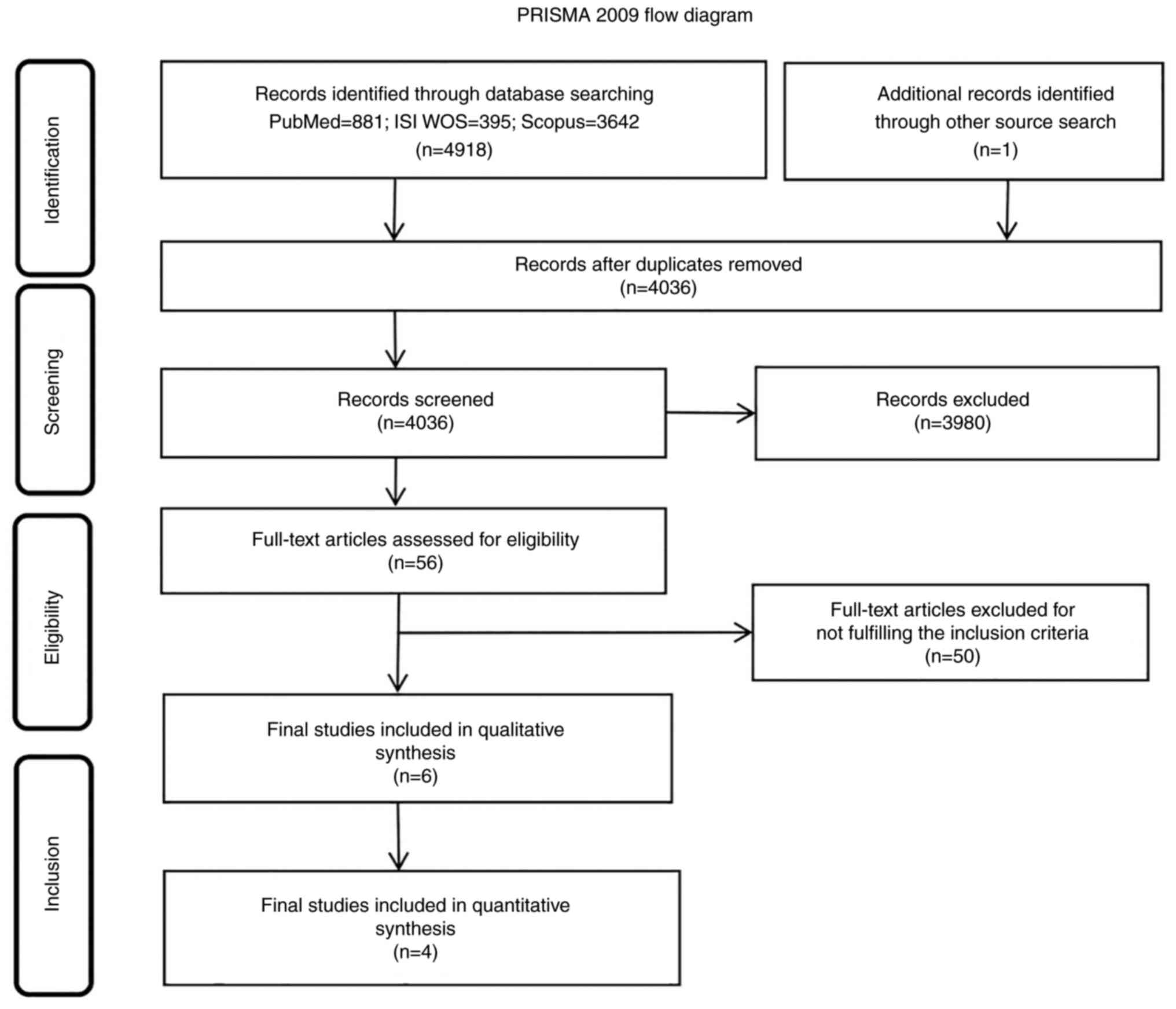
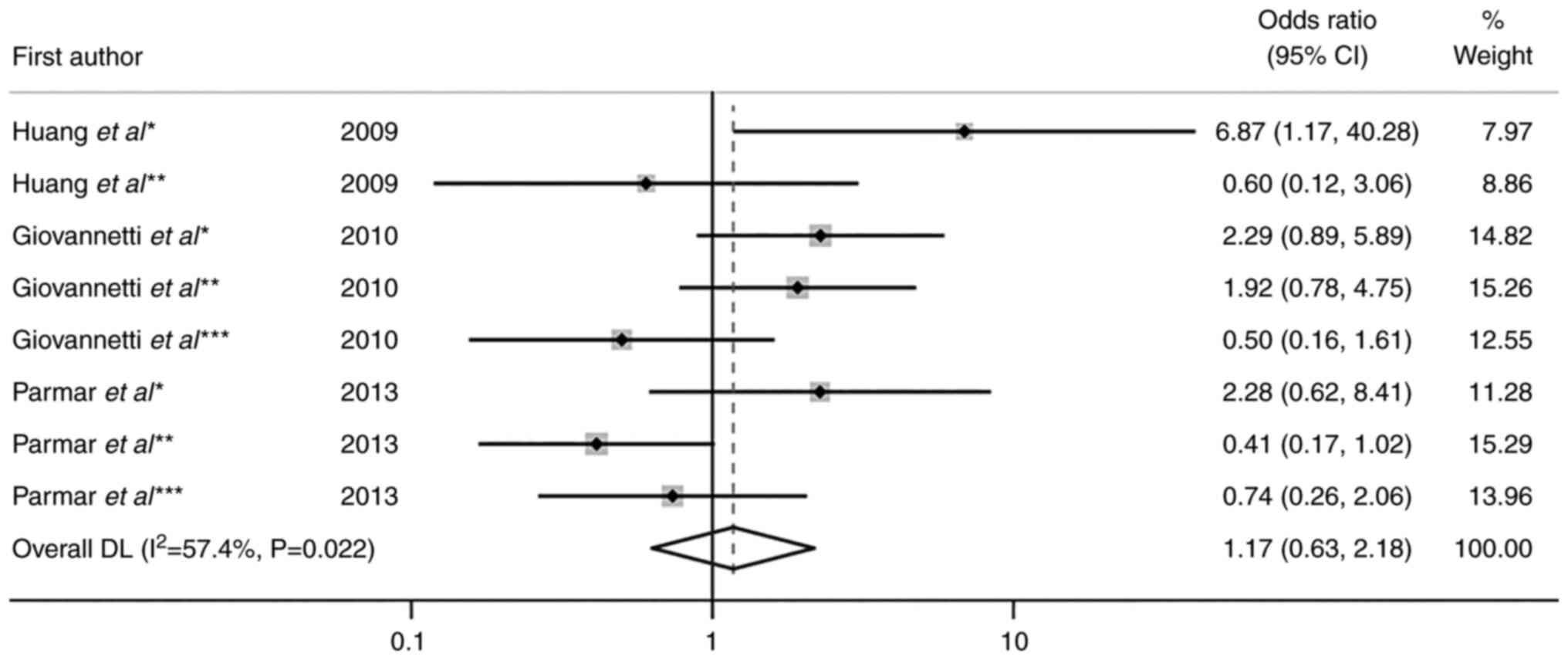
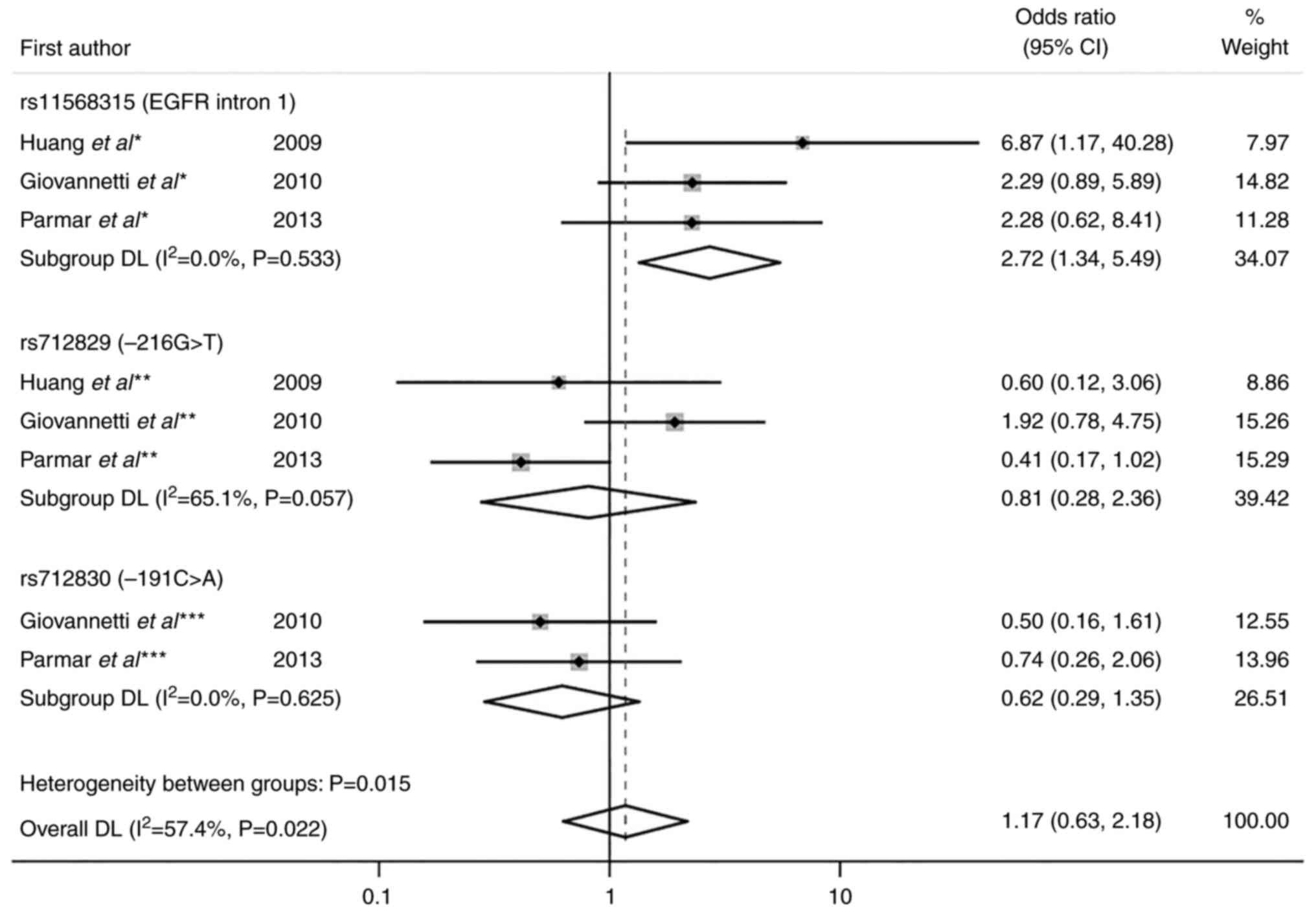
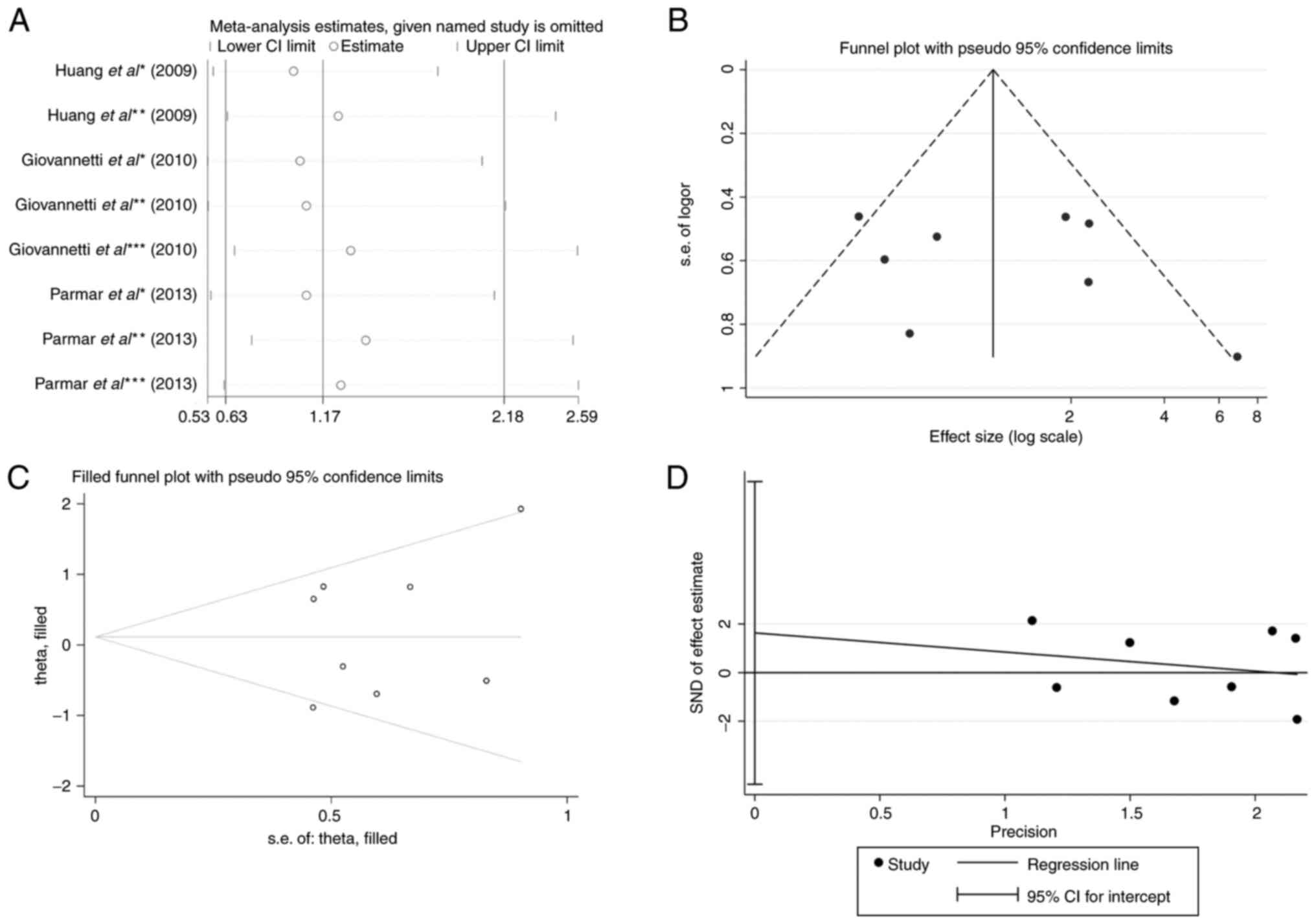
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics. CA Cancer J Clin. 71:7–33. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang ZY, Liu L, Mao C, Wu XY, Huang YF, Hu
XF and Tang JL: Chemotherapy with cetuximab versus chemotherapy
alone for chemotherapy-naive advanced non-small cell lung cancer.
Cochrane Database Syst Rev. 17:CD0099482014.PubMed/NCBI
|
|
4
|
Hirsch FR, Herbst RS, Olsen C, Chansky K,
Crowley J, Kelly K, Franklin WA, Bunn PA Jr, Varella-Garcia M and
Gandara DR: Increased EGFR gene copy number detected by fluorescent
in situ hybridization predicts outcome in non-small-cell lung
cancer patients treated with cetuximab and chemotherapy. J Clin
Oncol. 26:3351–3357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pirker R, Pereira JR, von Pawel J,
Krzakowski M, Ramlau R, Park K, de Marinis F, Eberhardt WEE,
Paz-Ares L, Störkel S, et al: EGFR expression as a predictor of
survival for first-line chemotherapy plus cetuximab in patients
with advanced non-small-cell lung cancer: Analysis of data from the
phase 3 FLEX study. Lancet Oncol. 13:33–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee CK, Davies L, Wu YL, Mitsudomi T,
Inoue A, Rosell R, Zhou C, Nakagawa K, Thongprasert S, Fukuoka M,
et al: Gefitinib or erlotinib vs chemotherapy for EGFR
mutation-positive lung cancer: Individual patient data
meta-analysis of overall survival. J Natl Cancer Inst.
109:djw2792017. View Article : Google Scholar
|
|
9
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yun CH, Mengwasser KE, Toms AV, Woo MS,
Greulich H, Wong KK, Meyerson M and Eck MJ: The T790M mutation in
EGFR kinase causes drug resistance by increasing the affinity for
ATP. Proc Natl Acad Sci U S A. 105:2070–2075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldberg SB, Oxnard GR, Digumarthy S,
Muzikansky A, Jackman DM, Lennes IT and Sequist LV: Chemotherapy
with Erlotinib or chemotherapy alone in advanced non-small cell
lung cancer with acquired resistance to EGFR tyrosine kinase
inhibitors. Oncologist. 18:1214–1220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amador ML, Oppenheimer D, Perea S, Maitra
A, Cusatis G, Iacobuzio-Donahue C, Baker SD, Ashfaq R, Takimoto C,
Forastiere A and Hidalgo M: An epidermal growth factor receptor
intron 1 polymorphism mediates response to epidermal growth factor
receptor inhibitors. Cancer Res. 64:9139–9143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu G, Gurubhagavatula S, Zhou W, Wang Z,
Yeap BY, Asomaning K, Su L, Heist R, Lynch TJ and Christiani DC:
Epidermal growth factor receptor polymorphisms and clinical
outcomes in non-small-cell lung cancer patients treated with
gefitinib. Pharmacogenomics J. 8:129–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung M, Cho BC, Lee CH, Park HS, Kang YS,
Kim SK, Chang J, Kim DJ, Rha SY, Kim JH and Lee JH: EGFR
polymorphism as a predictor of clinical outcome in advanced lung
cancer patients treated with EGFR-TKI. Yonsei Med J. 53:1128–1135.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Winther-Larsen A, Ebert EB, Meldgaard P
and Sorensen BS: EGFR gene polymorphism predicts improved outcome
in patients with EGFR mutation-positive non-small cell lung cancer
treated with erlotinib. Clin Lung Cancer. 20:161–166.e161. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jurisic V, Vukovic V, Obradovic J,
Gulyaeva LF, Kushlinskii NE and Djordjević N: EGFR polymorphism and
survival of NSCLC patients treated with TKIs: A systematic review
and meta-analysis. J Oncol. 2020:19732412020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peréz-Soler R and Saltz L: Cutaneous
adverse effects with HER1/EGFR-targeted agents: Is there a silver
lining? J Clin Oncol. 23:5235–5246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Segaert S and Van Cutsem E: Clinical
signs, pathophysiology and management of skin toxicity during
therapy with epidermal growth factor receptor inhibitors. Ann
Oncol. 16:1425–1433. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Agero AL, Dusza SW, Benvenuto-Andrade C,
Busam KJ, Myskowski P and Halpern AC: Dermatologic side effects
associated with the epidermal growth factor receptor inhibitors. J
Am Acad Dermatol. 55:657–670. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu JC, Sadeghi P, Pinter-Brown LC, Yashar
S and Chiu MW: Cutaneous side effects of epidermal growth factor
receptor inhibitors: Clinical presentation, pathogenesis, and
management. J Am Acad Dermatol. 56:317–326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bianchini D, Jayanth A, Chua YJ and
Cunningham D: Epidermal growth factor receptor inhibitor-related
skin toxicity: Mechanisms, treatment, and its potential role as a
predictive marker. Clin Colorectal Cancer. 7:33–43. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang CL, Yang CH, Yeh KH, Hu FC, Chen KY,
Shih JY, Lin ZZ, Yu CJ, Cheng AL and Yang PC: EGFR intron 1
dinucleotide repeat polymorphism is associated with the occurrence
of skin rash with gefitinib treatment. Lung Cancer. 64:346–351.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu G, Cheng D, Ding K, Maitre AL, Liu N,
Patel D, Chen Z, Seymour L, Shepherd FA and Tsao MS:
Pharmacogenetic analysis of BR.21, a placebo-controlled randomized
phase III clinical trial of erlotinib in advanced non-small cell
lung cancer. J Thorac Oncol. 7:316–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parmar S, Schumann C, Rüdiger S, Boeck S,
Heinemann V, Kächele V, Seeringer A, Paul T, Seufferlein T and
Stingl JC: Pharmacogenetic predictors for EGFR-inhibitor-associated
skin toxicity. Pharmacogenomics J. 13:181–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giovannetti E, Zucali PA, Peters GJ,
Cortesi F, D'Incecco A, Smit EF, Falcone A, Burgers JA, Santoro A,
Danesi R, et al: Association of polymorphisms in AKT1 and EGFR with
clinical outcome and toxicity in non-small cell lung cancer
patients treated with gefitinib. Mol Cancer Ther. 9:581–593. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim L, Saieg M, Di Maio M, Gallo C, Butts
C, Ciardiello F, Feld R, Cheng D, Gebbia V, Burgio MA, et al:
Biomarker analysis of the phase 3 TORCH trial for first line
erlotinib versus chemotherapy in advanced non-small cell lung
cancer patients. Oncotarget. 8:57528–57536. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma Y, Xin S, Huang M, Yang Y, Zhu C, Zhao
H, Zhang Y, Chen L, Zhao Y, Li J, et al: Determinants of Gefitinib
toxicity in advanced non-small cell lung cancer (NSCLC): A
pharmacogenomic study of metabolic enzymes and transporters.
Pharmacogenomics J. 17:325–330. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Petrelli F, Borgonovo K, Cabiddu M, Lonati
V and Barni S: Relationship between skin rash and outcome in
non-small-cell lung cancer patients treated with anti-EGFR tyrosine
kinase inhibitors: A literature-based meta-analysis of 24 trials.
Lung Cancer. 78:8–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wo H, He J, Zhao Y, Yu H, Chen F and Yi H:
The efficacy and toxicity of gefitinib in treating non-small cell
lung cancer: A meta-analysis of 19 randomized clinical trials. J
Cancer. 9:1455–1465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Xu Y, Tang W and Liu L: Efficacy
and safety of radiotherapy plus EGFR-TKIs in NSCLC patients with
brain metastases: A meta-analysis of published data. Transl Oncol.
11:1119–1127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: Preferred reporting items for systematic reviews and
meta-analyses: The PRISMA statement. BMJ. 339:b25352009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ottawa Hospital Research Institute, . NOS
Manual. Available from:. http://www.ohri.ca/programs/clinical_epidemiology/nos_manual.pdfMarch
8–2021
|
|
34
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Higgins JPT, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials revisited. Contemp Clin Trials. 45:139–145.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galbraith RF: A note on graphical
presentation of estimated odds ratios from several clinical trials.
Stat Med. 7:889–894. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Egger M, Smith GD, Schneider M and Minder
C: Bias in meta-analysis detected by a simple, graphical test. BMJ.
315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
National Cancer Institue, . Common
Terminology Criteria for Adverse Events (CTCAE) Version 4.
Available at. https://stacks.stanford.edu/file/druid:nw036fx4646/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdfFebruary
12–2021
|
|
41
|
Ruan Y, Jiang J, Guo L, Li Y, Huang H,
Shen L, Luan M, Li M, Du H, Ma C, et al: Genetic association of
curative and adverse reactions to tyrosine kinase inhibitors in
chinese advanced non-small cell lung cancer patients. Sci Rep.
6:233682016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nie Q, Yang XN, An SJ, Zhang XC, Yang JJ,
Zhong WJ, Liao RQ, Chen ZH, Su J, Xie Z and Wu YL: CYP1A1*2A
polymorphism as a predictor of clinical outcome in advanced lung
cancer patients treated with EGFR-TKI and its combined effects with
EGFR intron 1 (CA)n polymorphism. Eur J Cancer. 47:1962–1970. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tiseo M, Rossi G, Capelletti M, Sartori G,
Spiritelli E, Marchioni A, Bozzetti C, De Palma G, Lagrasta C,
Campanini N, et al: Predictors of gefitinib outcomes in advanced
non-small cell lung cancer (NSCLC): Study of a comprehensive panel
of molecular markers. Lung Cancer. 67:355–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chilingirova N, Hammoudeh Z, Balabanski L,
Ivanov S, Vazharova R, Nikolova D, Kurteva G, Toncheva D and
Chilingirov P: TruSight cancer sequencing panel reveals
pharmacogenetic variants associated with sensitivity to
chemotherapy in lung cancer. Memo-Magazine of European Medical
Oncology. 9:30–38. 2016. View Article : Google Scholar
|
|
45
|
Cusatis G, Gregorc V, Li J, Spreafico A,
Ingersoll RG, Verweij J, Ludovini V, Villa E, Hidalgo M, Sparreboom
A and Baker SD: Pharmacogenetics of ABCG2 and adverse reactions to
gefitinib. J Natl Cancer Inst. 98:1739–1742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rudin CM, Liu W, Desai A, Karrison T,
Jiang X, Janisch L, Das S, Ramirez J, Poonkuzhali B, Schuetz E, et
al: Pharmacogenomic and pharmacokinetic determinants of erlotinib
toxicity. J Clin Oncol. 26:1119–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tiseo M, Capelletti M, De Palma G,
Franciosi V, Cavazzoni A, Mozzoni P, Alfieri RR, Goldoni M, Galetti
M, Bortesi B, et al: Epidermal growth factor receptor intron-1
polymorphism predicts gefitinib outcome in advanced non-small cell
lung cancer. J Thorac Oncol. 3:1104–1111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cappuzzo F, Ligorio C, Jänne PA, Toschi L,
Rossi E, Trisolini R, Paioli D, Holmes AJ, Magrini E, Finocchiaro
G, et al: Prospective study of gefitinib in epidermal growth factor
receptor fluorescence in situ
hybridization-positive/phospho-Akt-positive or never smoker
patients with advanced non-small-cell lung cancer: The ONCOBELL
trial. J Clin Oncol. 25:2248–2255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
O'Byrne KJ, Bondarenko I, Barrios C,
Eschbach C, Martens U, Kortsik YH, Celik I, Stroh C and Pirker R:
Molecular and clinical predictors of outcome for cetuximab in
non-small cell lung cancer (NSCLC): Data from the FLEX study. J
Clin Oncol. 27:8007. 2009. View Article : Google Scholar
|
|
50
|
Su X, Lacouture ME, Jia Y and Wu S: Risk
of high-grade skin rash in cancer patients treated with
cetuximab-an antibody against epidermal growth factor receptor:
Systemic review and meta-analysis. Oncology. 77:124–133. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dubey S, Stephenson P, Levy DE, Miller JA,
Keller SM, Schiller JH, Johnson DH and Kolesar JM; Eastern
Cooperative Oncology Group, : EGFR dinucleotide repeat polymorphism
as a prognostic indicator in non-small cell lung cancer. J Thorac
Oncol. 1:406–412. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gebhardt F, Bürger H and Brandt B:
Modulation of EGFR gene transcription by secondary structures, a
polymorphic repetitive sequence and mutations-a link between
genetics and epigenetics. Histol Histopathol. 15:929–936.
2000.PubMed/NCBI
|
|
53
|
Han SW, Jeon YK, Lee KH, Keam B, Hwang PG,
Oh DY, Lee SH, Kim DW, Im SA, Chung DH, et al: Intron 1 CA
dinucleotide repeat polymorphism and mutations of epidermal growth
factor receptor and gefitinib responsiveness in non-small-cell lung
cancer. Pharmacogenet Genomics. 17:313–319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ichihara S, Toyooka S, Fujiwara Y, Hotta
K, Shigematsu H, Tokumo M, Soh J, Asano H, Ichimura K, Aoe K, et
al: The impact of epidermal growth factor receptor gene status on
gefitinib-treated Japanese patients with non-small-cell lung
cancer. Int J Cancer. 120:1239–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dienstmann R, Braña I, Rodon J and
Tabernero J: Toxicity as a biomarker of efficacy of molecular
targeted therapies: Focus on EGFR and VEGF inhibiting anticancer
drugs. Oncologist. 16:1729–1740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wacker B, Nagrani T, Weinberg J, Witt K,
Clark G and Cagnoni PJ: Correlation between development of rash and
efficacy in patients treated with the epidermal growth factor
receptor tyrosine kinase inhibitor erlotinib in two large phase III
studies. Clin Cancer Res. 13:3913–3921. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Perea S, Oppenheimer D, Amador M, Cusati
G, Baker S, Takimoto C, Maitra A, Iocobuzio-Donahue C and Hidalgo
M: Genotypic bases of EGFR inhibitors pharmacological actions. J
Clin Oncol. 22:3005. 2004. View Article : Google Scholar
|
|
58
|
Liu W, Innocenti F, Wu MH, Desai AA, Dolan
EM, Cook EH Jr and Ratain MJ: A functional common polymorphism in a
Sp1 recognition site of the epidermal growth factor receptor gene
promoter. Cancer Res. 65:46–53. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang HX, Tang Y, Wang L, Wei SX, Liu QX,
Li F and Yuan XL: EGFR-216 G/T polymorphism as a predictor of
clinical outcomes in advanced non-small cell lung cancer patients
treated with EGFR-TKIs: A meta-analysis. Int J Clin Exper Med.
9:10273–10280. 2016.
|
|
60
|
Biaoxue R, Hua L, Wenlong G and Shuanying
Y: Efficacy and safety of icotinib in treating non-small cell lung
cancer: A systematic evaluation and meta-analysis based on 15
studies. Oncotarget. 7:86902–86913. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yi L, Fan J, Qian R and Luo P: Efficacy
and safety of osimertinib in treating EGFR-mutated advanced NSCLC:
A meta-analysis. Int J Cancer. 145:284–294. 2019. View Article : Google Scholar : PubMed/NCBI
|