search terms were
used, including i) ‘CXCL12’ OR ‘SDF1’; ii) ‘colon’ OR ‘colorectal’
OR ‘rectal’ OR ‘anal’; and iii) ‘cancer’ OR ‘carcinoma’ OR
‘adenoma’ OR ‘adenocarcinoma’ OR ‘malignancy’ OR ‘tumor’ OR
‘tumour’ OR ‘neoplasm’. The search was limited to human studies
published as full-length articles. No restriction was applied
regarding the language of publication. As a supplementation, the
citations of the relevant original and review articles were
manually checked for possible studies of interest.
Study selection
The PICOS principle was used for study inclusion
with the following descriptions: P (patients): Adult patients with
a histologically confirmed diagnosis of CRC, regardless of the
cancer stage or treatments. I (exposure): Patients with higher
tumor expression levels of CXCL12. The methods for measuring tumor
CXCL12 expression levels and the cutoff for defining higher tumor
CXCL12 expression levels were in accordance with those applied in
the original studies. C (control): Patients with lower tumor
expression levels of CXCL12. O (outcomes): The primary outcome was
overall survival (OS) and the secondary outcome was
progression-free survival (PFS), compared with that of patients
with CRC and higher vs. lower tumor expression levels of CXCL12.
Generally, OS was defined as the time elapsed from treatment and to
the date of death from any cause, while PFS was defined as the
interval between initiation of the treatment and the first
recurrence or progression event. S (study design): Cohort studies,
including prospective and retrospective cohorts.
Reviews, preclinical studies, studies including
patients that did not have CRC, studies that did not evaluate tumor
expression levels of CXCL12 or studies that did not report the
survival outcomes were excluded.
Data collection and quality
assessing
Two independent assessors conducted the literature
search and analysis, data collection and study quality assessments
separately. If discrepancies were encountered, they were resolved
by discussion to reach a consensus. Data regarding study
information, patient demographic factors, cancer stage, methods for
measuring the tumor expression levels of CXCL12, cutoffs for
defining higher tumor expression levels of CXCL12, number of
patients with higher tumor expression levels of CXCL12 and
variables adjusted in the regression model for the analysis of the
association between CXCL12 and survival outcomes were collected.
Study quality assessment was achieved via the Newcastle-Ottawa
Scale (NOS) (34), with scoring
regarding the criteria for participant selection, comparability of
the groups and the validity of the outcomes. The scale ranged
between 1–9 stars, with a larger number of stars representing
higher study quality.
Statistical analysis
The main objective was to determine the relative
risks of OS and PFS of patients with CRC and higher vs. lower tumor
expression of CXCL12. These were presented as hazard ratios (HRs)
and confidence intervals (CIs). Using the 95% CIs or P-values, HRs
and standard errors (SEs) could be calculated, and a subsequent
logarithmical transformation was conducted to keep a stabilized
variance and normalized distribution. Between-study heterogeneity
was estimated with the Cochrane's Q test and the I2
statistic (35), with
I2>50% reflecting significant heterogeneity. A
random-effect model was applied to combine the results by
incorporating the influence of heterogeneity (33). The influence of each study on the
overall results was observed by performing sensitivity analyses
that omitted one study at a time (36). Subgroup analyses were also
performed to explore the influences of study characteristics on the
outcome. By construction of the funnel plots, the publication bias
was estimated based on the visual judgment of the symmetry of the
plots, supplemented by the Egger's regression asymmetry test
(37). RevMan (version 5.1;
Cochrane) and Stata (version 12.0; StataCorp LP) software were
applied for these analyses.
Results
Studies obtained
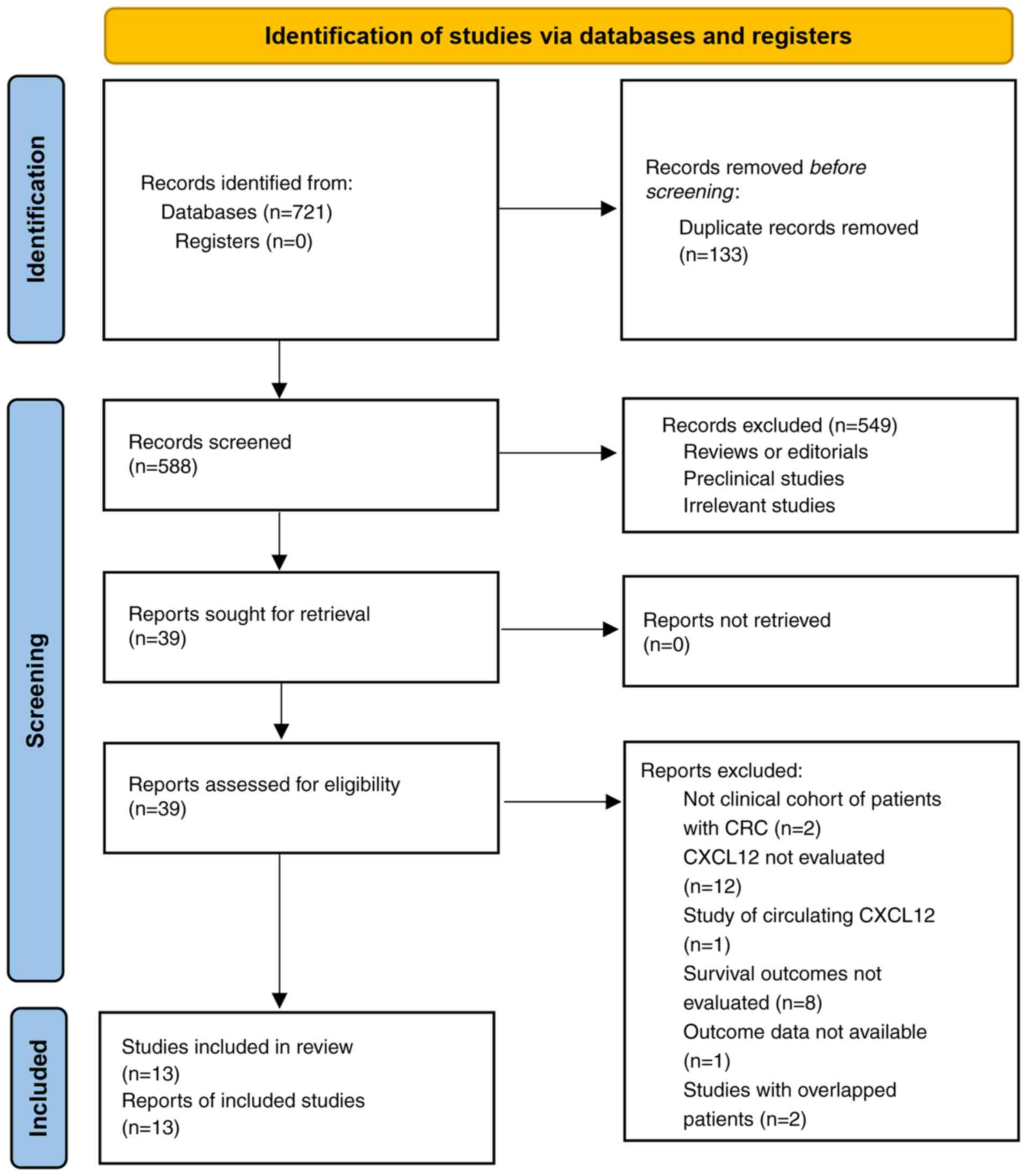
Fig. 1 shows the
process of the literature analysis. Briefly, the initial search of
the databases retrieved 721 articles, and 588 were left after
excluding duplicated records. An additional 549 articles were
excluded, as the contents of the titles and abstracts indicated
that they were not relevant to the aim of the meta-analysis, which
made a total of 39 studies for the full-text review. Finally, after
excluding 26 studies through full-text review, 13 studies (18–30)
were included. The reasons for the removal of the 26 studies are
also presented in Fig. 1. Since 1
report (27) included 2
independent cohort studies, a total of 14 cohort studies were
available for the meta-analysis.
Characteristics of the included
studies
As shown in Table
I, 14 cohort studies involving 2,060 patients with CRC
contributed to the meta-analysis. These studies were performed in
Japan, the United States, Italy, Tunisia, the Netherlands, Italy,
Norway, Switzerland and Korea. All were retrospective cohort
studies except for 1 study, which was a prospective cohort study
(26). Patients with rectal cancer
were included in 3 cohorts (20,23,30),
patients with colon cancer were included in 2 cohorts (27), while the remaining 9 cohorts
included patients with rectal or colon cancer (18,19,21,22,24–26,28,29).
Tumor expression levels of CXCL12 protein were assayed by
immunohistochemistry in most of the included studies except 2
studies, in which the reverse transcription-quantitative polymerase
chain reaction was applied to measure the tumor CXCL12 mRNA level
(20,28). Cutoffs for defining the higher
tumor expression levels of CXCL12 varied among the included
studies, such as CXCL12 protein expression in ≥50% of the tumor
cells, CXCL12 expression in ≥10% of the tumor cells or tumors with
detectable CXCL12 mRNA. Overall, 1,055 (51.2%) patients had higher
tumor expression levels of CXCL12. The mean expression levels of
CXCL12 in CRC varied between 25 and 81% among the included studies.
The median follow-up duration of the included studies varied
between 23 and 66 months. The outcome of OS was reported in 12
cohorts (18–26,28–30),
while the outcome of PFS was reported in 10 cohorts (19–23,26–28,30).
In 12 studies, multivariate models were applied to analyze the
association between CXCL12 and the survival outcomes, and variables
such as age, sex and cancer stage, among others, were adjusted
(18–23,25,27–30).
In two studies, univariate models were used for the analyses
without adjustment of the potential confounding factors (24,26).
For one of the included studies, the HRs for the association
between tumor CXCL12 expression levels and survival outcomes were
separately reported in patients with and without preoperative
chemoradiotherapy (PCRT), and these datasets were included into the
meta-analysis independently. The NOS of the included studies were 6
to 9 stars, suggesting moderate to good study quality (Table II).
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
| First author,
year | Country | Design | Diagnosis | Sample size | Mean age,
years | Men, % | Tumor stage | Methods for
measuring CXCL12 | Definition of
higher CXCL12 expression | No. of cases with
higher CXCL12 | Mean CXCL12
expression level, % | Outcome
reported | Median follow-up
duration, months | Variables
adjusted | (Refs.) |
|---|
| Yoshitake et al,
2008 | Japan | RC | Patients with CRC
who underwent surgery or endoscopic resection | 60 | 63.8 | 68.3 | I–IV | IHC | Greater or equal to
the expression level of endothelial 1 cells in the adjacent normal
colonic tissues | 38 | 63 | OS | 35 | Age, sex and cancer
stage | (18) |
| Akishima-Fukasawa
et al, 2009 | Japan | RC | Patients with CRC
who underwent surgery for curative resection | 165 | 61.8 | 61.2 | II–III | IHC | CXCL12 expression
in ≥50% of the tumor cells | 120 | 73 | OS and PFS | 61 | Age, sex, tumor
location, size, stage, lymphatic or blood vessel invasion and LN
metastasis | (19) |
| Saigusa et al,
2010 | Japan | RC | Patients with
rectal cancer underwent preoperative CRT | 53 | 62.4 | 81.1 | II–III | qRT-PCR | mRNA of CLCL12
detectable | 14 | 26 | OS and PFS | 40 | Age, sex and cancer
stage | (20) |
| Yopp et al,
2012 | USA | RC | Patients undergoing
partial hepatectomy for metastatic CRC | 75 | NR | 68 | IV | IHC | CXCL12 expression
in ≥50% of the tumor cells | 22 | 29 | OS and PFS | 42 | Age, sex, clinical
risk score and tumor distribution | (22) |
| Sakai et al,
2012 | Japan | RC | Patients with liver
metastases of CRC | 92 | NR | 63 | IV | IHC | CXCL12 expression
in ≥10% of the tumor cells | 51 | 55 | OS and PFS | 38 | Age, sex, tumor
size and number of metastases | (21) |
| D'Alterio et al,
2014 | Italy | RC | Patients with
locally advanced rectal cancer undergoing PCRT | 68 | 61 | 57.4 | I–III | IHC | CXCL12 expression
in ≥50% of the tumor cells | 44 | 65 | OS and PFS | 66 | Age, sex, tumor
histology and stage | (23) |
| Amara et al,
2015 | Tunisia | RC | Patients with
CRC | 124 | 61 | 56.5 | I–IV | IHC | CXCL12 expression
in ≥50% of the tumor cells | 89 | 72 | OS | 40 | None | (24) |
| de Cuba et al,
2016 | The
Netherlands | PC | Patients with
peritoneal metastases of CRC | 52 | 58 | 43.4 | IV | IHC | CXCL12 expression
in ≥50% of the tumor cells | 28 | 54 | OS | 23 | None | (26) |
| D'Alterio et al,
2016 | Italy | RC | Patients with liver
metastases of CRC | 33 | NR | 61 | IV | IHC | CXCL12 expression
in ≥50% of the tumor cells | 17 | 52 | OS and PFS | 28 | Age, sex, KRAS
mutational status, and number and size of metastases | (25) |
| Stanisavljevic et
al, 2016 (cohort 1) | Norway | RC | Patients with colon
cancer | 290 | 61.9 | 48 | II–III | IHC | CXCL12 expression
in ≥10% of the tumor cells | 192 | 66 | PFS | 30 | Age, sex, tumor
stage, LN metastasisand adjuvant therapy | (27) |
| (cohort 2) | Norway | RC | Patients with colon
cancer | 265 | 73.5 | 49 | I–III | IHC | CXCL12 expression
in ≥10% of the tumor cells | 214 | 81 | PFS | 30 | Age, sex, tumor
stage, LN metastasis and adjuvant therapy |
|
| Mitchell et al,
2019 | USA | RC | Patients with
CRC | 49 | 64 | 51 | I–III | RT-qPCR | mRNA of CLCL12
detectable | 29 | 59 | OS and PFS | 33 | Age, sex, tumor
stage and lymphatic or blood vessel invasion | (28) |
| Lalos et al,
2021 | Switzerland | RC | Patients with
CRC | 613 | 70 | 46.8 | I–III | IHC | CXCL12 expression
in ≥50% of the tumor cells | 156 | 25 | OS | 40 | Age, sex, tumor
grade, stage and lymphatic or blood vessel invasion | (29) |
| Kim et al,
2022 | Korea | RC | Patients with
locally advanced rectal cancer | 121 | NR | 65.3 | II–III | IHC | CXCL12 expression
in ≥10% of the tumor cells | 41 | 34 | OS and PFS | 45 | Age, sex, tumor
stage, R score and previous chemotherapy | (30) |
 | Table II.Detailed quality evaluation of the
included studies via the Newcastle-Ottawa Scale. |
Table II.
Detailed quality evaluation of the
included studies via the Newcastle-Ottawa Scale.
| Study | Representativeness
of the exposed cohort | Selection of the
non-exposed cohort | Ascertainment of
exposure | Outcome not present
at baseline | Control for age and
sex | Control for other
confounding factors | Assessment of
outcome | Long enough
follow-up duration | Adequacy of
follow-up of cohorts | Total | (Refs.) |
|---|
| Yoshitake et al,
2008 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | (18) |
| Akishima-Fukasawa
et al, 2009 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (19) |
| Saigusa et al,
2010 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (20) |
| Yopp et al,
2012 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (22) |
| Sakai et al,
2012 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (21) |
| D'Alterio et al,
2014 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (23) |
| Amara et al,
2015 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | (24) |
| de Cuba et al,
2016 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | (26) |
| D'Alterio et al,
2016 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (25) |
| Stanisavljevic et
al, 2016 (cohort 1) | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (27) |
| (cohort 2) | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
|
| Mitchell et al,
2019 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (28) |
| Lalos et al,
2021 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (29) |
| Kim et al,
2022 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (30) |
Tumor expression of CXCL12 and the OS
of patients with CRC
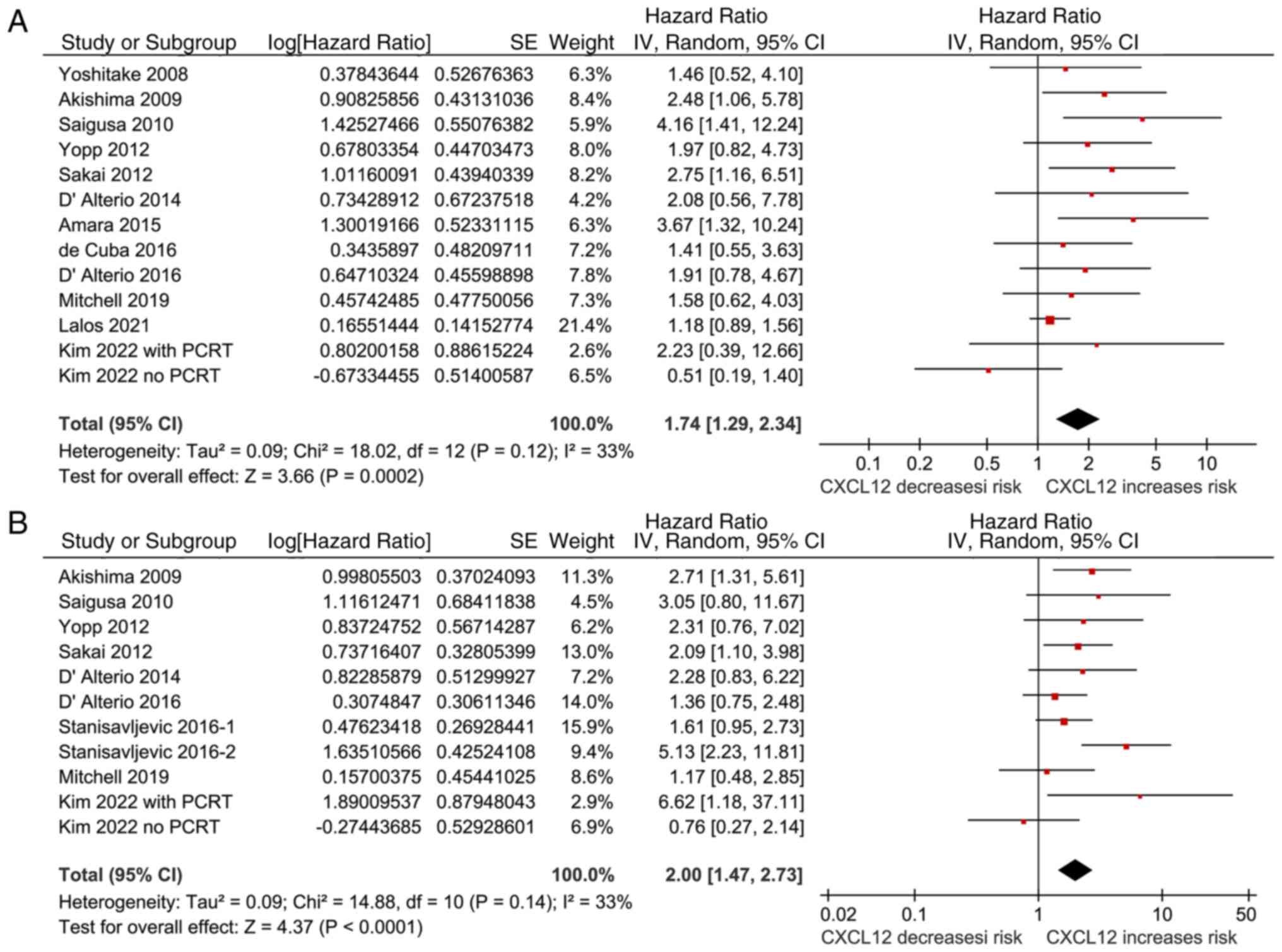
Pooled results of 12 cohorts (18–26,28–30)
showed that a higher tumor expression level of CXCL12 was
associated with the poor OS (HR, 1.74; 95% CI, 1.29-2.34;
P<0.001; I2, 33%) of patients with CRC (Fig. 2A). Sensitivity analyses performed
by excluding one study at a time showed consistent results (HR,
1.62-1.94; all P<0.05). Subgroup analyses showed that the
association between higher cancer expression levels of CXCL12 and
poor OS was not significantly affected by study country, tumor
location, tumor stage, methods for measuring tumor CXCL12 levels or
the models for the analyses of the association (all P>0.05;
Table III). Moreover,
sensitivity analyses limited to retrospective studies showed
similar results (12 studies; HR, 1.79; 95% CI, 1.30-2.47; P=0.004;
Table III).
 | Table III.Results of subgroup and sensitivity
analyses for the association between CXCL12 and overall survival of
patients with colorectal cancer. |
Table III.
Results of subgroup and sensitivity
analyses for the association between CXCL12 and overall survival of
patients with colorectal cancer.
| Study
characteristics | Dataset number | HR (95% CI) | I2, % | P-value for
subgroup effect | P-value for
subgroup difference |
|---|
| Country |
|
|
|
| 0.41 |
|
Asian | 6 | 1.89
(1.04-3.44) | 50 | 0.04 |
|
|
Non-Asian | 7 | 1.44
(1.12-1.86) | 5 | 0.005 |
|
| Tumor location |
|
|
|
| 0.97 |
| Rectal
cancer | 4 | 1.70
(0.61-4.78) | 64 | 0.31 |
|
| Rectal
or colon cancer | 9 | 1.67
(1.27-2.19) | 18 | <0.001 |
|
| Cancer stage |
|
|
|
| 0.48 |
|
I–III | 7 | 1.57
(0.99-2.50) | 48 | 0.05 |
|
| IV | 4 | 1.98
(1.27-3.10) | 0 | 0.003 |
|
| Methods for
measuring CXCL12 |
|
|
|
| 0.42 |
|
IHC | 11 | 1.64
(1.21-2.24) | 31 | 0.002 |
|
|
RT-qPCR | 2 | 2.47
(0.96-6.35) | 43 | 0.06 |
|
| Method for
analysis |
|
|
|
| 0.57 |
|
Univariate model | 2 | 2.23
(0.87-5.68) | 45 | 0.09 |
|
|
Multivariate model | 11 | 1.68
(1.22-2.30) | 33 | 0.001 |
|
| Design |
|
|
|
|
|
| RC
only | 12 | 1.79
(1.30-2.47) | 39 | 0.004 |
|
Tumor expression of CXCL12 and the PFS
of patients with CRC
Results of the meta-analysis with 10 cohorts
(19–23,26–28,30),
which were all retrospective studies with multivariate analyses,
showed that a higher tumor expression level of CXCL12 was
associated with poor PFS in patients with CRC (HR, 2.00; 95% CI,
1.47-2.73; P<0.001; I2, 33%; Fig. 2B). Sensitivity analyses performed
by excluding one study at a time did not significantly affect the
results (HR, 1.78-2.13; all P<0.05). Subgroup analyses showed
that the association between higher cancer expression levels of
CXCL12 and poor PFS was not significantly affected by
characteristics such as study country, tumor location, cancer stage
or the methods for measuring tumor CXCL12 levels (all P>0.05;
Table IV).
 | Table IV.Results of subgroup analyses for the
association between CXCL12 and progression-free survival of
patients with colorectal cancer. |
Table IV.
Results of subgroup analyses for the
association between CXCL12 and progression-free survival of
patients with colorectal cancer.
| Study
characteristics | Datasets
number | HR (95% CI) | I2, % | P-value for
subgroup effect | P-value for
subgroup difference |
|---|
| Country |
|
|
|
| 0.74 |
|
Asian | 5 | 2.16
(1.26-3.70) | 35 | 0.005 |
|
|
Non-Asian | 6 | 1.92
(1.27-2.90) | 40 | 0.002 |
|
| Tumor location |
|
|
|
| 0.76 |
| Rectal
cancer | 4 | 2.10
(0.90-4.87) | 47 | 0.09 |
|
| Colon
cancer | 2 | 2.74
(0.88-8.51) | 81 | 0.08 |
|
| Colon
or rectal cancer | 5 | 1.80
(1.30-2.51) | 0 | 0.005 |
|
| Cancer stage |
|
|
|
| 0.49 |
|
I–III | 8 | 2.15
(1.38-3.36) | 47 | <0.001 |
|
| IV | 3 | 1.74
(1.15-2.61) | 0 | 0.008 |
|
| Methods for
measuring CXCL12 |
|
|
|
| 0.64 |
|
IHC | 9 | 2.07
(1.46-2.93) | 39 | <0.001 |
|
|
RT-qPCR | 2 | 1.65
(0.67-4.06) | 27 | 0.28 |
|
Publication bias
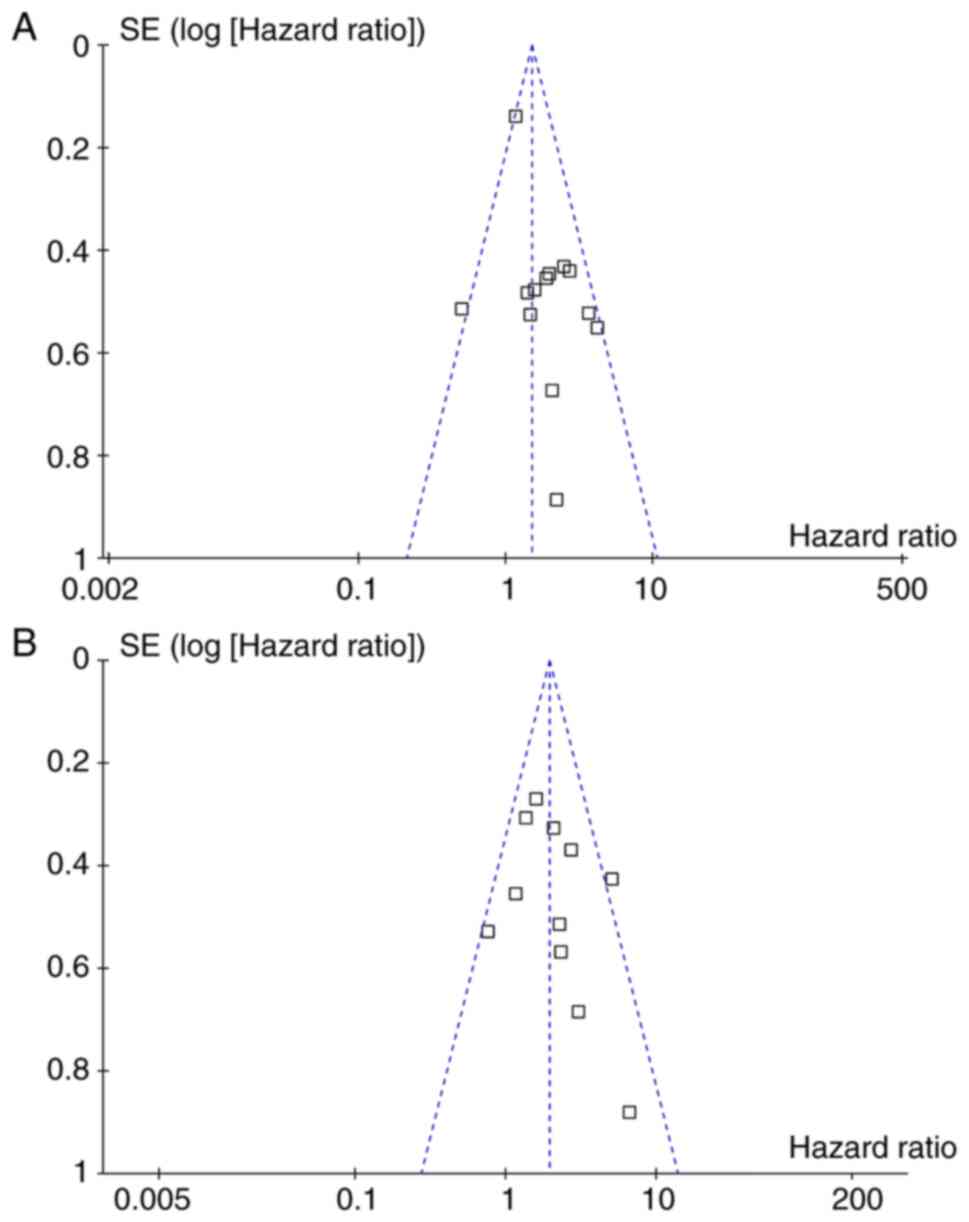
Fig. 3A and B
display the funnel plots for the outcomes of OS and PFS. Visual
inspection revealed symmetry of the plots, reflecting a low risk of
publication biases. Egger's regression tests also indicated low
risks of publication biases (P=0.18 and P=0.31, respectively).
Discussion
In this meta-analysis, by pooling the results of 14
cohort studies from 13 reports, the results showed that a higher
tumor expression level of CXCL12 was associated with the poor OS
and PFS of patients with CRC. The results were consistent for
sensitivity analyses by excluding one study at a time, and for
subgroup analyses according to multiple study characteristics, such
as the study country, tumor location, cancer stage and methods for
measuring tumor CXCL12. Taken together, these findings suggest that
a higher level of CXCL12 expression in tumors may be a predictor of
poor prognosis in patients with CRC.
An early meta-analysis in 2017 included 32 studies
and showed that high expression levels of CXCL12 were associated
with poor OS, but not poor PFS, in patients with various solid
malignancies (38). However,
significant heterogeneity was observed in this meta-analysis, and
further subgroup analyses with 6 studies of patients with CRC
failed to show a significant association between the tumor
expression level of CXCL12 and the survival outcomes (38). Another meta-analysis, also
published in 2017, suggested that a higher tumor expression level
of CXCL12 may be associated with poor OS (39). However, only 2 studies before 2011
were included, which made the results less convincing (39). The present meta-analysis has
several strengths compared with the previous meta-analyses. First,
the focus was on patients with CRC only and updated studies were
included, and the results showed that tumor expression levels of
CXCL12 may be a predictor of poor OS and PFS in patients with CRC.
Second, the robustness of the findings was evidenced by consistent
results of sensitivity and subgroup analyses, which indicated that
the results were not mainly driven by either of the included
cohorts and were not significantly affected by multiple study
characteristics. Furthermore, sensitivity analyses limited to
studies with multivariate analyses showed a significant association
between high CXCL12 expression levels and poor survival in patients
with CRC, which implies that the association may not be confounded
by factors such age, sex and cancer stage. Taken together, these
findings suggest that high expression levels of CXCL12 may be a
predictor of poor survival of patients with CRC.
The potential mechanisms underlying the association
between the high tumor expression of CXCL12 and the poor survival
of patients with CRC are not yet fully determined. An early
preclinical study showed that CXCL12 could activate multiple
signals, including extracellular signal-regulated kinase-1/2,
stress-activated protein kinase/c-Jun NH2-terminal kinase and
matrix metalloproteinase-9 (40),
which mediate the reorganization of the actin cytoskeleton,
resulting in increased cancer cell migration and invasion in CRC. A
subsequent study showed that silencing the CXCL12 gene could
significantly inhibit the proliferation, invasion and angiogenesis
ability of colon carcinoma cells through downregulation of the
mitogen-activated protein kinase-related signaling pathway
(41). Moreover, CXCL12 has also
been involved in the inflammation-induced progression of CRC. For
example, the CXCL12/CXCR4 signaling pathway was shown to play a
critical role in promoting the progression of inflammatory
colorectal cancer by recruiting immunocytes and enhancing
cytoskeletal remodeling (42). In
addition, high tumor expression levels of CXCL12 were shown to
reduce the sensitivity of CRC to radiotherapy by upregulating the
expression of survivin (43).
Collectively, the aforementioned results suggest that CXCL12 plays
a key role in the progression of CRC. Another important question is
whether interventions lowering the expression of CXCL12 in CRC
could improve the clinical outcomes of the patients. An ongoing
clinical trial evaluating the safety and efficacy of anti-CXCL12
(NOX-A12) in patients with advanced-stage pretreated metastatic CRC
and pancreatic cancer (OPERA trial, Keynote-559; ClinicalTrials.gov
identifier, NCT03168139) is expected to give an answer.
In the present meta-analysis, the HRs of the
included datasets for the association between CXCL12 and survival
outcomes were all >1 except for one dataset (Kim et al
2022; no PCRT) (30), which showed
the HRs for the association were <1. Sensitivity analyses
performed by excluding these datasets showed that it did not
significantly affect the results (OS: HR, 1.79; 95% CI, 1.38-2.34;
P<0.001; I2, 18%; PFS: HR, 2.12; 95% CI, 1.58-2.84;
P<0.001; I2, 22%). However, between-study
heterogeneity was slightly reduced, as evidenced by reduced
I2 for both OS and PFS after removing the datasets,
suggesting that this dataset at least partly explains the source of
the heterogeneity. The reasons for the discrepancy between this
dataset and the other included studies are currently unknown. In
the study by Kim et al (30), it was shown that a higher
expression level of CXCL12 may be associated with poor PFS in
patients with CRC who received PCRT, but not in those who did not
receive PCRT, suggesting that the association between CXCL12 and
the survival of patients with CRC may be modified by the different
treatment modalities. However, the present study was unable to
determine the influence of anticancer modality on the
aforementioned association in this meta-analysis, as most of the
included studies did not provide stratified results according to
the treatment modalities. Large-scale studies are needed to
determine if the association between CXCL12 levels and the survival
of patients with CRC is consistent in patients who receive
different anticancer treatments.
The present meta-analysis also has certain
limitations. Firstly, although the statistical heterogeneity
observed in both the outcomes of OS and PFS was not significant
(both I2 values of 33%), there may be clinical
heterogeneity among the included studies, which could be a result
of differences in patient comorbidities, anticancer treatments and
methods for measuring CXCL12. Furthermore, as an outcome of
patients with cancer, PFS is highly associated with the cancer
stage and treatments. Although the HRs for PFS were pooled with the
most adequately adjusted models in individual studies in order to
minimize the influence of possible confounding factors on the
association, the results may be confounded by differences of study
characteristics such as cancer stages and treatment modalities.
However, pooling the data of HRs for PFS in prognostic
meta-analyses has been well applied in previous studies (44–46).
In addition, most of the included studies were retrospective, which
may confound the results by possible recall and selection biases.
Large-scale prospective cohort studies are needed to confirm the
findings of the present study. Finally, a causative association
between high tumor expression levels of CXCL12 and poor survival in
patients with CRC could not be derived from the present study, as
it is a meta-analysis based on observational studies. As
aforementioned, clinical trials are warranted to determine the
possible influence of anti-CXCL12 on clinical outcomes in patients
with CRC.
In conclusion, results of the meta-analysis
indicated that a higher tumor expression level of CXCL12 is
associated with the poor survival of patients with CRC. Studies are
warranted to determine if CXCL12-targeted intervention could
improve the prognosis of patients with CRC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ and GL designed the study, searched the
literature, evaluated the study quality, extracted the study data,
performed statistical analyses and interpreted the results. SZ
drafted the manuscript. GL critically revised the manuscript. SZ
and GL confirm the authenticity of all the raw data. Both authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giaquinto AN, Miller KD, Tossas KY, Winn
RA, Jemal A and Siegel RL: Cancer statistics for African
American/Black People 2022. CA Cancer J Clin. 72:202–229. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burnett-Hartman AN, Lee JK, Demb J and
Gupta S: An update on the epidemiology, molecular characterization,
diagnosis, and screening strategies for early-onset colorectal
cancer. Gastroenterology. 160:1041–1049. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Biller LH and Schrag D: Diagnosis and
treatment of metastatic colorectal cancer: A Review. JAMA.
325:669–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bien J and Lin A: A review of the
diagnosis and treatment of metastatic colorectal cancer. JAMA.
325:2404–2405. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Portella L, Bello AM and Scala S: CXCL12
Signaling in the tumor microenvironment. Adv Exp Med Biol.
1302:51–70. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smit MJ, Schlecht-Louf G, Neves M, van den
Bor J, Penela P, Siderius M, Bachelerie F and Mayor F Jr: The
CXCL12/CXCR4/ACKR3 Axis in the Tumor Microenvironment: Signaling,
crosstalk, and therapeutic targeting. Annu Rev Pharmacol Toxicol.
61:541–563. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Britton C, Poznansky MC and Reeves P:
Polyfunctionality of the CXCR4/CXCL12 axis in health and disease:
Implications for therapeutic interventions in cancer and
immune-mediated diseases. FASEB J. 35:e212602021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huynh C, Dingemanse J, Meyer Zu
Schwabedissen HE and Sidharta PN: Relevance of the
CXCR4/CXCR7-CXCL12 axis and its effect in pathophysiological
conditions. Pharmacol Res. 161:1050922020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Janssens R, Struyf S and Proost P: The
unique structural and functional features of CXCL12. Cell Mol
Immunol. 15:299–311. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lopez-Gil JC, Martin-Hijano L, Hermann PC
and Sainz B Jr: The CXCL12 crossroads in cancer stem cells and
their niche. Cancers (Basel). 13:4692021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mortezaee K: CXCL12/CXCR4 axis in the
microenvironment of solid tumors: A critical mediator of
metastasis. Life Sci. 249:1175342020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi Y, Riese DJ II and Shen J: The Role of
the CXCL12/CXCR4/CXCR7 chemokine axis in cancer. Front Pharmacol.
11:5746672020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mezzapelle R, Leo M, Caprioglio F, Colley
LS, Lamarca A, Sabatino L, Colantuoni V, Crippa MP and Bianchi ME:
CXCR4/CXCL12 activities in the tumor microenvironment and
implications for tumor immunotherapy. Cancers (Basel). 14:23142022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goita AA and Guenot D: Colorectal cancer:
The contribution of CXCL12 and its receptors CXCR4 and CXCR7.
Cancers (Basel). 14:18102022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khare T, Bissonnette M and Khare S:
CXCL12-CXCR4/CXCR7 axis in colorectal cancer: Therapeutic target in
preclinical and clinical studies. Int J Mol Sci. 22:73712021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshitake N, Fukui H, Yamagishi H,
Sekikawa A, Fujii S, Tomita S, Ichikawa K, Imura J, Hiraishi H and
Fujimori T: Expression of SDF-1 alpha and nuclear CXCR4 predicts
lymph node metastasis in colorectal cancer. Br J Cancer.
98:1682–1689. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akishima-Fukasawa Y, Nakanishi Y, Ino Y,
Moriya Y, Kanai Y and Hirohashi S: Prognostic significance of
CXCL12 expression in patients with colorectal carcinoma. Am J Clin
Pathol. 132:202–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saigusa S, Toiyama Y, Tanaka K, Yokoe T,
Okugawa Y, Kawamoto A, Yasuda H, Inoue Y, Miki C and Kusunoki M:
Stromal CXCR4 and CXCL12 expression is associated with distant
recurrence and poor prognosis in rectal cancer after
chemoradiotherapy. Ann Surg Oncol. 17:2051–2058. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakai N, Yoshidome H, Shida T, Kimura F,
Shimizu H, Ohtsuka M, Takeuchi D, Sakakibara M and Miyazaki M:
CXCR4/CXCL12 expression profile is associated with tumor
microenvironment and clinical outcome of liver metastases of
colorectal cancer. Clin Exp Metastasis. 29:101–110. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yopp AC, Shia J, Butte JM, Allen PJ, Fong
Y, Jarnagin WR, DeMatteo RP and D'Angelica MI: CXCR4 expression
predicts patient outcome and recurrence patterns after hepatic
resection for colorectal liver metastases. Ann Surg Oncol. 19
(Suppl 3):S339–S346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Alterio C, Avallone A, Tatangelo F,
Delrio P, Pecori B, Cella L, Pelella A, D'Armiento FP, Carlomagno
C, Bianco F, et al: A prognostic model comprising pT stage, N
status, and the chemokine receptors CXCR4 and CXCR7 powerfully
predicts outcome in neoadjuvant resistant rectal cancer patients.
Int J Cancer. 135:379–390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amara S, Chaar I, Khiari M, Ounissi D,
Weslati M, Boughriba R, Hmida AB and Bouraoui S: Stromal cell
derived factor-1 and CXCR4 expression in colorectal cancer promote
liver metastasis. Cancer Biomark. 15:869–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
D'Alterio C, Nasti G, Polimeno M, Ottaiano
A, Conson M, Circelli L, Botti G, Scognamiglio G, Santagata S, De
Divitiis C, et al: CXCR4-CXCL12-CXCR7, TLR2-TLR4, and PD-1/PD-L1 in
colorectal cancer liver metastases from neoadjuvant-treated
patients. Oncoimmunology. 5:e12543132016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Cuba EM, de Hingh IH, Sluiter NR,
Kwakman R, Coupé VM, Beliën JA, Verwaal VJ, Meijerink WJ, Delis-van
Diemen PM, Bonjer HJ, et al: Angiogenesis-Related markers and
prognosis after cytoreductive surgery and hyperthermic
intraperitoneal chemotherapy for metastatic colorectal cancer. Ann
Surg Oncol. 23:1601–1608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stanisavljevic L, Assmus J, Storli KE, Leh
SM, Dahl O and Myklebust MP: CXCR4, CXCL12 and the relative
CXCL12-CXCR4 expression as prognostic factors in colon cancer.
Tumour Biol. 37:7441–7452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mitchell A, Hasanali SL, Morera DS, Baskar
R, Wang X, Khan R, Talukder A, Li CS, Manoharan M, Jordan AR, et
al: A chemokine/chemokine receptor signature potentially predicts
clinical outcome in colorectal cancer patients. Cancer Biomark.
26:291–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lalos A, Tulek A, Tosti N, Mechera R,
Wilhelm A, Soysal S, Daester S, Kancherla V, Weixler B, Spagnoli
GC, et al: Prognostic significance of CD8+ T-cells density in stage
III colorectal cancer depends on SDF-1 expression. Sci Rep.
11:7752021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim S, Yeo MK, Kim JS, Kim JY and Kim KH:
Elevated CXCL12 in the plasma membrane of locally advanced rectal
cancer after neoadjuvant chemoradiotherapy: A potential prognostic
marker. J Cancer. 13:162–173. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Page MJ, Moher D, Bossuyt PM, Boutron I,
Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: PRISMA 2020 explanation and elaboration: Updated
guidance and exemplars for reporting systematic reviews. BMJ.
372:n1602021. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Higgins J, Thomas J, Chandler J, Cumpston
M, Li T, Page MJ and Welch VA: Cochrane Handbook for Systematic
Reviews of Interventions version 6.2. The Cochrane Collaboration.
www.training.cochrane.org/handbook2021
|
|
34
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Tugwell P and Losos M: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp2010
|
|
35
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Patsopoulos NA, Evangelou E and Ioannidis
JP: Sensitivity of between-study heterogeneity in meta-analysis:
Proposed metrics and empirical evaluation. Int J Epidemiol.
37:1148–1157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Samarendra H, Jones K, Petrinic T, Silva
MA, Reddy S, Soonawalla Z and Gordon-Weeks A: A meta-analysis of
CXCL12 expression for cancer prognosis. Br J Cancer. 117:124–135.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li YP, Pang J, Gao S, Bai PY, Wang WD,
Kong P and Cui Y: Role of CXCR4 and SDF1 as prognostic factors for
survival and the association with clinicopathology in colorectal
cancer: A systematic meta-analysis. Tumour Biol.
39:10104283177062062017.PubMed/NCBI
|
|
40
|
Brand S, Dambacher J, Beigel F, Olszak T,
Diebold J, Otte JM, Göke B and Eichhorst ST: CXCR4 and CXCL12 are
inversely expressed in colorectal cancer cells and modulate cancer
cell migration, invasion and MMP-9 activation. Exp Cell Res.
310:117–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma J, Su H, Yu B, Guo T, Gong Z, Qi J,
Zhao X and Du J: CXCL12 gene silencing down-regulates metastatic
potential via blockage of MAPK/PI3K/AP-1 signaling pathway in colon
cancer. Clin Transl Oncol. 20:1035–1045. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu X, Wang D, Wang X, Sun S, Zhang Y, Wang
S, Miao R, Xu X and Qu X: CXCL12/CXCR4 promotes inflammation-driven
colorectal cancer progression through activation of RhoA signaling
by sponging miR-133a-3p. J Exp Clin Cancer Res. 38:322019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang D, Jiao C, Zhu Y, Liang D, Zao M,
Meng X, Gao J, He Y, Liu W, Hou J, et al: Activation of
CXCL12/CXCR4 renders colorectal cancer cells less sensitive to
radiotherapy via up-regulating the expression of survivin. Exp Biol
Med (Maywood). 242:429–435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fang Y, Zheng T and Zhang C: Prognostic
Role of the C-Reactive Protein/Albumin ratio in patients with
gynecological cancers: A meta-analysis. Front Oncol. 11:7371552021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim MS, Chun SW, Dho YS, Seo Y, Lee JH,
Won JK, Kim JW, Park CK, Park SH and Kim YH: Histopathological
predictors of progression-free survival in atypical meningioma: A
single-center retrospective cohort and meta-analysis. Brain Tumor
Pathol. 39:99–110. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peng Q, Liu L, Li T, Lei C and Wan H:
Prognostic impact of prognostic nutritional index on renal cell
carcinoma: A meta-analysis of 7,629 patients. PLoS One.
17:e02651192022. View Article : Google Scholar : PubMed/NCBI
|