Introduction
Esophageal cancer is the seventh most common
malignant tumor, ranking sixth in global cancer-associated
mortality (1,2). In terms of histological subtypes,
adenocarcinoma is commonly observed in Europe and the United
States, whereas squamous cell carcinoma is the primary subtype in
China (2,3). Early esophageal cancer is defined as
cancer confined to the mucosa (T1a) or submucosa (T1b), regardless
of the presence of lymph node metastasis (LNM) (4–6). In
previous years, endoscopic treatment, namely endoscopic mucosal
resection or endoscopic submucosal dissection (ESD), has been
increasingly regarded as a treatment option for early esophageal
cancer (7,8).
The proportion of early (T1) stage detection has
increased due to the improvement of endoscopic detection (9). Endoscopic resection (ER) of early
tumors is the first step in patient management (9,10).
ER is curative in most types of intramucosal (T1a) cancer and
cancer that partially invades the submucosa (T1b) (11). Esophagectomy with lymph node
dissection is not clearly indicated as the first choice in patients
with ‘non-curative’ or ‘potentially curative’ ER (11). Based on previous studies,
esophagectomy results in a 5–10% mortality rate (12,13).
However, due to high morbidity and mortality as a result of
complications with esophagectomy, certain patients do not receive
surgical treatment (14–17).
According to the guidelines of the European Society
of Gastrointestinal Endoscopy, if there are poorly differentiated
lesions, lymphovascular invasion (LVI) or positive vertical
margins, further treatment is recommended (11). By contrast, the Japanese
Gastroenterology Endoscopy Society recommends additional treatment
for patients with submucosal infiltration following ER of
esophageal cancer, but there is still no consensus on what to do in
these cases (18). To date,
adjuvant management following non-curative or high-risk ER is not
standardized. Therefore, the present study aimed to propose an
additional treatment strategy for patients with non-curative
resection (non-CR) and only positive lateral margin (LM+) following
ER of early esophageal squamous cell carcinoma (ESCC).
Materials and methods
Patients
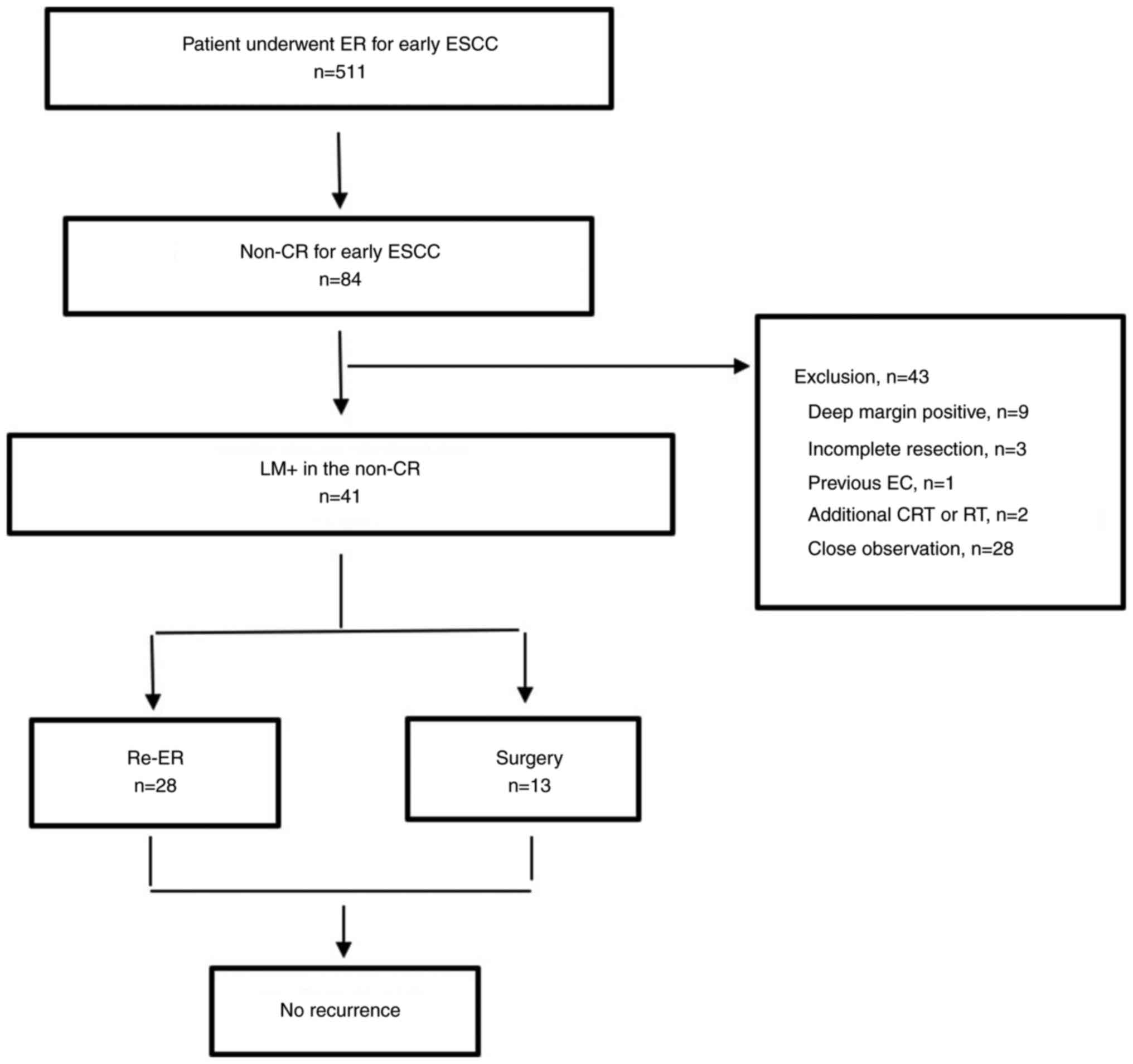
The present study retrospectively analyzed 511
patients (31–85 years) with early ESCC who underwent ER in the
Fourth Hospital of Hebei Medical University (Shijiazhuang, China)
from January 2000 to June 2022, of whom 84 had non-CR. The baseline
characteristics, methods of endoscopic treatment and histological
features in the medical records were reviewed.
The study included only patients with LM+ and non-CR
(n=41). The exclusion criteria were as follows: i) Positive deep
margin; ii) incomplete resection; iii) previous history of
esophageal cancer; iv) additional history of chemotherapy or
radiotherapy and v) clinical observation without additional
treatment (Fig. 1). All patients
provided written informed consent. The present study was approved
by the Ethics Committee of the Fourth Hospital of Hebei Medical
University (2020YB318).
Complete resection was defined as en bloc resection
with margins macroscopically and microscopically free of tumor.
Incomplete resection was defined as the presence of cancer cells in
the lateral (anterior, posterior, proximal or distal) and deep
margin. CR was achieved when a resected specimen met the
requirements of complete resection without submucosal invasion or
LVI. If the resection did not meet the criteria of CR, it was
defined as non-CR (19–21). LM+ was defined as cancer cells
histologically apparent in the LMs of the dissected specimens.
Single LM+ referred to one direction (anterior, posterior, proximal
or distal) of invasion and multiple LM+ to >2 directions. In
addition, residual cancer was defined as the presence of cancer
cells in pathological specimens following additional surgery or
re-ER.
Endoscopic procedure and
follow-up
Endoscopic treatment and follow-up were performed as
previously described (22). For
re-ER, Lugol's iodine staining was used to evaluate lesion size.
Argon plasma coagulation (FiAPC® probes were used for
the flexible endoscope; ERBE Elektromedizin GmbH) was used to mark
~5 mm outside the boundary of the lesion (22). This was the resection range of
re-ER.
Histopathological evaluation
Endoscopically resected specimens were serially
sectioned at 2 mm intervals. The protocol for detailed
histopathological evaluation was as previously described (22).
Additional treatment following ER
Of the 41 patients who received ER, 13 (31.7%)
underwent additional surgery and 28 (68.3%) underwent re-ER. The
clinicians chose re-ER or additional surgery after evaluating the
clinical and pathological factors of each patient (from inspecting
the final pathology report and the patient condition), which
included age, underlying disease and consent to additional surgery.
Patients who refused additional treatment underwent close
observation. Due to lack of consensus in the literature, the final
choice of treatment was based on the doctor's evaluation (11,18).
Re-ER and additional surgery were performed <3 months or 1 month
following initial ER, respectively.
Statistical analysis
Categorical variables were analyzed using
χ2 or the Fisher's exact test. Continuous variables are
reported as median and range or mean ± standard deviation and were
compared using Student's t or the Mann-Whitney U test. P<0.05
was considered to indicate a statistically significant difference.
All analyses were performed using SPSS version 19.0 (IBM
Corp.).
Results
Clinicopathological features of
patients
The mean age of all patients was 64.5±8.5 years.
Table I summarizes the baseline
clinicopathological characteristics of patients with non-CR who
were only LM+ following ER of early ESCC. There were 28 patients
(68.3%) with single LM+ and 13 patients (31.7%) with multiple LM+.
Following additional surgery or re-ER, 27 resected specimens
(65.9%) showed residual cancer.
 | Table I.Baseline clinicopathological
characteristics of patients. |
Table I.
Baseline clinicopathological
characteristics of patients.
| Characteristic | Number of
patients |
|---|
| Sex |
|
|
Male | 24 |
|
Female | 17 |
| Age, mean ± SD,
years | 64.5±8.5 |
| Tumor location |
|
| Upper
esophagus | 2 |
| Middle
esophagus | 25 |
| Lower
esophagus | 14 |
| Tumor size, cm |
|
|
<2.5 | 16 |
|
≥2.5 | 25 |
| Treatment
method |
|
|
EMR | 13 |
|
ESD | 28 |
| Resection
state |
|
| En
bloc | 33 |
|
Piecemeal | 8 |
| WHO
classification |
|
|
Well-differentiated | 15 |
|
Moderately differentiated | 16 |
| Poorly
differentiated | 10 |
| Final
pathology |
|
| No
residual cancer | 14 |
|
Residual cancer | 27 |
| Lateral margin
multiplicity |
|
|
Single | 28 |
|
Multiple | 13 |
Comparison of additional treatment
methods after ER
A comparison of the two additional treatment methods
following non-CR is shown in Table
II. The mean age of patients in the re-ER group was higher than
that in the surgery group (69.5±9.5 vs. 62.5±7.8 years; P=0.023).
Patients with multiple LM+ after ER were most often treated with
surgery (re-ER (n=28), 21.4% vs. surgery (n=13), 53.8%; P=0.038).
Patients with well-differentiated lesions were more likely to
receive re-ER than additional surgery (P=0.003). Residual cancer
was more common in the additional surgery group but there was not a
statistically significant difference (re-ER (n=28), 60.7% vs.
surgery (n=13), 76.9%; P=0.308). There were also no statistically
significant differences between the two groups in terms of sex,
tumor location, size, tumor shape or residual cancer at final
pathology.
 | Table II.Comparison of additional treatment
following ER for early esophageal squamous cell carcinoma. |
Table II.
Comparison of additional treatment
following ER for early esophageal squamous cell carcinoma.
| Characteristic | Re-ER, n=28 | Surgery, n=13 | P-value |
|---|
| Age, mean ± SD,
years | 69.5±9.5 | 62.5±7.8 | 0.023 |
| Sex, n |
|
| 0.756 |
|
Male | 18 | 9 |
|
|
Female | 10 | 4 |
|
| Tumor location,
n |
|
| 0.830 |
| Upper
esophagus | 1 | 1 |
|
| Middle
esophagus | 17 | 8 |
|
| Lower
esophagus | 10 | 4 |
|
| Tumor size, mean ±
SD, mm | 26.2±12.6 | 27.1±10.8 | 0.059 |
| Tumor shape, n |
|
| 0.781 |
|
Elevated | 9 | 3 |
|
|
Flat | 12 | 7 |
|
|
Depressed | 7 | 3 |
|
| WHO classification,
n |
|
| 0.003 |
|
Well-differentiated | 10 | 5 |
|
|
Moderately differentiated | 15 | 1 |
|
| Poorly
differentiated | 3 | 7 |
|
| Final pathology,
n |
|
| 0.308 |
| No
residual cancer | 11 | 3 |
|
|
Residual cancer | 17 | 10 |
|
| Lateral margin
multiplicity, n |
|
| 0.038 |
|
Single | 22 | 6 |
|
|
Multiple | 6 | 7 |
|
Pathological features of residual
lesions following additional treatment after ER
Table III lists
pathological features of residual lesions following non-CR (only
LM+) with re-ER and additional surgery. In patients with poorly
differentiated cancer, the proportion of residual cancer cells
increased significantly (No residual cancer (n=14), 14.3% vs.
Residual cancer (n=27), 29.6%; P=0.030). Residual cancer was also
significantly higher in the multiple LM+ group than in the single
LM+ group (single LM+, 7.1% vs. multiple LM+, 44.4%; P=0.015).
 | Table III.Pathological features of residual
lesions following additional treatment post-endoscopic
resection. |
Table III.
Pathological features of residual
lesions following additional treatment post-endoscopic
resection.
| Pathological
feature | No residual cancer,
n=14 | Residual cancer,
n=27 | P-value |
|---|
| Tumor size, mean ±
SD, mm | 26.1±11.8 | 26.6±10.9 | 0.779 |
| Tumor shape, n |
|
| 0.862 |
|
Elevated | 3 | 4 |
|
|
Flat | 6 | 13 |
|
|
Depressed | 5 | 10 |
|
| Lateral margin
multiplicity, n |
|
| 0.015 |
|
Single | 13 | 15 |
|
|
Multiple | 1 | 12 |
|
| WHO classification,
n |
|
| 0.030 |
|
Well-differentiated | 9 | 6 |
|
|
Moderately differentiated | 3 | 13 |
|
| Poorly
differentiated | 2 | 8 |
|
Clinical results of additional
treatments after ER
Themean follow-up time of all enrolled patients was
36.1±24.1 months. At the time of writing, there had been no
recurrence, although in the additional surgery group, two patients
had anastomotic leakage and one patient had respiratory
failure.
Clinicopathological features of
patients with additional surgery
The clinicopathological characteristics of 13
patients who underwent additional surgery are shown in Table IV. Among these 13 patients, LNM
was present in three cases (23.1%). There was no significant
difference between the LNM and non-LNM subgroups in terms of age,
sex or tumor location, size, shape, circumference or
differentiation.
 | Table IV.Factors associated with LN metastasis
in the esophagectomy group. |
Table IV.
Factors associated with LN metastasis
in the esophagectomy group.
|
| LN metastasis |
|
|---|
|
|
|
|
|---|
| Characteristic | Present, n=3 | Absent, n=10 | P-value |
|---|
| Age, mean ± SD,
years | 63.5±8.8 | 62±6.9 | 0.889 |
| Sex, n |
|
| 0.913 |
|
Male | 2 | 7 |
|
|
Female | 1 | 3 |
|
| Tumor location |
|
| 0.850 |
| Upper
esophagus | 0 | 1 |
|
| Middle
esophagus | 2 | 6 |
|
| Lower
esophagus | 1 | 3 |
|
| Tumor size, mean ±
SD, mm | 27.6±11.8 | 28.1±9.1 | 0.563 |
| Tumor shape |
|
| 0.550 |
|
Elevated | 1 | 2 |
|
|
Flat | 2 | 5 |
|
|
Depressed | 0 | 3 |
|
| Tumor circumference
relative to esophageal lumen |
|
| 0.701 |
|
<1/4 | 0 | 1 |
|
|
1/4-3/4 | 3 | 8 |
|
|
≥3/4 | 0 | 1 |
|
| WHO classification,
n |
|
| 0.084 |
|
Well-differentiated | 0 | 5 |
|
|
Moderately differentiated | 1 | 0 |
|
| Poorly
differentiated | 2 | 5 |
|
Discussion
With the advancement of endoscopic equipment, ER has
been widely used to treat early esophageal cancer (23,24).
Moreover, ER has been shown to be effective for early esophageal
cancer and can be used to histologically evaluate submucosal
infiltration and LVI (7,8). This can help decide whether to
recommend additional treatment following radical (R0) ER. ER is
mostly curative in early esophageal cancer (11). However, the best adjuvant treatment
methods following non-CR are still unclear (11,18),
so subsequent curative treatment strategies need to be established.
Xu et al (25) found that
repeated esophageal ESD provides an alternative choice for
recurrent superficial ESCC but did not assess early repeated ESD
immediately following non-CR (confirmed pathologically). The focus
of the present study was to propose treatment strategies for
patients with only LM+ following non-CR.
Previous studies have shown that, when considering
the age and complications (diabetes, cardiovascular and
cerebrovascular diseases) of patients, additional ER is an option
for patients with non-CR, as the overall survival rate and
incidence of adverse events of these patients are not significantly
different from those of patients undergoing surgery (26,27).
Toya et al (28)
recommended close follow-up as an alternative to surgery as there
was no difference in the cancer-specific survival rate between the
patients in these two groups (close follow-up or surgery) of their
study. Additional surgical treatment after non-CR considering
patient age and complications is controversial (27). Similarly, the present study found
that older patients were more likely to choose re-ER as subsequent
therapy. Due to perioperative risks and/or short life expectancy,
old age is an important reason for forgoing additional surgery
following non-CR (29–31). The aforementioned studies suggested
that older patients choose conservative treatment over surgical
treatment.
In the research of gastric cancer, certain scholars
have found that the poorer the differentiation type, the more
directions of invasion the tumor has and the total length of LM
affected by the tumor is significantly associated with a non-CR of
residual tumor caused by LM+ (32–35).
Similarly, in the present study, in patients with non-CR of ESCC,
residual cancer was more common when there was a poorly
differentiated histology and multiple LM+.
Certain studies have shown that patients with ESCC
and deep mucosal infiltration (pT1a-m3), submucosal involvement
(pT1b-m1-3) or LVI are considered to be at high risk of LNM and
esophagectomy and lymph node dissection are recommended (36,37).
However, in other studies, the incidence of LNM in esophageal
resection specimens is 0–30%, the perioperative mortality rate is
0–14% and the incidence of serious complications is 26–43%
(13,38–43).
These findings are consistent with the results of the present
study. In the present study, three patients who underwent
additional surgery (3/13, 23.1%) had serious complications.
Therefore, the present results do not support recommendations for
additional surgery.
In addition, three patients (3/13, 23.1%) had LNM.
Searching for accurate risk factors for LNM will help to determine
whether additional surgical treatment is needed. Previous studies
have shown that surgery is not the best option for patients with
early esophageal cancer whose ER is non-curative (44,45).
Most notably, esophagectomy with lymphadenectomy cannot prevent
tumor recurrence and the 5-year survival rate of T1N1 esophageal
cancer following esophagectomy is <40% (46,47).
Therefore, organ preservation strategy in the management of
patients with early esophageal cancer has been implemented in daily
clinical practice when patients refuse or are not suitable for
surgery, but evaluation of whether other alternatives can bring
greater benefits is needed. The positive margin may serve a role in
patient prognosis (22) but a more
detailed prognostic analysis needs to be confirmed by a multicenter
study with long-term follow-up.
The present study investigated the optimal treatment
strategy of only LM+ ESCC with non-curative ER. Younger patients
with multiple LM+ and poorly differentiated histological lesions
often chose additional surgical treatment. By contrast, older
patients with single LM+ involvement and well-differentiated
lesions were more likely to receive re-ER. There was no recurrence
or metastasis in patients who received re-ER during the limited
follow-up period (36.1±24.1 months) of the study. These results
suggested that additional endoscopic treatment for patients with
only LM+ following ER may be sufficient to remove residual tumors.
Although some scholars have suggested that ER combined with
radiotherapy and chemotherapy may be effective (48,49),
the present study did not include patients who had received
radiotherapy and chemotherapy so similar conclusions cannot be
made.
The present study had certain limitations. Firstly,
it was retrospective and single center, the number of cases was
small and other endoscopic treatments (such as argon plasma
coagulation and laser, photodynamic and microwave coagulation
therapy) were not involved. Further studies on endoscopic treatment
is required to conclude whether ER is useful. Secondly, the
follow-up time was short, which may lead to bias in judging
recurrence and metastasis of these patients. A multicenter long
follow-up study is needed to verify the results of the present
study. Thirdly, additional surgery was performed more often in
patients with poor prognosis and/or multiple LM+, thereby
potentially affecting the results.
In conclusion, re-ER may be adequate for patients
with only LM+ and non-curative ER, especially older patients and
those for whom surgical treatment is not recommended. This strategy
is feasible, at least in the medium-term. Further studies,
including large-scale, population-based, multicenter and
prospective studies, should be conducted to evaluate additional
endoscopic treatment strategies for patients with LM+ early ESCC
after non-curative ER.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Key Topics of Medical
Science Research of Hebei Provincial Health Commission (grant no.
20190765).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF and BQL designed the study. YF and SG confirm the
authenticity of all the raw data. YF and WW collected clinical and
pathological data of patients. SG and YF analyzed the data. BQL and
WW contributed to the interpretation of results. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All patients agreed to participate in the present
study and signed an informed consent form. The study was approved
by the Ethics Committee of the Fourth Hospital of Hebei Medical
University (2020YB318) (Shijiazhuang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bosetti C, Levi F, Ferlay J, Garavello W,
Lucchini F, Bertuccio P, Negri E and La Vecchia C: Trends in
oesophageal cancer incidence and mortality in Europe. Int J Cancer.
122:1118–1129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oyama T, Takahasi A, Takeuchi M, Ishihara
R, Tanaka M, Odfa I and Abe S: Long-term prognosis of superficial
esophageal cancers at least 50 mm in diameter treated by endoscopic
submucosal dissection. United European Gastroenterol J.
2:A4222014.
|
|
7
|
Phoa KN, Pouw RE, Bisschops R, Pech O,
Ragunath K, Weusten BL, Schumacher B, Rembacken B, Meining A,
Messmann H, et al: Multimodality endoscopic eradication for
neoplastic Barrett oesophagus: Results of an European multicentre
study (EURO-II). Gut. 65:555–562. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pech O, May A, Manner H, Behrens A, Pohl
J, Weferling M, Hartmann U, Manner N, Huijsmans J, Gossner L, et
al: Long-term efficacy and safety of endoscopic resection for
patients with mucosal adenocarcinoma of the esophagus.
Gastroenterology. 146:652–660.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guan CT, Song GH, Li BY, Gong YW, Hao CQ,
Xue LY, Chen WQ and Chen W: Endoscopy screening effect on stage
distributions of esophageal cancer: A cluster randomized cohort
study in China. Cancer Sci. 109:1995–2002. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Merkow RP, Bilimoria KY, Keswani RN, Chung
J, Sherman KL, Knab LM, Posner MC and Bentrem D: Treatment trends,
risk of lymph node metastasis, and outcomes for localized
esophageal cancer. J Natl Cancer Inst. 106:dju133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon
T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P,
Bialek A, et al: Endoscopic submucosal dissection: European society
of gastrointestinal endoscopy (ESGE) guideline. Endoscopy.
47:829–854. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Degisors S, Pasquer A, Renaud F, Béhal H,
Hec F, Gandon A, Vanderbeken M, Caranhac G, Duhamel A, Piessen G,
et al: Are thoracotomy and/or intrathoracic anastomosis still
predictors of postoperative mortality after esophageal cancer
surgery?: A nationwide study. Ann Surg. 266:854–862. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dermine S, Leconte M, Leblanc S, Dousset
B, Terris B, Berger A, Berger A, Rahmi G, Lepilliez V, Plomteux O,
et al: Outcomes of esophagectomy after noncurative endoscopic
resection of early esophageal cancer. Ther Adv Gastroenterol.
12:17562848198925562019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wright CD, Kucharczuk JC, O'Brien SM, Grab
JD and Allen MS; Society of Thoracic Surgeons General Thoracic
Surgery Database, : Predictors of major morbidity and mortality
after esophagectomy for esophageal cancer: A society of thoracic
surgeons general thoracic surgery database risk adjustment model. J
Thorac Cardiovasc Surg. 137:587–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kohn GP, Galanko JA, Meyers MO, Feins RH
and Farrell TM: National trends in esophageal surgery-are outcomes
as good as we believe? J Gastrointest Surg. 13:1900–1910;
discussion 1910-2. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Funk LM, Gawande AA, Semel ME, Lipsitz SR,
Berry WR, Zinner MJ and Jha AK: Esophagectomy outcomes at
low-volume hospitals: The association between systems
characteristics and mortality. Ann Surg. 253:912–917. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raymond DP, Seder CW, Wright CD, Magee MJ,
Kosinski AS, Cassivi SD, Grogan EL, Blackmon SH, Allen MS, Park BJ,
et al: Predictors of major morbidity or mortality after resection
for esophageal cancer: A society of thoracic surgeons general
thoracic surgery database risk adjustment model. Ann Thorac Surg.
102:207–214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishihara R, Arima M, Iizuka T, Oyama T,
Katada C, Kato M, Goda K, Goto O, Tanaka K, Yano T, et al:
Endoscopic submucosal dissection/endoscopic mucosal resection
guidelines for esophageal cancer. Dig Endosc. 32:452–493. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JS, Youn YH, Park JJ, Kim JH and Park
H: Clinical outcomes of endoscopic submucosal dissection for
superficial esophageal squamous neoplasms. Clin Endosc. 49:168–175.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi JY, Park YS, Jung HY, Ahn JY, Kim MY,
Lee JH, Choi KS, Kim DH, Choi KD, Song HJ, et al: Feasibility of
endoscopic resection in superficial esophageal squamous carcinoma.
Gastrointest Endosc. 73:881–889, 889.e1-2. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sano T and Aiko T: New Japanese
classifications and treatment guidelines for gastric cancer:
Revision concepts and major revised points. Gastric Cancer.
14:97–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng Y, Wei W, Guo S and Li BQ: Associated
risk factor analysis and the prognostic impact of positive
resection margins after endoscopic resection in early esophageal
squamous cell carcinoma. Exp Ther Med. 24:4572022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ono S, Fujishiro M, Niimi K, Goto O,
Kodashima S, Yamamichi N and Omata M: Long-term outcomes of
endoscopic submucosal dissection for superficial esophageal
squamous cell neoplasms. Gastrointest Endosc. 70:860–866. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Repici A, Hassan C, Carlino A, Pagano N,
Zullo A, Rando G, Strangio G, Romeo F, Nicita R, Rosati R and
Malesci A: Endoscopic submucosal dissection in patients with early
esophageal squamous cell carcinoma: Results from a prospective
Western series. Gastrointest Endosc. 71:715–721. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu JQ, Zhang ZC, Chen WF, Xu MD, Chen SY,
Zhong YS, Zhang YQ, Hu JW, Cai MY, Yao LQ, et al: Repeat endoscopic
submucosal dissection as salvage treatment for local recurrence of
esophageal squamous cell carcinoma after initial endoscopic
submucosal dissection. Gastrointest Endosc. 96:18–27.e1. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bae SY, Jang TH, Min BH, Lee JH, Rhee PL,
Rhee JC and Kim JJ: Early additional endoscopic submucosal
dissection in patients with positive lateral resection margins
after initial endoscopic submucosal dissection for early gastric
cancer. Gastrointest Endosc. 75:432–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamanouchi K, Ogata S, Sakata Y, Tsuruoka
N, Shimoda R, Nakayama A, Akutagawa T, Shirai S, Takeshita E,
Yamamoto K, et al: Effect of additional surgery after noncurative
endoscopic submucosal dissection for early gastric cancer. Endosc
Int Open. 4:E24–E29. 2016.PubMed/NCBI
|
|
28
|
Toya Y, Endo M, Nakamura S, Akasaka R,
Kosaka T, Yanai S, Kawasaki K, Koeda K, Sugai T and Matsumoto T:
Clinical outcomes of curative endoscopic submucosal dissection with
negative resected margins for gastric cancer. Gastrointest Endosc.
6:1218–1224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oda I, Gotoda T, Sasako M, Sano T, Katai
H, Fukagawa T, Shimoda T, Emura F and Saito D: Treatment strategy
after non-curative endoscopic resection of early gastric cancer. Br
J Surg. 95:1495–1500. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kusano C, Iwasaki M, Kaltenbach T, Conlin
A, Oda I and Gotoda T: Should elderly patients undergo additional
surgery after noncurative endoscopic resection for early gastric
cancer? Long-term comparative outcomes. Am J Gastroenterol.
106:1064–1069. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pyo JH, Lee H, Min BH, Lee JH, Kim KM, Yoo
H, Ahn S, An JY, Choi MG, Lee JH, et al: Comparison of long-term
outcomes after non-curative endoscopic resection in older patients
with early gastric cancer. Ann Surg Oncol. 24:2624–2631. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hwang JJ, Park KJ, Park YS, Lee HS, Yoon
H, Shin CM, Kim N and Lee DH: A scoring system for patients with a
tumor-positive lateral resection margin after endoscopic resection
of early gastric cancer. Surg Endosc. 30:2751–2758. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoon H, Kim SG, Choi J, Im JP, Kim JS, Kim
WH and Jung HC: Risk factors of residual or recurrent tumor in
patients with a tumor-positive resection margin after endoscopic
resection of early gastric cancer. Surg Endosc. 27:1561–1568. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim TK, Kim GH, Park DY, Lee BE, Jeon TY,
Kim DH, Jo HJ and Song GA: Risk factors for local recurrence in
patients with positive lateral resection margins after endoscopic
submucosal dissection for early gastric cancer. Surg Endosc.
29:2891–2898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sekiguchi M, Suzuki H, Oda I, Abe S,
Nonaka S, Yoshinaga S, Taniguchi H, Sekine S, Kushima R and Saito
Y: Risk of recurrent gastric cancer after endoscopic resection with
a positive lateral margin. Endoscopy. 46:273–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamashina T, Ishihara R, Nagai K, Matsuura
N, Matsui F, Ito T, Fujii M, Yamamoto S, Hanaoka N, Takeuchi Y, et
al: Long-term outcome and metastatic risk after endoscopic
resection of superficial esophageal squamous cell carcinoma. Am J
Gastroenterol. 108:544–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takubo K, Aida J, Sawabe M, Kurosumi M,
Arima M, Fujishiro M and Arai T: Early squamous cell carcinoma of
the oesophagus: The Japanese viewpoint. Histopathology. 51:733–742.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kitagawa Y, Uno T, Oyama T, Kato K, Kato
H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, et al:
Esophageal cancer practice guidelines 2017 edited by the Japan
esophageal society: Part 2. Esophagus. 16:25–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang S, Huang Y, Xie J, Zhuge L, Shao L,
Xiang J, Zhang Y, Sun Y, Hu H, Chen S, et al: Does delayed
esophagectomy after endoscopic resection affect outcomes in
patients with stage T1 esophageal cancer? A propensity score-based
analysis. Surg Endosc. 32:1441–1448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ikeda A, Hoshi N, Yoshizaki T, Fujishima
Y, Ishida T, Morita Y, Ejima Y, Toyonaga T, Kakechi Y, Yokosaki H
and Azuma T: Endoscopic submucosal dissection (ESD) with additional
therapy for superficial esophageal cancer with submucosal invasion.
Intern Med. 54:2803–2813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Molena D, Schlottmann F, Boys JA, Blackmon
SH, Dickinson KJ, Dunst CM, Hofstetter WL, Lada MJ, Louie BE, Mungo
B, et al: Esophagectomy following endoscopic resection of
submucosal esophageal cancer: A highly curative procedure even with
nodal metastases. J Gastrointest Surg. 21:62–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Plum PS, Hölscher AH, Pacheco Godoy K,
Schmidt H, Berlth F, Chon SH, Alakus H and Bollschweiler E:
Prognosis of patients with superficial T1 esophageal cancer who
underwent endoscopic resection before esophagectomy-A propensity
score-matched comparison. Surg Endosc. 32:3972–3980. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Suzuki G, Yamazaki H, Aibe N, Masui K,
Sasaki N, Shimizu D, Kimoto T, Shiozaki A, Dohi O, Fujiwara H, et
al: Endoscopic submucosal dissection followed by chemoradiotherapy
for superficial esophageal cancer: Choice of new approach. Radiat
Oncol. 13:2462018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hirasawa K, Kokawa A, Oka H, Yahara S,
Sasaki T, Nozawa A and Tanaka K: Superficial adenocarcinoma of the
esophagogastric junction: Long-term results of endoscopic
submucosal dissection. Gastrointest Endosc. 72:960–966. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Motoyama S, Jin M, Matsuhashi T, Nanjo H,
Ishiyama K, Sato Y, Yoshino K, Sasaki T, Wakita A, Saito H, et al:
Outcomes of patients receiving additional esophagectomy after
endoscopic resection for clinically mucosal, but pathologically
submucosal, squamous cell carcinoma of the esophagus. Surg Today.
43:638–642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pennathur A, Farkas A, Krasinskas AM,
Ferson PF, Gooding WE, Gibson MK, Schuchert MJ, Landreneau RJ and
Luketich JD: Esophagectomy for T1 esophageal cancer: Outcomes in
100 patients and implications for endoscopic therapy. Ann Thorac
Surg. 87:1048–1055. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dubecz A, Kern M, Solymosi N, Schweigert M
and Stein HJ: Predictors of lymph node metastasis in surgically
resected T1 esophageal cancer. Ann Thorac Surg. 99:1879–1886. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kawaguchi1 G, Sasamoto R, Abe A, Ohta A,
Sato H, Tanaka K, Maruyama K, Kaizu M, Ayukawa F, Yamana N, et al:
The effectiveness of endoscopic submucosal dissection followed by
chemoradiotherapy for superficial esophageal cancer. Radiat Oncol.
10:312015. View Article : Google Scholar
|
|
49
|
Kam TY, Kountouri M, Roth A, Frossard JL,
Huber O, Mönig S and Zilli T: Endoscopic resection with adjuvant
chemo-radiotherapy for superficial esophageal squamous cell
carcinoma: A critical review. Crit Rev Oncol Hemat. 124:61–65.
2018. View Article : Google Scholar : PubMed/NCBI
|