Introduction
Multiple primary cancers (MPCs) refer to two or more
primary malignancies occurring simultaneously or successively in
the same patient. The frequency of MPCs varies between 2 and 17%,
depending on different criteria (1). The International Association of
Cancer Registries and International Agency for Research on Cancer
recommend a time-lapse of 6 months to distinguish between
synchronous and metachronous MPCs (2). Synchronous MPCs are less common
compared with metachronous MPCs. In a retrospective study that
included 8,204 patients with breast cancer with MPCs, 4 patients
suffered from synchronous cancer in the lungs or thorax, thus
suggesting that the morbidity of synchronous breast and lung cancer
(SBLC) was <0.05% in patients with breast cancer and MPCs
(3). Another study reported a
slightly higher percentage (0.56%), since among 1,066 patients with
breast cancer, 6 were diagnosed with SBLC (4). Besides, SBLC cases are commonly
reported as case reports lacking systemic analysis. The clinical
symptoms of synchronous MPCs may overlap with each other, thus
resulting in the masking of the clinical manifestations of the
second primary cancer. Therefore, improving the understanding of
synchronous MPCs is of great importance for avoiding misdiagnosis
or missed diagnosis. Next-generation sequencing (NGS) provides
significant information in terms of the diagnosis, treatment and
potential etiological clues of the disease. In the present study,
the case of a patient who initially presented with breast cancer
and metastatic lesions in the cervical lymph nodes is reported.
Further evaluation revealed the presence of synchronous primary
lung adenocarcinoma. Therefore, NGS across 689 genes associated
with the development, treatment and prognosis of solid tumors for
the breast, lung and lymph node lesions was performed aiming to
raise awareness of SBLC in genetics. To the best of our knowledge,
there is no similar case previously reported in the literature.
Case report
General clinical conditions
A 70-year-old female was first referred to The First
Hospital of Jilin University (Changchun, China) due to enlarged
lymph nodes in the left side of the neck in January 2017. The
patient was healthy prior to the onset of the this symptom. The
patient's living and working conditions were of good quality, and
there was no tobacco, alcohol or drug addiction. The patient had no
anemia, inflammatory syndrome or other clinical manifestations.
Tumor marker detection showed no abnormalities.
Methods
Ultrasound of the neck and mammary gland, as well as
positron emission tomography-computed tomography (PET-CT), was
performed when the patient was first admitted to the hospital. A
left breast and left cervical lymph node biopsy were then performed
for diagnostic purposes. When the disease progressed 62 months
after her first visit, the patient underwent a CT scan of the lungs
and a biopsy of the right lung nodule. The breast, lymph node and
lung specimens were fixed with 10% formalin at room temperature for
24 h, then embedded with paraffin and assessed for
immunohistochemical examination, gene variations and fluorescence
in situ hybridization (FISH) for further diagnosis and
treatment.
The sections (5 µm) for immunohistochemical
examination were heated at 70°C for 30 min in an electric
thermostatic drying oven (Shanghai Yiheng Scientific Instrument
Co., Ltd.), dewaxed with xylene at room temperature, rehydrated in
a descending alcohol series at room temperature, and endogenous
peroxidase activity was blocked using 3% H2O2
at room temperature for 10 min, followed by blocking with 10%
normal goat serum (ab7481; Abcam) at 37°C for 30 min. Subsequently,
the sections were incubated with primary antibodies stock solution
(undiluted), including CK7 (MAB-0828; Fuzhou Maixin Biotech Co.,
Ltd.), Napsin A (MAB-0704; Fuzhou Maixin Biotech Co., Ltd.), TTF-1
(MAB-0677; Fuzhou Maixin Biotech Co., Ltd.), ER (Kit-0012; Fuzhou
Maixin Biotech Co., Ltd.), PR (Kit-0013; Fuzhou Maixin Biotech Co.,
Ltd.), HER2 (Kit-0043; Fuzhou Maixin Biotech Co., Ltd.), CA15-3
(MAB-0241; Fuzhou Maixin Biotech Co., Ltd.) and GCDFP15 (MAB-0230;
Fuzhou Maixin Biotech Co., Ltd.) antibodies at 37°C for 2 h.
Finally, the sections were incubated with secondary antibody stock
solution (undiluted) at 37°C for 30 min and dyed with DAB for 5 min
using MaxVision III ultra DAB (Kit-0038; Fuzhou Maixin Biotech Co.,
Ltd.). The sections were then observed under a light microscope
(Olympus Corporation).
Amplification refractory mutation system-PCR
(ARMS-PCR) was performed in nine genes. DNA and RNA were extracted
from tissues using a formalin-fixed paraffin-embedded (FFPE)
DNA/RNA Kit (cat. no. 20150082; Amoy Diagnostics Co., Ltd.).
ARMS-PCR was performed using a Multi-Gene Mutations Detection Kit
(cat. no. 20183401043; Amoy Diagnostics Co., Ltd.) and detected
using Mx3000P Real-Time QPCR System (Agilent Technologies, Inc.),
using the negative and positive controls from the kit as the
quality control. The thermocycling conditions were set up according
to the manufacturer's instructions (stage 1, 42°C for 5 min, 95°C
for 5 min for 1 cycle; stage 2, 95°C for 25 sec, 64°C for 20 sec,
72°C for 20 sec for 10 cycles; stage 3, 93°C for 25 sec, 60°C for
35 sec, 72°C for 20 sec for 36 cycles). The cycle threshold of each
reaction was read directly on the Mx3000P Real-Time QPCR System and
interpreted according to the positive threshold defined in the
manual.
NGS was performed in 689 genes. The sequencing was
performed by BGI Genomics using a QIAamp DNA FFPE Tissue Kit (cat.
no. 56404; Qiagen Inc.) and a MagPure Buffy Coat DNA Midi KF Kit
(cat. no. D3537-02; Magen Biotechnology Co., Ltd.) for preparing
the DNA sample. Agarose gel electrophoresis was used to verify the
quality of the processed samples. The type of sequencing was 100 bp
paired end and was performed using an MGISEQ-2000RS NGS Kit (cat.
no. 1000012554; BGI Genomics). The loading concentrations of the
final library were 81.2, 33.8 and 85.4 ng/µl for the breast, lymph
node and lung specimens, respectively, as measured by a Qubit
fluorometer (Thermo Fisher Scientific, Inc.).
FISH was performed in the lung and lymph node tissue
specimens to detect ret proto-oncogene (RET) fusions as a
gold standard. After dewaxing with xylene, FFPE lung and lymph node
tissue sections (3 µm) were pretreated through proteolytic
digestion using FFPE FISH PreTreatment Kit 1 (cat. no. KA2691;
Abnova). Denaturation (75°C for 5 min), hybridization (37°C for 16
h), washes and counterstaining were performed using a kinesin
family member 5B (KIF5B)/RET SY Translocation FISH Probe Kit (cat.
no. FT0006; Abnova) according to the manufacturer's instructions.
The sections were then detected under a fluorescence microscope at
×1,000 magnification (Olympus Corporation).
Results
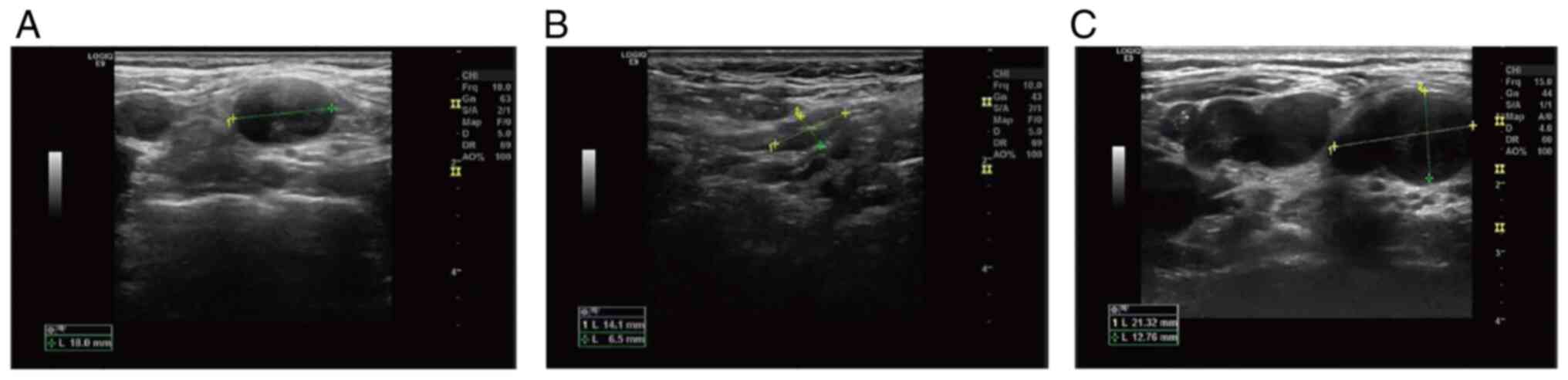
The ultrasound of the left side of the neck revealed
numerous hypoechoic areas, several of which were fused with each
other, without a normal cortex and medulla structure. The border
was clear and the internal echo was uneven. Some of these areas
exhibited a strong mottling echo (Fig.
1). The ultrasound of the mammary gland revealed an 11.0×6.6-mm
anechoic mass on the lateral side of the left mammillary papilla.
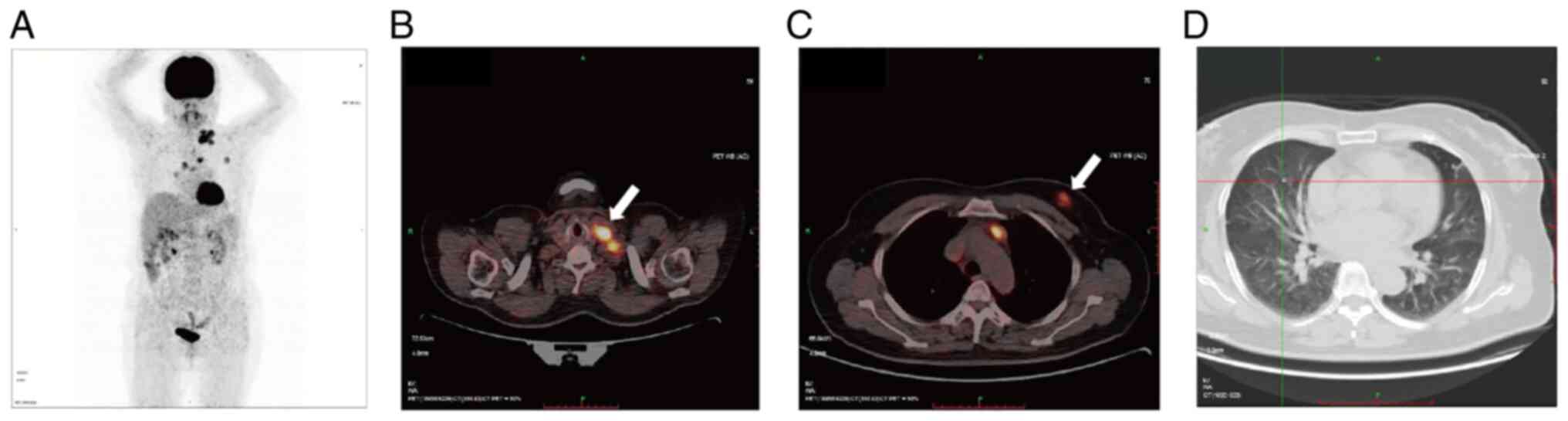
The boundary was clear and the shape was regular. PET-CT showed
that the aforementioned lesions in the breast and neck were
metabolically active (Fig. 2). The
right lung and upper lobe of the left lung presented with several
small nodules without metabolic activity, thus indicating
inflammation (Fig. 2).
Pathological morphology and immunohistochemical examination of the
left breast suggested breast invasive ductal carcinoma (Table I; Fig.
3). In addition, morphology in the left cervical lymph node
revealed a poorly differentiated tumor in the lymphoid tissue and
immunohistochemical results were of little help in identifying the
source of the tumor (Table I;
Fig. 3). Therefore, the left
cervical lymph node tissue specimens and peripheral blood were sent
for mutational analysis. The analysis showed that the cells
expressed wild-type EGFR, with no ALK or ROS1
gene rearrangements. Based on the aforementioned results, the
cervical lymph node lesions were considered as metastases arising
from breast cancer. The patient was treated with letrozole (2.5 mg
once a day; endocrine therapy) from February 2017 for breast cancer
until March 2020. During therapy, the neck mass was significantly
reduced in size (Fig. 1). In March
2020, the cervical lymph nodes were enlarged for the second time,
thus indicating disease progression (Fig. 1). Therefore, the patient was
treated with an adjusted treatment strategy with a combination of
palbonix (125 mg once a day) and fulvestrant (250 mg once a month)
until November 2021, when they were diagnosed with enlarged
bilateral pulmonary nodules. The patient was then treated with
chidamide (30 mg twice a week) plus exemestane (25 mg once a day)
for the next month, until a CT scan showed that the bilateral
pulmonary nodules were further enlarged. This finding indicated
disease progression for the third time. A right lung biopsy
revealed a poorly differentiated carcinoma and the
immunohistochemistry results supported the diagnosis of primary
lung adenocarcinoma (Table I;
Fig. 3). Next, the patient was
treated with albumin-bound paclitaxel (400 mg was administered
twice 3 weeks apart) from January 2022 for both breast and lung
cancer, and has survived with tumors for 8 months up to the time of
writing the study.
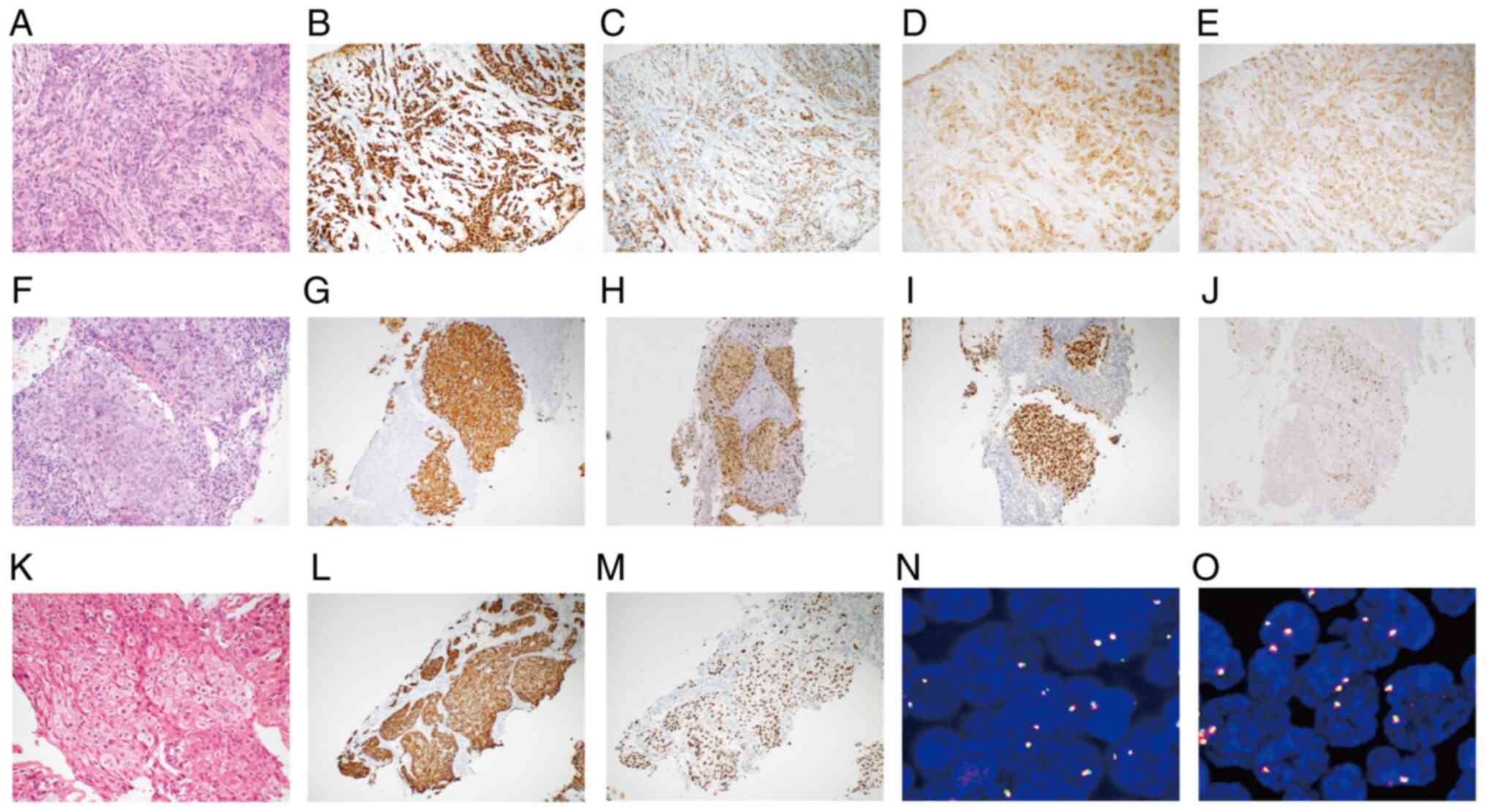 | Figure 3.Histopathological, immunohistochemical
and fluorescence in situ hybridization characterization of
the breast, lymph node and lung lesions. (A) H&E staining of
the breast lesion (magnification, ×200). (B-E) Immunohistochemical
expression of (B) estrogen receptor, (C) progesterone receptor, (D)
gross cystic disease fluid protein 15 and (E) CA15-3 in the breast
lesion (magnification, ×100). (F) H&E staining of the lymph
node lesion (magnification, ×200). (G-J) Immunohistochemical
expression of (G) CK7, (H) Napsin A, (I) TTF1 and (J) CA15-3 in the
lymph node lesion (magnification, ×100). (K) H&E staining of
the lung lesion (magnification, ×200). (L and M)
Immunohistochemical expression of (L) CK7 and (M) TTF1 in the lung
lesion (magnification, ×100). (N) KIF5B-RET fusions in the
lymph node lesion (magnification, ×1,000). (O) KIF5B-RET
fusions in the lung lesion (magnification, ×1,000). (A-J) July
2017. (K-M) November 2021. (N and O) August 2022. H&E,
hematoxylin and eosin; CK, cytokeratin; TTF1, thyroid transcription
factor 1; CA15-3, cancer antigen 15–3; KIF5B, kinesin family member
5B; RET, ret proto-oncogene. |
 | Table I.Immunohistochemistry results in the
breast, lymph node and lung. |
Table I.
Immunohistochemistry results in the
breast, lymph node and lung.
| Immunohistochemistry
marker | Breast | Lymph node | Lung |
|---|
| CK7 | NA | + | + |
| Napsin A | NA | + | - |
| TTF1 | - | + | + |
| ER | + (90%) | - | - |
| PR | + (70%) | - | - |
| HER2a | 2+ | 0 | 0 |
| CA15-3 | + | + | NA |
| GCDFP15 | + | - | NA |
Gene detection and analysis
The breast, lymph node and lung tissue specimens
were evaluated for gene mutations and fusions in nine genes using
ARMS-PCR. The analysis revealed RET gene fusions, without
EGFR, HER2, KRAS, NRAS, BRAF and PIK3CA gene
mutations, or ALK and ROS1 gene fusions, in both the
lung and lymph node specimens. Neither gene mutations nor fusions
were detected in the breast specimen. Subsequently, NGS across 689
genes in each specimen was performed. The Database for Annotation,
Visualization and Integrated Discovery Bioinformatics Resource
(v2022q3; http://david.ncifcrf.gov/) was
utilized to assess disease-related enrichment (P<0.05;
FDR<0.1) of lymph node mutant genes and Gene Ontology Biological
Process (GO BP) enrichment (P<0.05; FDR<0.1) of the breast
mutant genes, as well as lung mutant genes, which were identified
based on NGS results. The analysis identified 26 somatic
alterations in 27 genes in the lymph node specimen, 32 in 27 genes
in the breast specimen and nine somatic mutations in nine genes in
the lung specimen (Table II). No
germline mutations were detected in any specimen. RET gene
fusions were identified in both the lung and lymph node specimens.
Lymph node mutated genes were enriched in nine terms and the top
three were ‘lung cancer’, ‘lymphoma’ and ‘bladder cancer’ [Table III; P<0.05; false discovery
rate (FDR)<0.1]. In addition, the mutated genes in the lung
specimen, including RET, CCNE1, MYC, NSD1, PTCH1 and
ATAD2, were enriched in the ‘positive regulation of
transcription, DNA-templated’ (Table
IV; P<0.05; FDR<0.1). Finally, breast mutated genes,
including EGFR, STK11, ERBB2, PRKD1, MTOR, MAP2K1, RAD50 and
EPHA2, were enriched in eight significant terms. The top
three were ‘protein autophosphorylation’, ‘peptidyl-threonine
phosphorylation’ and ‘positive regulation of kinase activity’
(Table IV; P<0.05;
FDR<0.1).
 | Table II.Gene variations in the breast, lung
and lymph node. |
Table II.
Gene variations in the breast, lung
and lymph node.
| A, Breast |
|---|
|
|---|
| Gene | Variation | Transcript | Mutation abundance or
copy number |
|---|
| MTOR |
p.E1799K(c.5395G>A) | NM_004958.3 | 0.43% |
| EGFR |
p.T790M(c.2369C>T) | NM_005228.3 | 0.42% |
| IDH2 |
p.R140W(c.418C>T) | NM_002168.2 | 0.40% |
| MCL1 | copy number
amplification | NM_021960.4 | 4.82 |
| HLA-A |
p.Q250Hfs*47(c.750_751delGGinsC) | NM_002116.7 | 23.84% |
| RBM10 |
p.S288*(c.863C>G) | NM_005676.4 | 1.24% |
| PMS1 |
p.E543*(c.1627G>T) | NM_000534.4 | 0.93% |
| MAP2K1 |
p.D67N(c.199G>A) | NM_002755.3 | 0.55% |
| SMARCA4 |
p.T910M(c.2729C>T) | NM_001128849.1 | 0.49% |
| ERBB2 |
p.R896C(c.2686C>T) | NM_004448.2 | 0.42% |
| STK11 |
p.D194N(c.580G>A) | NM_000455.4 | 0.40% |
| TRRAP |
p.H913Q(c.2739C>G) | NM_003496.3 | 23.37% |
| CDC27 |
p.H615N(c.1843C>A) | NM_001114091.1 | 14.87% |
| CTNND2 |
p.V428I(c.1282G>A) | NM_001332.2 | 10.37% |
| ELF3 |
p.S266N(c.797G>A) | NM_001114309.1 | 10.28% |
| MUC6 |
p.T1411K(c.4232C>A) | NM_005961.2 | 3.78% |
| AMER1 |
p.V663L(c.1987G>C) | NM_152424.3 | 3.12% |
| MUC16 |
p.T11030A(c.33088A>G) | NM_024690.2 | 2.30% |
| PRKD1 |
p.P35_A36insGP(c.101_106dupGGCCCG) | NM_002742.2 | 1.79% |
| EP300 |
p.Q2108L(c.6323A>T) | NM_001429.3 | 1.40% |
| CIC |
p.G525A(c.1574G>C) | NM_015125.3 | 1.33% |
| MUC16 |
p.L11003I(c.33007C>A) | NM_024690.2 | 1.24% |
| MDC1 |
p.C1599G(c.4795T>G) | NM_014641.2 | 1.15% |
| MDC1 |
p.E1380D(c.4140G>C) | NM_014641.2 | 1.09% |
| RAD50 |
p.Y569K(c.1705_1707delTATinsAAA) | NM_005732.3 | 0.97% |
| RAD50 |
p.F570Y(c.1709T>A) | NM_005732.3 | 0.95% |
| PMS1 |
p.N542H(c.1624A>C) | NM_000534.4 | 0.94% |
| TFE3 |
p.R196L(c.587G>T) | NM_006521.4 | 0.90% |
| MDC1 |
p.S1508P(c.4522T>C) | NM_014641.2 | 0.86% |
| ADGRA2 |
p.P395R(c.1184C>G) | NM_032777.9 | 0.86% |
| SPRED1 |
p.E373Q(c.1117G>C) | NM_152594.2 | 0.73% |
| EPHA2 |
p.N831I(c.2492A>T) | NM_004431.3 | 0.68% |
|
| B, Lung |
|
|
KIF5B-RET | Fusion |
NM_004521.2-NM_020975.4 | 27.41% |
| MYC | Copy number
amplification | NM_002467.4 | 4.95 |
| NSD1 | Copy number
amplification | NM_022455.4 | 4.82 |
| ATAD2 | Copy number
amplification | NM_014109.3 | 4.68 |
| CCNE1 | Copy number
amplification | NM_001238.2 | 4.29 |
| PTCH1 |
p.P24L(c.71C>T) | NM_000264.3 | 17.36% |
| MSH4 |
p.L399V(c.1195C>G) | NM_002440.3 | 13.32% |
| KMT2C |
p.R973G(c.2917A>G) | NM_170606.2 | 12.26% |
| KMT2C |
p.R886C(c.2656C>T) | NM_170606.2 | 10.24% |
|
| C, Lymph
node |
|
|
KIF5B-RET | Fusion |
NM_004521.2-NM_020975.4 | 5.46% |
| TP53 |
p.P301Qfs*44(c.902delC) | NM_000546.5 | 0.31% |
| MCL1 | Copy number
amplification | NM_021960.4 | 5.71 |
| HLA-A |
p.Q250Hfs*47(c.750_751delGGinsC) | NM_002116.7 | 19.18% |
| PTPRS |
p.R979*(c.2935C>T) | NM_002850.3 | 0.73% |
| STAT5A | c.1169+1G>A | NM_003152.3 | 0.65% |
| MYC | Copy number
amplification | NM_002467.4 | 5.64 |
| ZNF217 | Copy number
amplification | NM_006526.2 | 5.19 |
| CDKN1B | Copy number
amplification | NM_004064.3 | 5.11 |
| SRSF2 | Copy number
amplification | NM_003016.4 | 4.83 |
| DAXX | Copy number
amplification | NM_001141970.1 | 4.81 |
| KLF6 | Copy number
amplification | NM_001300.5 | 4.57 |
| CDC27 |
p.H615N(c.1843C>A) | NM_001114091.1 | 17.01% |
| MSH4 |
p.L399V(c.1195C>G) | NM_002440.3 | 12.24% |
| PTCH1 |
p.P24L(c.71C>T) | NM_000264.3 | 8.90% |
| KMT2A |
p.R2707W(c.8119C>T) | NM_001197104.1 | 1.01% |
| NUDT18 |
p.P153L(c.458C>T) | NM_024815.3 | 0.78% |
| DOCK2 |
p.S1170L(c.3509C>T) | NM_004946.2 | 0.77% |
| BCL6 |
p.R40C(c.118C>T) | NM_001706.4 | 0.76% |
| POLG |
p.R869Q(c.2606G>A) | NM_002693.2 | 0.75% |
| ZNRF3 |
p.E775K(c.2323G>A) | NM_001206998.1 | 0.74% |
| PRDM1 |
p.P467L(c.1400C>T) | NM_001198.3 | 0.72% |
| TRRAP |
p.V3197M(c.9589G>A) | NM_003496.3 | 0.71% |
| SLX4 |
p.R1062H(c.3185G>A) | NM_032444.2 | 0.70% |
| KLHL6 |
p.E568K(c.1702G>A) | NM_130446.2 | 0.61% |
| RECQL4 |
p.R780W(c.2338C>T) | NM_004260.3 | 0.61% |
 | Table III.Disease enrichment terms of varied
genes from the lymph node specimen. |
Table III.
Disease enrichment terms of varied
genes from the lymph node specimen.
| Term | P-value | Genes | FDR |
|---|
| Lymphoma |
5.36×10−6 | CDKN1B, BCL6,
MYC, HLA-A, TP53 | 0.004937 |
| Bladder cancer |
1.95×10−4 | RECQL4, RET,
CDKN1B, BCL6, MYC, TP53, POLG | 0.087424 |
| Lung cancer |
3.61×10−4 | RET, KLF6,
CDKN1B, BCL6, MYC, HLA-A, TP53 | 0.087424 |
| Head and neck
cancer |
4.72×10−4 | RECQL4, CDKN1B,
HLA-A, TP53 | 0.087424 |
| Chronic renal
failure|Kidney |
5.34×10−4 | CDKN1B, BCL6,
MYC, MSH4, | 0.087424 |
| Failure,
Chronic |
| HLA-A, PRDM1,
TP53, POLG |
|
| Lymphoma,
B-Cell|Lymphoma, |
5.70×10−4 | BCL6, MYC,
TP53 | 0.087424 |
|
Follicular|Lymphoma, Large B-Cell, |
|
|
|
| Diffuse |
|
|
|
| Pharmacogenetic
studies |
7.58×10−4 | KMT2A, MYC,
HLA-A, TP53 | 0.090843 |
| Leukemia |
8.21×10−4 | CDKN1B, KMT2A,
HLA-A, TP53 | 0.090843 |
| Precursor Cell
Lymphoblastic |
8.88×10−4 | STAT5A, DAXX,
HLA-A, TP53 | 0.090843 |
|
Leukemia-Lymphoma |
|
|
|
 | Table IV.GO-biological process enrichment
terms of varied genes from the breast and lung specimens. |
Table IV.
GO-biological process enrichment
terms of varied genes from the breast and lung specimens.
| A, Breast |
|---|
|
|---|
| Term | P-value | Genes | FDR |
|---|
| GO:0046777~protein
autophosphorylation |
9.03×10−5 | STK11, ERBB2,
PRKD1, EGFR, MTOR |
2.33×10−2 |
|
GO:0018107~peptidyl-threonine
phosphorylation |
1.16×10−4 | MAP2K1, STK11,
PRKD1, MTOR |
2.33×10−2 |
| GO:0033674~positive
regulation of kinase activity |
1.26×10−4 | RAD50, ERBB2,
EGFR, EPHA2 |
2.33×10−2 |
|
GO:1904837~beta-catenin-TCF complex
assembly |
6.89×10−4 | TRRAP, EP300,
SMARCA4 |
6.65×10−2 |
|
GO:0070372~regulation of ERK1 and ERK2
cascade |
6.89×10−4 | ERBB2, EGFR,
EPHA2 |
6.65×10−2 |
|
GO:0018108~peptidyl-tyrosine
phosphorylation |
7.19×10−4 | MAP2K1, ERBB2,
EGFR, EPHA2 |
6.65×10−2 |
|
GO:0045765~regulation of angiogenesis |
8.94×10−4 | ADGRA2, ERBB2,
EPHA2 |
7.08×10−2 |
|
GO:1901796~regulation of signal
transduction by p53 class mediator |
11.45×10−4 | STK11, RAD50,
EP300, MTOR |
7.94×10−2 |
|
| B, Lung |
|
| Term | P-value | Genes | FDR |
|
| GO:0045893~positive
regulation of transcription, DNA-templated |
1.38×10−6 | RET, CCNE1, MYC,
NSD1, PTCH1, ATAD2 |
2.86×10−4 |
|
| GO, Gene
Ontology. |
Discussion
When dealing with MPCs, distinguishing synchronous
primary tumors from metastatic ones can be challenging but
significant, since there are different treatment strategies for
each type of cancer. Although no metastatic lung tumor from breast
cancer has been identified as pure ground glass opacity (5), more than one-half of primary lung
cancer cases are classified as partly or purely solid tumors based
on pulmonary CT scan (4).
Therefore, it is difficult to distinguish a primary lung cancer
from a metastatic tumor based on CT imaging features alone.
Therefore, pathological analysis is considered the gold standard
for the diagnosis. The majority of SBLC cases are breast invasive
ductal carcinoma with lung adenocarcinoma (4). However, when both tumors show a
poorly differentiated morphology, it is difficult to identify their
origin. Although immunohistochemistry can offer some useful clues,
due to the existence of triple-negative breast cancer, estrogen
receptor-, progesterone receptor- and human epidermal growth factor
receptor 2-negative results in lung lesions cannot completely
exclude the possibility of breast cancer metastasis. Thyroid
transcription factor-1 and Napsin A are two specific immunological
markers for lung adenocarcinoma. However, their negative expression
cannot completely exclude the possibility of primary lung
adenocarcinoma. Therefore, the application of immunohistochemistry
in the differential diagnosis is limited. NGS can reveal whether
the lesions show the same clonality and therefore a primary tumor
can be distinguished from metastases. In the present case, at the
time of the initial visit, the lung nodules were small and showed
low metabolism on the PET-CT scan. The mutational analysis of
EGFR, ALK and ROS1 in the lymph nodes revealed no
common gene mutations or fusions associated with lung cancer.
Therefore, the lymph node lesions were considered as metastases
from breast cancer and the patient was treated with endocrine
therapy. However, NGS was performed after disease progression and
the mutated genes in the lymph node specimen were enriched in ‘lung
cancer’, but not in breast cancer. Furthermore, although it has
been reported that RET rearrangements are driver factors in
several types of human cancer (6),
in the present study, the KIF5B-RET fusion was only detected
in the lung and lymph node specimens, but not in the breast
specimens. Based on the aforementioned findings, it was
hypothesized that the lymph node lesion was metastasis from the
lung, thus indicating that the lung cancer was concomitant with the
breast cancer. Although, it cannot be denied that histology,
immunohistochemistry and radiological examination are useful in the
differential diagnosis of SBLC, NGS displays a more significant
value in determining the source of the tumor and in the early
diagnosis of the second primary tumor.
The treatment of MPCs depends on the respective
stage and aggressiveness of each primary cancer. The treatment of
the more aggressive cancer precedes that of the other (7). Sometimes both types of cancer can be
treated simultaneously, and therefore concomitant surgery for both
breast and lung cancer is considered as a feasible and safe
approach (8). Although the female
patient in the present study suffered from synchronous MPC, lung
cancer was diagnosed 3 years after the first visit and the lymph
node lesion was reduced in size during endocrine therapy. The
present study demonstrated that endocrine therapy for breast cancer
was also effective for the metastatic cervical lymph nodes and
could regulate the progression of lung cancer in the early stages
of the disease. This finding was consistent with previous studies
suggesting that the short-term use of hormone replacement therapy
and oral contraceptives could significantly reduce the occurrence
of lung cancer. However, long-term and continuous use could enhance
the risk of lung cancer (9). The
treatment strategy for SBLC should be individualized and based on
multidisciplinary discussions.
Although several possible mechanisms for multiple
primary breast and lung cancers have been suggested (1,2),
some of the risk factors, such as radiotherapy and endocrine
therapy, cannot explain the development of synchronous cancers. NGS
provides significant information not only for the diagnosis and
treatment, but also for the possible etiology of the disease. GO BP
enrichment analysis of lung mutated genes revealed that the
‘positive regulation of transcription, DNA-templated’ was
associated with lung cancer based on the NGS results. In addition,
KIF5B-RET fusions were detected in both the lung and lymph
node specimens. KIF5B-RET fusions are present in 1–2% of
lung adenocarcinomas. It has been reported that the fusion
transcripts of KIF5B-RET are likely to form a homodimer
through the coiled coil domain of KIF5B, thus resulting in
the aberrant phosphorylation of RET kinase (10). Similarly, the results also
identified ‘protein phosphorylation’ as a significantly enriched
term in the breast GO BP. The top three significantly enriched
terms were ‘protein autophosphorylation’, ‘peptidyl-threonine
phosphorylation’ and ‘positive regulation of kinase activity’.
EGFR T790M mutation was detected in the breast specimen by
NGS, but not by ARMS-PCR. We hypothesize that this discrepancy may
be due to the low mutation abundance (0.42% in NGS), which was
below the lower limit of detection in ARMS-PCR. T790M is a common
acquired mutation in patients with non-small cell lung cancer
(NSCLC) initially treated with an EGFR tyrosine kinase
inhibitor (11). Although the
primary T790M mutation is commonly identified in NSCLC, reports of
this mutation in breast cancer are rare. In view of the fact that
in the current case report breast cancer was accompanied by lung
cancer, whether the occurrence of T790M was a random phenomenon or
it was closely associated with the occurrence of SBLC should be
further investigated in more cases.
In conclusion, the diagnosis and treatment of SBLC
remains a challenge due to the lack of specific screening and
well-established treatment guidelines. NGS could provide more
detailed information for the diagnosis and treatment of SBLC. In
addition, based on a large sample size, NGS could provide the
possible underlying mechanisms of SBLC in the future. Nevertheless,
further research is needed to increase our understanding in this
area, while multidisciplinary discussion on individualized
management is also required.
Acknowledgements
Not applicable.
Funding
This study was supported by the Norman Bethune Medical
Engineering and Instrument Center Foundation (grant no.
BQEGCZX2021020), the Department of Science and Technology of Jilin
Province (grant no. 20200201434JC) and 12th Youth Development
Foundation of the First Hospital of Jilin University (grant no.
JDYY11202130).
Availability of data and materials
The dataset used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XD designed and guided the paper. XD and DW confirm
the authenticity of all the raw data. DW analyzed the data and
wrote the manuscript. JY obtained PET-CT images. LG was responsible
for pathological diagnosis and obtained pathological images. XW and
ZT performed the experiments. All authors commented on the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was granted an exemption from requiring
ethics approval from the Ethics Committee of Jilin University
(Changchun, China).
Patient consent for publication
The patient provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vogt A, Schmid S, Heinimann K, Frick H,
Herrmann C, Cerny T and Omlin A: Multiple primary tumours:
Challenges and approaches, a review. ESMO Open. 2:e0001722017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Copur MS and Manapuram S: Multiple primary
tumors over a lifetime. Oncology (Williston Park).
33:6293842019.PubMed/NCBI
|
|
3
|
Lee J, Park S, Kim S, Kim J, Ryu J, Park
HS, Kim SI and Park BW: Characteristics and survival of breast
cancer patients with multiple synchronous or metachronous primary
cancers. Yonsei Med J. 56:1213–1220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shoji F, Yamashita N, Inoue Y, Kozuma Y,
Toyokawa G, Hirai F, Ito K, Tagawa T, Okamoto T and Maehara Y:
Surgical resection and outcome of synchronous and metachronous
primary lung cancer in breast cancer patients. Anticancer Res.
37:5871–5876. 2017.PubMed/NCBI
|
|
5
|
Tanaka K, Shimizu K, Ohtaki Y, Nakano T,
Kamiyoshihara M, Kaira K, Rokutanda N, Horiguchi J, Oyama T and
Takeyoshi I: Diagnosis and surgical resection of solitary pulmonary
nodules in patients with breast cancer. Mol Clin Oncol. 1:117–123.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takahashi M, Kawai K and Asai N: Roles of
the RET Proto-oncogene in cancer and development. JMA J. 3:175–181.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanawade P, Misra S, Yathiraj P and
Agarwal JP: A rare case of multicentric carcinoma left breast
synchronous with carcinoma right lung: Therapeutic challenge in
radiotherapy. J Cancer Res Ther. 11:6652015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohamed S, Jawad F, Darr A, Christensen TD
and Steyn R: Synchronous primary lung and breast carcinoma removed
via a single incision. J Surg Case Rep. 2020:rjz3482020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pesatori AC, Carugno M, Consonni D, Hung
RJ, Papadoupolos A, Landi MT, Brenner H, Müller H, Harris CC, Duell
EJ, et al: Hormone use and risk for lung cancer: A pooled analysis
from the international lung cancer consortium (ILCCO). Br J Cancer.
109:1954–1964. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kohno T, Ichikawa H, Totoki Y, Yasuda K,
Hiramoto M, Nammo T, Sakamoto H, Tsuta K, Furuta K, Shimada Y, et
al: KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 18:375–377.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li W, Qiu T, Guo L, Ling Y, Gao Y, Ying J
and He J: Primary and acquired EGFR T790M-mutant NSCLC patients
identified by routine mutation testing show different
characteristics but may both respond to osimertinib treatment.
Cancer Lett. 423:9–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update.
Arch Pathol Lab Med. 142:1364–1382. 2018. View Article : Google Scholar : PubMed/NCBI
|