Introduction
Fluorouracil-based chemotherapy (combined with
oxaliplatin or irinotecan) plus anti-epidermal growth factor
receptor/vascular endothelial growth factor (anti-EGFR/VEGF)
therapy is the standard first-line treatment for metastatic
colorectal cancer, with an overall median survival of about 30
months (1–3). Progression-free survival (PFS) of
first-line treatment is about a year (3,4);
thus, second- or third-line treatment assumes great importance.
Cetuximab (5), bevacizumab
(6), ramucirumab (7) and aflibercept (8) show survival benefits when used as
second-line chemotherapeutants. RAS is a predictive marker
for anti-EGFR therapy; however, no promising biomarkers have been
established for anti-VEGF therapy.
Angiogenesis is regulated by interactions between
vascular endothelial growth factors (VEGFs) and VEGF receptors
(VEGFRs) and is essential for cancer growth and metastasis
(9–11). VEGF-A is the central regulator of
tumor angiogenesis, endothelial proliferation, and survival
(12,13). VEGF-A binds with high affinity to
two structurally similar tyrosine kinase receptors, VEGFR-1 and
VEGFR-2, both of which are expressed in tumor vasculature (14). Blockade of the VEGF-A/VEGFR-2
interaction inhibits tumor angiogenesis and growth. Plasminogen
activator inhibitor-1 (PAI-1) has angiogenic activity and
contributes to tumor progression, tumor invasion, and metastasis
(15). High levels of PAI-1
degrade prognoses of patients with various types of cancers
(16), including colorectal cancer
(17).
At present, three anti-VEGF drugs are available to
block the VEGF pathway in different ways. Bevacizumab is a
humanized monoclonal antibody that binds to VEGF-A and blocks its
activation (4). Ramucirumab is a
humanized IgG1 monoclonal antibody that recognizes VEGFR-2,
preventing binding of agonists, VEGF-A, VEGF-C, and VEGF-D, and
blocking VEGFR-2 activation (7).
Aflibercept is a recombinant fusion protein containing a
VEGF-binding domain, and it antagonizes the activity of VEGF-A,
VEGF-B, and placental growth factor (PlGF) (8). Bevacizumab is used for first-line to
third-line treatment, and ramucirumab or aflibercept in combination
with FOLFIRI is an effective second-line treatment for patients
with metastatic colorectal cancer. However, dynamics and
contributions of angiogenic biomarkers to anti-VEGF therapy have
not been well established. In this retrospective study, we
evaluated those dynamics and contributions to anti-VEGF
therapy.
Materials and methods
Patients and study design
We conducted a retrospective study of patients with
metastatic colorectal cancer who were treated with anti-VEGF-drugs
between May 2015 and July 2021. This study included two cohorts.
Cohort 1 comprised patients who were treated with cytotoxic agents
and bevacizumab as first-line chemotherapy, and Cohort 2 included
patients who were treated with cytotoxic agents and anti-VEGF drugs
(bevacizumab, ramucirumab, or aflibercept) as second-line
chemotherapy. We included patients who participated in a bio-bank
project at our institution. This project was approved by local
ethics review boards (28-03-738) and written informed consent was
obtained from all patients who participated in this project.
Inclusion criteria were: histologically confirmed adenocarcinoma of
the colon or rectum, patients 20–80 years of age, Eastern
Cooperative Oncology Group performance status of 0–1, and adequate
organ function (white blood cell count ≥3.0×109 cells/l,
≥1.5×109 neutrophils/l, platelets ≥100×109/l,
hemoglobin ≥10.0 g/dl, serum bilirubin ≤1.5× upper limit of normal;
alanine aminotransferase and aspartate amino transferase ≤2.5×
upper limit of normal, and serum creatinine ≤1·5× upper limit of
normal), known RAS and BRAF status (mutant or
wild-type), and blood samples stocked in the bio-bank. The presence
of at least one measurable reference lesion following the Response
Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was also
required. Patients with a history of another malignancy within the
past 5 years were excluded. All Cohort 1 patients were chemo-naïve.
Eligible patients of Cohort 2 had to have experienced disease
progression within 6 months of the last dose of first-line
combination therapy with oxaliplatin and a fluoropyrimidine for
metastatic disease and had to have received at least one cycle of
doublet therapy. Exclusion criteria included brain metastases,
poorly controlled hypertension, or any arterial thrombotic or
thromboembolic events within 12 months prior to starting
chemotherapy. The study was conducted according to ethical
guidelines of the Declaration of Helsinki, and the protocol was
approved by local ethics review boards (B-2021-467). Information
about the right to opt-out was posted on the websites of Main
hospital of Nippon Medical School
Sample collection
In the bio-bank project, blood samples (10 ml in BD
Vacutainer EDTA tube: Becton Dickinson) were obtained from
participants every 1–2 months during chemotherapy. Blood samples
were centrifuged at 1,900 g for 10 min and the upper layer of each
sample was transferred to another tube and stored at −80°C until
analysis.
Measurement of VEGF-A, VEGF-D, PlGF,
and PAI-1
Plasma samples stored from the start to the end of
chemotherapy every 2 months were used for measurement of angiogenic
factors. VEGF-A, VEGF-D, PlGF, and PAI-1 were measured using
commercially available enzyme-linked immunosorbent assay (ELISA)
kits (VEGF-A: Human VEGF Quantikine kit, VEGF-D: VEGF-D Duoset
ELISA kit, PlGF: Human PlGF Quantikine kit, PAI-1: Human Serpin
E1/PAI-1 Duoset ELISA kit. All kits were from R & D Systems,
Minneapolis, MN, USA) and were used according to the manufacturer's
protocols. To measure PAI-1 concentrations, samples were diluted
200-fold.
Evaluation of clinical responses
Tumor responses were assessed by computed tomography
(CT) following RECIST 1.1 criteria, 3 months after starting
chemotherapy. After the initial assessment, CT was performed every
3 months until disease progression. Patients who achieved complete
responses (CR) or partial responses (PR) were categorized as
responders, and those who achieved stable disease (SD) or
progressive disease (PD) were considered non-responders.
Carcinoembryonic antigen (CEA) and CA19-9 were assayed monthly
throughout chemotherapy. The normal CEA level was <5.0 ng/ml and
the normal CA19-9 level was <37 U/ml.
Statistical analysis
Statistical analysis was performed using R version
4.1.2 (The R Foundation for Statistical Computing, Vienna,
Austria). The Mann-Whitney U test was used to compare differences
in each angiogenic factor. Multiple comparisons of the dynamics of
each angiogenic factors were tested with the Dunn's test after the
Kruskal-Wallis test. To evaluate impacts of VEGFs on the
cytoreductive effect, patients were divided into high, medium-high,
medium-low, and low groups with quartile values for each angiogenic
factor. Clinical responses were tested using Fisher's exact tests.
To evaluate impact of VEGFs on survival, patients were divided into
two groups, high and low, with the median as the cut-off for each
angiogenic factor. Progression free survival (PFS) and overall
survival (OS) were tested using Kaplan-Meier analysis followed by
the log-rank test and two-stage test.
Results
Patients
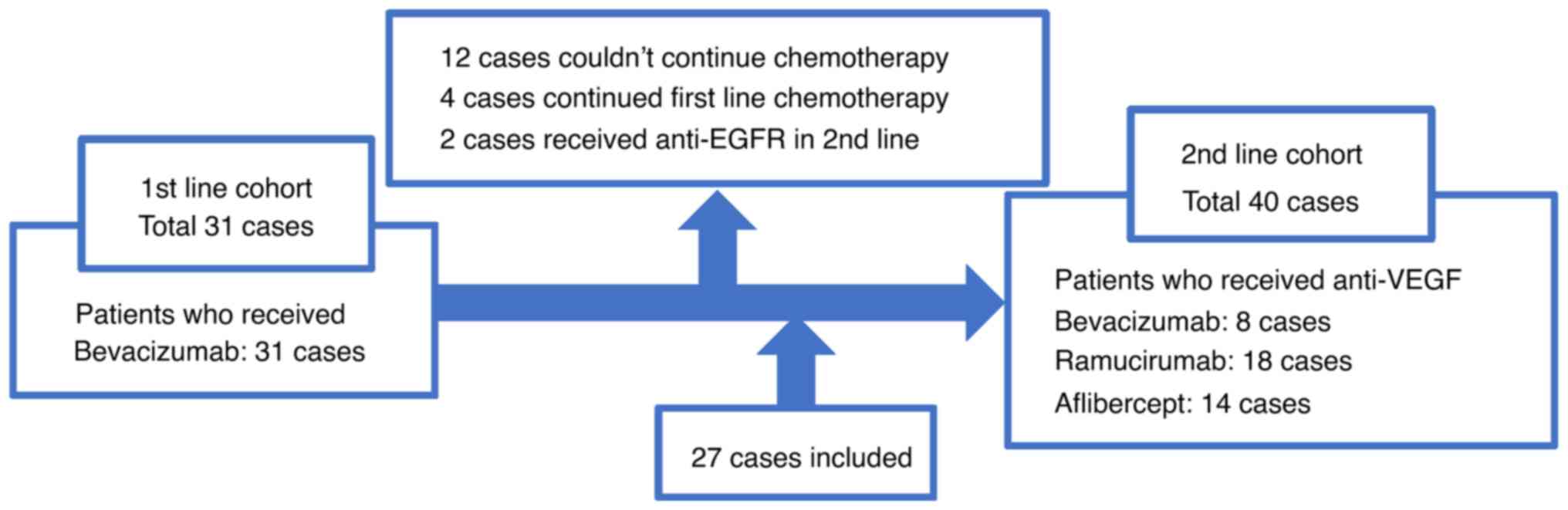
Thirty-one patients were included in Cohort 1 and 40
patients in Cohort 2 (Fig. 1).
Twelve of 31 Cohort 1 patients had not received second-line
chemotherapy. Four Cohort 1 patients were continuing first-line
chemotherapy. Two patients were administered anti-EGFR agents as
second-line chemotherapy, and the remaining 13 patients were
included in Cohort 2. Patient characteristics are shown in Table I and adverse events are listed in
Table SI. In Cohort 1, 17
patients had one metastatic site, 9 patients had 2 metastatic
sites, 5 patients had three or more metastatic sites total numbers
of cases; liver: 18 cases, lung: 9 cases, peritoneum: 11 cases,
others such as lymph node or bone: 12 cases). In Cohort 2, 26
patients had one metastatic site, 10 patients had 2 metastatic
sites, and 4 patients had three or more metastatic sites (total
number of cases: liver: 23 cases, lung: 12 cases, peritoneum: 9
cases, others: 12 cases). Twenty-two patients (71.0%) with
RAS mutations were included in Cohort 1 and 22 more (55.0%)
in Cohort 2. No patients with BRAF mutations were included
in either cohort. Among 40 patients belonging to Cohort 2, 8
patients received bevacizumab, 18 received ramucirumab, and 14
received aflibercept. In first-line chemotherapy, 21 of the 40
Cohort 2 patients received bevacizumab and 19 received anti-EGFR
drugs or no molecular target drugs.
 | Table I.Baseline characteristics. |
Table I.
Baseline characteristics.
| Variables | Cohort 1
(N=31) | Cohort 2
(N=40) |
|---|
| Median age, years
(IQR) | 64 (12.5) | 64 (12.0) |
| Sex, n (male:
female) | 15:16 | 26:14 |
| ECOG performance
status, n (0:1) | 19:12 | 30:10 |
| Tumor location, n
(right: left) | 9:22 | 7:33 |
| CEA, n (<10: ≥10
ng/ml) | 11:20 | 12:28 |
| Number of
metastatic sites, n (1:2: ≥3) | 17:9:5 | 26: 10:4 |
| RAS status
(tissue), n (wild-type: mutant) | 9:22 | 18:22 |
| BRAF status
(tissue), n (wild-type: mutant) | 31:0 | 40:0 |
| First line
chemotherapy, n |
|
|
| FOLFOX
+ bevacizumab | - | 21 |
| FOLFOX
+ anti-EGFRs or none | - | 19 |
| PFS, n (<6: ≥6
months) | 9:22 | 15:25 |
| Biomarker |
|
|
| Median
VEGF-A, pg/ml (IQR) | 56.7 (130.8) | 342.9 (682.1) |
| Median
VEGF-D, pg/ml (IQR) | 339.8 (534.0) | 459.5 (610.3) |
| Median
PlGF, pg/ml (IQR) | 8.3 (5.1) | 16.9 (12.4) |
| Median
PAI-1, ng/ml (IQR) | 16.1 (14.6) | 17.8 (16.5) |
Cohort 1
Outcomes of chemotherapy
In Cohort 1, median cycles of chemotherapies
administered were 14 (IQR: 16). Six patients (19%) achieved a CR,
12 (39%) achieved a PR, 9 (29%) experienced SD, and four (13%)
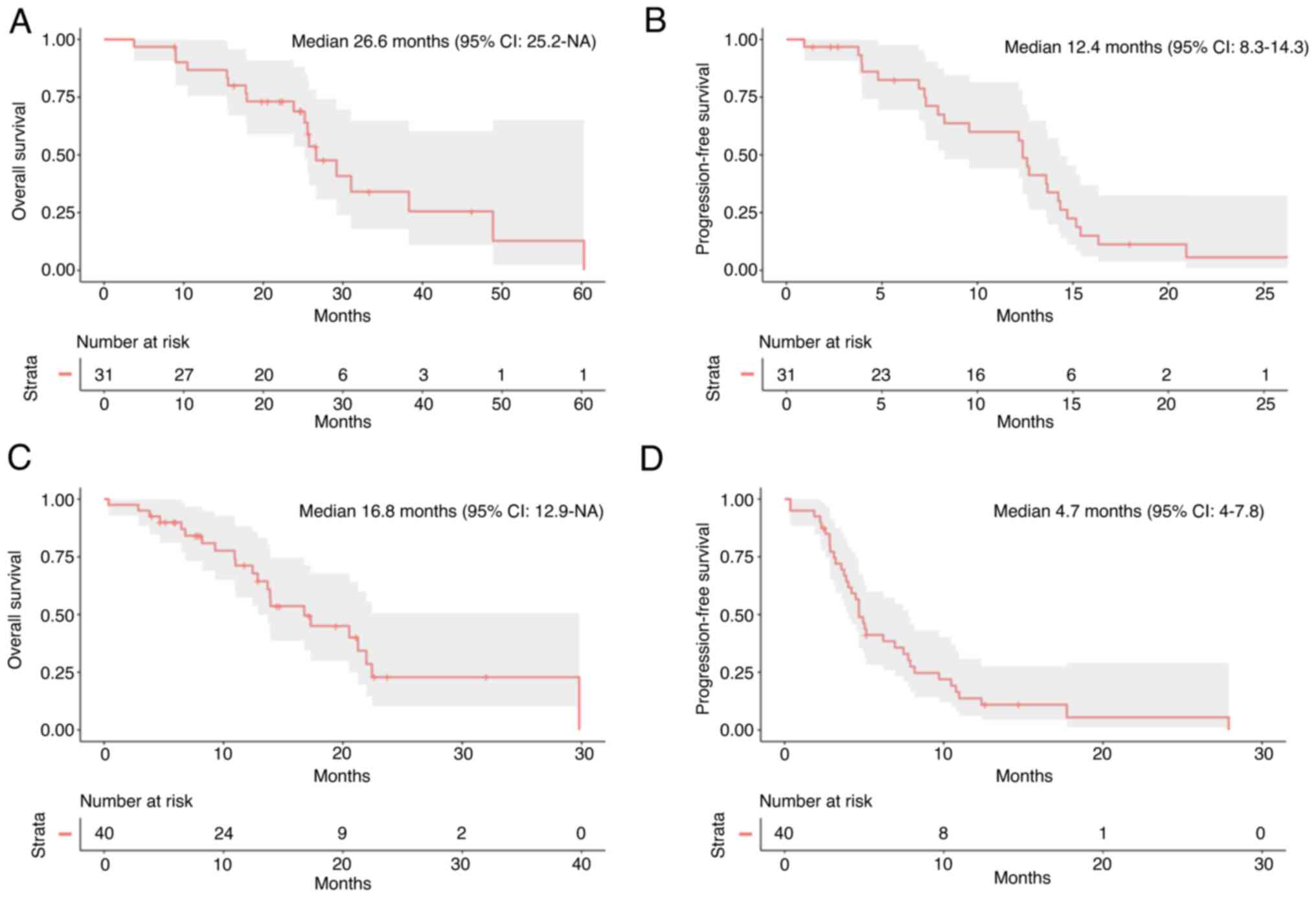
experienced PD. Median follow-up was 26.5 months (IQR 10.0). Median
OS was 26.6 months and median PFS was 12.4 months (Fig. 2A and B).
VEGF-A, VEGF-D, PlGF, and PAI-1
levels
At the start of first-line chemotherapy, there were
no correlations between the four angiogenic factors.
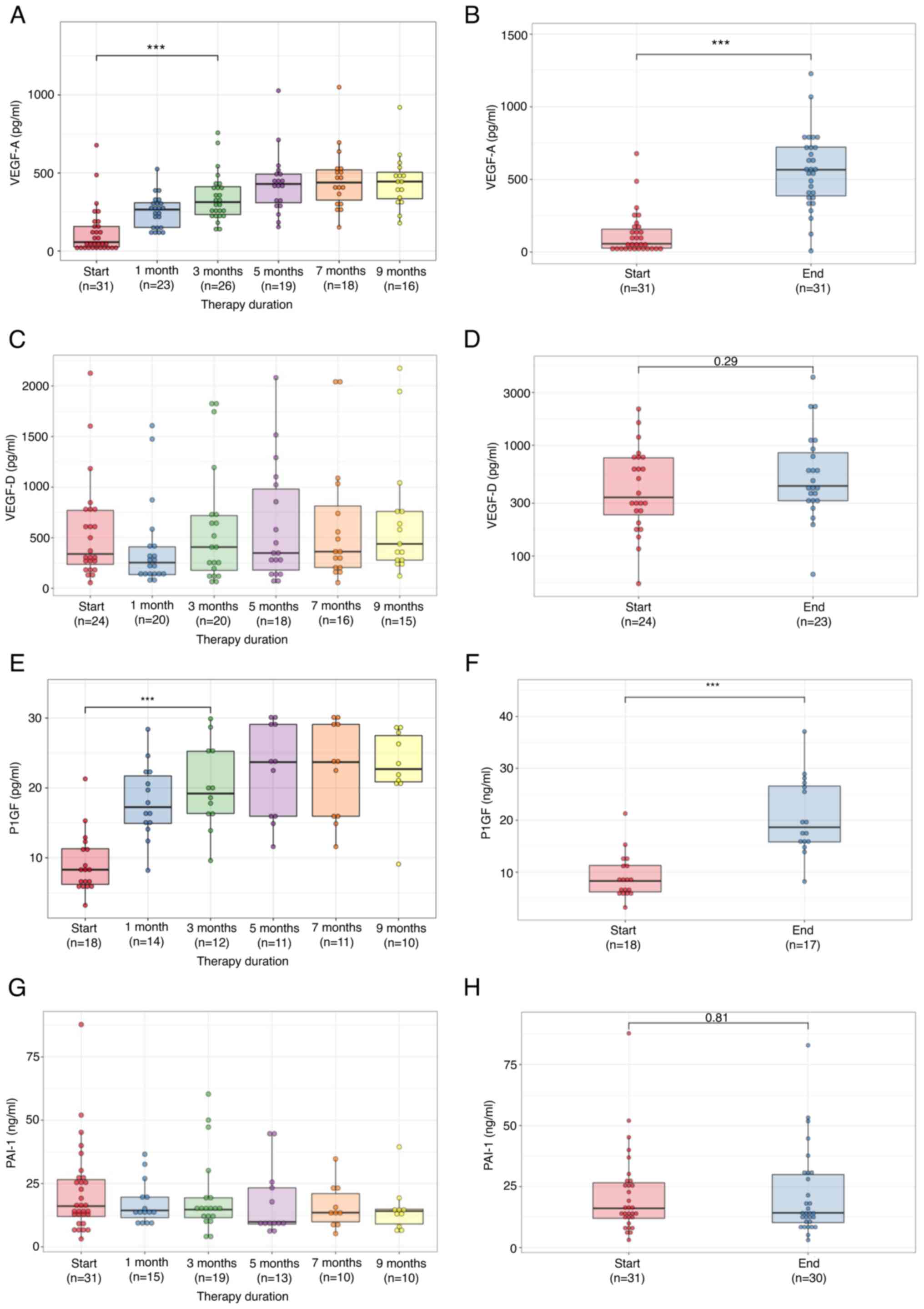
Median VEGF-A level before treatment was 56.7 pg/ml
(IQR: 131.0). There was no relationship between VEGF-A level and
tumor RAS status, or the number of metastatic sites and
tumor location (right or left side). VEGF-A level increased
significantly one month after starting chemotherapy and continued
to rise during treatment (Fig.
3A). At the end of chemotherapy, VEGF-A level (median: 566.8
pg/ml, IQR: 335.9) was significantly higher than before
chemotherapy (P<0.0001, Fig.
3B).
Median VEGF-D level before treatment was 340.0 pg/ml
(IQR: 534.0). There was no relationship between VEGF-D level and
tumor RAS status, or the number of metastatic sites and
tumor side. Bevacizumab had no impact on VEGF-D (Fig. 3C), and VEGF-D level at the end of
therapy (median: 429.4 pg/ml, IQR: 543.0) was the same as before
chemotherapy (P=0.29, Fig.
3D).
Median PlGF level before chemotherapy was 8.3 pg/ml
(IQR: 5.1). There was no relationship between PlGF level and tumor
RAS status, or between the number of metastatic sites and
tumor side. PlGF level increased significantly one month after
starting chemotherapy and continued to rise during chemotherapy
(Fig. 3E). PlGF levels at the end
of therapy (median: 18.6 pg/ml, IQR: 11.3) were significantly
higher than before (P<0.0001, Fig.
3F).
Median PAI-1 level before treatment was 16.1 ng/ml
(IQR: 14.6). It had no relationship with VEGF-A level, tumor
RAS status, the number of metastatic sites, or tumor side.
Bevacizumab had no impact on PAI-1 levels (Fig. 3G) and PAI-1 levels at the end of
therapy (median: 14.2 ng/ml, IQR: 19.7) were unchanged (P=0.81,
Fig. 3H).
Impact of VEGF-A, VEGF-D, PlGF, and
PAI-1 levels on the cytoreductive effect and survival
With regard to VEGF-A levels, 72.2% (13/18) of
responders (CR or PR) belonged to the medium-low and medium-high
quartiles. Conversely, 23.1% (3/13) of non-responders (SD or PD)
belonged to the medium-low and medium-high quartiles (Table II, P=0.05). VEGF-D, PlGF, and
PAI-1 levels did not influence the cytoreductive effect (Table II). There were no differences in
dynamics of those four angiogenic factors between responders and
non-responders (Fig. S1).
 | Table II.Impact of angiogenic factors on the
cytoreductive effect. |
Table II.
Impact of angiogenic factors on the
cytoreductive effect.
| Angiogenic
factors | Low | Medium low | Medium high | High | P-value |
|---|
| VEGF-A |
|
|
|
| 0.05 |
|
Responder | 2 | 7 | 6 | 3 |
|
|
Non-responder | 6 | 1 | 2 | 4 |
|
| VEGF-D |
|
|
|
| 0.46 |
|
Responder | 4 | 5 | 4 | 2 |
|
|
Non-responder | 2 | 1 | 2 | 4 |
|
| PlGF |
|
|
|
| 0.73 |
|
Responder | 3 | 3 | 3 | 2 |
|
|
Non-responder | 1 | 1 | 2 | 3 |
|
| PAI-1 |
|
|
|
| 0.25 |
|
Responder | 4 | 6 | 6 | 2 |
|
|
Non-responder | 4 | 2 | 2 | 5 |
|
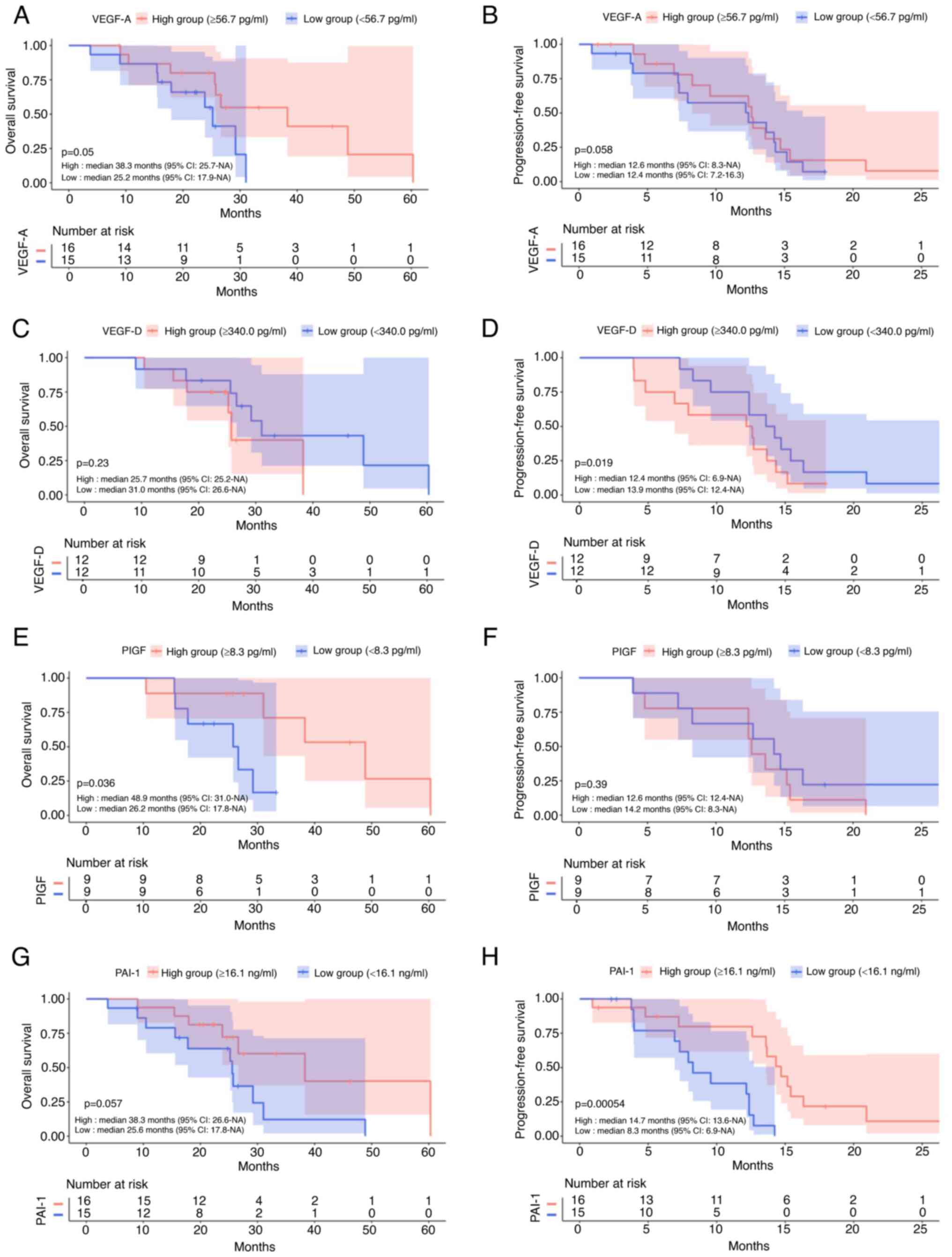
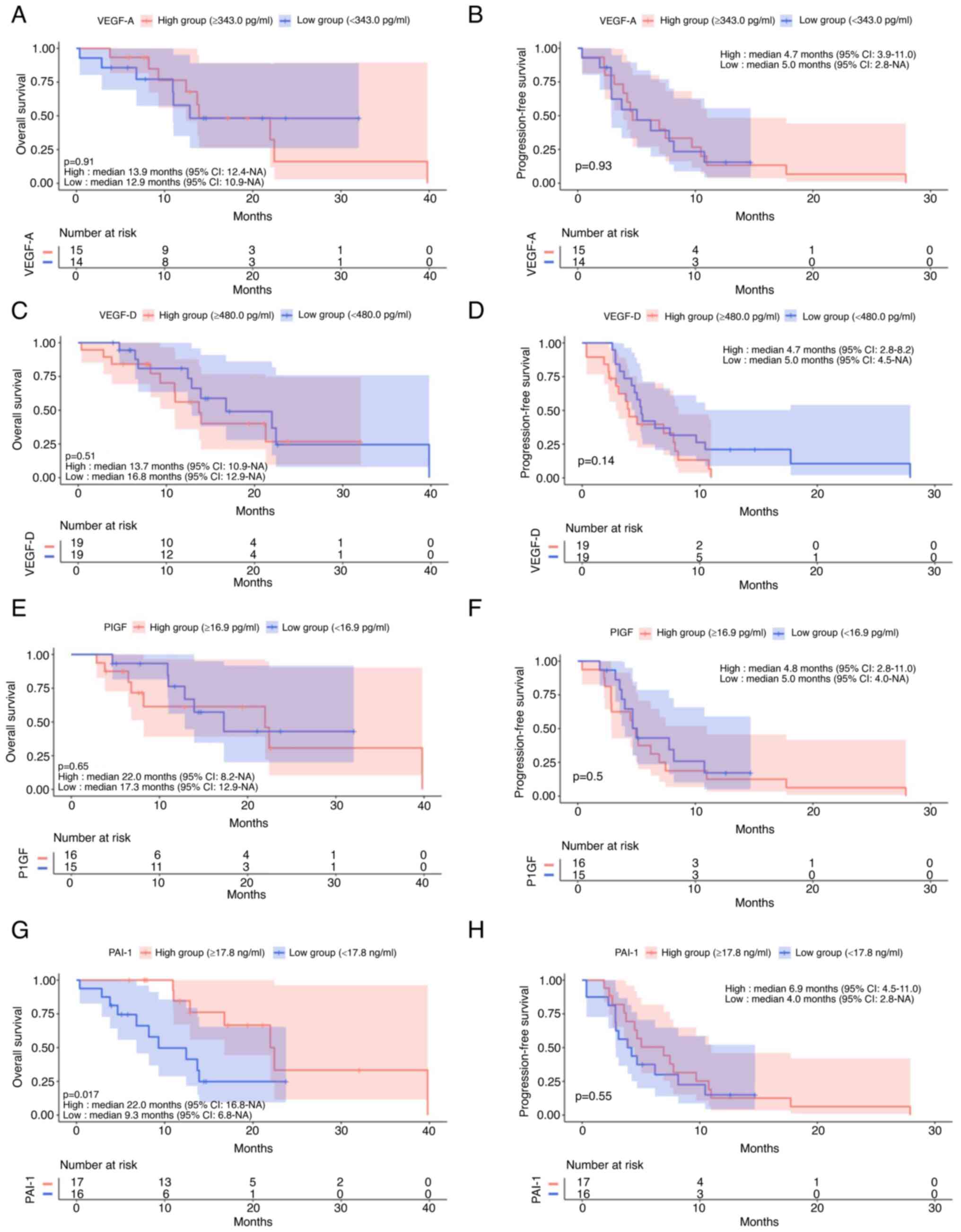
The low VEGF-A group had significantly shorter OS
(P=0.05, Fig. 4A). The low PlGF
group had significantly shorter OS (P=0.036, Fig. 4E) by log-rank test; however,
non-significant by two-stage test (P=0.86). PFS was not affected by
VEGF-A and PlGF (P=0.58, P=0.39, Fig.
4B and F). PAI-1 level had a small impact on OS (P=0.057,
Fig. 4G), and the low PAI-1 group
had significantly reduced PFS (P=0.0005, Fig. 4H). VEGF-D level did not affect OS
or PFS (P=0.23, P=0.19, Fig. 4C and
D).
Cohort 2
Outcomes of chemotherapy
In Cohort 2, the median cycles of chemotherapy were
8 with ramucirumab (IQR: 11.5) and with aflibercept (IQR: 9.25).
One patient (2.5%) achieved CR, 3 (7.5%) achieved PR, 25 (62.5%)
experienced SD, and 11 (27.5%) experienced PD. Median follow-up was
12.5 months. Median OS was 16.8 months (Fig. 2C) and median PFS was 4.7 months
(Fig. 2D).
VEGF-A, VEGF-D, PlGF, and PAI-1
levels
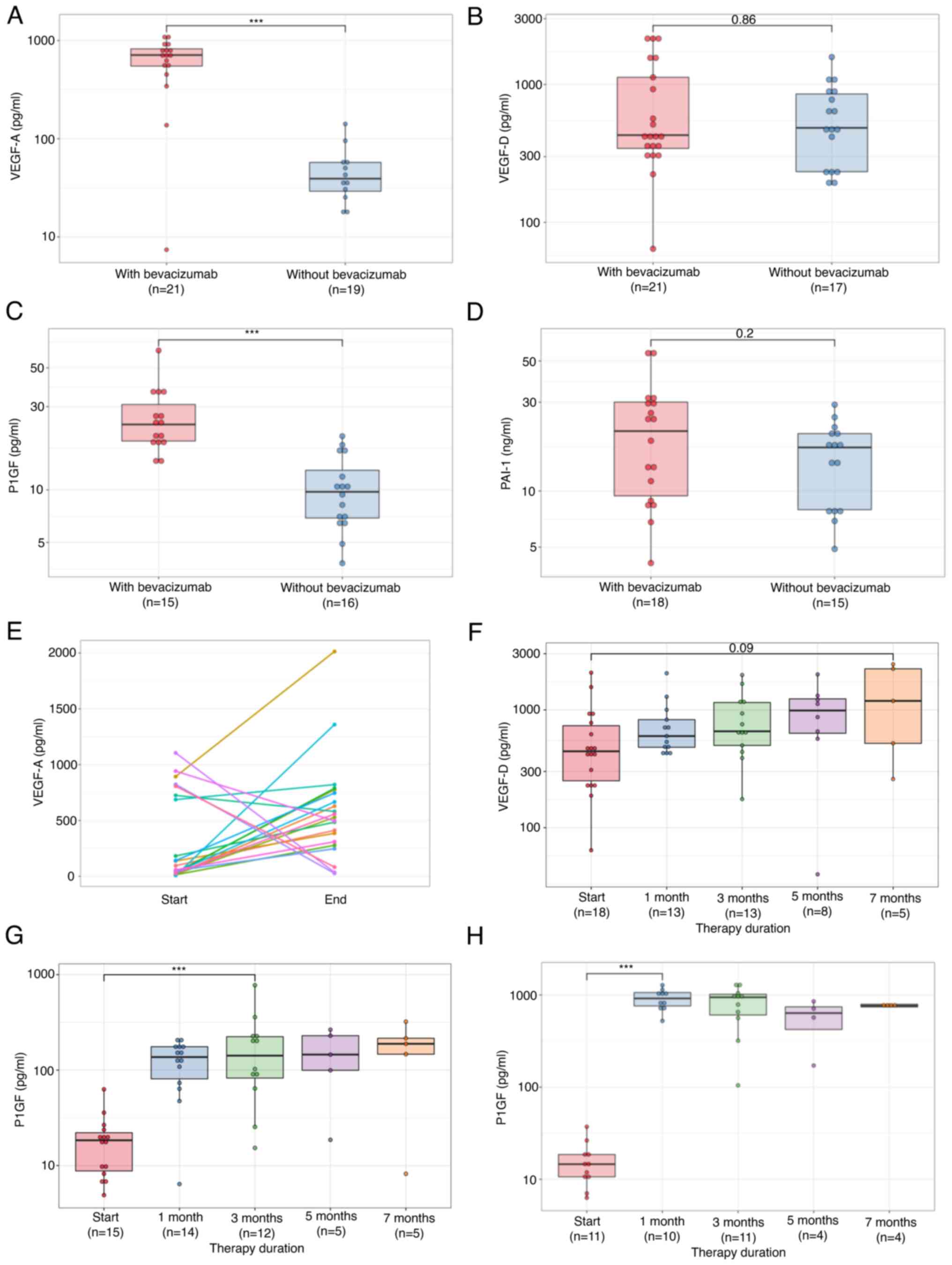
Median VEGF-A, VEGF-D, PlGF and PAI-1 levels of all
Cohort 2 patients before treatment were 342.9 pg/ml (IQR: 682.1),
480.0 pg/ml (IQR: 610.2), 16.9 pg/ml (IQR: 12.4) and 17.8 ng/ml
(IQR: 16.5). VEGF-A and PlGF levels of patients before second-line
chemotherapy treated with bevacizumab during first-line
chemotherapy were significantly higher than those of patients
treated without bevacizumab (P<0.00001, P<0.0001 Fig. 5A and C). Conversely, VEGF-D and
PAI-1 levels before second-line chemotherapy of patients treated
with bevacizumab in first-line chemotherapy were equal to those of
patients treated without bevacizumab (P=0.86, P=0.2, Fig. 5B and D).
In 5 of 7 patients with high levels of VEGF-A at the
start of second-line therapy, VEGF-A level decreased at the end
thereof. In all patients with low levels of VEGF-A at the start of
second-line therapy, VEGF-A level increased until the end of
treatment (Fig. 5E).
In patients treated with ramucirumab, VEGF-D
increased over time (Fig. 5F);
however, in patients treated with bevacizumab or aflibercept, there
was no obvious increase. Also, PlGF increased gradually in patients
treated with ramucirumab and aflibercept (Fig. 5G and H). Only 8 patients received
bevacizumab as a second-line. In these patients, PlGF increased;
however, the difference was not significant.
Impact of VEGF-A, VEGF-D, PlGF, and
PAI-1 levels on survival
VEGF-A (P=0.91, P=0.93, Fig. 6A and B), VEGF-D (P=0.51, P=0.14,
Fig. 6C and D) and PlGF (P=0.65,
P=0.5, Fig. 6E and F) levels had
no impact on OS or PFS. Low PAI-1 patients had significantly
shorter OS (P=0.017, Fig. 6G);
however, PAI-1 level had no impact on PFS (P=0.55, Fig. 6H).
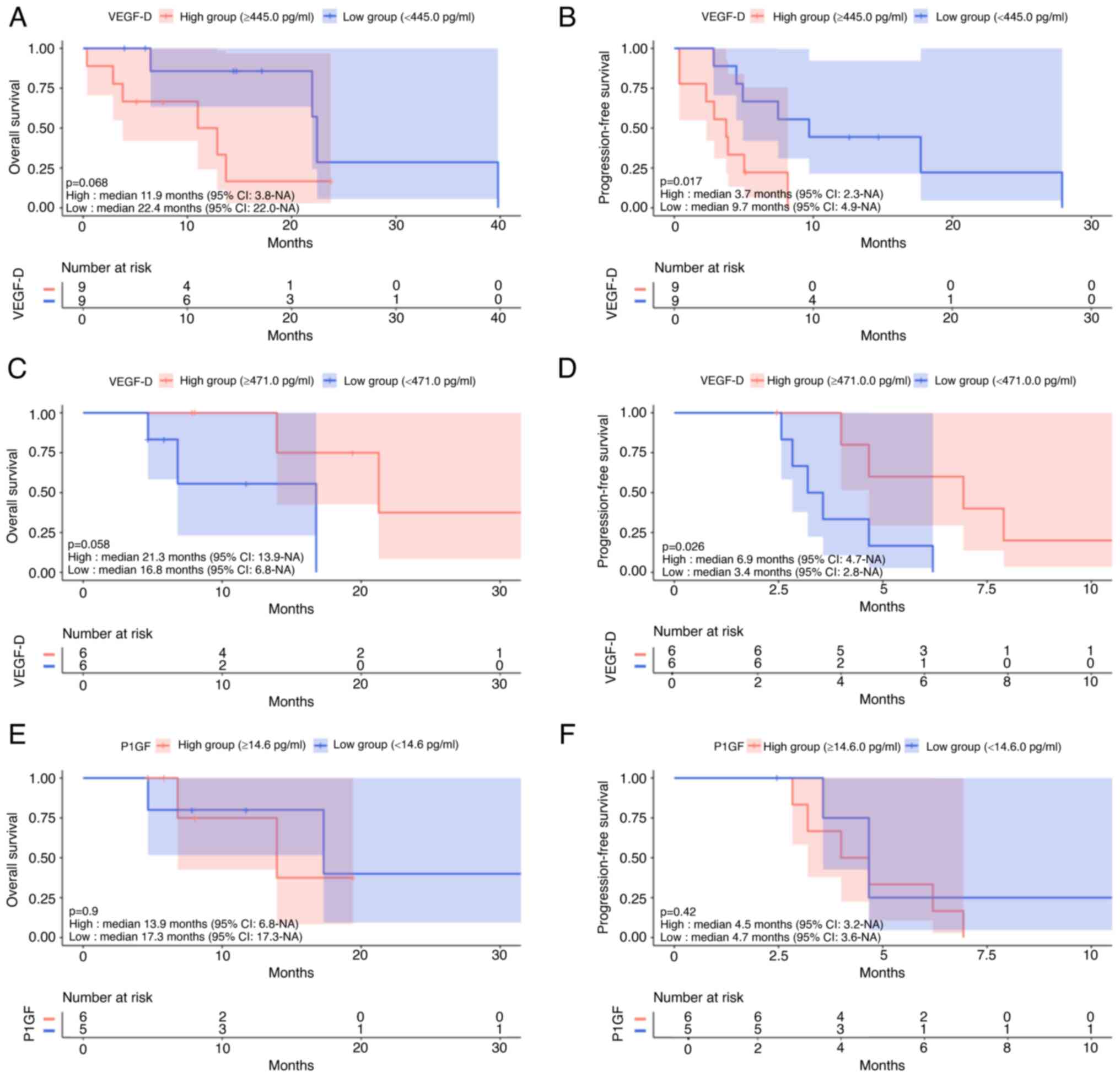
In patients treated with ramucirumab, patients with
high VEGF-D levels had non-significantly shorter OS (P=0.068,
Fig. 7A) and significantly shorter
PFS (P=0.017, Fig. 7B). VEGF-A,
PlGF, and PAI-1 had no impact on OS (P=0.61, P=0.79, P=0.41) and
PFS (P=0.85, P=0.27, P=0.30). In patients treated with aflibercept,
the high VEGF-D group had non-significantly longer OS (P=0.058,
Fig. 7C) and significantly longer
PFS (P=0.026, Fig. 7D). VEGF-A,
PlGF, and PAI-1 had no impact on OS (P=0.61, P=0.90, P=0.26) or PFS
(P=0.41, P=0.42, P=0.059). Fig. 7E and
F show that PlGF levels had no impact on OS or PFS of patients
who were treated with aflibercept.
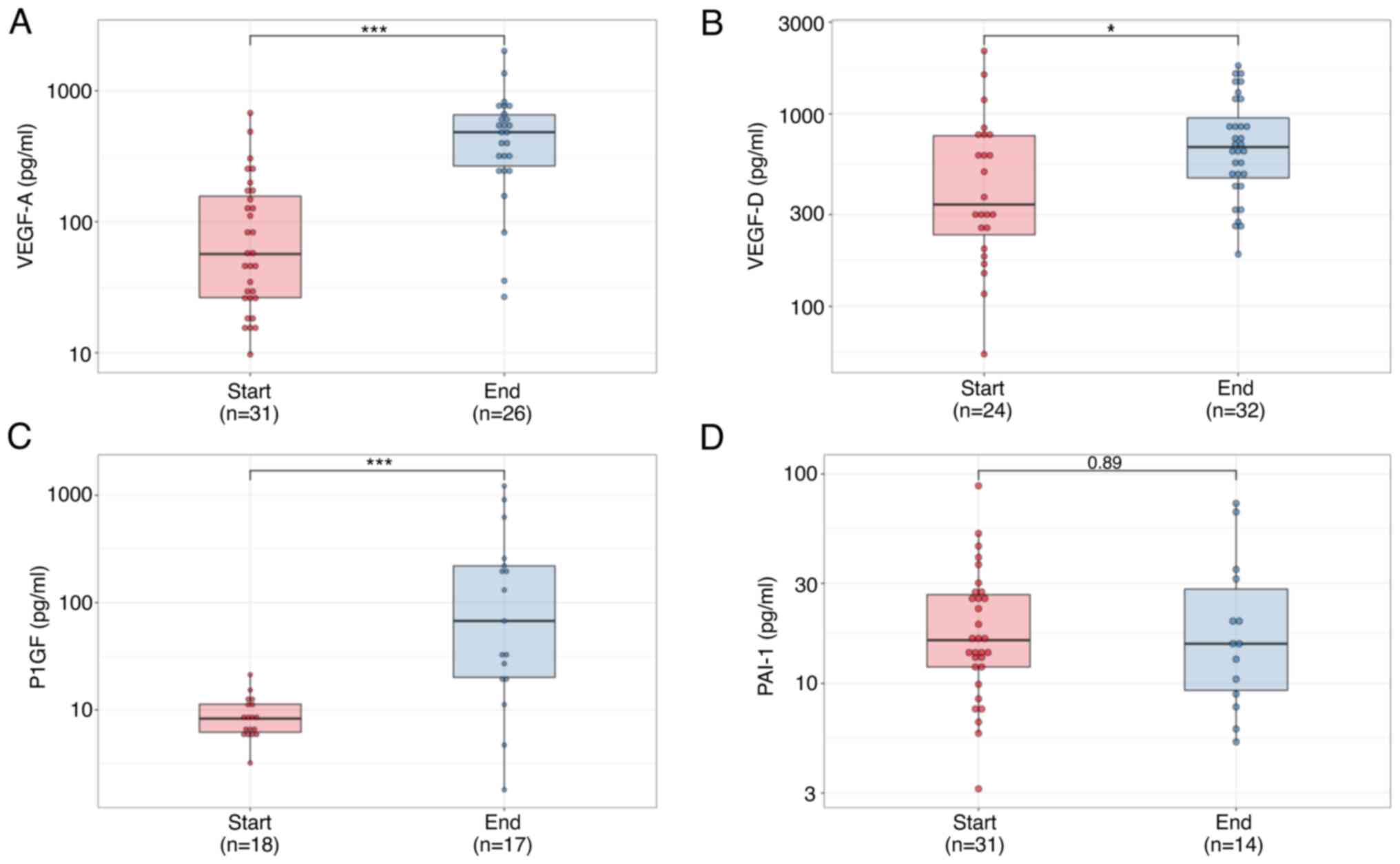
VEGF-A, VEGF-D, PlGF, and PAI-1 levels
at the end of second-line chemotherapy
At the end of second-line chemotherapy, VEGF-A,
VEGF-D, and PlGF levels were significantly higher than baseline
(P<0.0001, P=0.022, P<0.0001 Fig. 8A-C); however, PAI-1 levels did not
increase (P=0.89, Fig. 8D).
Discussion
In the present study, there were three valuable
findings. First, bevacizumab increases VEGF-A and PlGF levels, but
not VEGF-D or PAI-1 levels. Conversely, anti-EGFRs have no impact
on levels of these four growth factors. Second, anti-VEGF drugs
have greater benefit for patients with high PAI-1 levels before
starting first- and second-line chemotherapy, and PAI-1 level is
not affected by anti-VEGF drugs. Third, VEGF-D may be a useful
biomarker for drug selection in second-line chemotherapy.
It is noteworthy that VEGF and PlGF levels increase
one month after starting bevacizumab. It has been reported that
VEGF-A and PlGF levels are high in patients who had received prior
bevacizumab (18). However, the
present study shows that VEGF and PlGF levels increase one month
after starting bevacizumab and maintain high levels during drug
administration. Bevacizumab inhibits angiogenesis by binding VEGF-A
(19). Thus, bevacizumab is less
beneficial for patients with low VEGF-A levels before chemotherapy,
as indicated in Cohort 1. However, the sustainable increase of
VEGF-A induced by bevacizumab may provoke acquired resistance to
bevacizumab. Indeed, most responders had medium-low or medium-high
levels of VEGF-A before starting chemotherapy, indicating that
adequate VEGF-A levels provide benefits for patients treated with
bevacizumab. There appears to be no benefit if the initial VEGF-A
levels are too high or too low. Conversely, high VEGF-A levels may
not provide a benefit in patients treated with ramucirumab. This
hypothesis is supported by the fact that ramucirumab has better
outcomes in bevacizumab-naïve patients than in patients whose
first-line treatment included bevacizumab (20). We believe that the VEGF-A increase
induced by bevacizumab has an unfavorable impact on efficacy of
ramucirumab.
Anti-VEGF drugs have greater benefit for patients
with high PAI-1 levels before chemotherapy in either the first or
second line. This is the first study showing that high PAI-1 levels
are a favorable prognostic factor for patients receiving
second-line chemotherapy with ramucirumab or aflibercept. It has
been reported that high PAI-1 levels are a poor prognostic factor
for stage I–IV colorectal cancer patients who are not receiving
chemotherapy (17). Previous
studies have already reported that high PAI-1 levels are an
unfavorable prognostic factor for patients receiving bevacizumab
(21–23), in contrast to results of the
present study. Tumor angiogenesis requires PAI-1 (24), and PAI-1 has a dose-dependent
effect on tumor angiogenesis (25). Inhibition of PAI-1 limits tumor
angiogenesis (26), indicating
that patients with high PAI-1 levels have hyper-vascular tumors
that can respond to anti-VEGFs. However, patients with high PAI-1
levels had better PFS, but the same OS during the first line.
Conversely, in the present study, patients with high PAI-1 levels
had better OS, but similar PFS in the second line. Thus, we need
further studies to clarify or resolve this contradiction.
In the present study, we measured PAI-1 repeatedly
and showed that bevacizumab had no impact on PAI-1 levels. In
previous studies, bevacizumab decreased PAI-1 levels in patients
with lung cancer (21), metastatic
solid cancers (22), or colorectal
cancer (23); however, PAI-1
levels were measured only once after starting chemotherapy in these
studies. PAI-1 antigen is mainly detected in fibroblasts and
endothelial cells (27),
indicating that PAI-1 levels are strongly affected by the
microenvironment of cancer cells; thus, an increase or decrease of
PAI-1 has a complex mechanism. In the present study, tumor
progression or shrinkage had no effect on PAI-1 levels and there
was no association between VEGF-A and PAI-1 levels. Thus, PAI-1
inhibitors are accepted as anti-cancer drugs by virtue of their
anti-angiogenic effects.
VEGF-D may be a useful biomarker for drug selection
in second-line chemotherapy. Ramucirumab has less benefit for
patients with high VEGF-D levels and ramucirumab increases VEGF-D.
Conversely, aflibercept has greater benefit for patients with high
VEGF-D levels without increasing VEGF-D. VEGF-D has no effect on
benefits of bevacizumab and is not affected by bevacizumab. In the
present study, patients with low VEGF-D levels had significantly
better PFS and non-significantly better OS. Tabernero et al
(28) reported that ramucirumab
has a favorable impact on patients with high levels of VEGF-D
(>115 pg/ml) before chemotherapy, but no other studies have
reported an association between ramucirumab and VEGF-D levels. In
the present study, VEGF-D level was ≤115 pg/ml in only one of 18
patients who were treated with ramucirumab. As with the association
between bevacizumab and VEGF-A level, too high a level of VEGF-D
may restrict the efficacy of ramucirumab. Ramucirumab increased
VEGF-D levels one month after starting chemotherapy and sustained
the elevation during the second line; however, bevacizumab and
aflibercept did not. Although no studies, including that by
Tabernero et al (28),
reported VEGF-D dynamics after starting chemotherapy, including
ramucirumab, this increase is easy to understand in that VEGF-D
elevation caused acquired resistance to ramucirumab. The fact that
VEGF-A level did not show a distinctive trend after administration
of ramucirumab, supports this hypothesis. Interestingly, patients
with high levels of VEGF-D had greater benefit from aflibercept. In
the biomarker study, VELOUR, VEGF-D was not measured (18); thus, the present study is the first
to report a clear association between VEGF-D level and efficacy of
aflibercept. PlGF had no impact on the effect of aflibercept,
similar to the results of a previous study (18).
Results of the present study suggest that moderate
levels of VEGF-A are necessary for a favorable effect of
bevacizumab, and moderate levels of VEGF-D are necessary for
reasonable efficacy of ramucirumab. Bevacizumab inhibits
angiogenesis by blocking VEGF-A, and ramucirumab inhibits it by
blocking VEGF-D. Not surprisingly, bevacizumab has little effect in
patients with low VEGF-A levels and ramucirumab does not help
patients with low VEGF-D levels. However, it is surprising that
bevacizumab may be less effective for patients with very high
levels of VEGF-A, and ramucirumab, likewise, may be less useful for
patients with very high levels of VEGF-D.
This study had several limitations. This is a
retrospective, single-center study that included a small number of
patients. Thus, regimens of chemotherapy other than anti-VEGFs were
not standardized. It is unclear whether VEGF-A, VEGF-D, PlGF, or
PAI-1 impacted the prognosis of patients receiving anti-EGFR
therapy, because those were the only patients we included. The
present study failed to identify an optimal level of VEGF-A and
VEGF-D because it included small numbers of patients; thus, further
study is needed.
In conclusion, there is an optimal level of VEGF-A
for a favorable effect of bevacizumab and also of VEGF-D for
positive outcomes with ramucirumab. VEGF-A and PlGF levels are
increased by bevacizumab and VEGF-D levels are increased by
ramucirumab. Anti-VEGF drugs have benefits for patients with high
PAI-1 levels and PAI-1 levels are not affected by anti-VEGFs. These
biomarkers may be useful for predicting drug efficacy and
interpreting resistance to anti-VEGF drugs. Presently, two
prospective studies (the Brave Ace study and the Ukit study) which
evaluate the utility of biomarkers, including VEGFs, in patients
treated with anti-VEGF drugs in second-line chemotherapy, are
ongoing. These two studies have larger sample sizes compared with
our study; thus, they may provide additional information on the
efficacy of VEGFs.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on request.
Authors' contributions
TY, SKu and HY designed the study. TY, SKu, HS, AM,
RO, SS, YY, GT, TI, KT, TM, KY, SKa and KU acquired, analyzed and
interpreted the data. TY and SKu wrote the manuscript. AM and RO
were involved in drafting the manuscript. HY revised the manuscript
critically. TY and SKu confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Our bio-bank project was approved by Medical Ethics
Committee of Nippon Medical School (Bunkyo-ku, Tokyo, Japan;
28-03-738) and written informed consent was obtained from all
patients. The present study was approved by the Medical Ethics
Committee of Nippon Medical School (Bunkyo-ku, Tokyo, Japan;
B-2021-467). Information about the right to opt-out for the present
study was posted on the websites of Nippon Medical School. All
experimental procedures were performed according to regulations and
internal biosafety and bioethics guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
VEGF
|
vascular endothelial growth factor
|
|
EGFR
|
epidermal growth factor receptor
|
|
mCRC
|
metastatic colorectal cancer
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
RECIST
|
Response Evaluation Criteria in Solid
Tumors
|
References
|
1
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lenz HJ, Ou FS, Venook AP, Hochster HS,
Niedzwiecki D, Goldberg RM, Mayer RJ, Bertagnolli MM, Blanke CD,
Zemla T, et al: Impact of consensus molecular subtype on survival
in patients with metastatic colorectal cancer: Results From
CALGB/SWOG 80405 (Alliance). J Clin Oncol. 37:1876–1885. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwartzberg LS, Rivera F, Karthaus M,
Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS and Go WY: PEAK: A
randomized, multicenter phase II study of panitumumab plus modified
fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab
plus mFOLFOX6 in patients with previously untreated, unresectable,
wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol.
32:2240–2247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sobrero A, Lenz HJ, Eng C, Scheithauer W,
Middleton G, Chen W, Esser R, Nippgen J and Burris H: Extended RAS
analysis of the phase III EPIC trial: Irinotecan + Cetuximab versus
irinotecan as second-line treatment for patients with metastatic
colorectal cancer. Oncologist. 26:e261–e269. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bennouna J, Sastre J, Arnold D, Österlund
P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C,
et al: Continuation of bevacizumab after first progression in
metastatic colorectal cancer (ML18147): A randomised phase 3 trial.
Lancet Oncol. 14:29–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tabernero J, Yoshino T, Cohn AL,
Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy
DC, Van Cutsem E, Grothey A, et al: Ramucirumab versus placebo in
combination with second-line FOLFIRI in patients with metastatic
colorectal carcinoma that progressed during or after first-line
therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine
(RAISE): A randomised, double-blind, multicentre, phase 3 study.
Lancet Oncol. 16:499–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tabernero J, Van Cutsem E, Lakomý R,
Prausová J, Ruff P, van Hazel GA, Moiseyenko VM, Ferry DR,
McKendrick JJ, Soussan-Lazard K, et al: Aflibercept versus placebo
in combination with fluorouracil, leucovorin and irinotecan in the
treatment of previously treated metastatic colorectal cancer:
Prespecified subgroup analyses from the VELOUR trial. Eur J Cancer.
50:320–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neufeld G, Cohen T, Gengrinovitch S and
Poltorak Z: Vascular endothelial growth factor (VEGF) and its
receptors. FASEB J. 13:9–22. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrara N: Molecular and biological
properties of vascular endothelial growth factor. J Mol Med (Berl).
77:527–543. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leung DW, Cachianes G, Kuang WJ, Goeddel
DV and Ferrara N: Vascular endothelial growth factor is a secreted
angiogenic mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inoue M, Hager JH, Ferrara N, Gerber HP
and Hanahan D: VEGF-A has a critical, nonredundant role in
angiogenic switching and pancreatic beta cell carcinogenesis.
Cancer Cell. 1:193–202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Vries C, Escobedo JA, Ueno H, Houck K,
Ferrara N and Williams LT: The fms-like tyrosine kinase, a receptor
for vascular endothelial growth factor. Science. 255:989–991. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Placencio VR and DeClerck YA: Plasminogen
activator inhibitor-1 in cancer: Rationale and insight for future
therapeutic testing. Cancer Res. 75:2969–2974. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Binder BR and Mihaly J: The plasminogen
activator inhibitor ‘paradox’ in cancer. Immunol Lett. 118:116–124.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nielsen HJ, Christensen IJ, Sørensen S,
Moesgaard F and Brünner N: Preoperative plasma plasminogen
activator inhibitor type-1 and serum C-reactive protein levels in
patients with colorectal cancer. The RANX05 colorectal cancer study
group. Ann Surg Oncol. 7:617–623. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Van Cutsem E, Paccard C, Chiron M and
Tabernero J: Impact of prior bevacizumab treatment on VEGF-A and
PlGF levels and outcome following second-line aflibercept
treatment: Biomarker Post Hoc analysis of the VELOUR trial.
Clin Cancer Res. 26:717–725. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Presta LG, Chen H, O'Connor SJ, Chisholm
V, Meng YG, Krummen L, Winkler M and Ferrara N: Humanization of an
anti-vascular endothelial growth factor monoclonal antibody for the
therapy of solid tumors and other disorders. Cancer Res.
57:4593–4599. 1997.PubMed/NCBI
|
|
20
|
Suzuki T, Shinozaki E, Osumi H, Nakayama
I, Ota Y, Ichimura T, Ogura M, Wakatsuki T, Ooki A, Takahari D, et
al: Second-line FOLFIRI plus ramucirumab with or without prior
bevacizumab for patients with metastatic colorectal cancer. Cancer
Chemother Pharmacol. 84:307–313. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niki M, Yokoi T, Kurata T and Nomura S:
New prognostic biomarkers and therapeutic effect of bevacizumab for
patients with non-small-cell lung cancer. Lung Cancer (Auckl).
8:91–99. 2017.PubMed/NCBI
|
|
22
|
Liu Y, Starr MD, Brady JC, Rushing C, Pang
H, Adams B, Alvarez D, Theuer CP, Hurwitz HI and Nixon AB:
Modulation of circulating protein biomarkers in cancer patients
receiving bevacizumab and the anti-endoglin antibody, TRC105. Mol
Cancer Ther. 17:2248–2256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bal O, Ekinci AS, Dogan M, Atay C, Demirci
A, Oksuzoglu B and Kilic S: The prognostic and predictive
significance of plasma type 1 plasminogen activator inhibitor and
endoglin in metastatic colorectal cancer patients treated with
bevacizumab-containing chemotherapy. J Cancer Res Ther. 15:48–53.
2019.PubMed/NCBI
|
|
24
|
Bajou K, Noël A, Gerard RD, Masson V,
Brunner N, Holst-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen
D and Foidart JM: Absence of host plasminogen activator inhibitor 1
prevents cancer invasion and vascularization. Nat Med. 4:923–928.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bajou K, Maillard C, Jost M, Lijnen RH,
Gils A, Declerck P, Carmeliet P, Foidart JM and Noel A:
Host-derived plasminogen activator inhibitor-1 (PAI-1)
concentration is critical for in vivo tumoral angiogenesis and
growth. Oncogene. 23:6986–6990. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takayama Y, Hattori N, Hamada H, Masuda T,
Omori K, Akita S, Iwamoto H, Fujitaka K and Kohno N: Inhibition of
PAI-1 limits tumor angiogenesis regardless of angiogenic stimuli in
malignant pleural mesothelioma. Cancer Res. 76:3285–3294. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Naitoh H, Eguchi Y, Ueyama H, Kodama M and
Hattori T: Localization of urokinase-type plasminogen activator,
plasminogen activator inhibitor-1, 2 and plasminogen in colon
cancer. Jpn J Cancer Res. 86:48–56. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tabernero J, Hozak RR, Yoshino T, Cohn AL,
Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy
DC, Prausová J, et al: Analysis of angiogenesis biomarkers for
ramucirumab efficacy in patients with metastatic colorectal cancer
from RAISE, a global, randomized, double-blind, phase III study.
Ann Oncol. 29:602–609. 2018. View Article : Google Scholar : PubMed/NCBI
|