Introduction
Colorectal cancer (CRC) is one of the most common
carcinomas worldwide, and its clinical and therapeutic management
and prognostic stratification are important issues in oncology. It
has been well recognized that the tumor microenvironment plays an
important role in cancer progression and invasion in various types
of carcinomas, including CRC (1,2). The
fibrotic stroma response is histopathologically observed around
carcinoma cell nests to a greater or lesser extent [i.e.
desmoplastic reaction (DR)] in various types of carcinomas. In CRC,
DR has received attention for its prognostic significance (3–10),
and other prognostic indicators such as tumor budding (TB)
(11) and tumor deposits (TDs)
have similarly received attention (12). The prognostic significance of DR in
patients with CRC was first reported by Ueno et al (13,14),
and was classified into three categories: immature, intermediate,
and mature DR. They clearly demonstrated that the presence of
immature type DR indicated significantly poor overall and
disease-free survival, following intermediate and mature types in
patients with stage II and III CRC (4,6–10).
Thus, DR has been used as a useful prognostic indicator in
surgically resected CRC specimens, especially for determining the
treatment strategy after surgical resection (3).
TD is also an important prognostic indicator in
patients with CRC and is also called ‘extramural tumor deposits
without lymph node structures̛ (12,15).
TD is defined as discrete macroscopic or microscopic nodules
composed of carcinoma cells located in the extramural fatty tissue,
discontinuous from the primary tumor, and without lymph node
structures (15). The presence of
TDs is significantly correlated with a higher incidence of liver
and lung metastases and poorer disease-free and overall survival
(12). Thus, according to the
recent classification, the presence of TD is regarded to change
lymph node status to pN1c, if all lymph nodes are negative for
metastasis.
Although it has been reported that immature type DR
is significantly correlated with higher pT stage, presence of
lymphovascular invasion, and presence of lymph node metastases
(7,10), the relationship between DR and TD
in patients with CRC has not yet been examined. Thus, the present
study aimed to clarify the relationship between DR type and the
presence of TD.
Materials and methods
Patient selection
We selected consecutive patients with CRC who
underwent surgical resection in the Department of Surgery at Kansai
Medical University Hospital between January 2016 and December 2021.
Patients with pT1 or pT2 were excluded from this study because DR
was defined as tumors with pT3 or pT4 (3). Accordingly, 443 patients with pT3 or
pT4 CRC were included in this study. Patients who had undergone
neoadjuvant chemotherapy and/or radiation therapy were excluded
from the study.
This retrospective, single-institution study was
conducted according to the principles of the Declaration of
Helsinki, and the study protocol was approved by the Institutional
Review Board of Kansai Medical University Hospital (Approval
#2021197). All data were anonymized. The institutional review board
waived the requirement for informed consent because of the
retrospective study design, as medical records and archived samples
were used with no risk to the participants. Moreover, the present
study did not include minors. Information regarding this study,
such as the inclusion criteria and the opportunity to opt out, was
provided through the institutional website (https://www.kmu.ac.jp/hirakata/hospital/2671t800001356c-att/a1642567101597.pdf).
Histopathological analysis
Surgically resected specimens were fixed with
formalin, sectioned, and stained with hematoxylin and eosin. Two
researchers (TK and MI) independently evaluated the
histopathological features of all the tumor slides. The TNM
Classification of Malignant Tumours, Eighth edition was used.
DR was classified into immature, intermediate, and
mature types according to the definition by Ueno et al
(3,7,9).
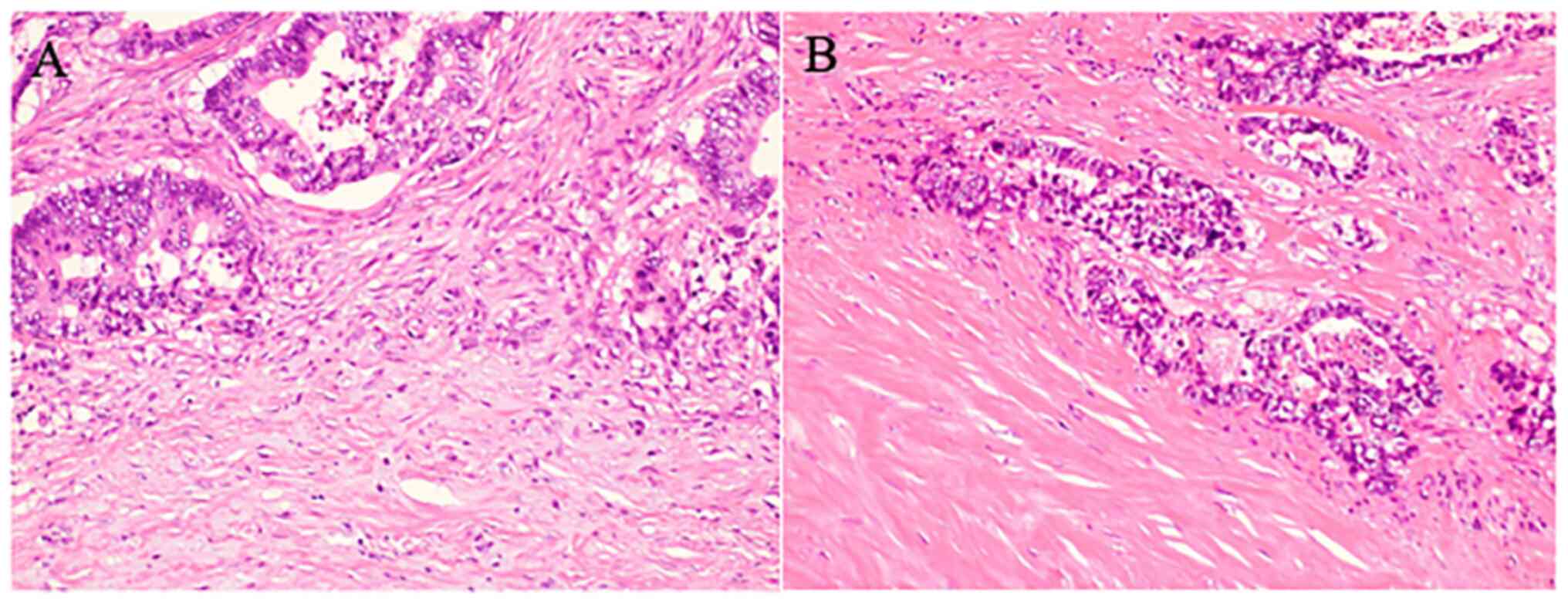
Briefly, the immature type is histopathologically characterized by
the presence of myxoid stroma (defined as stroma accompanied by an
amorphous mucoid material) greater than a microscopic field of ×400
magnification, at the invasive front of the tumor (Fig. 1A). The intermediate type is defined
by the presence of keloid-like collagen, (thick bundles of
hypocellular collagen showing hyalinization) without myxoid stroma
(Fig. 1B); the absence of myxoid
stroma and keloid-like collagen is regarded as the mature type.
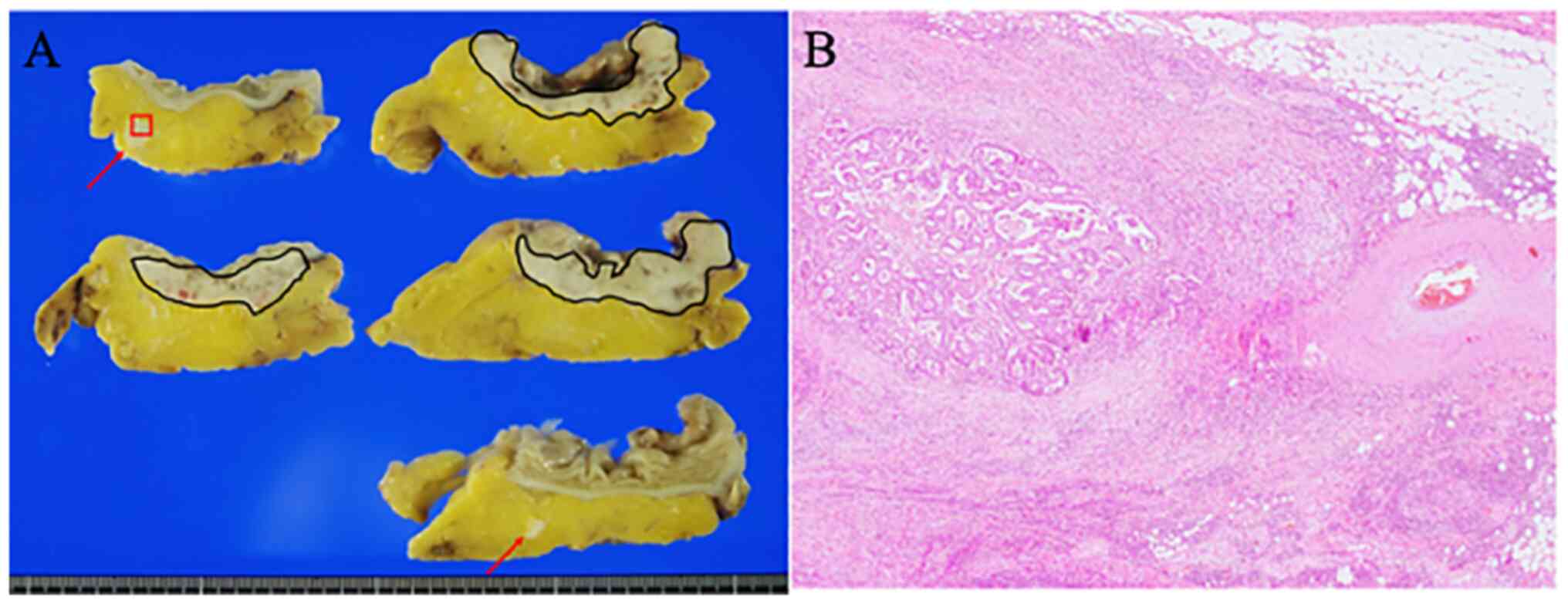
Typical histopathological features of TD are shown
in Fig. 2. TB was evaluated in
accordance with the 2016 International Tumor Budding Consensus
Conference (16). TB was defined
as a single tumor cell or a cluster of up to four tumor cells in
one hotspot at the invasive front of a field measuring 0.785
mm2 (16). Tumors with
0–4 buds were classified as TB1, 5–9 buds as TB2, and those with
more than 10 buds as TB3 (16).
Statistical analyses
All analyses were performed using JMP, version 13.0
(SAS Institute). Correlations between the two groups were analyzed
using the χ2 test or Fisher's exact test for categorical
variables. Mann-Whitney U test was used for continuous variables.
Logistic regression analysis was performed to detect the odds
ratios between DR type and other clinicopathological indicators.
Statistical significance was set at P<0.05.
Results
Patient characteristics
Table I summarizes
the clinicopathological features of the present cohort. This study
included 205 (46.3%) women and 238 (53.7%) men. The median age at
the time of surgery was 73 years (range: 21–99 years). 293 (66.1%)
patients were classified as pT3, and 150 (33.9%) patients as pT4.
The tumor locations were as follows: 230 patients had tumors in the
right colon (51.9%), 156 patients (35.2%) in the left colon, and 57
patients in the rectum (12.9%). Lymph node metastasis was observed
in 226 patients (51.0%).
 | Table I.Clinicopathological features between
immature and intermediate/mature desmoplastic reactions. |
Table I.
Clinicopathological features between
immature and intermediate/mature desmoplastic reactions.
|
| Desmoplastic reaction
category |
|
|---|
|
|
|
|
|---|
| Variables | Immature, n=282
(%) | Intermediate/Mature,
n=161 (%) | P-value |
|---|
| Sex |
|
| 0.4902 |
| Male | 148 (52.5) | 90 (55.9) |
|
|
Female | 134 (47.5) | 71 (44.1) |
|
| Median age, years
(range) | 72.5 (21–96) | 74 (37–99) | 0.2291 |
| Location |
|
| 0.1845 |
| Right
side | 140 (49.7) | 90 (55.9) |
|
| Left
side | 100 (35.5) | 56 (34.8) |
|
|
Rectum | 42 (14.8) | 15 (9.3) |
|
| pT |
|
| <0.0001 |
| pT3 | 160 (56.7) | 133 (82.6) |
|
|
pT4a/b | 122 (43.3) | 28 (17.4) |
|
| pN |
|
| <0.0001 |
|
Negative | 108 (38.3) | 109 (67.7) |
|
|
Positive | 174 (61.7) | 52 (32.3) |
|
| pStagea |
|
| <0.0001 |
| II | 109 (38.7) | 109 (68.6) |
|
| III | 148 (52.5) | 50 (31.0) |
|
| Tumor
differentiation |
|
| 0.6115 |
| Well | 55 (19.5) | 30 (18.6) |
|
|
Moderate | 168 (59.6) | 104 (64.6) |
|
| Poor | 35 (12.4) | 18 (11.2) |
|
|
Mucinous | 24 (8.5) | 9 (5.6) |
|
| Lymphatic
invasion |
|
| 0.0007 |
|
Positive | 234 (83.0) | 111 (68.9) |
|
|
Negative | 48 (17.0) | 50 (31.1) |
|
| Venous
invasion |
|
| <0.0001 |
|
Positive | 275 (97.5) | 134 (83.2) |
|
|
Negative | 7 (2.5) | 27 (16.8) |
|
| TB |
|
| <0.0001 |
|
TB1 | 72 (25.5) | 73 (45.3) |
|
|
TB2/TB3 | 210 (74.5) | 88 (54.7) |
|
| Perineural
invasion |
|
| <0.0001 |
|
Positive | 222 (78.7) | 76 (47.2) |
|
|
Negative | 60 (21.3) | 85 (52.8) |
|
| Tumor deposits |
|
| <0.0001 |
|
Positive | 81 (28.7) | 12 (7.5) |
|
|
Negative | 201 (71.3) | 149 (92.5) |
|
Immature, intermediate, and mature types of DR were
noted in 282 patients (63.7%), 91 patients (20.5%), and 70 patients
(15.8%), respectively. TD was observed in 93 (21.0%) patients.
Table I summarizes the
clinicopathological parameters of immature and intermediate/mature
types of DR. Immature type DR was significantly correlated with a
higher pT stage (P<0.0001), pStage (P<0.0001), presence of
lymph node metastasis (P<0.0001), and lymphatic (P=0.0007),
venous (P<0.0001), and perineural invasion (P<0.0001), as
well as higher TB (P<0.0001) compared to intermediate/mature
types of DR. Moreover, immature type DR was significantly
correlated with the presence of TDs (P<0.0001).
Odds ratios between immature type DR
and other clinicopathological indicators
Multivariate analyses were used to analyze the odds
ratio (OR) between immature type DR and other clinicopathological
indicators (Table II). This
analysis revealed that the presence of perineural and venous
invasion, a higher pT stage (pT4), and TDs were significantly
correlated with immature type DR (OR, 4.905; 3.539; 3.285; 2.884,
P=0.00001, 0.00029, 0.00052, and 0.00131, respectively); however,
histological subtypes, higher budding (TB2 and TB3), lymphatic
invasion were not significantly correlated with immature type
DR.
 | Table II.Odds ratios between immature type
desmoplastic reaction and other clinicopathological indicators. |
Table II.
Odds ratios between immature type
desmoplastic reaction and other clinicopathological indicators.
| Variable | Odds ratio | P-value |
|---|
| Perineural
invasion | 4.905 | 0.00001 |
| Venous
invasion | 3.539 | 0.00029 |
| pT4 | 3.285 | 0.00052 |
| Tumor deposits | 2.884 | 0.00131 |
| Tumor
differentiation (Poor) | 0.863 | 0.13701 |
| Tumor
differentiation (Mucinous) | 0.656 | 0.22085 |
| Tumor budding
2/3 | 0.293 | 0.50925 |
| Lymphatic
invasion | 0.041 | 0.91065 |
Discussion
The present study demonstrated for the first time
that there is a significant correlation between TD and
immature-type DR in CRC. Previous studies have shown a significant
correlation between immature type DR and a higher TB and the
presence of lymphovascular invasion and lymph node metastasis
(4,9,10).
TD is a histopathological parameter that indicates poor prognosis
in CRC (12,15). The mechanism of TD formation has
been investigated, and a study revealed that more than half of the
TDs have perineural and/or intravascular connections (17). Moreover, immature type DR was
significantly correlated with the presence of lymphovascular and
perineural invasion in the present cohort, as well as in previous
reports (4,9,10).
Therefore, there might be a close relationship between
lymphovascular and perivascular invasion, TD, and immature type
DR.
TB is also an important prognostic indicator in CRC,
and the prognostic significance of TB has also been reported in
other types of carcinomas, regardless of the histological type of
cancer (both adenocarcinoma and squamous cell carcinoma) (18–22).
For example, Noda et al recently analyzed the significance
of DR, TB, tumor-infiltrating lymphocytes, and depth of invasion of
oral squamous cell carcinoma to determine its ability to predict
extranodal extension, which is an important indicator of poor
prognosis for this type of carcinoma (21). They clearly demonstrated that both
a higher TB and immature type DR were significantly correlated with
the presence of lymphovascular invasion and lymph node metastasis.
In addition, higher TB was an independent indicator for extranodal
extension of oral squamous cell carcinoma using multivariate
analysis, and the presence of TB and immature type DR in biopsy
specimens was a useful indicator for predicting extranodal
extension of oral squamous cell carcinoma (21). A significant correlation between
higher TB and immature type DR was noted in the present cohort of
CRC patients, which corresponded to previous reports regarding CRC
(4,9,10).
TB is speculated to be closely related to the
epithelial-mesenchymal transition (EMT) of carcinoma cells
(16). These results suggest that
both higher TB and immature type DR are correlated with EMT in
carcinoma cells of CRC patients (discussed below), leading to
poorer prognosis in patients with CRC.
It has been speculated that the development of
immature type DR is related to the tumor microenvironment,
especially cancer-associated fibroblasts (CAFs) (3,10,23).
CAFs are important components of the cancer stroma in various types
of carcinomas, including CRC. A previous study showed that
tenascin-C and fibronectin, which are involved in cancer
angiogenesis, are frequently present in immature type DR of CRC
(10). Moreover, a significant
correlation between high periostin expression, a protein that is
related to EMT in cancer, and immature type DR in CRC tissues has
been recently reported (23).
Although the detailed molecular mechanism for the formation of
immature type DR remains unclear, the tumor microenvironment,
including CAFs, plays an important role in the development of DR.
CAFs are considered to play important roles in EMT in carcinoma
cells. Therefore, a close relationship between lymphovascular
invasion, DR, TB, and TD may exist. Additional studies are needed
to clarify the detailed molecular mechanism governing this
association, leading to novel therapeutic targets for patients with
CRC, especially for those with immature type DR.
Accurate risk stratification for predicting the
recurrence and/or metastasis after surgical resection in patients
with CRC is a very important issue for post-operative treatment
strategy. The present study for the first time demonstrated the
close correlation between immature-type DR and the presence of TD.
This result provides information regarding one of the mechanisms
between immature-type DR and poor prognosis. Additional studies are
needed to clarify the molecular mechanism between immature-type DR
and poor prognosis.
The present study has several limitations. First,
and most importantly, this study was a single-institute
retrospective analysis, which led to bias in statistical power of
the study. Second, this study examined the relationship between DR
and TD. The prognostic significance of DR and TD in the present
cohort was not analyzed because the follow-up period was less than
5 years. Therefore, additional studies with larger numbers of CRC
patients are needed to clarify the correlation between DR and TD,
and their prognostic significance.
In conclusion, the present study demonstrated a
significant correlation between immature type DR and the presence
of TD in patients with pT3 and pT4 CRC. A close relationship
between lymphovascular invasion, DR, TB, and TD is also suggested
based on this study. However, additional studies are needed to
analyze the detailed mechanism underlying the development of
immature type DR in CRC. These studies provide important
information for the risk stratification of metastasis and/or
recurrence in patients with CRC and may provide needed information
to novel treatment strategies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated and analyzed in this study are
included in this published article.
Authors' contributions
TK and MI conceived and designed the study. TK and
MI performed the histopathological analysis. TK and MI confirm the
authenticity of all the raw data. TK, MI, HM, MasH, MadH, YH and MS
acquired and analyzed the data. TK and MI drafted the manuscript,
tables and figures. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
The Declaration of Helsinki, and the study protocol was approved by
the Institutional Review Board of the Kansai Medical University
Hospital (protocol no. 2021197; Hirakata, Japan). All data are
completely anonymized. The Institutional Review Board waived the
requirement of informed consent due to the retrospective design of
the study, with no risk of identity exposure for the patients. The
present study did not include any minors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
DR
|
desmoplastic reaction
|
|
TB
|
tumor budding
|
|
TD
|
tumor deposit
|
References
|
1
|
Farc O and Cristea V: An overview of the
tumor microenviroment, from cells to complex networks (Review). Exp
The Med. 21:962021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Chen D and Shen M: Tumor
microenvironment shapes colorectal cancer progression, metastasis,
and treatment responses. Front Med (Lausanne). 9:8690102022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ueno H, Kajiwara Y, Ajioka Y, Sugai T,
Sekine S, Ishiguro M, Takashima A and Kanemitsu Y:
Histopathological atlas of desmoplastic reaction characterization
in colorectal cancer. Jpn J Clin Oncol. 51:1004–1012. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueno H, Ishiguro M, Nakatani E, Ishikawa
T, Uetake H, Murotani K, Matsui S, Teramukai S, Sugai T, Ajioka Y,
et al: Prognostic value of desmoplastic reaction characterisation
in stage II colon cancer: Prospective validation in a Phase 3 study
(SACURA Trial). Br J Cancer. 124:1088–1097. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ueno H, Konishi T, Ishikawa Y, Shimazaki
H, Ueno M, Aosasa S, Saiura A, Shinto E, Kajiwara Y, Mochizuki S,
et al: Primary tumor histology affects oncological outcomes
independently of the anatomical extent of disease in colorectal
liver metastasis. JMA J. 3:240–250. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nearchou IP, Kajiwara Y, Mochizuki S,
Harrison DJ, Caie PD and Ueno H: Novel internationally verified
method reports desmoplastic reaction as the most significant
prognostic feature for disease-specific survival in stage II
colorectal cancer. Am J Surg Pathol. 43:1239–1248. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueno H, Kanemitsu Y, Sekine S, Ishiguro M,
Ito E, Hashiguchi Y, Kondo F, Shimazaki H, Kajiwara Y, Okamoto K,
et al: A multicenter study of the prognostic value of desmoplastic
reaction categorization in stage II colorectal cancer. Am J Surg
Pathol. 43:1015–1022. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ueno H, Sekine S, Oshiro T, Kanemitsu Y,
Hamaguchi T, Shida D, Takashima A, Ishiguro M, Ito E, Hashiguchi Y,
et al: Disentangling the prognostic heterogeneity of stage III
colorectal cancer through histologic stromal categorization.
Surgery. 163:777–783. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ueno H, Kanemitsu Y, Sekine S, Ishiguro M,
Ito E, Hashiguchi Y, Kondo F, Shimazaki H, Mochizuki S, Kajiwara Y,
et al: Desmoplastic pattern at the tumor front defines
poor-prognosis subtypes of colorectal cancer. Am J Surg Pathol.
41:1506–1512. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ueno H, Shinto E, Shimazaki H, Kajiwara Y,
Sueyama T, Yamamoto J and Hase K: Histologic categorization of
desmoplastic reaction: Its relevance to the colorectal cancer
microenvironment and prognosis. Ann Surg Oncol. 22:1504–1512. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Studer L, Blank A, Bokhorst JM, Nagtegaal
ID, Zlobec I, Lugli A, Fischer A and Dawson H: Taking tumour
budding to the next frontier - a post International tumour budding
consensus conference (ITBCC) 2016 review. Histopathology.
78:476–484. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagtegaal ID, Knijn N, Hugen N, Marshall
HC, Sugihara K, Tot T, Ueno H and Quirke P: Tumor deposits in
colorectal cancer: Improving the value of modern staging-a
systematic review and meta-analysis. J Clin Oncol. 35:1119–1127.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ueno H, Jones AM, Wilkinson KH, Jass JR
and Talbot IC: Histological categorisation of fibrotic cancer
stroma in advanced rectal cancer. Gut. 53:581–586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ueno H, Jones A, Jass JR and Talbot IC:
Clinicopathological significance of the ‘keloid-like’ collagen and
myxoid stroma in advanced rectal cancer. Histopathology.
40:327–334. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ueno H, Mochizuki H, Shirouzu K, Kusumi T,
Yamada K, Ikegami M, Kawachi H, Kameoka S, Ohkura Y, Masaki T, et
al: Multicenter study for optimal categorization of extramural
tumor deposits for colorectal cancer staging. Ann Surg.
255:739–746. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lugli A, Kirsch R, Ajioka Y, Bosman F,
Cathomas G, Dawson H, El Zimaity H, Fléjou JF, Hansen TP, Hartmann
A, et al: Recommendations for reporting tumor budding in colorectal
cancer based on the International tumor budding consensus
conference (ITBCC) 2016. Mod Pathol. 30:1299–1311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldstein NS and Turner JR: Pericolonic
tumor deposits in patients with T3N+MO colon adenocarcinomas:
Markers of reduced disease free survival and intra-abdominal
metastases and their implications for TNM classification. Cancer.
88:2228–2238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lugli A, Zlobec I, Berger MD, Kirsch R and
Nagtegaal ID: Tumour budding in solid cancers. Nat Rev Clin Oncol.
18:101–115. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li ZW, He L, Zheng Z, Zhang Q, Xu YT, Chen
JY, Shi J, Huang WB and Fan XS: Combined assessment of tumour cell
nest size and desmoplastic reaction as an excellent prognostic
predictor in oesophageal squamous cell carcinoma. Histopathology.
80:1112–1120. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakaguchi T, Satoi S, Hashimoto D,
Yamamoto T, Yamaki S, Hirooka S, Ishida M, Ikeura T, Inoue K,
Naganuma M, et al: High tumor budding predicts a poor prognosis in
resected duodenal adenocarcinoma. Surg Today. 52:931–940. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noda Y, Ishida M, Ueno Y, Fujisawa T, Iwai
H and Tsuta K: Novel pathological predictive factors for extranodal
extension in oral squamous cell carcinoma: A retrospective cohort
study based on tumor budding, desmoplastic reaction,
tumor-infiltrating lymphocytes, and depth of invasion. BMC Cancer.
22:4022022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kosaka H, Ishida M, Ueno M, Komeda K,
Hokutou D, Iida H, Hirokawa F, Matsui K, Sekimoto M and Kaibori M:
Tumor budding may be a promising prognostic indicator in
intrahepatic cholangiocarcinoma: A multicenter retrospective study.
Ann Gastroenterol Surg. 2022.(In press). View Article : Google Scholar
|
|
23
|
Sueyama T, Kajiwara Y, Mochizuki S,
Shimazaki H, Shinto E, Hase K and Ueno H: Periostin as a key
molecule defining desmoplastic environment in colorectal cancer.
Virchows Arch. 478:865–874. 2021. View Article : Google Scholar : PubMed/NCBI
|