Introduction
Among females, breast cancer is the most common
malignancy worldwide and it is also the leading cause of
cancer-associated death (1).
Statistical results indicate that human epidermal growth factor
receptor 2 (HER2)-positive breast cancer accounts for 15–20%
(2) and this subtype of breast
cancer accounts for 22.6% in China (3). HER2-positive breast cancer is
comparatively more aggressive and has a poor prognosis (4). Although the use of anti-HER2 therapy
has significantly improved the prognosis of HER2-positive advanced
breast cancer, when drug resistance occurs, the treatment effect
will be poor and the tumor will progress (5,6).
Therefore, it is required to adopt new treatments to overcome drug
resistance.
Tumor angiogenesis is closely related to tumor
growth, invasion and metastasis. Therefore, anti-angiogenesis may
be one of the effective methods to treat cancer (7–9).
Bevacizumab, an anti-angiogenic monoclonal antibody, has
demonstrated anti-tumor activity in advanced breast cancer
(10–13). In addition, anti-angiogenic drugs
have been applied in the treatment of advanced breast cancer
(14–16).
Apatinib is a highly selective tyrosine kinase
inhibitor (TKI) that targets vascular endothelial growth factor
receptor-2 (VEGFR2). Apatinib was first used for the treatment of
advanced gastric cancer (17). Two
phase II clinical studies indicated that apatinib monotherapy had
good efficacy in the treatment of pretreated advanced breast cancer
(18,19). An observational study suggested
that apatinib combined with chemotherapy has potential efficacy in
the treatment of previously treated advanced breast cancer
(20). However, studies on the use
of apatinib for the treatment of HER2-positive advanced breast
cancer with brain metastasis are scarce. In the present study,
apatinib combined with trastuzumab and albumin-bound paclitaxel
were used to treat a patient with refractory HER2-positive breast
cancer with brain metastasis, and good efficacy was achieved. This
case is reported below.
Case report
In April 2014, a 42-year-old Chinese female
underwent radical surgery for left breast cancer at an external
hospital and the axillary lymph nodes on the same side were
removed. Pathological inspection revealed invasive non-specific
breast carcinoma (Grade III). The size of the tumor was 1.0×1.0 cm,
19 lymph node metastases were detected in 23 left axillary lymph
nodes and the postoperative stage was pT1N3M0, stage IIIC. The
immunohistochemistry results were as follows: Estrogen receptor
(ER), 85%+; progesterone receptor (PR), 15%+; HER-2, 2+; Ki-67,
45%+; fluorescence in situ hybridization (FISH) detection,
HER-2 gene amplification (+). The patient received six cycles of
adjuvant chemotherapy with paclitaxel combined with lobaplatin,
followed by one-month endocrine therapy with tamoxifen. The patient
refused to receive adjuvant radiotherapy and anti-HER2 targeted
therapy.
In March 2021, multiple metastases in the bone and
lymph node were detected by computed tomography (CT) and brain
magnetic resonance imaging (MRI) revealed multiple brain
metastases. The patient underwent left axillary lymph node biopsy
and the pathological diagnosis was metastasis of breast cancer with
the following immunohistochemical results: ER, 90%+; PR, 15%+;
HER-2, 2+; Ki67, 60%+; and FISH detection, HER-2 gene amplification
(+). The patient then received capecitabine plus pyrotinib for 8
months as the first-line treatment. The best tumor response of
intracranial and extracranial lesions was stable disease based on
CT and MRI examination. In November 2021, reexamination by brain
MRI indicated that the left parieto occipital lobe metastatic tumor
was enlarged and the evaluation indicated progressive disease. The
patient received second-line treatment with pyrotinib plus
trastuzumab (Herceptin®) and capecitabine. One month
later, the brain metastases progressed again. Table I provides the medical timeline of
the patient's treatment prior to presenting at our hospital.
 | Table I.Summary of the timeline of the
patient's past medical history. |
Table I.
Summary of the timeline of the
patient's past medical history.
| Treatment time | Therapy | Response
evaluation | DFS or PFS |
|---|
| April 2014-October
2014 | Radical surgery and
adjuvant therapy (TP for 6 cycles, tamoxifen for 1 month) | - | 83 months |
| March 2021-November
2021 | Capecitabine +
pyrotinib | SD | 8 months |
| November
2021-December 2021 | Herceptin +
capecitabine + pyrotinib | PD | 1 month |
In December 2021, the patient was admitted to our
hospital, the National Cancer Center/National Clinical Research
Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese
Academy of Medical Sciences and Peking Union Medical College
(Shenzhen, China), for the first time, after having received
second-line palliative chemotherapy at other hospitals. The patient
complained of headache and vomiting. CT of the whole body revealed
multiple lymph node and bone metastases, as well as suspected lung
metastasis. Brain MRI indicated two huge brain metastases (one in
the right temporooccipital parietal lobe and the other in the left
occipital parietal lobe), involving adjacent meninges, accompanied
by obvious edema; the midline structure shifted to the left and the
right lateral ventricle and the third ventricle moved to the left
under pressure.
As the brain metastases of this patient had
progressed significantly after using small molecule anti-HER2 TKI
drugs, the brain metastases were now huge and accompanied by
obvious edema. After multi-disciplinary discussion, consideration
was given to the following points: i) The radiotherapy department
had no indication for radiotherapy, so it was recommended to
perform a neurosurgery consultation to consider palliative surgery;
ii) the neurosurgery department indicated that it may be able to
perform palliative surgery, but the risk is relatively high and the
patient's condition should be fully informed; iii) after careful
consideration, the patient and the patient's family refused
surgical treatment of the patient. Finally, the combined treatment
plan of apatinib [250 mg per os (po) once per day (qd)] plus
trastuzumab (Zercepac®) [6 mg/kg, intravenous drip, day
1 of every 3 weeks] and albumin-bound paclitaxel (130
mg/m2, intravenous drip, days 1 and 8 of every 3 weeks)
was formulated and the patient began to receive the third-line
treatment from mid-December 2021. The patient also received
conventional intervention with mannitol and dexamethasone for
dehydration. After one cycle of combined treatment, the patient
complained that the severity of the headache and vomiting had
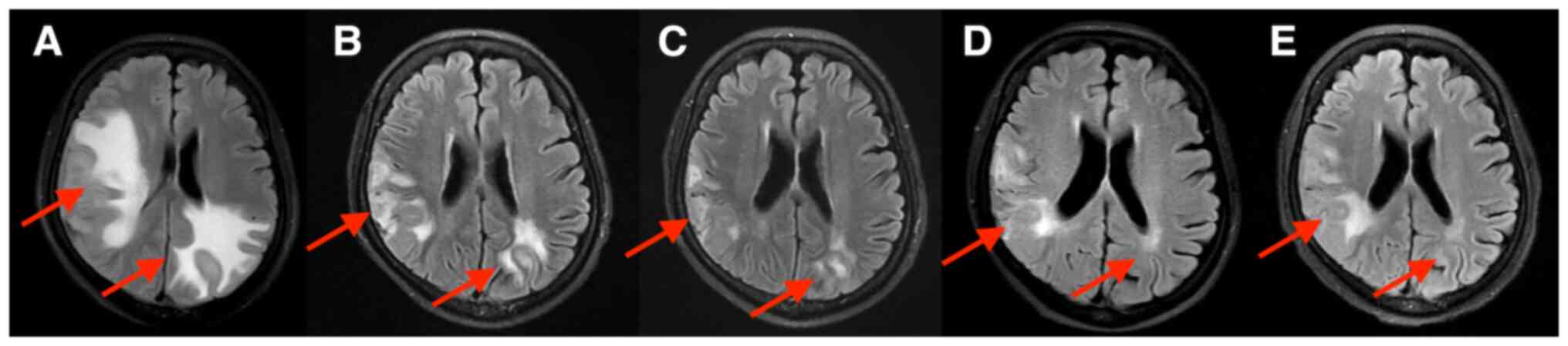
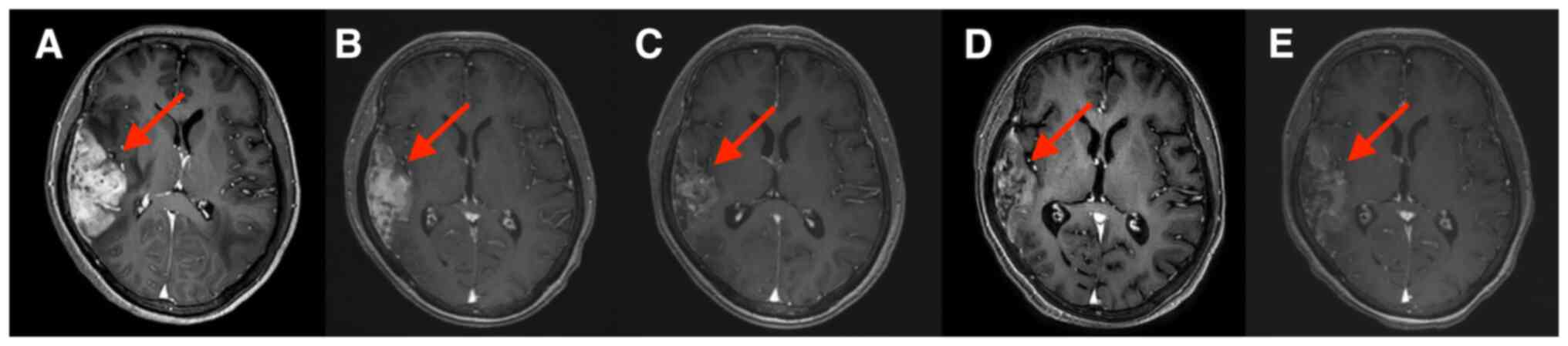
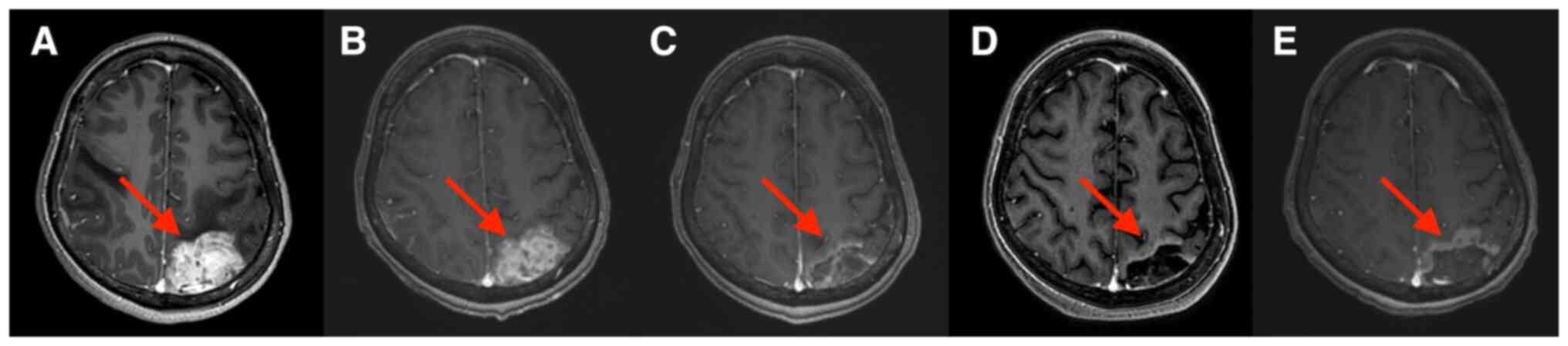
decreased. Brain MRIs are provided in Fig. 1, Fig.
2, Fig. 3. Brain MRI
examination after 2 cycles of combination treatment indicated that
peritumoral brain edema was significantly reduced (Fig. 1B) and two brain metastases were
significantly reduced (Figs. 2B
and 3B). After 4, 6 and 8 cycles
of combination treatment, further reductions of peritumoral brain
edema were found (Fig. 1C-E). The
right temporooccipital parietal lobe metastasis (Fig. 2C-E) and the left occipital parietal
lobe metastasis were further reduced after 4, 6 and 8 cycles of
combination treatment (Fig. 3C-E),
and the efficacy was rated as partial response. The patient
tolerated the treatment well and no grade 3 or 4 adverse events
were observed during the whole treatment period. In September 2022,
brain MRI indicated progress in brain metastases and systemic CT
suggested that extracranial metastases remained stable. The
progression-free survival was 9 months for the third-line
treatment.
When the brain metastases of this patient progressed
again, neurosurgery and radiotherapy experts were invited for
consultation again, but the patient and the patient's family still
refused the surgery and did not consider radiotherapy for the time
being. Therefore, the medical anti-tumor treatment plan was
adjusted as follows: Apatinib (250 mg po qd) plus inetetamab
(Cipterbin®, anti-HER2 recombinant human monoclonal
antibody; 6 mg/kg, intravenous drip, day 1 of every 3 weeks, with 8
mg/kg for the first cycle) and oral vinorelbine (80
mg/m2, po, days 1 and 8 of every 3 weeks, with 60
mg/m2 for the first cycle). To date, three cycles of the
aforementioned treatment with a combination of apatinib, inetetamab
and oral vinorelbine have been performed and a comprehensive review
and evaluation will be performed before the next cycle. At present,
the patient has no obvious headache, vomiting or other
complaints/symptoms.
Discussion
At present, the treatment options for brain
metastasis of breast cancer include local treatment, systematic
treatment or combination of multiple treatments. Local treatment
includes surgery, stereotactic radiosurgery (SRS) and whole-brain
radiation therapy (WBRT). Systemic therapy includes chemotherapy
and targeted therapy. Certain studies have indicated that surgery
may improve the overall survival (OS) of patients with brain
metastases from breast cancer, but it is usually only applicable to
patients with obvious symptoms, good general condition and limited
brain metastasis lesions (21,22).
Most patients with brain metastases from breast cancer receive
radiotherapy (23). Patients with
good performance and localized brain metastases receive SRS, while
patients with poor performance or extensive brain metastases
usually receive WBRT (23). One
study indicated that WBRT combined with SRS had no benefit
regarding OS, while increasing the risk of neurocognitive toxicity
compared with SRS alone (24).
Therefore, the latest consensus guidelines recommend complete
resection or SRS for limited brain metastases, and adjuvant WBRT
should not be conducted subsequently (25). In terms of systematic treatment,
the application of chemotherapy in brain metastasis from breast
cancer is limited. However, due to the use of anti-HER2 therapy in
HER2-positive subtypes, systematic therapy has achieved good
results. The exploratory study on central nervous system metastasis
in the EMILIA phase III study shows that T-DM1 may prolong the OS
of patients with brain metastasis of breast cancer (26). The subgroup analysis of the
DESTINY-Breast 01 study on brain metastasis of breast cancer
indicated that trastuzumab deruxtecan (T-DXd, DS8201) has achieved
encouraging therapeutic benefits for patients with brain metastasis
of breast cancer (27).
Small-molecule anti-HER2 TKI drugs, such as
lapatinib, neratinib, tucatinib and pyrotinib, may also be used for
the systematic treatment of brain metastases in patients with
HER2-positive breast cancer. However, the treatment of
HER2-targeted drug-resistant patients is an important clinical
challenge. The present study reported a case in which the
combination of apatinib with trastuzumab and albumin-bound
paclitaxel effectively controlled refractory brain metastases after
the failure of pyrotinib therapy. The reduction of the brain
metastases from breast cancer and alleviation of the brain edema
lasted 9 months.
Angiogenesis is closely related to HER2 signal
transduction at the molecular level (28). A previous study indicated that
VEGFR2-positive stromal vessel counts were higher in HER2-positive
breast cancer compared with other subtypes. These data indicate
that the influence of HER2 on tumorigenesis may be partially
mediated by the stimulation of angiogenesis, which provides a
strong theoretical basis for the use of anti-angiogenic drugs in
HER2-positive breast cancer (29).
In another preclinical study, the authors observed that HER2
overexpression was associated with upregulation of VEGF in human
breast cancer cell lines (30).
Several clinical studies suggested that trastuzumab combined with
bevacizumab was effective in the treatment of HER2-positive
advanced breast cancer (31–33).
In addition, a phase II study suggested that lapatinib combined
with bevacizumab was effective in patients with HER2-positive
advanced breast cancer (34).
Due to the existence of the blood-brain barrier
(BBB), which has low permeability and strong expression of multiple
specific efflux transporters, the entry of numerous drugs into the
brain is limited (35). Therefore,
numerous anti-tumor drugs cannot achieve any therapeutic effects on
brain metastases.
Apatinib is a highly selective and effective VEGFR
TKI, which has high affinity for VEGFR2. The molecular weight of
apatinib is small, and thus, it may easily pass through the BBB and
its potential effect on brain metastasis may be better than that of
other agents. Furthermore, apatinib is administered orally, which
avoids injection, reduces hospitalization and the drug is taken
orally every day to maintain a stable blood concentration. Two
phase II clinical studies demonstrated the efficacy of apatinib in
patients with advanced triple negative and non-triple negative
breast cancer (18,19). Of note, three patients with brain
metastasis were included in the study of triple negative breast
cancer above, but the therapeutic effect on brain metastasis with
apatinib was not described in detail. The mechanism of apatinib
penetrating the BBB remains elusive and there is also a lack of
large randomized controlled studies to confirm its efficacy in
patients with breast cancer with brain metastasis. However, in
certain cases, the beneficial effect of apatinib on brain tumors
has been reported. Apatinib has been reported to be effective in
the treatment of brain glioma (36). One case report indicated that
apatinib was effective in the treatment of brain edema caused by
brain metastases (37). In
addition, two case reports demonstrated the efficacy of apatinib
monotherapy or in combination for the treatment of brain metastases
of advanced triple negative breast cancer (38,39).
However, to the best of our knowledge, no previous case report on
apatinib for the treatment of a patient with HER2-positive breast
cancer with brain metastasis, particularly after anti-HER2
treatment resistance, has been published to date.
The present case was a patient with HER2-positive
advanced breast cancer that was progressing with small molecule
anti-HER2 TKI drugs, with a heavy brain metastasis load and obvious
brain edema. The radiotherapy department was unable to perform
brain radiotherapy, the neurosurgery department determined that the
operation was risky and the patient and the patient's family
refused the operation. It was only possible to treat the brain
metastases by using drugs. Compared with standard paclitaxel,
albumin-bound paclitaxel has better efficacy and higher safety in
the treatment of advanced breast cancer (40). A study indicated that albumin is
able to penetrate the BBB and drugs conjugated with albumin can
also penetrate the BBB, and thus, anti-tumor drugs conjugated with
albumin may serve a role in the treatment of intracranial tumors
(41).
Considering the molecular subtype, brain metastases
and previous resistance to small-molecule TKI drugs of the patient
of the present study, the regimen of apatinib combined with
trastuzumab and albumin-bound paclitaxel was finally adopted.
Surprisingly, this combination regimen produced remarkable results.
After two courses of treatment, the patient's headache and vomiting
symptoms were significantly diminished, brain MRI indicated that
brain metastases were significantly reduced and brain edema was
significantly alleviated. This may be due to the synergistic
anti-tumor effect of apatinib and albumin-bound paclitaxel through
the BBB.
In conclusion, the present study reported on the
treatment of apatinib combined with trastuzumab and albumin-bound
paclitaxel for a patient with HER2-positive advanced breast cancer
with brain metastases who developed resistance to anti-HER2 drugs.
The curative effect of the combined treatment of this patient is
good. The symptoms of headache and vomiting were significantly
improved and the brain metastases were significantly reduced. This
case demonstrates the therapeutic effect of apatinib on brain
metastases of HER2-positive breast cancer. In the future, further
large-scale clinical trials are needed to clarify the role of
apatinib in patients with refractory brain metastasis of breast
cancer and HER2-targeted drug resistance.
Acknowledgements
Not applicable.
Funding
This work was supported by funds from Shenzhen Basic Research
Program (2018, grant no. JCYJ20180306171227129), the National
Natural Science Foundation of China (grant no. 81671750, 2016) and
the National Natural Science Foundation of Guangdong Province
(grant no. 2016A030312008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH and CD designed the study and wrote the
manuscript. JH, XC and LS confirmed the authenticity of all the raw
data analyzed and interpreted the data. XC and JG obtained medical
images (MRI scans) and analyzed patient data. YM and HZ contributed
to the study conception, overall design and quality control. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the case report, including publication
of all clinical details and diagnostic images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loibl S and Gianni L: HER2-positive breast
cancer. Lancet. 389:2415–2429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Q, Li J, Zheng S, Li JY, Pang Y,
Huang R, Zhang BN, Zhang B, Yang HJ, Xie XM, et al: Breast cancer
stage at diagnosis and area-based socioeconomic status: A
multicenter 10-year retrospective clinical epidemiological study in
China. BMC Cancer. 12:1222012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heinemann V, Di Gioia D, Vehling-Kaiser U,
Harich HD, Heinrich B, Welt A, Ziske C, Deutsch G, Pihusch R, Kölbl
H, et al: A prospective multicenter phase II study of oral and i.v.
vinorelbine plus trastuzumab as first-line therapy in
HER2-overexpressing metastatic breast cancer. Ann Oncol.
22:603–608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cameron D, Piccart-Gebhart MJ, Gelber RD,
Procter M, Goldhirsch A, de Azambuja E, Castro G Jr, Untch M, Smith
I, Gianni L, et al: 11 years' follow-up of trastuzumab after
adjuvant chemotherapy in HER2-positive early breast cancer: Final
analysis of the HERceptin Adjuvant (HERA) trial. Lancet.
389:1195–1205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Wang J, Fan Y, Luo Y, Zhang P, Li
Q, Ma F, Yuan P, Chen S, Li Q, et al: Primary trastuzumab
resistance after (Neo)adjuvant Trastuzumab-containing treatment for
patients with HER2-positive breast cancer in real-world practice.
Clin Breast Cancer. 21:191–198. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Viallard C and Larrivee B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banerjee S, Dowsett M, Ashworth A and
Martin LA: Mechanisms of disease: angiogenesis and the management
of breast cancer. Nat Clin Pract Oncol. 4:536–550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Y, Ren X, Hou Z, Wang N, Jiang Y and
Luan Y: Engineering a photosensitizer nanoplatform for amplified
photodynamic immunotherapy via tumor microenvironment modulation.
Nanoscale Horiz. 6:120–131. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miller K, Wang M, Gralow J, Dickler M,
Cobleigh M, Perez EA, Shenkier T, Cella D and Davidson NE:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. N Engl J Med. 357:2666–2676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robert NJ, Diéras V, Glaspy J, Brufsky AM,
Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, et
al: RIBBON-1: Randomized, double-blind, placebo-controlled, phase
III trial of chemotherapy with or without bevacizumab for
first-line treatment of human epidermal growth factor receptor
2-negative, locally recurrent or metastatic breast cancer. J Clin
Oncol. 29:1252–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brufsky AM, Hurvitz S, Perez E, Swamy R,
Valero V, O'Neill V and Rugo HS: RIBBON-2: A randomized,
double-blind, placebo-controlled, phase III trial evaluating the
efficacy and safety of bevacizumab in combination with chemotherapy
for second-line treatment of human epidermal growth factor receptor
2-negative metastatic breast cancer. J Clin Oncol. 29:4286–4293.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cobleigh MA, Langmuir VK, Sledge GW,
Miller KD, Haney L, Novotny WF, Reimann JD and Vassel A: A phase
I/II dose-escalation trial of bevacizumab in previously treated
metastatic breast cancer. Semin Oncol. 30:117–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baselga J, Segalla JG, Roché H, Del Giglio
A, Pinczowski H, Ciruelos EM, Filho SC, Gómez P, Van Eyll B,
Bermejo B, et al: Sorafenib in combination with capecitabine: An
oral regimen for patients with HER2-negative locally advanced or
metastatic breast cancer. J Clin Oncol. 30:1484–1491. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burstein HJ, Elias AD, Rugo HS, Cobleigh
MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo
SE, et al: Phase II study of sunitinib malate, an oral
multitargeted tyrosine kinase inhibitor, in patients with
metastatic breast cancer previously treated with an anthracycline
and a taxane. J Clin Oncol. 26:1810–1816. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu N, Si Y, Yue J, Sun T, Wang X, Jia Z,
Gao S, Li Q, Shao Y, Wang J, et al: Anlotinib has good efficacy and
low toxicity: A phase II study of anlotinib in pre-treated HER-2
negative metastatic breast cancer. Cancer Biol Med. 18:849–859.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
placebo-controlled phase III trial of apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or gastroesophageal junction. J Clin Oncol.
34:1448–14454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu X, Zhang J, Xu B, Jiang Z, Ragaz J,
Tong Z, Zhang Q, Wang X, Feng J, Pang D, et al: Multicenter phase
II study of apatinib, a novel VEGFR inhibitor in heavily pretreated
patients with metastatic triple-negative breast cancer. Int J
Cancer. 135:1961–1969. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu X, Cao J, Hu W, Wu C, Pan Y, Cai L,
Tong Z, Wang S, Li J, Wang Z, et al: Multicenter phase II study of
apatinib in non-triple-negative metastatic breast cancer. BMC
Cancer. 14:8202014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu A, Yuan P, Wang J, Fan Y, Luo Y, Cai
R, Zhang P, Li Q, Ma F and Xu B: Apatinib combined with
chemotherapy in patients with previously treated advanced breast
cancer: An observational study. Oncol Lett. 17:4768–4778.
2019.PubMed/NCBI
|
|
21
|
Patchell RA, Tibbs PA, Walsh JW, Dempsey
RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS and Young
B: A randomized trial of surgery in the treatment of single
metastases to the brain. N Engl J Med. 322:494–500. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalkanis SN, Kondziolka D, Gaspar LE,
Burri SH, Asher AL, Cobbs CS, Ammirati M, Robinson PD, Andrews DW,
Loeffler JS, et al: The role of surgical resection in the
management of newly diagnosed brain metastases: A systematic review
and evidence-based clinical practice guideline. J Neurooncol.
96:33–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mills MN, Figura NB, Arrington JA, Yu HM,
Etame AB, Vogelbaum MA, Soliman H, Czerniecki BJ, Forsyth PA, Han
HS and Ahmed KA: Management of brain metastases in breast cancer: A
review of current practices and emerging treatments. Breast Cancer
Res Treat. 180:279–300. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown PD, Jaeckle K, Ballman KV, Farace E,
Cerhan JH, Anderson SK, Carrero XW, Barker FG II, Deming R, Burri
SH, et al: Effect of radiosurgery alone vs. radiosurgery with whole
brain radiation therapy on cognitive function in patients with 1 to
3 brain metastases: A randomized clinical trial. JAMA. 316:401–409.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soffietti R, Abacioglu U, Baumert B, Combs
SE, Kinhult S, Kros JM, Marosi C, Metellus P, Radbruch A, Villa
Freixa SS, et al: Diagnosis and treatment of brain metastases from
solid tumors: Guidelines from the european association of
neuro-oncology (EANO). Neuro Oncol. 19:162–174. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krop IE, Lin NU, Blackwell K, Guardino E,
Huober J, Lu M, Miles D, Samant M, Welslau M and Diéras V:
Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in
patients with HER2-positive metastatic breast cancer and central
nervous system metastases: A retrospective, exploratory analysis in
EMILIA. Ann Oncol. 26:113–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jerusalem G, Park YH, Yamashita T, Hurvitz
SA, Modi S, Andre F, Krop IE, Gonzàlez Farré X, You B, Saura C, et
al: Trastuzumab deruxtecan in HER2-positive metastatic breast
cancer patients with brain metastases: A DESTINY-Breast01 subgroup
analysis. Cancer Discov. 12:2754–2762. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alameddine RS, Otrock ZK, Awada A and
Shamseddine A: Crosstalk between HER2 signaling and angiogenesis in
breast cancer: Molecular basis, clinical applications and
challenges. Curr Opin Oncol. 25:313–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nasir A, Holzer TR, Chen M, Man MZ and
Schade AE: Differential expression of VEGFR2 protein in HER2
positive primary human breast cancer: Potential relevance to
anti-angiogenic therapies. Cancer Cell Int. 17:562017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yen L, You XL, Al Moustafa AE, Batist G,
Hynes NE, Mader S, Meloche S and Alaoui-Jamali MA: Heregulin
selectively upregulates vascular endothelial growth factor
secretion in cancer cells and stimulates angiogenesis. Oncogene.
19:3460–3469. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schwartzberg LS, Badarinath S, Keaton MR
and Childs BH: Phase II multicenter study of docetaxel and
bevacizumab with or without trastuzumab as first-line treatment for
patients with metastatic breast cancer. Clin Breast Cancer.
14:161–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin NU, Seah DS, Gelman R, Desantis S,
Mayer EL, Isakoff S, Dipiro P, Krop IE, Come SE, Weckstein D, et
al: A phase II study of bevacizumab in combination with vinorelbine
and trastuzumab in HER2-positive metastatic breast cancer. Breast
Cancer Res Treat. 139:403–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martin M, Makhson A, Gligorov J,
Lichinitser M, Lluch A, Semiglazov V, Scotto N, Mitchell L and
Tjulandin S: Phase II study of bevacizumab in combination with
trastuzumab and capecitabine as first-line treatment for
HER-2-positive locally recurrent or metastatic breast cancer.
Oncologist. 17:469–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rugo HS, Chien AJ, Franco SX, Stopeck AT,
Glencer A, Lahiri S, Arbushites MC, Scott J, Park JW, Hudis C, et
al: A phase II study of lapatinib and bevacizumab as treatment for
HER2-overexpressing metastatic breast cancer. Breast Cancer Res
Treat. 134:13–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He C, Cai P, Li J, Zhang T, Lin L, Abbasi
AZ, Henderson JT, Rauth AM and Wu XY: Blood-brain
barrier-penetrating amphiphilic polymer nanoparticles deliver
docetaxel for the treatment of brain metastases of triple negative
breast cancer. J Control Release. 246:98–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang H, Chen F, Wang Z and Wu S:
Successful treatment with apatinib for refractory recurrent
malignant gliomas: A case series. Onco Targets Ther. 10:837–845.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song Y MD, PhD, Liu B MD, PhD; Guan M
Master of Medicine, ; Liu M: Successful treatment using apatinib in
intractable brain edema: A case report and literatures review.
Cancer Biol Ther. 19:1093–1096. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu T, Liu C, Li Q, Xiong J, Ma Y, Wu G and
Zhao Y: Apatinib + CPT-11 + S-1 for treatment of refractory brain
metastases in patient with triple-negative breast cancer: Case
report and literature review. Medicine (Baltimore). 97:e03492018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li T, Wang SB, Lei KJ, Jiang MQ and Jia
YM: Significant response of low-dose apatinib monotherapy in brain
metastases of triple-negative breast cancer: A case report.
Medicine (Baltimore). 98:e141822019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gradishar WJ, Tjulandin S, Davidson N,
Shaw H, Desai N, Bhar P, Hawkins M and O'Shaughnessy J: Phase III
trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel in women with breast
cancer. J Clin Oncol. 23:7794–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin T, Zhao P, Jiang Y, Tang Y, Jin H, Pan
Z, He H, Yang VC and Huang Y: Blood-brain-barrier-penetrating
albumin nanoparticles for biomimetic drug delivery via
albumin-binding protein pathways for antiglioma therapy. ACS Nano.
10:9999–10012. 2016. View Article : Google Scholar : PubMed/NCBI
|