Introduction
The (MAPK) family is a highly conserved protein
family, the members of which participate in several cytokine
pathways (1–3). P38 has four isoforms encoded by
different genes in mammalian cells: P38 α (MAPK14), P38 β (MAPK11),
P38 γ (MAPK12), and P38 δ (MAPK13) (3). The MAPK signaling pathway partakes in
numerous core biological functions such as in the regulation of
cell proliferation, inflammation, survival, innate immunity, and
other cellular processes related to cancer progression and
development (4,5). Classical MAPKs primarily include P38,
JNK1/2/3, and other subtypes, which have been studied in depth
(6–8).
Studies suggest that MAPK12 is expressed in multiple
tissues and promotes tumorigenesis and tumor progression (9). For example, high MAPK12 expression
promoted epithelial-mesenchymal transition (EMT) in breast cancer
cells, and the downregulation of MAPK12 inhibited EMT (10,11).
MAPK12 overexpression increased the number of cancer stem cells
(CSCs), whereas MAPK12 knockdown decreased the proportion of CSCs
in breast cancer cells (12). Chen
et al (13) found that
overexpression of MAPK12 enhanced the transformation to a malignant
phenotype in renal cell carcinoma (RCC) cells, and MAPK12 may be a
novel therapeutic target for the management of RCC.
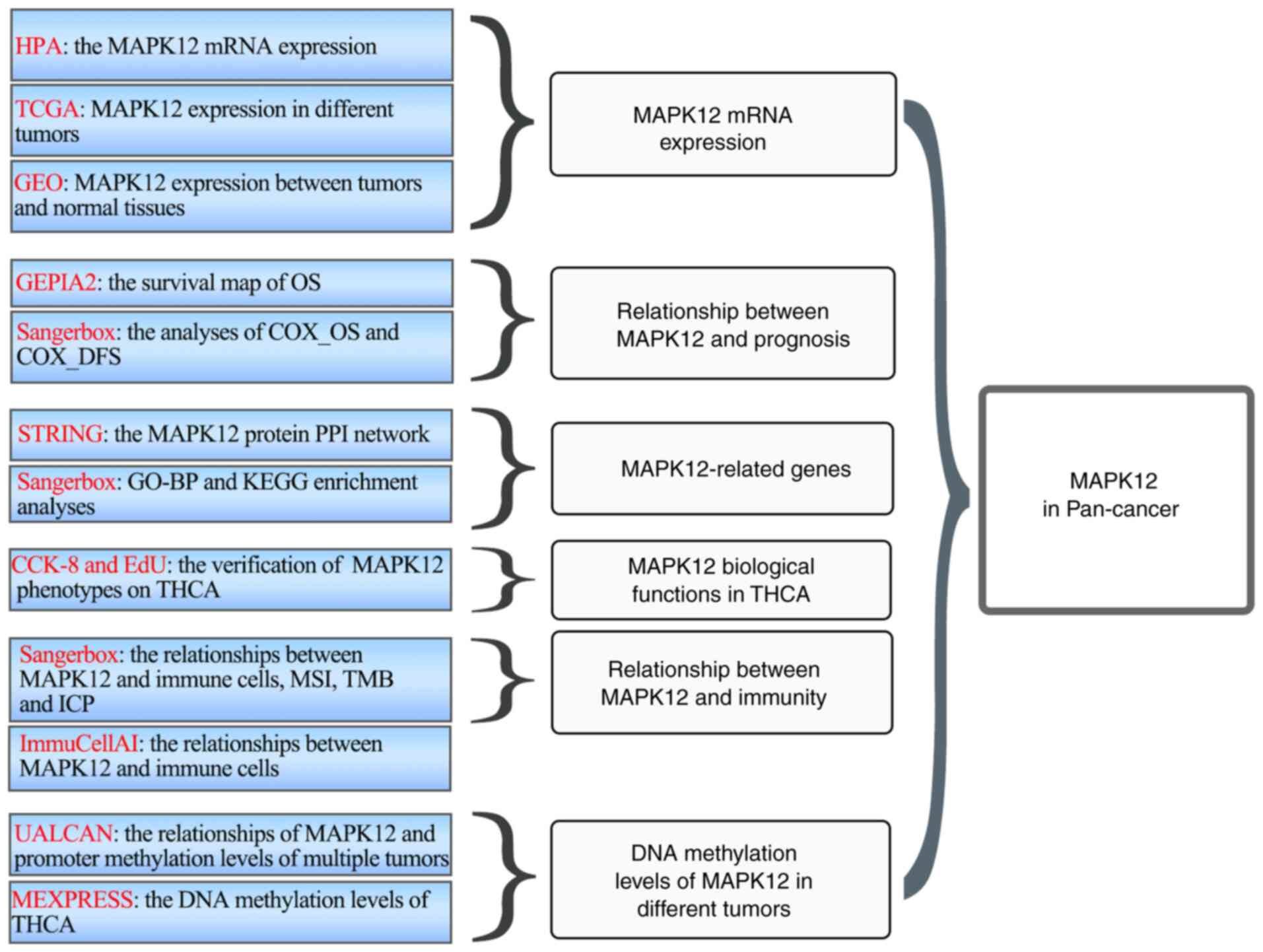
Here, systematic bioinformatics analysis was used to
explore the functions and effects of MAPK12 in a variety of
cancers. The significance of abnormal MAPK12 expression in multiple
cancer types was comprehensively studied using mRNA expression
analysis, patient prognostic indicators, functional analyses of
MAPK12-related genes, tumor immunity, and methylation patterns.
Additionally, the relationship between MAPK12 expression and
thyroid carcinoma (THCA) proliferation was determined using
cytotoxicity and EdU assays in vitro.
Materials and methods
Expression analysis
The mRNA expression profiles of MAPK12 in various
normal tissues were obtained from the Human Protein Atlas (HPA)
website (https://www.proteinatlas.org). Data
sources are Tumor-Node-Metastasis standardized. The ‘Gene’ module
of SangerBox (14), a web-based
program (http://www.sanger box.com), was used
to examine the mRNA expression levels of MAPK12 in normal and
cancer tissues as reported by TCGA (https://cancergenome.nih.gov/abouttcga/overview). The
GSE33630 (15), GSE27155 (16), and GSE65144 (17) datasets were downloaded to analyze
the MAPK12 mRNA expression differences between normal thyroid cells
and thyroid carcinoma cells.
Survival analysis
The relationship between MAPK12 mRNA expression and
overall survival (OS) in each tumor type in the TCGA database was
analyzed using the GEPIA2 website (18). Patients were divided into MAPK12
low and MAPK12 high cohorts based on MAPK12 expression levels. Cox
regression analysis was used in SangerBox to study the effects of
MAPK12 expression on the OS and disease-free survival (DFS) of
patients with different types of tumors.
MAPK12-related gene enrichment
analysis
First, the ‘similar Gene’ module in GEPIA was used
to analyze the top 100 genes that were most commonly associated
with MAPK12 pan-cancer, and we screened the top 50 genes. A network
map of MAPK12 and the 50 genes was created using STRING (https://string-db.org/) (19). Gene Ontology (GO) (20,21)
and Kyoto Encyclopedia of Genes and Genomes (KEGG) (22) were used to perform enrichment
analysis of these 100 MAPK12-related genes. The TCGA-THCA database
was downloaded, and the genes that most significantly correlated
with MAPK12 were screened based on thresholds of R>0.35 and
P<0.05 using R-project (http://www.R-project.org/) and R studio (http://www.rstudio.com/). GO and KEGG enrichment
analyses were performed for the screened differentially expressed
genes using the GeneDenovo tool (https://www.omicshare.com/tools/).
Immune-related analysis
The EPIC and QUANTISEQ (https://www.epicimmunea tlas.org) datasets from TCGA
(https://icbi.i-med.ac.at/software/quantiseq/doc/index.html)
were used for the immune analysis of all types of infiltrating
immune cells. The relationship between the expression levels of
MAPK12 mRNA and immune checkpoint (ICP), microsatellite instability
(MSI), and tumor mutational burden (TMB) in different cancer types
in TCGA were analyzed using the immuno-analysis module of the
SangerBox website (http://vip.sangerbox.com/home.html). The ImmuCellAI
(http://bioinfo.life.hust.edu.cn/ImmuCellAI#!/) portal
was used to analyze the relationship between MAPK12 expression and
immune-related cells in THCA using the dataset from TCGA. The
P-values and partial correlation were obtained using the Spearman
rank correlation test.
Methylation analysis
The MAPK12 promoter methylation level differences in
tumor tissues and normal tissues were analyzed using UALCAN
(http://ualcan.path.uab.edu). The
transcripts per kilobase of exon model per million mapped reads
(TPM) was used to normalize the methylation expression value of raw
data from TCGA. The MEXPRESS website (https://mexpress.be/) (23) was used to obtain the DNA promoter
methylation patterns of MAPK12 in THCA.
Cell culture
The human normal thyroid cell line HTORI-3 and human
THCA cell lines (TPC-1, K-1, and HTH-83) were obtained from ATCC.
All cell lines were tested for mycoplasma, and STR cell
identification was performed. All cell lines used in this study
were cultured in DMEM supplemented with 10% FBS (both from Thermo
Fisher Scientific, Inc.) and maintained in an incubator at 37°C,
supplied with 5% CO2, and 95% humidity.
Transfection
The MAPK12 small interfering (si)RNAs were purchased
from Shanghai GenePharma Co., Ltd. siRNAs were used to knock down
the expression of MAPK12 in HTH-83 and K-1 cells. The
pcDNA3.1-MAPK12 plasmid was purchased from Genewiz, Inc. and used
to overexpress MAPK12. Transient transfection of 3 µl si-MAPK12 (20
µM) or 2 µl MAPK12 plasmid (1,000 ng/µl) was performed in 6-well
plates with a cell density of 2×105 cells using
Lipofectamine 3000 (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. The empty vector (pcDNA3.1) and
si-NC were also used as negative controls. Transfected cells were
harvested 48 h after transfection for subsequent analysis and
detection. The MAPK12 siRNA sequences were
#1:'5′-AAGUAACACGCUUCCAUUCTT'3′ and
#2:'5′-UACAAAAGGGUCUAUUUCCTT'3′; the si-NC sequence was sense:
UUCUCCGAACGUGUCACGUTT and antisense: ACGUGACACGUUCGGAGAATT.
Reverse transcription-quantitative
(RT-q) PCR
The total RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.). The GoScript RT system
(Promega Corporation) was used to synthesize cDNA according to the
manufacturer's protocol. The MAPK12 mRNA expression status was
detected using GoTaq®qPCR Master Mix (Promega
Corporation) on an ABI QuantStudio 3. The PCR system was 20 µl in
total, including 2X SYBR Green qPCR Master Mix (10 µl; Bimake),
cDNA (2 µl), primer mix (2 µl), DNase/RNase-free water (6 µl), and
the following thermocycling conditions were used: 95°C for 3 min,
40 cycles of 95°C for 15 sec and 60°C for 1 min. The dissolution
curve program was: 95°C for 15 sec, 60°C for 1 min, and 95°C for 1
sec. The relative expression levels of the target gene were
calculated using the 2−∆∆Cq method (24). The oligonucleotide primers used for
qPCR were: MAPK12 forward, 5′-CCCTGGATGACTTCACGGAC-3′ and reverse,
5′-GCTTCAGGTCCCTCAGCC-3′; GAPDH forward,
5′-GGTGGTCTCCTCTGACTTCAACA-3′ and reverse,
5′-GTTGCTGTAGCCAAATTCGTTGT-3′.
Western blotting
The plates were carefully washed twice with PBS.
Total protein was extracted using RIPA buffer (Beijing Solarbio
Science & Technology Co., Ltd.) containing protease inhibitors
(Beijing Solarbio Science & Technology Co., Ltd.). The protein
concentration was detected using a BCA kit (Beijing Solarbio
Science & Technology Co., Ltd.) according to the manufacturer's
instructions. The protein lysates (20 µg) were loaded on a 10% SDS
gel, resolved using SDS-PAGE, and transferred to PVDF membranes
(MilliporeSigma). Then membranes were blocked using TBS containing
5% BSA (cat. no. A7906, MilliporeSigma) at room temperature for 2 h
followed by incubation with primary antibodies against MAPK12
(1:2,000; cat. no. 9212; Cell Signaling Technology, Inc.) or GAPDH
(1:5,000; cat. no. 97166; Cell Signaling Technology, Inc.)
overnight at 4°C, followed by incubation with the corresponding
HRP-conjugated secondary antibody at 1 h (1:3,000; cat. no. 7074;
Cell Signaling Technology, Inc.). The protein bands were visualized
and detected using an enhanced chemiluminescence system (Bio-Rad
Laboratories, Inc.). GAPDH was used as a loading control.
Cytotoxicity assay
A cytotoxicity assay (Dojindo Molecular
Technologies, Inc.) was used to detect cell proliferation. A total
of 2×103 cells/well were plated in 96-well plates with 3
replicate wells/group. The treatment group was treated with
siMAPK12 knockdown. After 0, 1, 2, 3, 4, and 5 days, 100 µl
serum-free solution (ApexBio) containing 10% cytotoxicity reagent
was added, and cells were further incubated at 37°C for 1 h. The
optical density values were obtained at 450 nm using a microplate
reader (Thermo Fisher Scientific, Inc.).
EdU assay
An EdU assay was used to detect cell proliferation.
A total of 2×105 THCA cells/well (HTH-83, K-1, or TPC1)
were plated in 96-well plates. After 24 h, the adherent cells were
transfected. After 48 h, 100 µl EdU medium containing 10 µM
(Guangzhou RiboBio Co., Ltd.) was added to each well for 2 h, and
the culture medium was washed and fixed with 100 µl cell fixator
(cat. no. P1110; Beijing Solarbio Science & Technology Co.,
Ltd.) at room temperature for 30 min. The fixed cells were
permeabilized with 100 µl PBS containing 0.5% Triton X-100,
followed by staining using an Apollo staining reaction solution
(Guangzhou RiboBio Co., Ltd.) in the dark for 30 min at 37°C. A
Hoechst 33342 reaction solution (100 µl 1×; Guangzhou RiboBio Co.,
Ltd.) was used for 10 min at 37°C. The dyed plate was placed under
an inverted fluorescence microscope (×100 magnification) to obtain
fluorescence images.
Statistical analysis
Statistical analyses were automatically calculated
using the aforementioned online tools. Comparisons between two
groups were made using an unpaired t-test. Comparisons between
multiple groups were made by one-way ANOVA and comparisons between
CCK8 groups were made using two-way ANOVA, followed by Bonferroni's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
MAPK12 expression patterns
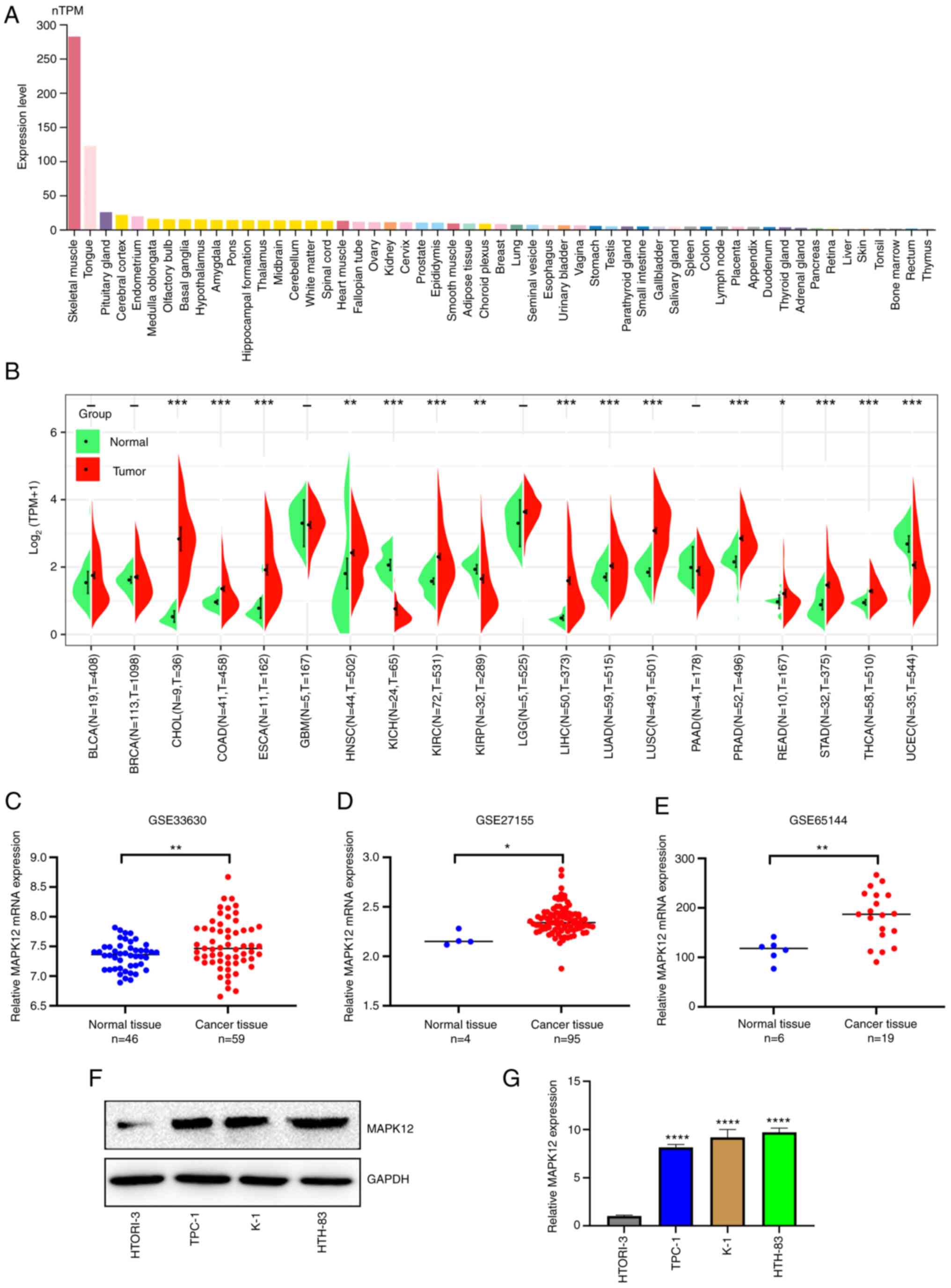
The flowchart of this study is shown in Fig. 1. The HPA database revealed that
MAPK12 mRNA expression was highest in skeletal muscle, followed by
the tongue (nTPM >100; Fig.
2A). MAPK12 mRNA expression levels were detectable but low
(nTPM <20) in most other normal human tissues (Fig. 2A). To understand and analyze the
differences in mRNA expression levels of MAPK12 in normal tissues
compared with the respective tumor tissues, the expression profiles
of several cancers were obtained from TCGA and the differences in
expression of MAPK12 mRNA in normal tissues and tumor tissues were
determined. The mRNA expression levels of MAPK12 in all TCGA tumor
datasets are shown in Fig. 2B.
MAPK12 mRNA expression levels were higher in several TCGA tumor
datasets compared with the corresponding normal tissues. The
expression of MAPK12 mRNA was significantly higher in 12 cancers:
Cholangiocarcinoma (CHOL), colon adenocarcinoma (COAD), esophageal
carcinoma (ESCA), head and neck cancer (HNSC), kidney renal clear
cell carcinoma (KIRC), liver hepatocellular carcinoma (LIHC), lung
adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC),
prostate adenocarcinoma (PRAD), rectum adenocarcinoma (READ),
stomach adenocarcinoma (STAD), and THCA. However, MAPK12 mRNA
expression was lower in kidney chromophobe (KICH), kidney renal
papillary cell carcinoma (KIRP), and uterine corpus endometrial
carcinoma (UCEC) (Fig. 2B).
The expression levels of MAPK12 in THCA were further
studied to add to the relevance of disciplinary research. First,
three GEO datasets were obtained: GSE33630, GSE27155, and GSE65144.
MAPK12 expression levels were higher in all three databases
compared with the normal tissues (Fig.
2C-E). Three THCA cell lines and a normal thyroid follicular
cell line were used to verify the expression of MAPK12 RNA and
protein. The expression levels of MAPK12 protein and mRNA were
higher in the THCA cell lines compared with the normal thyroid
follicle cells (Fig. 2F-G).
In conclusion, these results suggested that MAPK12
expression was upregulated in several tumors, including THCA.
MAPK12 expression is associated with
prognosis across several types of cancer, including THCA
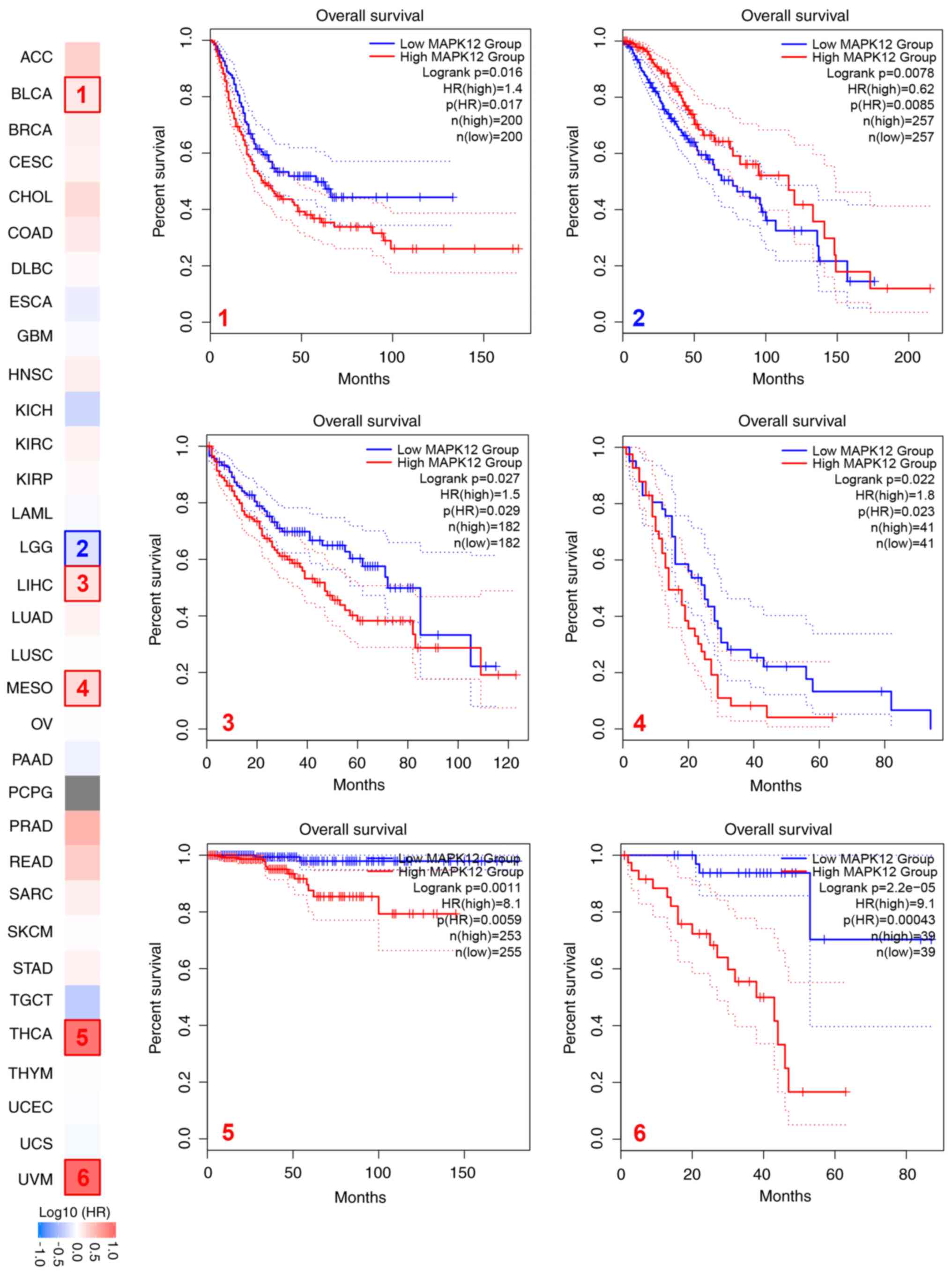
First, the correlation between MAPK12 mRNA levels
and prognosis using patient survival-related information obtained
from TCGA was determined, and the OS curves were plotted. The
results showed that higher levels of MAPK12 mRNA in multiple cancer
types were associated with a poorer prognosis and shorter survival
in patients with multiple tumors, including bladder urothelial
carcinoma (BLCA), LIHC, mesothelioma (MESO), THCA, and uveal
melanoma (UVM) (Fig. 3). Cox
regression analysis was used to further analyze the relationship
between MAPK12 mRNA levels with OS and DFS in tumor patients. The
results showed that high mRNA levels of MAPK12 were associated with
a shorter OS in the pan-kidney cohort (KIPAN), LAML, HNSC, LIHC,
lung adenocarcinoma (LUAD), BLCA, LAML, COADREAD, COAD, ACC, MESO,
THCA, and UVM, and a shorter DFS in KIPAN, STES, HNSC, BRCA, KIRP,
BLCA, ACC, COAD, COADREAD, UVM, MESO, and THCA (Fig. S1A and B).
Overall, the analyses suggested that high levels of
MAPK12 mRNA were associated with a poorer prognosis pan-cancer,
including in THCA.
Enrichment analysis of MAPK12-related
genes
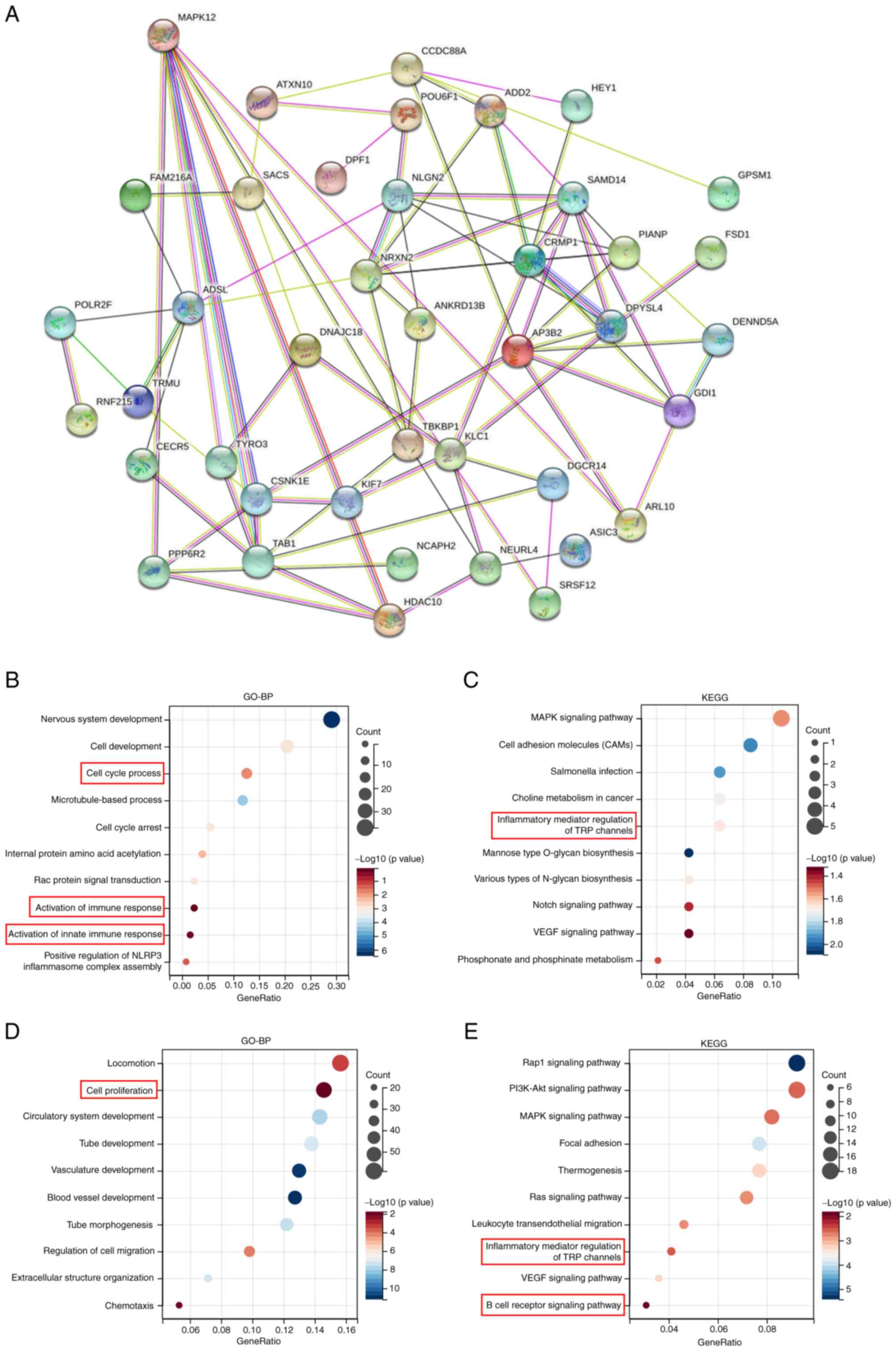
To further examine the molecular biological
mechanism of MAPK12 function in tumors, MAPK12 expression-related
proteins were identified, a protein-protein interaction network was
constructed, and functional enrichment analysis of the MAPK12
expression-related genes obtained above was performed. First, the
interaction of the top 50 proteins associated with MAPK12 in the
form of a network diagram was shown using STRING (Fig. 4A). Second, the top 100 genes with a
significant correlation with the MAPK12 gene in the generalized
carcinoma dataset from TCGA were determined using GEPIA2. Third,
GO-biological process (BP) and KEGG functional enrichment analyses
were performed using the top 100 positively related genes (Fig. 4B and C). The results showed that
the top 100 genes were enriched in cell proliferation-related
pathways and immune-related pathways, including ‘cell cycle
process’ and ‘activation of immune response’.
The THCA dataset from TCGA was downloaded and
analyzed regarding MAPK12-related genes based on thresholds of
R>0.35 and P<0.05. The related genes were analyzed using
GO-BP and KEGG functional enrichment. The results were similar to
that of the pan-cancer analysis. MAPK12 THCA was also enriched in
cell proliferation, and in immune-related functions and pathways
(Fig. 4D and E).
Based on these results, it was speculated that
MAPK12 promoted the development of tumors pan-cancer, particularly
in THCA, by influencing cell proliferation and the tumor immune
microenvironment (TIM). Therefore, the underlying mechanism was
further examined.
MAPK12 expression is associated with
THCA cell proliferation in vitro
Although the functional enrichment analysis results
confirmed that MAPK12 promoted cancer progression in THCA,
experimental verification was required to confirm the
bioinformatics results. Three classical THCA cell lines, TPC-1,
HTH-83, and K-1, were selected for the cell proliferation
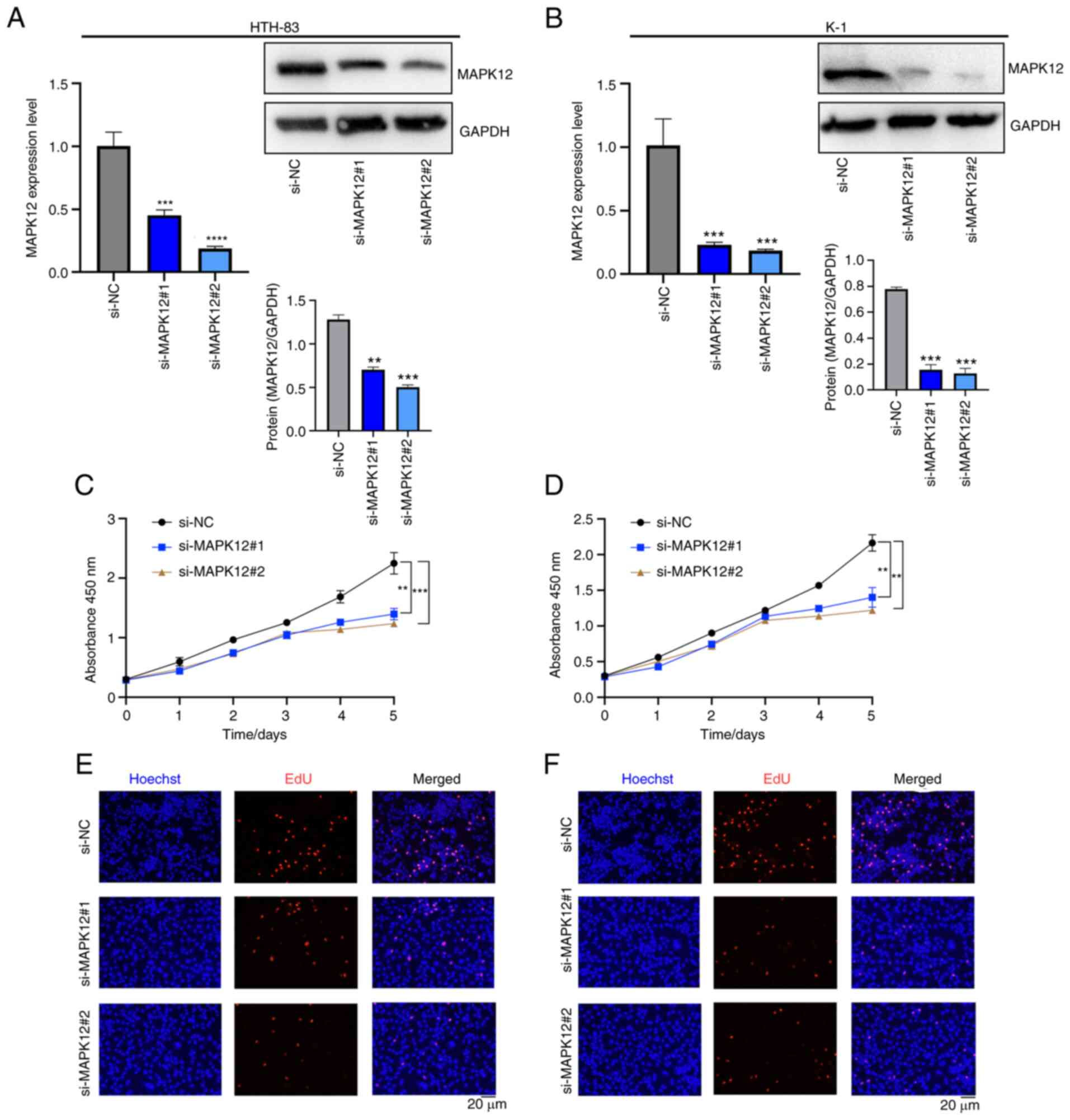
experiments. MAPK12 expression in HTH-83 and K-1 cell lines was
knocked down using siRNA. The MAPK12 expression levels in the TPC-1
cell line were upregulated using a pcDNA.31-MAPK12 plasmid. The
efficiencies of MAPK12 knockdown or overexpression were confirmed
using RT-qPCR and western blot analyses (Figs. 5A, B, and S2A). Cytotoxicity and EdU assays were
performed, and the results showed that knockdown of MAPK12 led to a
significant decrease in cell proliferation, whereas overexpression
of MAPK12 resulted in an increase in cell proliferation (Figs. 5C-F, S2B and C). Together, it was
experimentally demonstrated that MAPK12 played a role in tumor
occurrence and development by regulating cell proliferation.
MAPK12 expression is associated with
the TIM pan-cancer, including in THCA
The enrichment analysis of MAPK12-related genes also
suggested that MAPK12 influenced the development of tumors by
influencing the TIM. Therefore, the impact of MAPK12 on TIM
pan-cancer and in THCA was assessed.
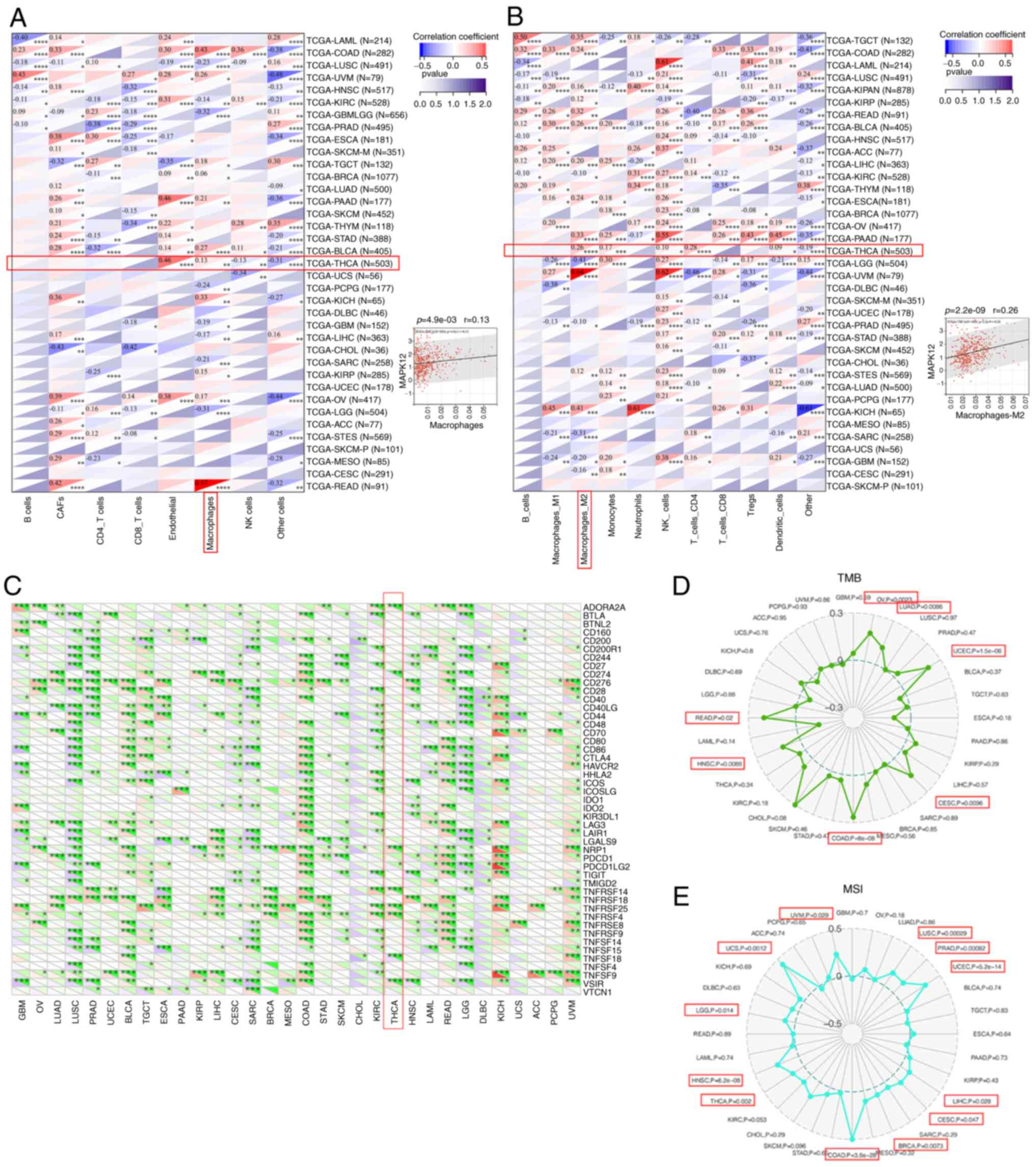
First, the correlation between MAPK12 mRNA levels
and the abundance of tumor immune cells that had invaded diffuse
carcinoma tissues was determined. Tumor-infiltrating lymphocytes
(TILs) are an important component of the TIM and are generally
associated with the development of tumors. TILs are key predictors
of metastatic lymph node status and prognosis in patients with
cancer. First, the relationship between TIL abundance and MAPK12
mRNA levels was determined using the EPIC and QUANTISEQ datasets.
MAPK12 mRNA levels showed significant correlations with multiple
TIIs/TILs in THCA (Fig. 6A and B).
MAPK12 expression levels also showed a positive correlation with
various TILs in the TCGA-THCA dataset (Fig. S3). Macrophages are a significant
constituent of the innate immune system and play an indispensable
role in activating the body's first-line defense against infection
and cancer (25). Therefore, the
infiltration levels of macrophages were assessed. A positive
correlation was obtained in the EPIC database between macrophages
and MAPK12 mRNA expression in THCA. Macrophages polarize to
antitumor M1 and protumor M2 macrophages. Therefore, the QUANTISEQ
database was used to further analyze the levels of invading M1 and
M2 macrophages (26) and found
that the M2 invasion levels were positively correlated with the
MAPK12 mRNA expression levels in THCA. Therefore, it was
hypothesized that THCA tumor cells secreted chemokines to increase
the infiltration of tumor-promoting M2 macrophages to increase the
degree of tumor malignancy.
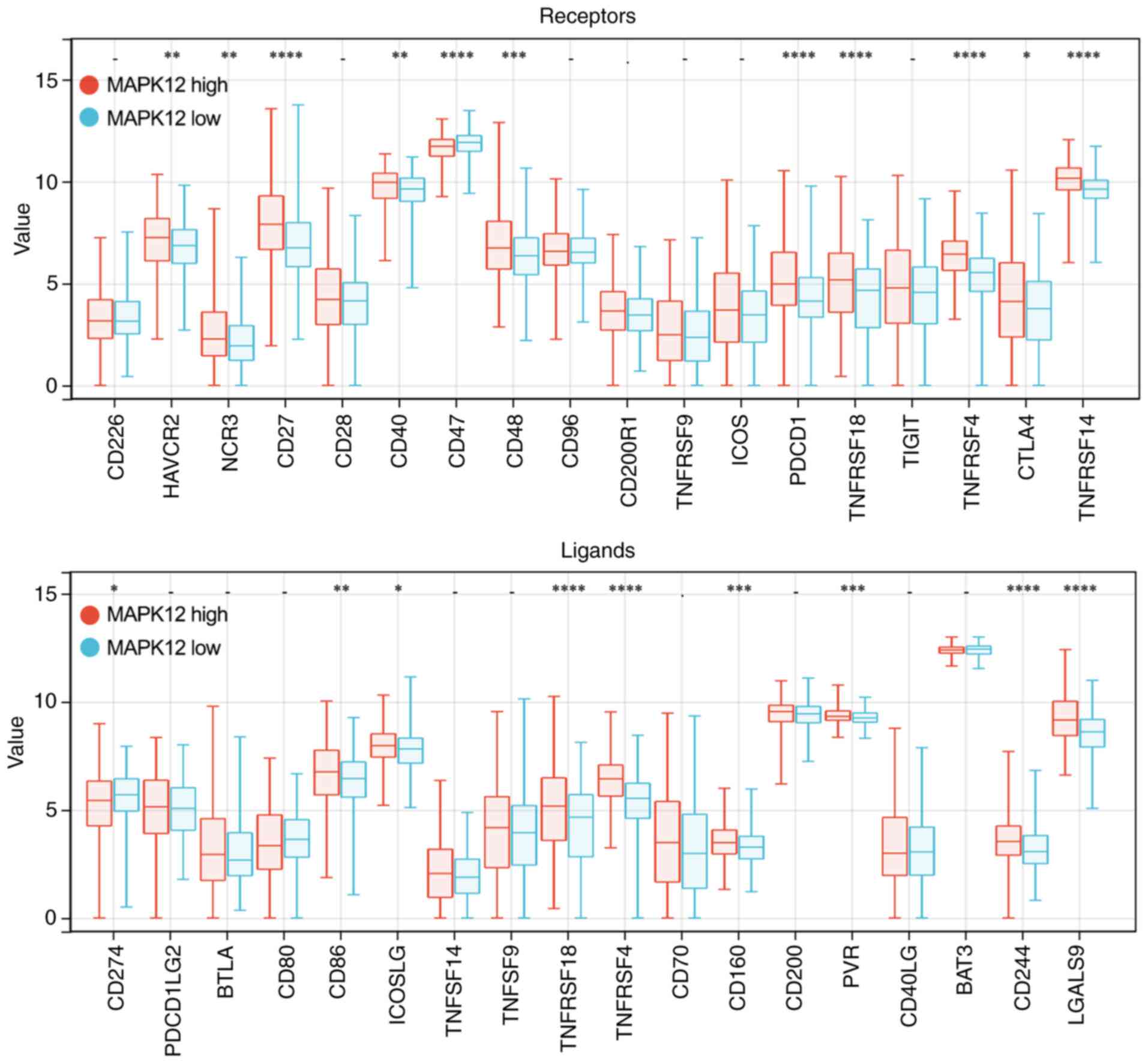
We analyzed whether MAPK12 affected the sensitivity
of cancer patients to immunotherapy. ICP proteins are often
regarded as promising therapeutic targets in the field of cancer
immunotherapy (27–29). Therefore, the relationship between
ICP gene expression levels and MAPK12 in various cancer types were
determined. MAPK12 mRNA levels were positively correlated with
multiple ICP genes in THCA (Fig.
6C). Moreover, MAPK12 expression in THCA showed a positive
association with several ICP receptors including HAVCR2, NCR3,
CD27, CD40, CD47, CD48, PDCD1, TNFRSF18, TNFRSF4, CTLA4, and
TNFRSF14, as well as ICP ligands such as CD274, CD86, ICOSLG,
CD160, PVR, CD244, and LGALS9 (Fig.
7). TMB and MSI are also important indicators of whether
patients with cancer will benefit from immunotherapy (30,31).
The relationship between the expression levels of MAPK12 with TMB
and MSI in the TCGA dataset was studied using SangerBox. The
results suggested that MAPK12 affected the sensitivity of THCA
cells to immune checkpoint inhibitor therapy (Fig. 6D and E). Therefore, it was
hypothesized that MAPK12 altered the TIM in THCA tissues by
regulating the expression levels of ICP receptors and ligands.
These results suggest that MAPK12 mediates the activation of ICP
genes and is thus an ideal target for immunotherapy in THCA
patients.
In conclusion, MAPK12 may affect the TIM by
modulating the infiltration of immune cells within tumors and the
sensitivity of multiple tumors to immunotherapy. Therefore, MAPK12
may serve as an immunotherapeutic target.
Determination of the MAPK12
methylation levels pan-cancer
To further study the mechanism of abnormal MAPK12
expression, we also analyzed the DNA methylation patterns of the
MAPK12 gene promoter. DNA methylation generally leads to increased
expression levels, and upregulation of oncogenes promotes tumor
development (32).
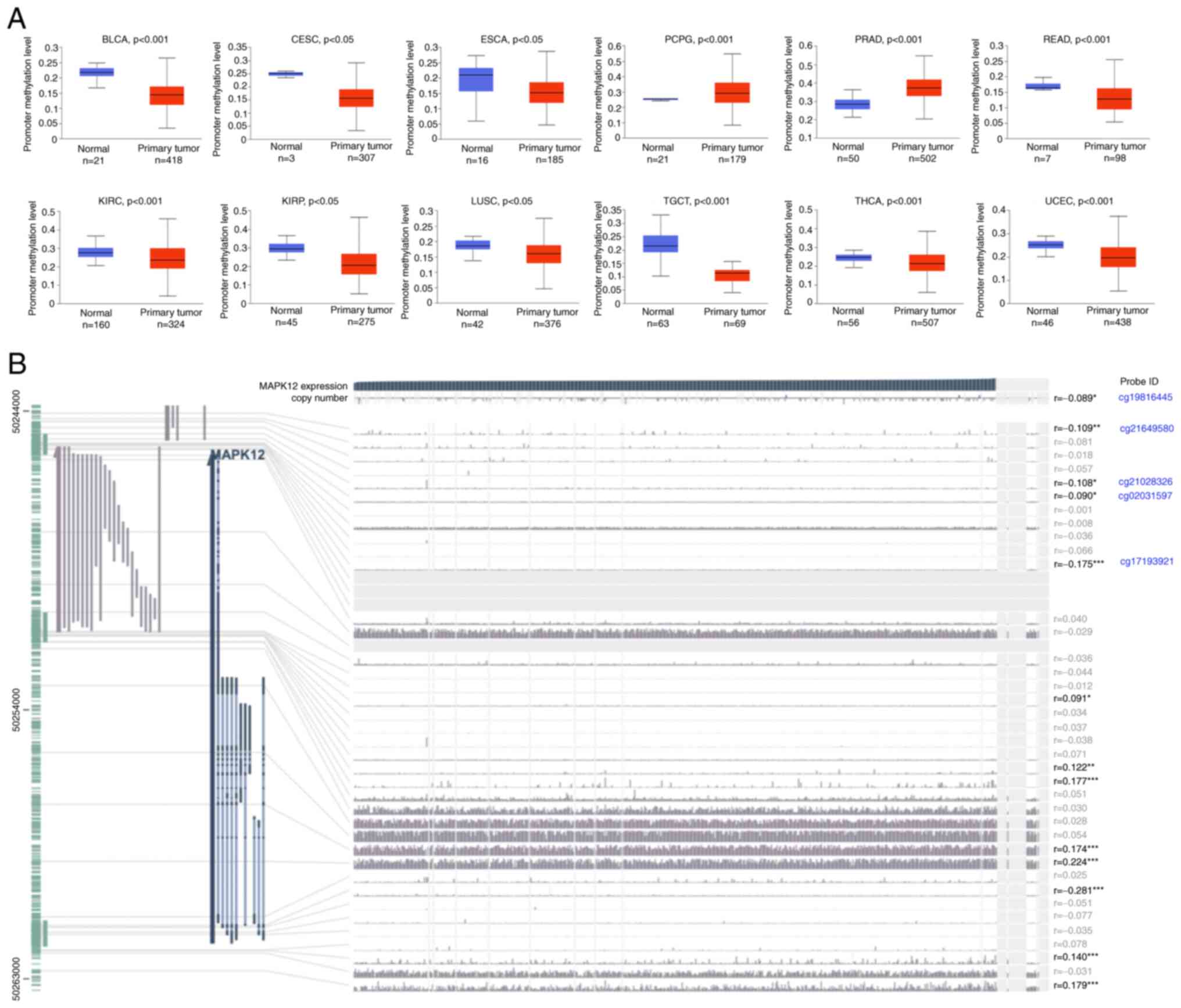
The UALCAN online tool was used to explore
methylation levels in the MAPK12 promoter region. MAPK12 promoter
methylation levels were lower in BLCA, CESC, ESCA, READ, KIRC,
KIRP, LUSC, TGCT, THCA, and UCEC (Fig.
8A). These results suggest that methylation of the MAPK12
promoter may lead to its upregulation in a variety of cancer
tissues. Next, the MEXPRESS methylation analytical tool was used to
analyze THCA promoter levels and the results showed that MAPK12
mRNA expression levels were negatively correlated with MAPK12
methylation levels in THCA. The mRNA levels of MAPK12 were
negatively correlated with the MAPK12 methylation levels at probe
ID: cg19816445 (r=−0.089, P<0.05), probe ID: cg21649580
(r=−0.109, P<0.001), probe ID: cg21028326 (r=−0.108, P<0.05),
probe ID: cg02031597 (r=−0.090, P<0.05), and probe ID:
cg17193921 (r=−0.175, P<0.0001) in THCA (Fig. 8B).
Taken together, these results suggest that the
carcinogenicity of high MAPK12 expression in multiple types of
cancer was due to hypomethylation of its promoter, particularly in
THCA.
Discussion
There are four subtypes of p38 MAPK encoded by
different genes in mammalian cells: P38α (MAPK14), P38β (MAPK11),
P38γ (MAPK12), and P38δ (MAPK13) (33). P38 MAPKs exhibit different
expression patterns in different tissues. P38α was detected in all
cells and tissues, and P38β was specifically overexpressed in brain
tissue, thymus tissue, and spleen tissues. P38β is expressed at low
levels in several tissues, such as the adrenal gland, and it is not
expressed in skeletal muscle. In contrast, P38γ is highly expressed
in skeletal muscle, whilst being expressed at very low levels in
other tissues (34–37). All P38 MAPKs are serine/threonine
kinases that are activated by a variety of inflammatory factors in
a variety of conditions.
MAPK12, also known as P38γ, ERK6, and SAPK3,
regulates some of the processes of malignant transformation in
several human cancer cell lines, such as proliferation, cell cycle
progression, and apoptosis (38,39).
Several researchers found that MAPK12 promoted the development and
progression of various types of cancer (40–42).
However, the role of MAPK12 in THCA metastasis is not known.
Therefore, data from TCGA was used to analyze the functional role
of MAPK12 in various tumors, particularly THCA. The analysis
performed in this study included the expression of MAPK12 at the
RNA level and the effect of differential expression on prognosis,
functional enrichment analysis of MAPK12-related genes, and further
analysis of its effect on tumor cell growth and proliferation, and
the TIM.
The present study found that MAPK12 was
overexpressed in several tumors. The mRNA and protein expression
levels of MAPK12 were higher in THCA cell lines compared with
normal thyroid follicles. Higher mRNA levels of MAPK12 were
associated with a worse OS in KIPAN, LAML, HNSC, LIHC, LUAD, BLCA,
LAML, COADREAD, COAD, ACC, MESO, THCA, and UVM, and a shorter DFS
in KIPAN, STES, HNSC, BRCA, KIRP, BLCA, ACC, COAD, COADREAD, UVM,
MESO, and THCA. GO-BP and KEGG enrichment analyses were performed
using the MAPK12-related genes following analysis of the THCA data
from TCGA, and the results showed that MAPK12-related genes were
enriched in cell proliferation and tumor immune-related functions
and pathways. MAPK12 is highly expressed in HNSC, and it promotes
the proliferation of ESCC cells and prevents their apoptosis in
vitro (24). Hou et al
(25) found that MAPK12 expression
was significantly elevated in human colorectal cancer tissues
relative to the corresponding normal epithelial tissues, and it
promoted the growth, proliferation, and migration of CRC cells
whilst inhibiting cellular apoptosis via the direct phosphorylation
of PTPH1. Xu et al (26)
showed that MAPK12 was positively correlated with the grade of
glioma and may be a tumorigenic factor that promotes the growth and
progression of glioma. Based on these results, it was hypothesized
that MAPK12 promoted the development of tumors pan-cancer,
particularly in THCA, by affecting cell proliferation and antitumor
immunity.
The association between MAPK12 mRNA levels and
immune cell infiltration based on the GO and KEGG results of
MAPK12-related genes was assessed. As an important component of the
TIM, tumor-infiltrating immune cells are generally associated with
the occurrence, progression, treatment, and/or metastasis of tumors
(43). The MAPK12 mRNA levels were
significantly correlated with multiple TIIs/TILs in THCA. A
positive correlation was observed between macrophage numbers and
MAPK12 mRNA expression levels in THCA in the EPIC database. As
macrophages can polarize to antitumor M1 and protumor M2
macrophages (27), the levels of
invading M1 and M2 macrophages were analyzed and the results showed
that the level of invading M2 macrophages was positively correlated
with the expression levels of MAPK12 mRNA in THCA. Therefore, it
was hypothesized that tumor cells secreted chemokines to increase
the infiltration of tumor M2 macrophages in THCA, which increased
the degree of tumor malignancy. Whether MAPK12 affected the
sensitivity of cancer patients to immunotherapy was next assessed.
ICP, MSI, and TMB analyses showed that MAPK12 may be an ideal
target for the treatment of THCA patients, especially in
immunotherapy.
In conclusion, the results of the present study
suggest that MAPK12 may be a promising prognostic marker and a
potential factor for predicting sensitivity to immunotherapy in
patients with malignant tumors, particularly THCA.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Science and Technology
Department of Sichuan Province, China (grant no. 2021YJ0160).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CF and YG conceived and designed the study. JW and
ZS performed the experiments. LR, BZ and YZ analyzed the results.
TL and XY wrote the manuscript and performed some of the
experiments. CF and YG confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACC
|
adrenocortical carcinoma
|
|
BLCA
|
bladder urothelial carcinoma
|
|
BRCA
|
breast invasive carcinoma
|
|
CHOL
|
cholangiocarcinoma
|
|
COAD
|
colon adenocarcinoma
|
|
DFS
|
disease free survival
|
|
ESCA
|
esophageal carcinoma
|
|
GBM
|
glioblastoma multiforme
|
|
HNSC
|
head and neck cancer
|
|
ICP
|
immune checkpoint
|
|
KICH
|
kidney chromophobe
|
|
KIRC
|
kidney renal clear cell carcinoma
|
|
KIRP
|
kidney renal papillary cell
carcinoma
|
|
LAML
|
acute myeloid leukemia
|
|
LGG
|
brain lower grade glioma
|
|
LIHC
|
liver hepatocellular carcinoma
|
|
LUAD
|
lung adenocarcinoma
|
|
LUSC
|
lung squamous cell carcinoma
|
|
MAPK12
|
P38 mitogen-activated protein kinase
12
|
|
MESO
|
mesothelioma
|
|
MSI
|
microsatellite instability
|
|
OS
|
overall survival
|
|
PAAD
|
pancreatic adenocarcinoma
|
|
PRAD
|
prostate adenocarcinoma
|
|
READ
|
rectum adenocarcinoma
|
|
RFS
|
relapse-free survival
|
|
STAD
|
stomach adenocarcinoma
|
|
STES
|
stomach and esophageal carcinoma
|
|
THCA
|
thyroid carcinoma
|
|
TMB
|
tumor mutational burden
|
|
UCEC
|
uterine corpus endometrial
carcinoma
|
|
UVM
|
uveal melanoma
|
References
|
1
|
Sakurai K, Dainichi T, Garcet S, Tsuchiya
S, Yamamoto Y, Kitoh A, Honda T, Nomura T, Egawa G, Otsuka A, et
al: Cutaneous p38 mitogen-activated protein kinase activation
triggers psoriatic dermatitis. J Allergy Clin Immunol.
144:1036–1049. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mai L, Zhu X, Huang F, He H and Fan W: p38
mitogen-activated protein kinase and pain. Life Sci.
256:1178852020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zarubin T and Han J: Activation and
signaling of the p38 MAP kinase pathway. Cell Res. 15:11–18. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coulthard LR, White DE, Jones DL,
Mcdermott MF and Burchill SA: p38(MAPK): Stress responses from
molecular mechanisms to therapeutics. Trends Mol Med. 15:369–379.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uchida S, Yoshioka K, Kizu R, Nakagama H,
Matsunaga T, Ishizaka Y, Poon RY and Yamashita K: Stress-activated
mitogen-activated protein kinases c-Jun NH2-terminal kinase and p38
target Cdc25B for degradation. Cancer Res. 69:6438–6444. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garcia-Hernandez L, Garcia-Ortega MB,
Ruiz-Alcala G, Carrillo E, Marchal JA and Garcia MA: The p38 MAPK
components and modulators as biomarkers and molecular targets in
cancer. Int J Mol Sci. 23:3702021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peluso I, Yarla NS, Ambra R, Pastore G and
Perry G: MAPK signalling pathway in cancers: Olive products as
cancer preventive and therapeutic agents. Semin Cancer Biol.
56:185–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Messina S, Frati L, Leonetti C, Zuchegna
C, Di Zazzo E, Calogero A and Porcellini A: Dual-specificity
phosphatase DUSP6 has tumor-promoting properties in human
glioblastomas. Oncogene. 30:3813–3820. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luan Q, Jin L, Jiang C, Tay KH, Lai F, Liu
XY, Liu YL, Guo ST, Li CY, Yan XG, et al: RIPK1 regulates survival
of human melanoma cells upon endoplasmic reticulum stress through
autophagy. Autophagy. 11:975–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng G, Fan X, Hao M, Wang J, Zhou X and
Sun X: Higher levels of TIMP-1 expression are associated with a
poor prognosis in triple-negative breast cancer. Mol Cancer.
15:302016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi X, Yin N, Ma S, Lepp A, Tang J, Jing W,
Johnson B, Dwinell MB, Chitambar CR and Chen G: p38gamma MAPK is a
therapeutic target for triple-negative breast cancer by stimulation
of cancer stem-like cell expansion. Stem Cells. 33:2738–2747. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen XF, Pan YS, Zheng B and Lu Q:
p38gamma overexpression promotes renal cell carcinoma cell growth,
proliferation and migration. Biochem Biophys Res Commun.
516:466–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang D, Wang Y, Zou X, Shi Y, Liu Q, Huyan
T, Su J, Wang Q, Zhang F, Li X and Tie L: FOXO1 inhibition prevents
renal ischemia-reperfusion injury via cAMP-response element binding
protein/PPAR-γ coactivator-1α-mediated mitochondrial biogenesis. Br
J Pharmacol. 177:432–448. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie Z, Li X, He Y, Wu S, Wang S, Sun J, He
Y, Lun Y and Zhang J: Immune cell confrontation in the papillary
thyroid carcinoma microenvironment. Front Endocrinol (Lausanne).
11:5706042020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu X, Zhong P, Han Y, Huang Q, Wang J, Jia
C and Lv Z: Key candidate genes associated with BRAF(V600E) in
papillary thyroid carcinoma on microarray analysis. J Cell Physiol.
234:23369–23378. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan Z, Li L, Fang Q, Qian Y, Zhang Y, Zhu
J, Ge M and Huang P: Integrated bioinformatics analysis of master
regulators in anaplastic thyroid carcinoma. Biomed Res Int.
2019:97345762019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
The Gene Ontology Consortium, . The Gene
Ontology Resource: 20 years and still GOing strong. Nucleic Acids
Res. 47:D330–D338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanehisa M: A database for post-genome
analysis. Trends Genet. 13:375–376. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koch A, De Meyer T, Jeschke J and Van
Criekinge W: MEXPRESS: Visualizing expression, DNA methylation and
clinical TCGA data. BMC Genomics. 16:6362015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hou S, Suresh PS, Qi X, Lepp A, Mirza SP
and Chen G: p38γ Mitogen-activated protein kinase signals through
phosphorylating its phosphatase PTPH1 in regulating ras protein
oncogenesis and stress response. J Biol Chem. 287:27895–27905.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu Y, Sun Q, Yuan F, Dong H, Zhang H, Geng
R, Qi Y, Xiong X, Chen Q and Liu B: RND2 attenuates apoptosis and
autophagy in glioblastoma cells by targeting the p38 MAPK
signalling pathway. J Exp Clin Cancer Res. 39:1742020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kiaie SH, Sanaei MJ, Heshmati M, Asadzadeh
Z, Azimi I, Hadidi S, Jafari R and Baradaran B: Immune checkpoints
in targeted-immunotherapy of pancreatic cancer: New hope for
clinical development. Acta Pharm Sin B. 11:1083–1097. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei C, Wang B, Peng D, Zhang X, Li Z, Luo
L, He Y, Liang H, Du X, Li S, et al: Pan-cancer analysis shows that
ALKBH5 is a potential prognostic and immunotherapeutic biomarker
for multiple cancer types including gliomas. Front Immunol.
13:8495922022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonaventura P, Shekarian T, Alcazer V,
Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, Caux C and
Depil S: Cold tumors: A therapeutic challenge for immunotherapy.
Front Immunol. 10:1682019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Picard E, Verschoor CP, Ma GW and Pawelec
G: Relationships between immune landscapes, genetic subtypes and
responses to immunotherapy in colorectal cancer. Front Immunol.
11:3692020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goodman AM, Sokol ES, Frampton GM, Lippman
SM and Kurzrock R: Microsatellite-stable tumors with high
mutational burden benefit from immunotherapy. Cancer Immunol Res.
7:1570–1573. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koch A, Joosten SC, Feng Z, de Ruijter TC,
Draht MX, Melotte V, Smits KM, Veeck J, Herman JG, Van Neste L, et
al: Analysis of DNA methylation in cancer: Location revisited. Nat
Rev Clin Oncol. 15:459–466. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rouse J, Cohen P, Trigon S, Morange M,
Alonso-Llamazares A, Zamanillo D, Hunt T and Nebreda AR: A novel
kinase cascade triggered by stress and heat shock that stimulates
MAPKAP kinase-2 and phosphorylation of the small heat shock
proteins. Cell. 78:1027–1037. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lechner C, Zahalka MA, Giot JF, Møller NP
and Ullrich A: ERK6, a mitogen-activated protein kinase involved in
C2C12 myoblast differentiation. Proc Natl Acad Sci USA.
93:4355–4359. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Z, Jiang Y, Ulevitch RJ and Han J: The
primary structure of p38 gamma: A new member of p38 group of MAP
kinases. Biochem Biophys Res Commun. 228:334–340. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang Y, Gram H, Zhao M, New L, Gu J, Feng
L, Di Padova F, Ulevitch RJ and Han J: Characterization of the
structure and function of the fourth member of p38 group
mitogen-activated protein kinases, p38delta. J Biol Chem.
272:30122–30128. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu W, Liu R, Dai Y, Hong S, Dong H and
Wang H: The role of p38gamma in cancer: From review to outlook. Int
J Biol Sci. 17:4036–4046. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin J, Yang J, Xu X, Wang Y, Yu M and Zhu
Y: A robust 11-genes prognostic model can predict overall survival
in bladder cancer patients based on five cohorts. Cancer Cell Int.
20:4022020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roche O, Fernandez-Aroca DM,
Arconada-Luque E, Garcia-Flores N, Mellor LF, Ruiz-Hidalgo MJ and
Sanchez-Prieto R: p38beta and cancer: The beginning of the road.
Int J Mol Sci. 21:75242020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang K, Liu Y, Liu Z, Liu J, Liu X, Chen
X, Li C and Zeng Y: p38γ overexpression in gliomas and its role in
proliferation and apoptosis. Sci Rep. 3:20892013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu M, Wang S, Wang Y, Wu H, Frank JA,
Zhang Z and Luo J: Role of p38gamma MAPK in regulation of EMT and
cancer stem cells. Biochim Biophys Acta Mol Basis Dis.
1864:3605–3617. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mao X, Xu J, Wang W, Liang C, Hua J, Liu
J, Zhang B, Meng Q, Yu X and Shi S: Crosstalk between
cancer-associated fibroblasts and immune cells in the tumor
microenvironment: New findings and future perspectives. Mol Cancer.
20:1312021. View Article : Google Scholar : PubMed/NCBI
|