The energy of cells mainly comes from glycolysis and
the oxidative phosphorylation of glucose, where the former produces
pyruvate. It has long been hypothesized that pyruvate is
metabolized by mitochondrial oxidative phosphorylation to produce
water and carbon dioxide under aerobic conditions, while it is
converted to lactic acid by lactate dehydrogenase under anoxic
conditions during glycolysis. However, in the 1920s, German
physiologist Otto Warburg reported that the main source of energy
acquisition of tumor cells is anaerobic glycolysis of sugar, where
the sugar undergoes aerobic glycolysis and oxidative
phosphorylation even when the oxygen supply is sufficient (1,2).
This is known as the Warburg effect. This switching from oxidative
phosphorylation to glycolysis is considered to be a major feature
of tumors (3,4). Glycolysis is an oxygen-independent
process in which the rate-limiting step is controlled by a group of
enzymes, including phosphofructokinase, hexokinase, glucokinase and
pyruvate kinase (PK). Competitive PK is an important regulatory
protein involved in glucose catabolism (4,5–7), and
there are several different isoforms of PK in mammals, which differ
in allosteric regulation and tissue expression. Cancer cells tend
to preferentially express a specific competitive PK subtype, PKM2,
which plays an important role in tumor metabolism (8–10).
Previous studies have shown that human pathogenic
viruses have a reprogramming effect on tumor metabolism (11,12).
In some cases, the interaction between viral proteins and cell
proteins leads to the malignant transformation of cells, and the
metabolism changes accordingly to meet the energy needed for the
rapid proliferation of these tumor cells (13,14).
For example, HPV E7 oncoprotein can interact with SMAD2/3/4 and
cause SMAD3 suppression, which results in TGF-β signaling pathway
inhibition (15). Studies have
shown that PKM2 is highly expressed in embryonic and tumor cells,
and the role of PKM2 in cervical cancer has been a long-term
concern (16–18). Since the beginning of the 21st
century, it has been known that PKM2 plays an important role in the
metabolism of cervical cancer cells (19,20),
and the function of its non-metabolic pathway has also been studied
recently (21,22). As one of the most common types of
cancer threatening women's health (23), cervical cancer has always had
global attention, and human papillomavirus (HPV) persistence is the
main risk factor for cervical cancer (24). However, the specific regulatory
mechanism of PKM2 and HPV is not clear.
In general, competitive PK regulates the last step
of glycolysis and the conversion of PEP and ADP into pyruvate and
ATP, and has four different subtypes: L, R, M1 and M2 (6). PKM2 is the only subtype that can be
detected at the embryonic stage and exists in various
differentiated adult tissues, such as those of the brain and liver
(31). The PKM gene consists of 12
exons (32). Alternative splicing
of PKM mRNA can lead to the production of PKM1 (exon 9) and PKM2
(exon 10). PKM2 can exist in all three oligomeric states, including
monomer, dimer, and tetramer, among which the dimer form promotes
the Warburg effect (33). The
single PKM2 monomer is made of 531 amino acids (aas) and consists
of 4 domains: N (43 aas), A (244 aas), B (102 aas) and C (142 aas)
(34). The A domain of PKM2
represents the core of the monomer and is responsible for mediating
the interaction of subunits to form a dimer. PKM2 differs from PKM1
by a 56-aa stretch encoded by the alternatively spliced region.
This stretch of aas forms an allosteric pocket that allows binding
of FBP (35). After the binding of
FBP, the conformation of the PKM2 tetramer is changed from the
inactive T-state to the active R-state, which favors the binding of
PEP in the active site and enhances its enzymatic activity
(36). It has been shown that PKM2
not only plays a central role in metabolic reprogramming, but also
plays a direct regulatory role in gene expression and subsequent
cell cycle processes (37).
As a glycolytic enzyme, PKM2 is mainly located in
the cytoplasm; however, PKM2 in tumor cells accumulates in the
nucleus (34). Monomeric PKM2
translocates to the nucleus, acts as a histone kinase and
upregulates the expression of proto-oncogene c-Myc, thus promoting
the Warburg effect (38). In
addition, the deacetylation of residue K62 of PKM2 can promote the
transport of PKM2 to the nucleus and binding to β-catenin, thus
promoting the transcription of the cyclin D1 gene and the process
of the cell cycle (39).
Therefore, intranuclear PKM2 is essential for tumorigenesis.
Moreover, the direct interaction between lncRNA-AC020978 and PKM2
in non-small cell lung cancer can enhance the stability of PKM2
(40). Furthermore,
lncRNA-AC020978 can promote the nuclear translocation of PKM2 and
regulate the transcriptional activity of hypoxia-inducible factor α
(HIF-1α) enhanced by PKM2 (40).
It has been reported that the epidermal growth
factor receptor (EGFR)-mitogen-activated protein kinase
(MEK)/extracellular signal-regulated kinase (ERK) signaling pathway
phosphorylates PKM2 at S37 and promotes nuclear translocation in
hepatocellular carcinoma cells stimulated by EGF (41). It has also been observed that HPV16
E7 can promote the acetylation of K433 of PKM2 (42). This acetylation can in turn promote
the kinase activity and nuclear localization of PKM2. Thus, it can
be suggested that E7 plays a direct role in the nuclear
localization of PKM2, but this needs further exploration.
The expression of PKM2 is driven by several cellular
signaling pathways, including transcription factor 1 (SP1), HIF-1α
(43), mammalian target of
rapamycin (mTOR) (44), c-Myc
(45), nuclear factor κB (NF-κB)
(46) and peroxisome
proliferator-activated receptor γ (PPARγ) (47). The promoter of PKM2 is composed of
three cis-acting regions and three GC boxes (48). In previous research, five possible
binding sites of SP1 and SP3 were found in the PKM2 promoter. SP1
was shown to activate the transcription of the PKM2 gene
structurally by binding to the common DNA-binding site (GC box) in
the promoter of the PKM gene. SP3 cooperated with SP1 to enhance
the expression of PKM2 (49). The
expression of E6 and E7, and the downregulation of phosphatase and
tensin homolog deleted on chromosome ten (PTEN) and thioredoxin
interactions protein significantly promoted glucose transporter
protein 1, glucose uptake (50)
and the dephosphorylation of SP1, which in turn promoted the
expression of PKM2 (51). In
addition, the protein and mRNA expression levels of liver kinase B1
(LKB1) were downregulated by HPV16 E6/E7, while the deletion of
LKB1 upregulated the expression and activity of SP1. SP1 further
upregulated the expression of genomic amplification of human
telomerase gene (hTERC) at the mRNA and gene amplification levels.
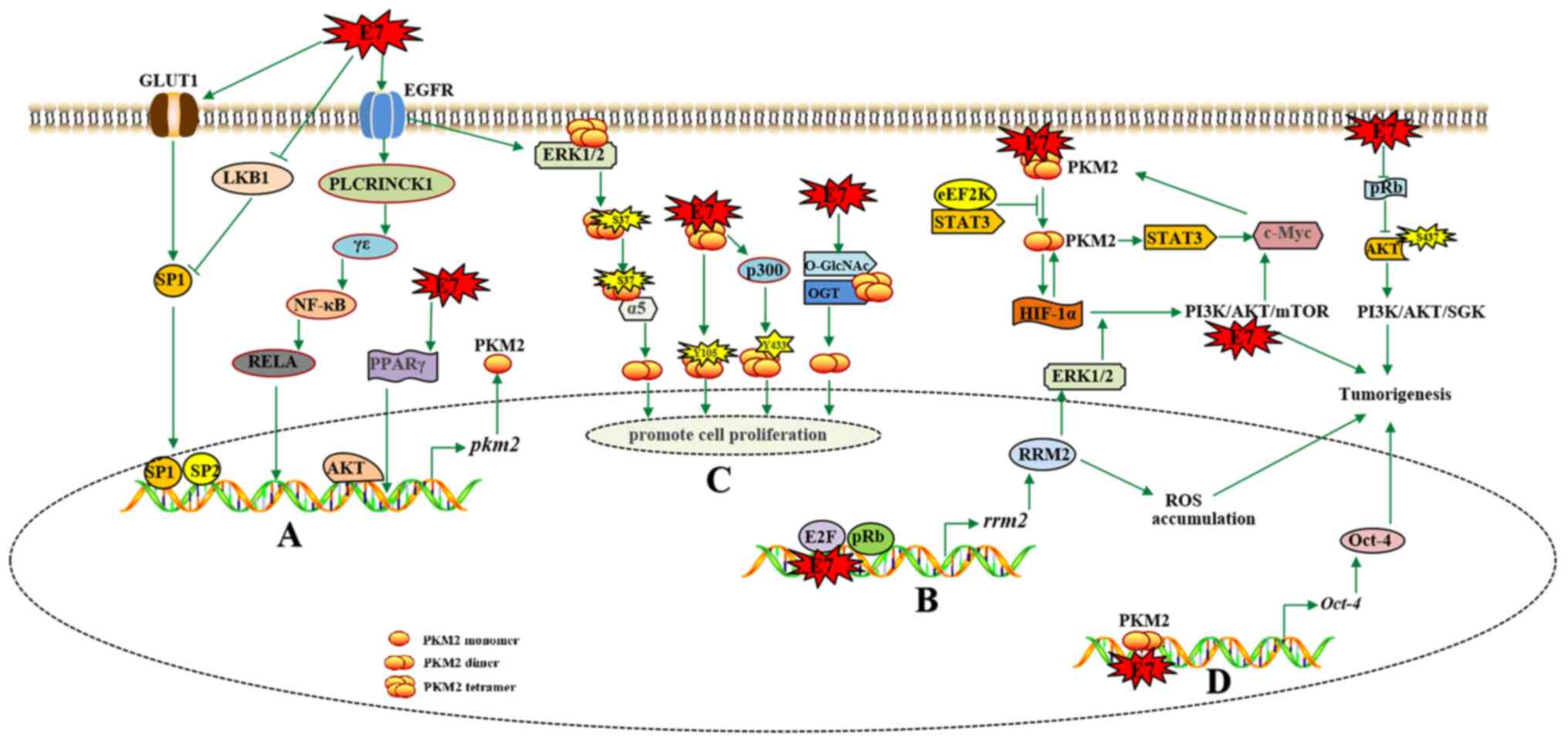
Based on this, the HPV-LKB1-SP1-hTERC axis was proposed (52). The aforementioned studies
demonstrated that E7 can not only enhance the activity of SP1 by
enhancing the absorption of glucose, but also by downregulating
LKB1 to increase the activity of SP1 and ultimately promote the
expression of PKM2 (Fig. 1).
When PI3K binds to growth factor receptors (such as
EGFR) in the PI3K/AKT/mTOR network, the structure of AKT changes
and is activated. This in turn phosphorylates and activates or
inhibits the activity of a series of downstream substrates (such as
apoptosis-related proteins Bad and Caspase 9), thus regulating cell
proliferation, differentiation, apoptosis and migration (59–61).
In hypoxic and normoxic HPV-positive cancer cells, the
PI3K/AKT/mTOR network plays a key role in the virus-host cell
interface (62). Under hypoxic
conditions, HIF-1α induces the activation of the PI3K/AKT/mTOR
signal pathway and regulates PKM2 by downregulating the expression
of c-myc and upregulating heterogeneous ribonucleoproteins (hnRNPs)
(63). In hypoxic HPV-positive
cancer cells, hypoxia can block the effect of E6/E7, which is
canceled by AKT and a high glucose supply (64). It is worth noting that the ability
of E7 to upregulate the activity of AKT depends on its ability to
bind to and inactivate pRb. It has been observed that knockout of
pRb with shRNA alone is sufficient to activate AKT activity in
differentiated keratinocytes (65). In addition, E7 may participate in
the PI3K/AKT/SGK signaling pathway by activating the
phosphorylation of the AKT S473 site (Fig. 1) (66).
In summary, SP1, HIF-1α, NF-κB and PPARγ can
regulate PKM2 in tumor cells and interact with HPV E7, which
indicates that E7 may play a key role in PKM2 transcription
(Fig. 1).
In addition, the activation of β-catenin enhances
the expression of c-Myc, thus promoting the Warburg effect
(78). It has been reported that
HPV16 can regulate the migration of β-catenin in HPV-associated
oropharyngeal squamous cell carcinoma (78). Furthermore, both E6 and E7 were
able to upregulate β-catenin and enhance thymocyte transcription
factor-mediated transcription in HPV16-positive oropharyngeal
carcinoma cells (79). This
evidence suggests that E7 may enhance the expression of c-Myc
through β-catenin, thus also regulating PKM2. However, both
low-risk and high-risk HPV E7 in cervical cancer cells can interact
with c-Myc, but only the interaction between high-risk HPV E7 and
c-Myc can functionally enhance the transcriptional activation
activity of c-Myc (80).
Post-translational modification of amino acids
endows proteins with other biochemical groups that adapt protein
function by changing the chemical properties of amino acids and/or
by causing structural changes (such as the establishment of
disulfide bonds). These modifications include phosphorylation,
glycosylation, ubiquitin, nitrosation, methylation, acetylation,
lipidation and proteolysis (81).
It has been discovered that PKM2 is phosphorylated
by a variety of tyrosine kinases and forms dimers to promote the
growth of cancer cells (10,82,83).
The phosphorylation of PKM2 is common in human cancer and can be
increased by HPV16 E7 (4,21,84).
Phosphorylated PKM2 Y105F mutants are highly expressed in cancer
cells, which can promote cell proliferation and tumorigenesis
(4). A recent study has shown that
highly upregulated gene in liver cancer (the most upregulated gene
in HCC, which was characterized as a novel mRNA-like ncRNA) acts as
an adaptor molecule to enhance the binding of lactate dehydrogenase
A and PKM2 to fibroblast growth factor receptor 1, increasing the
phosphorylation of these two enzymes and thus promoting glycolysis
(85). EGFR-activated ERK2
directly binds to PKM2 and phosphorylates S37, but does not
phosphorylate PKM1. Phosphorylated PKM2 recruits peptidylprolyl
cis/trans isomerase for isomerization of PKM2, thus promoting the
binding of PKM2 to the input protein α5 and translocation to the
nucleus (86). This nuclear
localization of PKM2 further promotes the Warburg effect (Fig. 1).
A high concentration of reactive oxygen species
(ROS) can destroy cell composition and damage cell vitality
(87). Therefore, controlling the
concentration of intracellular ROS is very important for cell
proliferation and survival. The sharp increase of intracellular ROS
leads to the oxidation of PKM2 at C358, thus inhibiting the
activity of PKM2 (88). The cell
then transfers from the glucose pathway to the pentose phosphate
pathway, resulting in a reduction potential sufficient to detoxify
ROS. Studies have shown that E7 can induce the accumulation of
intracellular ROS (89) by
inducing the upregulation of RRM2 and promoting the occurrence of
cervical cancer through angiogenesis induced by
ROS/ERK1/2/HIF-1α/VEGF (56).
Due to the accumulation of research on the
post-translational modification of PKM2, the non-metabolic effect
of PKM2 on tumor cells has been gradually revealed, and thus the
mechanism of E7 on PKM2 will open a new chapter.
It has been revealed that E7 may mimic the effect of
inhibitory amino acids (such as alanine or leucine) on PKM2
activity (93). In a high
glycolytic NIH3T3 cell line, E7 led to the transformation of PKM2
into its dimeric form and resulted in a decrease in the cellular PK
mass action ratio, the glycolysis flux rate and the
(ATP+GTP)/(UTP+CTP) ratio, and an increase of FBP level, glutamine
consumption and cell proliferation (30). In addition, it has been reported
that a low glycolytic NIH3T3 cell line is characterized by high
pyruvate and glutamine consumption and a large number of dimeric
forms of PKM2, which is consistent with high FBP levels, low
(ATP+GTP)/(CTP+UTP) ratios and high diffusivity (20). This interaction is very important
for the transformation potential of tumor cells (94).
PKM2 not only plays a glycolytic role in the cell
but also acts as a protein kinase. Yang et al (95) found that the activation of EGFR
induced PKM2 translocation to the nucleus, and the interaction of
PKM2 with β-catenin led to the inhibition of histone deacetylase 3
and an increase in cyclin D1 expression from the promoter, thus
promoting tumor cell proliferation.
Signal transducer and activator of transcription 3
(STAT3) is also a substrate of PKM2 kinase activity. Nuclear PKM2
phosphorylates STAT3 at Y705 and then activates MEK5 transcription
(96). In addition, CD276 induces
PKM2 phosphorylation through the STAT3 signal pathway, thus
promoting glucose metabolism in tumors (97). In lung cancer cells, eukaryotic
EF2K forms a complex with PKM2 and STAT3, and phosphorylates PKM2
at T129, resulting in a decrease in PKM2 dimerization.
Subsequently, PKM2 blocks STAT3 phosphorylation and STAT3-dependent
c-Myc expression (98). A study on
HPV16-positive cervical cancer cell lines (SiHa and CaSki) and
primary tumor tissues showed that STAT3 plays a role in the
occurrence and development of cervical cancer (99). The activity of STAT3 was positively
correlated with the expression of HPV16 E6 and E7, and negatively
correlated with the expression of p53 and pRb (100). E6 is mainly responsible for the
phosphorylation of STAT3 in HPV keratinocytes, while E5, E6 and E7
can induce STAT3 tyrosine phosphorylation in HPV cervical cancer
cells (101). However, the direct
dependence of STAT3 on the activation of PKM2 to induce the
formation of cervical cancer cells has not yet been studied.
PKM2 can interact with the transcription factor
encoded by the octamer-binding transcription factor-4 (Oct-4) gene,
which plays an important role in maintaining the pluripotent state
of embryonic stem cells (102).
The pituitary-specific Pit-1 (POU) DNA binding domain of Oct-4 is
necessary for interaction with PKM2, which positively regulates the
transactivation potential of Oct-4. In addition, ectopic expression
of PKM2 enhances Oct-4-mediated transcription (103). A study demonstrated that HPV E7
also specifically binds to the Oct-4 POU domain, and the expression
of E7 in differentiated cells can stimulate the transactivation of
Oct-4-mediated distal binding sites (104). However, whether the combination
of E7 and Oct-4 has an impact on PKM2 also needs further study
(Fig. 1).
The TIME has attracted increasing attention in
recent years, especially in clinics (105). With the progress of technology,
the understanding of the complexity and diversity of the TIME and
its impact on treatment response has deepened. The TIME is composed
of factors such as blood vessels, myeloid-derived suppressor cells,
antigen-presenting cells, lymphocytes, dendritic cells,
fibroblasts, extracellular matrix, cytokines and growth factors
(106). These components can
functionally sculpt the TIME by secreting various cytokines,
chemokines and other factors, resulting in the anticancer immune
response, and can have an important influence on the occurrence and
development of cancer. Previous studies have shown that PKM2 plays
an important role in the TIME by influencing the Warburg effect of
cancer cells, immune cells (such as lymphocytes, dendritic cells
and macrophages) and immune checkpoints [such as programmed cell
death ligand-1 (PDL1)] (34,107). However, it is not clear whether
PKM2 plays a vital role in cancer metabolism and immunity.
In the process of tumorigenesis, lymphocytes are
activated and proliferate, which is characterized by a significant
increase in aerobic glycolysis (108). Meanwhile, T-cell proliferation
increases the expression of PKM2 (especially in CD4+ T
cells), and the accumulation of PKM2 in the nucleus affects the
metabolic reprogramming of CD4+ T cells in turn
(109). A previous study has
shown that PKM2 controls T-cell activation induced by homocysteine
(8). In addition, TEPP-46 and
DASA-58, (two common activators of PKM2) can convert PKM2 dimer
into tetramer and inhibit PKM2 nuclear transfer (110,111). TEPP-46 can also restrict the
development of Th17 and Th1 cells in vitro and inhibits T
cell-mediated inflammation (112).
Nuclear localization of PKM2 is important for the
regulation of the TIME, and the role of HPV E7 is essential in this
process. PKM2 has been found to play a role in the differentiation
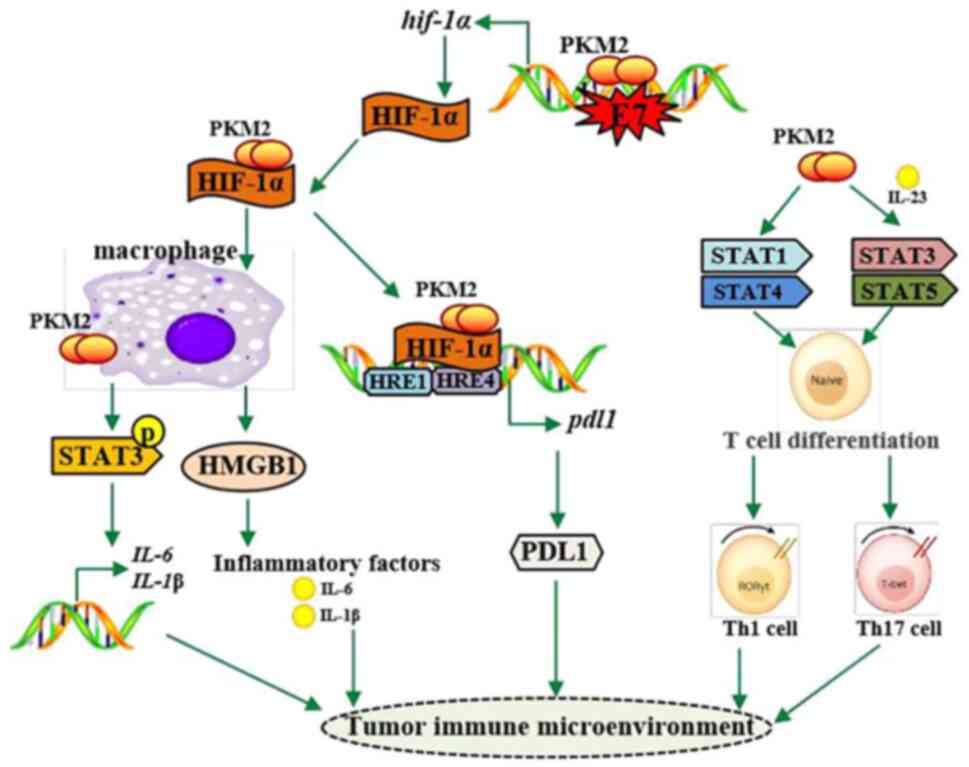
and function of immune cells (113,114). PKM2 can form transcriptional
complexes with HIF-1α, and then regulate the release of high
mobility group box-1 from activated macrophages. The latter can
release inflammatory factors to change the immune microenvironment
(115). In addition, nuclear
translocation of dimer PKM2 leads to phosphorylation of STAT3 in
lipopolysaccharide (LPS)-stimulated coronary artery disease
macrophages and promotes IL-1β and IL-6 transcription (116). Moreover, the transformation of
PKM2 from a dimeric to a tetrameric conformation effectively
inhibits LPS-induced nuclear translocation and subsequent
expression of IL-1β and a series of other HIF-1α-dependent genes,
which is important for the Warburg effect in macrophages (Fig. 2) (117).
It is worth noting that PKM2 also plays a key role
in the differentiation of different immune cells; for example, PKM2
participates in T-cell differentiation by regulating STAT1/STAT4
and then inducing type 1 T helper (Th1) cell formation (118). In addition, PKM2 interacts with
STAT3 to enhance its activation after migration to the nucleus,
thus increasing the differentiation of Th17 cells (113). Furthermore, IL-23 is an important
cytokine in the polarization of Th17, which can induce the
phosphorylation of PKM2 and STAT3 in T cells and promote their
nuclear translocation (119).
Moreover, previous studies have indicated that nuclear PKM2 can
also stimulate the proliferation of cancer cells by regulating the
activity of STAT1 and STAT5 (Fig.
2) (120,121).
Finally, as one of the most promising immunotherapy
methods, the use of immune checkpoint inhibitors, especially PDL1,
has attracted extensive attention in recent years. Immune
checkpoint inhibitors have been proven to have strong
immunomodulatory effects through their function as negative
regulatory factors of T cells (122). PDL1 can be expressed in a variety
of cell types, including cancer cells, and PKM2 is crucial for PDL1
expression according to the report by Luo et al (58). PKM2 and HIF-1α can bind to two
hypoxia-response elements (HRE1 or HRE4) of the PDL1 promoter at
the same time to promote the transcription of PDL1 (123). In cervical cancer, the high
expression of E7 can promote the nuclear localization and
phosphorylation of PKM2 thus promoting the proliferation of cancer
cells, and increase the activity of HIF-1α and then interact with
PKM2 to enhance immune surveillance of cancer escape (Fig. 2).
During energy metabolism, cervical cancer cells are
inclined to utilize glycolysis under aerobic conditions. As a
rate-limiting enzyme in glycolysis, PKM2 plays an important role in
the aerobic glycolysis pathway. Although HPV E7 can also promote
the Warburg effect, the mechanism is not clear. A large number of
studies have shown that there is some regulatory relationship
between E7 and PKM2, but the specific mechanism is still mostly
unknown and needs further study. Since the intracellular mechanisms
influenced by HPV E7 interaction with PKM2 are much more complex
than previously assumed, insights gained from the present review
may be helpful for further research and clinical treatment of
cervical cancer in the future.
Not applicable.
This study was supported by the National Natural Science
Foundation of China (grant no. 81802761), the Jinan Science and
Technology Bureau (grant nos. 202019040 and 202019033) and the Key
Research and Development Program of Shandong Province (grant no.
2019GSF107014).
Not applicable.
CG was the major contributor to writing the
manuscript. MJ, YS and JW revised the manuscript. YZ made
substantial contributions to conception and design and gave the
final approval of the version to be published. MJ involved in
revising the manuscript critically for important intellectual
content. All authors read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Warburg O and Minami S: Versuche an
überlebendem carcinom-gewebe. Klin Wochenschr. 2:776–777. 1923.
View Article : Google Scholar
|
|
2
|
Warburg O: Über den stoffwechsel der
carcinomzelle. Naturwissenschaften. 12:1131–1137. 1924. View Article : Google Scholar
|
|
3
|
Hoppe-Seyler K, Bossler F, Braun JA,
Herrmann AL and Hoppe-Seyler F: The HPV E6/E7 oncogenes: Key
factors for viral carcinogenesis and therapeutic targets. Trends
Microbiol. 26:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hitosugi T, Kang S, Vander Heiden MG,
Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, et
al: Tyrosine phosphorylation inhibits PKM2 to promote the Warburg
effect and tumor growth. Sci Signal. 2:ra732009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zahra K, Dey T, Ashish, Mishra SP and
Pandey U: Pyruvate kinase M2 and cancer: The role of PKM2 in
promoting tumorigenesis. Front Oncol. 10:1592020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Z: PKM2, function and expression and
regulation. Cell Biosci. 9:522019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malla R and Kamal MA: E6 and E7
oncoproteins: Potential targets of cervical cancer. Curr Med Chem.
28:8163–8181. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lü S, Deng J, Liu H, Liu B, Yang J, Miao
Y, Li J, Wang N, Jiang C, Xu Q, et al: PKM2-dependent metabolic
reprogramming in CD4+ T cells is crucial for
hyperhomocysteinemia-accelerated atherosclerosis. J Mol Med (Berl).
96:585–600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mazurek S, Boschek CB, Hugo F and
Eigenbrodt E: Pyruvate kinase type M2 and its role in tumor growth
and spreading. Semin Cancer Biol. 15:300–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nandi S, Razzaghi M, Srivastava D and Dey
M: Structural basis for allosteric regulation of pyruvate kinase M2
by phosphorylation and acetylation. J Biol Chem. 295:17425–17440.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyake-Stoner SJ and O'Shea CC: Metabolism
goes viral. Cell Metab. 19:549–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pant A, Dsouza L and Yang Z: Alteration in
cellular signaling and metabolic reprogramming during viral
infection. mBio. 12:e00635212021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thaker SK, Ch'ng J and Christofk HR: Viral
hijacking of cellular metabolism. BMC Biol. 17:592019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kori M and Arga KY: Pathways involved in
viral oncogenesis: New perspectives from virus-host protein
interactomics. Biochim Biophys Acta Mol Basis Dis. 1866:1658852020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
French D, Belleudi F, Mauro MV, Mazzetta
F, Raffa S, Fabiano V, Frega A and Torrisi MR: Expression of HPV16
E5 down-modulates the TGFbeta signaling pathway. Mol Cancer.
12:382013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martínez-Ramírez I, Carrillo-García A,
Contreras-Paredes A, Ortiz-Sánchez E, Cruz-Gregorio A and Lizano M:
Regulation of cellular metabolism by high-risk human
papillomaviruses. Int J Mol Sci. 19:18392018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim H, Jang H, Kim TW, Kang BH, Lee SE,
Jeon YK, Chung DH, Choi J, Shin J, Cho EJ and Youn HD: Core
pluripotency factors directly regulate metabolism in embryonic stem
cell to maintain pluripotency. Stem Cells. 33:2699–2711. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou PP, Luo LJ, Chen HZ, Chen QT, Bian XL,
Wu SF, Zhou JX, Zhao WX, Liu JM, Wang XM, et al: Ectosomal PKM2
promotes HCC by inducing macrophage differentiation and remodeling
the tumor microenvironment. Mol Cell. 78:1192–1206.e10. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mazurek S, Zwerschke W, Jansen-Dürr P and
Eigenbrodt E: Metabolic cooperation between different oncogenes
during cell transformation: Interaction between activated ras and
HPV-16 E7. Oncogene. 20:6891–6898. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mazurek S, Zwerschke W, Jansen-Dürr P and
Eigenbrodt E: Effects of the human papilloma virus HPV-16 E7
oncoprotein on glycolysis and glutaminolysis: Role of pyruvate
kinase type M2 and the glycolytic-enzyme complex. Biochem J.
356:247–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SA, Ho C, Troxler M, Lin CY and Chung
SH: Non-metabolic functions of PKM2 contribute to cervical cancer
cell proliferation induced by the HPV16 E7 oncoprotein. Viruses.
13:4332021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abudula A, Rouzi N, Xu L, Yang Y and
Hasimu A: Tissue-based metabolomics reveals potential biomarkers
for cervical carcinoma and HPV infection. Bosn J Basic Med Sci.
20:78–87. 2020.PubMed/NCBI
|
|
23
|
Wang R, Pan W, Jin L, Huang W, Li Y, Wu D,
Gao C, Ma D and Liao S: Human papillomavirus vaccine against
cervical cancer: Opportunity and challenge. Cancer Lett.
471:88–102. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Doorbar J, Quint W, Banks L, Bravo IG,
Stoler M, Broker TR and Stanley MA: The biology and life-cycle of
human papillomaviruses. Vaccine. 30 (Suppl 5):F55–F70. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
No authors listed. Human papillomavirus
vaccines: WHO position paper, May 2017. Wkly Epidemiol Rec.
92:241–268. 2017.(In English, French). PubMed/NCBI
|
|
27
|
Schwarz E, Freese UK, Gissmann L, Mayer W,
Roggenbuck B, Stremlau A and zur Hausen H: Structure and
transcription of human papillomavirus sequences in cervical
carcinoma cells. Nature. 314:111–114. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Münger K, Basile JR, Duensing S, Eichten
A, Gonzalez SL, Grace M and Zacny VL: Biological activities and
molecular targets of the human papillomavirus E7 oncoprotein.
Oncogene. 20:7888–7898. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scarth JA, Patterson MR, Morgan EL and
Macdonald A: The human papillomavirus oncoproteins: A review of the
host pathways targeted on the road to transformation. J Gen Virol.
102:0015402021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zwerschke W, Mazurek S, Massimi P, Banks
L, Eigenbrodt E and Jansen-Dürr P: Modulation of type M2 pyruvate
kinase activity by the human papillomavirus type 16 E7 oncoprotein.
Proc Natl Acad Sci USA. 96:1291–1296. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Israelsen WJ, Dayton TL, Davidson SM,
Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW,
et al: PKM2 isoform-specific deletion reveals a differential
requirement for pyruvate kinase in tumor cells. Cell. 155:397–409.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takenaka M, Noguchi T, Sadahiro S, Hirai
H, Yamada K, Matsuda T, Imai E and Tanaka T: Isolation and
characterization of the human pyruvate kinase M gene. Eur J
Biochem. 198:101–106. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mazurek S: Pyruvate kinase type M2: A key
regulator of the metabolic budget system in tumor cells. Int J
Biochem Cell Biol. 43:969–980. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alquraishi M, Puckett DL, Alani DS,
Humidat AS, Frankel VD, Donohoe DR, Whelan J and Bettaieb A:
Pyruvate kinase M2: A simple molecule with complex functions. Free
Radic Biol Med. 143:176–192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dombrauckas JD, Santarsiero BD and Mesecar
AD: Structural basis for tumor pyruvate kinase M2 allosteric
regulation and catalysis. Biochemistry. 44:9417–9429. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang P, Sun C, Zhu T and Xu Y: Structural
insight into mechanisms for dynamic regulation of PKM2. Protein
Cell. 6:275–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Otto AM: Warburg effect(s)-a biographical
sketch of Otto Warburg and his impacts on tumor metabolism. Cancer
Metab. 4:52016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang W and Lu Z: Nuclear PKM2 regulates
the Warburg effect. Cell Cycle. 12:3154–3158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang R, Shen M, Wu C, Chen Y, Lu J, Li J,
Zhao L, Meng H, Zhou X, Huang G, et al: HDAC8-dependent
deacetylation of PKM2 directs nuclear localization and glycolysis
to promote proliferation in hepatocellular carcinoma. Cell Death
Dis. 11:10362020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hua Q, Mi B, Xu F, Wen J, Zhao L, Liu J
and Huang G: Hypoxia-induced lncRNA-AC020978 promotes proliferation
and glycolytic metabolism of non-small cell lung cancer by
regulating PKM2/HIF-1α axis. Theranostics. 10:4762–4778. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Onodera Y, Nam JM and Bissell MJ:
Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and
O-GlcNAc pathways. J Clin Invest. 124:367–384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lv L, Xu YP, Zhao D, Li FL, Wang W, Sasaki
N, Jiang Y, Zhou X, Li TT, Guan KL, et al: Mitogenic and oncogenic
stimulation of K433 acetylation promotes PKM2 protein kinase
activity and nuclear localization. Mol Cell. 52:340–352. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tirpe AA, Gulei D, Ciortea SM, Crivii C
and Berindan-Neagoe I: Hypoxia: Overview on hypoxia-mediated
mechanisms with a focus on the role of HIF genes. Int J Mol Sci.
20:61402019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Magaway C, Kim E and Jacinto E: Targeting
mTOR and metabolism in cancer: Lessons and innovations. Cells.
8:15842019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Reyes-González JM and Vivas-Mejía PE:
c-MYC and epithelial ovarian cancer. Front Oncol. 11:6015122021.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Azoitei N, Becher A, Steinestel K, Rouhi
A, Diepold K, Genze F, Simmet T and Seufferlein T: PKM2 promotes
tumor angiogenesis by regulating HIF-1α through NF-κB activation.
Mol Cancer. 15:32016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Feng J, Dai W, Mao Y, Wu L, Li J, Chen K,
Yu Q, Kong R, Li S, Zhang J, et al: Simvastatin re-sensitizes
hepatocellular carcinoma cells to sorafenib by inhibiting
HIF-1α/PPAR-γ/PKM2-mediated glycolysis. J Exp Clin Cancer Res.
39:242020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Noguchi T, Yamada K, Inoue H, Matsuda T
and Tanaka T: The L- and R-type isozymes of rat pyruvate kinase are
produced from a single gene by use of different promoters. J Biol
Chem. 262:14366–14371. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schäfer D, Hamm-Künzelmann B and Brand K:
Glucose regulates the promoter activity of aldolase A and pyruvate
kinase M2 via dephosphorylation of Sp1. FEBS Lett. 417:325–328.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang JY, Li DY, He L, Qiu XS, Wang EH and
Wu GP: HPV 16 E6/E7 promote the glucose uptake of GLUT1 in lung
cancer through downregulation of TXNIP due to inhibition of PTEN
phosphorylation. Front Oncol. 10:5595432020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Netzker R, Weigert C and Brand K: Role of
the stimulatory proteins Sp1 and Sp3 in the regulation of
transcription of the rat pyruvate kinase M gene. Eur J Biochem.
245:174–181. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang JH, Wu MZ, Wang XB, Wang S, Qiu XS,
Wang EH and Wu GP: HPV16 E6/E7 upregulate hTERC mRNA and gene
amplification levels by relieving the effect of LKB1 on Sp1
phosphorylation in lung cancer cells. Ther Adv Med Oncol.
12:17588359209175622020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhu H, Wu J, Zhang W, Luo H, Shen Z, Cheng
H and Zhu X: PKM2 enhances chemosensitivity to cisplatin through
interaction with the mTOR pathway in cervical cancer. Sci Rep.
6:307882016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bodily JM, Mehta KP and Laimins LA: Human
papillomavirus E7 enhances hypoxia-inducible factor 1-mediated
transcription by inhibiting binding of histone deacetylases. Cancer
Res. 71:1187–1195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gu J, Li X, Zhao L, Yang Y, Xue C, Gao Y,
Li J, Han Q, Sun Z, Bai C and Zhao RC: The role of PKM2 nuclear
translocation in the constant activation of the NF-κB signaling
pathway in cancer-associated fibroblasts. Cell Death Dis.
12:2912021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang N, Zhan T, Ke T, Huang X, Ke D, Wang
Q and Li H: Increased expression of RRM2 by human papillomavirus E7
oncoprotein promotes angiogenesis in cervical cancer. Br J Cancer.
110:1034–1044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
David CJ, Chen M, Assanah M, Canoll P and
Manley JL: HnRNP proteins controlled by c-Myc deregulate pyruvate
kinase mRNA splicing in cancer. Nature. 463:364–368. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Luo W, Hu H, Chang R, Zhong J, Knabel M,
O'Meally R, Cole RN, Pandey A and Semenza GL: Pyruvate kinase M2 is
a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell.
145:732–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Horn D, Hess J, Freier K, Hoffmann J and
Freudlsperger C: Targeting EGFR-PI3K-AKT-mTOR signaling enhances
radiosensitivity in head and neck squamous cell carcinoma. Expert
Opin Ther Targets. 19:795–805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Freudlsperger C, Burnett JR, Friedman JA,
Kannabiran VR, Chen Z and Van Waes C: EGFR-PI3K-AKT-mTOR signaling
in head and neck squamous cell carcinomas: Attractive targets for
molecular-oriented therapy. Expert Opin Ther Targets. 15:63–74.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chiou TT, Chau YY, Chen JB, Hsu HH, Hung
SP and Lee WC: Rapamycin attenuates PLA2R activation-mediated
podocyte apoptosis via the PI3K/AKT/mTOR pathway. Biomed
Pharmacother. 144:1123492021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bossler F, Hoppe-Seyler K and Hoppe-Seyler
F: PI3K/AKT/mTOR signaling regulates the virus/host cell crosstalk
in HPV-positive cervical cancer cells. Int J Mol Sci. 20:21882019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Strickland SW and Vande Pol S: The human
papillomavirus 16 E7 oncoprotein attenuates AKT signaling to
promote internal ribosome entry site-dependent translation and
expression of c-MYC. J Virol. 90:5611–5621. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bossler F, Kuhn BJ, Günther T, Kraemer SJ,
Khalkar P, Adrian S, Lohrey C, Holzer A, Shimobayashi M, Dürst M,
et al: Repression of human papillomavirus oncogene expression under
hypoxia is mediated by PI3K/mTORC2/AKT signaling. mBio.
10:e02323–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang M, Qiao X, Cooper T, Pan W, Liu L,
Hayball J, Lin J, Cui X, Zhou Y, Zhang S, et al: HPV E7-mediated
NCAPH ectopic expression regulates the carcinogenesis of cervical
carcinoma via PI3K/AKT/SGK pathway. Cell Death Dis. 11:10492020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Menges CW, Baglia LA, Lapoint R and
McCance DJ: Human papillomavirus type 16 E7 up-regulates AKT
activity through the retinoblastoma protein. Cancer Res.
66:5555–5559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yang W, Xia Y, Cao Y, Zheng Y, Bu W, Zhang
L, You MJ, Koh MY, Cote G, Aldape K, et al: EGFR-induced and PKCε
monoubiquitylation-dependent NF-κB activation upregulates PKM2
expression and promotes tumorigenesis. Mol Cell. 48:771–784. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hu G, Liu W, Mendelsohn J, Ellis LM,
Radinsky R, Andreeff M and Deisseroth AB: Expression of epidermal
growth factor receptor and human papillomavirus E6/E7 proteins in
cervical carcinoma cells. J Natl Cancer Inst. 89:1271–1276. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang Q, Lenardo MJ and Baltimore D: 30
Years of NF-κB: A blossoming of relevance to human pathobiology.
Cell. 168:37–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Havard L, Delvenne P, Fraré P, Boniver J
and Giannini SL: Differential production of cytokines and
activation of NF-kappaB in HPV-transformed keratinocytes. Virology.
298:271–285. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Senba M and Mori N: Mechanisms of virus
immune evasion lead to development from chronic inflammation to
cancer formation associated with human papillomavirus infection.
Oncol Rev. 6:e172012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Senba M, Buziba N, Mori N, Fujita S,
Morimoto K, Wada A and Toriyama K: Human papillomavirus infection
induces NF-κB activation in cervical cancer: A comparison with
penile cancer. Oncol Lett. 2:65–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tontonoz P and Spiegelman BM: Fat and
beyond: The diverse biology of PPARgamma. Annu Rev Biochem.
77:289–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Panasyuk G, Espeillac C, Chauvin C,
Pradelli LA, Horie Y, Suzuki A, Annicotte JS, Fajas L, Foretz M,
Verdeguer F, et al: PPARγ contributes to PKM2 and HK2 expression in
fatty liver. Nat Commun. 3:6722012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang S, Liu F, Mao X, Huang J, Yang J,
Yin X, Wu L, Zheng L and Wang Q: Elevation of miR-27b by HPV16 E7
inhibits PPARγ expression and promotes proliferation and invasion
in cervical carcinoma cells. Int J Oncol. 47:1759–1766. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chen M, Zhang J and Manley JL: Turning on
a fuel switch of cancer: hnRNP proteins regulate alternative
splicing of pyruvate kinase mRNA. Cancer Res. 70:8977–8980. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Rampias T, Boutati E, Pectasides E, Sasaki
C, Kountourakis P, Weinberger P and Psyrri A: Activation of Wnt
signaling pathway by human papillomavirus E6 and E7 oncogenes in
HPV16-positive oropharyngeal squamous carcinoma cells. Mol Cancer
Res. 8:433–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hu Z, Müller S, Qian G, Xu J, Kim S, Chen
Z, Jiang N, Wang D, Zhang H, Saba NF, et al: Human papillomavirus
16 oncoprotein regulates the translocation of β-catenin via the
activation of epidermal growth factor receptor. Cancer.
121:214–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang YW, Chang HS, Lin CH and Yu WC:
HPV-18 E7 conjugates to c-Myc and mediates its transcriptional
activity. Int J Biochem Cell Biol. 39:402–412. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lu Z and Hunter T: Degradation of
activated protein kinases by ubiquitination. Annu Rev Biochem.
78:435–475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Christofk HR, Vander Heiden MG, Wu N,
Asara JM and Cantley LC: Pyruvate kinase M2 is a
phosphotyrosine-binding protein. Nature. 452:181–186. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhou Z, Li M, Zhang L, Zhao H, Şahin Ö,
Chen J, Zhao JJ, Songyang Z and Yu D: Oncogenic kinase-induced PKM2
tyrosine 105 phosphorylation converts nononcogenic PKM2 to a tumor
promoter and induces cancer stem-like cells. Cancer Res.
78:2248–2261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lin Y, Zhai H, Ouyang Y, Lu Z, Chu C, He Q
and Cao X: Knockdown of PKM2 enhances radiosensitivity of cervical
cancer cells. Cancer Cell Int. 19:1292019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang C, Li Y, Yan S, Wang H, Shao X, Xiao
M, Yang B, Qin G, Kong R, Chen R and Zhang N: Interactome analysis
reveals that lncRNA HULC promotes aerobic glycolysis through LDHA
and PKM2. Nat Commun. 11:31622020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo
F, Lyssiotis CA, Aldape K, Cantley LC and Lu Z: ERK1/2-dependent
phosphorylation and nuclear translocation of PKM2 promotes the
Warburg effect. Nat Cell Biol. 14:1295–1304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wellen KE and Thompson CB: Cellular
metabolic stress: Considering how cells respond to nutrient excess.
Mol Cell. 40:323–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Anastasiou D, Poulogiannis G, Asara JM,
Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW,
Auld DS, et al: Inhibition of pyruvate kinase M2 by reactive oxygen
species contributes to cellular antioxidant responses. Science.
334:1278–1283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liu Y, Guo JZ, Liu Y, Wang K, Ding W, Wang
H, Liu X, Zhou S, Lu XC, Yang HB, et al: Nuclear lactate
dehydrogenase A senses ROS to produce α-hydroxybutyrate for
HPV-induced cervical tumor growth. Nat Commun. 9:44292018.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wang Y, Liu J, Jin X, Zhang D, Li D, Hao
F, Feng Y, Gu S, Meng F, Tian M, et al: O-GlcNAcylation
destabilizes the active tetrameric PKM2 to promote the Warburg
effect. Proc Natl Acad Sci USA. 114:13732–13737. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zeng Q, Zhao RX, Chen J, Li Y, Li XD, Liu
XL, Zhang WM, Quan CS, Wang YS, Zhai YX, et al: O-linked
GlcNAcylation elevated by HPV E6 mediates viral oncogenesis. Proc
Natl Acad Sci USA. 113:9333–9338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li L, Peng G, Liu X, Zhang Y, Han H and
Liu ZR: Pyruvate kinase M2 coordinates metabolism switch between
glycolysis and glutaminolysis in cancer cells. iScience.
23:1016842020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Reinstein E, Scheffner M, Oren M,
Ciechanover A and Schwartz A: Degradation of the E7 human
papillomavirus oncoprotein by the ubiquitin-proteasome system:
Targeting via ubiquitination of the N-terminal residue. Oncogene.
19:5944–5950. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Alani RM and Münger K: Human
papillomaviruses and associated malignancies. J Clin Oncol.
16:330–337. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yang W, Xia Y, Ji H, Zheng Y, Liang J,
Huang W, Gao X, Aldape K and Lu Z: Nuclear PKM2 regulates β-catenin
transactivation upon EGFR activation. Nature. 480:118–122. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Gao X, Wang H, Yang JJ, Liu X and Liu ZR:
Pyruvate kinase M2 regulates gene transcription by acting as a
protein kinase. Mol Cell. 45:598–609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yue G, Tang J, Zhang L, Niu H, Li H and
Luo S: CD276 suppresses CAR-T cell function by promoting tumor cell
glycolysis in esophageal squamous cell carcinoma. J Gastrointest
Oncol. 12:38–51. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xiao M, Xie J, Wu Y, Wang G, Qi X, Liu Z,
Wang Y, Wang X, Hoque A, Oakhill J, et al: The eEF2 kinase-induced
STAT3 inactivation inhibits lung cancer cell proliferation by
phosphorylation of PKM2. Cell Commun Signal. 18:252020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Shukla S, Mahata S, Shishodia G, Pandey A,
Tyagi A, Vishnoi K, Basir SF, Das BC and Bharti AC: Functional
regulatory role of STAT3 in HPV16-mediated cervical carcinogenesis.
PLoS One. 8:e678492013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang W, Wu X, Hu L, Ma Y, Xiu Z, Huang B,
Feng Y and Tang X: Overexpression of human papillomavirus type 16
oncoproteins enhances epithelial-mesenchymal transition via STAT3
signaling pathway in non-small cell lung cancer cells. Oncol Res.
25:843–852. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Morgan EL and Macdonald A: Manipulation of
JAK/STAT signalling by high-risk HPVs: Potential therapeutic
targets for HPV-associated malignancies. Viruses. 12:9772020.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Christensen DR, Calder PC and Houghton FD:
GLUT3 and PKM2 regulate OCT4 expression and support the hypoxic
culture of human embryonic stem cells. Sci Rep. 5:175002015.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lee J, Kim HK, Han YM and Kim J: Pyruvate
kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4
in regulating transcription. Int J Biochem Cell Biol. 40:1043–1054.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Brehm A, Ohbo K, Zwerschke W, Botquin V,
Jansen-Dürr P and Schöler HR: Synergism with germ line
transcription factor Oct-4: Viral oncoproteins share the ability to
mimic a stem cell-specific activity. Mol Cell Biol. 19:2635–2643.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Park EG, Pyo SJ, Cui Y, Yoon SH and Nam
JW: Tumor immune microenvironment lncRNAs. Brief Bioinform.
23:bbab5042022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hinshaw DC and Shevde LA: The tumor
microenvironment innately modulates cancer progression. Cancer Res.
79:4557–4566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Adem S, Comakli V and Uzun N: Pyruvate
kinase activators as a therapy target: A patent review 2011–2017.
Expert Opin Ther Pat. 28:61–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wang T, Marquardt C and Foker J: Aerobic
glycolysis during lymphocyte proliferation. Nature. 261:702–705.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wang R, Dillon CP, Shi LZ, Milasta S,
Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger
J and Green DR: The transcription factor Myc controls metabolic
reprogramming upon T lymphocyte activation. Immunity. 35:871–882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Boxer MB, Jiang JK, Vander Heiden MG, Shen
M, Skoumbourdis AP, Southall N, Veith H, Leister W, Austin CP, Park
HW, et al: Evaluation of substituted N,N'-diarylsulfonamides as
activators of the tumor cell specific M2 isoform of pyruvate
kinase. J Med Chem. 53:1048–1055. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Jiang JK, Boxer MB, Vander Heiden MG, Shen
M, Skoumbourdis AP, Southall N, Veith H, Leister W, Austin CP, Park
HW, et al: Evaluation of thieno[3,2-b]pyrrole[3,2-d]pyridazinones
as activators of the tumor cell specific M2 isoform of pyruvate
kinase. Bioorg Med Chem Lett. 20:3387–3393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Angiari S, Runtsch MC, Sutton CE,
Palsson-McDermott EM, Kelly B, Rana N, Kane H, Papadopoulou G,
Pearce EL, Mills KHG and O'Neill LAJ: Pharmacological activation of
pyruvate kinase M2 inhibits CD4+ T cell pathogenicity
and suppresses autoimmunity. Cell Metab. 31:391–405.e8. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Damasceno LEA, Prado DS, Veras FP, Fonseca
MM, Toller-Kawahisa JE, Rosa MH, Públio GA, Martins TV, Ramalho FS,
Waisman A, et al: PKM2 promotes Th17 cell differentiation and
autoimmune inflammation by fine-tuning STAT3 activation. J Exp Med.
217:e201906132020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Deng J, Lü S, Liu H, Liu B, Jiang C, Xu Q,
Feng J and Wang X: Homocysteine activates B cells via regulating
PKM2-dependent metabolic reprogramming. J Immunol. 198:170–183.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W,
Kang R, Lotze MT, Billiar TR, Wang H, et al: PKM2 regulates the
Warburg effect and promotes HMGB1 release in sepsis. Nat Commun.
5:44362014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Shirai T, Nazarewicz RR, Wallis BB, Yanes
RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC,
Assimes TL, et al: The glycolytic enzyme PKM2 bridges metabolic and
inflammatory dysfunction in coronary artery disease. J Exp Med.
213:337–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Palsson-McDermott EM, Curtis AM, Goel G,
Lauterbach MAR, Sheedy FJ, Gleeson LE, van den Bosch MWM, Quinn SR,
Domingo-Fernandez R, Johnston DGW, et al: Pyruvate kinase M2
regulates Hif-1α activity and IL-1β induction and is a critical
determinant of the Warburg effect in LPS-activated macrophages.
Cell Metab. 21:3472015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang L, Deng Z, Sun Y, Zhao Y, Li Y, Yang
M, Yuan R, Liu Y, Qian Z, Zhou F and Kang H: The study on the
regulation of Th cells by mesenchymal stem cells through the
JAK-STAT signaling pathway to protect naturally aged sepsis model
rats. Front Immunol. 13:8206852022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lochmatter C, Fischer R, Charles PD, Yu Z,
Powrie F and Kessler BM: Integrative phosphoproteomics links IL-23R
signaling with metabolic adaptation in lymphocytes. Sci Rep.
6:244912016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Deng W, Zhu S, Zeng L, Liu J, Kang R, Yang
M, Cao L, Wang H, Billiar TR, Jiang J, et al: The circadian clock
controls immune checkpoint pathway in sepsis. Cell Rep. 24:366–378.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Park YS, Kim DJ, Koo H, Jang SH, You YM,
Cho JH, Yang SJ, Yu ES, Jung Y, Lee DC, et al: AKT-induced PKM2
phosphorylation signals for IGF-1-stimulated cancer cell growth.
Oncotarget. 7:48155–48167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Long L, Zhao C, Ozarina M, Zhao X, Yang J
and Chen H: Targeting immune checkpoints in lung cancer: Current
landscape and future prospects. Clin Drug Investig. 39:341–353.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Palsson-McDermott EM, Dyck L, Zasłona Z,
Menon D, McGettrick AF, Mills KHG and O'Neill LA: Pyruvate kinase
M2 is required for the expression of the immune checkpoint PD-L1 in
immune cells and tumors. Front Immunol. 8:13002017. View Article : Google Scholar : PubMed/NCBI
|