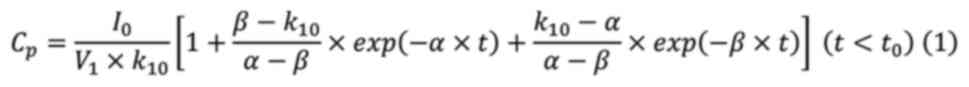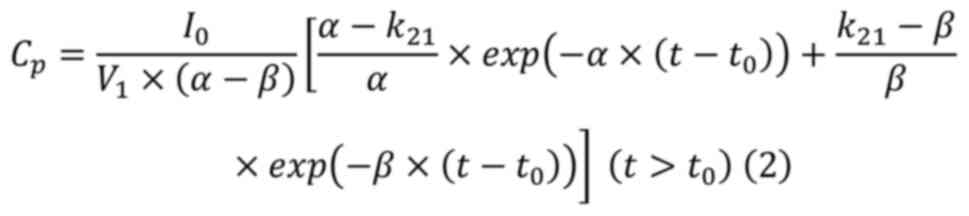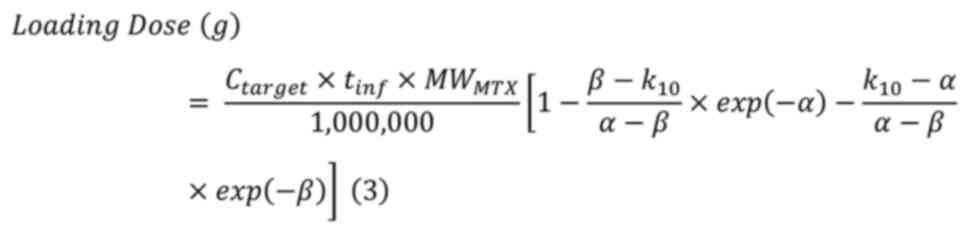Introduction
Methotrexate (MTX) inhibits the enzyme that converts
dihydrofolate to tetrahydrofolate and exhibits antineoplastic and
immunosuppressive effects (1,2). For
the antineoplastic effects of MTX, it is necessary to increase drug
delivery to cancer cells by passive membrane transport based on
concentration gradients. Therefore, high-dose MTX (HD-MTX) therapy
is used in clinical settings. However, administration of HD-MTX to
the body causes severe toxicity in normal cells, resulting in
lethal side effects. Therefore, leucovorin, an active folate, is
administered to reduce this toxicity (3–6).
Inhibition of the folate cycle of MTX in cancer cells is not
compensated by leucovorin because cancer cells do not have a
mechanism for leucovorin uptake (4,6–8). Due
to this advantage, HD-MTX therapy is widely used at present and
exhibits high efficacy in various carcinomas, especially
osteosarcoma (9–14).
MTX exhibits time-dependent antitumor effects, and
exposure time as well as the maximum concentration
(Cmax) are important in HD-MTX therapy (2,10,15).
When MTX is infused continuously for over 4–6 h, Cmax of
more than 700–1,000 µmol/l is associated with prolonged
disease-free survival, tumor necrosis, and improved 5-year survival
rates in patients with osteosarcoma (16–21).
However, in infusing for over 6 h, Cmax of more than
1,000 µmol/l is suggested to no longer improve the efficacy
(20). Therefore, in the clinical
field, where continuous infusion over 6 h is widely used,
increasing the Cmax to about 700–1,000 µmol/l for
successful treatment is recommended. In contrast, some reports
suggest that Cmax is not associated with clinical
efficacy (10,21–23).
Intra-individual variability in blood MTX levels has been pointed
to as a factor underlying these contradictory results (24,25).
Because of the large intra-individual variability in MTX clearance
depending on each course of HD-MTX therapy, it is thought that many
of the previous studies have not been able to assess adequate blood
MTX levels. Therefore, the importance of blood MTX concentrations
in efficacy remains inconclusive. However, dosing regimens in
HD-MTX therapy have been designed based on blood MTX levels, as
these levels may be the only predictive factor for efficacy.
Adverse events are also a major problem in HD-MTX
therapy, and even with leucovorin rescue, HD-MTX therapy remains
highly toxic. For safety, serum MTX concentrations of less than 10,
1, and 0.1 µmol/l at 24, 48, and 72 h, respectively, after starting
MTX administration are recommended (1,6,21,26–31).
Delayed MTX excretion not only causes serious adverse events such
as myelosuppression, renal dysfunction, hepatic dysfunction, and
mucositis but also makes it difficult to continue the treatment and
worsens patient prognosis (1,10).
Approximately 10% of deaths in patients with osteosarcoma are
reported to be caused by factors other than osteosarcoma, and MTX
is considered to be the most important causative drug related to
death (32). Therefore, less toxic
therapies for osteosarcoma that do not depend on HD-MTX therapy
have also been investigated (33,34),
however their clinical applicability has not been established.
Consequently, the safe administration of HD-MTX therapy, which has
a high risk of adverse effects, is crucial for patient prognosis
and requires that blood MTX levels be maintained within the
effective concentration range, followed by a rapid reduction to the
safety range.
The body surface area (BSA) method, which calculates
the dose based on BSA, is widely used in HD-MTX therapy and shows
that 8–12 g/m2 of MTX is required to achieve a
Cmax >700 µmol/l by continuous infusion for 6 h
(4,16,18,20,23,28,35).
However, the serum concentration of MTX varies by 5–10 times in
BSA-based dosing designs (21–23,36),
because the BSA method does not account for intra-individual
variability between courses (24,25),
in addition to inter-individual variability in MTX clearance due to
several factors, including renal function, gender, and age
(37–40). Thus, the high efficacy and safety of
HD-MTX therapy cannot be ensured in several cases. As an
individualized dosing method that also considers intra-individual
variability, methods utilizing pharmacokinetic (PK) parameters have
attracted research attention (41).
In 2010, to stabilize blood MTX levels in individuals with
osteosarcoma, Fujita et al (42) developed a dose-adjustment method
using the PK parameter (PK method) for each patient to calculate
MTX dose for loading (0–1 h) and maintenance (1–6 h) infusion by
analyzing individual PK parameters of the serum MTX concentration
profile from the previous course and showed its safety despite of
larger dose compared to traditional constant rate infusion for 0–6
h in nine patients with osteosarcoma. However, whether the PK
method can control the blood MTX concentration to the effective
range and safely administer MTX in comparison with the conventional
BSA method, remain known. To optimize the treatment of osteosarcoma
patients with HD-MTX therapy, appropriate evaluation of the effect
on blood levels and the safety of the PK method is necessary.
Therefore, in this study, to verify the utility of the PK method
for designing individualized dosing in HD-MTX therapy, the target
concentration achievement rate for efficacy and safety using the
BSA and PK methods was evaluated retrospectively.
Materials and methods
Subjects and HD-MTX regimen
Patients with osteosarcoma who underwent HD-MTX
therapy at the Department of Orthopedic Surgery, Gunma University
Hospital, from April 2004 to March 2020 were enrolled in this
study. During the HD-MTX therapy, the MTX dose in the first course
was determined by the BSA method; a total of 8–12 g/m2
was administered as an initial dose for 1 h and a maintenance dose
for 5 h. In the subsequent courses, loading and maintenance
infusion doses were calculated by the PK method using the PK
parameters of each patient, which were calculated based on their
serum concentration profiles from the previous course, according to
the report by Fujita et al (42). The target serum MTX concentration
was 700–1,000 µmol/l after 1–6 h, and less than 10, 1, and 0.1
µmol/l at 24, 48, and 72 h, respectively, from the start of
administration. The loading and maintenance doses can be slightly
adjusted according to the discretion of the attending physician.
Leucovorin rescue was initiated 24 h after starting HD-MTX therapy.
Leucovorin was started at a dose of 21 mg administered every 3 h
and adjusted according to the serum concentration of MTX at 48 and
72 h. After 72 h of MTX treatment, leucovorin was continued until
the serum MTX concentration reached 0.1 µmol/l. Sodium bicarbonate
was administered to maintain urine pH >7, and hydration and
acetazolamide were administered to maintain urine volume.
Data collection and assessment
Electronic medical records from Gunma University
Hospital were used to retrospectively survey patient history and
MTX-related laboratory data. The following characteristics were
surveyed: age, sex, height, weight, BSA, diagnosis, site of onset,
MTX dose, serum MTX concentration (a total of 10 points at 1, 2, 4,
6, 7, 9, 12, 24, 48, and 72 h after the start of MTX treatment, as
C1-C72), number of courses of HD-MTX therapy,
laboratory data [aspartate aminotransferase (AST), alanine
aminotransferase (ALT), and serum creatinine levels], and treated
patients with toxic MTX levels. As an efficacy index, the
achievement of the MTX effective concentration (700–1,000 µmol/l)
at Cmax and the mean concentration during maintenance
dose [Cmean (1–6)] were evaluated. The achievement
of the safety range (C24 <10 µmol/l, C48
<1 µmol/l, C72 <0.1 µmol/l), and the incidence of
hepatic and renal dysfunction within 1 week after MTX
administration were assessed to determine safety. AST, ALT, and
creatinine clearance (Ccr) were used as indices of hepatic and
renal dysfunction, respectively, and the Common Terminology
Criteria for Adverse Events version 5.0 was used to evaluate the
grade of the adverse event.
Calculation of dosage in the PK
method
The PK parameter was calculated on a 10-point scale
based on the serum MTX concentrations using the method described by
Fujita et al (42). Assuming
that the serum concentration profiles of MTX were represented by a
linear two-compartment model, the serum MTX concentrations before
and after the end of maintenance dose were applied to equations
(1) and (2), respectively, and the nonlinear
least-squares MULTI program was used to calculate α, β, the
elimination rate constant (k10), and distribution volume
of the central compartment (V1) (43).
Where Cp: Serum concentration;
I0: Infusion rate; V1: distribution volume of
central compartment; α and β: elimination rate constants at
distribution and terminal phase (the real solutions of the equation
[s2 + (k10 + k12 +
k21)][s + (k21
+ k10)]=0), respectively; k10: the
elimination rate constant; k12 and k21: inter
compartmental transfer rate constants; t: time after the start of
administration, and t0: the duration of infusion (6 h).
The loading and maintenance infusion doses were
calculated using the equations (3)
and (4). α and β were calculated
from equations (1) and (2), and CLtot was estimated by
dividing the total dose by the area under the concentration-time
curve (AUC) calculated using the trapezoidal method.
Ctarget was set at 700 µmol/l; tinf for
loading infusion and maintenance infusion was 1 and 5 h,
respectively, and MWMTX was 454.45.
Where Ctarget is the target
concentration; tinf is the infusion time;
MWMTX is the molecular weight of MTX, and
CLtot is the total body clearance.
Statistical analysis
Unpaired Student's t-test was used to compare the
mean values of the PK parameters of the BSA and PK methods. The
achievement rates of target concentrations of efficacy and safety
and the incidence of adverse events of each dosing method were
compared using Pearson's chi-square test or Fisher's exact test.
Adverse events were divided into two categories for analysis: Grade
≥2 adverse events, which required treatment, and grade ≤1 adverse
events, which did not require treatment. Logistic regression
analysis was used to correct for the effects of known factors (age,
sex, creatinine clearance immediately before MTX administration,
and MTX dosage) on the association of each dosing method with
delayed MTX excretion assessed by C24, C48,
and C72 and adverse events (37–40,44).
Statistical analysis was performed using the SPSS software version
26.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of patients
Patient characteristics are shown in Table I. A total of 43 patients were
included, with a median age of 17.0 years. In the first dose, 43
courses with the BSA method were performed. In the subsequent
courses, 200 courses of the PK method were performed.
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
|
Characteristics | Values |
|---|
| Sex, n (%) |
|
|
Male | 26 (60.5) |
|
Female | 17 (39.5) |
| Median age (range),
years | 17 (8–74) |
| Median body surface
area (range), m2 | 1.56
(0.71-1.98) |
| Median total number
of courses of HD-MTX therapy (range) | 5 (1–12) |
| Median serum
creatinine at diagnosis(range), mg/dl | 0.57
(0.22-1.67) |
| Median creatinine
clearance at diagnosis (range), ml/min | 135.38
(61.07-307.58) |
| Location, n
(%) |
|
| Lower
limb | 24 (55.8) |
| Upper
limb | 8 (18.6) |
|
Pelvis | 6 (14.0) |
|
Others | 5 (11.6) |
MTX concentration and clearance for
each dosing design
Table II and
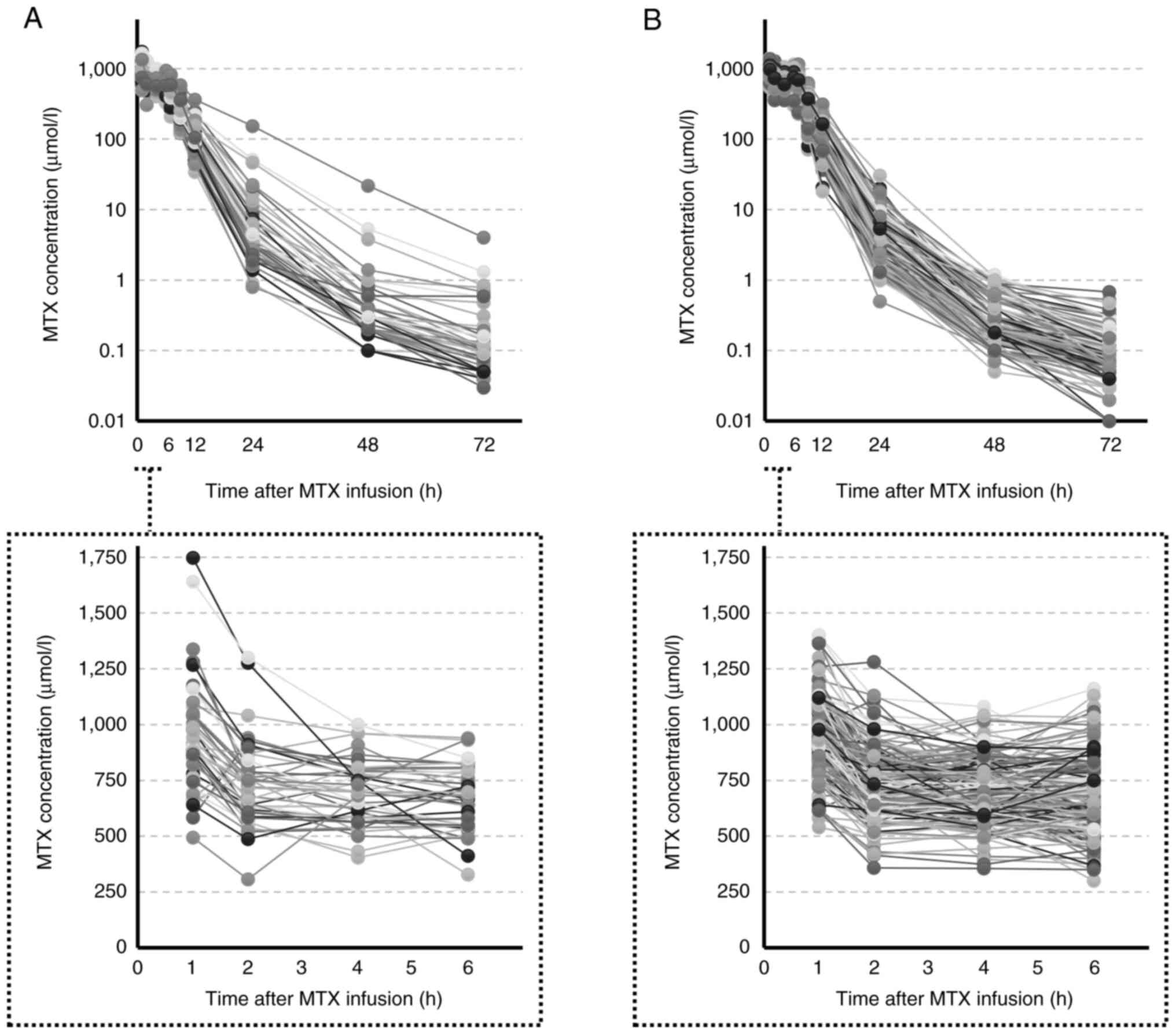
Fig. 1 show the dosing and blood
concentration profiles in the BSA and PK methods. There were no
significant differences in the MTX dosage and mean blood
concentration of the effective range between the BSA and PK
methods. The serum concentration was the highest immediately after
completion of loading infusion (at 1 h), and the coefficient of
variation was 26.7% in the BSA method and 17.4% in the PK method.
Similarly, for Cmax and Cmean (1–6),
the coefficients of variation of the BSA method were 23.2 and
18.5%, and those of the PK method were 17.2 and 16.3%,
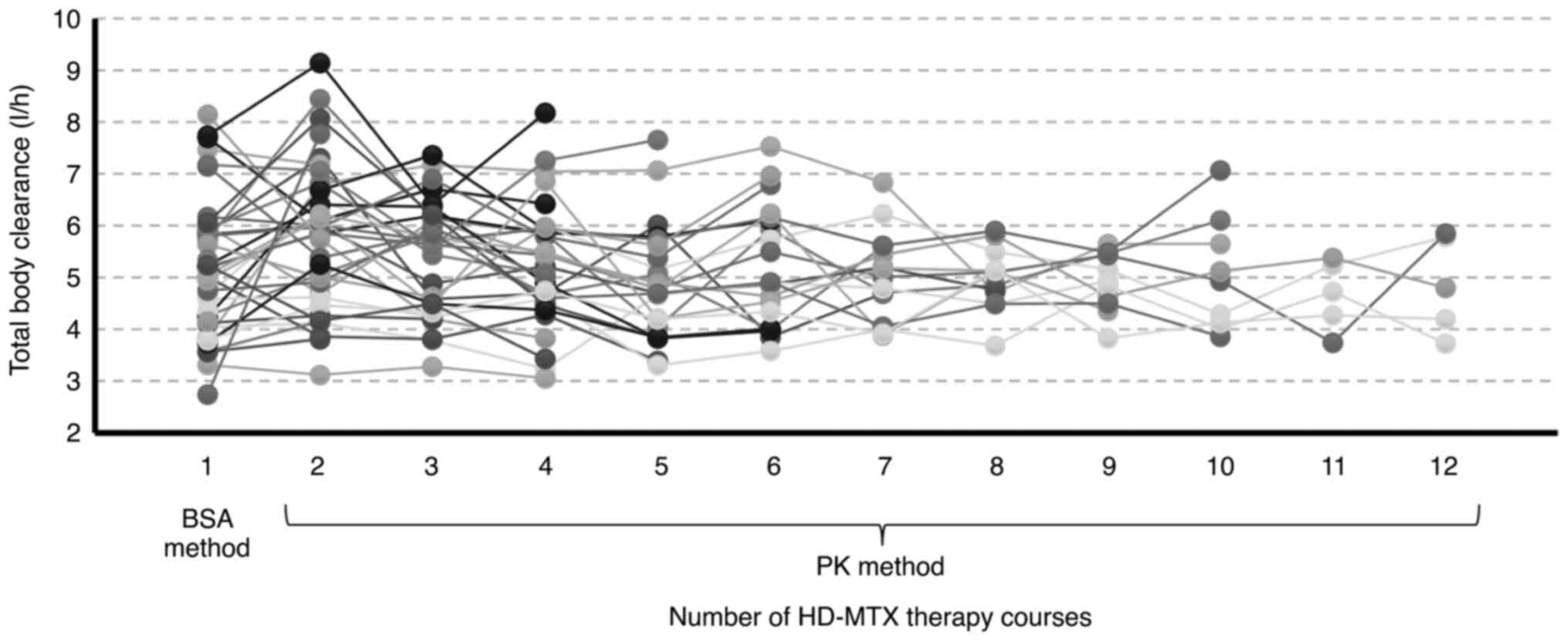
respectively. MTX clearance varied from 2.74-8.14 l/h in the first
course administered by the BSA method, with a 0.74-2.84-fold change
in MTX clearance after the second course compared to the first
course (Fig. 2).
 | Table II.Dosage and concentration of MTX for
each dosing design. |
Table II.
Dosage and concentration of MTX for
each dosing design.
| Parameters | BSA method
n=43 | PK method
n=200 | P-value |
|---|
| Loading dose,
g/m2 | 4.44±0.53
(11.9) | 4.30±0.71
(16.5) | 0.148 |
| Maintenance dose,
g/m2 | 5.45±0.62
(11.4) | 5.49±1.13
(20.6) | 0.766 |
| Total dose,
g/m2 | 9.90±0.80
(8.1) | 9.80±1.64
(16.7) | 0.551 |
| C1,
µmol/l | 945.8±252.4
(26.7) | 936.8±163.3
(17.4) | 0.824 |
| C2,
µmol/l | 734.4±190.5
(25.9) | 733.9±143.0
(19.5) | 0.985 |
| C4,
µmol/l | 694.6±138.6
(20.0) | 697.6±132.3
(19.0) | 0.897 |
| C6,
µmol/l | 666.6±142.6
(21.4) | 679.1±157.7
(23.2) | 0.637 |
| C24,
µmol/l | 11.04±24.56
(222.5) | 4.32±3.67
(85.0) | 0.081 |
| C48,
µmol/l | 1.07±3.36
(314.0) | 0.27±0.22
(81.5) | 0.128 |
| C72, µmol/l | 0.30±0.64
(213.3) | 0.09±0.08
(88.9) | 0.042 |
| Cmax,
µmol/l | 973.6±225.6
(23.2) | 941.8±162.1
(17.2) | 0.280 |
| Cmean
(1–6), µmol/l | 760.4±140.5
(18.5) | 762.2±124.5
(16.3) | 0.932 |
| AUC
(0–72), µmol/l × h | 6732±1569
(23.3) | 6493±1203
(18.5) | 0.349 |
| CLtot,
l/h | 5.14±1.30
(25.3) | 5.24±1.12
(21.4) | 0.593 |
Achievement rates of target concentrations are
shown in Table III. The rate of
achieving the target concentration (700–1,000 µmol/l) of Cmean
(1–6) was significantly higher in the PK
method than in the BSA method (P=0.030), but that of
Cmax was not significantly different (P=0.735). On the
contrary, zero cases of Cmax >1,500 µmol/l were found
in the PK method, which was significantly lower than the two cases
in the BSA method (P=0.033). The rates of reaching the safety range
for C24, C48, and C72 were
significantly higher in the PK method than in the BSA method at all
concentrations (P<0.001, P=0.003, and P=0.006, respectively). Of
the cases, wherein C48 became toxic, four courses of the
BSA method and two courses of the PK method required advanced
intervention with cholestyramine administration in addition to
usual leucovorin rescue therapy. The rate of advanced intervention
required was significantly lower with the PK method (P=0.010).
Furthermore, in addition to age and sex, the BSA method was
extracted as an independent factor for delayed MTX excretion
(C24>10 µmol/l, C48>1 µmol/l,
C72>0.1 µmol/l), and adjusted odds ratios were 3.534
(95% CI: 1.326–9.434, P=0.012), 8.065 (95% CI: 2.020–32.29,
P=0.003), and 2.299 (95% CI: 1.107–4.762, P=0.025) (Table SI).
 | Table III.The achievement rate of target MTX
concentration. |
Table III.
The achievement rate of target MTX
concentration.
|
Characteristics | BSA method
n=43 | PK method
n=200 | P-value |
|---|
| Efficacy |
|
|
|
|
Cmax |
|
|
|
|
700-1,000
µmol/l | 27 (62.8) | 131 (65.5) | 0.735a |
|
<700
µmol/l | 2 (4.7) | 12 (6.0) | 1.000b |
|
>1,000
µmol/l | 14 (32.6) | 57 (28.5) | 0.596a |
|
Cmean (1–6) |
|
|
|
|
700-1,000
µmol/l | 22 (51.1) | 137 (68.5) | 0.030a |
|
<700
µmol/l | 18 (41.9) | 56 (28.0) | 0.073a |
|
>1,000
µmol/l | 3 (7.0) | 7 (3.5) | 0.388b |
| Safety |
|
|
|
|
C24 <10
µmol/l | 33 (76.7) | 187 (93.5) |
<0.001a |
|
C48 <1
µmol/l | 37 (86.0) | 196 (98.0) | 0.003b |
|
C72 <0.1
µmol/l | 20 (46.5) | 137 (68.5) | 0.006a |
Adverse events
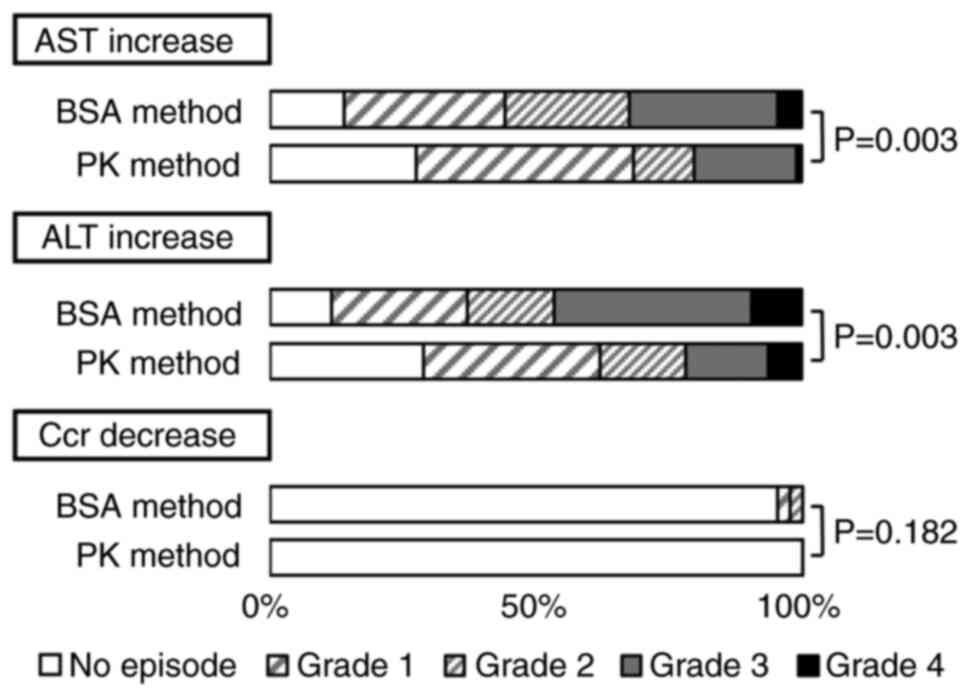
The incidences of hepatic and renal dysfunction in
the BSA and PK methods are shown in Fig. 3. Increase in AST and ALT within 1
week after HD-MTX administration were significantly lower in the PK
method than in the BSA method (P=0.003 and 0.003). Furthermore, in
addition to age and sex, the BSA method was extracted as an
independent factor for increased AST and ALT levels, and adjusted
odds ratios were 2.941 (95% CI: 1.404-6.173, P=0.004), and 3.205
(95% CI: 1.490-6.897, P=0.003), respectively (Table SII). Although there was no
significant difference in the decrease in Ccr (P=0.182), none of
the patients with Ccr decreased when the PK method was used. Since
there were few cases of decreased Ccr, a multivariate analysis
could not be performed.
Discussion
In the BSA method, widely used for dose
calculation, serum MTX concentrations of patients vary widely
because of large inter-individual and intra-individual variability
(21–25), and administration of HD-MTX is
difficult in many cases. A dosing method based on individual PK
parameters is one of the choices. To our knowledge based on our
findings, although this has not been validated in an in
vitro study, the utilization of PK parameters has been
clinically proven to be useful in anticancer therapy (41,42,45–47).
However, the utility of the PK method for osteosarcoma compared to
conventional methods has not been validated. Thus, we examined
whether the use of the PK method helped achieve the target
concentrations for efficacy and safety and confirmed its usefulness
in patients with osteosarcoma.
In the PK method, the mean values of C1
after loading infusion and Cmean (1–6)
during maintenance infusion were similar to those of the BSA
method, and the serum concentration during maintenance infusion
tended to decrease with time. Therefore, to maintain
C1-C6 at 700–1,000 µmol/l, it may be
necessary to change to the more appropriate PK model to predict the
increased maintenance dose. Evaluating by Cmean (1–6),
the control rate within the target concentration was significantly
higher in the PK method than in the BSA method (P=0.030). In
addition, significantly fewer patients had a concentration of more
than 1,500 µmol/l, a known poor prognostic factor (10,23),
in the PK method compared to the BSA method (P=0.033). This may be
due to the smaller variation in C1-C6 in the
PK method as compared to the BSA method. Regarding safety, the
rates of reaching the safety range for C24,
C48, and C72 were significantly higher than
those by the BSA method (P<0.001, P=0.003, and P=0.006,
respectively). Furthermore, the incidence of hepatic dysfunction
caused by MTX was also significantly lower than that found by the
BSA method (P=0.005 and 0.001), suggesting that the PK method was
safer than the BSA method. Consequently, although it is difficult
to increase the maintenance dose with the BSA method due to delayed
MTX excretion and the risk of adverse events, the maintenance dose
can be increased to maintain the C1-C6
concentration at 700–1,000 µmol/l while avoiding adverse events
using the PK method. On the other hand, maintenance of high MTX
concentrations has only been demonstrated in some in vitro
and in vivo studies (2,10,15),
and the clinical usefulness and target values of Cmean
(1–6) need to be verified in detail in
the future.
MTX clearance varies with its repeated
administration (24,25), and this study also confirmed a
0.47-2.84-fold change in MTX clearance compared to the clearance
after the first administration. Despite this change in MTX
clearance, we thought that the PK method was able to control the
target therapeutic concentration range safely compared to the BSA
method because of the individualized dosing method that considers
more immediate prior MTX clearance. For individual differences in
MTX concentrations, the population PK analysis of MTX by Dupuis
et al (48) and Lui et
al (49) reported that the
contribution of BSA was small and that of individual patient
clearance was large, consistent with our data. Moreover, our
results are comparable to those of Pauley et al (45) that validated the utility of an
individualized dosing design for acute lymphocytic leukemia (ALL)
utilizing changes in MTX clearance in the previous course, similar
to the approach used in this study. However, even with the PK
method, it was difficult to control the target concentration for
patients with large intra-individual variability between courses.
In recent years, a method to adjust MTX dosage in real-time by
analyzing blood MTX levels during continuous infusion in patients
with ALL has also been investigated (46,47).
In a patient with ALL who received MTX 3–5 g/m2 over 24
h, Shen et al (47) reported
that adjusting the infusion time, using the concentration at 16 h
after the start of MTX administration as a reference, not only
improved safety but also ensured the therapeutic target
concentration compared to the fixed-dose regimen. Foster et
al (46) found similar results
to Shen et al (47), using
MTX concentration at 2 and 6–8 h after MTX administration to adjust
the subsequent infusion time. While these methods do not require
complex PK analysis unlike our method, their application to HD-MTX
therapy for patients with osteosarcoma given continuous infusion at
4 or 6 h is very complicated. Moreover, it is an unsuitable method
for upward dose adjustment. Therefore, the PK method may be
considered the optimal dosing design for HD-MTX therapy in patients
with osteosarcoma at this time. Although the factors of
inter-individual variability are being clarified, the
intra-individual variability factors in MTX clearance for each
course are still unknown, and we believe that elucidating these
factors will improve the PK method to a more accurate
individualized dosing method.
This study has the following limitations. First, it
is a single-center retrospective survey; thus, multiple biases are
possible and no causal effect can be proved. Second, many blood
samples is required, and the procedure is challenging to perform.
Because the necessity of blood collection at all points has not
been mentioned, the number of blood collection points needs to be
revised. In addition, to reduce the burden on patients and health
personnel, it is necessary to further verify the necessity of
switching to the PK method in patients for whom C24,
C48, and C72 enter the safe range, and
Cmean (1–6) and Cmax reach the
effective concentration range by the BSA method.
In conclusion, this study demonstrated for the
first time that the PK method significantly reduced the incidence
of adverse events as well as increased the rate of achieving the
effective serum concentration range and safety range as compared to
the BSA method in patients with osteosarcoma who require higher
doses of MTX than other diseases. Therefore, the PK method is very
useful for HD-MTX therapy.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Emiri Takahashi
and Dr Yuta Takahashi (Faculty of Pharmacy, Takasaki University of
Health and Welfare, Gunma, Japan), for their guidance and help.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
AN and TA contributed to the study design and
drafting of the manuscript. HY, AK and TS collected and analyzed
the clinical data. AN and TA confirmed the authenticity of all the
raw data. TY, KO and KY were involved in data interpretation and
discussion. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committees of
Gunma University (approval no. HS2020-002) and Takasaki University
of Health and Welfare (approval no. 2007). This study was a
retrospective study using data from the past 16 years, and it was
difficult to obtain informed consent from all subjects. Hence,
based on the approval of the Ethics Committee, an opt-out approach
was adopted instead of obtaining consent from all participants. The
study was widely publicized, and sufficient time was allowed for
the study subjects to declare their willingness of refusal to
participate in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Widemann BC and Adamson PC: Understanding
and managing methotrexate nephrotoxicity. Oncologist. 11:694–703.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rizvi SAA, Shahzad Y, Saleh AM and
Muhammad N: Dose issues in cancer chemotherapy. Oncology.
98:520–527. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tishler M, Caspi D, Fishel B and Yaron M:
The effects of leucovorin (folinic acid) on methotrexate therapy in
rheumatoid arthritis patients. Arthritis Rheum. 31:906–908. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jaffe N and Gorlick R: High-dose
methotrexate in osteosarcoma: Let the questions surcease-time for
final acceptance. J Clin Oncol. 26:4365–4366. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shea B, Swinden MV, Ghogomu ET, Ortiz Z,
Katchamart W, Rader T, Bombardier C, Wells GA and Tugwell P: Folic
acid and folinic acid for reducing side effects in patients
receiving methotrexate for rheumatoid arthritis. J Rheumatol.
41:1049–1060. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang R, Mei S and Zhao Z: Leucovorin
(folinic acid) rescue for high-dose methotrexate: A review. J Clin
Pharm Ther. 47:1452–1460. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilmanns W, Sauer H and Schalhorn A:
Biochemical control of high-dose methotrexate/leucovorin rescue
therapy. Recent Results Cancer Res. 74:42–49. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Visentin M, Zhao R and Goldman ID: The
antifolates. Hematol Oncol Clin North Am. 26629–648. (ix)2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meyers PA, Gorlick R, Heller G, Casper E,
Lane J, Huvos AG and Healey JH: Intensification of preoperative
chemotherapy for osteogenic sarcoma: Results of the memorial
sloan-kettering (T12) protocol. J Clin Oncol. 16:2452–2458. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crews KR, Liu T, Rodriguez-Galindo C, Tan
M, Meyer WH, Panetta JC, Link MP and Daw NC: High-dose methotrexate
pharmacokinetics and outcome of children and young adults with
osteosarcoma. Cancer. 100:1724–1733. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anninga JK, Gelderblom H, Fiocco M, Kroep
JR, Taminiau AHM, Hogendoorn PCW and Egeler RM: Chemotherapeutic
adjuvant treatment for osteosarcoma: Where do we stand? Eur J
Cancer. 47:2431–2445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang B and Zhang Y, Li R, Li J, Lu X and
Zhang Y: The efficacy and safety comparison of first-line
chemotherapeutic agents (high-dose methotrexate, doxorubicin,
cisplatin, and ifosfamide) for osteosarcoma: A network
meta-analysis. J Orthop Surg Res. 15:512020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rathore R and Van Tine BA: Pathogenesis
and current treatment of osteosarcoma: Perspectives for future
therapies. J Clin Med. 10:11822021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rajeswari B, Guruprasad CS, Nair M,
Prasanth VR, Sugath BS and Thankamony P: High dose methotrexate
containing regimen in pediatric non-metastatic extremity
osteosarcoma patients: Experience from a tertiary cancer center in
India. Pediatr Hematol Oncol. 39:225–232. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pinedo HM and Chabner BA: Role of drug
concentration, duration of exposure, and endogenous metabolites in
determining methotrexate cytotoxicity. Cancer Treat Rep.
61:709–715. 1977.PubMed/NCBI
|
|
16
|
Ferrari S, Sassoli V, Orlandi M, Strazzari
S, Puggioli C, Battistini A and Bacci G: Serum methotrexate (MTX)
concentrations and prognosis in patients with osteosarcoma of the
extremities treated with a multidrug neoadjuvant regimen. J
Chemother. 5:135–141. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Graf N, Winkler K, Betlemovic M, Fuchs N
and Bode U: Methotrexate pharmacokinetics and prognosis in
osteosarcoma. J Clin Oncol. 12:1443–1451. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Delepine N, Delepine G, Cornille H, Brion
F, Arnaud P and Desbois JC: Dose escalation with pharmacokinetics
monitoring in methotrexate chemotherapy of osteosarcoma. Anticancer
Res. 15:489–494. 1995.PubMed/NCBI
|
|
19
|
Bacci G, Ferrari S, Picci P, Zolezzi C,
Gherlinzoni F, Iantorno D and Cazzola A: Methotrexate serum
concentration and histological response to multiagent primary
chemotherapy for osteosarcoma of the limbs. J Chemother. 8:472–478.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bacci G, Ferrari S, Delepine N, Bertoni F,
Picci P, Mercuri M, Bacchini P, Brach del Prever A, Tienghi A,
Comandone A and Campanacci M: Predictive factors of histologic
response to primary chemotherapy in osteosarcoma of the extremity:
Study of 272 patients preoperatively treated with high-dose
methotrexate, doxorubicin, and cisplatin. J Clin Oncol. 16:658–663.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin F, Juan Y, Zheng SE, Shen Z, Tang LN,
Zhao H and Yao Y: Relationship of serum methotrexate concentration
in high-dose methotrexate chemotherapy to prognosis and
tolerability: A prospective cohort study in Chinese adults with
osteosarcoma. Curr Ther Res Clin Exp. 70:150–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zelcer S, Kellick M, Wexler LH, Shi W,
Sankaran M, Lo S, Healey J, Huvos AG, Meyers PA and Gorlick R:
Methotrexate levels and outcome in osteosarcoma. Pediatr Blood
Cancer. 44:638–642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang B, Yao H, Xie X, Yin J, Zou C, Huang
G and Shen J: Relationship of peak serum methotrexate concentration
to prognosis and drug tolerance in non-metastatic extremity
osteosarcomas. Cancer Chemother Pharmacol. 82:221–227. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawakatsu S, Nikanjam M, Lin M, Le S,
Saunders I, Kuo DJ and Capparelli EV: Population pharmacokinetic
analysis of high-dose methotrexate in pediatric and adult oncology
patients. Cancer Chemother Pharmacol. 84:1339–1348. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arshad U, Taubert M, Seeger-Nukpezah T,
Ullah S, Spindeldreier KC, Jaehde U, Hallek M, Fuhr U, Vehreschild
JJ and Jakob C: Evaluation of body-surface-area adjusted dosing of
high-dose methotrexate by population pharmacokinetics in a large
cohort of cancer patients. BMC Cancer. 21:7192021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stoller RG, Hande KR, Jacobs SA, Rosenberg
SA and Chabner BA: Use of plasma pharmacokinetics to predict and
prevent methotrexate toxicity. N Engl J Med. 297:630–634. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perez C, Wang YM, Sutow WW and Herson J:
Significance of the 48-h plasma level in high-dose methotrexate
regimens. Cancer Clin Trials. 1:107–111. 1978.PubMed/NCBI
|
|
28
|
Hegyi M, Gulácsi A, Cságoly E, Csordás K,
Eipel OT, Erdélyi DJ, Müller J, Nemes K, Lautner-Csorba O and
Kovács GT: Clinical relations of methotrexate pharmacokinetics in
the treatment for pediatric osteosarcoma. J Cancer Res Clin Oncol.
138:1697–1702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park JA and Shin HY: Influence of genetic
polymorphisms in the folate pathway on toxicity after high-dose
methotrexate treatment in pediatric osteosarcoma. Blood Res.
51:50–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Howard SC, McCormick J, Pui CH, Buddington
RK and Harvey RD: Preventing and managing toxicities of high-dose
methotrexate. Oncologist. 21:1471–1482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Traivaree C, Likasitthananon N,
Monsereenusorn C and Rujkijyanont P: The effect of intravenous
hydration strategy on plasma methotrexate clearance during
intravenous high-dose methotrexate administration in pediatric
oncology patients. Cancer Manag Res. 10:4471–4478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bielack SS, Blattmann C, Borkhardt A,
Csóka M, Hassenpflug W, Kabíčková E, Kager L, Kessler T, Kratz C,
Kühne T, et al: Osteosarcoma and causes of death: A report of 1520
deceased patients from the cooperative osteosarcoma study group
(COSS). Eur J Cancer. 176:50–57. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shaikh AB, Li F, Li M, He B, He X, Chen G,
Guo B, Li D, Jiang F, Dang L, et al: Present advances and future
perspectives of molecular targeted therapy for osteosarcoma. Int J
Mol Sci. 17:5062016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rathore R, Caldwell KE, Schutt C,
Brashears CB, Prudner BC, Ehrhardt WR, Leung CH, Lin H, Daw NC,
Beird HC, et al: Metabolic compensation activates pro-survival
mTORC1 signaling upon 3-phosphoglycerate dehydrogenase inhibition
in osteosarcoma. Cell Rep. 34:1086782021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kwong DL, Ha SY, Chau KY, Choi PH, Chan
GC, Kwong PW and Lau YL: Multidisciplinary management of
osteosarcoma: Experience in Hong Kong. Pediatr Hematol Oncol.
15:229–236. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Watanabe M, Fukuoka N, Takeuchi T,
Yamaguchi K, Motoki T, Tanaka H, Kosaka S and Houchi H: Developing
population pharmacokinetic parameters for high-dose methotrexate
therapy: Implication of correlations among developed parameters for
individual parameter estimation using the bayesian least-squares
method. Biol Pharm Bull. 37:916–921. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fukuhara K, Ikawa K, Morikawa N and
Kumagai K: Population pharmacokinetics of high-dose methotrexate in
Japanese adult patients with malignancies: A concurrent analysis of
the serum and urine concentration data. J Clin Pharm Ther.
33:677–684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wippel B, Gundle KR, Dang T, Paxton J,
Bubalo J, Stork L, Fu R, Ryan CW and Davis LE: Safety and efficacy
of high-dose methotrexate for osteosarcoma in adolescents compared
with young adults. Cancer Med. 8:111–116. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Young EP, Cheng WS, Bernhardt MB, Wang LL,
Rainusso N and Foster JH: Risk factors associated with delayed
methotrexate clearance and increased toxicity in pediatric patients
with osteosarcoma. Pediatr Blood Cancer. 67:e281232020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Abe K, Maeda-Minami A, Ishizu T, Iwata S,
Kobayashi E, Shimoi T, Kawano Y, Hashimoto H, Yamaguchi M, Furukawa
T, et al: Risk factors for hepatic toxicity of high-dose
methotrexate in patients with osteosarcoma. Anticancer Res.
42:1043–1050. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Porta-Oltra B and Merino-Sanjuán M:
Personalized pharmacotherapy in oncology: Application of
pharmacokinetic-pharmacodynamic criteria. Farm Hosp. 45:45–55.
2021.PubMed/NCBI
|
|
42
|
Fujita Y, Nakamura T, Aomori T, Nishiba H,
Shinozaki T, Yanagawa T, Takagishi K, Watanabe H, Okada Y, Nakamura
K, et al: Pharmacokinetic individualization of high-dose
methotrexate chemotherapy for the treatment of localized
osteosarcoma. J Chemother. 22:186–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yamaoka K, Tanigawara K, Nakagawa T and
Uno T: A pharmacokinetic analysis program (multi) for
microcomputer. J Pharmacobiodyn. 4:879–885. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Misaka KO, Suga Y, Staub Y, Tsubata A,
Shimada T, Sai Y and Matsushita R: Risk factors for delayed
elimination of methotrexate in children, adolescents and young
adults with osteosarcoma. In Vivo. 34:3459–3465. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pauley JL, Panetta JC, Crews KR, Pei D,
Cheng C, McCormick J, Howard SC, Sandlund JT, Jeha S, Ribeiro R, et
al: Between-course targeting of methotrexate exposure using
pharmacokinetically guided dosage adjustments. Cancer Chemother
Pharmacol. 72:369–378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Foster JH, Thompson PA, Bernhardt MB,
Margolin JF, Hilsenbeck SG, Jo E, Marquez-Do DA, Scheurer ME and
Schafer ES: A prospective study of a simple algorithm to
individually dose high-dose methotrexate for children with leukemia
at risk for methotrexate toxicities. Cancer Chemother Pharmacol.
83:349–360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shen YQ, Wang ZJ, Wu XY, Li K, Wang ZJ, Xu
WF, Zhou F and Jin RM: Dose-individualization efficiently maintains
sufficient exposure to methotrexate without additional toxicity in
high-dose methotrexate regimens for pediatric acute lymphoblastic
leukemia. Curr Med Sci. 42:769–777. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dupuis C, Mercier C, Yang C,
Monjanel-Mouterde S, Ciccolini J, Fanciullino R, Pourroy B, Deville
JL, Duffaud F, Bagarry-Liegey D, et al: High-dose methotrexate in
adults with osteosarcoma: A population pharmacokinetics study and
validation of a new limited sampling strategy. Anti Cancer Drugs.
19:267–273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lui G, Treluyer JM, Fresneau B,
Piperno-Neumann S, Gaspar N, Corradini N, Gentet JC, Marec Berard
P, Laurence V, Schneider P, et al: A pharmacokinetic and
pharmacogenetic analysis of osteosarcoma patients treated with
high-dose methotrexate: Data from the OS2006/sarcoma-09 trial. J
Clin Pharmacol. 58:1541–1549. 2018. View Article : Google Scholar : PubMed/NCBI
|