Introduction
The GLOBOCAN 2020 estimates of cancer burden suggest
that there were ~4.57 million new cases of cancer in China in 2020.
These include >410,000 patients with breast cancer (BC),
accounting for 9.1% of all newly diagnosed cancers in China and
19.9% of all newly diagnosed cancers in Chinese women, making it
the most prevalent cancer among women. In addition, the estimated
number of deaths from BC was >110,000, accounting for ~3.9% of
all cancer-associated deaths in China and 9.9% of all Chinese
female deaths, ranking fourth in female cancer mortality (1). The incidence of BC and its associated
deaths are increasing annually, seriously endangering the lives and
health of Chinese women. Developments in medical technology have
facilitated advances in BC treatment, from a single surgical
treatment to a systemic, comprehensive mode of treatment based on
surgery and including chemotherapy and endocrine, targeted,
radiation and immune therapies. Therefore, BC is among the
malignant tumors with the most clinical treatments and the greatest
likelihood of a curative effect (2).
Serum lipids are essential blood-borne lipid
components in the human body. Along with proteins and nucleic
acids, lipids are important biological membrane constituents and
cellular building materials. Furthermore, lipids store energy, are
crucial for cell metabolism and are critical signaling agents for
numerous cellular functions (3).
The modulation of lipid metabolism, such as lipid absorption,
production and hydrolysis, is essential for maintaining cellular
homeostasis (4).
The tumor microenvironment (TME) is crucial for the
metabolic adaptation of tumor cells. Various substrates in addition
to glucose are necessary to meet the nutritional requirements of
highly proliferative tumor cells. Lipids have been suggested to be
the most important alternative fuel supporting tumor cell
proliferation. Tumor cell-based lipid metabolism and its
significance in tumor progression and metastasis have gained
extensive attention (5). Tumor
cells use lipid metabolism to produce biofilm components, energy
and signaling molecules required for proliferation, survival,
invasion and metastasis, as well as to alter the TME and respond to
cancer treatment (6). Changes in
lipid metabolism, particularly fatty acid (FA) synthesis and
oxidation, have been identified as essential metabolic
reorganization phenomena within tumor cells (7). Previous studies have shown that serum
lipid metabolism is associated with colon, ovarian, prostate and BC
tumor malignancy (8,9).
The incidence of BC is affected by numerous factors,
including estrogen levels, heredity and lifestyle. Lifestyle is
considered an increasingly important factor in BC, with obesity,
metabolic syndrome (MS) and hyperlipidemia increasing BC risk
(10). However, the mechanism by
which abnormal serum lipid metabolism leads to BC remains unclear.
It has been suggested that the relationship between blood lipid
metabolism and BC occurrence and development is reflected in blood
lipid metabolism components such as 27-hydroxycholesterol,
phosphatidylinositol, adiponectin and leptin (11–13).
Indeed, some studies have indicated that serum lipid metabolism has
a key role in the prognosis and treatment of BC metastasis
(14,15).
BC exhibits clinical, morphological and molecular
heterogeneity. Since the advent of microarray technology, gene
expression studies have increasingly been used to elucidate the
behavior of breast tumors. The identified gene expression profiles
have provided an improved understanding of the complex
heterogeneity and biological behavior of BC. Molecular typing can
be used to further confirm these characteristics. In 2000, Perou
et al (16) studied BC
typing and classified BC into luminal, human epidermal growth
factor receptor 2 (HER2)-positive, basal cell-like and normal
breast-like types. However, obtaining intrinsic subcategories by
gene expression profiling is complex and expensive and its clinical
application is limited.
Methods that determine subcategories via
clinicopathological examination have become the basis for the
diagnosis and treatment of BC. Estrogen receptor (ER), progesterone
receptor (PR), HER2 and proliferation marker protein Ki-67 statuses
determined by pathological examination are associated with BC
prognosis (17). In 2011, a new BC
molecular subtyping standard was proposed at the St. Gallen
International BC Conference. In this standard, according to the ER,
PR, HER2 and Ki-67 expression of the tumor, BC is divided into the
following types: Luminal A type, which is ER and/or PR positive and
HER2 negative with low Ki-67 expression; luminal B (HER2-negative)
type, which is ER and/or PR positive and HER2 negative with high
Ki-67 expression; luminal B (HER2-positive) type, which is ER
and/or PR positive and HER2 positive; HER2 overexpression type,
which is ER and PR negative with HER2 upregulation or
amplification; and triple-negative type, which is ER, PR and HER2
negative (18). This diagnosis and
treatment consensus was the first to perform molecular BC typing
through routine pathological diagnosis results, confirming that
molecular typing can serve an important role in BC treatment
decision-making.
The clinicopathological BC subcategories vary in
invasiveness, treatment options and prognosis. Luminal
subcategories often exhibit enhanced prognosis compared with the
non-luminal subcategories because they are hormone
receptor-positive and more sensitive to hormonal intervention.
Since cases of HER2 overexpressing and triple-negative BC (TNBC)
often experience early and frequent recurrence and metastasis,
their prognosis is poor. The HER2 overexpressing type has an
improved prognosis compared with TNBC because it can benefit from
HER2-targeted therapy. TNBC is considered the most severe BC
subtype because it does not express ER, PR or HER2. Also, it is
more aggressive, has elevated recurrence and distant metastasis
risks and a worse outcome, relative to other BC subcategories.
Systemic treatment methods for TNBC are limited; since endocrine
and targeted therapies are ineffective, chemotherapy is currently
its primary treatment modality (19,20).
Ki-67 is a macromolecular nuclear protein that
serves as an antigen in Hodgkin's lymphoma and is closely
associated with cell proliferation. Ki-67 is involved in the cell
cycle and is present in all cell cycle phases with the exception of
G0 (17). Ki-67 levels
are lower in the G1 and S phases, peak during prophase
mitosis and decrease sharply during anaphase mitosis (21). Ki-67 has been widely employed as a
proliferation capacity indicator for human tumor cells for numerous
years, and its expression in normal cells is low. The faster the
tumor cell growth rate and the lower the tissue differentiation,
the higher the Ki-67 expression (22). Ki-67 is used as a proliferation
marker in BC tissue (23), with
its expression level reflecting tumor cell proliferation. Studies
have shown that the proliferation of BC cells is closely associated
with invasiveness, which is an indicator of patient prognosis.
Therefore, Ki-67 has been shown to be a key prognostic and
predictive marker for BC (24,25).
Researchers have performed several studies on the
association between circulating lipid concentrations and BC.
However, few studies have explored the correlations between
circulating lipid concentrations in BC subcategories and Ki-67
expression. To the best of our knowledge, there is only one study
on this topic, which reported that serum lipid levels differ among
BC molecular subcategories (26).
Therefore, the present study explored differences in preoperative
circulating lipid concentrations in patients with different BC
molecular subcategories and their correlations with Ki-67
expression. The aim was to identify BC diagnostic and prognostic
indicators and provide a more accurate reference and guidance for
the diagnosis, prognosis and individual treatment planning of
patients with BC.
Materials and methods
Research objects
The study included the clinical data of 170 patients
with an initial BC diagnosis at the Second Affiliated Hospital of
Shandong First Medical University (Ti'an, China) from January 2019
to January 2021. The patients all underwent surgical treatment and
were subsequently diagnosed with invasive BC by postoperative
pathological examination.
Patient inclusion criteria were: i) First radical
surgery for BC; ii) invasive non-specific BC, including invasive
ductal carcinoma, invasive lobular carcinoma, sclerosing carcinoma
and medullary carcinoma, confirmed by postoperative pathology; iii)
no neoadjuvant therapy before surgery; iv) measurement of blood
lipid levels within 1 week before surgery; v) no use of drugs
affecting blood lipid levels within half a year before surgery; vi)
no primary hyperlipidemia, diabetes, coronary heart disease,
thyroid disease, MS or other diseases affecting blood lipid levels;
v) complete clinical data.
The following patients were excluded from the study:
i) Those with other cancers; ii) those given BC-associated adjuvant
therapy before surgery; iii) those without original blood
biochemical test samples collected before surgery; iv) those with
hyperlipidemia, diabetes, coronary heart disease, MS or other
diseases affecting serum lipid levels; v) those who had taken
lipid-lowering drugs within half a year before surgery; vi) those
without complete clinical data.
Patient clinical information included age, body mass
index (BMI), menopausal condition (menopausal or non-menopausal),
preoperative serum lipid levels and pathological data. Serum lipid
levels and pathological examination results were extracted from
patients' laboratory test results. Patient pathological data
included ER, PR, HER2 and Ki-67 expression levels and pathological
tumor type.
This retrospective clinical investigation only
gathered existing clinical information; it did not intervene in the
therapeutic regimens of the patients and caused no risks to their
physiology. The researchers did their best to protect the
information provided by the patients and not to breach their
personal privacy. The requirement for ethics approval was waived
due to the retrospective nature of the study, and informed patient
consent was also waived by the Science Research Ethics Committee of
The Second Affiliated Hospital of Shandong First Medical University
(ref. no. 2022-088).
Serum lipid level determination
Fasting peripheral venous blood was collected from
all participants at hospital admission, and serum lipid levels were
determined using an automatic biochemical analyzer. Serum lipid
contents included total cholesterol (TC), low-density lipoprotein
cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C),
triglycerides (TG) and apolipoproteins A1 (ApoA1) and B (ApoB). The
normal ranges of these lipids were defined on the basis of the 2016
revised edition of the Chinese Adult Dyslipidemia Prevention and
Control Guidelines for stratifying circulating lipid concentrations
(27) and the reference ranges of
the serum lipid indicator tests used in the hospital's laboratory:
TC, 3.10-5.20 mmol/l; TG, 0.56-1.70 mmol/l; HDL-C, 1.29-1.55
mmol/l; LDL-C, 2.70-3.13 mmol/l; ApoA1, 1.0-1.60 g/l; and ApoB,
0.60-1.10 g/l. Dyslipidemia typically refers to elevated
circulating TC, TG and LDL-C concentrations and diminished
circulating HDL-C concentrations.
Immunohistochemical result
determination
Immunohisto-chemistry (IHC) was used to measure the
ER, PR, HER2 and Ki-67 levels in tumor tissues according to the
2021 Chinese Society of Clinical Oncology (CSCO) criteria for BC
diagnosis and treatment (27). The
positive ER and PR levels standards are ≥1% of tumor cells showing
nuclear staining; 20% PR positive cells is the standard threshold
used to define high and low PR expression. HER2 status is described
as follows: An IHC score of 3+ is considered HER2 positive; an IHC
score of 0 or 1+ is considered HER2 negative; an IHC score of 2+ is
considered uncertain and requires confirmation using fluorescence
in situ hybridization (FISH); a positive FISH outcome is
considered HER2 positive. Ki-67 positivity was defined as any
degree of brown staining infiltrating the cancer cell nuclei. The
expression of Ki-67 was calculated as the percentage of positively
stained cells, with >30% indicating high expression and <15%
indicating low expression. BCs were then stratified into five
subcategories according to their ER, PR, HER2 and Ki-67 expression
(Table I).
 | Table I.Subcategories of breast cancer. |
Table I.
Subcategories of breast cancer.
|
| Indicator
expression level |
|---|
|
|
|
|---|
| Subtype | HER2 | ER | PR | Ki-67 |
|---|
| HER2-positive | + | - | - | Any |
| (HR-negative) |
|
|
|
|
| HER2-positive | + | + | Any | Any |
| (HR-positive) |
|
|
|
|
| TNBC | - | - | - | Any |
| Luminal A | - | + | + | Low |
| Luminal B | - | + | - | High |
|
(HER2-negative) |
|
|
|
|
Other indicators
The height (m), weight (kg) and BMI of the patients
were routinely measured at admission. BMI (kg/m2) was
computed as the weight in kg divided by height squared in
m2. Menopause is generally described as the permanent
cessation of menstruation, indicating a persistent reduction in
estrogen synthesis by the ovaries. For the diagnosis of menopause,
it was necessary for a patient to meet at least one of the
following conditions: i) Bilateral oophorectomy; ii) aged ≥60
years; iii) aged <60 years with natural menopause for ≥12 months
without chemotherapy, tamoxifen, toremifen or ovarian castration
within the last year and serum follicle-stimulating hormone and
estradiol contents within the postmenopausal range; iv) aged <60
years, consuming tamoxifen or toremifene, and serum
follicle-stimulating hormone and estradiol levels within the
postmenopausal range for two consecutive measurements.
Statistical analysis
IBM SPSS Statistics Version 26 (IBM Corp.) was
employed for all data analyses. Data with a normal distribution are
presented as the mean ± standard deviation. Multi-group assessments
were conducted via one-way analysis of variance with Tukey's test
employed for post hoc analyses. Enumeration data are expressed as
the case number (n) and percentage (%) and were analyzed using the
Chi-square test. Linear correlation analyses were performed using
Pearson's correlation test. In all results, P<0.05 was
considered to indicate a statistically significant result.
Results
Clinical profile of each subgroup
None of the 170 patients with invasive BC had a
family history of genetic disease or a history of other cancers.
The age range of the patients was 30–83 years, with a median of 50
years and a mean of 50.71±9.96 years. The average BMI of the
patients was 25.43±3.28 kg/m2. A total of 92 patients
were premenopausal and 78 were postmenopausal. Age, BMI and
menopausal status did not show any significant differences among
the various subtypes (Tables II
and III). Based on the American
Joint Committee on Cancer TNM stages, 91 patients had stage I
cancer and 79 had stage II cancer. A Chi-square test indicated no
significant differences in BC subtype in the two stages (Table III).
 | Table II.Clinical profiles of patients in the
five breast cancer groups. |
Table II.
Clinical profiles of patients in the
five breast cancer groups.
| Subtype | Cases (n) | Age (mean ±
SD) | BMI
(kg/m2; mean ± SD) |
|---|
| HER2-positive | 18 | 53.44±8.77 | 24.63±3.12 |
| (HR-negative) |
|
|
|
| HER2-positive | 28 | 50.43±9.71 | 25.47±3.26 |
| (HR-positive) |
|
|
|
| TNBC | 22 | 53.00±11.31 | 25.77±3.66 |
| Luminal A | 37 | 49.62±9.46 | 25.91±3.43 |
| Luminal B | 65 | 49.91±10.21 | 25.23±3.15 |
|
(HER2-negative) |
|
|
|
| F-value |
| 0.848 | 0.582 |
| P-value |
| 0.497 | 0.676 |
 | Table III.TNM stages and menopausal status of
patients in the five breast cancer groups. |
Table III.
TNM stages and menopausal status of
patients in the five breast cancer groups.
|
|
| TNM stage [n
(%)] | Menopausal status
[n (%)] |
|---|
|
|
|
|
|
|---|
| Clinical
features | Cases (n) | I | II | Menopausal | Premenopausal |
|---|
| HER2-positive
(HR-negative) | 18 | 13 (72.2) | 5 (27.8) | 12 (66.7) | 6 (33.3) |
| HER2-positive
(HR-positive) | 28 | 22 (78.6) | 6 (21.4) | 14 (50.0) | 14 (50.0) |
| TNBC | 22 | 20 (90.9) | 2 (9.1) | 13 (59.1) | 9 (40.9) |
| Luminal A | 37 | 19 (51.4) | 18 (48.6) | 14 (37.8) | 23 (62.2) |
| Luminal B
(HER2-negative) | 65 | 17 (26.2) | 48 (73.8) | 25 (38.5) | 40 (61.5) |
|
χ2-value |
| 2.688 | 7.274 |
| P-value |
| 0.112 | 0.122 |
Serum lipid levels in each
subgroup
TG and ApoB levels were comparable among patients in
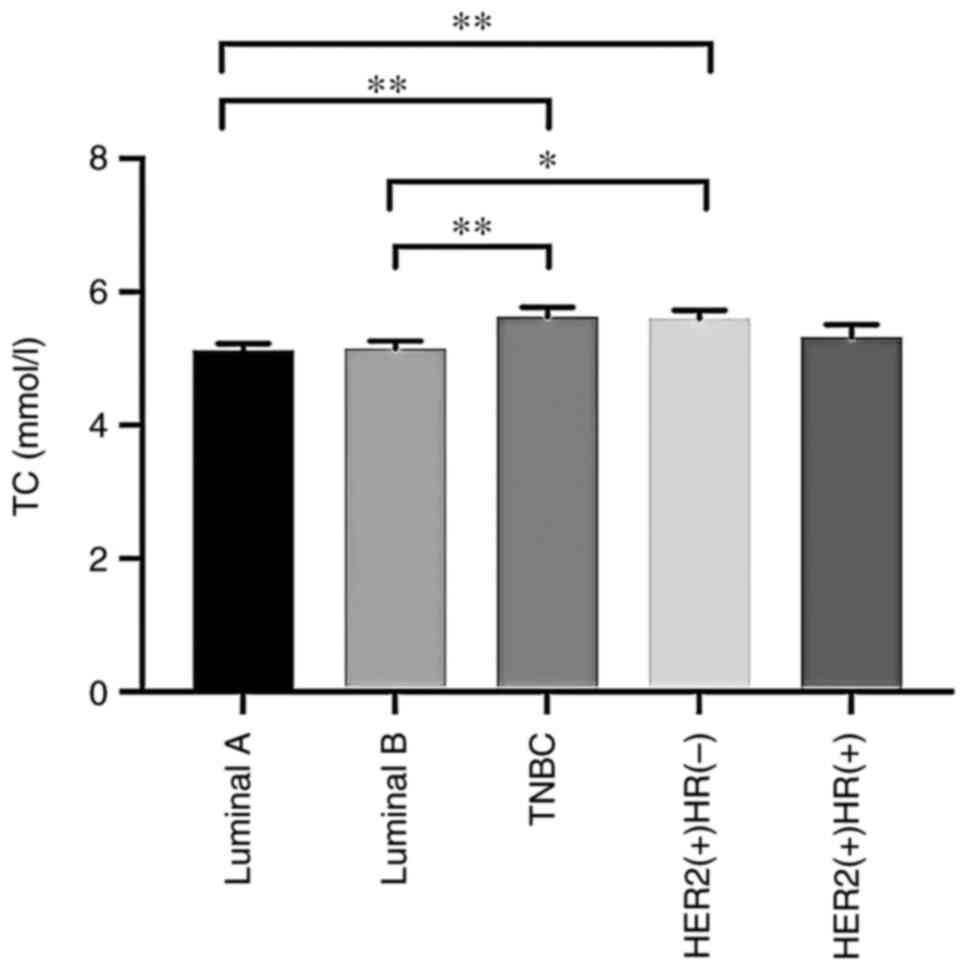
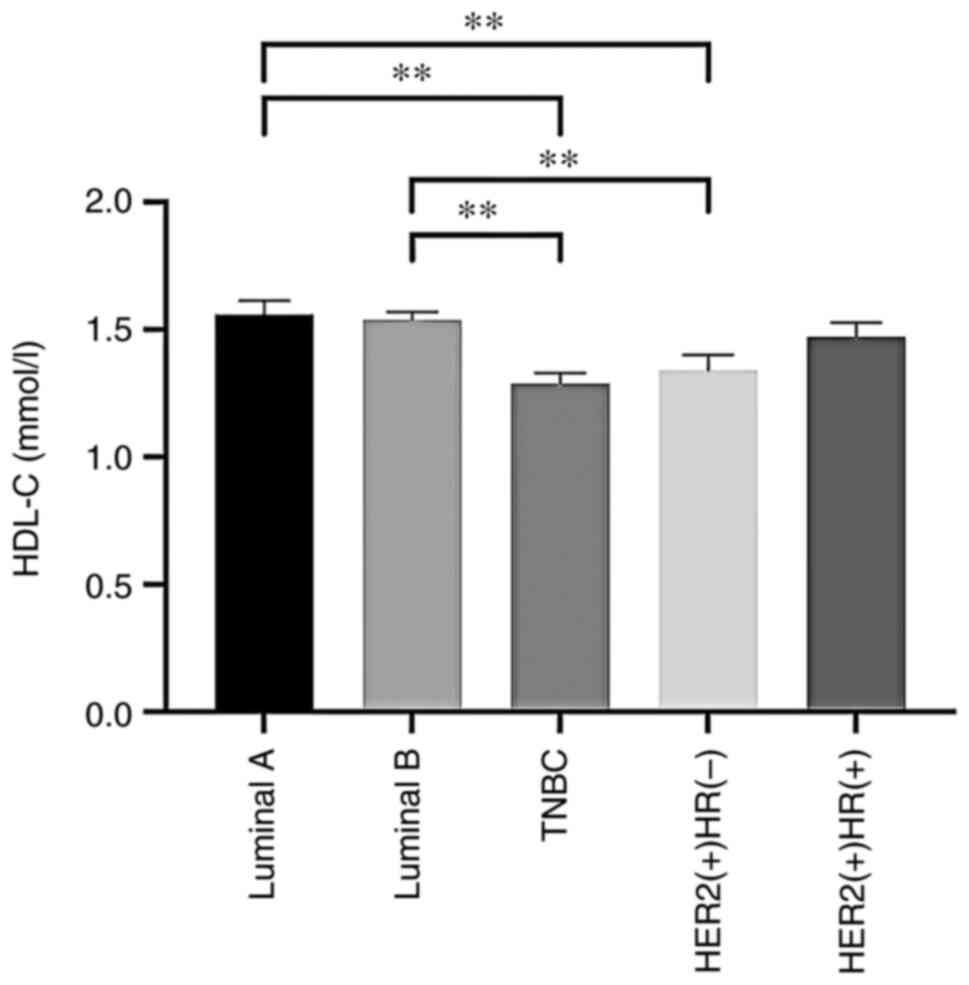
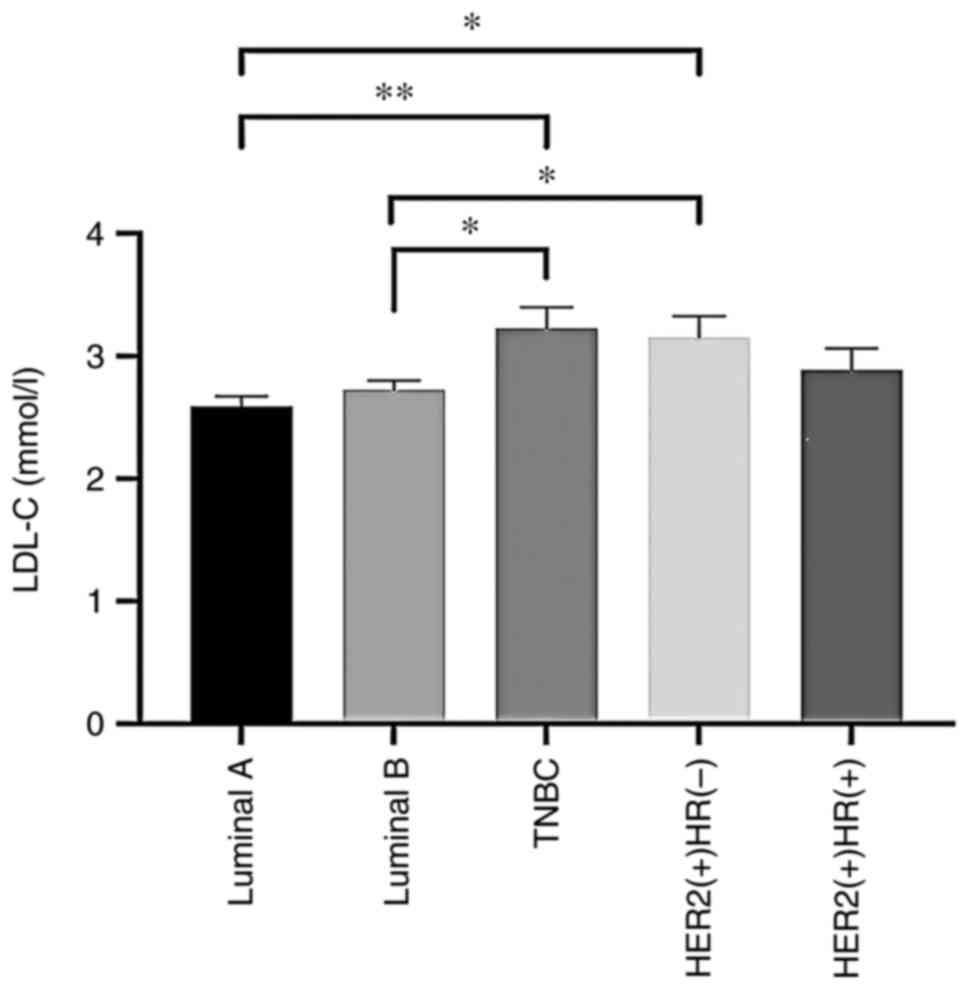
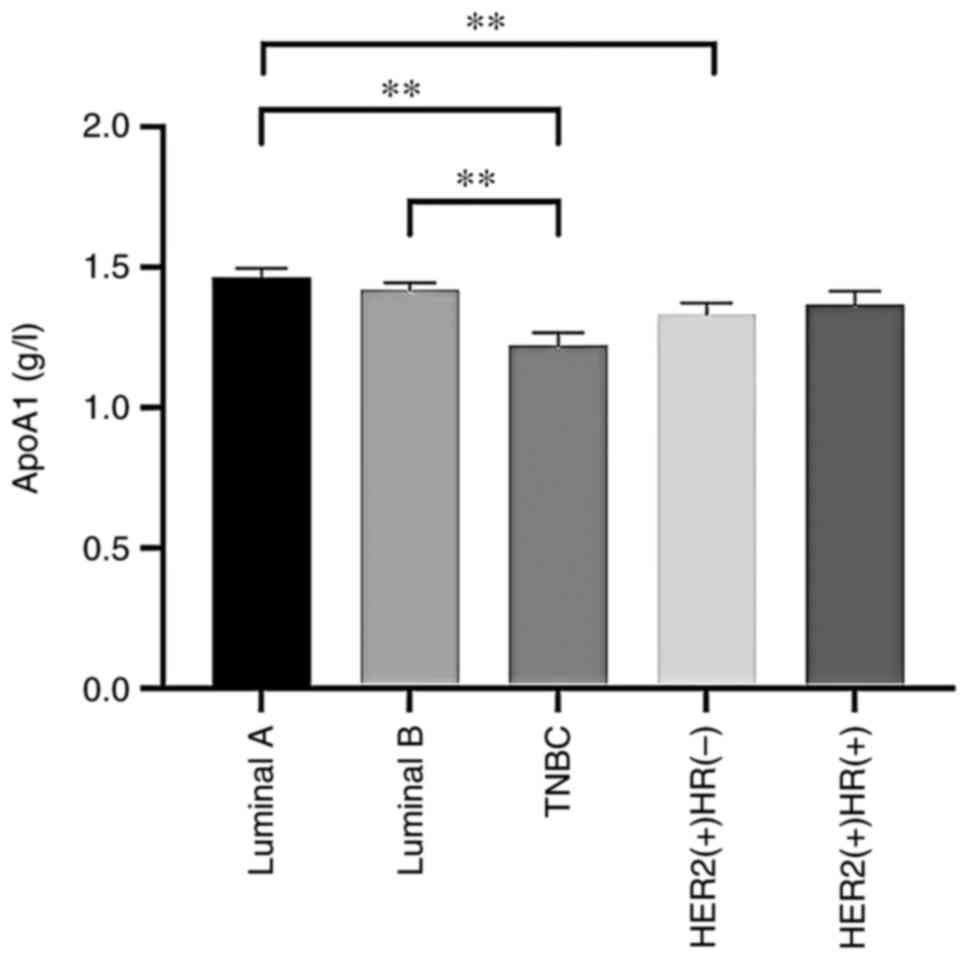
the different BC subtype cohorts (P>0.05; Table IV). However, TC, LDL-C, HDL-C and
ApoA1 levels differed significantly among patients in the different
BC subtype groups (P<0.05).
 | Table IV.Comparison of serum lipid levels of
patients in the five breast cancer groups. |
Table IV.
Comparison of serum lipid levels of
patients in the five breast cancer groups.
| Indicator | HER2-positive
(HR-negative) | HER2-positive
(HR-positive) | TNBC | Luminal A | Luminal B
(HER2-negative) | F-value | P-value |
|---|
| Cases (n) | 18 | 28 | 22 | 37 | 65 | - | - |
| TC (mmol/l) | 5.60±0.52 | 5.33±0.99 | 5.63±0.67 |
5.12±0.61a,b |
5.15±0.93a,b | 2.682 | 0.041 |
| TG (mmol/l) | 1.19±0.40 | 1.14±0.45 | 1.26±0.52 | 1.05±0.55 | 1.10±0.50 | 1.055 | 0.382 |
| HDL-C (mmol/l) | 1.34±0.26 | 1.47±0.29 | 1.29±0.20 |
1.56±0.32a,b |
1.54±0.26a,b | 5.443 | <0.001 |
| LDL-C (mmol/l) | 3.15±0.73 | 2.89±0.92 | 3.23±0.80 |
2.59±0.49a,b |
2.73±0.61a,b | 4.376 | <0.001 |
| ApoA1 (g/l) | 1.33±0.17 | 1.37±0.25 | 1.22±0.21 |
1.46±0.21a,b |
1.42±0.18a | 5.982 | <0.001 |
| ApoB (g/l) | 0.96±0.24 | 0.93±0.29 | 0.97±0.26 | 0.94±0.20 | 0.97±0.22 | 0.265 | 0.902 |
Serum TC and LDL-C levels were significantly
elevated in the TNBC and HER2-positive [hormone receptor
(HR)-negative] groups compared with the luminal A and B
(HER2-negative) groups. Serum HDL-C levels were significantly
reduced in TNBC and HER2-positive (HR-negative) cases compared with
luminal A and B (HER2-negative) cases. ApoA1 levels were
significantly diminished in patients in the TNBC and HER2-positive
(HR-negative) groups relative to those in the luminal A group.
Furthermore, ApoA1 levels in patients with TNBC were markedly
reduced compared with those in patients with luminal B
(HER2-negative) BC. Differences in TC, HDL-C, LDL-C and ApoA1
levels among patients with different subcategories of BC are
evident in Fig. 1, Fig. 2, Fig.
3, Fig. 4.
Correlations between serum lipid and
Ki-67 levels in BC patients
Among the 170 patients, 65 had high Ki-67 levels and
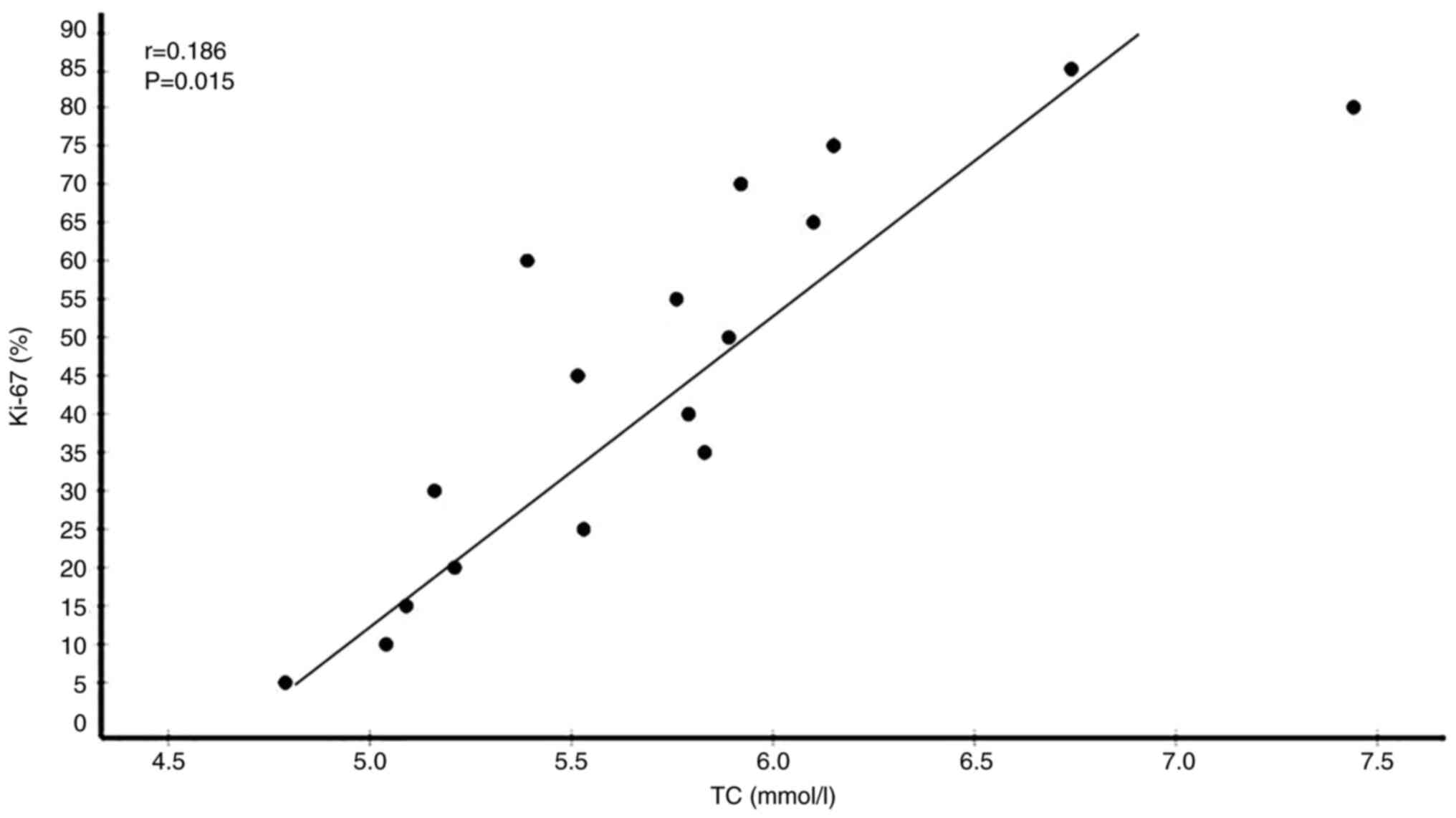
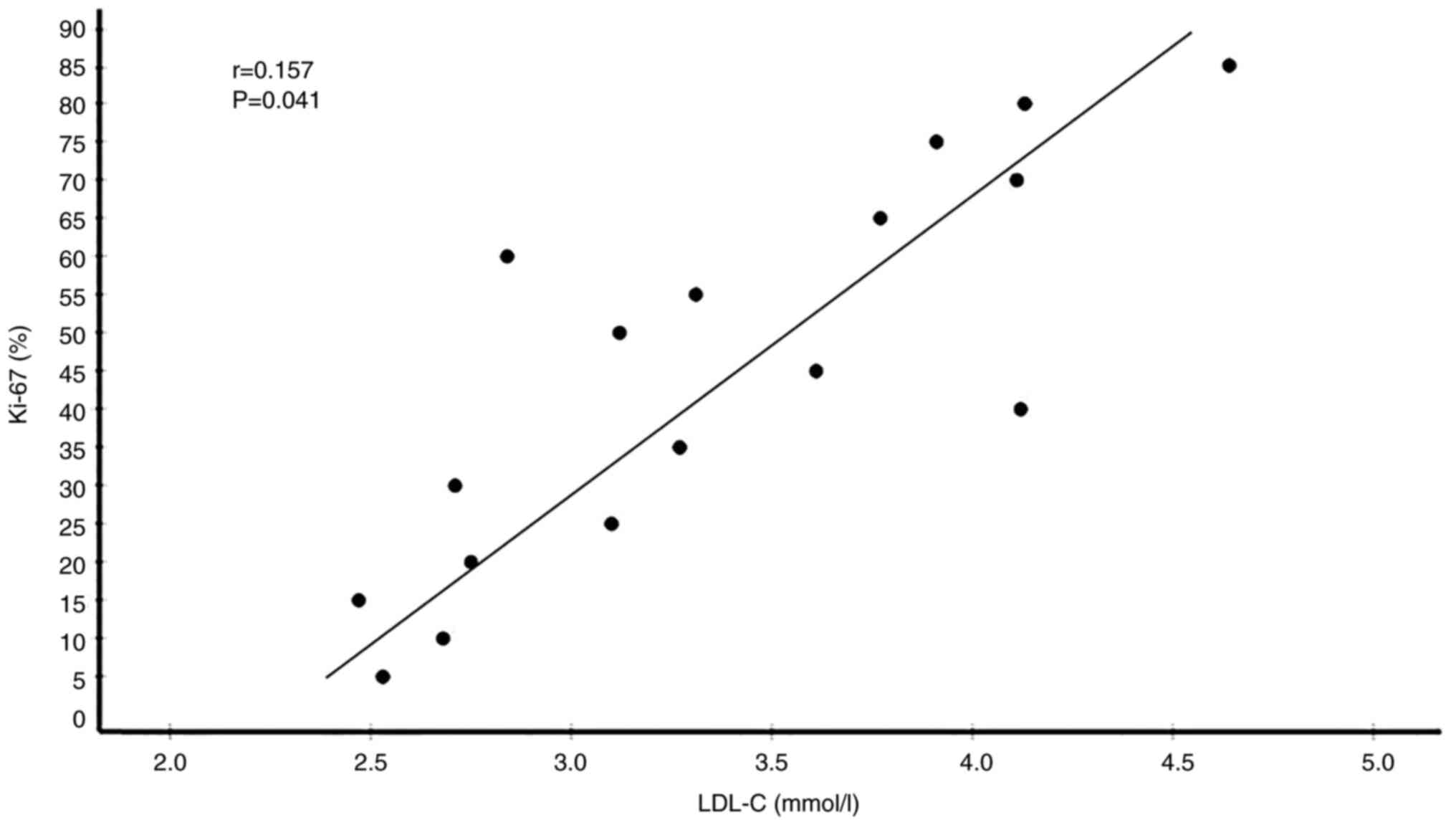
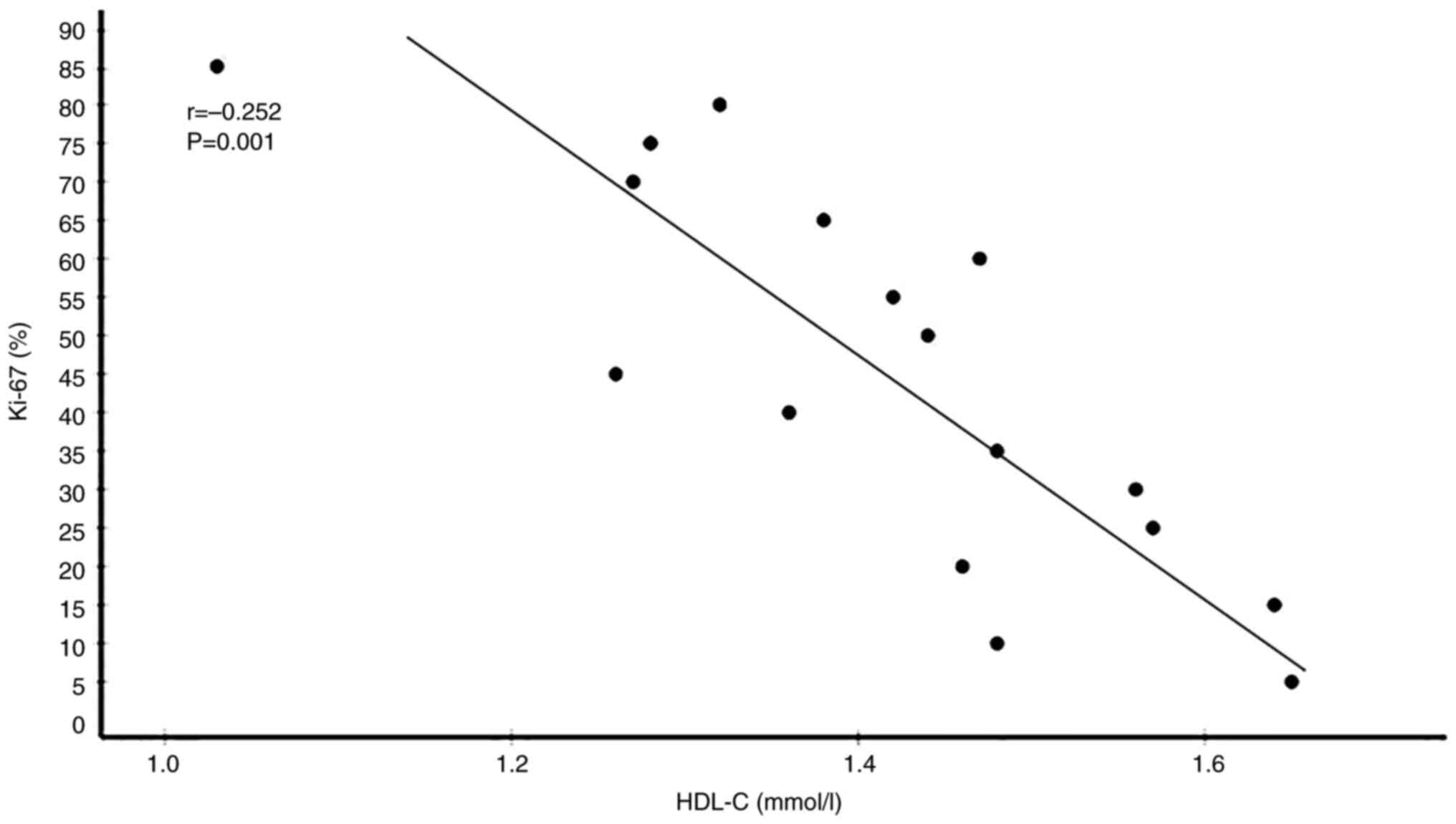
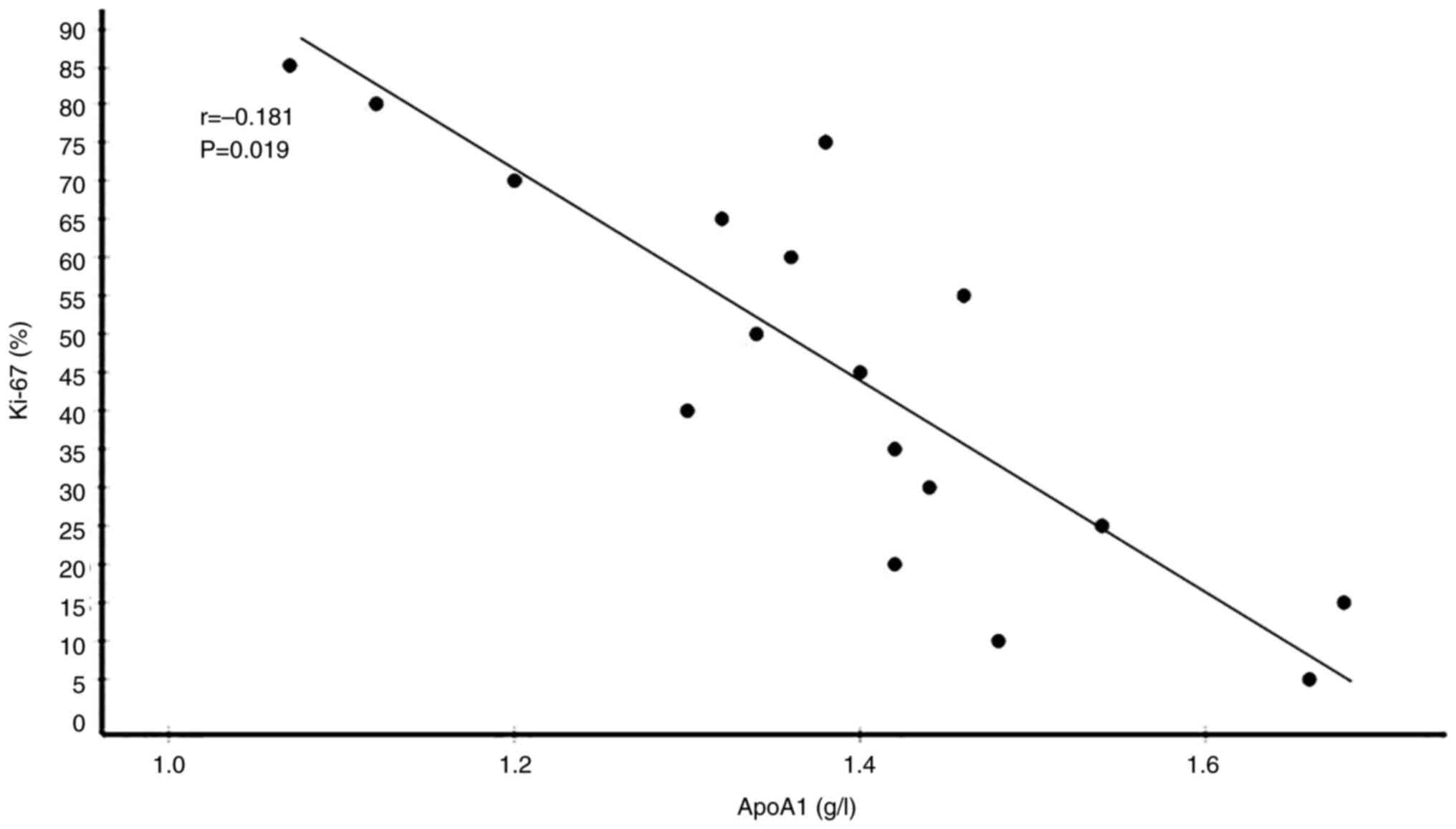
35 had low Ki-67 levels. Correlation analyses between serum lipid
and Ki-67 expression levels showed that circulating TC (r=0.186,
P=0.015) and LDL-C (r=0.157, P=0.041) were significantly positively
correlated with Ki-67 expression levels. By contrast, HDL-C
(r=−0.262, P=0.001) and ApoA1 (r=−0.181, P=0.019) were
significantly negatively correlated with Ki-67 expression levels.
However, ApoB (r=−0.038, P=0.625) and TG (r=−0.146, P=0.057) levels
were not significantly associated with Ki-67 expression levels
(Table V). Plots showing the
correlations of circulating TC, LDL-C, HDL-C and ApoA1 levels with
Ki-67 expression levels in patients with BC are presented in
Fig. 5, Fig. 6, Fig.
7, Fig. 8.
 | Table V.Correlations between serum lipid and
Ki-67 levels in patients with breast cancer. |
Table V.
Correlations between serum lipid and
Ki-67 levels in patients with breast cancer.
| Serum lipids | Levels (mean ±
SD) | r | P-value |
|---|
| TC (mmol/l) | 5.093±0.852 | 0.186 | 0.015a |
| TG (mmol/l) | 1.161±0.496 | −0.146 | 0.057 |
| HDL-C (mmol/l) | 1.482±0.265 | −0.262 | 0.001b |
| LDL-C (mmol/l) | 2.815±0.675 | 0.157 | 0.041a |
| ApoA1 (g/l) | 1.405±0.210 | −0.181 | 0.019a |
| ApoB (g/l) | 0.955±0.236 | −0.038 | 0.625 |
Discussion
Cancer is one of the foremost global public health
problems. Notable progress has been made in cancer diagnosis and
treatment in recent decades. Early cancer detection via the use of
predictive and diagnostic biomarkers is one of the most robust
methods for early-stage cancer identification and personalized
treatment. However, specific biomarkers with 100% diagnostic
accuracy are lacking due to tumor heterogeneity and genetic
instability induced by various carcinogenic factors (28,29).
Lipid metabolomics may provide an improved molecular definition of
tumors. The correlation between abnormal lipid metabolism and the
occurrence and development of malignant tumors is a current topic
of interest.
Lipids play numerous important roles in cell
survival, proliferation and apoptosis and contribute to chemical
energy storage, cell signal transduction, cell membrane and
cell-to-cell associations. These cellular activities are closely
associated with oncogenic pathways, particularly transformation,
progression and metastasis (28).
Studies have found aberrant levels of enzymes associated with lipid
production, storage, activation and destruction in BC, indicating
that the overexpression of these enzymes is closely associated with
BC tumor progression (30,31). Heterogeneity among different
molecular subcategories of BC is partly reflected by differences in
serum lipid metabolism. An analysis of raw metabolomic data from 92
patients with primary BC who received surgery at the National
Institute of Oncology in Milan, Italy, identified metabolic
differences between BC subcategories. The results revealed that the
magnitude of these metabolic changes was more pronounced in TNBC
and HER2-positive BCs than in luminal BCs. In particular, luminal B
subtype tumors showed a greater dependency on FA metabolism for
energy. By contrast, the HER2 overexpressing and TNBC subcategories
exhibited general alterations in glucose and glutamine metabolism
(30). In another study, Eiriksson
et al (32) showed that
while TNBC depends more on exogenous FA absorption and storage, the
luminal subtype upregulates FA production and oxidation while the
HER2-positive subtype depends on additional FA production and
enhanced FA storage and oxidation.
Heterogeneity among BC subcategories is also
associated with changes in the transcript and protein expression
levels of lipid metabolic enzymes (33,34)
and subtype-specific lipid profiles (35). The most widely studied during
carcinogenesis is FA synthetase (FASN), an essential enzyme for FA
biosynthesis. It is ubiquitous in various cancers, namely,
prostate, liver, ovarian, colon, endometrial and breast cancer, and
is associated with malignant transformation and poor outcomes
(33). A strong relationship has
been identified between FASN mRNA levels and HER2 overexpressing,
luminal A and luminal B subcategories, with IHC analyses of tumor
samples confirming that FASN protein levels were the highest in the
HER2 overexpressing subtype and most diminished in TNBC (34). FASN is often upregulated and
activated in HER2-positive BC and is critical for maintaining the
growth, proliferation and viability of HER2-positive BC cells
(36).
Acetyl-coenzyme A (CoA), critical for FA synthesis,
is generated from citrate and CoA via ATP-citrate lyase (ACLY)
catalysis. Upregulated ACLY levels and function have been observed
in numerous cancers, including BC, suggesting that ACLY is
important in cancer metabolism (37). ACLY mRNA has been shown to
be the most upregulated in HER2-overexpressing subcategories of BC
and downregulated in TNBC. Enzymes that release free FAs from TGs,
including monoglyceride lipase and fatty triglyceride lipase, have
also been found to be downregulated in TNBC. This finding indicates
that free FA mobilization predominates in luminal-type and
HER2-positive BCs (31). Kang
et al (35) examined 34
pairs of breast surgical tissue samples comprising BC and adjacent
normal tissue to distinguish cancerous and normal epithelial tissue
and classify different BC subcategories. Lipidomic analyses
revealed elevated phosphatidylcholine (PC) levels in BC, with
significantly higher PC levels in TNBC than in luminal and
HER2-positive subcategories. In addition, the study also found that
matrix-assisted laser desorption/ionization mass spectrometry lipid
profiles differed significantly between luminal, HER2-positive and
TNBC subcategories, which may have important prognostic
relevance.
A meta-analysis identified a strong link between
HDL-C levels and BC risk (38).
Other studies have reported that LDL-C levels are not associated BC
risk, whereas circulating LDL levels may predict BC progression
(39,40). However, few clinical investigations
have explored the association between circulating lipid
concentrations and different BC molecular subcategories. The
present study performed a retrospective analysis to investigate
differences in serum lipid levels between invasive BC molecular
subcategories. The results demonstrated that serum lipid levels
differed between BC subcategories. TC and LDL-C levels were
elevated while HDL-C levels were diminished in TNBC and
HER2-positive (HR-negative) BCs relative to the other types of BC.
In addition, ApoA1 levels were particularly high in patients with
luminal-type BC. However, the levels of TG and ApoB did not differ
among the BC subcategories.
Cholesterol is important in promoting cell
proliferation, migration and invasion, and so is essential in
cancer occurrence and development. During carcinogenesis, cancer
cells exhibit dysregulated cholesterol homeostasis and increased
cholesterol synthesis and uptake, which enables them to synthesize
cell membranes. These changes lead to the accumulation of
cholesterol in cancer cells, which increases tumor cell survival,
proliferation, metastasis and invasion, thereby promoting tumor
survival and development (41). At
present there is no clear evidence supporting an independent
dyslipidemic effect of cholesterol in BC pathogenesis. However,
clinical studies support a strong link between cholesterol and BC
(42–46): A prospective trial in South Korean
postmenopausal women assessed the relationship between total
circulating cholesterol and BC risk and found a positive
association in an age- and BMI-adjusted model (42). The first systematic review and
meta-analysis of prospective trials investigating the relationship
between cholesterol and BC by Touvier et al (38) showed a negative association between
prediagnostic TC levels and BC risk. A number of studies have
studied the association between cholesterol and BC types. For
example, one study observed that cholesterol biosynthesis was
significantly upregulated in TNBC tissues, and the high expression
of genes involved in cholesterol synthesis was associated with
short recurrence-free survival in patients with TNBC (43). In another study, Fagherazzi et
al (44) reported on 2,932
primary invasive BC cases after a 12-year follow-up in a
prospective trial of the French E3N cohort. The study showed that
women who used cholesterol-lowering drugs had a significantly lower
risk of luminal BC than women who did not use them. Furthermore, a
large French study collected 215 breast adipose tissue samples from
patients with invasive BC and identified increased breast fat
cholesterol levels in tissues obtained from patients with HER2
overexpressing and TNBC types. The authors concluded that increased
cholesterol levels in breast adipose tissue might increase the
aggressiveness of HER2-positive BC and TNBC (45). In the present study, patients with
TNBC had the highest serum TC levels, and the TC levels of the
patients with TNBC and HER2-positive (HR-negative) BC were
significantly higher compared with those of patients with luminal A
and B BC. Since HER2-positive BC and TNBC are more aggressive and
with worse outcome then luminal BC, abnormal TC levels might be
associated with enhanced aggressiveness and a worse outcome in
patients with BC.
HDL-C is a small, dense lipoprotein comprising
proteins and lipids that remove fat and cholesterol from cells and
return them to the liver for excretion or reuse, which is known as
reverse cholesterol transport. The levels of HDL-C are generally
considered to be inversely associated with cardiovascular risk, and
it has also been suggested that HDL-C function may be associated
with BC (40,46–48).
However, although most studies have found that circulating HDL-C
levels are inversely associated with BC risk, some studies have
reported opposing results, making this association unclear.
Notably, a meta-analysis of observational studies revealed an
inverse relationship between HDL-C levels and BC risk in
postmenopausal women (46). In
addition, Li et al (48)
retrospectively assessed the blood lipid data of 1,044 patients who
underwent BC surgery. They concluded that lower HDL-C levels were
significantly associated with a worse overall survival (OS).
Katzke et al (47) found a strong association between
HDL-C levels and BC risk. A large Mendelian randomization analysis
also suggested that elevated circulating HDL-C levels may be linked
to enhanced BC risk (40).
However, a Korean controlled study of 2,070 BC cases and controls
found a marked inverse association between HDL-C levels and BC
risk, with an increased risk of ER/PR negative BC compared with
ER/PR positive BC (49). Maiti
et al (50) investigated a
possible association between highly aggressive TNBC and MS,
collecting data on MS components and tumor profiles from 176
patients, including 86 with TNBC. A strong downregulation of HDL-C
levels was evident in the patients with TNBC. Another study
examined the association of MS and its components with TNBC and
non-TNBC by reviewing 1,391 patients, including 394 with TNBC and
855 with non-TNBC. The results showed that among the patients with
TNBC, those with lower HDL-C levels had worse 5-year
recurrence-free survival and OS rates, suggesting that patients
with TNBC and low serum HDL-C levels were at higher risk of death.
One possible mechanism is the inverse association between HDL-C and
angiotensin II, whose concentrations correlate positively with the
vascular endothelial growth factor signaling pathway in TNBC cells
(51). In the present study, serum
HDL-C levels were the lowest in patients with TNBC and
significantly lower in patients with TNBC and HER2-positive
(HR-negative) BC than in those with luminal A and B BC. Based on
the associations between HDL-C and molecular BC type reported in
most prior studies, it can be concluded that a significant inverse
relationship between HDL-C levels and TNBC exists. While elevated
HDL-C levels are not a prerequisite for TNBC development, they have
prognostic value in TNBC tumor recurrence and cancer-specific
mortality. Therefore, the monitoring and normalization of HDL
levels in TNBC subcategories may be particularly important.
LDL is mainly responsible for distributing
cholesterol to tissues and cells other than those in the liver. LDL
binds to the LDL receptor (LDLR) in most tissues. LDLR upregulation
often accompanies elevated serum LDL-C levels (52). In vitro analysis has shown
that cancer cells show the dysregulated expression of genes
associated with cholesterol regulation and metabolism, including
LDLR and HMG-CoA reductase (53). Indeed, numerous types of cancer
cells show elevated LDL-C uptake and LDLR levels (54). Antalis et al (55) revealed that ER-negative BC cells
contained more lipid droplets and had higher oleic acid uptake,
LDL-C uptake, cholesteryl ester to triacylglycerol ratios and
acyl-CoA-cholesterol acyltransferase 1 expression than ER-positive
BC cells. These data confirmed an association between lipid
accumulation and invasive behavior in ER-negative BC cell lines.
Therefore, LDL-C was suggested to be involved in the invasiveness
of ER-negative BC cells. In another study, dos Santos et al
(56) used well-established in
vitro and in vivo cholesterol enrichment models to
investigate the mechanisms by which LDL-C promotes BC growth and
invasiveness. The results revealed that LDL-C induced the
proliferation and migration of ER-negative cell lines from
different BC subcategories and stages; an association between high
LDL-C levels and HER2-positive BC was also identified.
The expression profile of the LDLR differs among BC
subcategories. The abundance of LDLR mRNA is reportedly
3–5-fold higher in MDA-MB-231 TNBC cells than in ER-positive MCF-7
cells. Moreover, LDL has been shown to accelerate MDA-MB-231 cell
proliferation but not MCF-7 cell proliferation. This difference may
be due to the capacity of TNBC cells to absorb, store and use
LDLR-mediated exogenous cholesterol. Elevated LDLR levels in TNBC
cells are associated with the invasive and metastatic properties of
these cells (57). Furthermore, in
a study using a mouse model of hyperlipidemia to establish the
significance of upregulated serum LDL-C and LDLR levels in tumor
cells in BC growth, LDLR expression was higher in TNBC and
HER2-positive cell lines than in ER-positive cells. Moreover, high
LDL-C levels increased the size of the tumors in the mice (52).
LDLR-related protein 1 (LRP1) is an LDLR family
member. A study of ductal BC found that LRP1 expression was
associated with aggressive TNBC and HER2-positive tumors but not
with HR-positive carcinomas, and that LRP1 upregulation was
associated with the proliferation and invasiveness of HER2 BC and
TNBC (58). Since LDLR
overexpression was often accompanied by increased serum LDL-C
levels in the aforementioned studies, this suggests an association
of abnormally elevated LDL-C levels with TNBC and HER2-positive
BCs, consistent with the present study; LDL-C levels were markedly
elevated in the patients in the TNBC and HER2-positive
subcategories compared with the luminal subcategories. However,
further studies are required to confirm whether there is an
association between high serum LDL-C levels and TNBC and
HER2-positive subcategories.
ApoA1 is the main protein component of HDL-C and has
an essential function in reverse cholesterol transport; it
retrieves cholesterol and phospholipids from peripheral tissues and
transports them to the liver for excretion. The role of ApoA1 in
cancer is a current topic of research interest. Reduced serum ApoA1
levels have been reported in patients with gallbladder, lung and
colon cancers (59–61). In addition, another study showed
that ApoA1-deficient mice rapidly developed tumors and were at a
significant survival disadvantage while ApoA1-expressing mice
exhibited a significant reduction in tumor progression and improved
survival in a dose-dependent manner (62).
Studies have reported mixed results concerning the
association between ApoA1 and BC. A study conducted by Borgquist
et al (60) showed that
elevated ApoA1 expression levels were associated with higher BC
incidence. A small case-control study also concluded that elevated
plasma ApoA1 levels were associated with a higher BC risk in
Chinese women (63). Conversely, a
large, nested case-control study of multiple plasma lipid marker
measurements prior to BC diagnosis showed that the BC risk was
reduced in patients with higher ApoA1 levels (11). Another study similarly detected a
negative association between ApoA1 levels and BC risk (64). Plasma ApoA1 binds to ATP-binding
cassette proteins to regulate ER and PR levels in BC in
vitro and in vivo by inducing the cell division control
protein 42 protein, which is essential for cancer metastasis, and
the PAK1 signaling pathway (65).
This indicates that ApoA1 contributes more to ER/PR-positive BC
than to ER/PR-negative BC.
A comparative study by Lin et al (66) found that plasma ApoA1 levels were
negatively associated with the incidence of invasive ductal
carcinoma and that most patients with ER/PR-positive invasive
ductal carcinoma had elevated plasma ApoA1 levels. By contrast, the
patients with TNBC more frequently had low ApoA1 levels. These
results suggest that tumors with high plasma ApoA1 levels are more
likely to be luminal-type BCs than other BC types. In the present
study, ApoA1 levels were markedly elevated in patients with luminal
subtype BC compared with other subcategories, suggesting that ApoA1
levels could be used as a predictive marker for prognosis and
hormone therapy sensitivity in HR-positive BC patients.
Ki-67 is a proliferation marker that is mainly used
to predict cancer prognosis and treatment response. The association
of Ki-67 with the prognosis of BC has been extensively studied
(67,68). Studies have shown that elevated
Ki-67 levels are linked with increased recurrence rates and poorer
survival in BC and have confirmed that Ki-67 is of independent
prognostic value (67,69). However, the current definition of
the critical value of Ki-67 is inconsistent and lacks validity. A
large meta-analysis that included 64,196 patients revealed that
Ki-67 was a stand-alone prognostic indicator for OS in BC patients
when a Ki-67 threshold of >25% was employed (70).
Various studies have investigated the function of
Ki-67 expression in different molecular BC subcategories. They have
shown a marked association between elevated Ki-67 levels and ER/PR
negativity and a positive correlation with HER2 positivity
(71,72). Soliman and Yussif (73) reported that upregulated Ki-67,
defined as >15% Ki-67-positive nuclei, was negatively associated
with ER and PR expression, and that 34% of HER2-positive BCs and
60% of TNBC cases had high Ki-67 expression. Hashmi et al
(74) found that Ki-67 positivity
in >90% of HER2-positive BCs and >14% of TNBCs. TNBC had the
highest Ki-67 index (50.9±23.7%), followed by the HER2-positive
type (42.6±21.6%). Another study confirmed that the Ki-67
expression levels in TNBC and HER2-positive subcategories of BC are
higher compared with those in luminal subcategories (75). As an independent BC prognostic
factor, Ki-67 indicates the increased invasiveness and recurrence
rate of the TNBC and HER2-positive subcategories, as well as a
reduced survival rate.
In the present study, circulating LDL-C and TC
levels correlated positively with Ki-67 expression levels, while
HDL-C and ApoA1 levels correlated negatively with Ki-67 expression
levels. These results suggest that abnormal circulating lipid
concentrations are associated with a poor outcome in patients with
BC. However, additional clinical studies are necessary to determine
whether they can be used as useful clinical indicators to predict
BC prognosis.
In summary, the present study demonstrated that
abnormal TC, LDL-C, HDL-C and ApoA1 levels are closely associated
with different molecular subcategories of BC and correlated with
Ki-67 expression levels. TC, LDL-C, HDL-C and ApoA1 abnormalities
were more common in TNBC and HER2-positive BC subcategories than in
luminal subcategories. The measurement of blood lipid levels in
patients with primary invasive BC is useful for the discovery of
diagnostic markers and therapeutic targets for BC, and provides new
information that may be helpful for the prognosis and specific
therapy of different molecular BC subcategories. It also enables
patients' prognosis and survival rates to be improved via the
adjustment of abnormal serum lipid levels. However, the present
investigation used a retrospective clinical observation design.
Consequently, the blood lipid levels of patients 2 and 6 months
after treatment were not collected for further analysis.
Furthermore, the relationship between lipid levels and survival
rate was not analyzed. Therefore, further studies are required to
explore these important issues.
The present study explored the association between
circulating lipid concentrations and BC patient prognosis. However,
certain fundamental factors affect lipogenesis. For example, a
study showed that the expression of oncogenic phosphatidylinositol
3-kinase (PI3K; H1047R) or KRAS (G12V) mutations in breast
epithelial cells promotes lipid synthesis via the convergent
activation of the mammalian target of rapamycin (mTOR) complex 1
downstream of these common oncogenes. These findings indicate that
PI3K/protein kinase B/mTOR pathway activation is critical for the
oncogenic network to produce additional lipids to accelerate
abnormal cancer cell growth and proliferation (76). A limitation of the present
retrospective study is that only the relationship between serum
lipid level differences among molecular BC subcategories and Ki-67
expression was explored; its mechanistic basis was not
investigated. In future clinical and experimental studies,
explorations of the correlations between PI3K or KRAS
genetic mutations and lipogenesis are planned.
In conclusion, the current study indicated that
elevated TC and LDL-C and diminished HDL-C and ApoA1 levels are
risk factors for TNBC and HER2-positive (HR-negative) BCs but not
luminal subtype BCs. It also revealed that circulating TC, LDL-C,
HDL-C and ApoA1 levels are correlated with Ki-67 expression, with
abnormal serum TC, LDL-C, HDL-C and ApoA1 levels being intricately
associated with worse BC patient outcomes. Based on these findings,
it is suggested that changes in serum lipid concentrations should
be closely monitored in BC patients, and TC, LDL-C, HDL-C and ApoA1
levels should be kept within the normal range.
Acknowledgements
Not applicable.
Funding
Funding was received from the National Natural Science
Foundation of China (grant no. 82074061), The National Key Research
and Development Program (grant no. 2017YFC1309204) and Beijing
Municipal Science and Technology Commission (grant no.
Z181100001718150).
Availability of data and materials
This datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
BQR designed the study. WWL and XBS conceived the
article, performed the literature search and data analysis, drafted
and critically revised the work, and confirm the authenticity of
the raw data. BQR reviewed and edited the manuscript. BW, ZPY, HZT,
SL, YYW and JXQ analyzed the data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Ethical approval was waived by The Science Research
Ethics Committee of The Second Affiliated Hospital of Shandong
First Medical University since researchers have open access to
relevant data for research purposes, the study used retrospective
clinical data and no ethical issues or other conflicts of interest
were encountered. A waiver of informed patient consent was also
obtained from the committee (ref. no. 2022-088).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gang H, Jingyi G, Shujiang R, Xiaobin L
and Fenli L: Advances in breast cancer treatment. J Front Med.
10:11–12. 2020.(In Chinese).
|
|
3
|
Long Y, Pan Z, Wenhua Z and Jingzhi G:
Progress in the key enzymes in tumor lipid metabolism. Cancer Res
Clin. 28:789–792. 2016.(In Chinese).
|
|
4
|
Röhrig F and Schulze A: The multifaceted
roles of fatty acid synthesis in cancer. Nat Rev Cancer.
16:732–749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maan M, Peters JM, Dutta M and Patterson
AD: Lipid metabolism and lipophagy in cancer. Biochem Biophys Res
Commun. 504:582–589. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bian X, Liu R, Meng Y, Xing D, Xu D and Lu
Z: Lipid metabolism and cancer. J Exp Med. 218:e202016062021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beloribi-Djefaflia S, Vasseur S and
Guillaumond F: Lipid metabolic reprogramming in cancer cells.
Oncogenesis. 5:e1892016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Halama A, Guerrouahen BS, Pasquier J,
Satheesh NJ, Suhre K and Rafii A: Nesting of colon and ovarian
cancer cells in the endothelial niche is associated with
alterations in glycan and lipid metabolism. Sci Rep. 7:399992017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kouba S, Ouldamer L, Garcia C, Fontaine D,
Chantome A, Vandier C, Goupille C and Potier-Cartereau M: Lipid
metabolism and calcium signaling in epithelial ovarian cancer. Cell
Calcium. 81:38–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sung H, Siegel RL, Torre LA,
Pearson-Stuttard J, Islami F, Fedewa SA, Goding Sauer A, Shuval K,
Gapstur SM, Jacobs EJ, et al: Global patterns in excess body weight
and the associated cancer burden. CA Cancer J Clin. 69:88–112.
2019.PubMed/NCBI
|
|
11
|
Lingquan K, Xin L, Hongyuan L, Guosheng R
and Kainan W: Progress on the study of breast cancer related
dyslipidemia. Chin J Endocrine Surg. 11:89–91. 962017.
|
|
12
|
Nelson ER: The significance of cholesterol
and its metabolite, 27-hydroxycholesterol in breast cancer. Mol
Cell Endocrinol. 466:73–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quiroga-Morales LA, Sat-Muñoz D,
Martínez-Herrera BE, Alcántara-Cadillo RR, Macías-López GG,
García-Cobián TA, Bañuelos-Rizo M and Balderas-Peña LMA: Obesity
and adipocytokines in breast cancer and benign breast disease. Rev
Med Inst Mex Seguro Soc. 56:246–254. 2018.(In Spanish). PubMed/NCBI
|
|
14
|
Qi L, Yanhui Z, Junzhe Y, Xian W, Xingmeng
W, Chaoran Y, Ruyu C and Xiaoan L: Research progress on the
relationship between serum lipid level and breast cancer treatment.
Chin J Surg Oncol. 11:168–171. 2019.(In Chinese).
|
|
15
|
Chen M, Zhao Y, Yang X, Zhao Y, Liu Q, Liu
Y, Hou Y, Sun H and Jin W: NSDHL promotes triple-negative breast
cancer metastasis through the TGFβ signaling pathway and
cholesterol biosynthesis. Breast Cancer Res Treat. 187:349–362.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bustreo S, Osella-Abate S, Cassoni P,
Donadio M, Airoldi M, Pedani F, Papotti M, Sapino A and Castellano
I: Optimal Ki67 cut-off for luminal breast cancer prognostic
evaluation: A large case series study with a long-term follow-up.
Breast Cancer Res Treat. 157:363–371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ; Panel members, : Strategies for
subtypes-dealing with the diversity of breast cancer: Highlights of
the St. Gallen international expert consensus on the primary
therapy of early breast cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin NU, Claus E, Sohl J, Razzak AR,
Arnaout A and Winer EP: Sites of distant recurrence and clinical
outcomes in patients with metastatic triple-negative breast cancer:
High incidence of central nervous system metastases. Cancer.
113:2638–2645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao MD, Lamichhane S, Lundgren S, Bofin A,
Fjøsne H, Giskeødegård GF and Bathen TF: Metabolic characterization
of triple negative breast cancer. BMC Cancer. 14:9412014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(review). Mol Med Rep. 11:1566–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan P, Xu B, Wang C, Zhang C, Sun M and
Yuan L: Ki-67 expression in luminal type breast cancer and its
association with the clinicopathology of the cancer. Oncol Lett.
11:2101–2105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Juríková M, Danihel Ľ, Polák Š and Varga
I: Ki67, PCNA, and MCM proteins: Markers of proliferation in the
diagnosis of breast cancer. Acta Histochem. 118:544–552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inwald EC, Klinkhammer-Schalke M,
Hofstädter F, Zeman F, Koller M, Gerstenhauer M and Ortmann O:
Ki-67 is a prognostic parameter in breast cancer patients: Results
of a large population-based cohort of a cancer registry. Breast
Cancer Res Treat. 139:539–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Denkert C, Budczies J, von Minckwitz G,
Wienert S, Loibl S and Klauschen F: Strategies for developing Ki67
as a useful biomarker in breast cancer. Breast. 24 (Suppl
2):S67–S72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baek AE and Nelson ER: The contribution of
cholesterol and its metabolites to the pathophysiology of breast
cancer. Horm Cancer. 7:219–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li JB and Jiang ZF: Chinese society of
clinical oncology breast cancer guideline version 2021: Updates and
interpretations. Zhonghua Yi Xue Za Zhi. 101:1835–1838. 2021.(In
Chinese). PubMed/NCBI
|
|
28
|
Perrotti F, Rosa C, Cicalini I, Sacchetta
P, Del Boccio P, Genovesi D and Pieragostino D: Advances in
lipidomics for cancer biomarkers discovery. Int J Mol Sci.
17:19922016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szász AM, Győrffy B and Marko-Varga G:
Cancer heterogeneity determined by functional proteomics. Semin
Cell Dev Biol. 64:132–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cappelletti V, Iorio E, Miodini P,
Silvestri M, Dugo M and Daidone MG: Metabolic footprints and
molecular subtypes in breast cancer. Dis Markers. 2017:76878512017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Monaco ME: Fatty acid metabolism in breast
cancer subtypes. Oncotarget. 8:29487–29500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eiriksson FF, Nøhr MK, Costa M,
Bödvarsdottir SK, Ögmundsdottir HM and Thorsteinsdottir M:
Lipidomic study of cell lines reveals differences between breast
cancer subtypes. PLoS One. 15:e2312892020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Furuta E, Okuda H, Kobayashi A and Watabe
K: Metabolic genes in cancer: Their roles in tumor progression and
clinical implications. Biochim Biophys Acta. 1805:141–152.
2010.PubMed/NCBI
|
|
34
|
Kim S, Lee Y and Koo JS: Differential
expression of lipid metabolism-related proteins in different breast
cancer subtypes. PLoS One. 10:e01194732015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang HS, Lee SC, Park YS, Jeon YE, Lee JH,
Jung SY, Park IH, Jang SH, Park HM, Yoo CW, et al: Protein and
lipid MALDI profiles classify breast cancers according to the
intrinsic subtype. BMC Cancer. 11:4652011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ligorio F, Pellegrini I, Castagnoli L,
Vingiani A, Lobefaro R, Zattarin E, Santamaria M, Pupa SM, Pruneri
G, de Braud F and Vernieri C: Targeting lipid metabolism is an
emerging strategy to enhance the efficacy of anti-HER2 therapies in
HER2-positive breast cancer. Cancer Lett. 511:77–87. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zaidi N, Swinnen JV and Smans K:
ATP-citrate lyase: A key player in cancer metabolism. Cancer Res.
72:3709–3714. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Touvier M, Fassier P, His M, Norat T, Chan
DS, Blacher J, Hercberg S, Galan P, Druesne-Pecollo N and
Latino-Martel P: Cholesterol and breast cancer risk: A systematic
review and meta-analysis of prospective studies. Br J Nutr.
114:347–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Orho-Melander M, Hindy G, Borgquist S,
Schulz CA, Manjer J, Melander O and Stocks T: Blood lipid genetic
scores, the HMGCR gene and cancer risk: A Mendelian randomization
study. Int J Epidemiol. 47:495–505. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Beeghly-Fadiel A, Khankari NK, Delahanty
RJ, Shu XO, Lu Y, Schmidt MK, Bolla MK, Michailidou K, Wang Q,
Dennis J, et al: A Mendelian randomization analysis of circulating
lipid traits and breast cancer risk. Int J Epidemiol. 49:1117–1131.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Meng Y, Wang Q and Lyu Z: Cholesterol
metabolism and tumor. Zhejiang Da Xue Xue Bao Yi Xue Ban. 50:23–31.
2021.PubMed/NCBI
|
|
42
|
Ha M, Sung J and Song YM: Serum total
cholesterol and the risk of breast cancer in postmenopausal Korean
women. Cancer Causes Control. 20:1055–1060. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ehmsen S, Pedersen MH, Wang G, Terp MG,
Arslanagic A, Hood BL, Conrads TP, Leth-Larsen R and Ditzel HJ:
Increased cholesterol biosynthesis is a key characteristic of
breast cancer stem cells influencing patient outcome. Cell Rep.
27:3927–3938.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fagherazzi G, Fabre A, Boutron-Ruault MC
and Clavel-Chapelon F: Serum cholesterol level, use of a
cholesterol-lowering drug, and breast cancer: Results from the
prospective E3N cohort. Eur J Cancer Prev. 19:120–125. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Goupille C, Ouldamer L, Pinault M,
Guimares C, Arbion F, Jourdan ML and Frank PG: Identification of a
positive association between mammary adipose cholesterol content
and indicators of breast cancer aggressiveness in a French
population. J Nutr. 151:1119–1127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ni H, Liu H and Rong G: Serum lipids and
breast cancer risk: A meta-analysis of prospective cohort studies.
PLoS One. 10:e01426692015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Katzke VA, Sookthai D, Johnson T, Kühn T
and Kaaks R: Blood lipids and lipoproteins in relation to incidence
and mortality risks for CVD and cancer in the prospective
EPIC-Heidelberg cohort. BMC Med. 15:2182017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li X, Tang H, Wang J and Xie X, Liu P,
Kong Y, Ye F, Shuang Z, Xie Z and Xie X: The effect of preoperative
serum triglycerides and high-density lipoprotein-cholesterol levels
on the prognosis of breast cancer. Breast. 32:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim Y, Park SK, Han W, Kim DH, Hong YC, Ha
EH, Ahn SH, Noh DY, Kang D and Yoo KY: Serum high-density
lipoprotein cholesterol and breast cancer risk by menopausal
status, body mass index, and hormonal receptor in Korea. Cancer
Epidemiol Biomarkers Prev. 18:508–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Maiti B, Kundranda MN, Spiro TP and Daw
HA: The association of metabolic syndrome with triple-negative
breast cancer. Breast Cancer Res Treat. 121:479–483. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fan Y, Ding X, Wang J, Ma F, Yuan P, Li Q,
Zhang P and Xu B: Decreased serum HDL at initial diagnosis
correlates with worse outcomes for triple-negative breast cancer
but not non-TNBCs. Int J Biol Markers. 30:e200–e207. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gallagher EJ, Zelenko Z, Neel BA, Antoniou
IM, Rajan L, Kase N and LeRoith D: Elevated tumor LDLR expression
accelerates LDL cholesterol-mediated breast cancer growth in mouse
models of hyperlipidemia. Oncogene. 36:6462–6471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Scheinman EJ, Rostoker R and Leroith D:
Cholesterol affects gene expression of the Jun family in colon
carcinoma cells using different signaling pathways. Mol Cell
Endocrinol. 374:101–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hoque M, Rentero C, Conway JR, Murray RZ,
Timpson P, Enrich C and Grewal T: The cross-talk of LDL-cholesterol
with cell motility: Insights from the Niemann pick type C1 mutation
and altered integrin trafficking. Cell Adh Migr. 9:384–391. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Antalis CJ, Uchida A, Buhman KK and
Siddiqui RA: Migration of MDA-MB-231 breast cancer cells depends on
the availability of exogenous lipids and cholesterol
esterification. Clin Exp Metastasis. 28:733–741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
dos Santos CR, Domingues G, Matias I,
Matos J, Fonseca I, de Almeida JM and Dias S: LDL-cholesterol
signaling induces breast cancer proliferation and invasion. Lipids
Health Dis. 13:162014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ye J, Xia X, Dong W, Hao H, Meng L, Yang
Y, Wang R, Lyu Y and Liu Y: Cellular uptake mechanism and
comparative evaluation of antineoplastic effects of
paclitaxel-cholesterol lipid emulsion on triple-negative and
non-triple-negative breast cancer cell lines. Int J Nanomedicine.
11:4125–4140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Catasus L, Gallardo A, Llorente-Cortes V,
Escuin D, Muñoz J, Tibau A, Peiro G, Barnadas A and Lerma E:
Low-density lipoprotein receptor-related protein 1 is associated
with proliferation and invasiveness in Her-2/neu and
triple-negative breast carcinomas. Hum Pathol. 42:1581–1588. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
van Duijnhoven FJ, Bueno-De-Mesquita HB,
Calligaro M, Jenab M, Pischon T, Jansen EH, Frohlich J, Ayyobi A,
Overvad K, Toft-Petersen AP, et al: Blood lipid and lipoprotein
concentrations and colorectal cancer risk in the European
prospective investigation into cancer and nutrition. Gut.
60:1094–1102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Borgquist S, Butt T, Almgren P, Shiffman
D, Stocks T, Orho-Melander M, Manjer J and Melander O:
Apolipoproteins, lipids and risk of cancer. Int J Cancer.
138:2648–2656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zuo M, Rashid A, Wang Y, Jain A, Li D,
Behari A, Kapoor VK, Koay EJ, Chang P, Vauthey JN, et al: RNA
sequencing-based analysis of gallbladder cancer reveals the
importance of the liver X receptor and lipid metabolism in
gallbladder cancer. Oncotarget. 7:35302–35312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zamanian-Daryoush M, Lindner D, Tallant
TC, Wang Z, Buffa J, Klipfell E, Parker Y, Hatala D,
Parsons-Wingerter P, Rayman P, et al: The cardioprotective protein
apolipoprotein A1 promotes potent anti-tumorigenic effects. J Biol
Chem. 288:21237–21252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Han C, Zhang HT, Du L, Liu X, Jing J, Zhao
X, Yang X and Tian B: Serum levels of leptin, insulin, and lipids
in relation to breast cancer in China. Endocrine. 26:19–24. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chang SJ, Hou MF, Tsai SM, Wu SH, Hou LA,
Ma H, Shann TY, Wu SH and Tsai LY: The association between lipid
profiles and breast cancer among Taiwanese women. Clin Chem Lab
Med. 45:1219–1223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Holm C, Rayala S, Jirström K, Stål O,
Kumar R and Landberg G: Association between Pak1 expression and
subcellular localization and tamoxifen resistance in breast cancer
patients. J Natl Cancer Inst. 98:671–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lin X, Hong S, Huang J, Chen Y, Chen Y and
Wu Z: Plasma apolipoprotein A1 levels at diagnosis are independent
prognostic factors in invasive ductal breast cancer. Discov Med.
23:247–258. 2017.PubMed/NCBI
|
|
67
|
Luporsi E, André F, Spyratos F, Martin PM,
Jacquemier J, Penault-Llorca F, Tubiana-Mathieu N, Sigal-Zafrani B,
Arnould L, Gompel A, et al: Ki-67: Level of evidence and
methodological considerations for its role in the clinical
management of breast cancer: Analytical and critical review. Breast
Cancer Res Treat. 132:895–915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Haroon S, Hashmi AA, Khurshid A,
Kanpurwala MA, Mujtuba S, Malik B and Faridi N: Ki67 index in
breast cancer: Correlation with other prognostic markers and
potential in pakistani patients. Asian Pac J Cancer Prev.
14:4353–4358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
de Azambuja E, Cardoso F, de Castro G Jr,
Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D,
Piccart-Gebhart MJ and Paesmans M: Ki-67 as prognostic marker in
early breast cancer: A meta-analysis of published studies involving
12,155 patients. Br J Cancer. 96:1504–1513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Petrelli F, Viale G, Cabiddu M and Barni
S: Prognostic value of different cut-off levels of Ki-67 in breast
cancer: A systematic review and meta-analysis of 64,196 patients.
Breast Cancer Res Treat. 153:477–491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Alco G, Bozdogan A, Selamoglu D, Pilanci
KN, Tuzlali S, Ordu C, Igdem S, Okkan S, Dincer M, Demir G and
Ozmen V: Clinical and histopathological factors associated with
Ki-67 expression in breast cancer patients. Oncol Lett.
9:1046–1054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kanyilmaz G, Yavuz BB, Aktan M, Karaağaç
M, Uyar M and Fındık S: Prognostic importance of Ki-67 in breast
cancer and its relationship with other prognostic factors. Eur J
Breast Health. 15:256–261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Soliman NA and Yussif SM: Ki-67 as a
prognostic marker according to breast cancer molecular subtype.
Cancer Biol Med. 13:496–504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hashmi AA, Hashmi KA, Irfan M, Khan SM,
Edhi MM, Ali JP, Hashmi SK, Asif H, Faridi N and Khan A: Ki67 index
in intrinsic breast cancer subtypes and its association with
prognostic parameters. BMC Res Notes. 12:6052019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mitrović O, Čokić V, Đikić D, Budeč M,
Vignjević S, Subotički T, Gulan M, Radović S and Furtula S:
Correlation between ER, PR, HER-2, Bcl-2, p53, proliferative and
apoptotic indexes with HER-2 gene amplification and TOP2A gene
amplification and deletion in four molecular subtypes of breast
cancer. Target Oncol. 9:367–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ricoult SJH, Yecies JL, Ben-Sahra I and
Manning BD: Oncogenic PI3K and K-Ras stimulate de novo lipid
synthesis through mTORC1 and SREBP. Oncogene. 35:1250–1260. 2016.
View Article : Google Scholar : PubMed/NCBI
|