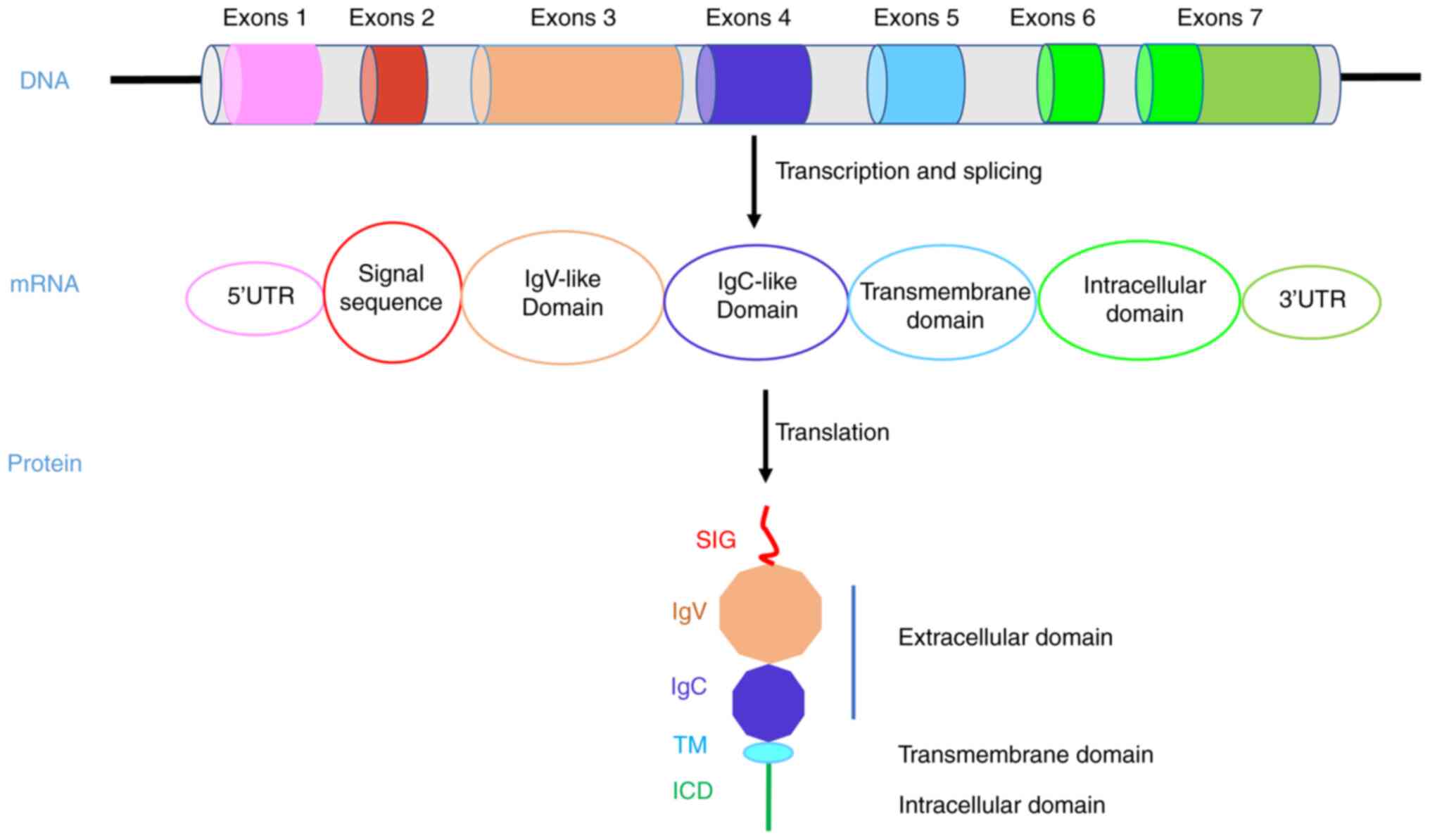
Programmed cell death ligand 1 (PD-L1), an essential
member of the B7 protein family, is well known to bind to
programmed cell death 1 (PD-1) to make tumor cells evade death from
the immune system (1). A number of
types of cancers (renal cell carcinoma (RCC), breast cancer,
colorectal cancer (CRC), stomach cancer, non-small cell lung cancer
(NSCLC), papillary thyroid cancer and testicular cancer) exhibit
high expression of PD-L1, which is correlated with poor prognosis
(2–8). At present, antibodies targeting the
PD-1/PD-L1 axis have been approved to be effective in some types of
cancers such as melanoma, NSCLC, RCC, Hodgkin's lymphoma, bladder
cancer, head and neck squamous cell carcinoma (HNSCC), Merkel-cell
carcinoma and microsatellite instable-high (MSI-H) or mismatch
repair-deficient (dMMR) solid tumors (9). Although PD-1/PD-L1 blockade therapy
has shown significant clinical benefits, its efficiency is only
≤40% across multiple cancer types (10,11).
Until now, most studies of PD-L1 in tumors have focused on its role
as an immune checkpoint. However, PD-L1 has a number of non-immune
functions in tumor cells. Several studies have also demonstrated
that PD-L1 possesses some intrinsic regulatory functions and can
play an important role in promoting tumorigenesis and progression
(12–15).
In recent years, the inherent function of PD-L1
mediated in tumor cells and the interaction with other carcinogenic
pathways has attracted more and more attention. PD-L1 is a
transmembrane protein that contains extracellular IgV and IgC
domains, a transmembrane domain (TM) and a short intracellular
domain (ICD) (16). The
extracellular domain is well known for binding with PD-1 to inhibit
T cell immune killing. However, there are few studies on the ICD of
PD-L1. For example, one study showed that PD-L1 can counteract the
cytotoxicity caused by IFNβ through its ICD and accelerate tumor
progression (17).
The immune function of PD-L1 has been well
demonstrated since it has been proved effective in a number of
tumors treatment. However, the therapeutic effect is still not
ideal, with an efficiency rate of ≤40%, which indicates that there
are still some mechanisms that have not been explored, such as
whether PD-L1 has a non-immune checkpoint function. The non-immune
functions of PD-L1 mainly include regulating tumor proliferation,
epithelial-mesenchymal transition (EMT), cell stem cells (CSCs),
cell metabolism, genome stability and drug resistance. It is of
great significance to study the intrinsic function of PD-L1 to
improve the antitumor therapeutic effect of the PD-L1 antibody.
Therefore, the present review mainly focused on the non-immune
functions of PD-L1.
PD-L1 is aberrantly highly expressed in a number of
types of human tumors and often high PD-L1 expression is associated
with poor patient prognosis. A meta-analysis of included studies
showed that high PD-L1 expression is associated with shorter
overall survival (OS) time and poorer prognosis in patients with
NSCLC (23). In an analysis of a
database containing 305 curatively resected esophageal cancers, it
was found that PD-L1+ cases have significantly poorer OS
compared with PD-L1− cases (24). In a study that included 94 patients
with glioblastoma (GBM), researchers using immunohistochemistry
analysis measurements found a high incidence of PD-L1 expression in
patients with GBM, but only in a small subgroup, and higher PD-L1
expression was associated with poorer long-term outcomes (5). In a meta-analysis of 8,419 patients
with gastric cancer (GC), researchers found that PD-L1 positivity
in patients with GC is associated with poor prognosis and poor OS;
however, there were no significant differences between PD-L1
expression and lymph node metastasis and overall TNM stage
(25). A systematic review study
including 13 clinical studies with 1,422 patients with cervical
cancer found that high PD-L1 expression is associated with the poor
OS but not with progression-free survival (PFS); overexpression of
PD-L1 in tumor cells and tumor-infiltrating immune cells predict
poor OS (26). In a meta-analysis
on RCC that included six studies and 1,323 cases, it was found that
higher levels of PD-L1 expression in RCC increases the risk of
death by 81%, representing a poor prognosis (27). A meta-analysis included 14,367
patients in 47 studies that focused on the relationship between
PD-L1 expression in primary breast cancer (PBC) and found that
PD-L1 expression in tumors is correlated with higher clinical risk
pathological parameters and poor prognosis in patients with PBC and
that patients with PD-L1+ tumors are significantly
associated with shorter disease-free survival (DFS) and OS
(28). In a meta-analysis of PD-L1
expression and CRC prognosis, which included clinical data from
4,344 patients in 12 studies, the results showed that PD-L1
overexpression is correlated with shorter OS and RFS/DFS ratios;
the study concludes that PD-L1 can be an effective biomarker for
negative prognosis and poor clinicopathological characteristics of
CRC (29). In a meta-analysis of
the association between PD-L1 expression and melanoma, including 13
articles with a total of 1,062 enrolled patients with melanoma, the
analysis revealed that high PD-L1 expression is not associated with
patient OS or PFS; however, PD-L1 overexpression is negatively
related to lymph node metastasis; this study suggests that PD-L1
expression cannot be used as a marker of prognosis in melanoma
patients (30).
Although PD-L1 expression is associated with poor
prognosis in patients with tumors in most cases, it must be noted
that the results are inconsistent in a number of studies.
Therefore, one needs to be aware that PD-L1 expression is diverse
in different types of tumors, PD-L1 expression is also diverse in
the population and the relationship between PD-L1 and clinical
tumor cases and patient prognosis is also variable. The role of
PD-L1 in different types of tumors may also be inconsistent and its
mechanism of action may be affected by a number of factors.
Therefore, more detailed studies are needed to elucidate the
mechanism of PD-L1 action in different tumors, so we can achieve
better results for immunotherapy and target therapy more
effectively.
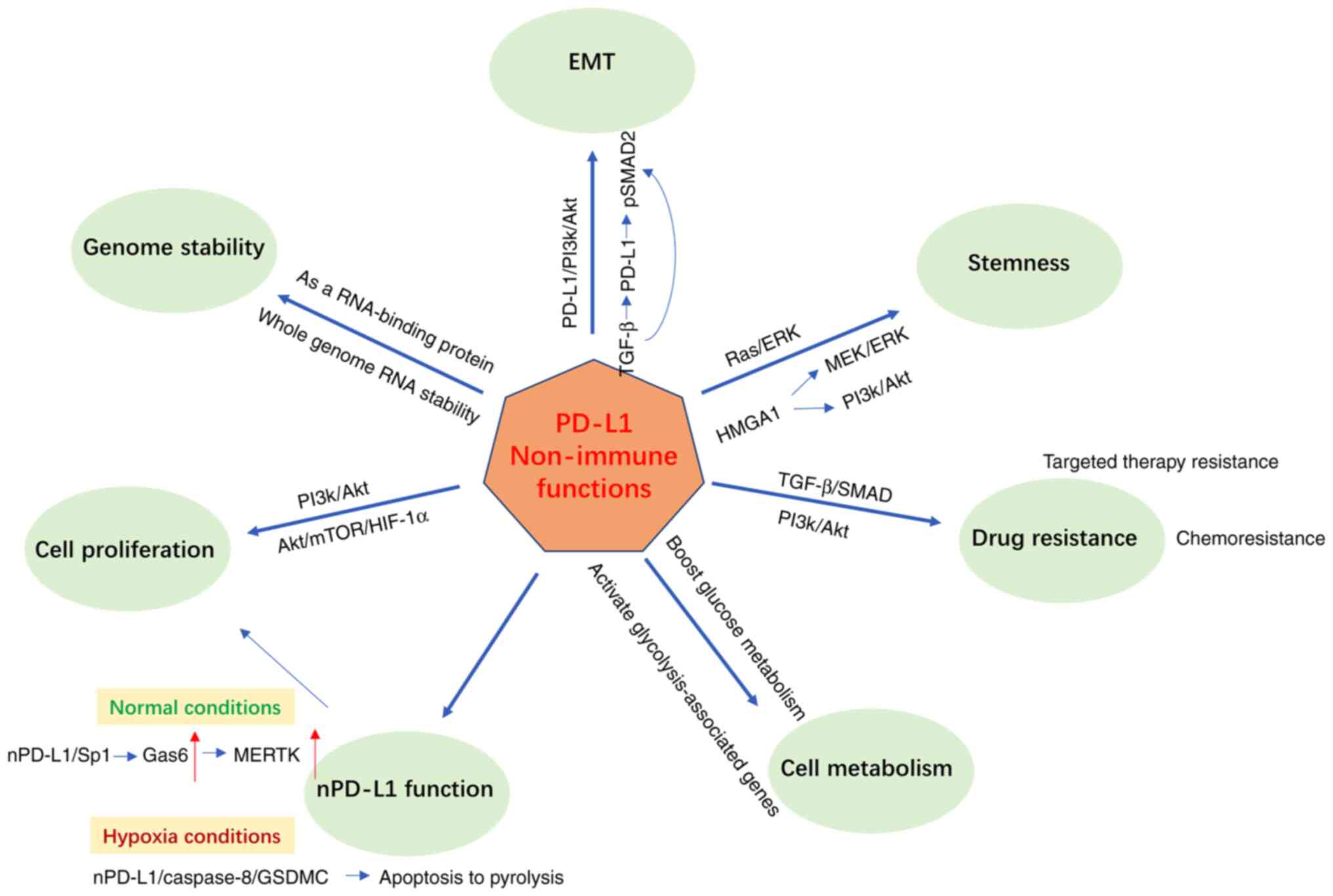
Based on the published studies, the non-immune
checkpoint functions of PD-L1 are mainly: Promoting tumor
proliferation, promoting EMT and stemness, regulating drug
resistance, regulating tumor metabolism, maintaining genomic
stability and entering the nucleus to perform functions. These are
described separately in this section. (Fig. 2).
The interaction between PD-L1 and PD1 has been
widely reported to interfere with the T cell receptor (TCR)
signaling transduction of T cells. PD-L1 is vital in inhibiting
T-cell-mediated immune response in cytotoxic T cells, leading to
immune killing escape and tumor progression in several malignancies
(31).
Studies have shown that PD-L1 can regulate cancer
cell growth, proliferation and suppress apoptosis without PD-1
involvement (12,32–39).
A study showed that the knockdown of PD-L1 expression in GC cells
can significantly suppress cell proliferation, migration, invasion,
apoptosis, cell cycle, tumorigenicity and cytotoxic sensitivity to
CIK therapy (32). Lotfinejad
et al (33) demonstrate
that PD-L1 knockdown can reduce triple-negative breast cancer
(TNBC) cell proliferation and induce apoptosis via intrinsic and
extrinsic apoptosis pathways. In a mouse sarcoma model, blocking
PD-L1 on tumors could interrupt tumor progression and cell
glycolysis; the mechanism is to suppress mTOR signals and decrease
the expression of some glycolytic enzymes (34). In ovarian cancer and melanoma,
Clark et al (35) observed
that PD-L1low cells proliferate more weakly than control
cells in vitro and PD-L1 attenuation also reduces mTORC1
activity. Fan et al (36)
found that Cbl-b could interact with STAT5a and cause its
ubiquitination, which downregulates PD-L1 expression and inhibits
cell proliferation, but miR-940 could target Cbl-b and then
upregulate PD-L1 expression and promote gastric cancer cell
proliferation. A study found that in TNBC and NSCLC, the cell
surface adhesion receptor CD44 was a critical positive regulator of
PD-L1; CD44 could bind to the regulatory region of PD-L1, which
contains the CD44-ICD binding site and activates PD-L1
transcription through its ICD; the activated PD-L1 could promote
tumor cell proliferation independent of T cell response (37). During cell division, PD-L1 is a
subunit of the adhesin complex: PD-L1 could compensate for the loss
of Sororin and compete with Wing Apart-Like (WAPL) for binding to
PDS5B, which secures proper sister chromatid cohesion and
segregation; depleting PD-L1 leads to multinuclear cells and
suppresses cell proliferation in vitro and tumor growth
in vivo in immunodeficient NSG mice (38). In NSCLC cells, activation of EGFR
could upregulate the expression of PD-L1 through IL-6/Janus kinase
(JAK)/STAT3 signal pathway and promote NSCLC cell proliferation
(39). Yang et al found
that PIM2-mediated phosphorylation of heat shock factor 1 (HSF1) at
Thr120 enhanced the stability of HSF1 protein and phosphorylation
of HSF1 could bind to the promoter of PD-L1, which strengthened
PD-L1 expression and promoted breast cancer cells proliferation
(12).
Although a number of basic studies have demonstrated
the ability of PD-L1 to promote tumor proliferation and progression
(Table I), clinicopathological
data have also confirmed that high PD-L1 expression is associated
with poor prognosis in most cases. However, the mechanism of PD-L1
promoting tumor proliferation and progression is not well studied.
Most of the studies found that PD-L1 high expression can show the
proliferative phenotypes of tumor cells, but how does PD-L1 promote
tumor proliferation? Does it promote the activation of
transcription factors and participate in post-transcriptional
modification (PTM) of certain oncogenes or tumor suppressors? These
may be the next breakthroughs for PD-L1 research.
The most common and effective cancer treatment
methods include surgery, chemotherapy and radiation therapy.
Chemotherapy is quite important in cancer treatment and can extend
the survival time of patients with a number of cancers. Advances in
biotechnology and intensive research on signaling pathways have led
to the rapid development of targeted therapy, which has also become
an important option for tumor treatment.
Although chemotherapy has a noticeable effect in the
early period of advanced tumor treatment, but, after a while, a
large proportion of chemoresistance might develop, which leads to
treatment failure and metastasis occurrence (40). Studies have shown that the high
expression of PD-L1 in cancer cells could cause chemotherapy
resistance in cancer therapy (Table
II). Following doxorubicin treatment, PD-L1 was observed to
transfer from membrane to nuclear concomitant with the
translocation of phosphorylated Akt and promote doxorubicin-induced
drug resistance (41). The
mechanism was that doxorubicin-dependent downregulation of cell
surface PD-L1 was accompanied by upregulation of PD-L1 in the
nucleus and this redistribution of PD-L1 occurred with a similar
translocation of phosphorylated Akt to the nucleus. PD-L1 was
considered an independent prognostic risk factor for osteosarcoma
as patients with high PD-L1 expression were observed to have a
lower five-year survival rate and knocking out PD-L1 in
osteosarcoma cells could increase doxorubicin and paclitaxel
sensitivities (42). A pair of
studies reported that PD-L1 could bind to NBS1 to form a complex
and lead to cisplatin resistance in HNSCC and knockdown of PD-L1 or
NBS1 could reverse this drug resistance. PD-L1 and IL-6 were
over-expressed on cisplatin-resistant HNSCC cells (43,44).
In cisplatin resistant NSCLC, researchers found that decreased COP1
could promote c-Jun accumulation, inhibit HDAC3 expression and
enhance PD-L1 acetylation, which would mediate or maintain the drug
resistance of cancer cells (45).
In ovarian cancer cells, Sp17high
(PD-L1+MHC-II−) cells showed enhanced
resistance to paclitaxel-induced cell death compared with
Sp17low (PD-L1−MHC-II+) cells
(46), which means Sp17 and PD-L1
are related to paclitaxel resistance. lncRNA FGD5-antisense 1
(FGD5-AS1) could negatively regulate miR-142 and promote cisplatin
resistance through miR-142-5p/PD-L1 axis (47). miR3609 could specifically bind to
the 3′ UTR region of PD-L1 and suppress PD-L1 expression to
sensitize breast cancer cells to doxorubicin (48).
Although targeted therapy is developing rapidly, the
problem of rapid drug resistance is a key obstacle to its further
development. PD-L1 has also been found to play a role in the
resistance of some targeted therapies. In EGFR-mutated NSCLC
cells, PD-L1 was correlated with the sensitivity of tyrosine kinase
inhibitors (TKIs) and PD-L1 could induce EMT by activating the
TGF-β/Smad signal pathway, leading to primary resistance to
gefitinib (49). PD-L1 expression
was found to be increased in gefitinib-resistant CRC cells, but
nano-diamino-tetras (NDAT)-induced low PD-L1 expression could
reverse tumor gefitinib resistance (50). In sorafenib-resistant hepatoma
cells, nuclear factor erythroid 2-related factor 2 inhibited the
expression of miR-1 and loss of miR-1 contributed to the PD-L1
upregulation and drug resistance (51).
Most of these studies on the role of PD-L1 in drug
resistance have also focused on the description of the phenotype,
while the underlying mechanisms have not been much studied. It was
hypothesized that PD-L1 may be involved in regulating the
expression or PTM of certain drug resistance-related genes to cause
the development of drug resistance. If the mechanism can be found,
it will help find a targeted drug resistance solution.
Studies have shown that PD-L1 plays a vital role in
promoting EMT and maintaining the stemness of cancer stem cells
(52–57) (Table
III).
PD-1 fusion protein-mediated stimulation of PD-L1
and the cytoplasmic domain of PD-L1 play a critical role in
promoting the EMT phenotype of Eca-109 cells (52). Upregulation of PD-L1 in skin
epithelial cells promotes EMT and accelerates carcinogenesis in
squamous cell carcinoma (53). The
significant association between PD-L1 expression and EMT phenotype
was maintained in EGFR-mutated pADCs in lung cancer cells
(54). A study found that CRC
characterized by a lack of CDX2 and prominent expression of ALCAM
frequently (71%) showed PD-L1 positivity, representing the
relations between PD-L1 and EMT (55). It was also found that EMT is
associated with the overexpression of PD-L1 in NSCLC (56). One study reports that
PD-L1+ cancer cells show the characteristics of EMT
(57). A survival analysis using
The Cancer Genome Atlas database shows that
PD-L1+/EMT− patients have a better prognosis
than PD-L1+/EMT+ patients in HNSCC (58). A study reports that PD-L1
expression increases in the induction of human breast EMT by
activating PI3K/Akt pathway and that PD-L1 can also regulate the
EMT state of breast cancer cells (59). PD-L1 can induce EMT and enhance the
stemness of renal cell carcinoma by upregulating SREBP-1c (60). In NSCLC, TGF-β1 can upregulate
PD-L1 expression at the transcriptional level through
phosphorylation of Smad2, M7824 is a novel bifunctional agent which
could target both PD-L1 and TGF-β1, using M7824 to treat NSCLC
could attenuate TGF-β1 mediated EMT (61).
PD-L1 is considered essential in maintaining the
stemness of breast cancer stem cells because it can upregulate the
expression of Oct4 and Nanog in a PI3K/Akt-dependent pathway and
directly promote BMI1 expression to affect the stemness of breast
CSCs (62). CD133+
cells in both cell lines and CRC tissues express a high level of
PD-L1 (57). Fang et al
(63) found that PD-L1 can promote
the proliferation of leukemia-inducing cells through
PD-L1/JNK/Cyclin D2 signaling pathway and prompt leukemia stem
cells to enter the cell cycle. PD-L1 can induce a stem cell-like
state and interact directly with high mobility group AT-hook 1
(HMGA1) to activate PI3K/Akt and MEK/ERK pathways in CRC to
maintain the self-renewal of CSCs (64).
Some studies show that non-coding RNAs and miRNAs
can regulate cancer stemness and EMT by binding with PD-L1. SNHG14
can sponge miR-5590-3p to upregulate ZEB1 and ZEB1
transcriptionally activate SNHG14 and PD-L1 to promote the immune
evasion of diffuse large B cell lymphoma cells (65). ZEB1, an EMT activator and
transcriptional repressor of miR-200, can relieve miR-200
repression of PD-L1, leading to lung adenocarcinoma metastasis
(66). In lymphoma cells, MALAT1
can sponge miR-195 to regulate the expression of PD-L1, knocking
down MALAT1 also suppresses the EMT-like process via the Ras/ERK
signaling pathway (67). In TNBC,
miR-200c can repress a number of genes encoding immunosuppressive
factors, including CD274/CD273, HMOX-1 and GDF15 to
reverse the classic EMT signature (68). miR-873 can inhibit PD-L1 expression
by directly binding to the 3′-UTR of CD274, which attenuates
the stemness and chemoresistance of breast cancer cells through the
PI3K/Akt signaling pathway (69).
Circular RNA circ-CPA4 can act as an RNA sponge for let-7 miRNA and
inhibit cell growth, migration and EMT by downregulating PD-L1 to
promote NSCLC cell death (70).
These studies have mainly focused on the role of
PD-L1 in cancer cell stemness maintenance and EMT and most of them
are limited to detecting the relationship between PD-L1 expression
changes and markers of stemness and EMT; however, little research
has been performed on the specific mechanisms. Whether PD-L1 could
regulate cancer metastasis or the mechanism behind this has not
been discovered, but is worth exploring, as several clinical
studies (3,15,36,70)
have confirmed the association between PD-L1 and lymph node
metastasis and distant metastasis of tumors.
Tumor cells can continuously adjust the metabolic
pathways to meet their energy requirements and respond to the
availability of nutrients. Warburg found that despite sufficient
oxygen, most solid tumor cells still choose the aerobic glycolysis
pathway rather than the oxidative phosphorylation pathway to adapt
to their microenvironment (71).
Studies show that PD-L1 can promote tumor progression by boosting
the glucose metabolism of tumor cells (Table IV). A study used
18F-Fluorodeoxyglucose Positron Emission
Tomography/Computed Tomography (18F FDG PET/CT) to
evaluate the metabolic effects of PD-L1 protein on lung cancer and
it was found that high PD-L1 expression can promote glucose
metabolism in NSCLC (72). PD-L1
can promote tumor cell glycolysis through Akt/mTOR signal pathway
and induce immune cells to consume glucose in the microenvironment.
Inhibiting the expression of PD-L1 can cause mTOR activity
inhibition to downregulate glycolytic enzymes, thereby inhibiting
glycolysis (34). In cervical
cancer, PD-L1 directly binds to integrin β4 and activates Akt/GSK3β
signaling pathway and promotes glucose metabolism (73). Retinoic acid-related orphan
receptor C (RORC) is found to negatively regulate the expression of
PD-L1 by binding to the PD-L1 promoter region, which can inhibit
the nuclear translocation of STAT3 and further inhibit the
proliferation and glucose metabolism of bladder tumor cells
(74). Ma et al (75) report that in acute myeloid leukemia
(AML) cell lines, glycolysis-associated genes ALDOA, PGK1,
LDHA and HK2 are highly expressed in the
PD-L1high cell line and overexpressed PD-L1 enhances
glucose consumption rate, accompanied by decreased apoptosis and S
phase cells. Feng et al (76) report that lactate-induced PD-L1 is
mediated by its receptor GPR81 and GPR81-mediated upregulation of
PD-L1 in glucose-stimulated lung cancer cells that recapitulated
the enhanced glycolysis is dependent on LDHA. In patients with
primary lung adenocarcinoma who received 18F-FDG PET/CT
before treatment, PD-L1 expression in the tumor was positively
correlated with 18F-fluorodeoxyglucose maximum
standardized uptake value (SUVmax), total lesion glycolysis (TLG),
HK2 and glucose transporter 1 (GLUT-1) expression (77).
These studies initially reveal the regulatory role
of PD-L1 in glucose metabolism, but a number of mechanisms remain
to be elucidated. Moreover, the regulation of PD-L1 in other
metabolic pathways has yet to be reported. As tumor metabolism is
not limited to glucose metabolism, it is hypothesized that PD-L1
may also play an important role in regulating other metabolic
pathways in tumor cells, so more in-depth and extensive research is
needed.
In addition to the numerous intrinsic functions
previously described, PD-L1 also has a role in regulating gene
stability. Tu et al (78)
demonstrate that PD-L1 can act as an RNA-binding protein in cells
to regulate the mRNA stability of NBS1, BRCA1 and a number of other
DNA damage-related genes; intracellular PD-L1 can prevent these
target RNAs from being degraded, thus increasing the resistance of
cells to DNA damage. This study also found that PD-L1 has the
ability to regulate whole genome RNA stability by RNA
immunoprecipitation and RNA-seq assays. Thus, it provides strong
evidence that PD-L1 possesses an intrinsically powerful gene
regulatory function. It also predicts that PD-L1 may become a
target to interfere with tumor radiotherapy resistance.
PD-L1 was previously widely considered to be
localized in the cytoplasm and cell membrane, but recently some
studies report the nuclear localization and role of PD-L1 in tumor
cells (Table V).
The distribution of PD-L1 in different tumor
specimens is diverse. Nuclear PD-L1 (nPD-L1) is expressed in RCC,
lung cancer and hepatocellular carcinoma tissues and nPD-L1 in
human esophageal cancer tissues is significantly correlated with
tumor invasion (79,80). According to some reports, the
expression of nPD-L1 is associated with a poor prognosis in some
tumors. Expression of nPD-L1 in cell-surface vimentin-positive
circulating tumor cells is significantly associated with the
short-term survival rate of CRC and prostate cancer (81). Doxorubicin treatment can
redistribute PD-L1 and increase the expression of nPD-L1 through
PI3K/Akt signaling pathway (41).
One study has shown that in NSCLC, KPNB1 binds to PD-L1 and
promotes its entry into the nucleus (79). At the same time, nPD-L1 can
integrate Sp1 to regulate the synthesis of Gas6, promote the
secretion of Gas6 and activate the MER proto-oncogene tyrosine
kinase signaling pathway to promote cell proliferation (79). In breast cancer, under hypoxic
conditions, pSTAT3 can physically interact with PD-L1 and upgrade
its nuclear translocation and enhance the transcription of the
gasdermin C (GSDMC) gene, GSDMC is cleaved explicitly by caspase 8
to switch cell apoptosis to pyrolysis and induce tumor necrosis
(82). This study showed a new
signal pathway of nPD-L1/caspase-8/GSDMC, which is required for
macrophage-derived TNFα-induced tumor necrosis. PD-L1 can be
acetylated and modified by p300 acetyltransferase at Lys 263 in the
cytoplasmic domain and blocking the acetylation of PD-L1 can damage
its nuclear translocation, reprogram the expression of immune
response-related genes and block the anti-tumor response to
PD-1/PD-L1 therapy (83).
These aforementioned studies report how PD-L1 enters
the nucleus and the functions it plays in the nucleus, but the
exact mechanism of PD-L1 action in the nucleus remains to be
elucidated. Therefore, further in-depth studies are needed to
clarify the nuclear membrane transfer process and the internal
effects of nPD-L1, which will help understand the non-immune
checkpoint functions of PD-L1 widely.
PD-L1 has an important immune checkpoint function.
Its role in tumor evasion of immune killing has been apparent, but
PD-L1 is highly expressed in various types of tumor and shows some
inherent non-immunological functions. PD-L1 has a number of
intrinsic functions, such as promoting tumor proliferation,
maintaining the stemness of cancer stem cells and EMT, regulating
tumor cell metabolism and promoting drug resistance in tumors
(Fig. 2). It also can perform
specific functions by entering the nucleus and regulating genome
stability.
At present, it is known that PD-L1 has these
non-immune checkpoint functions, but not the exact mechanism. For
example, the exact mechanism of PD-L1 to promote tumor progression
through non-immune checkpoint-dependent pathways, the mechanism of
PD-L1 to regulate the EMT process and maintain the stemness of
tumor stem cells, the functions of PD-L1 in cancer metastasis, the
specific role and function of PD-L1 after entering the nucleus and
possible role of PD-L1 in regulating other metabolic pathways in
tumors need to be explored widely and deeply. If these functions
and mechanisms can be studied carefully, it will help precisely to
target and intervene in the PD-L1 pathway from tumor prevention to
tumor recurrence and metastasis, providing more possibilities for
combining tumor immunotherapy with targeted therapy.
Not applicable.
The present study was supported by the Tongji Hospital
Foundation (grant no. 2021HGRY012) for XX.
Not applicable.
JD, LL, WZ and YW wrote the manuscript, WJ and XX
revised the manuscript, and XX reviewed the final version of the
manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Sun C, Mezzadra R and Schumacher TN:
Regulation and Function of the PD-L1 Checkpoint. Immunity.
48:434–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thompson RH, Gillett MD, Cheville JC,
Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen
L, et al: Costimulatory B7-H1 in renal cell carcinoma patients:
Indicator of tumor aggressiveness and potential therapeutic target.
Proc Natl Acad Sci USA. 101:17174–17179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muenst S, Schaerli AR, Gao F, Däster S,
Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE,
et al: Expression of programmed death ligand 1 (PD-L1) is
associated with poor prognosis in human breast cancer. Breast
Cancer Res Treat. 146:15–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kraft S, Fernandez-Figueras MT, Richarz
NA, Flaherty KT and Hoang MP: PDL1 expression in desmoplastic
melanoma is associated with tumor aggressiveness and progression. J
Am Acad Dermatol. 77:534–542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nduom EK, Wei J, Yaghi NK, Huang N, Kong
LY, Gabrusiewicz K, Ling X, Zhou S, Ivan C, Chen JQ, et al: PD-L1
expression and prognostic impact in glioblastoma. Neuro Oncol.
18:195–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia H, Shen J, Hu F, Chen S, Huang H, Xu Y
and Ma H: PD-L1 over-expression is associated with a poor prognosis
in Asian non-small cell lung cancer patients. Clin Chim Acta.
469:191–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang S, Yuan B, Wang Y, Li M, Liu X, Cao
J, Li C and Hu J: Clinicopathological and prognostic significance
of PD-L1 expression in colorectal cancer: A meta-analysis. Int J
Colorectal Dis. 36:117–130. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cha JH, Chan LC, Li CW, Hsu JL and Hung
MC: Mechanisms Controlling PD-L1 Expression in Cancer. Mol Cell.
76:359–370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yarchoan M, Hopkins A and Jaffee EM: Tumor
mutational burden and response rate to PD-1 Inhibition. N Engl J
Med. 377:2500–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pitt JM, Vetizou M, Daillere R, Roberti
MP, Yamazaki T, Routy B, Lepage P, Boneca IG, Chamaillard M,
Kroemer G and Zitvogel L: Resistance mechanisms to
immune-checkpoint blockade in cancer: Tumor-intrinsic and
-extrinsic factors. Immunity. 44:1255–1269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang T, Ren C, Lu C, Qiao P, Han X, Wang
L, Wang D, Lv S, Sun Y and Yu Z: Phosphorylation of HSF1 by PIM2
Induces PD-L1 expression and promotes tumor growth in breast
cancer. Cancer Res. 79:5233–5244. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim W, Chu TH, Nienhuser H, Jiang Z, Del
Portillo A, Remotti HE, White RA, Hayakawa Y, Tomita H, Fox JG, et
al: PD-1 Signaling promotes tumor-infiltrating myeloid-derived
suppressor cells and gastric tumorigenesis in mice.
Gastroenterology. 160:781–796. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao H, Zhang J and Ren X: PD-L1 regulates
tumorigenesis and autophagy of ovarian cancer by activating mTORC
signaling. Biosci Rep. 39:BSR201910412019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mu L, Wang Y, Su H, Lin Y, Sui W, Yu X and
Lv Z: HIF1A-AS2 promotes the proliferation and metastasis of
gastric cancer cells through miR-429/PD-L1 Axis. Dig Dis Sci.
66:4314–4325. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zak KM, Kitel R, Przetocka S, Golik P,
Guzik K, Musielak B, Dömling A, Dubin G and Holak TA: Structure of
the complex of human programmed death 1, PD-1, and Its Ligand
PD-L1. Structure. 23:2341–2348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gato-Canas M, Zuazo M, Arasanz H,
Ibañez-Vea M, Lorenzo L, Fernandez-Hinojal G, Vera R, Smerdou C,
Martisova E, Arozarena I, et al: PDL1 signals through conserved
sequence motifs to overcome interferon-mediated cytotoxicity. Cell
Rep. 20:1818–1829. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin DY, Tanaka Y, Iwasaki M, Gittis AG, Su
HP, Mikami B, Okazaki T, Honjo T, Minato N and Garboczi DN: The
PD-1/PD-L1 complex resembles the antigen-binding Fv domains of
antibodies and T cell receptors. Proc Natl Acad Sci USA.
105:3011–3016. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Jiang CC, Jin L and Zhang XD:
Regulation of PD-L1: A novel role of pro-survival signalling in
cancer. Ann Oncol. 27:409–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Azuma T, Yao S, Zhu G, Flies AS, Flies SJ
and Chen L: B7-H1 is a ubiquitous antiapoptotic receptor on cancer
cells. Blood. 111:3635–3643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang RSP, Decker B, Murugesan K, Hiemenz
M, Mata DA, Li G, Creeden J, Ramkissoon SH and Ross JS: Pan-cancer
analysis of CD274 (PD-L1) mutations in 314,631 patient samples and
subset correlation with PD-L1 protein expression. J Immunother
Cancer. 9:e0025582021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brody R, Zhang Y, Ballas M, Siddiqui MK,
Gupta P, Barker C, Midha A and Walker J: PD-L1 expression in
advanced NSCLC: Insights into risk stratification and treatment
selection from a systematic literature review. Lung Cancer.
112:200–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yagi T, Baba Y, Ishimoto T, Iwatsuki M,
Miyamoto Y, Yoshida N, Watanabe M and Baba H: PD-L1 expression,
tumor-infiltrating lymphocytes, and clinical outcome in patients
with surgically resected esophageal cancer. Ann Surg. 269:471–478.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hassen G, Kasar A, Jain N, Berry S, Dave
J, Zouetr M, Priyanka Ganapathiraju VLN, Kurapati T, Oshai S, Saad
M, et al: Programmed Death-Ligand 1 (PD-L1) positivity and factors
associated with poor prognosis in patients with gastric cancer: An
umbrella meta-analysis. Cureus. 14:e238452022.PubMed/NCBI
|
|
26
|
Wan X, Hu T, Wu H, Cheng X and Xu S:
Predictive values of PDL1 expression for survival outcomes in
patients with cervical cancer: A systematic review and
meta-analysis. Ginekol Pol. Aug 19–2022.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iacovelli R, Nole F, Verri E, Renne G,
Paglino C, Santoni M, Cossu Rocca M, Giglione P, Aurilio G, Cullurà
D, et al: Prognostic Role of PD-L1 expression in renal cell
carcinoma. A systematic review and meta-analysis. Target Oncol.
11:143–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang W, Ran R, Shao B and Li H:
Prognostic and clinicopathological value of PD-L1 expression in
primary breast cancer: A meta-analysis. Breast Cancer Res Treat.
178:17–33. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang L, Xue R and Pan C: Prognostic and
clinicopathological value of PD-L1 in colorectal cancer: A
systematic review and meta-analysis. Onco Targets Ther.
12:3671–3682. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Dong M, Shui Y, Zhang Y, Zhang Z,
Mi Y, Zuo X, Jiang L, Liu K, Liu Z, et al: A pooled analysis of the
prognostic value of PD-L1 in melanoma: Evidence from 1062 patients.
Cancer Cell Int. 20:962020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fife BT, Pauken KE, Eagar TN, Obu T, Wu J,
Tang Q, Azuma M, Krummel MF and Bluestone JA: Interactions between
PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop
signal. Nat Immunol. 10:1185–1192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Chen L, Xiong Y, Zheng X, Xie Q,
Zhou Q, Shi L, Wu C, Jiang J and Wang H: Knockdown of PD-L1 in
human gastric cancer cells inhibits tumor progression and improves
the cytotoxic sensitivity to CIK therapy. Cell Physiol Biochem.
41:907–920. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lotfinejad P, Kazemi T, Safaei S, Amini M,
Roshani Asl E, Baghbani E, Sandoghchian Shotorbani S, Jadidi
Niaragh F, Derakhshani A, Abdoli Shadbad M, et al: PD-L1 silencing
inhibits triple-negative breast cancer development and upregulates
T-cell-induced pro-inflammatory cytokines. Biomed Pharmacother.
138:1114362021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang CH, Qiu J, O'Sullivan D, Buck MD,
Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ,
et al: Metabolic competition in the tumor microenvironment is a
driver of cancer progression. Cell. 162:1229–1241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Clark CA, Gupta HB, Sareddy G, Pandeswara
S, Lao S, Yuan B, Drerup JM, Padron A, Conejo-Garcia J, Murthy K,
et al: Tumor-Intrinsic PD-L1 signals regulate cell growth,
pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer
Res. 76:6964–6974. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan Y, Che X, Hou K, Zhang M, Wen T, Qu X
and Liu Y: MiR-940 promotes the proliferation and migration of
gastric cancer cells through up-regulation of programmed death
ligand-1 expression. Exp Cell Res. 373:180–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kong T, Ahn R, Yang K, Zhu X, Fu Z, Morin
G, Bramley R, Cliffe NC, Xue Y, Kuasne H, et al: CD44 Promotes
PD-L1 expression and its tumor-intrinsic function in breast and
lung cancers. Cancer Res. 80:444–457. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu J, Qin B, Moyer AM, Nowsheen S, Tu X,
Dong H, Boughey JC, Goetz MP, Weinshilboum R, Lou Z and Wang L:
Regulation of sister chromatid cohesion by nuclear PD-L1. Cell Res.
30:590–601. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang N, Zeng Y, Du W, Zhu J, Shen D, Liu
Z and Huang JA: The EGFR pathway is involved in the regulation of
PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in
EGFR-mutated non-small cell lung cancer. Int J Oncol. 49:1360–1368.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kaufmann SH and Earnshaw WC: Induction of
apoptosis by cancer chemotherapy. Exp Cell Res. 256:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ghebeh H, Lehe C, Barhoush E, Al-Romaih K,
Tulbah A, Al-Alwan M, Hendrayani SF, Manogaran P, Alaiya A,
Al-Tweigeri T, et al: Doxorubicin downregulates cell surface B7-H1
expression and upregulates its nuclear expression in breast cancer
cells: Role of B7-H1 as an anti-apoptotic molecule. Breast Cancer
Res. 12:R482010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liao Y, Chen L, Feng Y, Shen J, Gao Y,
Cote G, Choy E, Harmon D, Mankin H, Hornicek F and Duan Z:
Targeting programmed cell death ligand 1 by CRISPR/Cas9 in
osteosarcoma cells. Oncotarget. 8:30276–30287. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shen B, Huang D, Ramsey AJ, Ig-Izevbekhai
K, Zhang K, Lajud SA, O'Malley BW and Li D: PD-L1 and MRN synergy
in platinum-based chemoresistance of head and neck squamous cell
carcinoma. Br J Cancer. 122:640–647. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang P, Liu J, Li W, Li S and Han X:
Lactoferricin B reverses cisplatin resistance in head and neck
squamous cell carcinoma cells through targeting PD-L1. Cancer Med.
7:3178–3187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang H, Fu C, Du J, Wang H, He R, Yin X,
Li H, Li X, Wang H, Li K, et al: Enhanced histone H3 acetylation of
the PD-L1 promoter via the COP1/c-Jun/HDAC3 axis is required for
PD-L1 expression in drug-resistant cancer cells. J Exp Clin Cancer
Res. 39:292020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gao Q, Xiang SD, Wilson K, Madondo M,
Stephens AN and Plebanski M: Sperm Protein 17 expression by murine
epithelial ovarian cancer cells and its impact on tumor
progression. Cancers (Basel). 10:2762018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhu F, Niu R and Shao X and Shao X:
FGD5AS1 promotes cisplatin resistance of human lung adenocarcinoma
cell via the miR1425p/PDL1 axis. Int J Mol Med. 47:523–532. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li D, Wang X, Yang M, Kan Q and Duan Z:
MiR3609 sensitizes breast cancer cells to adriamycin by blocking
the programmed death-ligand 1 immune checkpoint. Exp Cell Res.
380:20–28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Zeng Y, Liu T, Du W, Zhu J, Liu Z
and Huang JA: The canonical TGF-β/Smad signalling pathway is
involved in PD-L1-induced primary resistance to EGFR-TKIs in
EGFR-mutant non-small-cell lung cancer. Respir Res. 20:1642019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huang TY, Chang TC, Chin YT, Pan YS, Chang
WJ, Liu FC, Hastuti ED, Chiu SJ, Wang SH, Changou CA, et al: NDAT
Targets PI3K-Mediated PD-L1 upregulation to reduce proliferation in
gefitinib-resistant colorectal cancer. Cells. 9:18302020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li D, Sun FF, Wang D, Wang T, Peng JJ,
Feng JQ, Li H, Wang C, Zhou DJ, Luo H, et al: Programmed death
ligand-1 (PD-L1) Regulated by NRF-2/MicroRNA-1 regulatory axis
enhances drug resistance and promotes tumorigenic properties in
sorafenib-resistant hepatoma cells. Oncol Res. 28:467–481. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen L, Xiong Y, Li J, Zheng X, Zhou Q,
Turner A, Wu C, Lu B and Jiang J: PD-L1 expression promotes
epithelial to mesenchymal transition in human esophageal cancer.
Cell Physiol Biochem. 42:2267–2280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cao Y, Zhang L, Kamimura Y, Ritprajak P,
Hashiguchi M, Hirose S and Azuma M: B7-H1 overexpression regulates
epithelial-mesenchymal transition and accelerates carcinogenesis in
skin. Cancer Res. 71:1235–1243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim S, Koh J, Kim MY, Kwon D, Go H, Kim
YA, Jeon YK and Chung DH: PD-L1 expression is associated with
epithelial-to-mesenchymal transition in adenocarcinoma of the lung.
Hum Pathol. 58:7–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Inaguma S, Lasota J, Wang Z,
Felisiak-Golabek A, Ikeda H and Miettinen M: Clinicopathologic
profile, immunophenotype, and genotype of CD274 (PD-L1)-positive
colorectal carcinomas. Mod Pathol. 30:278–285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tieche CC, Gao Y, Buhrer ED, Hobi N,
Berezowska SA, Wyler K, Froment L, Weis S, Peng RW, Bruggmann R, et
al: Tumor initiation capacity and therapy resistance are
differential features of EMT-Related subpopulations in the NSCLC
cell line A549. Neoplasia. 21:185–196. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhi Y, Mou Z, Chen J, He Y, Dong H, Fu X
and Wu Y: B7H1 expression and epithelial-to-mesenchymal transition
phenotypes on colorectal cancer stem-like cells. PLoS One.
10:e01355282015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ock CY, Kim S, Keam B, Kim M, Kim TM, Kim
JH, Jeon YK, Lee JS, Kwon SK, Hah JH, et al: PD-L1 expression is
associated with epithelial-mesenchymal transition in head and neck
squamous cell carcinoma. Oncotarget. 7:15901–15914. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Alsuliman A, Colak D, Al-Harazi O, Fitwi
H, Tulbah A, Al-Tweigeri T, Al-Alwan M and Ghebeh H: Bidirectional
crosstalk between PD-L1 expression and epithelial to mesenchymal
transition: Significance in claudin-low breast cancer cells. Mol
Cancer. 14:1492015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang Y, Wang H, Zhao Q, Xia Y, Hu X and
Guo J: PD-L1 induces epithelial-to-mesenchymal transition via
activating SREBP-1c in renal cell carcinoma. Med Oncol. 32:2122015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
David JM, Dominguez C, McCampbell KK,
Gulley JL, Schlom J and Palena C: A novel bifunctional
anti-PD-L1/TGF-β Trap fusion protein (M7824) efficiently reverts
mesenchymalization of human lung cancer cells. Oncoimmunology.
6:e13495892017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Almozyan S, Colak D, Mansour F, Alaiya A,
Al-Harazi O, Qattan A, Al-Mohanna F, Al-Alwan M and Ghebeh H: PD-L1
promotes OCT4 and Nanog expression in breast cancer stem cells by
sustaining PI3K/AKT pathway activation. Int J Cancer.
141:1402–1412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fang X, Chen C, Xia F, Yu Z, Zhang Y,
Zhang F, Gu H, Wan J, Zhang X, Weng W, et al: CD274 promotes cell
cycle entry of leukemia-initiating cells through JNK/Cyclin D2
signaling. J Hematol Oncol. 9:1242016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wei F, Zhang T, Deng SC, Wei JC, Yang P,
Wang Q, Chen ZP, Li WL, Chen HC, Hu H and Cao J: PD-L1 promotes
colorectal cancer stem cell expansion by activating HMGA1-dependent
signaling pathways. Cancer Lett. 450:1–13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhao L, Liu Y, Zhang J, Liu Y and Qi Q:
LncRNA SNHG14/miR-5590-3p/ZEB1 positive feedback loop promoted
diffuse large B cell lymphoma progression and immune evasion
through regulating PD-1/PD-L1 checkpoint. Cell Death Dis.
10:7312019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chen L, Gibbons DL, Goswami S, Cortez MA,
Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, et al: Metastasis
is regulated via microRNA-200/ZEB1 axis control of tumour cell
PD-L1 expression and intratumoral immunosuppression. Nat Commun.
5:52412014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang QM, Lian GY, Song Y, Huang YF and
Gong Y: LncRNA MALAT1 promotes tumorigenesis and immune escape of
diffuse large B cell lymphoma by sponging miR-195. Life Sci.
231:1163352019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rogers TJ, Christenson JL, Greene LI,
O'Neill KI, Williams MM, Gordon MA, Nemkov T, D'Alessandro A,
Degala GD, Shin J, et al: Reversal of Triple-Negative Breast Cancer
EMT by miR-200c decreases tryptophan catabolism and a program of
immunosuppression. Mol Cancer Res. 17:30–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gao L, Guo Q, Li X, Yang X, Ni H, Wang T,
Zhao Q, Liu H, Xing Y, Xi T and Zheng L: MiR-873/PD-L1 axis
regulates the stemness of breast cancer cells. EBioMedicine.
41:395–407. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hong W, Xue M, Jiang J, Zhang Y and Gao X:
Circular RNA circ-CPA4/let-7 miRNA/PD-L1 axis regulates cell
growth, stemness, drug resistance and immune evasion in non-small
cell lung cancer (NSCLC). J Exp Clin Cancer Res. 39:1492020.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Takada K, Toyokawa G, Okamoto T, Baba S,
Kozuma Y, Matsubara T, Haratake N, Akamine T, Takamori S, Katsura
M, et al: Metabolic characteristics of programmed cell death-ligand
1-expressing lung cancer on (18) F-fluorodeoxyglucose positron
emission tomography/computed tomography. Cancer Med. 6:2552–2561.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang S, Li J, Xie J, Liu F, Duan Y, Wu Y,
Huang S, He X, Wang Z and Wu X: Programmed death ligand 1 promotes
lymph node metastasis and glucose metabolism in cervical cancer by
activating integrin β4/SNAI1/SIRT3 signaling pathway. Oncogene.
37:4164–4180. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cao D, Qi Z, Pang Y, Li H, Xie H, Wu J,
Huang Y, Zhu Y, Shen Y, Zhu Y, et al: Retinoic acid-related orphan
receptor C regulates proliferation, glycolysis, and chemoresistance
via the PD-L1/ITGB6/STAT3 signaling axis in bladder cancer. Cancer
Res. 79:2604–2618. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ma P, Xing M, Han L, Gan S, Ma J, Wu F,
Huang Y, Chen Y, Tian W, An C, et al: High PDL1 expression drives
glycolysis via an Akt/mTOR/HIF1α axis in acute myeloid leukemia.
Oncol Rep. 43:999–1009. 2020.PubMed/NCBI
|
|
76
|
Feng J, Yang H, Zhang Y, Wei H, Zhu Z, Zhu
B, Yang M, Cao W, Wang L and Wu Z: Tumor cell-derived lactate
induces TAZ-dependent upregulation of PD-L1 through GPR81 in human
lung cancer cells. Oncogene. 36:5829–5839. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cui Y, Li X, Du B, Diao Y and Li Y: PD-L1
in lung adenocarcinoma: Insights into the role of (18)F-FDG PET/CT.
Cancer Manag Res. 12:6385–6395. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tu X, Qin B, Zhang Y, Zhang C, Kahila M,
Nowsheen S, Yin P, Yuan J, Pei H, Li H, et al: PD-L1 (B7-H1)
Competes with the RNA exosome to regulate the DNA damage response
and can be targeted to sensitize to radiation or chemotherapy. Mol
Cell. 74:1215–1226.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Du W, Zhu J, Zeng Y, Liu T, Zhang Y, Cai
T, Fu Y, Zhang W, Zhang R, Liu Z and Huang JA: KPNB1-mediated
nuclear translocation of PD-L1 promotes non-small cell lung cancer
cell proliferation via the Gas6/MERTK signaling pathway. Cell Death
Differ. 28:1284–1300. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen L, Deng H, Lu M, Xu B, Wang Q, Jiang
J and Wu C: B7-H1 expression associates with tumor invasion and
predicts patient's survival in human esophageal cancer. Int J Clin
Exp Pathol. 7:6015–6023. 2014.PubMed/NCBI
|
|
81
|
Satelli A, Batth IS, Brownlee Z, Rojas C,
Meng QH, Kopetz S and Li S: Potential role of nuclear PD-L1
expression in cell-surface vimentin positive circulating tumor
cells as a prognostic marker in cancer patients. Sci Rep.
6:289102016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hou J, Zhao R, Xia W, Chang CW, You Y, Hsu
JM, Nie L, Chen Y, Wang YC, Liu C, et al: PD-L1-mediated gasdermin
C expression switches apoptosis to pyroptosis in cancer cells and
facilitates tumour necrosis. Nat Cell Biol. 22:1264–1275. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gao Y, Nihira NT, Bu X, Chu C, Zhang J,
Kolodziejczyk A, Fan Y, Chan NT, Ma L, Liu J, et al:
Acetylation-dependent regulation of PD-L1 nuclear translocation
dictates the efficacy of anti-PD-1 immunotherapy. Nat Cell Biol.
22:1064–1075. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bouillez A, Rajabi H, Jin C, Samur M,
Tagde A, Alam M, Hiraki M, Maeda T, Hu X, Adeegbe D, et al: MUC1-C
integrates PD-L1 induction with repression of immune effectors in
non-small-cell lung cancer. Oncogene. 36:4037–4046. 2017.
View Article : Google Scholar : PubMed/NCBI
|