Introduction
Lung cancer is one of the most malignant tumors and
is the leading cause of cancer-associated mortality worldwide
(1,2). Among patients with lung cancer,
non-small cell lung carcinoma (NSCLC) accounts for ~85% of all
cases (3,4). Despite the development of novel
therapies in the past few decades, the 5-year survival rate (4–17%
depending on stage and regional differences) of patients with NSCLC
remains markedly low (5); this is
due to the difficulty in early diagnosis, the lack of targeted
therapies, drug resistance and frequent relapses. Therefore,
identifying new biomarkers for early diagnosis and new therapies is
essential for the clinical treatment of NSCLC.
Bioinformatics analysis of the human genome showed
that <2% of the genome sequence corresponds to protein-coding
genes, whereas >70% is transcribed into non-coding RNAs (ncRNAs)
(6). Long ncRNAs (lncRNAs) are an
important type of ncRNAs, with a length of >200 nucleotides.
lncRNAs have been reported to play important roles in various tumor
types, such as lung, colorectal and breast cancer, through multiple
mechanisms (7,8). By comparative analysis of microarray
and next-generation sequencing data of NSCLC and normal tissues,
thousands of lncRNAs were identified to be differentially expressed
in NSCLC samples (9–12). Although a few of studies have shown
that several lncRNAs could promote or suppress the progression of
NSCLC (13), identifying novel
oncogenic lncRNAs remains critical for NSCLC diagnosis and
treatment.
Cancer/testis (CT) antigens are the protein products
of genes frequently expressed in multiple human cancer types and in
the normal testis (14,15). To date, only a few lncRNAs have
been reported to be CT genes (16–19).
The present study identified long intergenic non-protein coding RNA
1635 (LINC01635) as a potential novel CT lncRNA. The expression of
LINC01635 in lung cancer was investigated, and it was found that
LINC01635 was highly expressed in samples from patients with lung
cancer and in NSCLC cell lines. Functional studies showed that
LINC01635 regulated the proliferation and metastasis of NSCLC cells
in vitro and in vivo. Furthermore, it was also found
that LINC01635 could bind to microRNA (miRNA/miR)-455-5p in
vitro and that it regulated the expression of
miR-455-5p-targeting tumor-related genes in NSCLC cells, which
demonstrated its functional mechanism in lung cancer.
Materials and methods
lncRNA selection with CT expression
pattern from lung cancer RNA-expression data
Microarray dataset GSE113852 (20) was downloaded from the Gene
Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/), and LINC01635
expression profiling in normal tissue was analyzed according to the
data of the RNA-sequencing (RNA-seq) of normal tissues in the Human
Protein Atlas (https://www.ncbi.nlm.nih.gov/). The coding potential
of LINC01635 was assessed using the CPAT and CNCI online tools
(https://lncar.renlab.org/), and the
expression level of LINC01635 was also confirmed by analyzing
another microarray dataset (GSE101929) (21) using online tools (https://lncar.renlab.org/) (22). LINC01635 expression profiling in
different tumors and normal tissues was analyzed online by Gene
Expression Profiling Interactive Analysis using RNA-seq data from
The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression
(GTEx) databases (http://gepia.cancer-pku.cn/) (23).
Clinical samples
A total of 14 paired human NSCLC and adjacent normal
lung tissues were obtained from patients who underwent surgery or
biopsy at Lianshui County People's Hospital, Kangda College of
Nanjing Medical University (Lianshui, China) (mean age, 69 years;
range, 50–80 years) between December 2018 and December 2019. All
patients were diagnosed with NSCLC according to histopathological
evaluation. No radiotherapy or chemotherapy was performed prior to
sample collection. Tissue samples were immediately stored in
RNAlater solution (Invitrogen; Thermo Fisher Scientific, Inc.) at
−80°C. Written informed consents was obtained from all the enrolled
patients with NSCLC. The study protocol was approved by the
Research Ethics Committee of Lianshui County People's Hospital,
Kangda College of Nanjing Medical University (approval no.
2021602-2).
Cell culture
The human NSCLC A549, H1299, H1975 and PC9 cell
lines, and the human bronchial epithelial HBE135-E6E7 cell line
(HBE135) were obtained from the Institute of Biochemistry and Cell
Biology of Chinese Academy of Sciences. A549, H1975 and HBE135
cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.), while H1299 and PC9 cells were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.). Both media were
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin.
All cell lines were maintained in a humidified air atmosphere with
5% CO2 at 37°C.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from patient tissues and
cultured cells using TRIzol® reagent (Ambion; Thermo
Fisher Scientific, Inc.). A total of 1 µg of RNA was reversed
transcribed to cDNA with the PrimeScript RT Reagent Kit (Takara
Biotechnology Co., Ltd.) by using 6-mer random primers or a
miR-455-5p specific primer
(5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACCGATGT-3′) based on
the stem-loop primer method (24).
The reaction conditions were as follows: 42°C for 2 min, 25°C for 5
min, 42°C for 30 min and 85°C for 5 min. qPCR was then performed
using SYBR-Green Master Mix (Takara Biotechnology Co., Ltd.) in a
StepOnePlus™ Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The thermocycling conditions for qPCR were as
follows: 95°C for 5 min for 1 cycle, and 95°C for 10 sec and 60°C
for 30 sec for 40 cycles. The endogenous control was GAPDH or U6
(for miR-455-5p), and the expression levels were analyzed by the
2−∆∆Cq method (25).
The specific primers were designed with Primer-Blast (National
Center for Biotechnology; National Institutes of Health), and the
primer sequences are listed in Table
I. All primers were synthesized by General Biosystems, Inc.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Molecule | Primer sequence
(5′-3′) |
|---|
| GAPDH-F |
GGGAGCCAAAAGGGTCAT |
| GAPDH-R |
GAGTCCTTCCACGATACCAA |
| LINC01635-F |
GGGCCCCATTCTGAGGTTAC |
| LINC01635-R |
GCGCCAATACAAGGACCACT |
| SOX11-F |
GGTGGATAAGGATTTGGATTCG |
| SOX11-R |
GCTCCGGCGTGCAGTAGT |
| RAB18-F |
CAATGTGCCTTTGAAGAACTTGT |
| RAB18-R |
CTCCTTGGCCTTCTTCCCTG |
| TMED2-F |
ATGTATTCCTGTTCGTGCTT |
| TMED2-R |
CACATGGATGGAACATACAA |
| USP3-F |
AGGTGCTATGCTTACATTTG |
| USP3-R |
CTGTTCTCAGGCTCTAGTAAG |
| CDKN1B-F |
GCCGCAACCAATGGATCTCCTC |
| CDKN1B-R |
AGTCGCAGAGCCGTGAGCAA |
| CPEB1-F |
CACAGATAAGCACAAGTATC |
| CPEB1-R |
GACACAGAGAATCTTCTAG |
| UBE2V1-F |
AAAAGTCCCTCGCAATTTCC |
| UBE2V1-R |
CTGCCATTTTGCTAGCACTG |
| HDAC4-F |
AGAATGGCTTTGCTGTGGTC |
| HDAC4-R |
ATCTTGCTCACGCTCAACCT |
| STK24-F |
GCCTCCACCAAGATATTCCA |
| STK24-R |
AACAAGAAATCACAGTGCTGAGTC |
| MMP2-F |
CTGCGGTTTTCTCGAATCCATG |
| MMP2-R |
GTCCTTACCGTCAAAGGGGTATCC |
| MMP9-F |
GAGGCGCTCATGTACCCTATGTAC |
| MMP9-R |
GTTCAGGGCGAGGACCATAGAG |
| LINC00339-F |
TCTTTCCATTTTGCAGTTGGGC |
| LINC00339-R |
CTCCTCGGCCCATCATTTCAT |
| U6-F |
GCTTCGGCAGCACATATACTAAAAT |
| U6-R |
CGCTTCACGAATTTGCGTGTCAT |
| miR-455-5p-F |
GCCGCCTATGTGCCTTTGGACT |
| miR-455-5p-R |
GTGCAGGGTCCGAGGT |
Cloning of LINC01635
Primers were designed according to the prediction of
LINC01635 transcripts in the Ensembl website (https://www.ensembl.org/) and the human mRNA clone of
LINC01635 (GenBank ID: BC039533), and LINC01635 was cloned using
the cDNA of A549 cell lines by ExTaq (Takara Biotechnology Co.,
Ltd.) in a Mastercycler® Nexus X2 (Eppendorf). The
following thermocycling conditions were used: 94°C for 5 min for 1
cycle, and 94°C for 30 sec, 60°C for 30 sec and 72°C for 2 min for
35 cycles. The primer sequences were as follows:
5′-TTACCGTGCGGAGTTTTGGA-3′ (forward) and
5′-TTATGTGCCTATGAAATTGGAGTTG-3′ (reverse).
Small RNA transfection
LINC01635 small interfering (si)RNA (si-LINC01635),
negative control (NC) siRNA, miR-455-5p inhibitor and NC inhibitor
were purchased from General Biosystems and used for transiently
downregulating the expression of LINC01635 or miR-455-5p. The
sequences of the synthesized siRNAs and miRNA inhibitors were as
follows: 5′-GGAGUUUUGGAUACAUUCU-3′ (si-LINC01635),
5′-UUCUCCGAACGUGUCACGU-3′ (NC siRNA), 5′-UAUGUGCCUUUGGACUACAUCG-3′
(miR-455-5p inhibitor) and 5′-UCUACUCUUUCUAGGAGGUUGUGA-3′ (NC
inhibitor). miR-455-5p mimic and NC miRNA mimic were also purchased
from General Biosystems and used for transiently upregulating the
expression of miR-455-5p. The sequences of the synthesized miRNA
mimics were as follows: 5′-UAUGUGCCUUUGGACUACAUCG-3′ (miR-455-5p
mimic) and 5′-UCACAACCUCCUAGAAAGAGUAGA-3′ (NC mimic).
A549 and H1975 cells (1×105) were seeded
in 6-well plates and cultured at 37°C overnight. Next, the cells
were transfected with siRNAs (50 µM), miRNA mimics (50 µM) or miRNA
inhibitors (100 µM) for 24 h at 37°C using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. At
24 h post-transfection, the knockdown efficiency was detected by
RT-qPCR.
Cell proliferation assay
A cell proliferation assay was performed using Cell
Counting Kit-8 (CCK-8; Dojindo Laboratories, Inc.). A549 and H1975
cells transfected with siRNA-LINC01635 and NC siRNA were seeded at
2×103 cells/well in 96-well plates. Next, 10 µl CCK-8
reagent was added to each well, which contained 100 µl culture
medium. After 2 h, cell proliferation was monitored by measuring
the optical density at 450 nm on a microplate reader (BioTek
Elx800; BioTek Instruments, Inc.) every 24 h from 0 to 96 h
according to the manufacturer's instructions.
Transwell assay
Cell migration was analyzed by Transwell assay. A549
and H1975 cells were transfected with si-LINC01635, NC siRNA,
miR-455-5p inhibitor, NC inhibitor or siRNA + inhibitor, and then
re-suspended with 200 µl serum-free medium to a total of
2–5×104 A549 cells or 5×104 H1975 cells.
Transwell chambers (8-µm pore size) were placed into 24-well
plates, and 800 µl medium containing 10% FBS was added to the lower
chamber. After 24 h at 37°C, the upper chambers were fixed with
methanol for 30 min at room temperature, and then stained with 0.1%
crystal violet for 20 min at room temperature. Next, the upper
surface of the membrane was removed with cotton swabs. The
Transwell inserts were imaged under an optical inverted
microscope.
Western blotting
Total protein from transfected lung cancer cells was
extracted with RIPA lysis buffer (Beyotime Institute of
Biotechnology). The protein concentration was measured with a BCA
kit (Beyotime Institute of Biotechnology). The protein samples (50
µg per lane) were separated on 10% gels using SDS-PAGE and then
were electro-transferred onto polyvinylidene fluoride membranes
(MilliporeSigma), which were blocked with 5% skimmed milk for 1 h
at room temperature. Subsequently, the membranes were incubated
with primary antibodies against MMP2 (1:1,000; cat. no. A00286),
MMP9 (1:1,000; cat. no. PB0709) or GAPDH (internal control;
1:10,000; cat. no. A00227) (all Boster Biological Technology) at
4°C overnight. Next, the membranes were incubated with
HRP-conjugated AffiniPure mouse anti-rabbit IgG (H+L) (1:5,000;
cat. no. BM2006; Boster Biological Technology) for 1 h at room
temperature. After washing for 5 times (each for 5 min) using PBST
(0.05% Tween-20) at room temperature, the proteins were visualized
with the BeyoECL plus kit (Beyotime Institute of
Biotechnology).
Zebrafish xenograft models
Zebrafish were maintained in a fish culture system
(Haisheng Instruments, Inc.) at 28°C under a light-dark cycle of
10–14 h. Approximately 100 2-days post-fertilization (dpf)
transgenic Tg(fli1a:EGFP) zebrafish larvae (China Zebrafish
Resource Center) were used for cell injection, and the endothelial
cells of these transgenic zebrafish larvae were labeled with EGFP
(26). At 24 h post-transfection
of si-LINC01635 or NC siRNA (control) in cultured cells, including
A549 and H1975 cell lines, the cells were collected and stained for
5 min at 37°C and 15 min at 4°C with a fluorescent dye (CM-DiI;
Thermo Fisher Scientific, Inc.) for injection. The stained cells
were examined under a fluorescence microscope before injection. A
total of 300–400 cells labeled with CM-DiI were transplanted into
the perivitelline space (PVS) of 48-h-post-fertilization zebrafish
larvae under a pressure systems for ejection (Picosprizer III;
Parker Hannifin Corporation). At 1 day post-injection (dpi), the
injected larvae with similar tumor size of CM-DiI-positive cells
were selected and cultured at 34°C until the end of experiments. At
4 dpi, the intact zebrafish larvae were mounted using 1.2%
low-melting gel, and the yolk and trunk were imaged via a
stereomicroscope (MVX10; Olympus Corporation) or a confocal
microscope (FLUOVIEW FV3000; Olympus Corporation) using a 20X
water-immersion objective directly. The spatial resolution of the
images was 1,600×1,200 pixels for the MVX10 or 1,024×1,024 pixels
for the FLUOVIEW FV3000. After the imaging experiments, zebrafish
larvae were anesthetized with alcohol and then sacrificed by
hypothermia (−20°C).
miRNA binding prediction
miRNAs that could potentially bind to LINC01635 were
predicted by the online tools miRDB (http://mirdb.org/) and LncBase (http://www.microrna.gr/LncBase). The miRNAs that were
predicted by both tools were considered as candidates.
miRNA-targeting genes were predicted by the online tools miRDB
(http://mirdb.org/) and TargetScan (https://www.targetscan.org/). The tumor-related
targeted genes of interest were screened out individually by
scientific literature search using the key words ‘tumor’ and the
name of each miRNA in PubMed (https://pubmed.ncbi.nlm.nih.gov/).
Isolation of cytoplasmic and nuclear
RNA
A total of 1×107 A549 cells were
collected, and their cytoplasmic and nuclear RNA were extracted
respectively with the PARIS™ Kit (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Next, the cytoplasmic and nuclear RNA were
reverse-transcribed, respectively. Then, the expression levels of
cytoplasmic and nuclear RNA were detected by RT-qPCR, which
represented the localization of RNA. U6 was used for the positive
control of nuclear RNA, and GAPDH was used for the positive control
of cytoplasmic RNA.
Reporter plasmid construction and
luciferase reporter assay
The total sequence of LINC01635 transcript 1# was
cloned into a pmirGLO Dual-Luciferase miRNA Target Expression
Vector (E1330; Promega Corporation) to generate the reporter
plasmid. To construct a LINC01635 mutant reporter plasmid, the
putative binding site of miR-455-5p in LINC01635 was mutated by
PCR.
The plasmids and miR-455-5p mimic or NC mimic were
co-transfected into 2×104 293T cells (Saihongrui
Biotechnology Co., Ltd.) cultured in DMEM with 10% FBS) at 37°C for
48 h using Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The luciferase activity in co-transfected cells was
detected using the Dual-Luciferase Reporter Assay System (Promega
Corporation) and GloMax® Explorer Multimode Microplate
Reader (Promega Corporation). Firefly luciferase activity was used
as the main reporter activity, and Renilla luciferase
activity was used as the control for normalization.
Statistical analysis
Data are presented as the mean ± SEM from at least
three repetitions. Unpaired Student's t-test was used to perform
statistical analysis of two unpaired groups, while paired Student's
t-test was used to perform statistical analysis of two paired
groups (Microsoft Excel 2010; Microsoft Corporation). In addition,
one-way ANOVA was used to perform statistical analysis of multiple
groups (GraphPad Prism 8; GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Identification of LINC01635 as an
lncRNA with CT expression pattern
To screen for differentially expressed lncRNAs in
lung cancer, the gene expression profile of lung adenocarcinoma and
normal lung tissue was obtained from GEO dataset GSE113852, which
contained the expression data of 27 lung tumor samples and 27
normal lung samples. With Log2FC>2 as the screen
threshold, 168 genes were highly expressed in lung adenocarcinoma
compared with those in normal lung tissues (Fig. 1A). The expression of these genes in
physiological conditions was assessed by analyzing the HPA RNA-seq
normal tissue database, and only LINC01635 (ENSG00000228397;
Log2FC=2.36; P=8.58×10−8) was found to be highly
expressed in the testis (Fig. 1B).
LINC01635 was located on chromosome 1p36.12, upstream of human cell
division cycle 42 (CDC42) in the human genome, but its
transcriptional direction is opposite to that of CDC42. The lncRNA
property of LINC01635 was confirmed by evaluating its coding
potential (CPAT and CNCI tools) and predicting the secondary
structure (Fig. 1B). Furthermore,
a high LINC01635 expression level was also confirmed in lung cancer
samples by analyzing another GEO dataset using lnCAR online tools
(GSE101929; Fig. 1D). In addition,
the expression level of LINC01635 was analyzed in multiple cancer
types compared with corresponding normal tissues (TCGA and GTEx
data), and LINC01635 was highly expressed in multiple cancer types
and testis tissues (Fig. 1E). To
analyze the common high expression pattern of LINC01635 in
different human cancer types, its expression level in all cancer
types was compared with that in all normal tissues excluding
testis. LINC01635 was found to be significantly upregulated in
cancer (Fig. 1E).
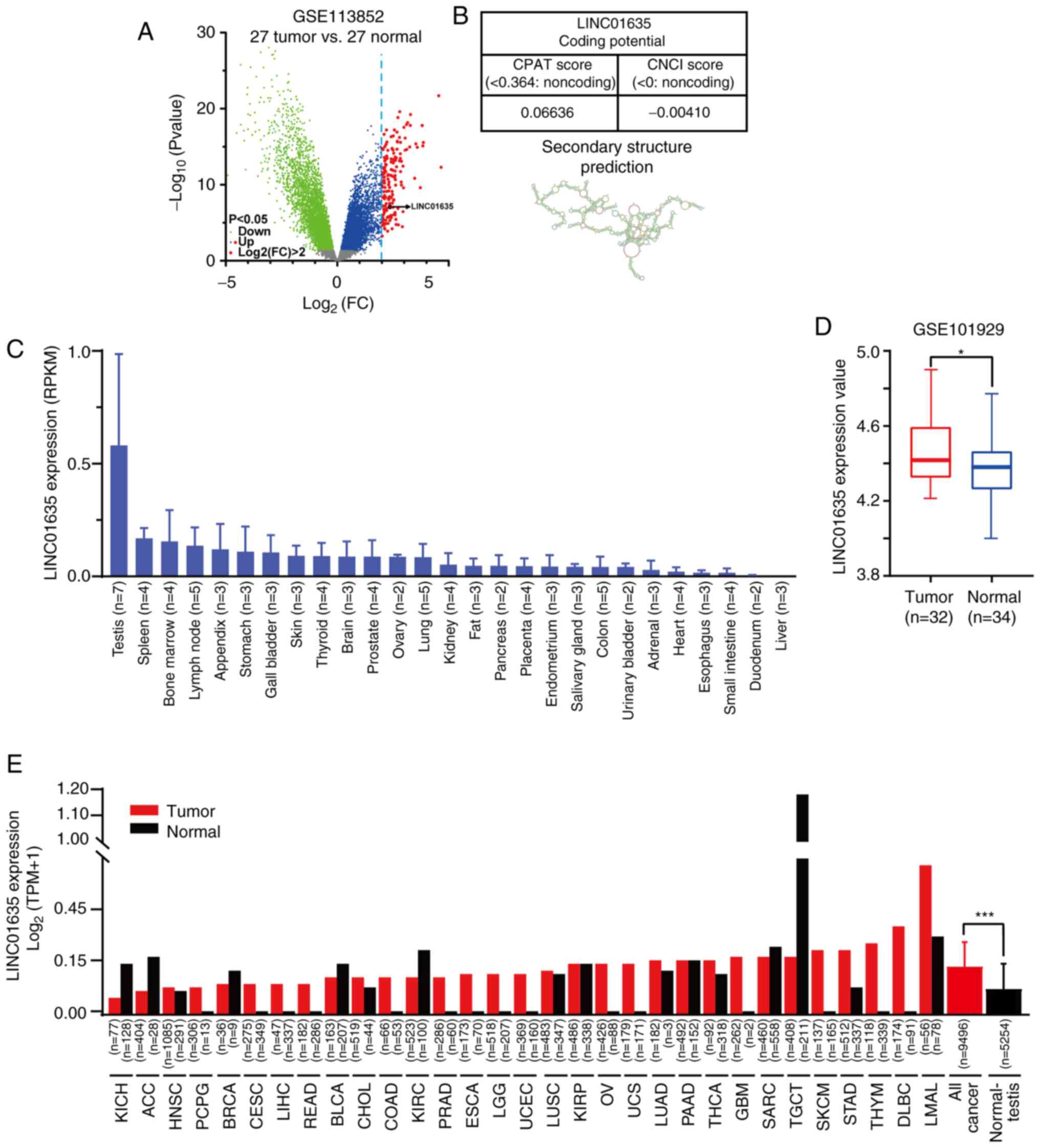 | Figure 1.LINC01635 is a long non-coding RNA
with a cancer/testis expression pattern. (A) The volcano map of
differentially expression genes of the GSE113852 dataset, which
contains the expression profiles of 27 lung adenocarcinoma and 27
normal lung tissue samples. The green dots represent downregulated
genes (P<0.05), the blue dots represent upregulated genes
(P<0.05) and the red dots represent upregulated genes
(P<0.05) for which the value of log2(FC) was >2. The log2(FC)
value of LINC01635 was 2.36, and the P-value was 8.58×10-8. (B)
Non-coding property prediction of LINC01635, including CPAT, CNCI
and Secondary Structure Prediction tools. (C) Expression in RPKM of
LINC01635 among the Human Protein Atlas RNA-seq normal tissue
datasets. (D) The overexpression of LINC01635 in lung cancer
tissues was confirmed by analyzing GSE101929 datasets using lnCAR
online tools. (E) Expression of LINC01635 in tumor and normal
tissue samples from GTEx and TCGA database. *P<0.05;
***P<0.001. LINC01635, long intergenic non-protein coding RNA
1635; CPAT, coding-potential assessment tool; CNCI,
coding-non-coding index; RPKM, reads per kilobase million; RNA-seq,
RNA-sequencing; lnCAR, lncRNAs from cancer arrays; TCGA, The Cancer
Genome Atlas; KICH, kidney chromophobe; ACC, adrenocortical
carcinoma; HNSC, head and neck squamous cell carcinoma; PCPG,
pheochromocytoma and paraganglioma; BRCA, breast invasive
carcinoma; CESC, cervical squamous cell carcinoma and endocervical
adenocarcinoma; LIHC, liver hepatocellular carcinoma; READ, rectal
adenocarcinoma; BLCA, bladder urothelial carcinoma; CHOL,
cholangiocarcinoma; COAD, colon adenocarcinoma; KIRC, kidney renal
clear cell carcinoma; PRAD, prostate adenocarcinoma; ESCA,
esophageal carcinoma; LGG, brain lower grade glioma; UCEC, uterine
corpus endometrial carcinoma; LUSC, lung squamous cell carcinoma;
KIRP, kidney renal papillary cell carcinoma; OV, ovarian serous
cystadenocarcinoma; UCS, uterine carcinosarcoma; LUAD, lung
adenocarcinoma; PAAD, pancreatic adenocarcinoma; THCA, thyroid
carcinoma; GBM, glioblastoma multiforme; SARC, sarcoma; TGCT,
testicular germ cell tumor; SKCM, skin cutaneous melanoma; STAD,
stomach adenocarcinoma; THYM, thymoma; DLBC, lymphoid neoplasm
diffuse large B-cell lymphoma; LAML, acute myeloid leukemia. |
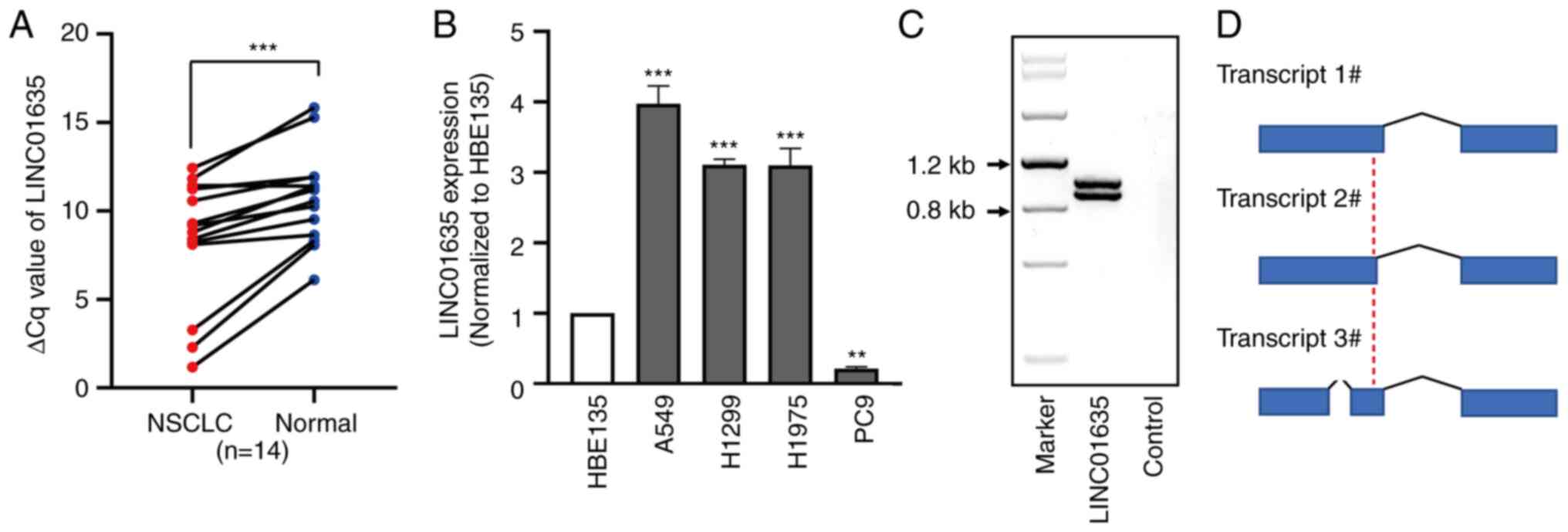
To validate the data from the bioinformatics
analysis, the expression of LINC01635 was firstly checked in
samples from human patients with NSCLC (Table II). All patients were diagnosed
with LAD NSCLC according to histopathological evaluation. No
radiotherapy or chemotherapy was performed before the surgery or
puncture. The ΔCq values
(CqLINC01636-CqGAPDH) in the lung cancer
tissues were lower than those in the normal lung tissues, which
showed that the expression level of LINC01635 was significantly
higher in the 14 NSCLC tissues than in the corresponding normal
lung tissues (P<0.05; Fig. 2A).
Next, the expression level of LINC01635 was examined in four human
lung cancer cell lines (A549, H1299, H1975 and PC9), and LINC01635
was found to be overexpressed in the A549, H1299 and H1975 cell
lines by 3.1- to 3.9-fold compared with that in human bronchial
epithelial HBE135 cell line, but was reduced to 21% in the PC9 cell
line compared with that in the HBE135 cell line (Fig. 2B). Next, according to the
prediction of full-length LINC01635 transcript sequences in the
Ensembl website, primers were designed to clone LINC01635 in the
A549 cell line and two bands of PCR products were found (Fig. 2C). Next, the PCR products were
cloned, and the sequencing results showed that LINC01635 in the
A549 cell line had three transcripts (Figs. 2D and S1). These results suggested that
LINC01635 could be a CT-lncRNA.
 | Table II.Characteristics of the patient tissue
samples. |
Table II.
Characteristics of the patient tissue
samples.
| Characteristic | Value |
|---|
| Mean age ± SD,
years | 69.00±7.71 |
| Sex, % (n/total
n) |
|
|
Male | 71.4 (10/14) |
|
Female | 28.6 (4/14) |
| Histological
classification, % (n/total n) |
|
|
Adenocarcinoma | 50.0 (7/14) |
|
Squamous | 50.0 (7/14) |
| TNM stage, %
(n/total n) |
|
| I | 28.6 (4/14) |
| II | 21.4 (3/14) |
|
III | 14.3 (2/14) |
| IV | 35.7 (5/14) |
| Lymph node
metastasis, % (n/total n) |
|
| N0 | 78.6 (11/14) |
| N1 | 21.4 (3/14) |
| N2 | 0.0 (0/14) |
| N3 | 0.0 (0/14) |
| Tumor location, %
(n/total n) |
|
|
Left | 42.9 (6/14) |
|
Right | 57.1 (8/14) |
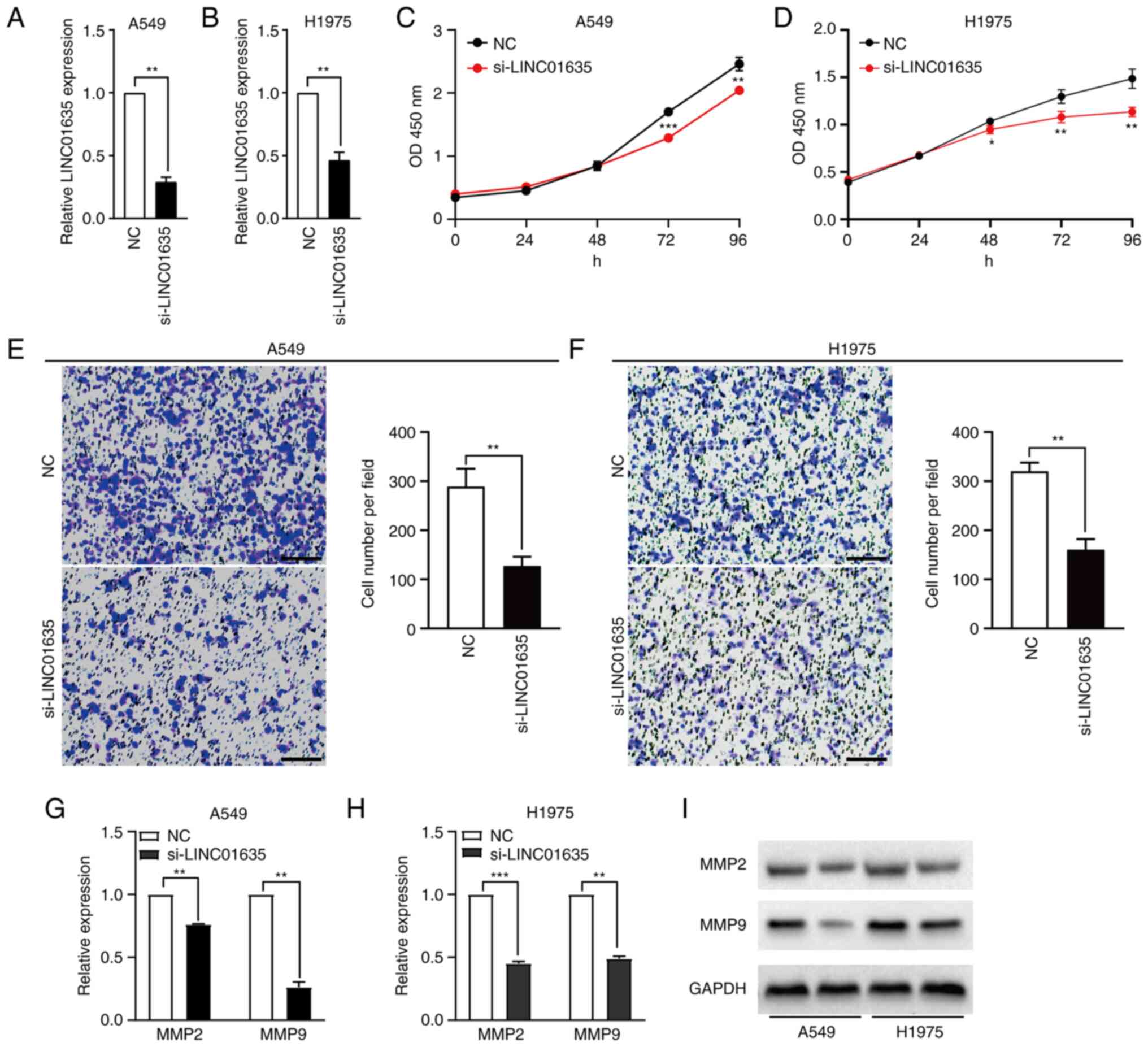
Silencing of LINC01635 suppresses the
proliferation and migration of lung cancer in cultured cells
To investigate the function of LINC01635 in NSCLC
cell lines, LINC01635 was knocked down in A549 and H1975 cell lines
by transfection with siRNA, which targeted the common sequence of
the three transcripts. After 24 h of transfection, the knockdown
efficiency of si-LINC01635 was 70.7% in the A549 cell line and
53.5% in the H1975 cell line, compared with transfection with NC
siRNA (Fig. 3A and B). It is worth
noting that the designed siRNA also targeted LINC00339, which was
partially overlapped but transcriptionally opposite to LINC01635.
The expression of LINC00339 was examined in the lung cell lines, as
well as the effect of the siRNA on LINC00339. LINC00339 was also
highly expressed in the A549 and H1975 cell lines, but the designed
siRNA could not efficiently downregulate the expression of
LINC00339 in either cell line (Fig.
S2). Next, the role of LINC01635 in the proliferation of NSCLC
cells was examined using CCK-8 assays. The results indicated that
knockdown of LINC01635 decreased the proliferation in the A549
after 72 h post-transfection and in H1975 cell lines after 48 h
post-transfection (Fig. 3C and D).
Furthermore, Transwell assays were performed and found that
knockdown of LINC01635 also significantly suppressed cell migration
in both the A549 and H1975 cell lines (Fig. 3E and F). The expression levels of
MMP2 and MMP9, migration-related markers, were also assessed and
were shown to be downregulated both at the transcriptional and
translational levels when LINC01635 was knocked down (Fig. 3G-I). These results demonstrated
that LINC01635 plays important roles in the proliferation and
migration of NSCLC cell lines.
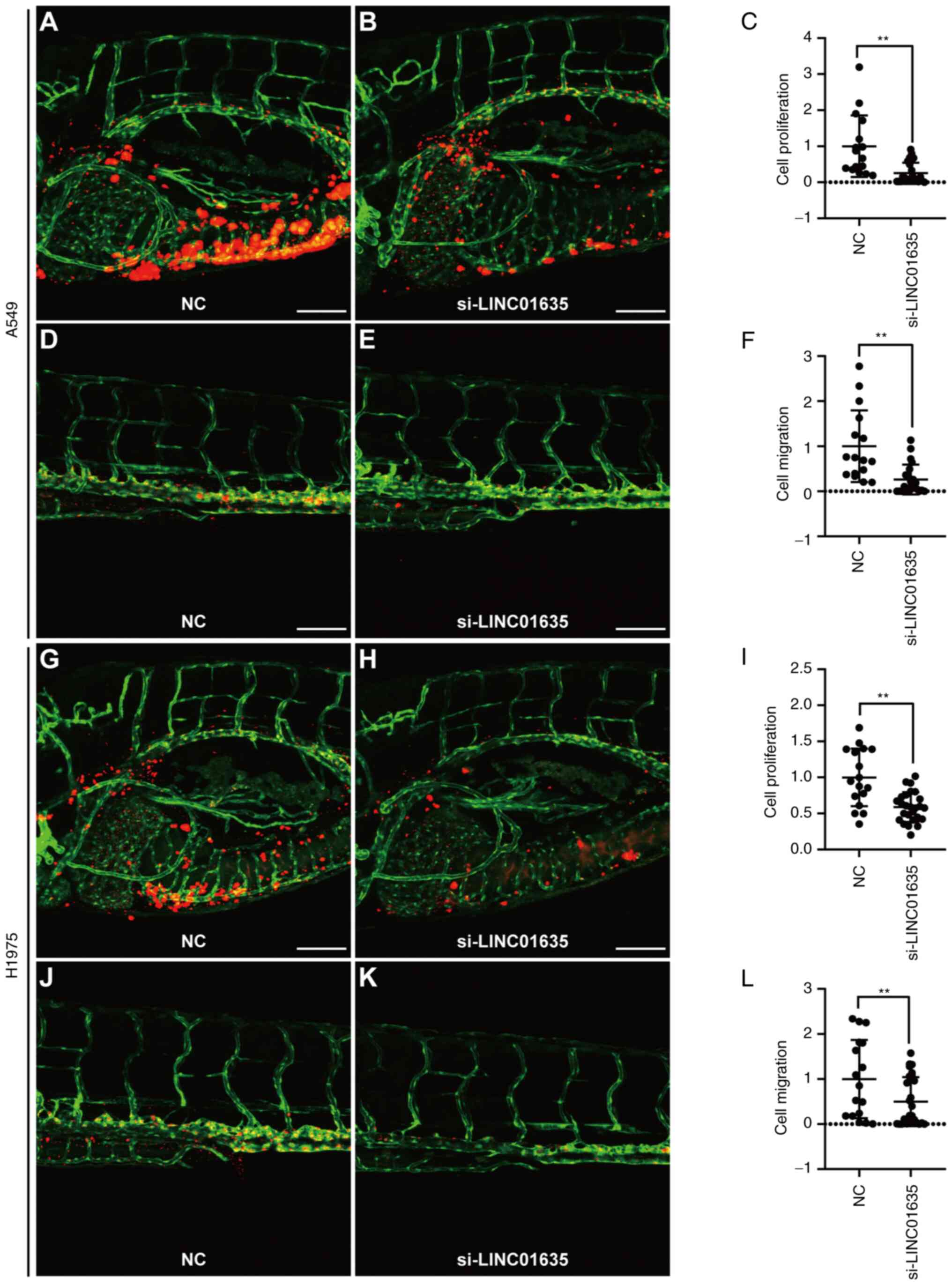
Silencing of LINC01635 suppresses the
proliferation and metastasis of lung cancer cells in zebrafish
xenograft models
To verify whether LINC01635 could regulate the
progression of lung cancer in vivo, zebrafish xenograft
models were used to examine proliferation and metastasis
simultaneously. The PVS of zebrafish larvae were implanted with
A549 or H1975 cells that were transfected with si-LINC01635 and
labeled by CM-DiI. At 1 dpi, the zebrafish larvae with a similar
tumor size at the PVS and no CM-DiI signal at other sites were
selected according to the CM-DiI-positive area (Fig. S3), and then cultured for further
analysis. At 4 dpi, the yolk and trunk of the injected zebrafish
larvae were imaged to assess the cell proliferation and metastasis,
respectively (27,28). Compared with that for NC siRNA
transfection, the CM-DiI-positive region was significantly smaller
both in the yolk and trunk when LINC01635 was silenced in A549
cells (Fig. 4A-F). Similar results
were also obtained in zebrafish xenograft using H1975 cells
(Fig. 4G-L). These results
demonstrated that LINC01635 regulates the proliferation and
metastasis of lung cancer in vivo.
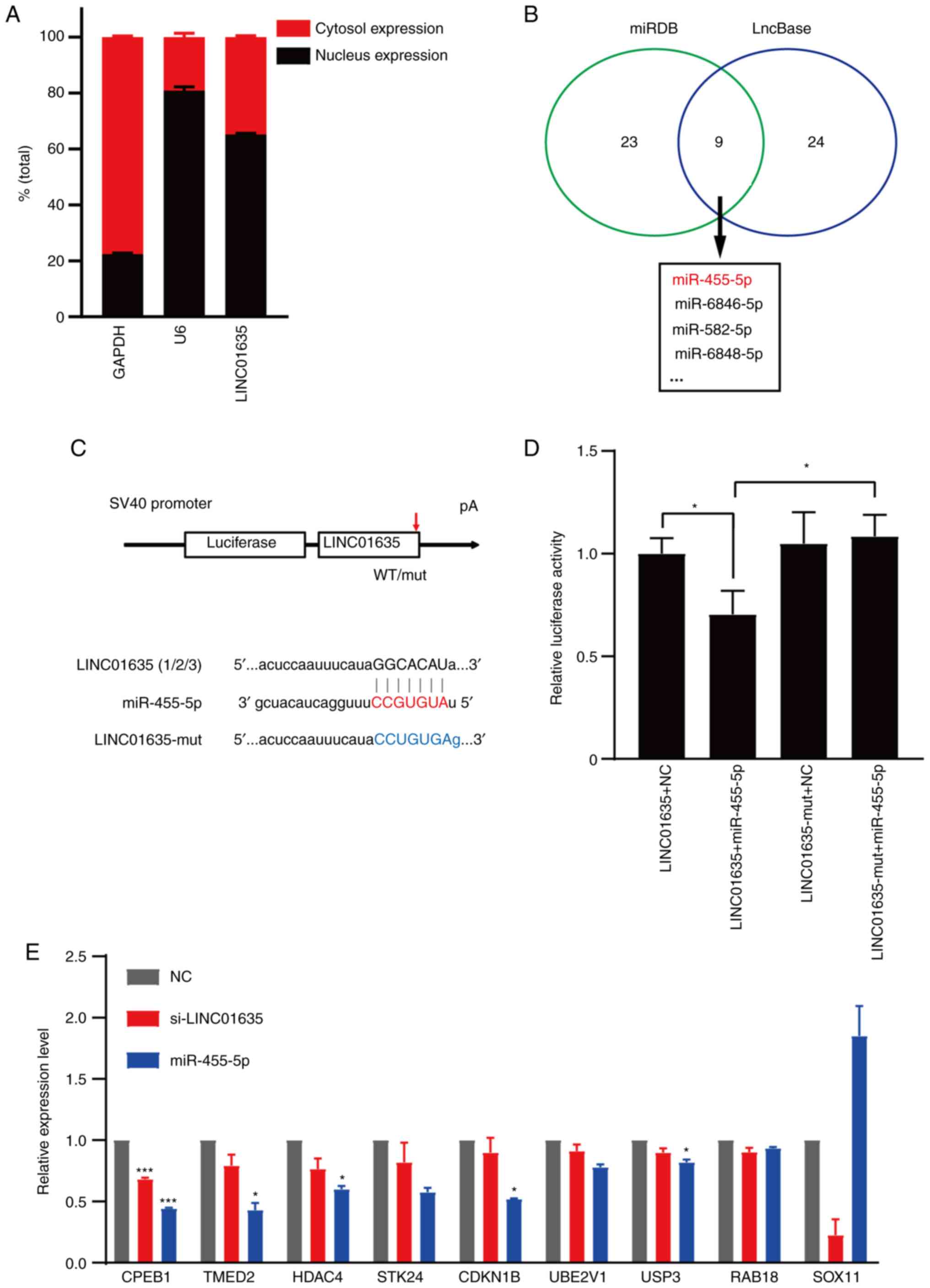
LINC01635 can bind with miR-455-5p and
regulates the expression of miR-455-5p-targeting tumor-related
genes
To explore the functional mechanism of LINC01635,
the subcellular location of LINC01635 was first studied and found
to be both in the nucleus and cytoplasm (Fig. 5A). As LINC01635 is located both in
the nucleus and cytoplasm of lung cancer cells, it might regulate
the downstream genes at the transcriptional and/or
post-transcriptional levels. To examine whether LINC01635 could
function at the post-transcriptional level by binding miRNAs, its
miRNA binging sites were predicted by miRDB (http://mirdb.org/) (29) and LncBase (30), with 9 microRNA binding sites
predicted by both softwares in total. Among these miRNAs,
miR-455-5p could bind with all of the transcripts of LINC01635 with
highest potential (rank 1; Fig.
5B) (31). To confirm the
binding possibility between LINC01635 and miR-455-5p,
dual-luciferase reporter plasmids were constructed that contained
the wild-type or mutant binding site of LINC01635 (Fig. 5C). The dual-luciferase assay
revealed that overexpression of miR-455-5p reduced the luciferase
activity of the reporter plasmid containing the LINC01635 sequence,
but not that of the reporter plasmid containing the LINC01635
mutant sequence (Fig. 5D). To
confirm whether the LINC01635 could regulate a series of
tumor-related genes through miR-455-5p, several
miR-455-5p-targeting genes (CPEB1, TMED2, HDAC4, STK24, CDKN1B,
UBE2V1, USP3, RAB18 and SOX11) that are involved in multiple cancer
types were examined (32–40). The majority of these genes were
downregulated when knocking down LINC01635 in lung cancer cells,
and similar regulation was also observed when overexpressing
miR-455-5p (Fig. 5E). These
results imply that LINC01635 could regulate the expression of
tumor-related genes by targeting miR-455-5p.
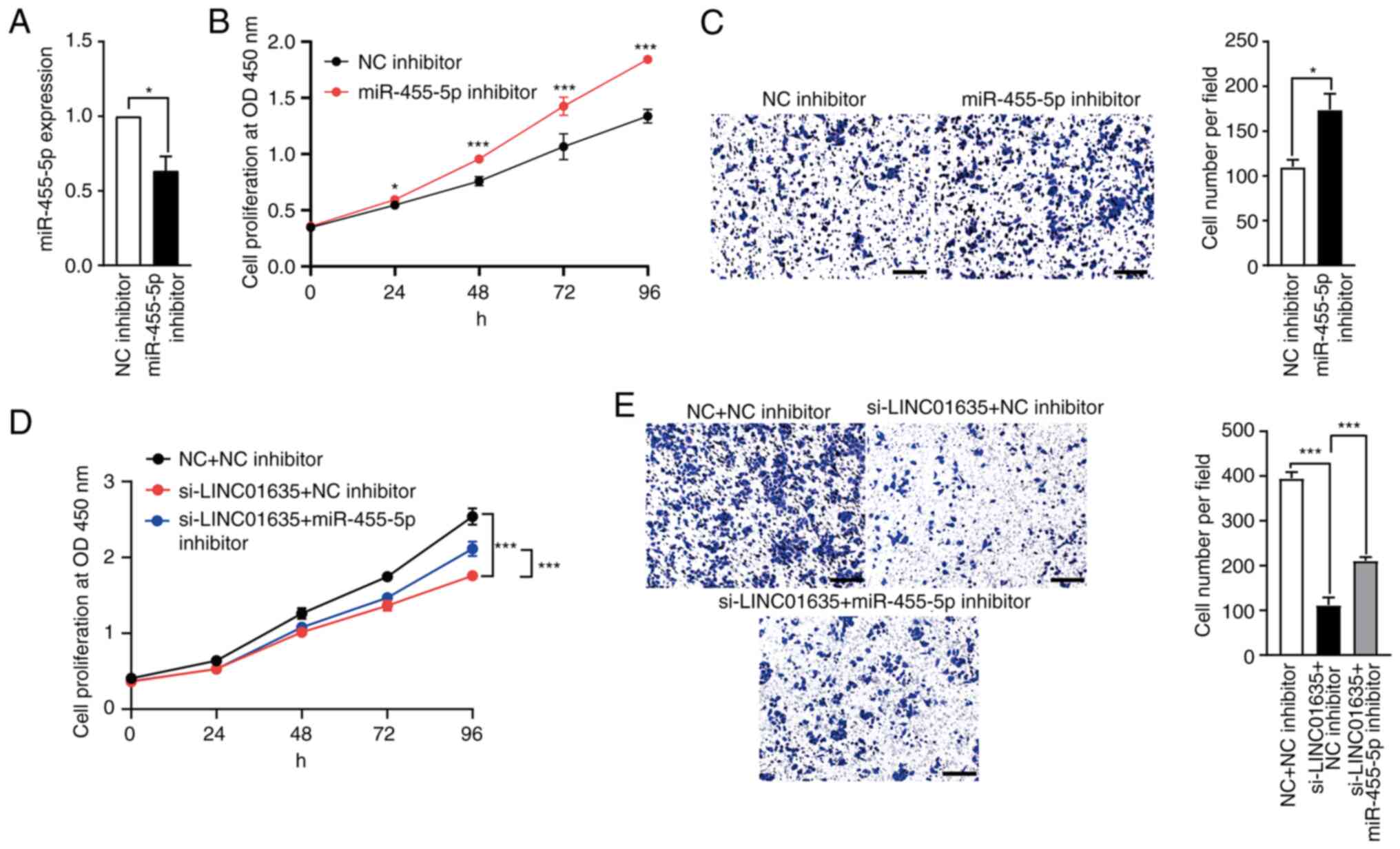
Knockdown of miR-455-5p partially
rescues the proliferation and migration of lung cancer cells, which
is suppressed by LINC01635 silencing
To study the roles of miR-455-5p in lung cancer
cells, the expression of miR-455-5p was first downregulated by
transfection with the miR-455-5p inhibitor (Fig. 6A). After transfection, the results
of CCK-8 and Transwell assays showed that miR-455-5p inhibition
promoted the proliferation and migration of the A549 cells
(Fig. 6B and C). To examine
whether miR-455-5p mediates the roles of LINC01635 in the
progression of lung cancer cells, si-LINC01635 and miR-455-5p
inhibitor were cotransfected into A549 cells simultaneously. The
knockdown of LINC01635 suppressed the growth and migration of lung
cancer cells, but miR-455-5p inhibitor transfection partially
counteracted the suppressive effects of LINC01635 knockdown both in
terms of the proliferation and migration of the lung cancer cells
(Fig. 6D and E). These results
indicate that LINC01635 could promote the progression of lung
cancer cells via the miR-455-5p-mediated pathway.
Discussion
An increasing number of studies have reported that
lncRNAs play important roles in the development and progression of
NSCLC through different signal pathways (13,41–43).
The present study found that LINC01635 was upregulated in lung
cancer tissues and cell lines. Functional experiments showed that
LINC01635 promoted the proliferation and metastasis of NSCLC cells
in vitro and in vivo. Moreover, LINC01635 was able to
bind miR-455-5p and regulated the expression of a series of
miR-455-5p-targeting tumor-related genes. These findings suggest
that LINC01635 could regulate NSCLC progression though binding with
miR-455-5p.
CT genes are a diverse group of genes that are
restrictively expressed in the testis under normal conditions, but
they are also expressed in ~40% of different types of cancer
(44). Similar to tumors, the
testes exhibit abundant cell division, migration and
immortalization (45), and lots of
testicular genes are considered as promising cancer biomarkers and
treatment targets (14). Moreover,
the similarities in cellular processes between gametogenesis and
tumorigenesis also provide valuable insights into understanding the
mechanism of tumorigenesis. CT-lncRNA represents a new direction
for the study of lncRNA mechanisms in tumor biology. For example,
Hosono et al (16)
demonstrated that THOR is a conserved CT-lncRNA and that it
promotes the progression of lung cancer through interaction with
IGF2BP1 (16). Tan et al
(19) showed that CT-lncRNA PACT6
facilitates the malignant phenotype of ovarian cancer through
binding with miR-143-3p (19). In
the present study, by screening the GEO database of lung cancer
tissues and the HPA RNA-seq normal tissue database, LINC01635 was
revealed to be a novel CT-lncRNA that promotes lung cancer
progression by binding with miR-455-5p. However, in the present
study, mainly the basic cellular functions of LINC01635 in lung
cancer cells were assessed through the examination of the migration
and invasion biomarkers MMP2 and MMP9 in cultured cell lines, and
the detailed molecular mechanism of LINC01635 shall be analyzed by
examining different biomarkers in future studies.
It has been reported that lncRNAs play important
roles in proliferation, differentiation, metastasis, metabolism and
apoptosis in cancer progression by regulating their target genes at
the transcriptional, post-transcriptional and epigenetic levels
(7,8). Different regulatory functions depend
on the subcellular locations and interactions with specific
molecules (46). In the nucleus,
lncRNAs generally function by modulating transcriptional programs
through chromatin interactions and remodeling by formatting the
scaffolding complex (47,48). In the cytoplasm, lncRNAs function
by regulating translational and/or post-transcriptional programs to
affect gene expression levels. In addition, numerous lncRNAs have
been identified as competing endogenous RNAs (ceRNAs) that can
regulate the expression of target genes at the post-transcriptional
level by competitive binding with miRNAs in the cytoplasm (49). The present study found LINC01635
located in both the nucleus and cytoplasm, which implies that
LINC01635 may play regulatory roles at both the transcriptional and
post-transcriptional levels. To examine whether LINC01635 could
function as a ceRNA, the binding sites of miRNAs in LINC01635 were
predicted by cross-comparison analysis between the miRDB and
LncBase database, and miR-455-5p was screened out with the highest
potential. Furthermore, the present data not only demonstrated that
miR-455-5p could bind with LINC01635 directly in vitro, but
also showed that miR-455-5p inhibition could partially rescue the
suppression effects caused by LINC01635 knockdown, which implies
that LINC01635/miR-455-5p may function as a ceRNA network.
The present study also examined the expression of a
series of miR-455-5p-targeting tumor-related genes following the
knockdown of LINC01635 or the overexpression of miR-455-5p, and
found that the changes in the expression of some genes was
associated. These genes may regulate tumor progression through
different pathways. CDKN1B can shift from cyclin-dependent kinase
inhibitor to oncogene by regulating the cell cycle in a
cyclin-dependent kinase-dependent or -independent manner (36,50).
SOX11, a transcription factor, mainly regulates progenitor and stem
cell behavior during embryogenesis, and it also expressed in a wide
variety of cancer types, such as neck cancer, malignant glioma,
ovarian cancer and breast cancer (40,51).
RAB18 is a member of the Ras oncogene superfamily, which promotes
cell invasion and inhibits cell apoptosis in various cancer types,
such as hepatocellular carcinoma and gastric cancer (39,52).
UBE2V1 is a member of the ubiquitin-conjugating E2 enzyme variant
proteins, and it has been reported as an oncogene that acts via
ubiquitination and degradation of SIRT1 (37). USP3 is a deubiquitinase that
accelerates tumor proliferation and epithelial-to-mesenchymal
transition (EMT) via deubiquitinating KLF5 (38). According to the association in
expression between LINC01635 and these genes, our future studies
shall focus on CPEB1, TMED2 and HDAC4, which might reveal the novel
mechanism of LINC01635/miR-455-5p regulatory pathways.
Zebrafish xenografts have been demonstrated as
effective models for tumor research (25,26).
Compared with mouse models, zebrafish xenografts have obvious
advantages. First, the zebrafish xenograft model offers a fast
in vivo evaluation method of tumor proliferation and
metastasis by using same group of transplanted zebrafish larvae.
When using mouse xenograft models, it requires two separate models
for evaluating the proliferation and metastasis of the tumor cells,
respectively. Second, with the help of transparent larvae, the
zebrafish xenograft model supplies intuitive studies at the
cellular level. The zebrafish xenograft model can be used to assess
proliferation and metastasis in only 1 week by transiently
transfecting siRNAs, instead of 2–4 weeks in mouse xenograft
models, which have to construct stable cell lines using shRNA
plasmids. Third, by combining different types of transgenic lines
that label different cell types, zebrafish xenograft can be used
for studying the tumor microenvironment in vivo, including
angiogenesis and immune reactions. In mouse xenograft models, it
usually requires additional staining steps for the quantification
in vitro. The results of the zebrafish xenograft model
experiments in the present study showed that the silencing of
LINC01635 decreased the proliferation and metastasis of the NSCLC
cells, which was consistent with the data from the cultured cells,
suggesting that a zebrafish xenograft is a good alternative in
vivo model for examining tumor biology.
In summary, the present study demonstrated LINC01635
is a novel CT-lncRNA and that it promotes the proliferation and
metastasis of lung cancer by regulating miR-455-5p-targeting
tumor-related genes. These findings indicate that LINC01635 could
be a potential biomarker and treatment target for lung cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Research Fund of Lianshui
County People's Hospital (2020), and The Program of Innovation and
Entrepreneurship Doctor of Jiangsu Province (2021).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. Microarray datasets GSE113852 (20) and GSE101929 (21) were downloaded from the Gene
Expression Omnibus database.
Authors' contributions
YZ conceived and designed the study. WS, JP, SG, JS,
LW, BT and JC acquired the data. JS, LW, BT and JC collected the
patient samples. SG, JS and LW created the zebrafish xenograft
model. BT and JC performed the reverse transcription-quantitative
PCR experiments. WS and JP performed the rest of the experiments,
and analyzed and interpreted the data. SG performed the statistical
analysis and wrote the manuscript. WS, JP and YZ revised the
manuscript. WS, JP, SG, JS, LW, BT, JC and YZ confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of The Lianshui County
People's Hospital, Kangda College of Nanjing Medical University
(Huai'an, China). All patients enrolled in this study were informed
of the study details and provided written informed consent. The use
of the clinical samples was approved by The Medical Ethics
Committee of Lianshui County People's Hospital, Kangda College of
Nanjing Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bade BC and Dela Cruz CS: Lung cancer
2020: Epidemiology, etiology, and prevention. Clin Chest Med.
41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Venkatesan P: IASLC 2020 world conference
on lung cancer. Lancet Respir Med. 8:e762020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carninci P, Kasukawa T, Katayama S, Gough
J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al:
The transcriptional landscape of the mammalian genome. Science.
309:1559–1563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagano T and Fraser P: No-nonsense
functions for long noncoding RNAs. Cell. 145:178–181. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanchez Calle A, Kawamura Y, Yamamoto Y,
Takeshita F and Ochiya T: Emerging roles of long non-coding RNA in
cancer. Cancer Sci. 109:2093–2100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Chen Z, An L, Wang Y, Zhang Z, Guo
Y and Liu C: Analysis of long non-coding RNA expression profiles in
non-small cell lung cancer. Cell Physiol Biochem. 38:2389–2400.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu M, Feng D, Zhang H, Xia W, Xu Y, Wang
J, Dong G, Zhang Y, Yin R and Xu L: Comprehensive analysis of
lncRNA expression profiles and identification of functional lncRNAs
in lung adenocarcinoma. Oncotarget. 7:16012–16022. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen J, Wang R, Zhang K and Chen LB: Long
non-coding RNAs in non-small cell lung cancer as biomarkers and
therapeutic targets. J Cell Mol Med. 18:2425–2436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu YJ, Du Y and Fan Y: Long noncoding RNAs
in lung cancer: What we know in 2015. Clin Transl Oncol.
18:660–665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Z, Lei T, Chen X, Gu J, Huang J, Lu B
and Wang Z: Long non-coding RNA in lung cancer. Clin Chim Acta.
504:190–200. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Whitehurst AW: Cause and consequence of
cancer/testis antigen activation in cancer. Annu Rev Pharmacol
Toxicol. 54:251–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Babatunde KA, Najafi A, Salehipour P,
Modarressi MH and Mobasheri MB: Cancer/testis genes in relation to
sperm biology and function. Iran J Basic Med Sci. 20:967–974.
2017.PubMed/NCBI
|
|
16
|
Hosono Y, Niknafs YS, Prensner JR, Iyer
MK, Dhanasekaran SM, Mehra R, Pitchiaya S, Tien J, Escara-Wilke J,
Poliakov A, et al: Oncogenic role of THOR, a conserved
cancer/testis long non-coding RNA. Cell. 171:1559–1572. e202017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin N, Wang C, Lu Q, Ma Z, Dai J, Ma H,
Jin G, Shen H and Hu Z: Systematic identification of long
non-coding RNAs with cancer-testis expression patterns in 14 cancer
types. Oncotarget. 8:94769–94779. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen S, Chen Y, Qian Q, Wang X, Chang Y,
Ju S, Xu Y, Zhang C, Qin N, Ding H, et al: Gene amplification
derived a cancer-testis long noncoding RNA PCAT6 regulates cell
proliferation and migration in hepatocellular carcinoma. Cancer
Med. 8:3017–3025. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan X, Shao Y, Teng Y, Liu S, Li W, Xue L,
Cao Y, Sun C, Zhang J, Han J, et al: The cancer-testis long
non-coding RNA PCAT6 facilitates the malignant phenotype of ovarian
cancer by sponging miR-143-3p. Front Cell Dev Biol. 9:5936772021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roth A, Boulay K, Groß M,
Polycarpou-Schwarz M, Mallette FA, Regnier M, Bida O, Ginsberg D,
Warth A, Schnabel PA, et al: Targeting LINC00673 expression
triggers cellular senescence in lung cancer. RNA Biol.
15:1499–1511. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mitchell KA, Zingone A, Toulabi L,
Boeckelman J and Ryan BM: Comparative transcriptome profiling
reveals coding and noncoding RNA differences in NSCLC from African
Americans and European Americans. Clin Cancer Res. 23:7412–7425.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng Y, Xu Q, Liu M, Hu H, Xie Y, Zuo Z
and Ren J: lnCAR: A comprehensive resource for lncRNAs from cancer
arrays. Cancer Res. 79:2076–2083. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Czimmerer Z, Hulvely J, Simandi Z,
Varallyay E, Havelda Z, Szabo E, Varga A, Dezso B, Balogh M,
Horvath A, et al: A versatile method to design stem-loop
primer-based quantitative PCR assays for detecting small regulatory
RNA molecules. PLoS One. 8:e551682013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu R, Fan C, Li H, Zhang Q and Fu YF:
Evaluation of putative reference genes for gene expression
normalization in soybean by quantitative real-time RT-PCR. BMC Mol
Biol. 10:932009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lawson ND and Weinstein BM: In vivo
imaging of embryonic vascular development using transgenic
zebrafish. Dev Biol. 248:307–318. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fior R, Póvoa V, Mendes RV, Carvalho T,
Gomes A, Figueiredo N and Ferreira MG: Single-cell functional and
chemosensitive profiling of combinatorial colorectal therapy in
zebrafish xenografts. Proc Natl Acad Sci USA. 114:E8234–E8243.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hason M and Bartůněk P: Zebrafish models
of cancer-new insights on modeling human cancer in a non-mammalian
vertebrate. Genes (Basel). 10:9352019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y and Wang X: miRDB: An online
database for prediction of functional microRNA targets. Nucleic
Acids Res. 48:D127–D131. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paraskevopoulou MD, Vlachos IS, Karagkouni
D, Georgakilas G, Kanellos I, Vergoulis T, Zagganas K, Tsanakas P,
Floros E, Dalamagas T, et al: DIANA-LncBase v2: Indexing microRNA
targets on non-coding transcripts. Nucleic Acids Res. 44:D231–D238.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xing Q, Xie H, Zhu B, Sun Z and Huang Y:
MiR-455-5p suppresses the progression of prostate cancer by
targeting CCR5. Biomed Res Int. 2019:63947842019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu M, Fang S, Song J, Chen M, Zhang Q,
Weng Q, Fan X, Chen W, Wu X, Wu F, et al: CPEB1 mediates
hepatocellular carcinoma cancer stemness and chemoresistance. Cell
Death Dis. 9:9572018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi-Peng G, Chun-Lin C, Huan W, Fan-Liang
M, Yong-Ning C, Ya-Di Z, Guang-Ping Z and Ye-Ping C: TMED2 promotes
epithelial ovarian cancer growth. Oncotarget. 8:94151–94165. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin K, Zhao W, Xie X, Pan Y, Wang K and
Zhang H: MiR-520b restrains cell growth by targeting HDAC4 in lung
cancer. Thorac Cancer. 9:1249–1254. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang N, Lin W, Shi X and Tao T: STK24
expression is modulated by DNA copy number/methylation in lung
adenocarcinoma and predicts poor survival. Future Oncol.
14:2253–2263. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Belletti B and Baldassarre G: Roles of
CDKN1B in cancer? Aging (Albany NY). 7:529–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen T, Cai LD, Liu YH, Li S, Gan WJ, Li
XM, Wang JR, Guo PD, Zhou Q, Lu XX, et al: Ube2v1-mediated
ubiquitination and degradation of Sirt1 promotes metastasis of
colorectal cancer by epigenetically suppressing autophagy. J
Hematol Oncol. 11:952018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu Y, Qin J, Li F, Yang C, Li Z, Zhou Z,
Zhang H, Li Y, Wang X, Liu R, et al: USP3 promotes breast cancer
cell proliferation by deubiquitinating KLF5. J Biol Chem.
294:17837–17847. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gong T, Zhou B, Liu M, Chen X, Huang S, Xu
Y, Luo R and Chen Z: RAB18 promotes proliferation and metastasis in
hepatocellular carcinoma. Am J Transl Res. 11:1009–1019.
2019.PubMed/NCBI
|
|
40
|
Huang J, Ji EH, Zhao X, Cui L, Misuno K,
Guo M, Huang Z, Chen X and Hu S: Sox11 promotes head and neck
cancer progression via the regulation of SDCCAG8. J Exp Clin Cancer
Res. 38:1382019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu XH, Liu ZL, Sun M, Liu J, Wang ZX and
De W: The long non-coding RNA HOTAIR indicates a poor prognosis and
promotes metastasis in non-small cell lung cancer. BMC Cancer.
13:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang Y, Xiao G, Chen Y and Deng Y: LncRNA
MALAT1 promotes migration and invasion of non-small-cell lung
cancer by targeting miR-206 and activating Akt/mTOR signaling.
Anticancer Drugs. 29:725–735. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nie FQ, Sun M, Yang JS, Xie M, Xu TP, Xia
R, Liu YW, Liu XH, Zhang EB, Lu KH and Shu YQ: Long noncoding RNA
ANRIL promotes non-small cell lung cancer cell proliferation and
inhibits apoptosis by silencing KLF2 and P21 expression. Mol Cancer
Ther. 14:268–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Scanlan MJ, Simpson AJ and Old LJ: The
cancer/testis genes: Review, standardization, and commentary.
Cancer Immun. 4:12004.PubMed/NCBI
|
|
45
|
Gordeeva O: Cancer-testis antigens: Unique
cancer stem cell biomarkers and targets for cancer therapy. Semin
Cancer Biol. 53:75–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bridges MC, Daulagala AC and Kourtidis A:
LNCcation: lncRNA localization and function. J Cell Biol.
220:e2020090452021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Melé M and Rinn JL: ‘Cat's Cradling’ the
3D genome by the Act of LncRNA transcription. Mol Cell. 62:657–664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Clemson CM, Hutchinson JN, Sara SA,
Ensminger AW, Fox AH, Chess A and Lawrence JB: An architectural
role for a nuclear noncoding RNA: NEAT1 RNA is essential for the
structure of paraspeckles. Mol Cell. 33:717–726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Razavipour SF, Harikumar KB and
Slingerland JM: p27 as a transcriptional regulator: New roles in
development and cancer. Cancer Res. 80:3451–3458. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tsang SM, Oliemuller E and Howard BA:
Regulatory roles for SOX11 in development, stem cells and cancer.
Semin Cancer Biol. 67:3–11. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu J, Zhang J, Li Y, Wang L, Sui B and
Dai D: MiR-455-5p acts as a novel tumor suppressor in gastric
cancer by down-regulating RAB18. Gene. 592:308–315. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lim W, Ridge CA, Nicholson AG and
Mirsadraee S: The 8 th lung cancer TNM classification and clinical
staging system: review of the changes and clinical implications.
Quant Imaging Med Surg. 8:709–718. 2018. View Article : Google Scholar : PubMed/NCBI
|