Introduction
Mesothelioma is a tumor with a poor prognosis that
occurs mostly from mesothelial cells in the pleura or peritoneum
(1). Mesothelioma is associated
with exposure to asbestos and has a poor prognosis; the median
survival is 9–12 months and the 5-year survival rate is 5%
(1,2). Mesothelioma is classified into three
morphologic subtypes, namely epithelioid, biphasic, and
sarcomatoid; the latter two subtypes have even shorter survival
times (1,3). Clinically, there are poor or
non-specific symptoms, and the latent period from asbestos exposure
to onset is long. Therefore, in many cases, mesothelioma tends to
be diagnosed at a later stage of the disease (1–3).
Recently, there have been reports that mesothelioma has an early
phase, known as mesothelioma in situ (MIS) (1–4). MIS
is defined as a single layer of atypical mesothelial cells
proliferating along the pleural surface (2,4); it
may be cured with appropriate therapies (1–3).
However, it is difficult to distinguish MIS from reactive surface
mesothelial proliferation based on routine morphology (5). Thus, the 2021 World Health
Organization (WHO) classification of tumors of the pleura included
the following criteria of MIS (4):
1) pleural effusion (non-resolving), 2) no thoracoscopic or imaging
evidence of tumor, 3) a single layer of mesothelial cells (with or
without atypia) on the pleural surface, 4) no histological features
of invasive growth, 5) loss of BRCA-1 associated protein-1 (BAP-1)
and/or methylthioadenosine phosphorylase (MTAP) based on
immunohistochemistry (IHC) and/or cyclin-dependent kinase inhibitor
2A/p16 (CDKN2A/p16) homozygous deletion based on fluorescence in
situ hybridization (FISH), and 6) multidisciplinary discussion
of the diagnosis.
There have been some cases of MIS published. Due to
the difficulty of detecting MIS, for many of these cases, the
diagnosis was made retrospectively, using previously collected
specimens, after the patient had progressed to mesothelioma. Here,
we present a case of MIS that was diagnosed at the initial
presentation based on cytology of pleural effusion. As far as we
know, this is the first report of MIS with overtly malignant
mesothelial cells in the first pleural effusion cytology.
Case report
The patient was a 74-year-old man, an ex-smoker. He
had been a mason from 23 to 60 years old of age, with exposure to
particles of cement containing asbestos and hexavalent chromium
without a dust respirator. He had no remarkable past medical
history. Until 2015, there had been no abnormality in his medical
checkups; however, a year later he went to a local hospital with a
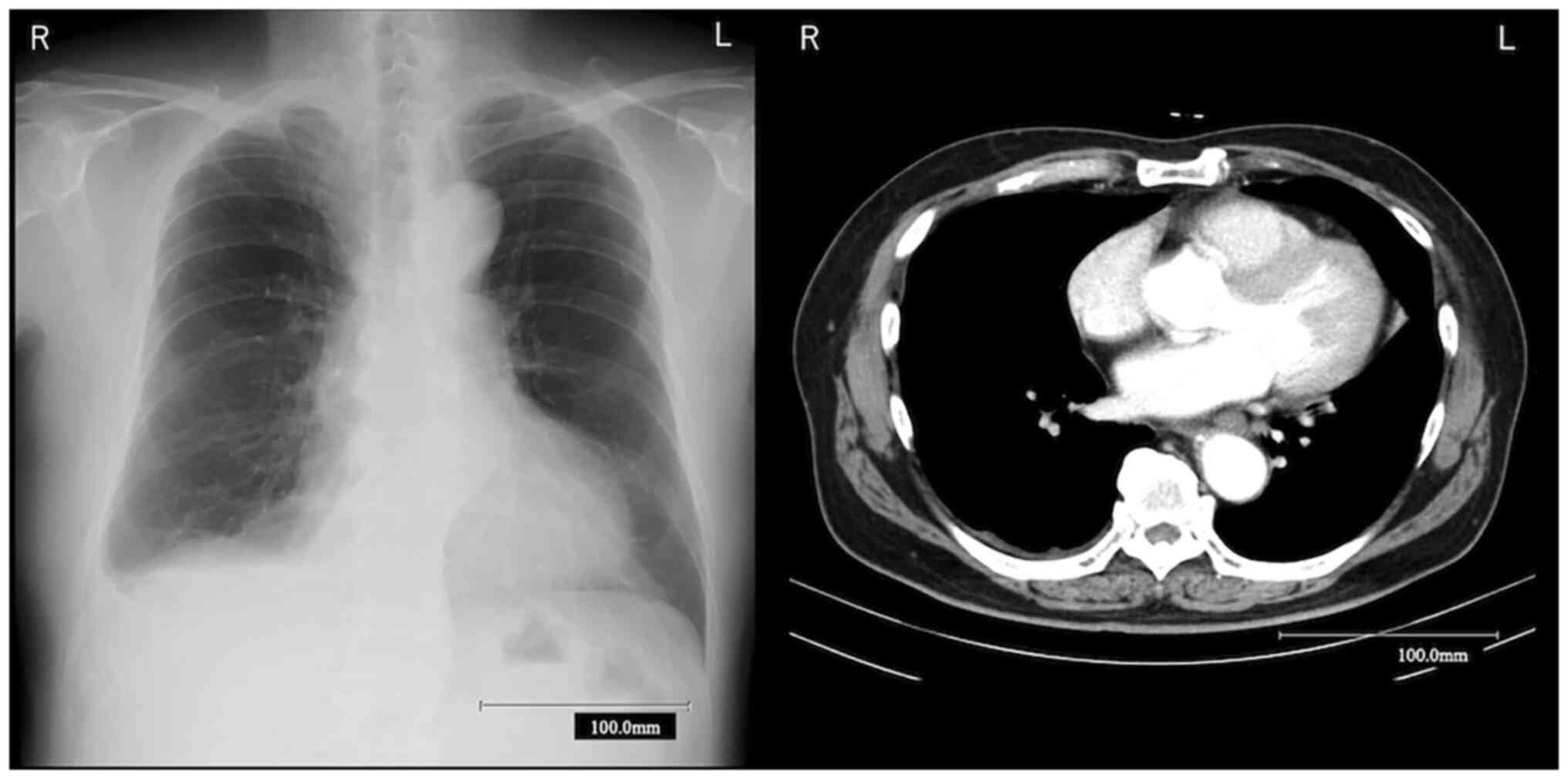
complaint of dyspnea. Because chest X-ray (CXR) showed right
pleural effusion (Fig. 1), he was
referred and admitted to Ibaraki Higashi National Hospital
(Tokai-mura, Japan). On presentation, there were no abnormal
physical findings. Blood examinations revealed normal laboratory
data and negative serum tumor markers. Chest contrast-enhanced
computed tomography (CT) presented only minimal right pleural
effusion (Fig. 1). Right pleural
effusion revealed by thoracentesis was exudative based on Light's
criteria, and the value of hyaluronic acid was normal. On the other
hand, cytology of pleural effusion was classified as class V
(overtly malignant) according to the Papanicolaou classification.
Note that the Papanicolaou smears had been borrowed from a previous
hospital and have already been returned, so we could not show the
image here. Immunohisto/immunocytochemical staining was performed
on 4-µm-thick sections mounted on glass slides. Endogenous
peroxidase activity was then blocked for 5 min at room temperature
using blocking reagents, and epitopes were activated by protease at
37°C or Tris-ethylenediaminetetraacetic acid (EDTA) buffer (pH 8.5)
at 95°C for different times for each antibody and incubated with
MTAP clone 2G4 (Abnova) (EDTA, 64 min), BAP-1 clone C-4 (Nichirei)
(EDTA, 32 min), sialylated protein HEG homolog 1 (HEG-1) Clone
SKM9-2 (Nichirei) (EDTA, 64 min), thyroid transcription factor-1
(TTF1) clone SP141 (Roche Diagnostics) (EDTA, 64 min), podoplanin
(D2-40) clone D2-40 (Roche Diagnostics) (EDTA, 64 min), epithelial
membrane antigen (EMA) Clone E29 (Agilent Technologies Japan)
(EDTA, 64 min), Desmin Clone D33 (Agilent Technologies) (EDTA, 36
min), carcinoembryonic antigen (CEA) Clone COL-1 (Nichirei) (EDTA,
36 min), p53 Clone DO7 (Roche Diagnostics) (EDTA, 32 min),
Calretinin clone SP65 (Roche Diagnostics) (EDTA, 64 min) and
epithelial specific antigen (Ber-EP4) Clone Ber-EP4 (Protease, 4
min). OptiView DAB IHC Detection Kit (Roche Diagnostics) or
ultraView Universal DAB Detection Kit (Roche Diagnostics) were used
according to the manufacturer's recommendations for the
visualization of each primary antibodies. In a cell block made
using the pleural effusion at our hospital, staining hematoxylin
and eosin (H&E), the mesothelioma cells had nuclear
enlargement, irregular nuclear membranes, frequent binucleation or
multinucleation, humps, and cellular pleomorphism. These features
indicated overtly malignant mesothelial cells (Fig. 2A and B). Based on IHC, the overtly
malignant mesothelial cells were positive for three markers, namely
D2-40 in the cytoplasmic membrane (Fig. 2C), calretinin in cytoplasm and
nucleus (Fig. 2D), and EMA in the
cytoplasm and membrane (Fig. 2E),
while they were negative for TTF-1, CEA, and desmin (Fig. 2F). Furthermore, although CDKN2A/p16
homozygous deletion was not confirmed by FISH, there was loss of
BAP-1 based on IHC (Fig. 2G). A
right pleural biopsy was performed for precise diagnosis. The
surgical findings did not show an obvious nodule in the thoracic
cavity and no thickening of the pleura. The sample was taken from
all layers of the right dorsal parietal pleura. The pathological
findings included mild cellular atypia with proliferation of mildly
atypical cuboidal or columnar cells derived from mesothelial cells
that formed a single layer in places (Fig. 3A and B). IHC for atypical cells was
positive for D2-40, calretinin, and EMA, and negative for desmin,
TTF-1, CEA, and p53. There findings were consistent with the
pleural effusion cytology. Furthermore, loss of BAP-1 was confirmed
(Fig. 3C), while MTAP was retained
with IHC and CKDN2A/p16 homozygous deletion was not identified with
FISH (Fig. 3D and E). Due to mild
cellular atypia, MIS rather than mesothelioma was suspected at the
time. The case retrospectively met the 2021 WHO criteria of MIS.
Unfortunately, the patient did not agree to undergo an operation
and quit attending his medical check-ups 4 months after the MIS
diagnosis.
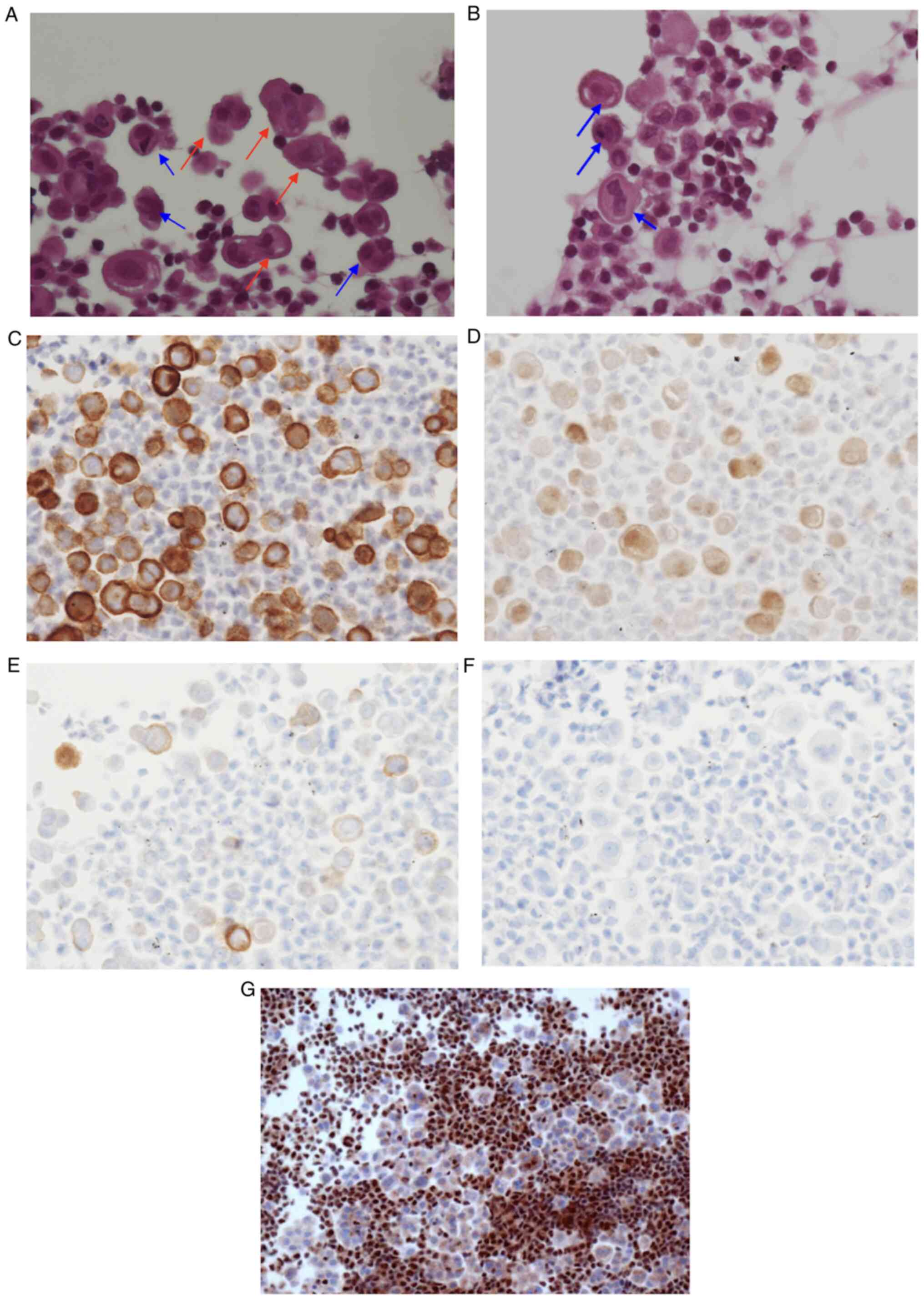 | Figure 2.Cytological specimen of right pleural
effusion showing malignant cells. Based on the cell block made
using pleural effusion, (A) H&E staining showed malignant cells
that formed glomerular or papillary clusters (magnification, ×400).
In detail, the cells presented nuclear enlargement, irregular
nuclear membranes, frequent binucleation or multinucleation
indicated by the blue arrows, humps indicated by the red arrows and
cellular pleomorphism. (B) H&E staining also showed nuclear
enlargement, binucleation or multinucleation indicated by blue
arrows (magnification, ×400). Immunohistochemically, the cells were
positive for (C) podoplanin (D2-40) in the cytoplasmic membrane
(magnification, ×400), (D) calretinin in the cytoplasm and nucleus
(magnification, ×400) and (E) EMA in the cytoplasm and membrane
(magnification, ×400), while they were negative for (F) desmin
(magnification, ×400) and (G) BAP-1 (magnification, ×400). BAP-1,
BRCA-1 associated protein-1; EMA, epithelial membrane antigen. |
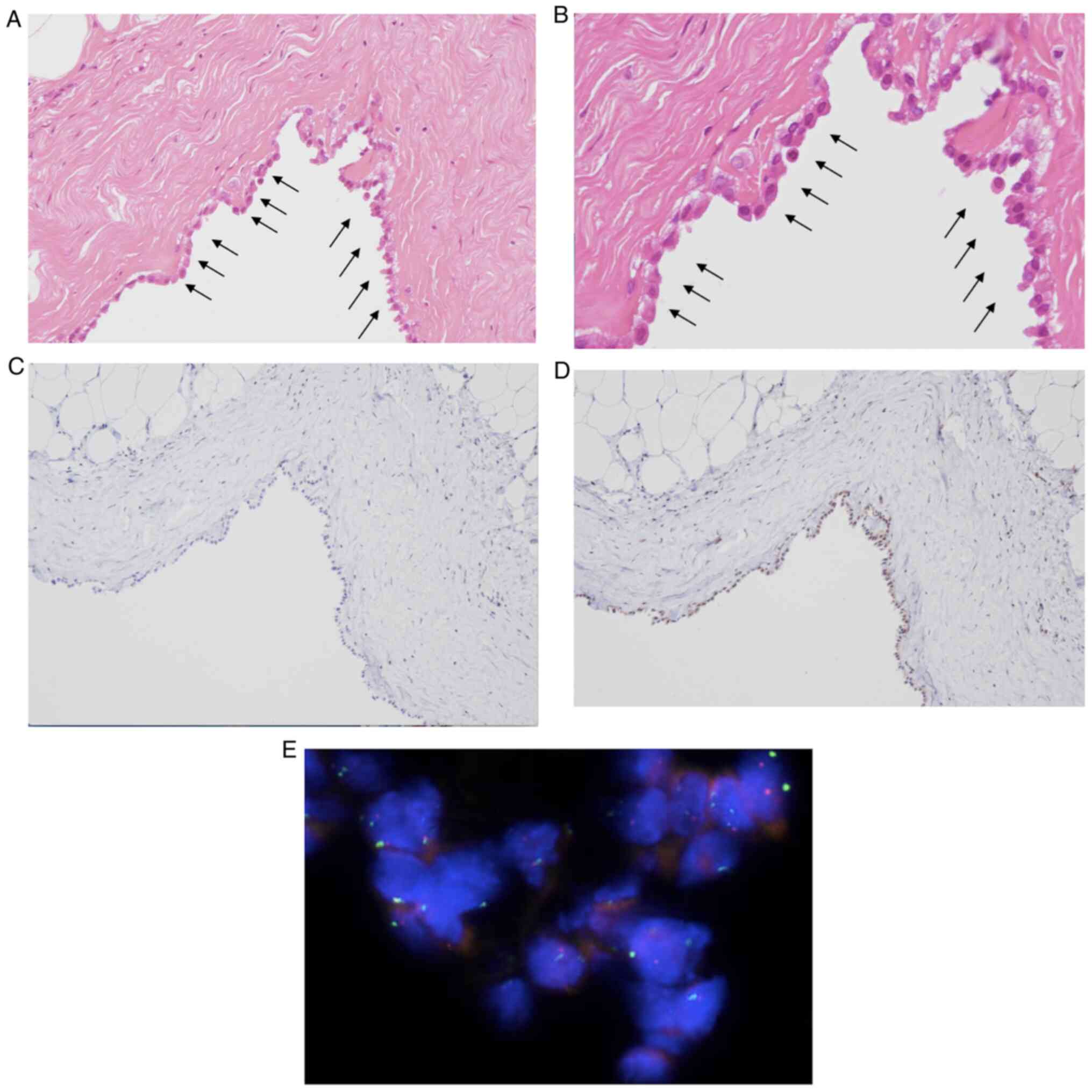 | Figure 3.Pathological finding in the first
right pleural biopsy showing mildly atypical mesothelial cells
forming a single layer indicated by black arrows based H&E
staining. (A) Magnification, ×100. (B) Magnification, ×200. (C)
Loss of BAP-1 (magnification, ×100) and (D) presence of MTAP
(magnification, ×100) based on immunohistochemistry, and (E)
presence of CDKN2A based on FISH (original magnification, ×63).
BAP-1, BRCA-1 associated protein-1; CDKN2A, cyclin-dependent kinase
inhibitor 2A; FISH, fluorescence in situ hybridization;
MTAP, methylthioadenosine phosphorylase. |
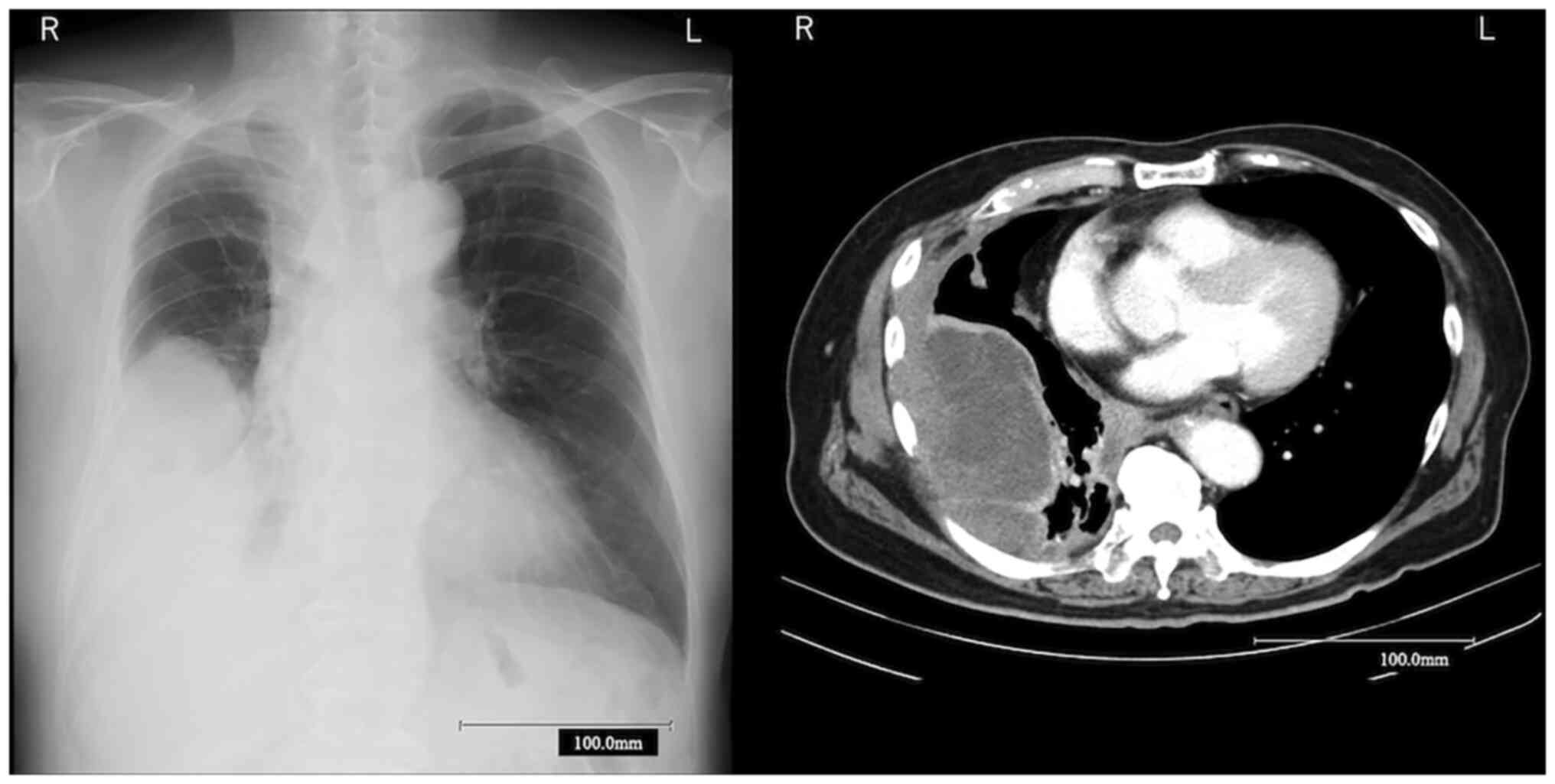
Forty-four months later, he was re-referred to our
hospital due to a complaint for dyspnea and worsening right pleural
effusion. Cytokeratin 19 fragment (CYFRA), a serum tumor marker,
was increased to 19.4 ng/ml. Chest CT revealed a large mass that
originated from the right pleura, diffuse pleural thickening, and a
mediastinal mass (Fig. 4). Because
progression to mesothelioma was suspected, CT-guided needle biopsy
from the right large mass was performed. Based on cytology of
needle lavage fluid, there were atypical cells with nuclear
enlargement in an isolated or accumulated state, suspected to be
malignant mesothelial cells (Fig.
5A). The pathological findings of biopsied specimen included
tumor cells (Fig. 5B) with IHC
positive for D2-40, calretinin, EMA, pankeratin, and HEG-1
(Fig. 5C), and negative for TTF-1,
CEA, desmin, and Ber-EP4. These findings fulfilled the diagnosis of
mesothelioma. Although there was no loss of MTAP based on IHC and
homozygous deletion of CDKN2A/p16 based on FISH (Fig. 5E and F), there was loss of BAP-1
based on IHC (Fig. 5D). After
diagnosis, the patient was started on chemotherapy with carboplatin
with pemetrexed; this continued for five courses. Subsequently, the
tumor progressed, and the patient was switched to nivolumab, but he
did not respond to treatment. His disease then worsened and he died
52 months after the initial diagnosis of MIS.
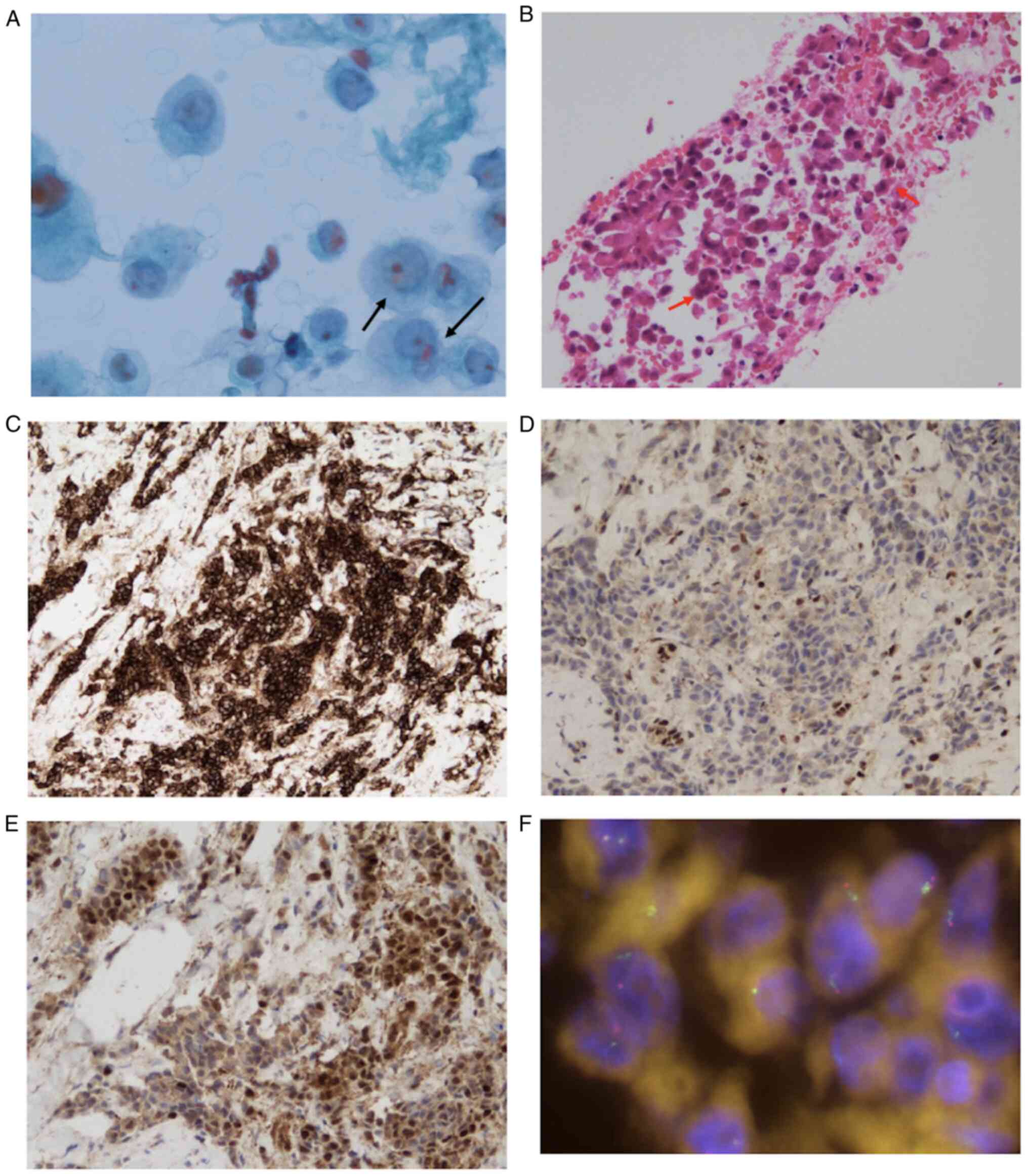 | Figure 5.CT-guided needle biopsy of the right
large mass was performed. (A) Cytology of needle lavage fluid
showing atypical cells with nuclear enlargement indicated by black
arrows (magnification, ×600). (B) The pathological findings based
on H&E staining included tumor cells with humps on the edge
indicated by red arrows (magnification, ×200) and (C) positive for
HEG-1 based on IHC (magnification, ×200). Also shown are the
results of right pleural biopsy, with (D) loss of BAP-1
(magnification, ×200) and (E) presence of MTAP (magnification,
×200) based on IHC, and (F) presence of CDKN2A based on FISH
(original magnification, ×63). BAP-1, BRCA-1 associated protein-1;
CDKN2A, cyclin-dependent kinase inhibitor 2A; FISH, fluorescence
in situ hybridization; HEG-1, sialylated protein HEG homolog
1; IHC, immunohistochemistry; MTAP, methylthioadenosine
phosphorylase. |
Discussion
We have presented a case of MIS that showed
obviously malignant mesothelial cells based on cytology of pleural
effusion. Based on our search of the literature, 17 cases of
pleural MIS have been reported (Table
I) (2,5–12).
According to available data from previous reports, MIS was
confirmed only in 8 cases before progression to mesothelioma
(2,5,6,8,10,11).
It is difficult to suspect MIS at the time of sampling due to
unremarkable clinical findings including symptoms, serum tumor
markers, radiology, and even pathology. On the other hand, all
cases had pleural effusion from the first examinations (Table I). Among them, with the available
information, in six cases cytology of pleural fluid had been
performed; there was mild or no cellular atypia (Table II) (2,5–10,12).
Moreover, two cases were confirmed to be malignant based on the
loss of BAP-1 expression (Table
II) (10,12). In our case, although biochemical
examinations including hyaluronic acid were normal, the initially
obtained pleural fluid cytology showed overtly malignant class V
mesothelial cells (based on the Papanicolaou classification), a
factor that played a key role in suspicion of MIS. Mesothelioma can
be diagnosed without ancillary tests such as loss of BAP-1
expression and/or homozygous deletion of CDKN2A by FISH when
overtly malignant features are identified (13). As far as we know, this is the first
report of MIS with overtly malignant mesothelial cells in the
initially obtained pleural fluid. It may be useful to perform
cytology of pleural effusion when considering the diagnosis of
MIS.
 | Table I.Clinical characteristics of 17
previously reported MIS cases and the present case. |
Table I.
Clinical characteristics of 17
previously reported MIS cases and the present case.
| First author/s,
year | Age, years | Sex | CT findings | BAP1/MTAP/CDKN2A | Periods from MIS to
mesothelioma | (Refs.) |
|---|
| Churg et al,
2018; | 70 | F | Right PE | Loss/loss/loss | 36 months | (5,6) |
| Churg et al,
2020 |
|
|
|
|
|
|
| Churg et al,
2020 | 71 | F | PE, smooth PT | Loss/retain/NA | 64 months | (6) |
| Churg et al,
2020 | 72 | F | PE, smooth PT |
Loss/retain/retain | 92 months | (6) |
| Churg et al,
2020 | 68 | M | PE |
Loss/retain/retain | 58 months | (6) |
| Churg et al,
2020 | 69 | M | PE | Loss/NA/retain | 69 months | (6) |
| Churg et al,
2020 | 79 | M | PE | Loss/NA/NA | 60 months | (6) |
| Churg et al,
2020 | 67 | M | Po resection, no
PE |
Loss/loss/retain | Stable for 12
months | (6) |
| Churg et al,
2020 | 68 | M | PE |
Loss/retain/retain | Stable for 120
months | (6) |
| Churg et al,
2020 | 76 | M | Po resection, no
PE |
Loss/retain/retain | Stable for 57
months | (6) |
| Churg et al,
2020 | 53 | F | Ascites |
Loss/loss/retain | Stable for 12
months | (6) |
| Haefliger et
al, 2021 | 57 | M | PE | Loss/NA/NA | NA | (7) |
| Minami et
al, 2020; | 73 | M | Right PE, Slightly
PT |
Retain/loss/loss | 25 months | (8,11) |
| Nishikubo et
al, 2022 |
|
|
|
|
|
|
| Hidaka et
al, 2020 | 50s | F | Right PE |
Loss/retain/retain | 168 months | (9) |
| Pulford et
al, 2020; | 74 | F | Right PE | Loss/retain/NA | Stable for 36
months | (2,10) |
| Klebe, 2022 |
|
|
|
|
|
|
| Pulford et
al, 2017 | 89 | M | PE | Loss/NA/NA | Died 24 months
later | (3) |
| Pulford et
al, 2017 | 79 | M | PE | Loss/NA/NA | Stable for 9
months | (3) |
| Churg et al,
2022 | 70 | NA | Right PE | Loss/NA/NA | NA | (12) |
| Present study | 74 | M | Right PE |
Loss/retain/retain | 44 months | - |
 | Table II.Cytological features of 6 previously
reported mesothelioma in situ cases and the present
case. |
Table II.
Cytological features of 6 previously
reported mesothelioma in situ cases and the present
case.
| First author/s,
year | Age, years | Sex | Findings of initial
pleural fluid cytology | BAP1 in initial
pleural fluid cytology | (Refs.) |
|---|
| Churg et al,
2018; | 70 | F | No atypical
cells | NA | (5,6) |
| Churg et al,
2020 |
|
|
|
|
|
| Haefliger et
al, 2021 | 57 | M | Mild atypical
mesothelial cells satellited by lymphocytes | NA | (7) |
| Minami et
al, 2020; | 73 | M | Atypical
epithelioid cells | NA | (8,11) |
| Nishikubo et
al, 2022 |
|
|
|
|
|
| Hidaka et
al, 2020 | 50s | F | No atypical
cells | NA | (9) |
| Pulford et
al, 2020; | 74 | F | Mild atypical
cells | Loss | (2,10) |
| Klebe, 2022 |
|
|
|
|
|
| Churg et al,
2022 | 70 | NA | Multiple balls of
slightly atypical mesothelial cells | Loss | (12) |
| Present study | 74 | M | Malignant
mesothelial cells | Loss | - |
BAP-1 is a tumor suppressor gene located at 3p21.1
(3,6); it acts as a nuclear deubiquitinating
agent, regulating especially chromatin remodeling to suppress cell
proliferation and apoptosis (3,4).
Loss of BAP-1 based on IHC has been reported in 60% of mesothelioma
cases and has a specificity of 100% to distinguish malignant from
benign mesothelial proliferation (2,4).
CDKN2A/p16 located at 9p21.3 is a tumor suppressor gene whose
product arrests the cell cycle in G1 (14). Homozygous deletion of CDKN2A/p16
results in uncontrolled cell proliferation, which is commonly
detected in mesothelioma (14).
This mutation has 100% specificity to differentiate between a
benign and a malignant tumor (14). On the other hand, MTAP located at
9p21.3 encodes an enzyme used for the salvage pathway of adenine
and methionine (14). Because MTAP
and CDKN2A/p16 are located on the same chromosome, it has been
reported that loss of MTAP based on IHC could be a surrogate marker
for CDKN2A homozygous deletion (15).
According to Table
II, three cases (including our case) demonstrated BAP-1 loss
based on IHC of pleural fluid, which could be a key factor to
diagnose MIS based on the initially obtained samples. According to
WHO, ancillary analyses such as IHC for BAP-1 and MTAP are
necessary for the diagnosis of mesothelioma and are expected to
become more widespread. Additionally, BAP-1 and MTAP may be used as
prognostic markers in mesothelioma (16). Nishikubo et al (11) reported that, among 13 patients with
MIS, the median progression-free survival for patients with CDKN2A
homozygous deletion or MTAP loss was 18 months; in patients who had
lost BAP-1 but retained CDKN2A and MTAP, the median progress-free
survival was 60 months. Although the authors did not elucidate the
mechanism, they hypothesized that BAP-1 loss occurs during the
early phase of the disease and MTAP/CDKN2A deletion occurs at a
later phase (6,17). In our patient, we confirmed
progression to mesothelioma 44 months later. This relatively slow
progression might have been because he had lost BAP-1 but retained
CDKN2A and MTAP.
In conclusion, we have presented a case of MIS with
malignant mesothelioma cells in pleural effusion. We suggest that
cytology of pleural fluid may play an important role in diagnosis
of MIS at the time of presentation. When atypical cells are
detected in pleural fluid, if available, IHC of BAP-1 and MTAP
should be performed.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YY, KHi, TS, NH and YM designed this case report.
HO, JK, KHa, SOI and TS acquired the data. TN, MS, KHa, SU and SOI
performed analysis of the data. YY, KHi, NH and YM drafted and
revised the manuscript. YY and YM submitted the final manuscript.
YM and KHi confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ibaraki
Higashi National Hospital ethical committee (Tokai-mura,
Japan).
Patient consent for publication
Written informed consent was obtained from the
patient's family for publication of this case report and
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BAP-1
|
BRCA-1 associated protein-1
|
|
Ber-EP4
|
epithelial specific antigen
|
|
CDKN2A/p16
|
cyclin-dependent kinase inhibitor
2A/p16
|
|
CEA
|
carcinoembryonic antigen
|
|
CXR
|
chest X-ray
|
|
CYFRA
|
cytokeratin 19 fragment
|
|
D2-40
|
podoplanin
|
|
EDTA
|
Tris-ethylenediaminetetraacetic
acid
|
|
EMA
|
epithelial membrane antigen
|
|
FISH
|
fluorescence in situ
hybridization
|
|
H&E
|
hematoxylin and eosin
|
|
HEG-1
|
sialylated protein HEG homolog 1
|
|
IHC
|
immunohistochemistry
|
|
MIS
|
mesothelioma in situ
|
|
MTAP
|
methylthioadenosine phosphorylase
|
|
TTF-1
|
thyroid transcription factor-1
|
|
WHO
|
World Health Organization
|
References
|
1
|
Beasley MB, Galateau-Salle F and Dacic S:
Pleural mesothelioma classification update. Virchows Arch.
478:59–72. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pulford E, Henderson DW and Klebe S:
Malignant mesothelioma in situ: Diagnostic and clinical
considerations. Pathology. 52:635–642. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pulford E, Huilgol K, Moffat D, Henderson
DW and Klebe S: Malignant mesothelioma, BAP1 immunohistochemistry,
and VEGFA: Does BAP1 have potential for early diagnosis and
assessment of prognosis? Dis Markers. 2017:13104782017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sauter JL, Dacic S, Galateau-Salle F,
Attanoos RL, Butnor KJ, Churg A, Husain AN, Kadota K, Khoor A,
Nicholson AG, et al: The 2021 WHO classification of tumors of the
pleura: Advances since the 2015 classification. J Thorac Oncol.
17:608–622. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Churg A, Hwang H, Tan L, Qing G, Taher A,
Tong A, Bilawich AM and Dacic S: Malignant mesothelioma in situ.
Histopathology. 72:1033–1038. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Churg A, Galateau-Salle F, Roden AC,
Attanoos R, von der Thusen JH, Tsao MS, Chang N, De Perrot M and
Dacic S: Malignant mesothelioma in situ: Morphologic features and
clinical outcome. Mod Pathol. 33:297–302. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haefliger S, Prince SS, Rebetez J, Borer H
and Bubendorf L: Putative malignant pleural mesothelioma in situ
(MPMIS) with sequential acquisition of genomic alterations on
fluorescence in situ hybridization (FISH) examination. Acta Cytol.
65:99–104. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Minami K, Jimbo N, Tanaka Y, Hokka D,
Miyamoto Y, Itoh T and Maniwa Y: Malignant mesothelioma in situ
diagnosed by methylthioadenosine phosphorylase loss and homozygous
deletion of CDKN2A: A case report. Virchows Arch. 476:469–473.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hidaka K, Takeda T, Kinoshita Y, Nabeshima
K, Tamiya S, Yoshikawa Y and Tsujimura T: Development of
mesothelioma in situ and its progression to invasive disease
observed in a patient with uncontrolled pleural effusions for 15
years. Pathol Int. 70:1009–1014. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klebe S: Progression of mesothelioma in
situ to invasive disease 4 years and 10 months after initial
diagnosis. Pathology. 54:384–386. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishikubo M, Jimbo N, Tanaka Y, Tachihara
M, Itoh T and Maniwa Y: Sarcomatoid mesothelioma originating from
mesothelioma in situ: Are methylthioadenosine phosphorylase loss
and CDKN2A homozygous deletion poor prognostic factors for
preinvasive mesothelioma? Virchows Arch. 481:307–312. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Churg A, Galateau-Salle F, Tan L and Qing
G: Cytological diagnosis of mesothelioma in situ versus invasive
mesothelioma. Pathology. 54:133–136. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Michael C, Hiroshima K, Hjerpe A, Michelow
P, Onal B and Segal A: Malignant-Primary (MAL-P) (Mesothelioma).
The international system for serous fluid cytopathology. Chandra A,
et al: Springer; Cham: pp. 63–98. 2020, View Article : Google Scholar
|
|
14
|
Churg A, Sheffield BS and Galateau-Salle
F: New markers for separating benign from malignant mesothelial
proliferations: Are we there yet? Arch Pathol Lab Med. 140:318–321.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng YY, Yuen ML, Rath EM, Johnson B,
Zhuang L, Yu TK, Aleksova V, Linton A, Kao S, Clarke CJ, et al:
CDKN2A and MTAP are useful biomarkers detectable by droplet digital
PCR in malignant pleural mesothelioma: A potential alternative
method in diagnosis compared to fluorescence in situ hybridisation.
Front Oncol. 10:5793272020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma GY, Shi S, Wang P, Wang XG and Zhang
ZG: Clinical significance of 9P21 gene combined with BAP1 and MTAP
protein expression in diagnosis and prognosis of mesothelioma
serous effusion. Biomed Rep. 17:662022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dacic S, Roy S, Lyons MA, von der Thusen
JH, Galateau-Salle F and Churg A: Whole exome sequencing reveals
BAP1 somatic abnormalities in mesothelioma in situ. Lung Cancer.
149:1–4. 2020. View Article : Google Scholar : PubMed/NCBI
|