Introduction
Renal cell carcinoma (RCC) is the leading type of
malignant tumor originating from the kidneys, and makes up about
90% of all malignant renal tumors (1). RCC develops from the epithelium of
the proximal tubules and collecting ducts in the kidneys. According
to the WHO classification, there are different subtypes of kidney
carcinomas: clear cell, papillary, chromophobe and collecting duct
carcinomas. The identification of renal cell carcinoma subtypes is
essential in clinics, because RCC constitutes a heterogeneous group
of tumors with prognostic uncertainty (2).
The incidence of RCC is increasing, and the
treatment results remain unsatisfactory. Although patients with
early disease can be treated with surgery at a high success rate,
nearly 50% of RCC patients die within 5 years after being diagnosed
(3). The decisive factor
determining the clinical outcome is the development of distant
metastases, and the 5-year survival rate of patients with
metastatic lesions is less than 10% (4). The use of non-targeted chemotherapy
for the treatment of metastatic-RCC patients' is associated with
indiscriminate toxicity (5).
Currently, there is no chemotherapy for advanced kidney carcinoma
providing high objective response rates. Chemotherapy in
combination with cytokines (IFN-alpha and IL-2) or only cytokines
have been studied, but this treatment is not effective and leads to
additive toxicity (3). Due to the
low efficiency of existing treatments, it is necessary to develop
new approaches for both the therapy and diagnosis of renal cell
carcinoma. Regarding new therapy approaches, some advances have
been achieved by targeting either the vascular endothelial growth
factor receptor or the mammalian target of rapamycin (5,6).
Another promising treatment approach that has emerged, is the use
of immune-checkpoint inhibitors (7). Still, there is an urgent need for the
identification of new molecular targets and biomarkers, as well as
the development of new targeted therapies for RCC (7).
One particularly promising molecular target for RCC
is the epithelial cell adhesion molecule (EpCAM). EpCAM is highly
expressed in multiple carcinoma types and promotes tumor
proliferation. Importantly, EpCAM overexpression has been detected
in a large fraction of RCC cases. There has also been a continuous
development and evaluation of EpCAM-targeting therapeutics which
include monoclonal antibodies or antibody fragments as well as
their drug- and toxin-conjugates (8–12),
the EpCAM-targeting trifunctional T-cell engaging antibody
catumaxomab (Removab®) (13), targeted toxins based on scaffold
proteins (14,15), and chimeric antigen
receptor-modified T cells (16).
Importantly, catumaxomab was successfully used in the treatment of
malignant ascites which originated from EpCAM-positive renal cell
carcinoma (17).
Clinical data suggests that EpCAM-targeted treatment
is efficient in tumors with high target expression (18,19).
However, the level of EpCAM expression is heterogeneous in RCC and
it was found that the overexpression of EpCAM depends on the tumor
cell histology. EpCAM expression was found in 36.3% of clear-cell
RCC, in 81.3% of papillary RCC (pRCC) and in 78.3% of chromophobe
RCC (cpRCC) (20). This
heterogeneous expression of EpCAM in renal carcinoma is an
essential problem for targeted-cancer therapy because it might
cause an overtreatment. Consequently, patient stratification for
therapy based on EpCAM expression in tumors is a high-priority
task. Typically, target expression is determined by the
immunohistochemical analysis of biopsy samples (18). The invasiveness of these procedures
makes it difficult to perform multiple biopsies. This, in turn,
prevents obtaining information about the heterogeneity of
expression in different metastases or changes in expression that
occur over time. The radionuclide-based molecular imaging of EpCAM
expression is a promising alternative to biopsy-based methods.
The previously known approach to imaging molecular
targets, which uses radiolabeled therapeutic monoclonal antibodies
(mAbs), has several disadvantages (21–23).
The large size of monoclonal antibodies (molecular weight of 150
kDa) causes a slow accumulation of the imaging probes in the tumor,
along with a slow decrease of their concentration in the blood.
Consequently, a reasonable imaging contrast might be achieved only
four to seven days after injection. Another factor, which decreases
the accuracy of imaging when using monoclonal antibodies, is the
enhanced permeability and retention (EPR) effect, i.e., unspecific
accumulation of macromolecules in tumors. This effect decreases the
specificity of imaging (24). The
use of engineered scaffold proteins (ESPs) as targeted probes in
radionuclide diagnostics is a promising option (25). A comparison of several formats of
targeting vectors suggests that imaging probes based on engineered
scaffold proteins provide higher contrast than probes based on
monoclonal antibodies or their derivatives (25). ESPs typically have high binding
specificities and affinities to their selected therapeutic targets.
In addition, their small size facilitates rapid localization in
tumors and prompt excretion from the blood. These features provide
a high contrast for imaging on the day of injection.
The structure of ESPs determines their affinity to
molecular targets. Designed ankyrin repeat proteins (DARPins) are
ESPs built from 4–6 blocks (each block containing 33 amino acids)
with a total molecular weight of 14–18 kDa (26). Previous studies have shown that
DARPin Ec1 binds to EpCAM with a very high affinity, 68 pM
(27).
Selecting a strategy for the radiolabeling of ESPs
requires special attention because these proteins are small (having
molecular weights between 4 and 20 kDa), and therefore labeling
could significantly change their physicochemical properties and
affect their biodistribution pattern.
The aim of this study was to assess the potential of
a DARPin Ec1 derivative, EpCAM-visualizing DARPin (EVD), for
imaging EpCAM in an in vivo RCC model. A residualizing
99mTc-label and non-residualizing 125I-label
were evaluated for this purpose.
Materials and methods
Materials and instruments
Iodine-125 in the form of sodium iodide was provided
by Perkin Elmer Sverige AB. Instant thin-layer chromatography
(iTLC) on iTLC silica gel strips (Varian) was used for measurements
of radiochemical yield and purity. A cyclone storage phosphor
system (Packard Instrument Company) with the OptiQuant image
analysis software (Perkin Elmer) was used for the quantitative
assessment of iTLC. Cell-associated activity during in vitro
studies and organ-associated activity in biodistribution
evaluations were measured using an automated gamma-spectrometer
(1480 Wizard). For animal studies, radioactivity was measured using
an ionization chamber VDC-405 (Veenstra Instruments BV) for
formulation of injected solutions. Cells were cultured in a
humidified incubator with 5% CO2 at 37°C in RPMI medium
(Biochrom) containing 10% fetal bovine serum (FBS) (Merck), 2 mM
L-glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin (all
from Biochrom).
Protein production and
radiolabeling
The EpCAM-specific DARPin Ec1 was designed using the
binding sequence published earlier (27). An amino acid sequence H-E-H-E-H-E
((HE)3-tag) was introduced to the N-terminus of DARPin
Ec1 for site-specific labelling using [99mTc]technetium
tricarbonyl and to improve its biodistribution. The
(HE)3-containing Ec1 was designated as EpCAM-visualizing
DARPin (EVD). The production of EVD has also been described
previously (28).
Mass-spectrometry analysis confirmed that the protein had the
correct mass, which demonstrated its authenticity.
EVD was indirectly radioiodinated by using
N-succinimidyl-para-(trimethylstannyl)benzoate as a precursor
according to a previously published protocol (28,29).
A stock solution of [125I]iodide in 0.01 M NaOH (5–15
µl, 17–42 MBq) was added to 10 µl of 0.1% acetic acid in water. A
solution of the precursor in 5% acetic acid in methanol (5 µl, 1
mg/ml) was added to this mixture followed by addition of
chloramine-T (20 µg, 5 µl in water). The oxidative
iododestannylation reaction was terminated after 5 min by the
addition of sodium metabisulfite (30 µg, 5 µl in water). A solution
of DARPin Ec1 (140 µg in a mixture of 40 µl of 0.05 M
phosphate-buffered saline, pH 7.5, and 140 µl of 0.07 M borate
buffer, pH 9.3) was added and the resulting solution was incubated
at room temperature for 30 min. 125I-EVD was purified
using a NAP-5 column (Cytiva, Uppsala, Sweden) which was
pre-equilibrated with 1% bovine serum albumin (BSA) in PBS and then
eluted with PBS. A mixture of acetone:water (4:1) was used as the
mobile phase for the development of iTLC strips to determine the
radiochemical yield and radiochemical purity of
125I-EVD.
Labelling of DARPin (HE)3-Ec1 using
[99mTc][Tc(CO)3(H2O)3]+
was performed as described earlier (28,29).
The eluate from a technetium generator (500 µl, 3 GBq of
[99mTc]TcO4−) was added to a CRS
kit. After the kit reconstitution, the solution was incubated at
100°C for 30 min. After incubation, the solution containing
[99mTc][Tc(CO)3(H2O)3]+
(12 µl) was mixed with a solution of EVD (40 µg) in 33 µl of PBS
and incubated at 60°C for 60 min. The radiolabeled DARPin EVD was
purified using NAP-5 columns pre-equilibrated and eluted with PBS.
PBS was used for the development of iTLC strips to determine the
radiochemical yield and radiochemical purity of
99mTc-EVD.
Binding specificity and cellular
processing assays
The EpCAM-expressing human renal cell carcinoma cell
line SK-RC-52 (American Type Culture Collection) was used for in
vitro studies. One day before the experiment, 1×106
cells per dish were seeded. Groups of three dishes were used per
data point.
The evaluation of 125I-EVD's and
99mTc-EVD's binding specificity to EpCAM-expressing
cells was performed using a saturation test. To saturate EpCAM
binding sites, a 100-fold (200 nM) excess of unlabeled DARPin Ec1
was added to a group of three cell-seeded dishes. An equal volume
of media only was added to the second group of three cell-seeded
dishes. The cells were incubated for 30 min at room temperature,
thereafter radiolabeled EVD was added to obtain a final
concentration of 2 nM. The cells were then incubated for 6 h at
room temperature, and afterwards the medium was collected. The
cells were washed and detached from the dishes by incubation with
trypsin. The cell suspension was collected, and the activity of
both the cells and media was measured to calculate the percentage
of cell-bound activity. The unpaired two-tailed t-test was used to
determine if a significant difference (P<0.05) between binding
to pre-saturated and unsaturated cells existed.
To evaluate the internalization of Ec1 by renal
carcinoma cells, 99mTc-EVD (which contained a
residualizing label) was used. The internalization of
99mTc-EVD was studied during continuous incubation using
the acid-wash method (30).
Radiolabeled 99mTc-EVD was added to the
cells to obtain a concentration of 2 nM. The cells were incubated a
37°C. After 1, 2, 4, 6 and 24 h of incubation, the media from the
plated cells was collected. The cells were additionally washed once
with fresh media. To collect the membrane-bound fraction, the cells
were treated with a 0.2 M glycine buffer containing 4 M urea at pH
2.0 (which was placed on ice for 5 min before use) which caused
dissociation of membrane-bound proteins. Afterwards, the cells were
washed and treated with 1 M NaOH for 30 min in order to collect the
fraction containing any internalized compound. The activity in
every fraction was measured. The maximum value of cell-bound
activity in each dataset was taken as 100% and the data were
normalized to that value.
Affinity measurements using a
LigandTracer
Binding affinities of the radiolabeled EVD to living
SK-RC-52 renal carcinoma cells were measured using the LigandTracer
instrument (Ridgeview Instruments). The TraceDrawer Software
(Ridgeview Instruments AB) was used for evaluating the kinetics
(31). The binding and
dissociation kinetics were measured at room temperature. After a
background measurement, increasing concentrations of radiolabeled
EVD (1.8 and 5.4 nM) were added to the cells to determine the
binding kinetics. After the association phase, the cell media was
replaced and the retention in the dissociation phase was measured.
The real time association and dissociation data were fitted into a
one-to-one Langmuir binding model using the TraceDrawer Software.
Both association and dissociation rates were determined, and the
equilibrium dissociation constant was calculated.
Animal studies
Animal studies were performed according to national
legislation on laboratory animal protection. The animal welfare was
ensured by following The Guide for Care and Use of Laboratory
Animals (32). After tumor
implantation, the tumor size and animal behavior was monitored
twice a week. To develop RCC xenografts, SK-RC-52 cells
(107 cells in 100 µl media) were subcutaneously
inoculated into the hind legs of female Balb/c nu/nu mice. The
experiments were performed two weeks after SK-RC-52 cells
implantation. At the time of experiment, the average mouse's weight
was 18±2 g. The average tumor weight was 0.2±0.1 g (the largest
tumor volume was 0.28 cm3). A group of four animals
point was used.
The biodistribution was measured using a dual-label
technique. A mixture of both 125I-EVD (20 kBq/mouse) and
99mTc-EVD (30 kBq/mouse) with a combined mass of 4
µg/mouse was injected as a solution in 100 µl PBS into the tail
vein of mice. The biodistribution was measured 3 h post-injection.
All animals were sedated by an intraperitoneal injection of a
lethal dose of anesthesia (ketamine [Ketalar, Pfizer], 200 mg/kg of
body weight, and xylazin [Rompun], 20 mg/kg of body weight). The
sufficient degree of sedation was evaluated by absence of the pedal
withdrawal reflex to toe pinch. The sedated animals were euthanized
by a heart puncture with following exsanguination. The organs and
tissues were collected, weighed and their activities were measured.
The activity of iodine-125 in each sample was measured in the
energy range between 18 and 85 keV. The activity of technetium-99m
was measured in the energy range between 110 and 160 keV. These
data were then used to calculate the percent of labelled compound
taken up per gram of sample relative to the injected dose
(%ID/g).
To test the specificity of EpCAM targeting in
vivo, the uptake of tracers in EpCAM-positive SK-RC-52 tumors
was compared to their uptake in EpCAM-negative xenografts produced
using the Ramos lymphoma cell line (American Type Culture
Collection). Lymphoma is an ideal negative control because it,
unlike malignancies of epithelial origin, does not express EpCAM.
On the other hand, Ramos lymphoma forms vascularized solid
xenografts and might reflect nonspecific tumor accumulation in
vivo. Ramos cells (107 cells) were subcutaneously
inoculated into the hind legs of another group of female Balb/c
nu/nu mice. The experiment was performed three weeks after Ramos
cells implantation. At the time of experiment, the average mouse's
weight was 18±2 g. The average tumor weight was 0.5±0.3 g (the
largest tumor volume was 0.803 cm3). A group of five
animals was used. The injected activity and protein mass were the
same as that used for mice bearing SK-RC-52 ×enografts. The animals
were euthanized after sedation (as aforementioned) by a heart
puncture with subsequent exsanguination 3 h after injection, the
xenografts were excised, and the uptake of the tracers was
measured.
SPECT/CT scans of mice (bearing SK-RC-52 ×enografts)
injected with 99mTc-EVD (4 µg, 4.9 MBq) or with
125I-EVD (4 µg, 2.5 MBq) in 100 µl PBS were performed
using a nanoScan SPECT/CT platform (Mediso Medical Imaging
Systems). The tumors size was 1.3×0.6 and 0.9×0.7 cm for animals
imaged using 99mTc-EVD and 125I-EVD,
respectively. Imaging was performed 3 h post-injection. Immediately
before imaging, the animals were euthanized by CO2
asphyxiation (displacement rate 35% per minute), which caused them
to urinate. In this way, the high activity from the urinary bladder
is eliminated, which is a frequent artifact in preclinical studies.
The animals' death was verified by lack of respiration and
heartbeat and lack of response to toe pinch. The animals were
positioned in the camera in a prone position, and imaging was
performed using the protocol described in (28). The scanning time was 30 min.
Statistical analysis
The data are presented as the mean ± standard
deviation of three samples for cell studies or four samples for
animal studies. Data analyses for in vitro experiments were
performed using an unpaired two-tailed t-test, the statistical
significance threshold was set at P<0.05. The biodistribution
data for the dual-label experiment were also analyzed using a
paired two-tailed t-test, the statistical significance threshold
was also set at P<0.05. The statistical analyses were performed
using GraphPad Prism (version 7.02; GraphPad Software, Inc.).
Results
Radiolabeling
EVD was labelled with
[99mTc][Tc(CO)3(H2O)3]+
using a triple histidine-glutamate tag, (HE)3-tag, as a
chelator to provide a residualizing label with a radiochemical
yield of 58±16% (n=3). Labeling of EVD with the
para-[125I]iodobenzoyl group was performed by
conjugating it as the N-hydroxysuccinimide ester
(N-succinimidyl-para-[125I]iodobenzoate) to the amino
acid groups of lysine with a radiochemical yield of 16±3% (n=4).
Purification using size-exclusion NAP-5 columns provided purities
over 98% for both compounds.
Binding to RCC cells in vitro
For the specificity test, cells of the human
carcinoma cell line SK-RC-52 were incubated with 2 nM of either
99mTc-EVD or 125I-EVD, and the
cell-associated activity was measured. In the control group, the
binding sites of EpCAM were saturated by incubating cells in a
100-fold molar excess of unlabeled Ec1.
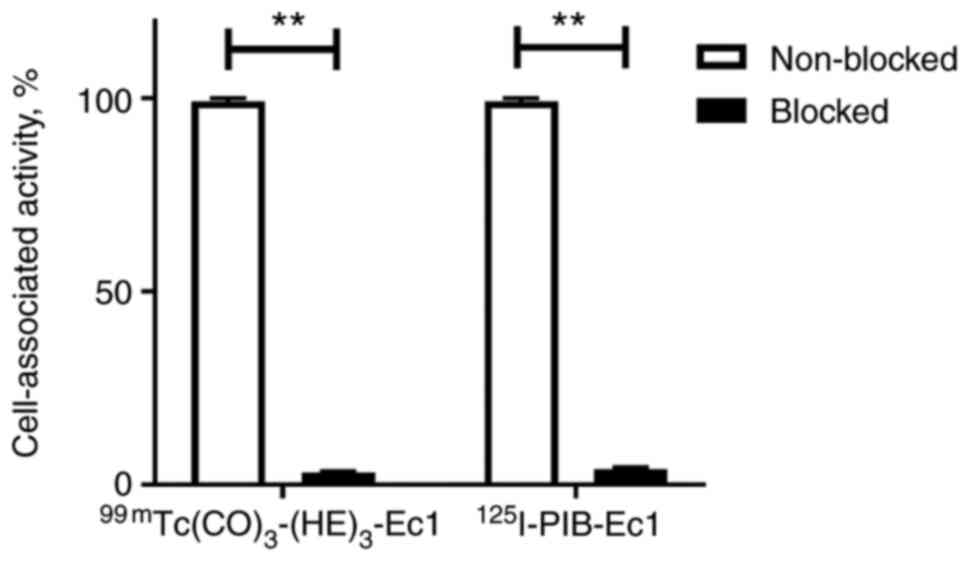
The blocking test showed that the pre-treatment of
SK-RC-52 renal carcinoma cells with a large excess of unlabeled Ec1
resulted in a highly significant (P<0.00005) reduction in
binding for both radiolabeled DARPins (Fig. 1).
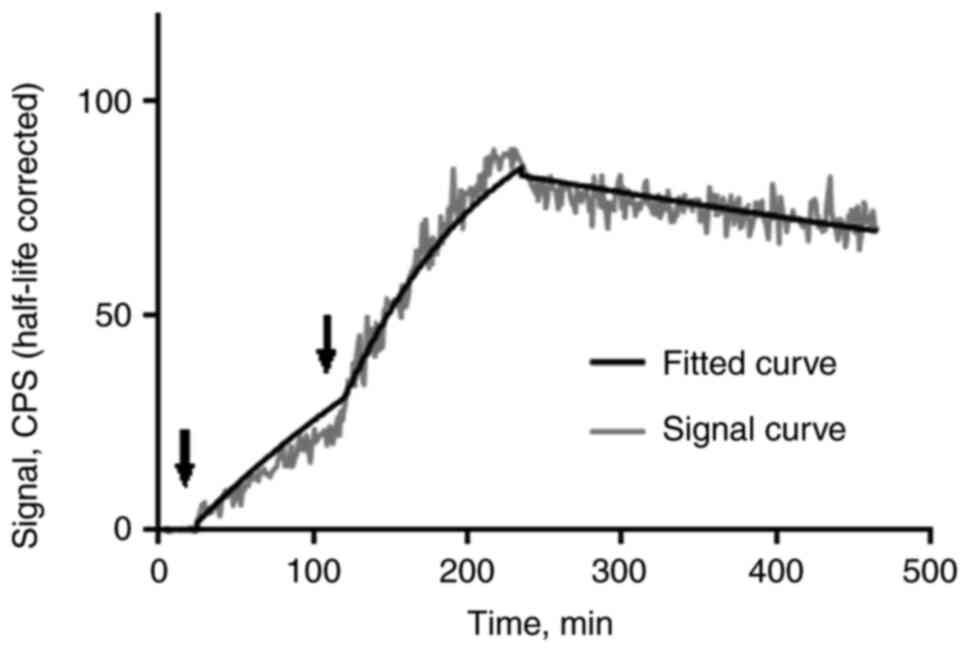
According to sensorgrams from the LigandTracer
instrument (Fig. 2), the binding
of 99mTc-EVD to EpCAM-expressing renal carcinoma cells
was rapid and showed an association rate constant or ka
value of 2.7(±0.2)x104 M−1.s−1.
Its dissociation was slow and showed a dissociation rate constant
or kd value of 1.1(±0.1)x10−5 s−1.
This resulted in a subnanomolar affinity or KD value of
400±28 pM.
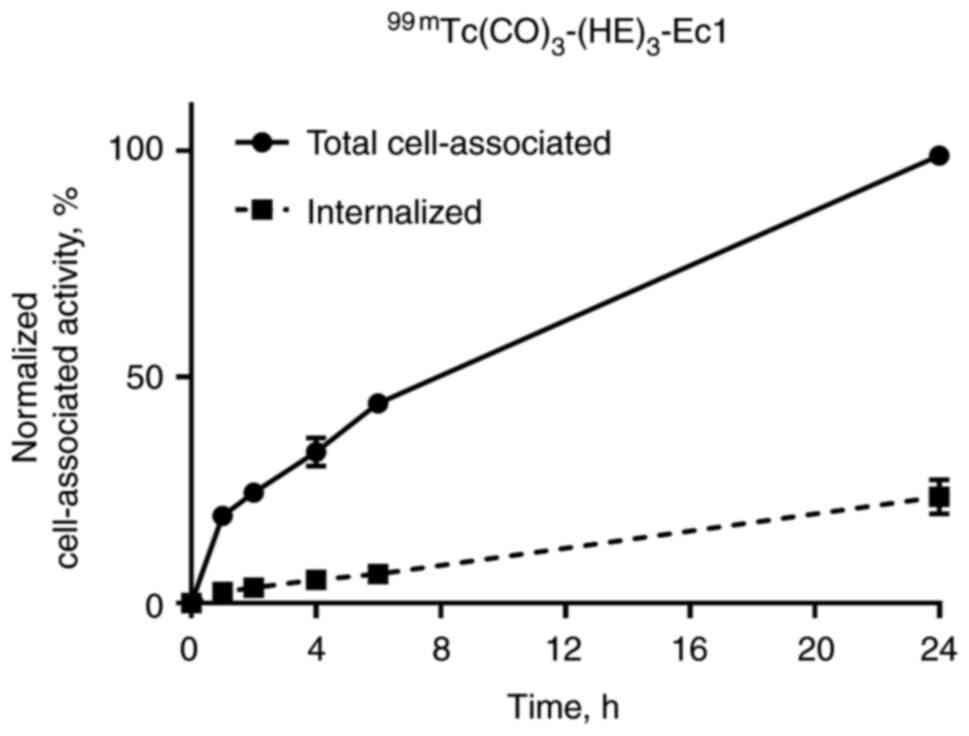
Internalization of 99mTc-EVD, after
binding to renal carcinoma cells, was evaluated using the acid wash
method. The data were normalized to the maximum value of cell-bound
activity, and the cellular processing data are presented in
Fig. 3. The total cell-associated
and internalized activity increased continuously for the
99mTc-labeled variant. The internalization of
99mTc-EVD was slow, and only 20% was internalized after
24 h of incubation.
In vivo studies
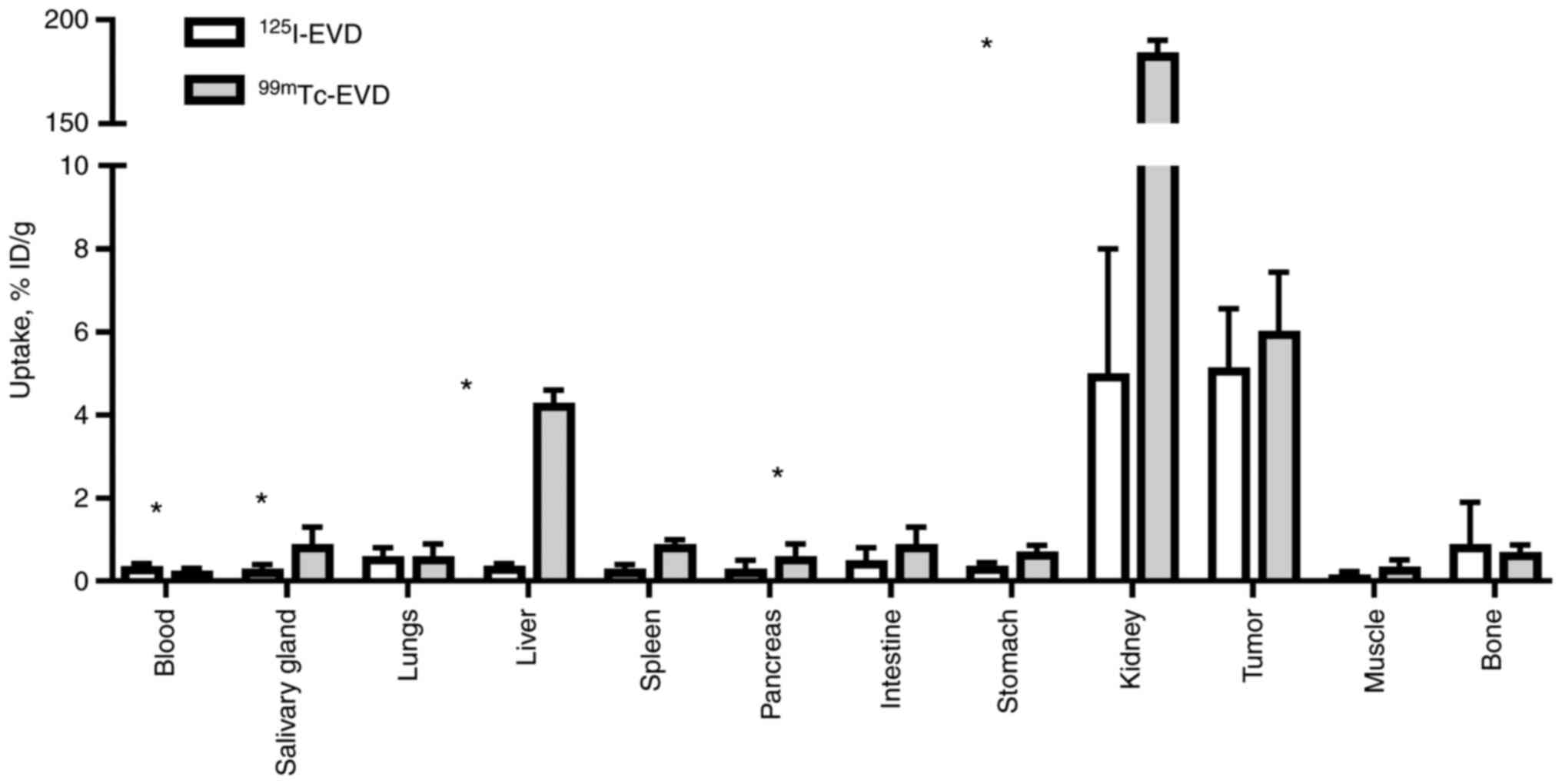
The biodistribution evaluation of
125I-EVD and 99mTc-EVD was measured in Balb/c
nu/nu mice bearing SK-RC-52 renal carcinoma xenografts 3 h
post-injection (pi). These data are presented in Figs. 4 and 5. Rapid blood clearance was observed for
both radiolabeled-EVD variants. The tumor uptake of
125I-EVD and 99mTc-EVD did not differ
significantly. However, there were significant differences
(P<0.05) in their distribution in normal tissues. The renal
uptake of 99mTc-EVD was much higher than the renal
uptake of its radioiodinated counterpart. Overall,
99mTc-EVD resulted in a significantly lower uptake in
the blood, but 125I-EVD provided a significantly lower
uptake in the salivary glands, liver, spleen, stomach, kidneys and
bones.
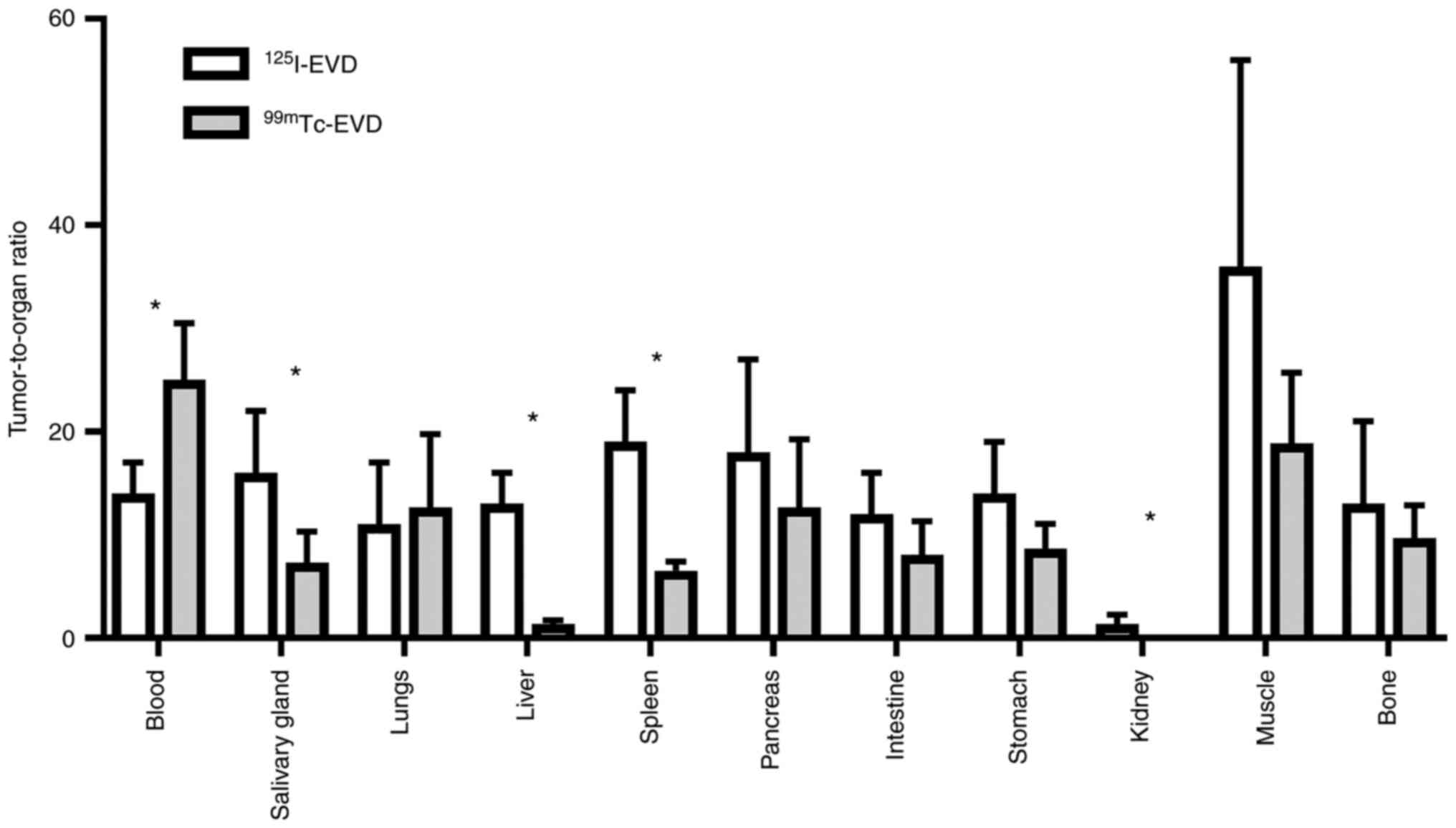
Based on these biodistribution features,
125I-EVD provided significantly higher tumor-to-salivary
gland, tumor-to-liver, tumor-to-spleen and tumor-to-kidney ratios
compared to 99mTc-EVD (Fig.
5). However, 99mTc-EVD provided a higher
tumor-to-blood ratio.
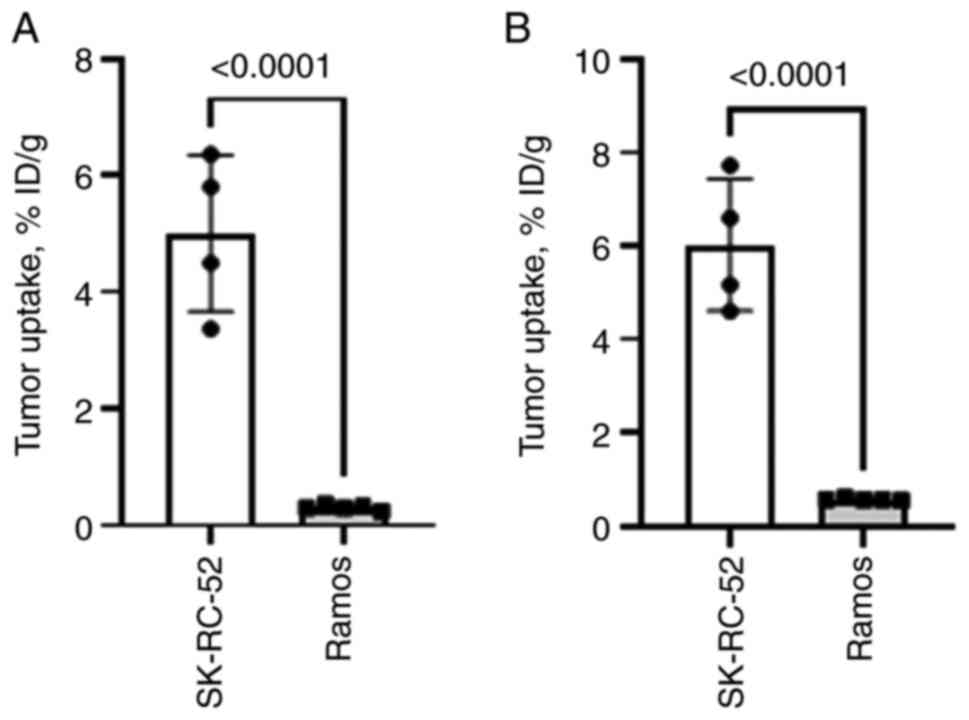
The uptake of both 125I-EVD and
99mTc-EVD in EpCAM-negative Ramos lymphoma xenografts
was significantly lower (P<0.0001) than in EpCAM-expressing
SK-RC-52 ×enografts (Fig. 6). This
suggests that the recognition of EpCAM is a prerequisite for the
accumulation of both tracers in tumors, i.e., the uptake is
EpCAM-specific.
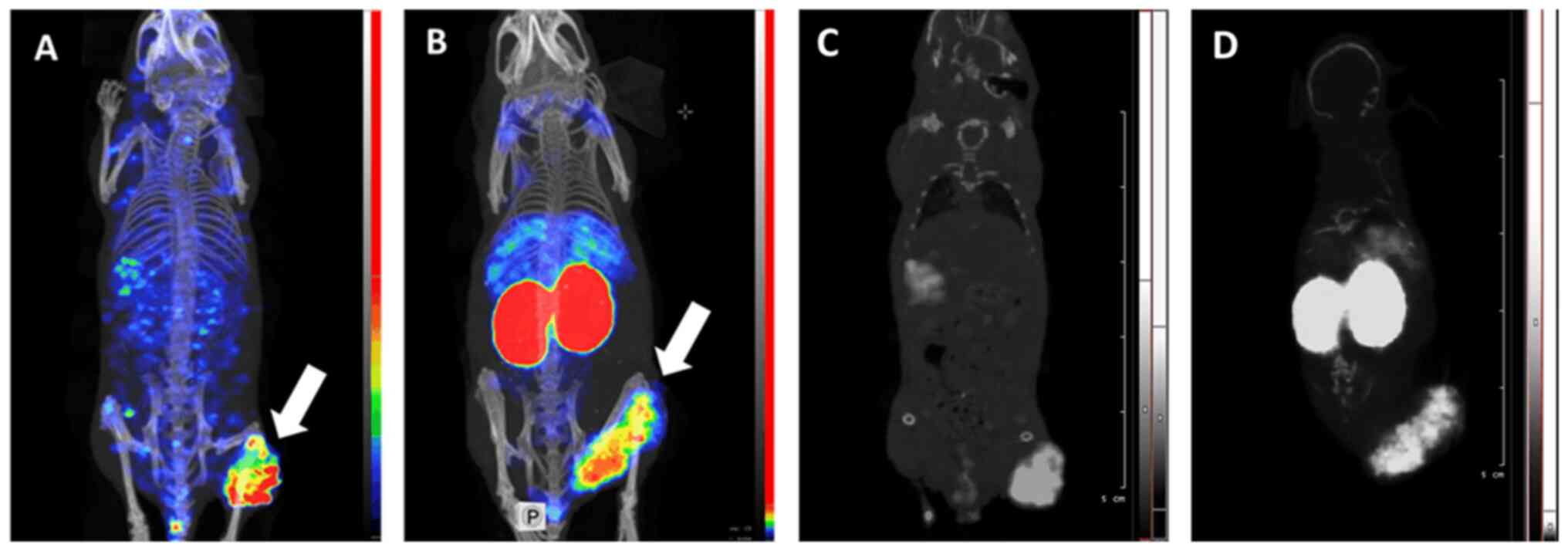
SPECT/CT imaging confirmed the findings of direct
ex vivo measurements (Fig.
7). Both 99mTc-EVD and 125I-EVD provided
a clear visualization of SK-RC-52 renal carcinoma xenografts.
Although there was some background activity in the whole body, in
the case of 125I-EVD, the contrast of the tumor
visualization was high. It has to be noted that some spots with
elevated activity accumulation (hot spots) were visible in the left
femoral muscle of the mouse injected with 125I-EVD. Most
likely, these hot spots are reconstruction artefacts, as the image
obtained using radioiodinated tracer is more pixelated. Most
importantly, 125I-EVD provided a much higher tumor
contrast with respect to the kidneys and liver, which are the major
metastatic sites for renal cell carcinoma. Overall,
125I-EVD was the better probe for imaging
EpCAM-expressing renal cell carcinomas.
Discussion
The overexpression of EpCAM, in an appreciable
proportion of RCC cases (including the most frequent clear cell and
papillary histological subtypes), makes it an attractive molecular
target for the treatment of this disease. Still, a large number of
patients have RCC tumors without sufficiently high levels of EpCAM
expression for targeted therapy. This necessitates the
stratification of patients for targeted anti-EpCAM treatment.
Radionuclide-based molecular imaging would potentially enable a
non-invasive analysis of EpCAM-expression levels in disseminated
RCC cases.
Single-photon emission computed tomography (SPECT)
offers the advantage of easy access around the world. Unlike
positron emission tomography (PET), it is relatively available at
hospitals in Africa, South America and Asia, where PET centers are
not so common. Thus, the development of a SPECT-compatible imaging
probe might have a greater impact on global healthcare.
The most suitable nuclides for SPECT imaging are
technetium-99m (T1/2=6 h, Eg=140 keV) and
iodine-123 (T1/2=13.3 h, E=159 keV). The energy of the
gamma-ray photons from these nuclides provides an optimal
combination of spatial resolution and photopeak registration
efficiency for modern gamma-cameras. An additional advantage of
technetium-99m is its production using the
99Mo/99mTc-generator, which makes it cheaper
and more readily available. However, the main criterion for
suitability of an imaging probe is the sensitivity of imaging that
it provides. The sensitivity depends on the imaging contrast, which
is determined by the tumor-to-background uptake ratios for the main
metastatic sites. The partial volume effect (which complicates
visualization of small metastases) can be reduced with high
tumor-to-background ratios.
Common metastatic sites for RCC include the lung (in
50–60 percent of metastatic cases), bone (in 30–40 percent), liver
(in 30–40 percent) and brain (in 5 percent) (33). Accordingly, the most suitable
tracer for imaging EpCAM in disseminated RCC should provide high
tumor-to-lung, tumor-to-bone and tumor-to-liver uptake ratios. The
tumor-to-brain ratio is less important because the brain uptake is
generally very low for protein-based imaging probes when the
blood-brain barrier is still intact. The judicious selection of a
labelling strategy (i.e., a radionuclide, a chelator or linker for
its coupling and the position of radionuclide coupling), which
provides the lowest uptake in the aforementioned organs, would
ensure the highest tumor-to-organ ratios and therefore result in
the highest imaging sensitivity.
The fact that the selected labeling strategy
determines the biodistribution of radiolabeled scaffold proteins is
well established (25,34). For example, it has been
demonstrated that an increase in negative charges on the N-terminus
of affibody molecules (by selecting an appropriate combination of
chelator and radionuclide) reduces their hepatic uptake (35). It has also been found that by using
the negatively charged (HE)3-tag as a chelator for
[99mTc][Tc(CO)3(H2O)3]+
at the N-terminus of DARPins G3 (36) or Ec1 (29), a significant decrease of activity
accumulation in the liver was observed. Results of previous studies
(36,37) suggest that the uptake of DARPins in
liver is not target-specific, but depends on unspecific interaction
of protein-surface amino acids and a chelator with hepatocytes. In
this case, it cannot be suppressed without re-engineering of the
DARPins. Considering the importance of detecting hepatic metastases
in RCC, the use of (HE)3-tag as a chelator was selected
for the 99mTc-labelling of EVD in this study.
An alternative strategy, which was also utilized in
this study, is based on the use of non-residualizing labels, i.e.,
combinations of a nuclide and a chelator or a linker, which can
diffuse through lysosomal and cytoplasmic membranes after
internalization and lysosomal degradation of the targeting protein.
Usually, such properties are demonstrated when proteins have been
labelled with bromine-76, iodine-123, iodine-124, iodine-125 or
iodine-131, because proteolysis of such proteins results in
lipophilic radiometabolites capable of diffusing through membranes.
It is essential when using this approach, that a targeted protein
is internalized rapidly by excretory organs (e.g., liver and
kidneys) but slowly by cancer cells. To test this approach in the
current study, we evaluated radioiodinated Ec1. We have used the
radionuclide iodine-125 (T1/2=59.4 d) as a surrogate for
iodine-123. These nuclides are identical from both a chemical and
biochemical point of view, but the longer half-life and
lower-energy electromagnetic radiation of iodine-125 make it more
convenient for development work. It should also be noted that while
leakage from excretory organs is a common feature of
non-residualizing radioiodine labels, when coupled to scaffold
proteins their influence on biodistribution is different. For
example, the use of [(4-hydroxyphenyl)-ethyl]maleimide (HPEM) as a
prosthetic group, for the radioiodination of both anti-HER2 DARPin
G3 and anti-EpCAM DARPin Ec1, resulted in a massive hepatobiliary
excretion and accumulation of activity in the gastrointestinal
tract (37,38). Since excess activity in the
gastrointestinal tract would complicate the imaging of abdominal
metastases in RCC, this method was excluded from the current
study.
One particular approach to radioiodination includes
so-called direct radioiodination, i.e., when an in-situ
oxidized radioiodine attacks the tyrosine residues of a protein.
The main radiometabolite of directly-iodinated proteins is
radioiodotyrosine, which has typical non-residualizing properties.
An advantage of direct radioiodination is usually the high
labelling yield. Although there is a risk that iodination of
tyrosines might reduce the binding strength of scaffold proteins
(39), directly-radioiodinated
DARPins have been shown to preserve their binding capacity
(29,40). An alternative method to
radioiodination is an indirect approach by conjugating a
radioiodobenzoyl (e.g., PIB) group. The yield of such labeling is
typically lower than the yield of direct radioiodination. However,
an advantage of this labeling approach is the rapid excretion of
radiometabolites from the blood without accumulation in any other
organ or tissue. A comparison between directly and indirectly
radioiodinated DARPins G3 and Ec1, has shown that the
redistribution of radiometabolites from the directly iodinated
variants resulted in appreciably elevated activity uptake in the
blood, organs expressing the Na/I-symporter and a number of other
tissues (including the gastrointestinal tract). However, the
indirectly iodinated variants (using radioactive-PIB) demonstrated
appreciably lower activity uptake in these organs and higher
tumor-to-organ ratios in the abdomen (29,41).
Therefore, indirect radioiodination using [125I]PIB was
selected for the imaging of EpCAM expression in RCC.
The labeling procedures for both
99mTc-EVD and 125I-EVD provided conjugates
with radiochemical purity over 98%, which makes their clinical
application possible. The binding of both conjugates to
EpCAM-expressing renal carcinoma cells was significantly reduced by
pre-saturating the EpCAM-binding sites. This demonstrated that
neither labeling procedure compromised the EpCAM-specific binding
character of the targeting protein. In order to evaluate the
internalization rate by RCC cells, we used 99mTc-EVD
with its residualizing label. If the non-residualizing radioiodine
label was used instead, it would have resulted in leakage of
radiometabolites from the cells and an underestimation of the
internalized activity. The internalized activity was found to be
below 15% of the total cell-bound activity after the first 6 h of
incubation, i.e., the internalization of 99mTc-EVD by
RCC cells was slow. Because of this slow internalization, the
retention of activity in tumors in vivo should not be
affected by the residualizing properties of the label, but rather,
it should mainly depend on the strength of the tracers' binding to
their molecular target (located on the membranes of malignant
cells). The binding affinity of radiolabeled Ec1 to SK-RC-52 renal
carcinoma cells was very high, 400±28 pM, which is a prerequisite
for successful targeting (with both 99mTc-EVD and
125I-EVD) to be achieved.
The results from the biodistribution experiments
confirmed equal targeting efficiency for both 99mTc-EVD
and 125I-EVD. The tumor uptake of both tracers did not
differ significantly at 3 h after injection (5.2±1.4%ID/g for
125I-EVD vs. 6.0±1.4%ID/g for 99mTc-EVD).
Importantly, the accumulation of both tracers in the SK-RC-52
×enografts was EpCAM-dependent. However, the main difference
observed was between the uptakes of tracers in normal tissues. The
blood-born activity was low, but the concentration was
significantly higher (P<0.05) for 125I-EVD
(0.37±0.06%ID/g) than it was for 99mTc-EVD
(0.25±0.06%ID/g). This difference is most likely due to the
retention pattern of radiometabolites in the kidneys. Previous
studies demonstrated that DARPins clear rapidly form blood via
kidneys, but they are reabsorbed in proximal tubules after
glomerular filtration. This reabsorption cannot be blocked with a
co-infusion of lysine or Gelofusine (42), which blocks sometimes for other
radiolabeled proteins or peptides. In the case of a residualizing
label, this phenomenon results in high renal uptake of activity
after injection of anti-HER2 or anti-EpCAM DARPins labeled with
residualizing radiometals (28,36,38).
In this study, metabolites from the residualizing
[99mTc][Tc(CO)3]+ label remained
in the kidneys after reabsorption and degradation (the renal uptake
was 184±6%ID/g) while the metabolites of the [125I]PIB
label left the kidneys (the renal uptake was 5±1%ID/g). It is
conceivable that a part of the renal metabolites from the
[125I]PIB label appears in the blood and contributes to
the total activity concentration in the blood. The difference in
radiometabolite retention is the most plausible cause of the
significantly higher (P<0.05) technetium-99m activity in the
salivary gland, liver, spleen and intestines. This difference in
the biodistribution is linked with the significantly higher
(P<0.05) tumor-to-salivary gland, tumor-to-liver,
tumor-to-spleen and tumor-to-intestinal wall ratios for
125I-EVD. These findings were confirmed by experimental
microSPECT imaging. The apparent higher imaging contrast for tumors
in abdominal tissues makes 125I-EVD a better imaging
probe for visualizing EpCAM expression in metastases of renal cell
carcinoma and for selecting patients for EpCAM-targeted
therapy.
In conclusion, the low retention of
125I-EVD radiometabolites in normal tissues ensures high
tumor-to-organ uptake ratios and results in a high imaging contrast
for the visualization of metastases of RCC. Radioiodinated DARPin
Ec1 is the most suitable imaging agent for the selection of RCC
patients for EpCAM-targeted therapy.
Acknowledgements
The authors would like to thank Mr. Tyran Günther
(Department of Materials Science and Engineering, Uppsala
University, Uppsala, Sweden) for proofreading the manuscript.
Funding
This research was funded by grants from the Swedish Cancer
Society Cancerfonden (20 0181; 20 0893 Pj; 21 1485 Pj), Ministry of
Science and Higher Education RF (075-15-2022-1103) and Swedish
Research Council Vetenskapsrådet (VR 2019-00994).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
VT, VB, AO and AV performed the experiments and
analyzed the data. AS and SMD performed the production and
purification of proteins. SMD participated in the molecular design,
supervised the production, purification and characterization of
protein, and coordinated the work. AV obtained funding,
participated in the study design, labelling chemistry development,
data treatment and interpretation, and coordinated the work. VT
obtained funding, and participated in the study design, data
treatment and interpretation. VT and VB wrote the first version of
the manuscript. AV and VT confirm the authenticity of all the raw
data. All authors have agreed to be held accountable for all
aspects of the research, including the accuracy and integrity of
all parts of the work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Animal studies were approved by the local ethics
committee for animal research in Uppsala (Uppsala djurförsöksetiska
nämnd), Sweden (ethical permission C5/16 from 26-02-2016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EpCAM
|
epithelial cell adhesion molecule
|
|
RCC
|
renal cell carcinoma
|
|
DARPin
|
designed ankyrin repeat protein
|
|
PIB
|
para-iodobenzoyl
|
|
pRCC
|
papillary RCC
|
|
cpRCC
|
chromophobe RCC
|
|
mAb
|
monoclonal antibody
|
|
EPR effect
|
enhanced permeability and retention
effect
|
|
ESP
|
engineered scaffold protein
|
|
iTLC
|
instant thin-layer chromatography
|
|
PBS
|
0.05 M phosphate-buffered saline, pH
7.5
|
|
PET
|
positron emission tomography
|
|
SPECT
|
single-photon emission computed
tomography
|
|
T1/2
|
half-life
|
|
HPEM
|
((4-hydroxyphenyl)-ethyl)maleimide
|
References
|
1
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryan CW, Vogelzang NJ and Stadler WM: A
phase II trial of intravenous gemcitabine and 5-fluorouracil with
subcutaneous interleukin-2 and interferon-alpha in patients with
metastatic renal cell carcinoma. Cancer. 94:2602–2609. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Went P, Dirnhofer S, Salvisberg T, Amin
MB, Lim SD, Diener PA and Moch H: Expression of epithelial cell
adhesion molecule (EpCam) in renal epithelial tumors. Am J Surg
Pathol. 29:83–88. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stewart GD, O'Mahony FC, Powles T, Riddick
AC, Harrison DJ and Faratian D: What can molecular pathology
contribute to the management of renal cell carcinoma? Nat Rev Urol.
8:255–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tran J and Ornstein MC: Clinical review on
the management of metastatic renal cell carcinoma. JCO Oncol Pract.
18:187–196. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Osawa T, Takeuchi A, Kojima T, Shinohara
N, Eto M and Nishiyama H: Overview of current and future systemic
therapy for metastatic renal cell carcinoma. Jpn J Clin Oncol.
49:395–403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tung I and Sahu A: Immune checkpoint
inhibitor in first-line treatment of metastatic renal cell
carcinoma: A review of current evidence and future directions.
Front Oncol. 11:7072142021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elias DJ, Kline LE, Robbins BA, Johnson HC
Jr, Pekny K, Benz M, Robb JA, Walker LE, Kosty M and Dillman RO:
Monoclonal antibody KS1/4-methotrexate immunoconjugate studies in
non-small cell lung carcinoma. Am J Respir Crit Care Med.
150:1114–1122. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Braun S, Hepp F, Kentenich CR, Janni W,
Pantel K, Riethmüller G, Willgeroth F and Sommer HL: Monoclonal
antibody therapy with edrecolomab in breast cancer patients:
Monitoring of elimination of disseminated cytokeratin-positive
tumor cells in bone marrow. Clin Cancer Res. 5:3999–4004.
1999.PubMed/NCBI
|
|
10
|
Punt CJ, Nagy A, Douillard JY, Figer A,
Skovsgaard T, Monson J, Barone C, Fountzilas G, Riess H, Moylan E,
et al: Edrecolomab alone or in combination with fluorouracil and
folinic acid in the adjuvant treatment of stage III colon cancer: A
randomised study. Lancet. 360:671–677. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Paolo C, Willuda J, Kubetzko S, Lauffer
I, Tschudi D, Waibel R, Plückthun A, Stahel RA and
Zangemeister-Wittke U: A recombinant immunotoxin derived from a
humanized epithelial cell adhesion molecule-specific single-chain
antibody fragment has potent and selective antitumor activity. Clin
Cancer Res. 9:2837–2848. 2003.PubMed/NCBI
|
|
12
|
Andersson Y, Inderberg EM, Kvalheim G,
Herud TM, Engebraaten O, Flatmark K, Dueland S and Fodstad Ø:
Immune stimulatory effect of anti-EpCAM immunotoxin-improved
overall survival of metastatic colorectal cancer patients. Acta
Oncol. 59:404–409. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seimetz D, Lindhofer H and Bokemeyer C:
Development and approval of the trifunctional antibody catumaxomab
(anti-EpCAM × anti-CD3) as a targeted cancer immunotherapy. Cancer
Treat Rev. 36:458–467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martin-Killias P, Stefan N, Rothschild S,
Plückthun A and Zangemeister-Wittke U: A novel fusion toxin derived
from an EpCAM-specific designed ankyrin repeat protein has potent
antitumor activity. Clin Cancer Res. 17:100–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu T, Vorobyeva A, Schulga A, Konovalova
E, Vorontsova O, Ding H, Gräslund T, Tashireva LA, Orlova A,
Tolmachev V and Deyev SM: Imaging-Guided therapy simultaneously
targeting HER2 and EpCAM with trastuzumab and EpCAM-Directed toxin
provides additive effect in ovarian cancer model. Cancers (Basel).
13:39392021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang BL, Li D, Gong YL, Huang Y, Qin DY,
Jiang L, Liang X, Yang X, Gou HF, Wang YS, et al: Preclinical
evaluation of chimeric antigen receptor-modified T cells specific
to epithelial cell adhesion molecule for treating colorectal
cancer. Hum Gene Ther. 30:402–412. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pilanc KN, Ordu Ç, Akpnar H, Balc C,
Başsülü N, Köksal Üİ, Elbüken F, Okutur K, Bülbül G, Sağlam S and
Demir G: Dramatic response to catumaxomab treatment for malign
ascites related to renal cell carcinoma with sarcomotoid
differentiation. Am J Ther. 23:e1078–e1081. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmidt M, Scheulen ME, Dittrich C, Obrist
P, Marschner N, Dirix L, Schmidt M, Rüttinger D, Schuler M,
Reinhardt C and Awada A: An open-label, randomized phase II study
of adecatumumab, a fully human anti-EpCAM antibody, as monotherapy
in patients with metastatic breast cancer. Ann Oncol. 21:275–282.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marschner N, Rüttinger D, Zugmaier G,
Nemere G, Lehmann J, Obrist P, Baeuerle PA, Wolf A, Schmidt M,
Abrahamsson PA, et al: Phase II study of the human anti-epithelial
cell adhesion molecule antibody adecatumumab in prostate cancer
patients with increasing serum levels of prostate-specific antigen
after radical prostatectomy. Urol Int. 85:386–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zimpfer A, Maruschke M, Rehn S, Kundt G,
Litzenberger A, Dammert F, Zettl H, Stephan C, Hakenberg OW and
Erbersdobler A: Prognostic and diagnostic implications of
epithelial cell adhesion/activating molecule (EpCAM) expression in
renal tumours: A retrospective clinicopathological study of 948
cases using tissue microarrays. BJU Int. 114:296–302. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bensch F, Lamberts LE, Smeenk MM,
Jorritsma-Smit A, Lub-de Hooge MN, Terwisscha van Scheltinga AGT,
de Jong JR, Gietema JA, Schröder CP, Thomas M, et al:
89Zr-Lumretuzumab PET Imaging before and during HER3 antibody
lumretuzumab treatment in patients with solid tumors. Clin Cancer
Res. 23:6128–6137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jauw YW, Zijlstra JM, de Jong D, Vugts DJ,
Zweegman S, Hoekstra OS, van Dongen GA and Huisman MC: Performance
of 89Zr-Labeled-Rituximab-PET as an Imaging Biomarker to Assess
CD20 Targeting: A pilot study in patients with relapsed/refractory
diffuse large B cell lymphoma. PLoS One. 12:e01698282017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ulaner GA, Lyashchenko SK, Riedl C, Ruan
S, Zanzonico PB, Lake D, Jhaveri K, Zeglis B, Lewis JS and
O'Donoghue JA: First-in-Human human epidermal growth factor
receptor 2-Targeted Imaging Using 89Zr-Pertuzumab PET/CT: Dosimetry
and clinical application in patients with breast cancer. J Nucl
Med. 59:900–906. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garousi J, Orlova A, Frejd FY and
Tolmachev V: Imaging using radiolabelled targeted proteins:
Radioimmunodetection and beyond. EJNMMI Radiopharm Chem. 5:162020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tolmachev VM, Chernov VI and Deyev SM:
Targeted nuclear medicine. Seek and destroy. Rus Chem Rev.
91:RCR50342022. View Article : Google Scholar
|
|
26
|
Plückthun A: Designed ankyrin repeat
proteins (DARPins): Binding proteins for research, diagnostics, and
therapy. Annu Rev Pharmacol Toxicol. 55:489–511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stefan N, Martin-Killias P, Wyss-Stoeckle
S, Honegger A, Zangemeister-Wittke U and Plückthun A: DARPins
recognizing the tumor-associated antigen EpCAM selected by phage
and ribosome display and engineered for multivalency. J Mol Biol.
413:826–843. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deyev SM, Vorobyeva A, Schulga A,
Abouzayed A, Günther T, Garousi J, Konovalova E, Ding H, Gräslund
T, Orlova A and Tolmachev V: Effect of a radiolabel biochemical
nature on tumor-targeting properties of EpCAM-binding engineered
scaffold protein DARPin Ec1. Int J Biol Macromol. 145:216–225.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vorobyeva A, Bezverkhniaia E, Konovalova
E, Schulga A, Garousi J, Vorontsova O, Abouzayed A, Orlova A, Deyev
S and Tolmachev V: Radionuclide molecular imaging of EpCAM
expression in triple-negative breast cancer using the scaffold
protein DARPin Ec1. Molecules. 25:47192020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wållberg H and Orlova A: Slow
internalization of anti-HER2 synthetic affibody monomer
111In-DOTA-ZHER2:342-pep2: Implications for development of labeled
tracers. Cancer Biother Radiopharm. 23:435–442. 2008.PubMed/NCBI
|
|
31
|
Tolmachev V, Orlova A and Andersson K:
Methods for radiolabelling of monoclonal antibodies. Methods Mol
Biol. 1060:309–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guide for the Care and Use of Laboratory
Animals. The National Academies Press; Washington, DC: 1996,
https://doi.org/10.17226/5140
|
|
33
|
Motzer RJ, Bander NH and Nanus DM:
Renal-cell carcinoma. N Engl J Med. 335:865–875. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tolmachev V and Orlova A: Affibody
molecules as targeting vectors for PET Imaging. Cancers (Basel).
12:6512020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hosseinimehr SJ, Tolmachev V and Orlova A:
Liver uptake of radiolabeled targeting proteins and peptides:
Considerations for targeting peptide conjugate design. Drug Discov
Today. 17:1224–1232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vorobyeva A, Schulga A, Konovalova E,
Güler R, Löfblom J, Sandström M, Garousi J, Chernov V, Bragina O,
Orlova A, et al: Optimal composition and position of
histidine-containing tags improves biodistribution of
99mTc-labeled DARPin G3. Sci Rep. 9:94052019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deyev SM, Xu T, Liu Y, Schulga A,
Konovalova E, Garousi J, Rinne SS, Larkina M, Ding H, Gräslund T,
et al: Influence of the position and composition of radiometals and
radioiodine labels on imaging of Epcam expression in prostate
cancer model using the DARPin Ec1. Cancers (Basel). 13:35892021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vorobyeva A, Sсhulga A, Konovalova E,
Güler R, Mitran B, Garousi J, Rinne S, Löfblom J, Orlova A, Deyev S
and Tolmachev V: Comparison of tumor-targeting properties of
directly and indirectly radioiodinated designed ankyrin repeat
protein (DARPin) G3 variants for molecular imaging of HER2. Int J
Oncol. 54:1209–1220. 2019.PubMed/NCBI
|
|
39
|
Wikman M, Steffen AC, Gunneriusson E,
Tolmachev V, Adams GP, Carlsson J and Ståhl S: Selection and
characterization of HER2/neu-binding affibody ligands. Protein Eng
Des Sel. 17:455–462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deyev S, Vorobyeva A, Schulga A, Proshkina
G, Güler R, Löfblom J, Mitran B, Garousi J, Altai M, Buijs J, et
al: Comparative evaluation of Two DARPin variants: Effect of
affinity, size, and label on tumor targeting properties. Mol Pharm.
16:995–1008. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vorobyeva A, Schulga A, Rinne SS, Günther
T, Orlova A, Deyev S and Tolmachev V: Indirect radioiodination of
DARPin G3 Using N-succinimidyl-Para-Iodobenzoate Improves the
Contrast of HER2 molecular imaging. Int J Mol Sci. 20:30472019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Altai M, Garousi J, Rinne SS, Schulga A,
Deyev S and Vorobyeva A: On the prevention of kidney uptake of
radiolabeled DARPins. EJNMMI Res. 10:72020. View Article : Google Scholar : PubMed/NCBI
|