Introduction
Cervical cancer is the fourth most common cancer in
women, with an estimated yearly incidence exceeding 600,000 and a
mortality rate of almost 340,000 in 2020 (1). Squamous cell carcinoma (SCC) and
adenocarcinoma are the two most common histological subtypes of
cervical cancer, accounting for almost 85 and 10% of all cervical
cancers, respectively (2). Other
histologies such as small cell, neuroendocrine, adenosquamous, and
glassy cell carcinomas (GCC) represent between 3–5% of cervical
cancers (3). They are commonly
associated with a higher risk of recurrence and death. GCC of the
cervix is a rare but aggressive subtype of cervical cancer,
considered a variation of Adenosquamous Carcinoma (ASC); It
accounts for less than 1–2% of all cervical cancers and is usually
diagnosed at a mean age 10 years younger than other histologies
(4). Possible associations were
suggested between GCC and high-risk human papillomavirus infection
(HPV 16, 18, 32) and recent or current pregnancies (5).
Histologically, GCC constitutes a part of the
spectrum of ASC, for which pathological diagnosis is based on
identifying a poorly differentiated ASC with no or rare squamous or
glandular differentiation and a high mitotic rate. It can be either
the predominant or focal component of the disease, with a cut-off
of 85%. GCC cells are large cells with a moderate-size cytoplasm
with fine granulations and a ground-glass appearance, large nuclei,
prominent nucleoli, and a distinct cell wall that stains eosin and
periodic acid-Schiff. This characteristic glassy appearance is
related to the abundance of chromatin in GCC cells (5–7).
Owing to the rarity of GCC of the cervix, large
retrospective, and prospective studies are lacking; thus, treatment
strategies for GCC are based on the treatment guidelines of SCC.
Radical hysterectomy preceded by bilateral pelvic lymph node
dissection is the recommended treatment for early stages GCC
cervical cancers (8); However,
despite its aggressiveness, radical trachelectomy was also proposed
as an acceptable conservative approach to treating early-stage
cervical cancer in young patients wishing to preserve their
fertility (9). This procedure has
gained acceptance secondary to the promising oncologic and
obstetrical outcomes (10,11).
Case report
We present the cases of two early-stage glassy cell
cervical cancer treated conservatively.
The study conforms to the French ethical standards,
and the 2008 Helsinki declaration and signed informed consent were
obtained from both the patients included in this study.
The first patient was a 37-year-old woman, gravida
2, para 1, who was admitted for post-coital vaginal bleeding for
over three months. Physical examination showed a cervical tumor
with no vaginal involvement. Biopsies confirmed the diagnosis of
squamous cell carcinoma. Pelvic MRI showed a 15 mm cervical lesion
(FIGO IB1) with no associated lymphadenopathy or signs of
metastatic spread. She underwent pelvic lymphadenectomy with a
negative frozen section analysis of the resected lymph nodes and a
radical trachelectomy. Definite pathology analysis confirmed the 29
disease-free lymph nodes and a 16 mm slightly differentiated
squamous cell carcinoma, limited to the left part of the cervix,
with a minimum of 3 mm lateral safety margin. No vaginal or
paracervical involvement and no lymphovascular space involvement
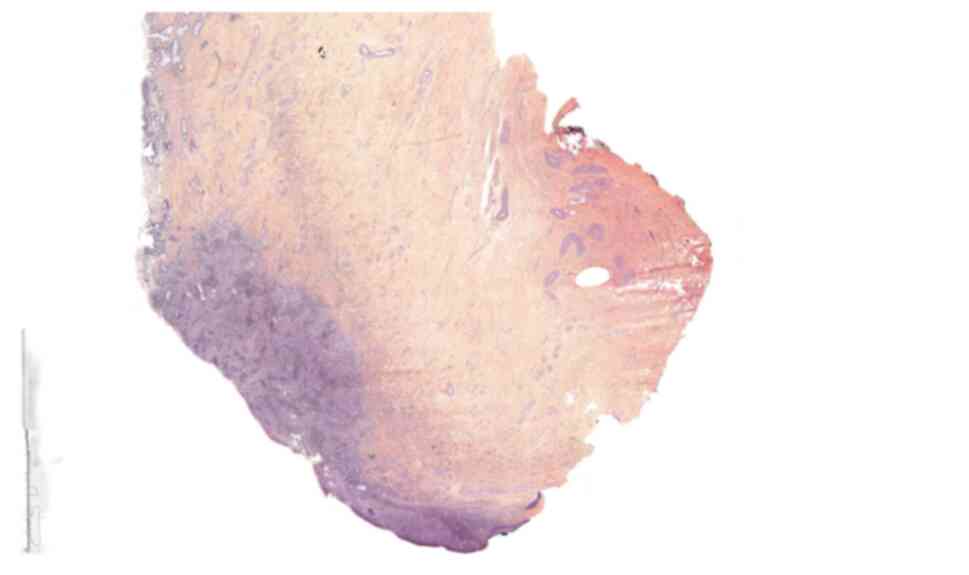
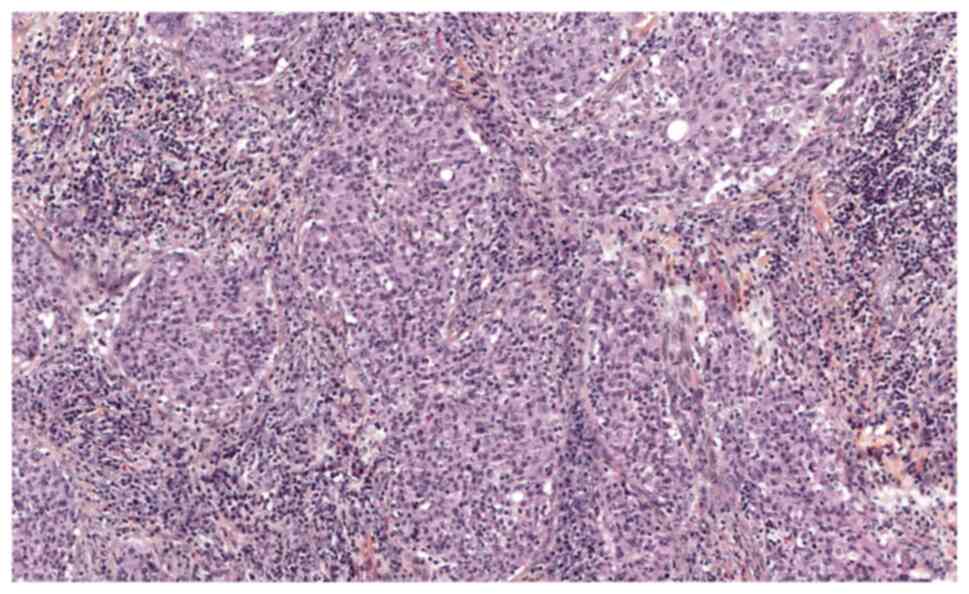
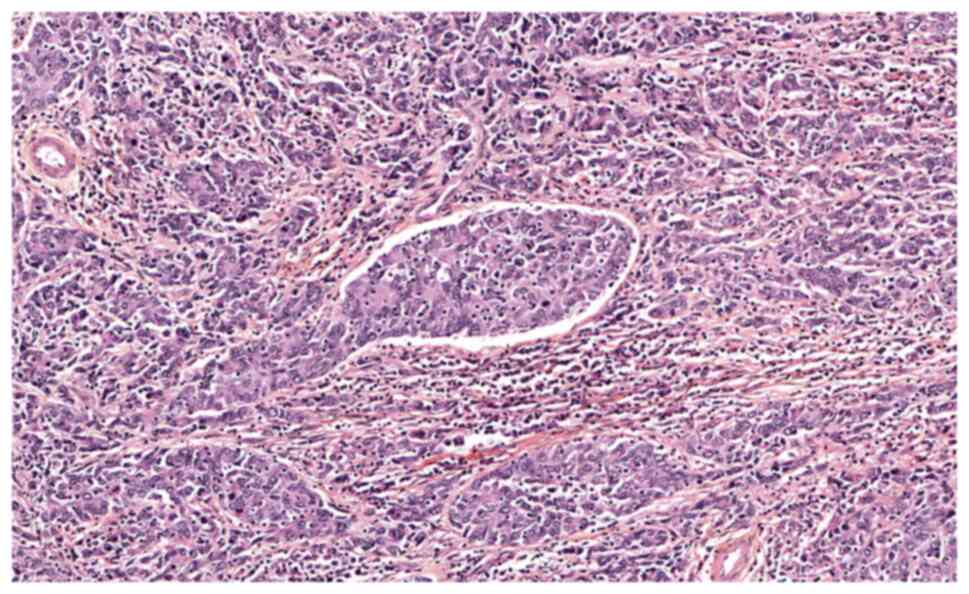
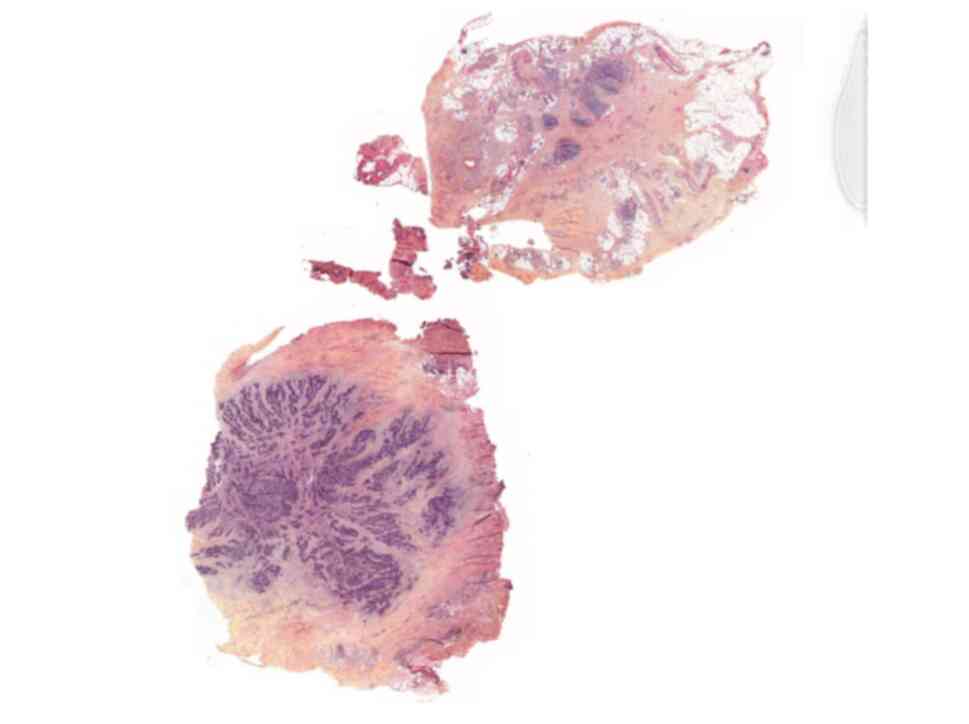
(LVSI) were found on the surgical specimens (Figs. 1 and 2). The patient's case was presented at
the multidisciplinary tumor board, and surveillance was installed.
Three months postoperatively, the patient's clinical exam revealed
vaginal recurrence with left lateral extension to the pelvic wall.
Pelvic MRI showed a 74 mm lesion with bilateral paracervical
extension, involvement of the upper third of the vagina, and
extension to the fascia recti. A positron emission tomography (PET)
scan revealed isolated uterine hypermetabolism. The biopsy of this
lesion confirmed the recurrence of a non-keratinizing SCC (Fig. 3). Reread of the trachelectomy
pathology specimens concluded a major GCC with a minor SCC
component (Figs. 1 and 2), whereas the biopsy of the recurrent
lesion showed only SCC. The patient's case was discussed in the
tumor board, and treatment with chemoradiation was recommended.
After administering a total dose of 45 Gy during concurrent pelvic
chemoradiation, post-treatment MRI confirmed the regression of the
recurrent lesion with the persistence of a 17 mm lesion. Therefore,
the patient received an amount of 15 Gy of vaginal and uterine
brachytherapy. Clinical examination and MRI performed respectively
at four months and then at 5 years after treatment completion
confirmed total remission.
The second patient was a 23-year-old woman, gravida
one, para one, who underwent her first gynecological exam 4 years
after the delivery because of disabling vaginismus. On physical
exam, the cervix was suspicious, and several biopsies were
performed. Pathology analysis found a slightly differentiated SCC
(HPV 16 positive; P53 negative). Pelvic MRI showed a 14 mm (FIGO
stage IB1) cervical lesion with no associated lymphadenopathy or
other signs of metastatic spread. After the tumor board discussion,
the patient underwent pelvic lymphadenectomy with a frozen section
analysis followed by a radical trachelectomy. Results of definite
pathology confirmed 13 disease-free lymph nodes and a 21 mm
slightly-differentiated adenosquamous carcinoma with a morphologic
appearance of GCC without lymphovascular invasion and minimal
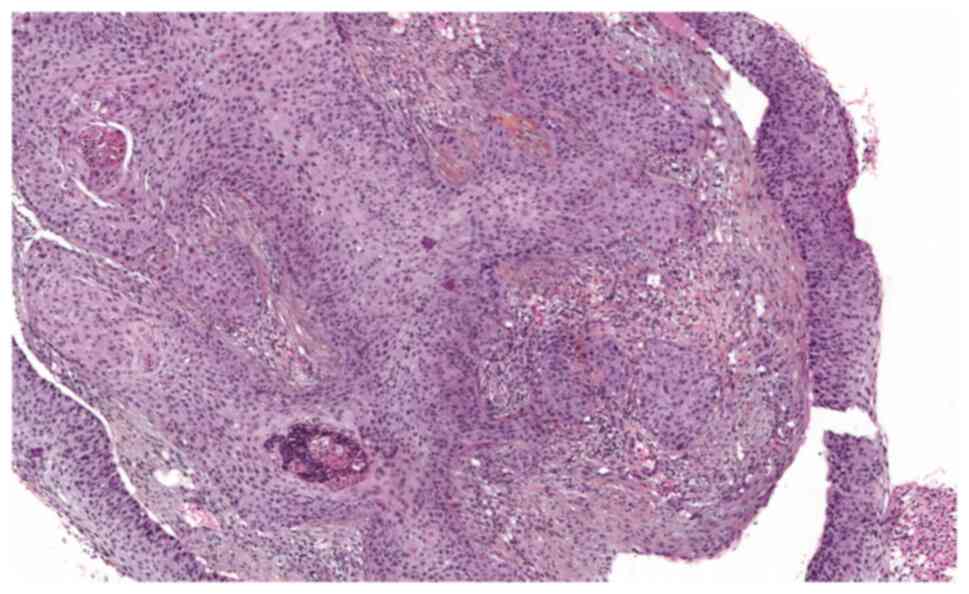
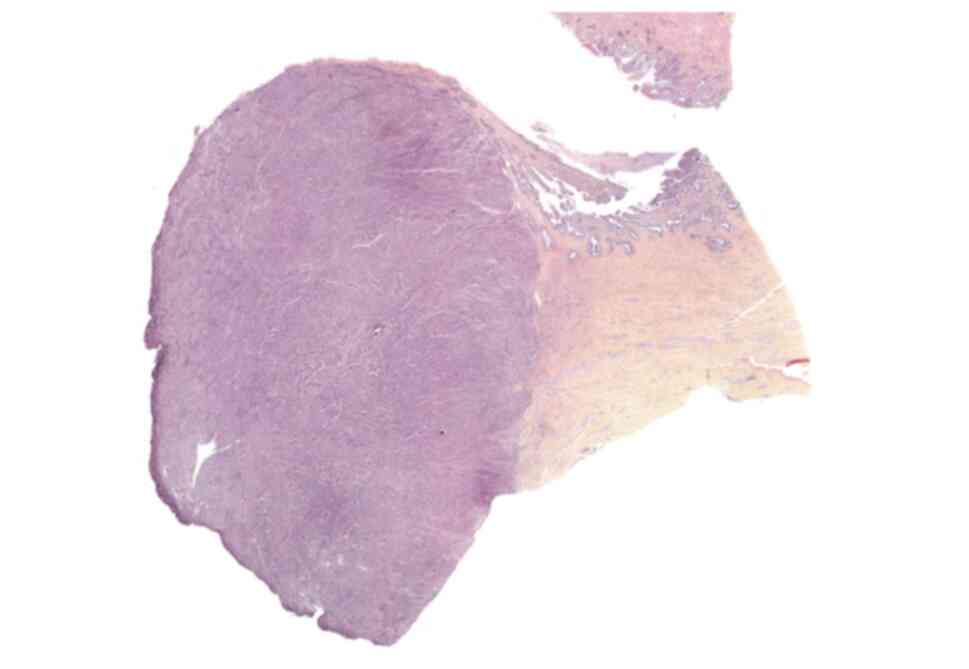
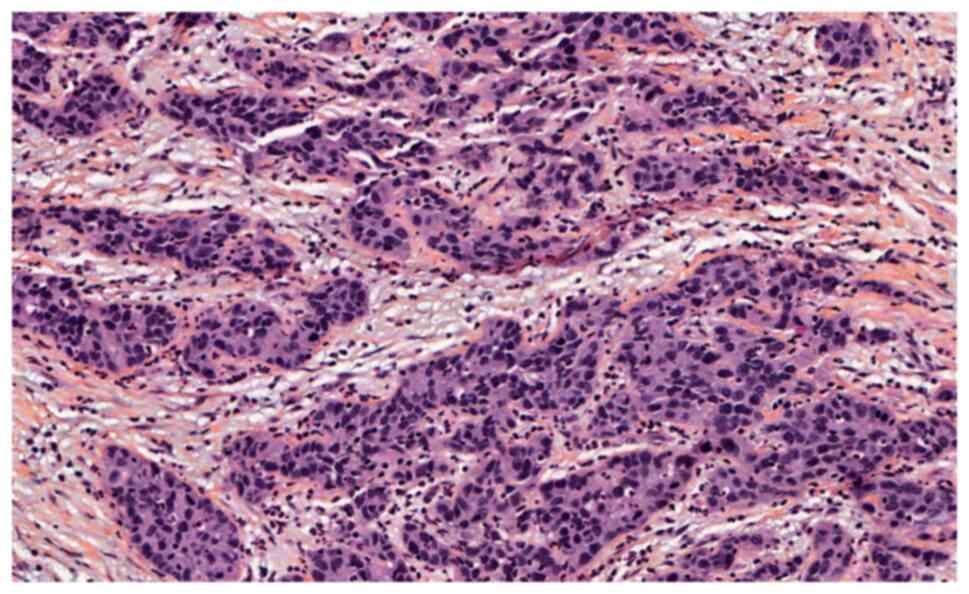
lateral safety margins of 3 mm (Figs.
4 and 5).
Due to the aggressiveness of this histological
subtype, a close follow-up of the patient was put in place that
consisted of a Pap smear and HPV testing performed at two months,
followed by a pelvic MRI at four months after treatment, and
contraception for at least one year. The HPV testing returned
positive for HPV16, and the pelvic MRI was normal four months
postoperatively. The pelvic MRI performed one year after treatment
completion showed a suspicious 13 mm right internal iliac
adenopathy, confirmed on the PET CT scan. The CT scan-guided biopsy
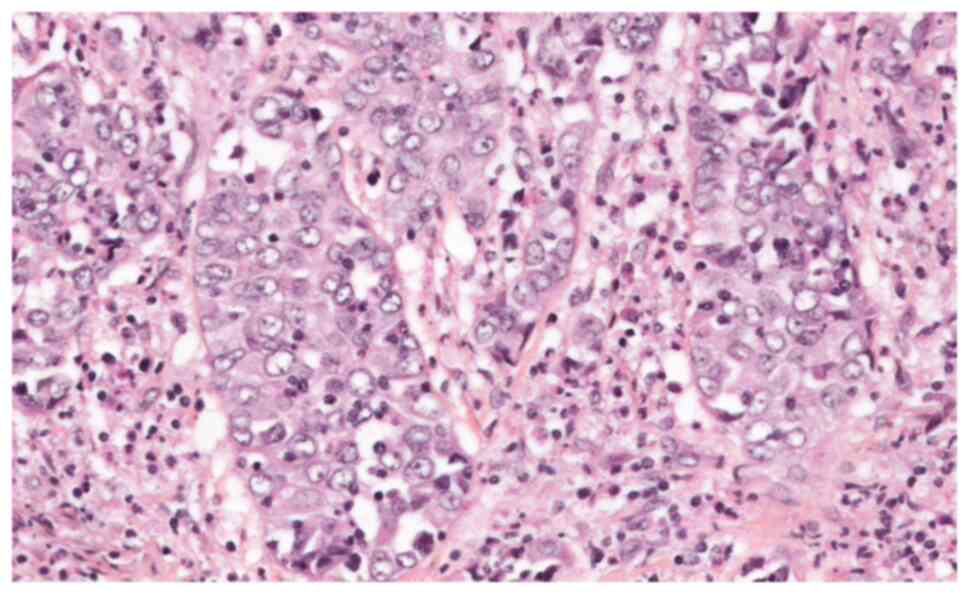
of this adenopathy confirmed the recurrence of GCC, despite the
normal Scc level (Figs. 6 and
7). After discussion in the tumor
board meeting, a diagnostic laparoscopy before paraaortic
lymphadenectomy was performed. We found suspicious pelvic lateral
and peri-splenic lesions that were biopsied, contraindicating
lymphadenectomy. The patient underwent six cycles of
Carboplatin-Paclitaxel and Bevacizumab chemotherapy. The CT scan
imaging, after three cycles of chemotherapy, showed the complete
regression of the right internal iliac adenopathy but the
persistence of the peri-splenic lesions. However, the PET CT scan
performed after 6 cycles showed no suspicious lesions. The patient
then underwent another diagnostic laparoscopy and laparoscopic
para-aortic lymphadenectomy, revealing 16 disease-free lymph nodes
and disease-free biopsies of the peri-splenic lesions. Concomitant
pelvic chemoradiation with a total dose of 45 Gy was performed. The
patient's clinical, biological, and MRI evaluation showed no
recurrence after 6 years of follow-up.
Discussion
GCC was first described by Cherry and Glucksman
(7) in 1956 as a rare subtype of
cervical cancer with distinctive characteristics, considered a
variant of adenosquamous carcinoma (ASC). According to the fifth
edition of the WHO classification of female genital tumors, this
rare, poorly differentiated adenosquamous carcinoma grows in sheets
of large cells with polygonal and abundant finely granular
eosinophilic glass-type cytoplasm. Nuclei are vesicular with
prominent nucleoli and numerous mitotic figures. Dense
lymphoplasmacytic and eosinophilic inflammatory cells infiltrate
the surrounding stroma characteristically. Intercellular bridges,
dyskeratosis, and intracellular glycogen are lacking. GCC is
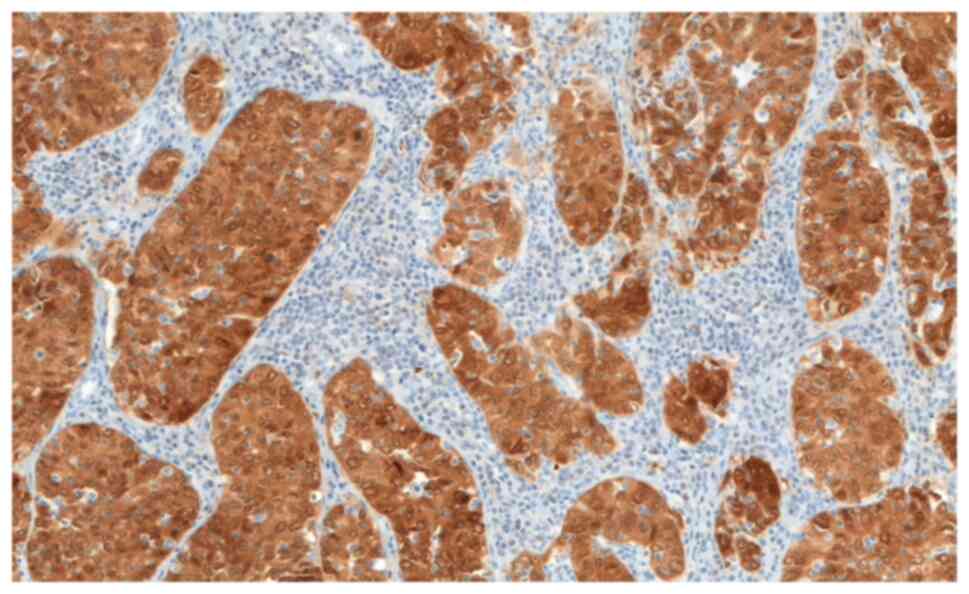
immunoreactive for P16 (Figs. 8
and 9) (12). GCC accounts for almost 5% of all
cervical cancers, 40% of which are diagnosed in reproductive-aged
women (4), with a median age
ranging between 28 years and 41 years, as described by Boustani
et al (2), Hopkins and
Morley (11) and Guitarte et
al (13), respectively
(2,11,13).
Incidence at a younger age, with most cases presenting at early
stages (stage I–II) associated with the tendency to delay
motherhood nowadays, shed light on conservative and
fertility-preserving strategies (2). Thus, a radical trachelectomy
associated with pelvic lymphadenectomy is considered an acceptable
fertility-sparing approach for treating selected patients with
stage I cervical cancer (10).
However, there is very little data on the conservative treatment of
early-stage GCC, and this approach is still controversial (14,15).
GCC's rapid growth and poor differentiation are
translated by increased aggressiveness, poorer prognosis, frequent
distant metastases, and a lower response to conventional treatment
modalities such as surgery, radiation, and chemotherapy (16). A meta-analysis of 292 patients
showed a low 5 years survival rate of 54.8% and a median overall
survival of 25 months (13). The
rarity of this entity and the absence of extensive studies led
initially to the adoption of the SCC guidelines in GCC treatment.
However, the treatment modalities of GCC were further tailored
throughout the years. In 1992, Lotocki et al (17) demonstrated the effectiveness of
associating surgery and radiation in treating stage I GCC. They
showed a five-year survival of 45% in patients treated with surgery
alone (radical hysterectomy and lymphadenectomy) compared to 87% in
patients who underwent the bimodal treatment (17). Piura et al (8) showed in their study that multimodal
treatment, including radical hysterectomy, lymphadenectomy, and
concomitant chemoradiation is an efficient approach. Wang et
al (4) showed that stage I
patients treated with primary radical hysterectomy followed by
pelvic radiation and monthly combined chemotherapy (paclitaxel and
cisplatin) presented a DFS rate of 93% after a median follow-up of
28 months.
In their meta-analysis of 292 patients, Guitarte
et al (13) showed that the
treatment modalities for stage I were not standardized, with 44% of
patients being treated with surgery alone, 32% treated with surgery
followed by radiotherapy, and only 11% received trimodal treatment
associating chemotherapy to the previous protocol. Recurrence rates
for stage I disease were 32% for patients treated only surgically
and 21% for those who received multimodal treatment. Boustani et
al (2) evaluated the
systematic preoperative brachytherapy in early stages GCC. They
found that almost 70% had a complete histological response at the
time of surgery, suggesting that the radiosensitivity of GCC is not
drastically different from that of other cervical carcinoma
(2).
The aggressiveness and high risk of recurrence of
GCC led to the exclusion of GCC patients from most cervical cancer
studies. Very few reports evoke fertility preservation in GCC
patients. Ferrandina et al (18) described a case of a 30 years-old
woman diagnosed with a stage IB GCC treated with cold knife
conization and pelvic lymphadenectomy. The patient refused
additional treatment, and clinical follow-up showed no recurrence
after 38 months. However, our experience is in line with the data
in the literature. It highlights the importance of multimodal
treatment even in early-stage GCC, contrary to what was described
by Ferrandina et al (18).
In conclusion, GCC, a rare cervical cancer subtype,
is frequently diagnosed in younger patients. Due to the rarity of
this tumor, specific guidelines are lacking, and patients are
treated following SCC guidelines. However, our experience with
these two patients and the data in the literature confirm that
conservative management is inadequate for early GCC patients. The
multimodal approach associating radiation, surgery, and
chemotherapy should remain the standard of care irrespective of the
disease stage until further extensive studies are performed.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FN, DH, HEH, EL and CP contributed to the conception
and design of the study. HEH, MC, TD and AS contributed to the
acquisition of data and its interpretation. HEH, DH, FN, EL, MC and
TD contributed to the drafting of the manuscript. FN, EL, CP and DH
contributed to revising the manuscript. All authors agree to be
accountable for all aspects of the work. HEH and FN confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study conformed to the French ethical standards,
and the 2008 Helsinki Declaration and written informed consent was
obtained from both patients included in the present study.
Patient consent for publication
The patients provided written informed consent for
their information to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boustani J, Achkar S, Bertaut A, Genestie
C, Gouy S, Pautier P, Morice P, Haie-Meder C and Chargari C: Glassy
cell carcinoma of the uterine cervix: 20-Year experience from a
comprehensive cancer center. Cancer Radiother. 25:207–212. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cannistra SA and Niloff JM: Cancer of the
uterine cervix. N Engl J Med. 334:1030–1038. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Q, Hu Y, He Y, Wang T and Ghimire P:
Glassy cell carcinoma of cervix: An analysis for 20 cases and
literatures review. Transl Cancer Res. 9:2357–2362. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zolciak-Siwinska A and Jonska-Gmyrek J:
Glassy cell carcinoma of the cervix: A literature review. Eur J
Obstet Gynecol Reprod Biol. 179:232–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Littman P, Clement PB, Henriksen B, Wang
CC, Robboy SJ, Taft PD, Ulfelder H and Scully RE: Glassy cell
carcinoma of the cervix. Cancer. 37:2238–2246. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cherry CP and Glucksmann A: Incidence,
histology, and response to radiation of mixed carcinomas
(adenoacanthomas) of the uterine cervix. Cancer. 9:971–979. 1956.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piura B, Rabinovich A, Meirovitz M and
Yanai-Inbar I: Glassy cell carcinoma of the uterine cervix. J Surg
Oncol. 72:206–210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dargent D, Martin X, Sacchetoni A and
Mathevet P: Laparoscopic vaginal radical trachelectomy: A treatment
to preserve the fertility of cervical carcinoma patients. Cancer.
88:1877–1882. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Q, Li W, Kanis MJ, Qi G, Li M, Yang
X and Kong B: Oncologic and obstetrical outcomes with
fertility-sparing treatment of cervical cancer: A systematic review
and meta-analysis. Oncotarget. 8:46580–46592. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hopkins MP and Morley GW: Glassy cell
adenocarcinoma of the uterine cervix. Am J Obstet Gynecol.
190:67–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
World Health Organization, . Female
Genital Tumours. World Health Organization Classification of
Tumours. 5th edition. Vol 4. International Agency for Research on
Cancer; Lyon: 2020
|
|
13
|
Guitarte C, Alagkiozidis I, Mize B,
Stevens E, Salame G and Lee YC: Glassy cell carcinoma of the
cervix: A systematic review and meta-analysis. Gynecol Oncol.
133:186–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stolnicu S, Hoang L, Hanko-Bauer O, Barsan
I, Terinte C, Pesci A, Aviel-Ronen S, Kiyokawa T, Alvarado-Cabrero
I, Oliva E, et al: Cervical adenosquamous carcinoma: Detailed
analysis of morphology, immunohistochemical profile, and clinical
outcomes in 59 cases. Mod Pathol. 32:269–279. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weed JC Jr, Graff AT, Shoup B and Tawfik
O: Small cell undifferentiated (neuroendocrine) carcinoma of the
uterine cervix. J Am Coll Surg. 197:44–51. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoon N, Kim JY and Kim HS: Clinical
outcomes of advanced-stage glassy cell carcinoma of the uterine
cervix: A need for reappraisal. Oncotarget. 7:78448–78454. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lotocki RJ, Krepart GV, Paraskevas M,
Vadas G, Heywood M and Fung FK: Glassy cell carcinoma of the
cervix: A bimodal treatment strategy. Gynecol Oncol. 44:254–259.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferrandina G, Salutari V, Petrillo M,
Carbone A and Scambia G: Conservatively treated glassy cell
carcinoma of the cervix. World J Surg Oncol. 6:922008. View Article : Google Scholar : PubMed/NCBI
|