Introduction
CD20-negative diffuse large B-cell lymphoma (DLBCL)
accounts for 1–3% of DLBCL cases (1). Commonly reported variants of
CD20-negative DLBCL include plasmablastic lymphoma (PBL), primary
effusion lymphoma (PEL), large B-cell lymphoma arising from human
herpesvirus 8 (HHV8)-associated multicentric Castleman disease
(MCD) and anaplastic lymphoma kinase (ALK)-positive DLBCL. Most
CD20-negative DLBCL cases develop in the oral cavity,
gastrointestinal tract, and other human body cavities (2–4).
These lymphomas are associated with highly aggressive pathologies,
chemotherapeutic resistance and poor prognoses (2). There is currently no effective
treatment for this disease. Primary central nervous system lymphoma
(PCNSL) originates in the CNS and accounts for 1–2% of all
non-Hodgkin's lymphomas. Over 90% of PCNSL cases are histologically
typed as DLBCL and express the pan-B cell markers CD20, CD19, CD22
and CD79a (5). In conjunction with
a review of the relevant literature, the present study reports the
case of a patient with CD20-negative PCNSL and the course of its
diagnosis and treatment, in order to improve our understanding of
the characteristics of this rare disorder.
Case report
In April 2020, a 65-year-old woman presented to The
Affiliated Hospital of Yangzhou University (Yanzhou, China) with a
persistent headache that had lasted for >10 days. The patient
had a history of hypertension of >10 years, but no personal or
familial history of neurological disorders or genetic disorders,
and no recent history of drug use. On admission, the blood pressure
was 158/89 mmHg and vital signs were stable. No abnormalities were
observed on cardiopulmonary, abdominal or neurological examination.
Laboratory tests suggested that the results of routine blood and
blood biochemical examinations were normal. Serum tests for human
immunodeficiency virus (HIV), Epstein-Barr virus (EBV)-DNA, HHV8,
hepatitis B and hepatitis C were negative. Cranial magnetic
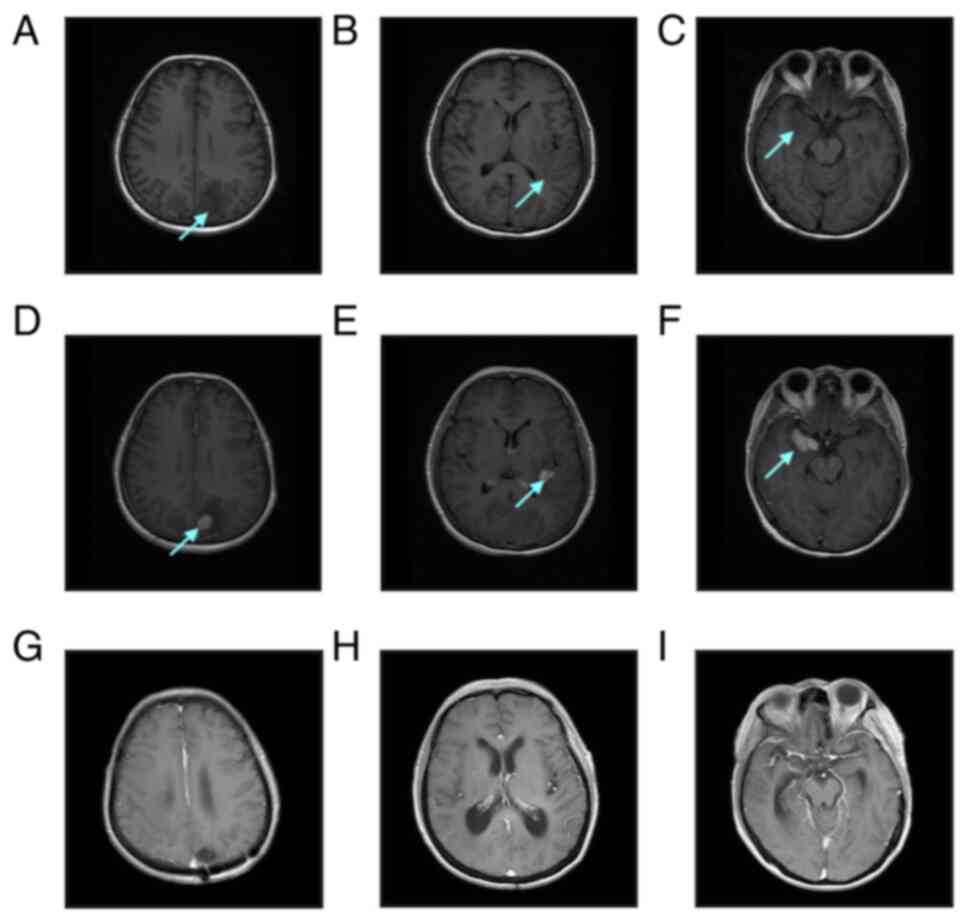
resonance imaging (MRI) showed multiple soft-tissue masses of
varying sizes in both parietal lobes, the left basal ganglia and
both temporal lobes, with a maximum size of ~3.1×2.1 cm, surrounded
by irregular edematous signals and slightly narrowed adjacent
ventricles, sulci and cisterns (Fig.
1A-F). Whole-body positron emission tomography-computed
tomography (PET/CT) results suggested multiple mixed density
shadows in the brain, abnormally increased fluorodeoxyglucose
metabolism and a maximum standardized uptake value value of 29.6,
indicating the presence of a tumor. No significant abnormalities
were found in other parts of the body. A left parieto-occipital
intracerebral tumor resection, expanded meningeal repair and
cranioplasty were performed in April 2020, at 23 days
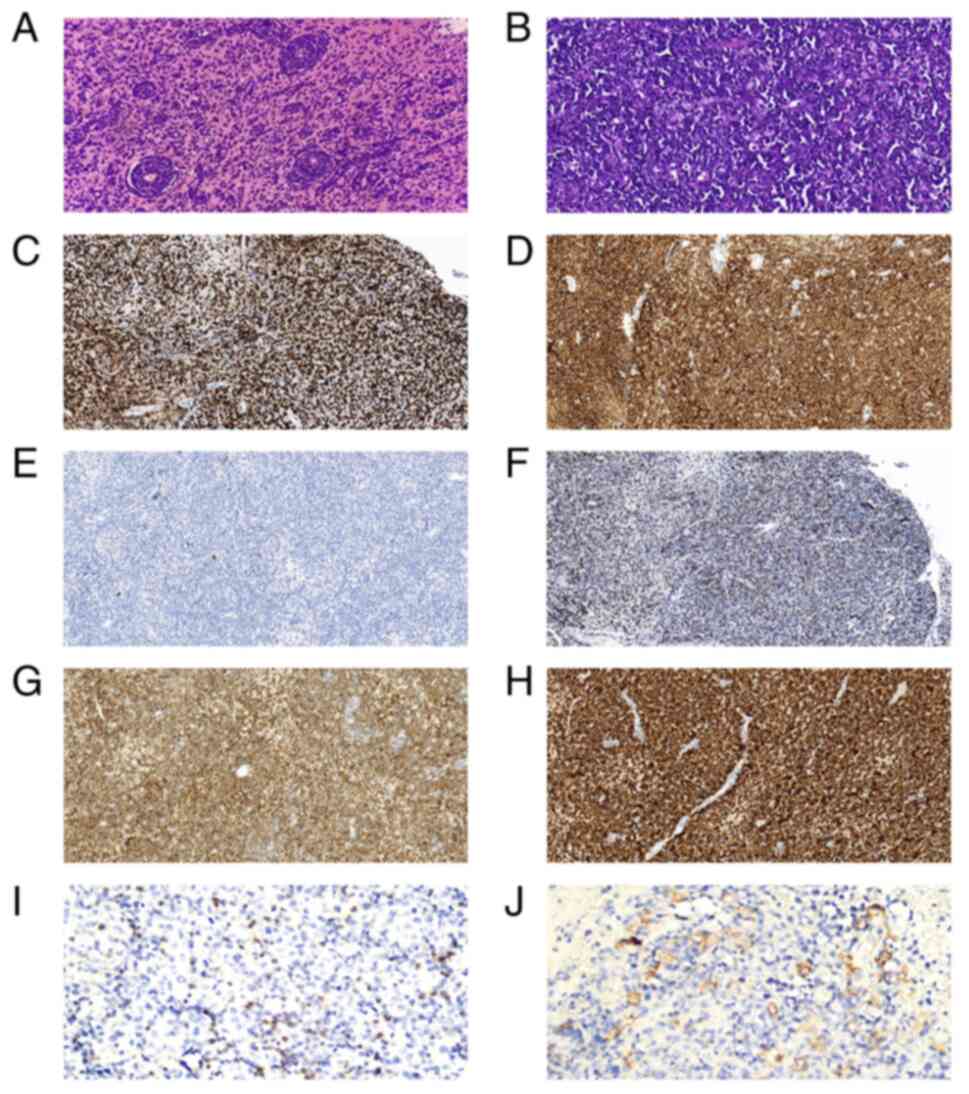
post-admission. Immunohistochemical analysis (Data S1) revealed that the tumor cells
were positive for CD19, CD79α, Bcl-2 (~92%), c-Myc (~50%), multiple
myeloma oncogene 1 (Mum-1), lymphocyte common antigen and paired
box protein 5, while showing negative results for CD20, CD10, CD38,
CD56, CD138, Bcl-6, programmed cell death protein 1 (PD-1),
programmed cell death receptor 1 ligand 1 (PD-L1), Epstein-Barr
encoding region, terminal deoxynucleotidyl transferase and ALK.
Microscopic examination revealed scattered positivity for CD3 and
CD8, but no CD4 signal. The Ki-67 proliferation index was >80%.
In the tumor microenvironment, <10% of tumor-associated
macrophages expressed PD-L1, with an intermediate intensity of
positive cells. The number of PD-1-positive tumor-infiltrating
lymphocytes (TILs) was 30–40 cells in the high-power field
microscopy, and the intensity of positive cells was intermediate
(Fig. 2 and Data S1). Next-generation sequencing
(Data S1) of the brain tumor
tissue showed a CD79B p.Y196C missense mutation at 77.64% variant
allele frequency (VAF), as well as an MYD88 p.L265P missense
mutation at 31.75% VAF, a TP53 p.R110P missense mutation at 20.41%
VAF and an IGHD3-10-IGHJ3 gene rearrangement at 90.69% VAF.
Fluorescence in situ hybridization (Data S1) of the tumor tissue was negative
for the IGH/BCL-2, Bcl-6, c-Myc translocation and
TP53 gene deletion. Upon examination of the peripheral
blood, the gene rearrangement of IGH and TCR was negative.
Morphological examination and flow cytometry (Data S1) of the bone marrow revealed no
abnormalities. Additionally, a bone marrow biopsy showed no
evidence of lymphoma. Based on these results, the patient was
diagnosed with CD20-negative PCNSL that was double positive for
c-Myc and Bcl-2.
First, the patient was treated with high-dose
methotrexate (HD-MTX) at 3.0 g/m2 on day 1 and
administered mannitol to lower cranial pressure, and analgesic
symptomatic treatment. After the first cycle of HD-MTX, the
patient's headache was not significantly relieved and was
accompanied by limb weakness. A cranial MRI examination was
performed again, revealing multiple irregular nodular enhancement
shadows in both parietal lobes and the right temporal lobe, with
more and larger lesions than had been previously observed,
increased edema around some of the lesions, new right ventricular
compression narrowing and a midline left deviation. The regimen was
then adjusted to HD-MTX combined with cytarabine (Ara-c) and
temozolomide chemotherapy at the following doses: 3.5
g/m2 MTX on day 1, 1.0 g/m2 Ara-c every 12 h
on days 2 and 3, and 220 mg temozolomide every day on days 1–5. The
patient's headache was relieved by this therapy. However, the
headache worsened in the intervals between chemotherapy,
accompanied by vomiting and a loss of vision. The patient
experienced left-sided limb weakness and impaired voluntary
walking; a physical examination revealed that the muscle strength
of the left limb was at grade 3. A third cranial MRI was performed,
and the results showed that both sides of the parietal occupancy
were larger than before, the extent of the surrounding brain
parenchymal edema had increased, and the right ventricle and
lateral fissure pool were significantly compressed. On assessing
the patient's symptoms and MRI results, it was concluded that the
patient's disease was progressing. The treatment regimen was
changed to a Bruton tyrosine kinase (BTK) inhibitor combined with a
PD-1 inhibitor, specifically zanubrutinib at 320 mg/day and
tislelizumab at 200 mg on day 1 for 21 days in one cycle. The
patient's headache and limb weakness were significantly relieved.
After two courses of treatment (5 months after the first
admission), the results of the cranial MRI review suggested that
the patient's disease had reached complete remission (CR) (Fig. 1G-I). The patient continued to
receive the same regimen for consolidation and as maintenance
treatment, and a cranial MRI was performed every 3 months. In the
20 months of follow-up since the CR diagnosis, the patient's
condition has ultimately remained stable and the patient is in good
general condition with no significant toxic side effects.
Discussion
The CD20 protein is expressed in most B-cell tumors,
and its expression levels vary amongst different types of diseases
(4). Loss of CD20 expression can
occur in primary large B-cell lymphomas. Known subtypes include
PBL, PEL, ALK-positive DLBCL and large B-cell lymphoma arising in
HHV8-associated MCD (2,3). These variants are frequently
associated with HIV, EBV and HHV8 infections. The tumor cells are
large with vesicular nuclei, and immature plasma cells or
plasmablasts that express CD138, CD38, EMA and MUM1 are present
amongst them. Most cases have extranodal invasion, more aggressive
clinical features, and resistance to both rituximab and
conventional chemotherapy (3,6).
Some patients even experience rapid progression of their disease,
resulting in no opportunity for them to receive chemotherapy
(3,6). In this case, the primary mass was
focally intracranial, and immunohistochemistry did not support the
diagnosis of primary PBL, with negative serum tests for HIV,
EBV-DNA and HHV8, unlike the four subtypes of primary CD20-negative
large B-cell lymphoma. PCNSL has been identified as a distinct
subtype of DLBCL according to the WHO lymphoma classification, and
the majority of cases express CD20 and have a poor prognosis
(5). Therefore, it is hypothesized
that CD20-negative PCNSL is more aggressive and patients may have a
shorter survival time. In the present case, the patient had a
rapidly progressing disease and showed primary resistance to
chemotherapy.
The reason for the loss of CD20 expression remains
unclear. CD20 is a non-glycosylated protein encoded by the MS4A1
gene located on chromosome 11q12.2 (7). Mutations in MS4A1, resulting in an
altered protein conformation, have been proposed to be the
molecular mechanism responsible for the CD20-negative phenotype
(6). In an in vitro trial,
Rushton et al (8)
demonstrated that missense mutations in the MS4A1 gene within the
transmembrane region resulted in the loss of CD20 expression, and
that patients carrying the mutated gene were negative for CD20
expression. The study also found that MS4A1-harboring subclones
might cause relapsing disease progression in a patient who had
received multiple rounds of treatment. It has also been proposed
that CD20 C-terminal deletion mutations in non-Hodgkin's lymphoma
are associated with the loss of CD20 expression (9). Terui et al (9) found that downregulation or negativity
of CD20 expression was closely associated with C-terminal deletion
mutation. C-terminal deletion may mask CD20 expression on the cell
surface or affect the time when CD20 is exposed to the cell
surface. This previous study also found that the C-terminal CD20
deletion mutant mRNA was produced, but protein expression was not
detectable at the cell surface. Further studies are required to
investigate the effects of CD20 deficiency.
The current first-line treatment for PCNSL is
HD-MTX-based chemotherapy. However, the median survival time of
treated patients is short, and most patients relapse within a short
period of time (5). In the present
case, the disease continued to progress even after the
administration of MTX and Ara-C standardized chemotherapy. Multiple
clinical studies have shown that for patients with PCNSL as well as
secondary CNS lymphoma, the administration of ibrutinib alone or in
combination with chemotherapy is up to 50–90% effective, with a
good overall response rate (ORR) and CR rate (10–12).
In several clinical studies, patients with PCNSL, especially those
with CD79B and MYD88 mutations, as well as CNS DLBCL, received
ibrutinib alone or in combination with chemotherapy (11,13–15).
The ORR was 50–90%, indicating a suitable CR rate and treatment
effect (11). However, ibrutinib
monotherapy for recurrent/refractory PCNSL has a low response rate
and short median progression-free survival (PFS) time (14,16).
Immune checkpoint inhibitor (ICI)-PD-1 inhibitors
have shown good antitumor effects in several malignant tumors
(17–20). PD-1 inhibitors can reactivate
cytotoxic T cell-mediated antitumor activity by blocking the
binding between PD-1 expressed on T cells and PD-L1 expressed on
tumor cells, thereby preventing the immune escape of tumors
(13). Due to the large molecular
weight of the anti-PD-1 monoclonal antibody, it rarely crosses the
blood-brain barrier (BBB). Lymph vessels linking the CNS and deep
cervical lymph nodes have been identified. Thus, T cells in the
deep cervical lymph nodes can enter the intracranial and
cerebrospinal fluid through the blood, while T cells and antigens
in the CNS can enter the peripheral lymphatic system and activate T
cells in the lymphoid tissue (13). By establishing a melanoma tumor
transplantation model with an intracranial plus extracranial
(subcutaneous) tumor, Taggart et al (21) found that the application of ICIs
(PD-1 inhibitors or CTLA-4 blockers) induced the activation and
release of CD8+ T cells in extracranial tumors and
enhanced the intracranial transport of CD8+ T cells by
upregulating vascular cell adhesion molecule 1 (VCAM-1) and
intercellular adhesion molecule 1 (ICAM-1) to achieve intracranial
antitumor effects. Therefore, it was proposed that the intracranial
efficacy of anti-PD-1 mAb is primarily achieved by increasing the
intracranial transport of extracranially activated CD8+
T cells. CD4+ T cells are the first to enter the neural tissues as
‘pioneers’ regulating the entry of other T cells (including
CD8+ T cells), as well as antibodies (22). It has been suggested that another
possible mechanism for the enhanced efficacy of
anti-PD-1/PD-L1-based treatments is the local production of IFN-γ
by CD4+ T cells upon entry into the BBB. IFN-γ can upregulate the
expression of VCAM-1 and ICAM-1 to loosen tight junctions between
brain microvascular endothelial cells, increase BBB permeability,
and facilitate the entry of other lymphocytes and circulating
therapeutic antibodies into the CNS (13,22).
Anti-PD-1 monoclonal antibodies have been used in a
variety of tumors to prolong patient survival time and have shown
therapeutic efficacy in previous clinical trials on DLBCL. A phase
Ib clinical trial found that among 11 DLBCL cases treated with
nivolumab, the ORR was 36%, with two patients achieving CR
(23). In another phase II study,
patients with relapsed/refractory DLBCL, who were ineligible for
autologous hematopoietic cell transplantation (auto-HCT) or who had
experienced auto-HCT failure, received nivolumab treatment. Despite
the low response rate to treatment, it was beneficial in extending
the survival time of refractory patients (24). In a study by Nayak et al
(25), four patients with
relapsed/refractory PCNSL were treated with an anti-PD-1 monoclonal
antibody, and three achieved CR with a PFS time of >13 months.
These clinical trials provide a practical basis for the application
of anti-PD-1 monoclonal antibodies in PCNSL.
However, no significant clinical effect has been
observed for a combination of anti-PD-1 monoclonal antibodies and
BTK inhibitors in solid tumors (17–20).
There have been some studies on BTK inhibitors in combination with
anti-PD-1 monoclonal antibodies for the treatment of B-cell
lymphoma. Hanna et al (26)
observed that ibrutinib effectively controlled the progression of
chronic lymphocytic leukemia (CLL) while also reducing the
activation, proliferation and efficacy of CD8+ T cells.
However, the combination of ibrutinib with an anti-PD-1 monoclonal
antibody can reduce CLL progression by increasing the percentage
and function of effector CD8+ T cells. In a trial of
PD-1 monoclonal antibodies in combination with a BTK inhibitor for
platinum-resistant metastatic urothelial cancer, an increase in
CD8+ T cells was observed for the combination treatment,
despite no significant improvement in patient prognosis (19). However, in experiments using mouse
models of lymphoma and CLL, combination therapy achieved better
therapeutic results. In a study by Sagiv-Barfi et al
(27), PD-L1 inhibitor monotherapy
only modestly improved the survival of mice with
ibrutinib-insensitive A20 lymphoma cells. However, when combined
with ibrutinib, approximately one-half of the tumors in the mice
were cured, and tumor growth in the remaining mice was
significantly reduced. The study also found evidence of
tumor-specific T cells in the urine of mice treated with the
combination treatment.
Both TILs and, potentially, tumoricidal macrophages,
depend on aerobic glycolysis. Tumor cells can affect the metabolic
structure of immune cells by depleting nutrients or directly
competing for metabolic nutrients (especially glucose) (28). Tumor cells evade immunity through
metabolic competition. Metabolic competition can limit T cell
function and activity, and mediate T-cell hyporeactivity, resulting
in tumor immune escape (28).
Chang et al (29) found
that blocking PD-L1 in tumor cells can inhibit mTOR activity and
reduce the expression of glycolytic enzymes, thereby inhibiting
glycolysis. Suppressed glycolytic capacity can leave more glucose
available for T cells in the tumor extracellular environment to
enhance their activity. Qorraj et al (28) found that the use of BTK inhibitors
in CLL may exacerbate immune metabolic defects in monocytes.
However, blocking PD-1/PD-L1 signaling could reverse these immune
metabolic dysfunctions, including T-cell dysfunction.
In a clinical study of an orelabrutinib-based
regimen for PCNSL, all four patients who were treated with a
combination of orelabrutinib, camrelizumab (PD-1 monoclonal
antibody) and fotemustine, with an ORR of 100%, had a 6-month PFS
rate of 100% (30). In a
prospective phase II study (NCT04899427), a combination of a BTK
inhibitor (orelabrutinib) and PD-1 monoclonal antibody (sintilimab)
was used for the treatment of relapsed/refractory PCNSL in 13
enrolled patients, and they had an ORR of 61.5%; four patients
achieved CR, one patient achieved unconfirmed CR and three achieved
partial remission (31). Several
clinical trials of BTK inhibitors in combination with immune
checkpoint inhibitors for PCNSL are currently underway, including
acalabrutinib combined with durvalumab (NCT04462328) and nivolumab
combined with ibrutinib (NCT03770416). As of June 2022, the patient
in the present study had undergone 23 cycles of the BTK inhibitor
combined with anti-PD-1 monoclonal antibody therapy, achieving CR
with no significant adverse effects. The effectiveness of the
combination of the two targeted drugs in treating PCNSL and the
mechanism of the combination of the two drugs should be further
confirmed by basic medical research and clinical trials.
In conclusion, by reviewing relevant basic medical
research and the few current clinical trials, we propose that BTK
inhibitor combined with PD1 monoclonal antibody is feasible for the
treatment of PCNSL.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LF, XG, FM, ZJ and ZW confirm the authenticity of
all the raw data. LF, XG, FM contributed to the analysis of the
clinical data, as well as the writing of the manuscript. ZJ and ZW
contributed to the collection of clinical and laboratory data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The patient provided written informed consent for
participation in the present study.
Patient consent for publication
The patient provided written informed consent for
the publication of all the data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Devi K, Ali N and Ahmed A: Case report of
primary CD20 negative diffuse large B-cell lymphoma. Oxf Med Case
Reports. 2021:omab1142021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qunaj L, Castillo JJ and Olszewski AJ:
CD20-negative large B-cell lymphomas: Analysis of the National
cancer database. Blood. 128:42262016. View Article : Google Scholar
|
|
3
|
Katchi T and Liu D: Diagnosis and
treatment of CD20 negative B cell lymphomas. Biomark Res. 5:52017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tomita A: Genetic and epigenetic
modulation of CD20 Expression in B-Cell Malignancies: Molecular
mechanisms and significance to rituximab resistance. J Clin Exp
Hematop. 56:89–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chihara D and Dunleavy K: Primary central
nervous system lymphoma: Evolving biologic insights and recent
therapeutic advances. Clin Lymphoma Myeloma Leuk. 21:73–79. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gamonet C, Bole-Richard E, Delherme A,
Aubin F, Toussirot E, Garnache-Ottou F, Godet Y, Ysebaert L,
Tournilhac O, Caroline D, et al: New CD20 alternative splice
variants: Molecular identification and differential expression
within hematological B cell malignancies. Exp Hematol Oncol.
5:72016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pavlasova G and Mraz M: The regulation and
function of CD20: An ‘enigma’ of B-cell biology and targeted
therapy. Haematologica. 105:1494–1506. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rushton CK, Arthur SE, Alcaide M, Cheung
M, Jiang A, Coyle KM, Cleary KLS, Thomas N, Hilton LK, Michaud N,
et al: Genetic and evolutionary patterns of treatment resistance in
relapsed B-cell lymphoma. Blood Adv. 4:2886–2898. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Terui Y, Mishima Y, Sugimura N, Kojima K,
Sakurai T, Mishima Y, Kuniyoshi R, Taniyama A, Yokoyama M, Sakajiri
S, et al: Identification of CD20 C-terminal deletion mutations
associated with loss of CD20 expression in non-Hodgkin's lymphoma.
Clin Cancer Res. 15:2523–2530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldwirt L, Beccaria K, Ple A, Sauvageon H
and Mourah S: Ibrutinib brain distribution: A preclinical study.
Cancer Chemother Pharmacol. 81:783–789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lionakis MS, Dunleavy K, Roschewski M,
Widemann BC, Butman JA, Schmitz R, Yang Y, Cole DE, Melani C,
Higham CS, et al: Inhibition of B cell receptor signaling by
ibrutinib in primary CNS lymphoma. Cancer Cell. 31:833–843.e5.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Li Y, Zhuang Z, Wang W, Wei C,
Zhao D, Zhou D and Zhang W: Preliminary evaluation of
zanubrutinib-containing regimens in DLBCL and the cerebrospinal
fluid distribution of zanubrutinib: A 13-case series. Front Oncol.
11:7604052021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou S, Xie J, Huang Z, Deng L, Wu L, Yu J
and Meng X: Anti-PD-(L)1 immunotherapy for brain metastases in
non-small cell lung cancer: Mechanisms, advances, and challenges.
Cancer Lett. 502:166–179. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soussain C, Choquet S, Blonski M, Leclercq
D, Houillier C, Rezai K, Bijou F, Houot R, Boyle E, Gressin R, et
al: Ibrutinib monotherapy for relapse or refractory primary CNS
lymphoma and primary vitreoretinal lymphoma: Final analysis of the
phase II ‘proof-of-concept’ iLOC study by the Lymphoma study
association (LYSA) and the French oculo-cerebral lymphoma (LOC)
network. Eur J Cancer. 117:121–130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rubenstein JL: Biology of CNS lymphoma and
the potential of novel agents. Hematology Am Soc Hematol Educ
Program. 2017:556–564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grommes C, Pastore A, Palaskas N, Tang SS,
Campos C, Schartz D, Codega P, Nichol D, Clark O, Hsieh WY, et al:
Ibrutinib unmasks critical role of bruton tyrosine kinase in
primary CNS lymphoma. Cancer Discov. 7:1018–1029. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taylor MH, Betts CB, Maloney L, Nadler E,
Algazi A, Guarino MJ, Nemunaitis J, Jimeno A, Patel P,
Munugalavadla V, et al: Safety and efficacy of pembrolizumab in
combination with acalabrutinib in advanced head and neck squamous
cell carcinoma: Phase 2 Proof-of-Concept Study. Clin Cancer Res.
28:903–914. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Overman M, Javle M, Davis RE, Vats P,
Kumar-Sinha C, Xiao L, Mettu NB, Parra ER, Benson AB, Lopez CD, et
al: Randomized phase II study of the Bruton tyrosine kinase
inhibitor acalabrutinib, alone or with pembrolizumab in patients
with advanced pancreatic cancer. J Immunother Cancer.
8:e0005872020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang T, Harrison MR, O'Donnell PH, Alva
AS, Hahn NM, Appleman LJ, Cetnar J, Burke JM, Fleming MT, Milowsky
MI, et al: A randomized phase 2 trial of pembrolizumab versus
pembrolizumab and acalabrutinib in patients with platinum-resistant
metastatic urothelial cancer. Cancer. 126:4485–4497. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim KH, Cho J, Ku BM, Koh J, Sun JM, Lee
SH, Ahn JS, Cheon J, Min YJ, Park SH, et al: The First-week
proliferative response of peripheral blood PD-1+CD8+ T
cells predicts the response to Anti-PD-1 therapy in solid tumors.
Clin Cancer Res. 25:2144–2154. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taggart D, Andreou T, Scott KJ, Williams
J, Rippaus N, Brownlie RJ, Ilett EJ, Salmond RJ, Melcher A and
Lorger M: Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain
metastases depends on extracranial disease and augmentation of
CD8+ T cell trafficking. Proc Natl Acad Sci USA.
115:E1540–E1549. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eguren-Santamaria I, Sanmamed MF, Goldberg
SB, Kluger HM, Idoate MA, Lu BY, Corral J, Schalper KA, Herbst RS
and Gil-Bazo I: PD-1/PD-L1 Blockers in NSCLC brain metastases:
Challenging paradigms and clinical practice. Clin Cancer Res.
26:4186–4197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lesokhin AM, Ansell SM, Armand P, Scott
EC, Halwani A, Gutierrez M, Millenson MM, Cohen AD, Schuster SJ,
Lebovic D, et al: Nivolumab in patients with relapsed or refractory
hematologic malignancy: Preliminary results of a phase Ib study. J
Clin Oncol. 34:2698–2704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ansell SM, Minnema MC, Johnson P,
Timmerman JM, Armand P, Shipp MA, Rodig SJ, Ligon AH, Roemer MGM,
Reddy N, et al: Nivolumab for relapsed/refractory diffuse large
B-Cell lymphoma in patients ineligible for or having failed
autologous transplantation: A single-arm, phase II study. J Clin
Oncol. 37:481–489. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nayak L, Iwamoto FM, LaCasce A, Mukundan
S, Roemer MGM, Chapuy B, Armand P, Rodig SJ and Shipp MA: PD-1
blockade with nivolumab in relapsed/refractory primary central
nervous system and testicular lymphoma. Blood. 129:3071–3073. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanna BS, Yazdanparast H, Demerdash Y,
Roessner PM, Schulz R, Lichter P, Stilgenbauer S and Seiffert M:
Combining ibrutinib and checkpoint blockade improves
CD8+ T-cell function and control of chronic lymphocytic
leukemia in Em-TCL1 mice. Haematologica. 106:968–977. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng
PP, Chang BY and Levy R: Therapeutic antitumor immunity by
checkpoint blockade is enhanced by ibrutinib, an inhibitor of both
BTK and ITK. Proc Natl Acad Sci USA. 112:E966–E972. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qorraj M, Bruns H, Böttcher M, Weigand L,
Saul D, Mackensen A, Jitschin R and Mougiakakos D: The PD-1/PD-L1
axis contributes to immune metabolic dysfunctions of monocytes in
chronic lymphocytic leukemia. Leukemia. 31:470–478. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang CH, Qiu J, O'Sullivan D, Buck MD,
Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ,
et al: Metabolic competition in the tumor microenvironment is a
driver of cancer progression. Cell. 162:1229–1241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu JJ, Wang WH, Dong M, Ma SS, Zhang XD,
Zhu LN, Niu ST, Ding MJ, Zhang JM, Zhang L, et al:
Orelabrutinib-bruton tyrosine kinase inhibitor-based regimens in
the treatment of central nervous system lymphoma: A retrospective
study. Invest New Drugs. 40:650–659. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Wang W, Zhao DQ, Zhang W and Zhou
DB: Preliminary results of a phase II study of orelabrutinib in
combination with anti-PD-1 monoclonal antibody in refractory or
relapsed primary CNS lymphoma. Proceedings of the EHA2022. EHA
Library; pp. S2242022
|