Introduction
In men, prostate cancer (PCa) is the second leading
cause of death worldwide (1,2). Early
detection and diagnosis are the most important steps to possibly
curing PCa before it metastasizes (3,4).
Different treatment modalities are important due to the resistance
of cancer cells to chemo- and antiandrogenic therapies in advanced
stages of the disease (5–7). With recent developments in the field
of oncolytic and phage-based cancer therapies, there is a growing
interest in novel approaches for cancer treatment. Recently,
bacteriophages have been considered alternative nanoparticles for
targeting, recognizing, and even killing cancer cells through phage
engineering or adeno-associated virus/phage hybrids (8). Numerous research groups worldwide have
reported the development of various phages, such as M13, MS2, T4,
and T7, and have studied their important applications (9–18). MS2
virus-like particles (VLPs) can carry cargoes of small interfering
RNA (siRNA) and modified RNA in the presence of specific nucleic
acids, which makes them an ideal vehicle for targeted therapeutic
drug delivery and imaging (11). In
breast cancer, bacteriophage MS2 RNA-free capsids have been
conjugated with anti-EGFR antibodies to target upregulated
receptors using in vitro and in vivo models (12). In addition to its production
efficiency, safety and non-toxicity, an MS2 VLP-based messenger RNA
vaccine against PCa induced potent humoral and cell-mediated immune
responses and slowed tumor growth, thus showing promising results
(13). Considering its in
vivo safety, the bacteriophage MS2-L2 VLP has been used for
oral immunization against various oral and vaginal human
papillomavirus infections as well as head and neck, and cervical
cancer (14). Similarly, VLPs are
used to target abnormal cells in vivo by targeting surface
peptides, e.g., a single chain fragment variable that binds to cell
surface receptors [such as androgen receptors (ARs) or G
protein-coupled receptors (GPCRs)] can be modified (15). In addition, nanovectors, such as MS2
VLPs, are used to target cancer cells for personalized therapy and
to deliver anticancer components, such as siRNAs or long non-coding
RNAs, for chemotherapy, immunotherapy, and radiotherapy; in this
process, these VLPs interfere with RNA expression in cancer cells
(16,17). MS2 bacteriophages target tumor
tissues through internalizing Arg-Gly-Asp (RGD) motif peptides,
which are ligands of integrins, and are used for the targeted
delivery of apoptosis-inducing agents, such as thallium (I) nitrate
(TlNO3 or NO3Tl) and thallium (I) ions
(Tl+) in tumor tissues (18). Since these agents penetrate
bacteriophage MS2 particles and bind to their RNA molecules without
inducing any side effects, they are particularly effective for
clinical applications (18).
Based on our previously reported work with PC3 and
LNCaP cells and their interactions with T4 and M13 phage (19–23);
we found that LNCaP cells more closely represent metastasis
Prostate cancer than PC3 cells due to their androgen independence.
Hence, we selected LNCaP cells for MS2 phage interaction studies.
Hence in the present study, key findings relating to the
interaction of the natural bacteriophage, MS2, with the PCa cell
line, LNCaP, were reported. Considering the importance of
bacteriophages for natural phage therapy and previous reports of
the interaction of T4 and M13 bacteriophages with PCa cell lines
(PC3 and LNCaP) affecting cell migration and viability and
modulating genes for cancer progression (19–23),
the present study is of great importance to understand the direct
effects of bacterial RNA viruses on PCa cell progression. Their
effects on cell viability and genes that are involved in cancer
cell proliferation [such as AR, AKT, PI3K, MAPK1, MAPK3,
heat shock protein 90 (HSP90), heat shock protein 27
(HSP27), and peroxisome proliferator-activated receptor-γ
coactivator 1α (PGC1A)], and adhesion, migration, and
invasion [such as integrins; integrin α5 (ITGA5), integrin
αV (ITGAV), integrin β1 (ITGB1), integrin β3
(ITGB3) and integrin β5 (ITGB5)] were investigated.
The results suggested that treatment affects cell metabolism and
renders LNCaP cells dependent on AR/SRC signaling and AKT, and
fibroblast growth factor (FGF)/MAPK signaling pathways, which can
be easily treated with drugs, suggesting the possibility of using
phages in combination therapies.
Materials and methods
LNCaP cell culture
LNCaP cells (ATCC CRL-1740) were obtained from
American Type Culture Collection. The cell culture protocol was as
described previously (23). Cells
were grown in a 25-cm2 culture flask (Qiagen, Inc.)
containing RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
50 µg/ml penicillin, 50 µg/ml streptomycin and 0.5 µg/ml
amphotericin B (Gibco; Thermo Fisher Scientific, Inc.), and were
incubated in 5% CO2 in a CO2 incubator at
37°C until 70% confluency. The cells were maintained in standard
culture conditions (37°C, 5% CO2 and 95% relative
humidity). Cells were treated with 0.05% trypsin (Gibco; Thermo
Fisher Scientific, Inc.) for 5 min at 37°C to detach the cells from
the flask surface and transferred to a new 75-cm2
culture flask (Qiagen, Inc.). During the experiment, the medium was
changed every 2 days, and the cells were observed daily with an
inverted microscope (Zeiss AG). Once cells reached 90% confluency,
they were treated with 0.05% trypsin and transferred to new culture
flasks or 24-well culture plates (Corning, Inc.) for hematoxylin
and eosin staining, MS2 phage treatments, and cell viability
experiments.
LNCaP cell exposure to bacteriophage
MS2
Bacteriophage MS2 (ZeptoMetrix®, LCC) was
recovered at a concentration of 5.0×1010 plaque forming
units (pfu)/ml in pre-made SM buffer (Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Phages were not
grown in bacterial culture due to possible contamination with
nucleic acids, lipopolysaccharides, or endotoxins. Phages were
centrifuged at 10,000 × g for 30 min at 4°C and then filtered
through a 0.22-µm cellulose acetate membrane filter
(MilliporeSigma). The resulting phage cells were further diluted in
a cell culture medium to reach 1×107 pfu/ml (20–23)
for LNCaP cancer cell treatment at 37°C for 24–48 h. This dilution
series reduced trace impurities (including endotoxins) from the
manufacturer-supplied stock solution (20–23).
MTT reduction assay
The LNCaP cells (5×104) were seeded in
24-well plates. When the cells reached 70% confluence, they were
treated with bacteriophage MS2 (107 pfu/ml). After 4,
24, and 48 h of exposure, cell viability was determined using the
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
(MilliporeSigma) reduction method according to the manufacturer's
instructions (24,25). The resulting purple formazan was
dissolved in 200 µl of dimethyl sulfoxide. The reaction was
performed in a 96-well plate and read using a spectrophotometer
(Asys Hitech GmbH; Harvard Bioscience, Inc.) at 550 nm to determine
the percentage of cell viability relative to control cells.
Hematoxylin and eosin staining
LNCaP cells were grown in 12-well plates with
coverslips, as previously described in the LNCaP cell culture
subsection. After reaching 30% confluency, LNCaP cells were exposed
to vehicle PBS or bacteriophage MS2 treatment at 30°C at the
highest concentration of 1×107 pfu/ml for 24 and 48 h,
washed with PBS, and fixed with 10% formaldehyde in PBS for 30 min
at 30°C. The cells were then washed with PBS and stained with 1%
hematoxylin (3–5 min) and 1% eosin (10 min) at room temperature.
Coverslips were dried in ethanol, and the samples were mounted on
glass slides using Permount™ (Thermo Fisher Scientific, Inc.) and
observed using a Leica light DMLB microscope (Leica Microsystems
GmbH).
RNA extraction and cDNA synthesis for
reverse transcription-quantitative PCR (RT-qPCR)
LNCaP cells were treated with the bacteriophage MS2
at a concentration of 1×107 cells at 37°C for 24 h. For
total RNA extraction, the culture medium was aspirated, and the
cells were washed with PBS. Total RNA was extracted using an
AllPrep DNA/RNA/protein extraction kit (Qiagen, Inc.) according to
the manufacturer's instructions. Extracted total RNA was quantified
using a NanoVue™ instrument (GE Healthcare). Finally, 2 µg total
RNA was reverse transcribed using the High-Capacity RNA-to-cDNA™
kit (Thermo Fisher Scientific, Inc) in a 20 µl reaction according
to the manufacturer's instructions.
Power SYBR™ Green/ROX qPCR 2X Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used for qPCR. The
total reaction volume per sample was 10 µl [5.0 µl Power SYBR
Green, 0.8 µl (800 nM) of each forward and reverse oligonucleotide
(Table I), 3.2 µl nuclease-free
water, 1 µl cDNA] and was performed in triplicate in 384-well
plates. The reaction was performed in a QuantStudio™ 12K Flex
thermal cycler (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and the results were analyzed using the QuantStudio™ 12K Flex
Real-Time PCR System v1.1 (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reaction consisted of the following cycles:
Step 1, 50°C for 2 min and 95°C for 2 min; step 2, 95°C for 1 sec
and step 3, 60°C for 30 sec; steps 2 and 3 were repeated 40 times;
dissociation curve with incubation at 95°C for 15 sec and 60°C for
1 min followed by a temperature gradient from 60 to 95°C at a rate
of 0.15°C per sec.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Genes | Forward primers
(5′-3′) | Reverse primers
(5′-3′) |
|---|
| ACTB |
GATTCCTATGTGGGCGACGA |
TGTAGAAGGTGTGGTGCCAG |
| AKT |
CATCGCTTCTTTGCCGGTATC |
ACTCCATGCTGTCATCTTGGTC |
| AR |
GACATGCGTTTGGAGACTGC |
CAATCATTTCTGCTGGCGCA |
| GAPDH |
GAATGGGCAGCCGTTAGGAA |
ATCACCCGGAGGAGAAATCG |
| HSP90 |
AGGGGGAAAGGGGAGTATCT |
ATGTCAACCCTTGGAGCAGC |
| HSP27 |
CGCGGAAATACACGCTGCC |
GACTCGAAGGTGACTGGGATG |
| ITGA5 |
GGGTGGTGCTGTCTACCTC |
GTGGAGCGCATGCCAAGATG |
| ITGAV |
AGGCACCCTCCTTCTGATCC |
CTTGGCATAATCTCTATTGCCTGT |
| ITGB1 |
GCCAAATGGGACACGCAAGA |
GTGTTGTGGGATTTGCACGG |
| ITGB3 |
CTGCCGTGACGAGATTGAGT |
CCTTGGGACACTCTGGCTCT |
| ITGB5 |
GGGCTCTACTCAGTGGTTTCG |
GGCTTCCGAAGTCCTCTTTG’ |
| MAPK1 |
TCAGCTAACGTTCTGCACCG |
ACTTGGTGTAGCCCTTGGA’ |
| MAPK3 |
ATCTTCCAGGAGACAGCACG |
TTCTAACAGTCTGGCGGGAG’ |
| PGC1A |
GAAGGGTACTTTTCTGCCCCT |
CTTCTTCCAGCCTTGGGGAG’ |
| PI3K |
AGAGCCCCGAGCGTTT |
TCGTGGAGGCATTGTTCTGA |
| STAT3 |
GCTTCCTGCAAGAGTCGAATG |
TGTAGAAGGCGTGATTCTTCCC |
The 2−ΔΔCq method was used to calculate
gene expression (26). This is
based on the exponential PCR reaction, according to the formula
QR=2−ΔΔCq, where QR represents the level of gene
expression, Cq represents the amplification cycle in which each
sample undergoes exponential amplification, ΔCq refers to the
difference between the Cq of the amplified sample for the target
gene and the Cq of the same amplified sample for the reference
gene, and ΔΔCq represents the difference between the ΔCq of the
sample of interest at a given time point and the ΔCq of the
reference sample. Fold change was calculated using
2−ΔΔCq, and the log2 fold change
(log2FC) was calculated. The results are presented as
the log2FC.
Reactions were performed using GAPDH as an
endogenous control in triplicate for 14 target genes: ACTB, AKT,
AR, HSP27, HSP90, ITGA5, ITGAV, ITGB1, ITGB3, ITGB5, MAPK1, MAPK3,
PGC1A, and PI3K, using an ABI 7900 Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Values for all samples were
normalized to the ratio between the target gene and mean Cq
obtained for the reference gene GAPDH. The forward and
reverse primers used are listed in Table I.
Bioinformatic analysis
The Enrichr (https://maayanlab.cloud/Enrichr/) gene set knowledge
discovery server was used to predict gene ontology-based
predictions for GO biological processes and GO cellular component
prediction (27), to support
evaluation of the association between gene expression and cancer
cell progression. The STRING functional protein association server
(https://string-db.org/) was used to assess
protein-protein interaction through gene expression associated with
cancer cell signaling protein pathways (28). The network of protein-protein
interactions in Homo sapiens was constructed using the
string protein-protein interaction network v11.5, using upregulated
genes in LNCaP cells (based on gene expression results) for
studying their protein-protein interactions with highest confidence
level setting. Interactions of proteins were mapped using the
highest confidence cut-off (0.7-0.9). In the resulting protein
association network, proteins were presented as nodes connected by
lines with varying thicknesses representing the highest confidence
level (0.7-0.9).
Statistical analysis
Results are presented as mean ± standard deviation
or percent survival in graphs with at least three replicates. The
Shapiro-Wilk normality test was used to test for normal data
distribution. For results that passed the test, the Mann-Whitney
test was used whereas for those which did not pass the normality
test, Dunn's multiple comparison test was used. The unpaired t-test
was performed to determine the significance of the results
obtained. All statistical analyses were performed using GraphPad
Prism (version 5.00; GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
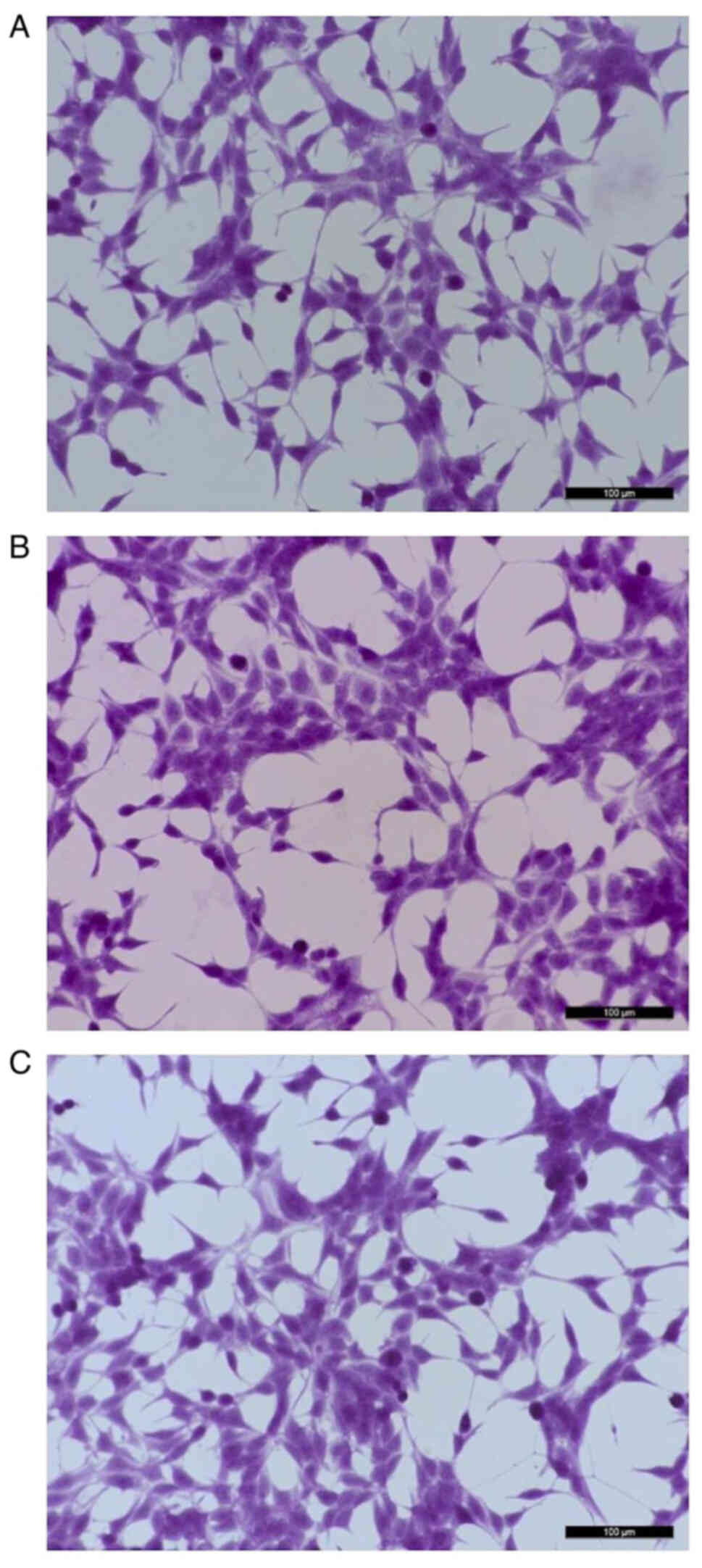
Cell morphology
LNCaP cells exposed to bacteriophage MS2 at
1×107 pfu/ml showed no marked morphological changes at
24 h compared with untreated cells. In particular, no
spindle-shaped nucleus formation was observed following the
staining of LNCaP cells treated with MS2 phages. Similarly, no
marked morphological changes were observed after 48 h of exposure
to the bacteriophage MS2 (Fig.
1).
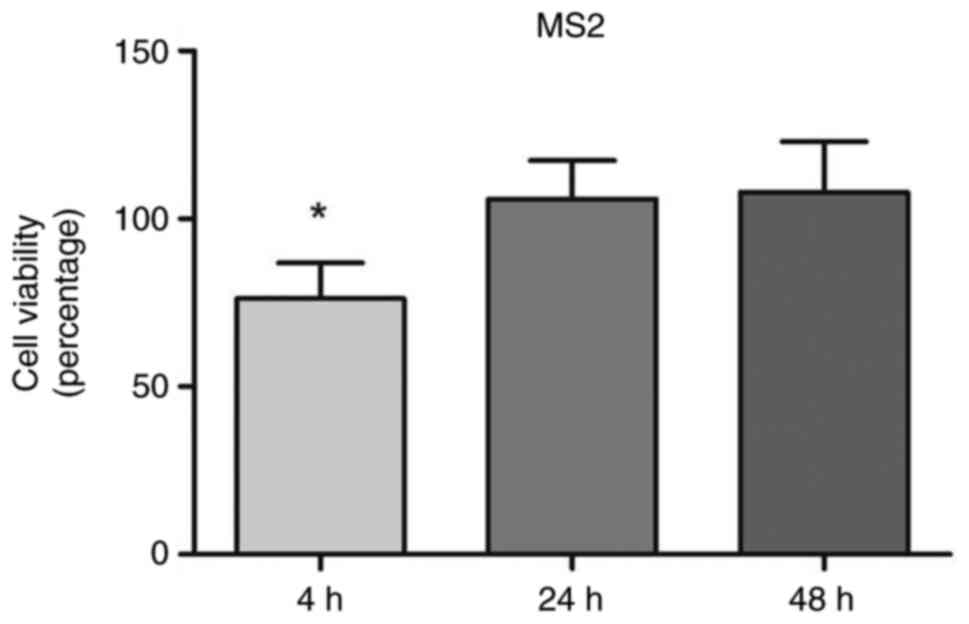
MTT reduction assay to measure cell
viability
Exposure to MS2 transiently reduced PCa cell
viability. The MS2 bacteriophage exposure initially affected the
viability of prostate cancer cells as compared with the cells
treated with MS2 phages for 4, 24, and 48 h, they temporarily
reduced the viability of LNCaP cells by 25% after 4 h of treatment.
The cell viability was measured at 4, 24, and 48 h for control and
phage-treated cells. Data are presented as percentages relative to
untreated control cells at each time point. The bacteriophage MS2
didn't affect the viability at 24 and 48 h of exposure. After 24
and 48 treatments, no significant difference in the viability of
LNCaP cells was noted (Fig. 2).
Gene expression profiles after
exposure to bacteriophage MS2
Gene expression analysis of LNCaP cells after
exposure to the bacteriophage MS2 revealed that some integrin genes
were upregulated. After 24 h of exposure to bacteriophage MS2, the
ITGA5 and ITGB1 genes showed significantly increased
gene expression compared with untreated cells. Similarly, MS2
treatment significantly increased the expression levels of AKT,
AR, MAPK1, MAPK3, PGC1A, and STAT3. Furthermore,
important cancer progression-related genes, such as HSP27,
HSP90, ITGAV, ITGB3, ITGB5, and PI3K, were not
significantly affected by bacteriophage MS2 treatment of LNCaP
cells (Fig. 3).
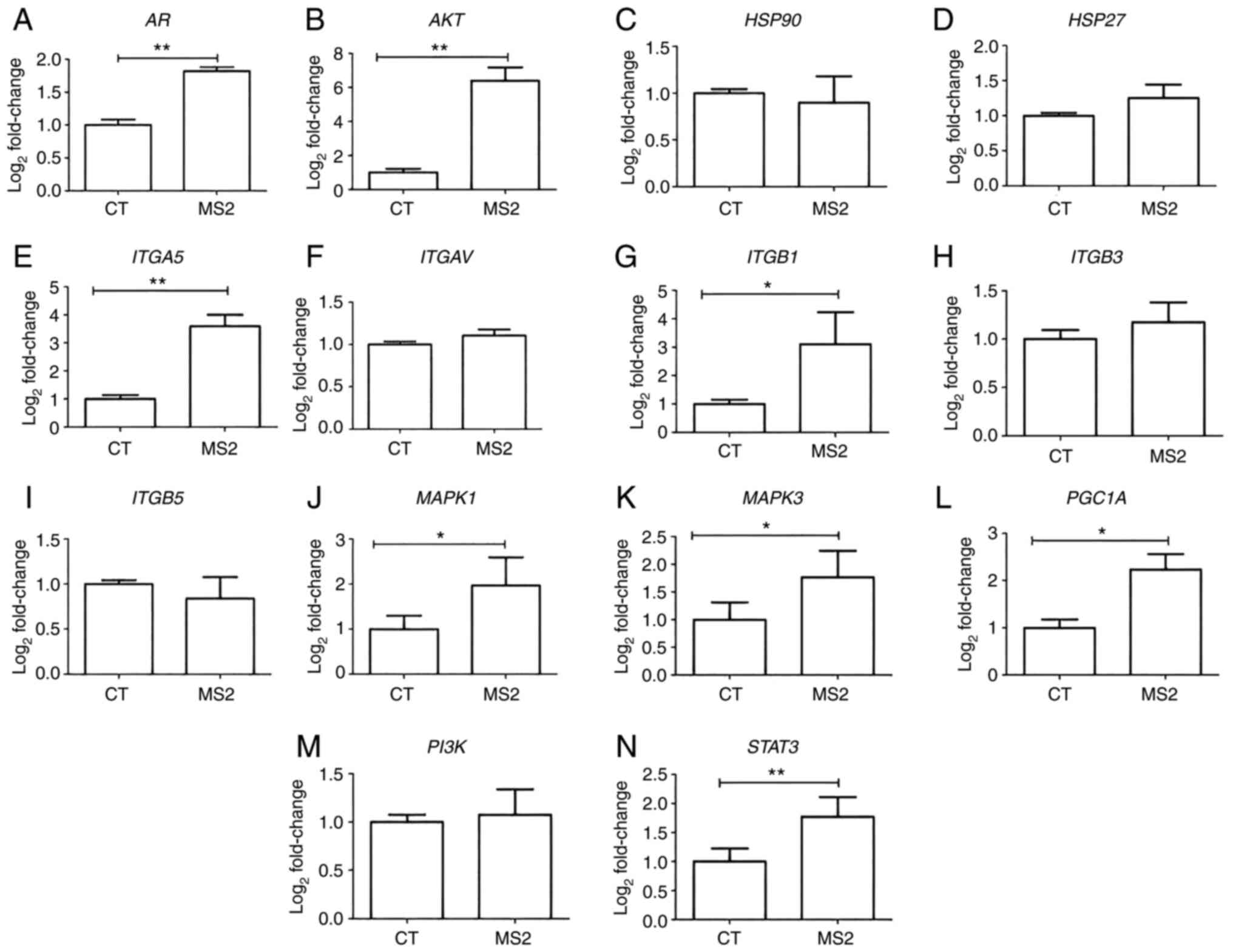 | Figure 3.Gene expression of the LNCaP prostate
cancer cells after 24 h of treatment with bacteriophage MS2. The
effect on the gene expression of LNCaP cells after interaction with
bacteriophage MS2 was compared with that of untreated CT cells. (A)
AR, (B) AKT, (C) HSP90, (D) HSP27, (E)
ITGA5, (F) ITGAV, (G) ITGB1, (H) ITGB3,
(I) ITGB5, (J) MAPK1, (K) MAPK3, (L)
PGC1A, (M) PI3K, and (N) STAT3. Relative
values of gene expression (median) are shown as
Log2Fold-Change. *P<0.05 and **P<0.01 vs. control
group within the same observation period, as determined using the
non-parametric Kruskal-Wallis test. AR, androgen receptor; CT,
control; HSP27, heat shock protein 27; HSP90, heat
shock protein 90; ITGA5, integrin α5; ITGAV, integrin
αV; ITGB1, integrin β1; ITGB3, integrin β3;
ITGB5, integrin β5; PGC1A, peroxisome
proliferator-activated receptor-γ coactivator 1α. |
Bioinformatic analysis
The bioinformatics analysis based on upregulated
genes was performed by using Enrichr (https://maayanlab.cloud/Enrichr/) gene set knowledge
discovery server to predict gene ontology-based predictions for GO
biological processes and GO cellular component prediction (27). The STRING functional protein
association server was also used to understand the association and
co-regulation of proteins expressed by these overexpressed genes
through protein-protein interactions studies. We predicted
‘caveolin-mediated endocytosis’ (P-value: 0.000001399; Fig. S1) as a potential MS2 bacteriophage
entry pathway from upregulated genes in LNCaP cells (27). Similarly for bacteriophages T4 and
M13, ‘integrin-mediated signaling pathway’ (P-value: 0.000007588)
and ‘caveolin-mediated endocytosis’ (P-value: 0.000001799; Fig. S1) were predicted. Also, through GO
cellular component prediction focal adhesion, cell-substrate
junction, caveloa, plasma membrane raft, early and late endosomes
were predicted for MS2, T4, as well as M13 phages validating the
caveolin-mediated endocytosis for these phages.
Discussion
Cellular receptors, including ARs, integrins,
β-arrestins, and GPCRs, are key players in tumor growth,
angiogenesis, and metastasis, and are indirectly regulated by
phage-cancer cell interactions, which are involved in regulating
numerous cellular functions, including proliferation, survival, and
mortality (19–23). Furthermore, since numerous GPCRs
(e.g., VPAC1 and VPAC2) serve as valuable biomarkers for cancer
screening, inhibition of GPCRs may offer opportunities to develop
novel mechanism-based strategies for cancer prevention and
treatment (29). As different
adhesion GPCRs are differentially regulated during cancer
progression, their role in different types of cancer is yet to be
defined, and thus, requires further research (29,30). G
protein-coupled receptor kinases (GRKs) and arrestins are involved
in the regulation of intracellular signal transductions associated
with different GPCRs and control various cellular processes
(29,30). For example, GRKs 1–7 serves a role
in regulating various stages of cancer progression and
physiological processes, such as insulin resistance and
inflammation (29,30).
Molecular dynamic simulations have enabled the
development of rational anticancer peptides (ACPs), which improved
the understanding of their interaction with different targets
through protein-protein binding (31). The US Food and Drug Association and
the European Medicines Agency have approved several ACPs for
clinical use; however, for ACPs to be effective, they should
overcome limitations associated with short plasma half-lives,
degradation by proteinases, stability and immunogenicity (8–17,31,32).
Expression of anticancer peptides/tumor targeting
peptides/tumor-specific internalizing peptides are possible by
presenting them on bacteriophage surfaces through the modification
of coat protein genes (32). It can
increase the stability of the bound peptides and further enhance
the interactions of engineered phages with GPCR receptor/integrins
and other surface proteins/receptors to facilitate the
understanding of their interaction between the different signaling
pathways of the transmembrane receptor/proteins (32,33).
In the present study, it was observed that the
interaction of LNCaP cells with bacteriophage MS2 did not markedly
affect cell morphology or spindle formation. Similar observations
were made after bacteriophages T4 and M13 interacted with LNCaP
cells at different time intervals and phage concentrations as they
altered the cancer progression-related gene expression, viability
and cell migration (21,23). Regardless of whether phages affect
the cytoskeleton of cancer cells, phages can be internalized and
affect cancer cell signaling through various pathways (34,35).
Similarly, in the present study, the interaction of bacteriophage
MS2 with LNCaP cells affected cancer cell viability at different
time intervals; however, viability was significantly affected only
up to 4 h, and viability was restored after 24 and 48 h. These
observations also fit well with those for bacteriophages T4 and M13
(23). Therefore, phage size and
structure may have differential effects on the proliferation,
migration, and viability of LNCaP cancer cells as reported
previously (20–23). Also, bacteriophage MS2 impairs cell
viability for 4 h as demonstrated during the present study. In
addition, cell-penetrating peptides displayed on the bacteriophage
M13, such as HIV-1 transactivator protein-derived TAT peptide, can
enter live mammalian breast cancer cells and are destroyed within 2
h, which is also consistent with a previous viability study
(35).
AR upregulation has been linked to the
upregulation of estrogen receptor β, and AR is responsible
for the activation of the Raf1-MEK signaling pathway, leading to
MAPK activation (36). Similarly,
the activation of AR can promote the activation of AKT metabolism,
leading to the activation of mTOR (37,38),
since overexpression of AR was associated with upregulation
of AKT as observed in the present study demonstrated by the
elevated gene expression of AR and AKT following MS2
phage exposure. Membrane-bound GPCRs also respond to androgen,
which may increase apoptosis and phosphorylation of ERK and
decrease cell migration and metastasis (20–23,39).
In addition, AR regulates SRC expression via microRNA
(miR)-203, prostate epithelial cell proliferation via miR-221,
proliferation and viability via miR-96, migration, metastasis, and
invasion via miR-541 and apoptosis via miR-125b by controlling the
expression of genes, such as kallikrein-related peptidase 3 and
prostate-specific membrane antigen, two important markers of
prostate differentiation (39–42).
Zhang et al (42)
demonstrated the delivery of microRNA-21-sponge and
pre-microRNA-122 by MS2 virus-like particles to target
hepatocellular carcinoma cells. In a previous study, the
semi-adherent relative upsurge method, a simple gap-filling method
to study the migration of adherent and semi-adherent cancer cells,
was used to investigate the effects of bacteriophages T4 and M13 on
androgen-dependent (LNCaP) and androgen-independent (PC3) cancer
cell lines (20–23). The migration of these cancer cells
was strongly influenced by the type of phage involved in the
interaction experiments (21,22).
In the present study, AR, AKT, MAPK1 and
MAPK3, and other crucial genes, such as ITGA5, ITGB1,
PGC1A, and STAT3, were significantly upregulated
compared with the levels in the untreated control groups after 24 h
of treatment with bacteriophage MS2 in LNCaP cells. Based on the
upregulated gene profile in LNCaP cells, mechanisms by which LNCaP
cells can activate survival mechanisms can be predicted.
Considering the difference in gene expression patterns in LNCaP and
PC3 cells concerning integrins, ARs, AKT, HSPs, MAPKs, PGC1A
and PI3K, following the interaction between bacteriophages
T4 and M13, it is clear that not only phage size, phage
concentration and/or the exposure time, but also natural peptide
display and genomic makeup affect the migration, viability and gene
expression of cancer cells in cancer progression (20–23).
In addition, since phage internalization is now a well-established
concept (38), it was hypothesized
that the effect of the phage genetic nature is also an important
factor in altering cancer progression genes in both LNCaP and PC3
cells, as reported previously (Tables
II and III) (19,21,22).
 | Table II.Effect of bacteriophages T4, M13 and
MS2 separately on the expression of cancer progression genes in
LNCaP and PC3 cells. |
Table II.
Effect of bacteriophages T4, M13 and
MS2 separately on the expression of cancer progression genes in
LNCaP and PC3 cells.
| First author/s,
year | Interacting
bacteriophage | Cancer progression
gene | LNCaP | PC3 | (Refs.) |
|---|
| Sanmukh et
al, 2018; Sanmukh et al, 2021 | T4 phage | AKT | Upregulated | Upregulated | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| AR | Downregulated | Downregulated | (21,22) |
| Sanmukh et
al, 2017; Sanmukh et al, 2018; Sanmukh et al,
2021 |
| HSP90 | No alteration | Downregulated | (19,21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| HSP27 | No alteration | Downregulated | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| ITGA5 | Upregulated | Upregulated | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| ITGAV | No alteration | Upregulated | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| ITGB1 | Upregulated | No alteration | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| ITGB3 | Upregulated | Upregulated | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| ITGB5 | Upregulated | Upregulated | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| MAPK1 | Upregulated | Data not
available | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| MAPK3 | Upregulated | Data not
available | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| PGC1A | Downregulated | Data not
available | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| PI3K | Upregulated | Data not
available | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| STAT3 | Upregulated | Data not
available | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 | M13 phage | AKT | Upregulated | Upregulated | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| AR | Downregulated | Downregulated | (21,22) |
| Sanmukh et
al, 2017; Sanmukh et al, 2018; Sanmukh et al,
2021 |
| HSP90 | No alteration | Downregulated | (19,21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| HSP27 | No alteration | Downregulated | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| ITGA5 | Upregulated | Upregulated | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| ITGAV | No alteration | Upregulated | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| ITGB1 | Upregulated | No alteration | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| ITGB3 | Upregulated | Upregulated | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| ITGB5 | Upregulated | Upregulated | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| MAPK1 | Upregulated | Data not
available | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| MAPK3 | Upregulated | Data not
available | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| PGC1A | Downregulated | Data not
available | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| PI3K | Upregulated | Data not
available | (21,22) |
| Sanmukh et
al, 2018; Sanmukh et al, 2021 |
| STAT3 | Upregulated | Data not
available | (21,22) |
| Present study | MS2 phage | AKT | Upregulated | Data not
available | - |
| Present study |
| AR | Upregulated | Data not
available | - |
| Present study |
| HSP90 | No alteration | Data not
available | - |
| Present study |
| HSP27 | No alteration | Data not
available | - |
| Present study |
| ITGA5 | Upregulated | Data not
available | - |
| Present study |
| ITGAV | No alteration | Data not
available | - |
| Present study |
| ITGB1 | Upregulated | Data not
available | - |
| Present study |
| ITGB3 | No alteration | Data not
available | - |
| Present study |
| ITGB5 | No alteration | Data not
available | - |
| Present study |
| MAPK1 | Upregulated | Data not
available | - |
| Present study |
| MAPK3 | Upregulated | Data not
available | - |
| Present study |
| PGC1A | Upregulated | Data not
available | - |
| Present study |
| PI3K | No alteration | Data not
available | - |
| Present study |
| STAT3 | Upregulated | Data not
available | - |
 | Table III.Effect of interaction between
bacteriophages and prostate cancer cell lines. |
Table III.
Effect of interaction between
bacteriophages and prostate cancer cell lines.
| First author/s,
year | Interacting
bacteriophage | Effect on cancer
cell lines | LNCaP cells | PC3 cells | (Refs.) |
|---|
| Kantoch and
Mordarski, 1958; | T4 phage | Phage binding | Yes | Yes | (20,72–75) |
| Hart et al,
1994; |
|
|
|
|
|
| Tobia et al,
2012; |
|
|
|
|
|
| Dąbrowska et
al, 2014; |
|
|
|
|
|
| Sanmukh et
al, 2017 |
|
|
|
|
|
| Sanmukh et
al, 2021; |
| Morphology | No significant | Marked
alteration | (22,23) |
| Sanmukh et
al, 2021 |
|
| alteration | (spindle shaped
cell formation) |
|
| Kantoch, 1958; |
| Cell | Reduced | Reduced | (20,76–78) |
| Lehti et al,
2017; |
| proliferation |
|
|
|
| Porayath et
al, 2018; |
|
|
|
|
|
| Sanmukh et
al, 2017 |
|
|
|
|
|
| Bloch, 1940; |
| Migration/ | Restricted | Restricted | (20,22,79–82) |
| Szczaurska-Nowak
et al, |
| invasion |
|
|
|
| 2009; Dabrowska
et al, 2009; |
|
|
|
|
|
|
Kurzepa-Skaradzinska et al, |
|
|
|
|
|
| 2013; Sanmukh et
al, 2017; |
|
|
|
|
|
| Sanmukh et
al, 2021 |
|
|
|
|
|
| Merril et
al, 1972; |
| Cell | Decreased | Data not | (23,83,84) |
| Eriksson et
al, 2009; |
| viability | during | available |
|
| Sanmukh et
al, 2021 |
|
| initial 4 h |
|
|
| Kantoch and
Mordarski, 1958; | M13 phage | Phage | Yes | Yes | (20,72–75) |
| Dąbrowska et
al, 2014; |
| binding |
|
|
|
| Sanmukh et
al, 2017 |
|
|
|
|
|
| Sanmukh et
al, 2021; |
| Morphology | No | Marked
alteration | (22,23) |
| Sanmukh et
al, 2021 |
|
| significant | (spindle shaped
cell formation) |
|
|
|
|
| alteration |
|
|
| Kantoch, 1958; |
| Cell | Reduced | Reduced | (20,76–78) |
| Lehti et al,
2017; |
| proliferation |
|
|
|
| Porayath et
al, 2018; |
|
|
|
|
|
| Sanmukh et
al, 2017 |
|
|
|
|
|
| Bloch, 1940; |
| Migration/ | Restricted | Restricted | (20,22,79–82) |
| Szczaurska-Nowak
et al, 2009; |
| invasion |
|
|
|
| Dabrowska et
al, 2009; |
|
|
|
|
|
|
Kurzepa-Skaradzinska et al,
2013; |
|
|
|
|
|
| Sanmukh et
al, 2017; |
|
|
|
|
|
| Sanmukh et
al, 2021 |
|
|
|
|
|
| Merril et
al, 1972; |
| Cell viability | Decreased | Data not | (23,83,84) |
| Eriksson et
al, 2009; |
|
| during | available |
|
| Sanmukh et
al, 2021 |
|
| initial 24 h |
|
|
Viral entry is mediated by different modes depending
on the virus type (34,35), and VLPs are used to deliver various
drug molecules for therapeutic applications (43). Similarly, MS2 VLPs enter mammalian
cells via caveolin-1-mediated ITGB1 endocytosis and alter gene
expression (44). Caveolin-1 mRNA
and protein are upregulated in metastatic murine and human PCa
cells, and caveolin-1 internalization mediates integrin-dependent
signaling pathways (44,45). MS2 is also taken up by mammalian
cells using such pathways (44–46).
Therefore, the present study is beneficial for understanding the
impact of bacterial viruses, which make up the majority of the
human microbiome, on cancer progression as well as bacteriophage
therapies in humans.
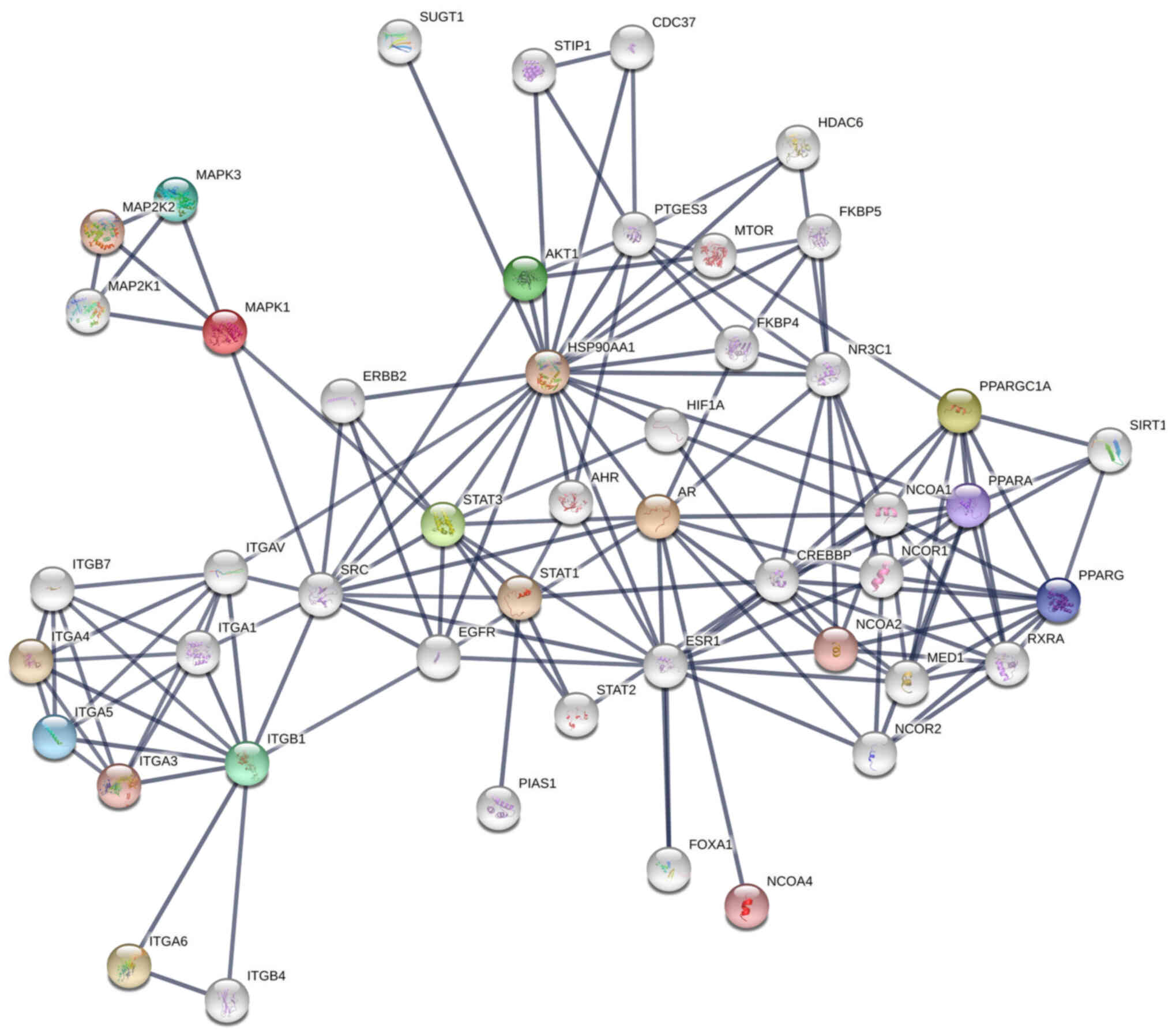
Considering these variations, the protein-protein
interaction map shown in Fig. 4 can
be proposed for the co-regulation of cancer progression genes in
LNCaP cells after interaction with bacteriophage MS2 (47). Similarly, the protein-protein
interaction map for the co-regulation of cancer genes in LNCaP
cells after interaction with bacteriophages T4 and M13, which were
reported previously, is shown in Fig.
S2 (20,22,23,47).
From these interactions, it appears that the genetic nature of
these phages (DNA or RNA), which is independent of the natural
phage presentation by T4, M13, and MS2, also influences LNCaP
cancer cell signaling. SRC, a proto-oncogenic tyrosine-protein
kinase (or non-receptor protein tyrosine kinase), interacts with
most of these proteins, and its activation involves various
cellular receptors, including immune receptors, integrin bound to
the extracellular matrix, adhesion receptors, platelet-derived
growth factor receptor, GPCRs and cytokine receptors (39). The initial interaction of phages
with various cellular transmembrane proteins facilitates
internalization and modification of cancer progression gene
expression by the phage genome, as reported previously (Fig. 5) (35,39).
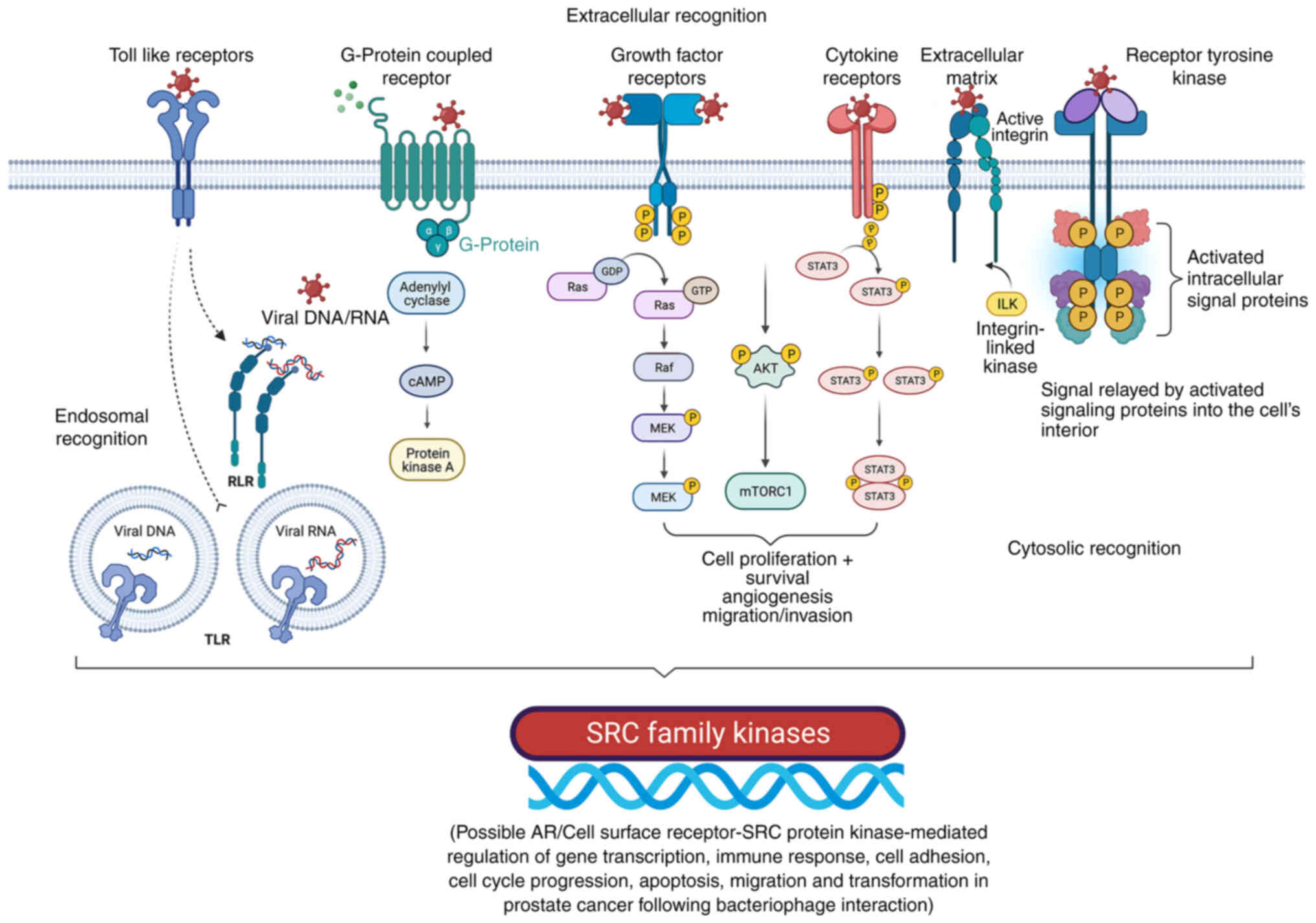 | Figure 5.Cell surface receptor-mediated
interaction with bacteriophages is associated with SRC family
kinases involved in cancer progression, gene upregulation, and
other cellular events. Bacteriophages may interact with various
cell membrane receptors. Cell surface receptors, such as Toll-like
receptors, G-protein coupled receptors, growth factor receptors,
cytokine receptors, extracellular matrix (e.g., integrins), and
receptor tyrosine kinases could be involved in phage interactions
with cancer cells and are responsible for the extracellular
recognition of bacteriophages. Following different routes of
internalization (e.g., endocytosis), bacteriophages appear to have
endosomal recognition. In addition, cytosolic recognition of
bacteriophages within cancer cells after degradation/decay of the
phage proteins and the genome (DNA/RNA) is possible. Adapted from
‘Non-phagocytic Nanoparticle internalization pathways’, by
BioRender.com (2022). Retrieved from https://app.biorender.com/illustrations/62e7f61bf2dd732bb630d9eb.
P, phosphate group; AR, androgen receptor; ILK, integrin-linked
kinase; TLR, toll-like receptors. |
Additionally, ITGA5 and ITGB1 are considered
fibronectin receptors and potential targets for solid tumor
treatment, as their upregulation is associated with poor prognosis
in colon, breast, ovarian, lung, and brain tumors (47). Since α5β1 recognizes and adheres to
extracellular ligands containing the tripeptide
arginine-glycine-aspartate motif, it can interact with numerous
extracellular matrix molecules, such as VEGFR-1, fibrinogen,
fibronectin, and fibrillin (48,49).
In addition, transmembrane proteins, such as CD97, CD87, and CD154,
contain these tripeptides, thus α5β1 interacts with them and
contributes to adhesion, intracellular signaling, angiogenesis,
chemotherapy, and radiation resistance (48).
The α5 integrin has also been associated with bone
metastasis in breast cancer due to its upregulation, which is
considered an important factor contributing to mortality and
morbidity in patients with breast cancer (49). Similarly, gene knockout studies of
β1 integrins in MDA-MB-231 breast cancer cells have shown
that they increase EGFR phosphorylation and decrease AKT
phosphorylation, suggesting that they are involved in AKT signaling
(50,51). Furthermore, α5β1 integrin maintains
pro-survival signaling through continuous AKT activation and
upregulates proliferation through EGFR activation in squamous cell
carcinomas (47–52).
Upregulation of MAPK1 and MAPK3 after
bacteriophage MS2 interaction shows that autophagy and compensatory
signaling pathways, such as the Ras/Raf/MEK/ERK signaling pathway,
are activated in LNCaP cancer cells (52,53).
Mammalian DNA and RNA viruses have previously been reported to
markedly influence the MAPK-ERK cascade through various cellular
receptors, which are also regulated by G proteins (53,54).
Therefore, targeting the PI3K/AKT/mTOR and Ras/MEK/ERK/FGF
signaling pathways together is recommended in prostate cancer and
other types of cancer, such as breast cancer (52–55).
Direct effects of DNA and RNA viruses on cellular signaling
cascades were previously reported; therefore, the effects of
bacteriophages T4, M13, and MS2 on gene expression changes in PC3
and LNCaP cell lines cannot be ignored (22,23).
In addition, since MAPK upregulation is associated with
castration-resistant PCa, it is recommended to target the FGF/MAPK
signaling pathways in AR-independent PCa to effectively combat PCa
metastasis (36–38,52–55).
Upregulation of PGC1A is associated with PCa
growth and metastasis, regulating estrogen receptor α
(ERRα)-dependent transcripts, and is responsible for suppressing
metastasis (56). Since PGC1A-ERRα
contributes to disease stratification (56) and treatment, these findings are
critical for the development of phage-based treatment therapies.
The bacteriophages T4, M13 and MS2, when separately used for the
treatment of PC3 and LNCaP cells, affected cell viability and PGC1A
gene expression, which demonstrates the direct effect on
mitochondrial function/biogenesis and requires further
investigation for the development of phage-based therapies against
prostate cancer (56).
Finally, the upregulation of STAT3 is directly
related to the progression of metastasis (57), while its inhibition promotes
apoptosis in PCa (58). The
expression of STAT3 and basic FGF (which is a potent
angiogenic regulator) are also coregulated, which has been
confirmed by gene knockout studies and further validates the
FGF/MAPK signaling pathway as a target in AR-independent PCa
(59–62).
In addition, the Enrichr server (https://maayanlab.cloud/Enrichr/), which is used
for large-scale enrichment analysis of gene sets, was used to
predict potential bacteriophage entry pathways from upregulated
genes in LNCaP cells (27). In this
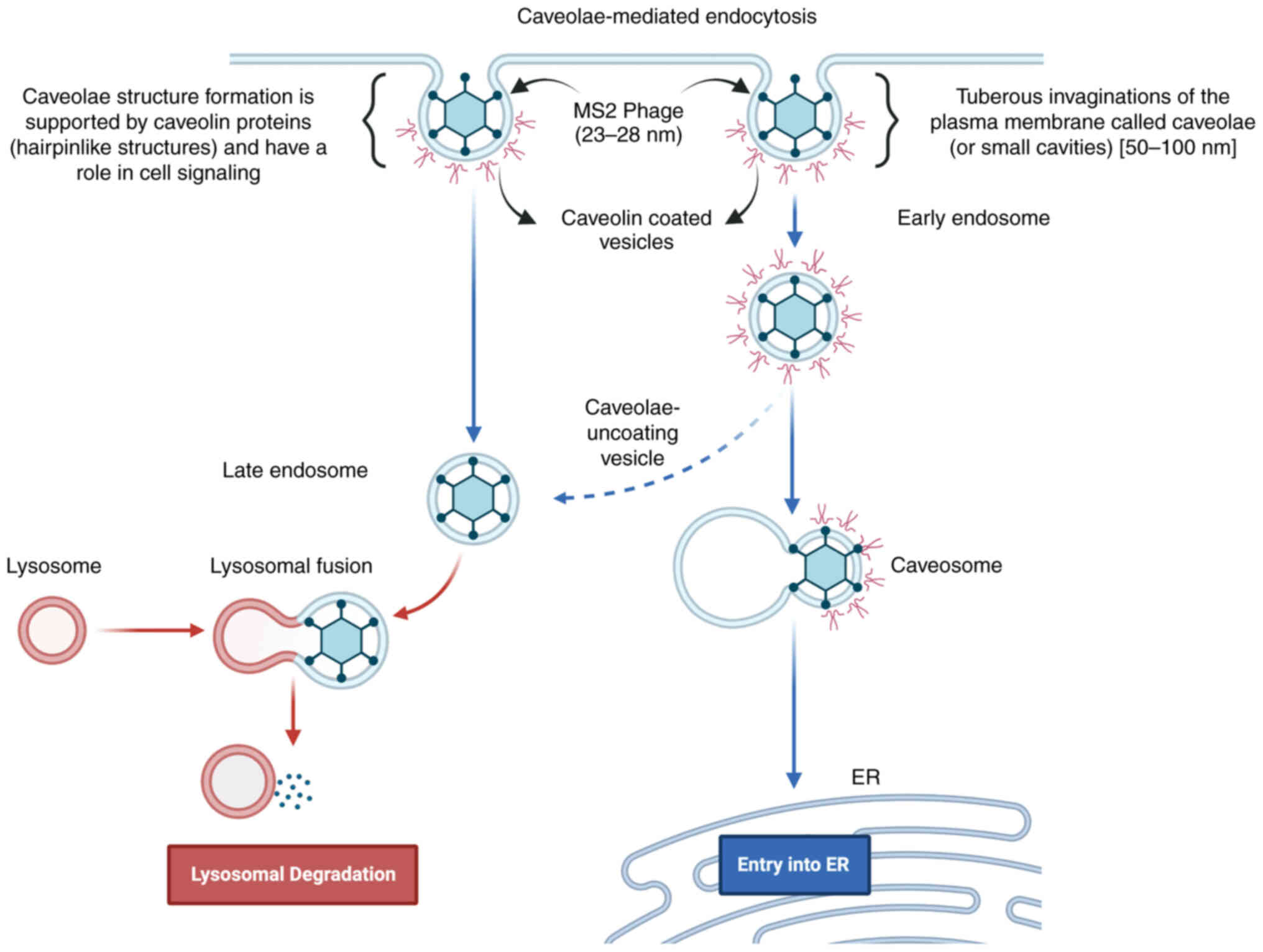
prediction, ‘caveolin-mediated endocytosis’ was the entry pathway
for bacteriophage MS2 (P=0.000001399; Fig. S1) based on upregulation of AKT,
AR, ITGA5, ITGB1, MAPK1, MAPK3, PGC1A, and STAT3 genes
(27,44–46).
This prediction also fits well with the bacteriophage MS2 size
scale (23–28 nm) as the caveolin-mediated endocytic process
involves 50–60 nm tuberous invaginations of the plasma membrane,
named ‘caveolae’ (small cavities), which are the best possible mode
as far as the size is concerned for bacteriophage MS2
internalization (27,44–46).
The mechanisms underlying these effects require further
experimental validation. Similarly, bacteriophage M13 is reported
to get internalized through clathrin-dependent endocytosis, which
has a larger dimension (880 nm in length and 6 nm in diameter)
(34). But, through GO biological
processes Enrichr web server-based gene set enrichment analysis for
upregulated cancer progression genes in LNCaP cells treated with
bacteriophages T4 and M13, ‘integrin-mediated signaling pathway’
(P=0.000007588) and ‘caveolin-mediated endocytosis’ (P=0.000001799)
were predicted for both these bacteriophages based on the
upregulated gene sets of AKT, ITGA5, ITGB1, ITGB3, ITGB5, MAPK1,
MAPK3, PI3K, and STAT3 genes. The gene set analysis was
performed based on overexpressed genes in LNCaP cells from our
studies to predict GO biological processes. Similarly, through GO
cellular component prediction focal adhesion, cell-substrate
junction, caveloa, plasma membrane raft, early and late endosomes
were predicted for MS2, T4, as well as M13 phages validating the
caveolin-mediated endocytosis for these phages. Bacteriophage MS2
internalization in cancer cells through caveolin-mediated
endocytosis was predicted based on the results of the present
study, as shown in Fig. 6, which
also confirmed previous reports (11,27,44–46).
Phage display library screening has attracted
attention as an inexpensive method for drug discovery (63). Several short peptides presented on
the surface of phages against, for example, a membrane receptor,
offer a much larger scale analysis compared with synthetic peptide
libraries (63–65). This number can be further increased
when considering non-peptide mimetics designed based on these short
peptides. This is particularly important in oncology drug
development, where large-scale drug screening is required due to a
variety of different drug targets and signaling pathways that may
simultaneously be involved in tumor growth and progression
(63–65). Based on the early report of
bacteriophage lambda-holin protein reducing tumor growth rates in
mammary cancer cell xenograft models (64), it appears that phages and phage
proteins may be useful for cancer gene therapy.
Similarly, GPCR drug targets involved in cancer
include lysophosphatidic acid receptors (LPA1-6; involved in
Rho-dependent signaling pathways) (31,65–67),
protease-activated receptors (involved in Hippo/yes-associated
protein 1 signaling pathways and angiogenesis) (68), frizzled receptors, parathyroid
hormone 1 receptor (involved in the Wnt signaling pathway),
chemokine receptors (69),
endothelin receptors (involved in crosstalk with EGFR and β-catenin
stabilization), prostaglandin receptors (involved in the
cyclooxygenase pathway), bradykinin receptors (involved in
crosstalk with EGFR, Ras, Raf, and ERK), sphingosine receptor 1
phosphate (involved in crosstalk with Ras-ERK, PI3K/Akt/Rac, Rho
and STAT3), angiotensin II receptor type 1 (involved in crosstalk
with TNF-α, ERK1/2, NF-κB, STAT) and gastrin-releasing peptide
receptor (involved in crosstalk with NF-κB, p38-MAPK and PI3K/AKT),
which are among the most commonly targeted in cases of PCa
(63,70–72).
Adhesion GPCRs, which until recently had not been extensively
studied in terms of structure and ligand determination, serve an
important role in regulating cell adhesion, migration,
proliferation, and tumor survival (31,66,67).
Peptide libraries containing phage display peptides have been used
to target LPA1 receptors (65),
protease-activated (69), chemokine
(69), frizzled (70,71)
and sphingosine 1-phosphate receptor 1 (72).
The importance of phage-displayed peptides in phage
engineering, the natural peptide display effect of bacteriophage
MS2 observed in the present study, and previous studies of
bacteriophages T4 and M13 (20–23)
suggest that along with other adjuvant therapies, targeting
adhesion GPCRs, integrins and other receptors appear to be
effective against multiple types of cancer, including ovarian,
breast and prostate cancer (31,66,67).
Phages can be engineered to express surface peptides and transport
cargo, making them prime candidates for fighting cancer, due to
their ubiquity (10,63,73,74).
In conclusion, the natural bacteriophage MS2
interacts directly with LNCaP cells and their surface receptors to
induce marked changes in gene expression in LNCaP cancer cells.
This, in turn, affects the viability of LNCaP cells. As such
interactions have been demonstrated to affect cancer cell
metabolism and direct gene expression, upregulation of the AR,
AKT and MAPK genes suggests that these genes affected
the AR, AKT, and MAPK signaling pathways (23). Such effects may be beneficial in
light of existing therapies that target the inhibitors of the
AKT/MAPK/FGF signaling pathway. To demonstrate that bacteriophage
MS2 is effective in fighting PCa, further studies are needed to
analyze and display modified phage surface peptides against cancer
cell receptors and proteins, such as G-proteins, GPCRs, and
integrins. Based on previous reports that analyzed the interactions
between DNA phages (T4 and M13) (20–23)
and the present study which demonstrated the interaction between
RNA (MS2) phages with LNCaP cells, it can be concluded that phages,
specifically MS2, utilize caveolin-mediated endocytosis and alter
PCa cell signaling pathways. The wide range of applications against
antibiotic-resistant bacterial pathogens makes phage engineering an
ideal technique for simultaneously targeting cancer cells and
antibiotic-resistant bacterial pathogens.
Supplementary Material
Supporting Data
Acknowledgments
The article is part of a Ph.D. thesis developed by
SGS at the Institute of Biosciences of Botucatu, Sao Paulo State
University (Botucatu, Brazil).
Funding
Funding was received from the National Council for Scientific
and Technological Development (CNPq; grant nos. 465699/2014-6 and
310805/2018-0) and São Paulo Research Foundation (FAPESP; grant
nos. 2014/50938-8 and 2019/19644-1). The present study was also
carried out with the support of the Coordenação de Aperfeiçoamento
de Pessoal de Nível Superior, Brasil (CAPES; finance code 001;
grant no. 963-14-2).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
SGS conducted most of the experiments and analyses.
SGS, NJDS, CNB, and MDC generated data/performed analyses. SGS,
NJDS, CNB, MDC, PPDR, FKD, HFC, DL, TF, and SLF confirm the
authenticity of all the raw data. SGS and SLF drafted the
manuscript. FKD, PPDR, and SLF supervised the project. FKD, HFC,
DL, TF, and SLF were responsible for overseeing the manuscript.
SGS, NJDS, CNB, PPDR, FKD, HFC, DL, TF, and SLF were responsible
for writing and revising the manuscript and contributed
significantly to the study design, data collection, data analysis,
and interpretation of data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AR
|
androgen receptor
|
|
FGF
|
fibroblast growth factor
|
|
GPCR
|
G-protein coupled receptor
|
|
HSP27
|
heat shock protein 27
|
|
HSP90
|
heat shock protein 90
|
|
ITGA5
|
integrin α5
|
|
ITGAV
|
integrin αV
|
|
ITGB1
|
integrin β1
|
|
ITGB3
|
integrin β3
|
|
ITGB5
|
integrin β5
|
|
miR
|
microRNA
|
|
PFU
|
plaque forming unit
|
|
PGC1A
|
peroxisome proliferator-activated
receptor-γ coactivator 1α.
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakr WA, Grignon DJ, Crissman JD, Heilbrun
LK, Cassin BJ, Pontes JJ and Haas GP: High grade prostatic
intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma
between the ages of 20–69: An autopsy study of 249 cases. In Vivo.
8:439–443. 1994.PubMed/NCBI
|
|
4
|
Nelson WG, De Marzo AM and Isaacs WB:
Prostate cancer. N Engl J Med. 349:366–381. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nuhn P, De Bono JS, Fizazi K, Freedland
SJ, Grilli M, Kantoff PW, Sonpavde G, Sternberg CN,
Yegnasubramanian S and Antonarakis ES: Update on systemic prostate
cancer therapies: Management of metastatic castration-resistant
prostate cancer in the era of precision oncology. Eur Urol.
75:88–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sumanasuriya S and De Bono J: Treatment of
advanced prostate cancer-a review of current therapies and future
promise. Cold Spring Harb Perspect Med. 8:a0306352018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barquilha CN, Santos NJ, Monção CCD,
Barbosa IC, Lima FO, Justulin LA, Pértega-Gomes N and Felisbino SL:
Sulfiredoxin as a potential therapeutic target for advanced and
metastatic prostate cancer. Oxid Med Cell Longev. 2020:21485622020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Przystal JM, Waramit S, Pranjol MZI, Yan
W, Chu G, Chongchai A, Samarth G, Olaciregui NG, Tabatabai G,
Carcaboso AM, et al: Efficacy of systemic temozolomide-activated
phage-targeted gene therapy in human glioblastoma. EMBO Mol Med.
11:e84922019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren S, Fengyu Zuo S, Zhao M, Wang X, Wang
X, Chen Y, Wu Z and Ren Z: Inhibition of tumor angiogenesis in lung
cancer by T4 phage surface displaying mVEGFR2 vaccine. Vaccine.
29:5802–5811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shadidi M, Sørensen D, Dybwad A, Furset G
and Sioud M: Mucosal vaccination with phage-displayed tumour
antigens identified through proteomics-based strategy inhibits the
growth and metastasis of 4T1 breast adenocarcinoma. Int J Oncol.
32:241–247. 2008.PubMed/NCBI
|
|
11
|
Ashley CE, Carnes EC, Phillips GK, Durfee
PN, Buley MD, Lino CA, Padilla DP, Phillips B, Carter MB, Willman
CL, et al: Cell-specific delivery of diverse cargos by
bacteriophage MS2 virus-like particles. ACS Nano. 5:5729–5745.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aanei IL, ElSohly AM, Farkas ME,
Netirojjanakul C, Regan M, Taylor Murphy S, O'Neil JP, Seo Y and
Francis MB: Biodistribution of antibody-MS2 viral capsid conjugates
in breast cancer models. Mol Pharm. 13:3764–3772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Sun Y, Jia T, Zhang R, Zhang K and
Wang L: Messenger RNA vaccine based on recombinant MS2 virus-like
particles against prostate cancer. Int J Cancer. 134:1683–1694.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhai L, Yadav R, Kunda NK, Anderson D,
Bruckner E, Miller EK, Basu R, Muttil P and Tumban E: Oral
immunization with bacteriophage MS2-L2 VLPs protects against oral
and genital infection with multiple HPV types associated with head
& neck cancers and cervical cancer. Antiviral Res. 166:56–65.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lino CA, Caldeira JC and Peabody DS:
Display of single-chain variable fragments on bacteriophage MS2
virus-like particles. J Nanobiotechnology. 15:132017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang L, Wang G, Jia T, Zhang L, Li Y, Han
Y, Zhang K, Lin G, Zhang R, Li J and Wang L: Armored long
non-coding RNA MEG3 targeting EGFR based on recombinant MS2
bacteriophage virus-like particles against hepatocellular
carcinoma. Oncotarget. 7:23988–24004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Briolay T, Petithomme T, Fouet M,
Nguyen-Pham N, Blanquart C and Boisgerault N: Delivery of cancer
therapies by synthetic and bio-inspired nanovectors. Mol Cancer.
20:552021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kolesanova EF, Melnikova MV, Bolshakova
TN, Rybalkina EY and Sivov IG: Bacteriophage MS2 as a tool for
targeted delivery in solid tumor chemotherapy. Acta Naturae.
11:98–101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanmukh SG and Felisbino SL:
Bacteriophages in cancer biology and therapies. Clin Oncol.
2:12952017.
|
|
20
|
Sanmukh SG, Dos Santos SAA and Felisbino
SL: Natural bacteriophages T4 and M13 down-regulates Hsp90 gene
expression in human prostate cancer cells (PC-3) representing a
potential nanoparticle against cancer. Virol Res J. 1:21–23.
2017.
|
|
21
|
Sanmukh SG and Felisbino SL: Development
of pipette tip gap closure migration assay (s-ARU method) for
studying semi-adherent cell lines. Cytotechnology. 70:1685–1695.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sanmukh SG, Santos NJ, Barquilha CN, dos
Santos SAA, Duran BOS, Delella FK, Moroz A, Justulin LA, Carvalho
HF and Felisbino SL: Exposure to bacteriophages T4 and M13
increases integrin gene expression and impairs migration of human
PC-3 prostate cancer cells. Antibiotics (Basel). 10:12022021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sanmukh SG, Dos Santos NJ, Barquilha CN,
Cucielo MS, de Carvalho M, Dos Reis PP, Delella FK, Carvalho HF and
Felisbino SL: Bacteriophages M13 and T4 increase the expression of
anchorage-dependent survival pathway genes and down regulate
androgen receptor expression in LNCaP prostate cell line. Viruses.
13:17542021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berridge MV and Tan AS: Characterization
of the cellular reduction of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT):
Subcellular localization, substrate dependence, and involvement of
mitochondrial electron transport in MTT reduction. Arch Biochem
Biophys. 303:474–482. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie Z, Bailey A, Kuleshov MV, Clarke DJB,
Evangelista JE, Jenkins SL, Lachmann A, Wojciechowicz ML,
Kropiwnicki E, Jagodnik KM, et al: Gene set knowledge discovery
with enrichr. Curr Protoc. 1:e902021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szklarczyk D, Gable AL, Nastou KC, Lyon D,
Kirsch R, Pyysalo S, Doncheva NT, Legeay M, Fang T, Bork P, et al:
The STRING database in 2021: Customizable protein-protein networks,
and functional characterization of user-uploaded gene/measurement
sets. Nucleic Acids Res. 49(D1): D605–D612. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Langer I, Jeandriens J, Couvineau A,
Sanmukh S and Latek D: Signal transduction by VIP and PACAP
receptors. Biomedicines. 10:4062022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peterson YK and Luttrell LM: The diverse
roles of arrestin scaffolds in G protein-coupled receptor
signaling. Pharmacol Rev. 69:256–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gad AA and Balenga N: The Emerging Role of
adhesion GPCRs in cancer. ACS Pharmacol Transl Sci. 3:29–42. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liscano Y, Oñate-Garzón J and Delgado JP:
Peptides with Dual antimicrobial-anticancer activity: Strategies to
overcome peptide limitations and rational design of anticancer
peptides. Molecules. 25:42452020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hwang JS, Kim SG, Shin TH, Jang YE, Kwon
DH and Lee G: Development of anticancer peptides using artificial
intelligence and combinational therapy for cancer therapeutics.
Pharmaceutics. 14:9972022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ripa I, Andreu S, López-Guerrero JA and
Bello-Morales R: Membrane rafts: Portals for viral entry. Front
Microbiol. 12:6312742021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim A, Shin TH, Shin SM, Pham CD, Choi DK,
Kwon MH and Kim YS: Cellular internalization mechanism and
intracellular trafficking of filamentous M13 phages displaying a
cell-penetrating transbody and TAT peptide. PLoS One. 7:e518132012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peterziel H, Mink S, Schonert A, Becker M,
Klocker H and Cato AC: Rapid signalling by androgen receptor in
prostate cancer cells. Oncogene. 18:6322–6329. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liao RS, Ma S, Miao L, Li R, Yin Y and Raj
GV: Androgen receptor-mediated non-genomic regulation of prostate
cancer cell proliferation. Transl Androl Urol. 2:187–196.
2013.PubMed/NCBI
|
|
38
|
Heinlein CA and Chang C: The roles of
androgen receptors and androgen-binding proteins in nongenomic
androgen actions. Mol Endocrinol. 16:2181–2187. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Siu MK, Chen WY, Tsai HY, Yeh HL, Yin JJ,
Liu SY and Liu YN: Androgen receptor regulates SRC expression
through microRNA-203. Oncotarget. 7:25726–25741. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Taheri M, Khoshbakht T, Jamali E,
Kallenbach J, Ghafouri-Fard S and Baniahmad A: Interaction between
non-coding RNAs and androgen receptor with an especial focus on
prostate cancer. Cells. 10:31982021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim KH, Dobi A, Shaheduzzaman S, Gao CL,
Masuda K, Li H, Drukier A, Gu Y, Srikantan V, Rhim JS and
Srivastava S: Characterization of the androgen receptor in a benign
prostate tissue-derived human prostate epithelial cell line:
RC-165N/human telomerase reverse transcriptase. Prostate Cancer
Prostatic Dis. 10:30–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang J, Li D, Zhang R, Peng R and Li J:
Delivery of microRNA-21-sponge and pre-microRNA-122 by MS2
virus-like particles to therapeutically target hepatocellular
carcinoma cells. Exp Biol Med (Maywood). 246:2463–2472. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Foglizzo V and Marchiò S: Bacteriophages
as therapeutic and diagnostic vehicles in cancer. Pharmaceuticals
(Basel). 14:1612021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Echarri A and Del Pozo MA: Caveolae
internalization regulates integrin-dependent signaling pathways.
Cell Cycle. 5:2179–2182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shi F and Sottile J: Caveolin-1-dependent
beta1 integrin endocytosis is a critical regulator of fibronectin
turnover. J Cell Sci. 121:2360–2371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tahir SA, Yang G, Ebara S, Timme TL, Satoh
T, Li L, Goltsov A, Ittmann M, Morrisett JD and Thompson TC:
Secreted caveolin-1 stimulates cell survival/clonal growth and
contributes to metastasis in androgen-insensitive prostate cancer.
Cancer Res. 61:3882–3885. 2001.PubMed/NCBI
|
|
47
|
Xing Y, Wen Z, Gao W, Lin Z, Zhong J and
Jiu Y: Multifaceted functions of host cell caveolae/caveolin-1 in
virus infections. Viruses. 12:4872020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schaffner F, Ray AM and Dontenwill M:
Integrin α5β1, the fibronectin receptor, as a pertinent therapeutic
target in solid tumors. Cancers (Basel). 5:27–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hou J, Yan D, Liu Y, Huang P and Cui H:
The roles of integrin α5β1 in human cancer. Onco Targets Ther.
13:13329–13344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pantano F, Croset M, Driouch K,
Bednarz-Knoll N, Iuliani M, Ribelli G, Bonnelye E, Wikman H, Geraci
S, Bonin F, et al: Integrin alpha5 in human breast cancer is a
mediator of bone metastasis and a therapeutic target for the
treatment of osteolytic lesions. Oncogene. 40:1284–1299. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hou S, Isaji T, Hang Q, Im S, Fukuda T and
Gu J: Distinct effects of β1 integrin on cell proliferation and
cellular signaling in MDA-MB-231 breast cancer cells. Sci Rep.
6:184302016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Morozevich GE, Kozlova NI, Ushakova NA,
Preobrazhenskaya ME and Berman AE: Integrin α5β1 simultaneously
controls EGFR-dependent proliferation and Akt-dependent
pro-survival signaling in epidermoid carcinoma cells. Aging (Albany
NY). 4:368–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Butler DE, Marlein C, Walker HF, Frame FM,
Mann VM, Simms MS, Davies BR, Collins AT and Maitland NJ:
Inhibition of the PI3K/AKT/mTOR pathway activates autophagy and
compensatory Ras/Raf/MEK/ERK signalling in prostate cancer.
Oncotarget. 8:56698–56713. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
DuShane JK and Maginnis MS: Human DNA
virus exploitation of the MAPK-ERK cascade. Int J Mol Sci.
20:34272019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mukherjee R, McGuinness DH, McCall P,
Underwood MA, Seywright M, Orange C and Edwards J: Upregulation of
MAPK pathway is associated with survival in castrate-resistant
prostate cancer. Br J Cancer. 104:1920–1928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bluemn EG, Coleman IM, Lucas JM, Coleman
RT, Hernandez-Lopez S, Tharakan R, Bianchi-Frias D, Dumpit RF,
Kaipainen A, Corella AN, et al: Androgen receptor
pathway-independent prostate cancer is sustained through FGF
signaling. Cancer Cell. 32:474–489.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liang H and Ward WF: PGC-1alpha: A key
regulator of energy metabolism. Adv Physiol Educ. 30:145–151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Abdulghani J, Gu L, Dagvadorj A, Lutz J,
Leiby B, Bonuccelli G, Lisanti MP, Zellweger T, Alanen K, Mirtti T,
et al: Stat3 promotes metastatic progression of prostate cancer. Am
J Pathol. 172:1717–1728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Barton BE, Karras JG, Murphy TF, Barton A
and Huang HF: Signal transducer and activator of transcription 3
(STAT3) activation in prostate cancer: Direct STAT3 inhibition
induces apoptosis in prostate cancer lines. Mol Cancer Ther.
3:11–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bishop JL, Thaper D and Zoubeidi A: The
multifaceted roles of STAT3 signaling in the progression of
prostate cancer. Cancers (Basel). 6:829–859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhao M, Gao FH, Wang JY, Liu F, Yuan HH,
Zhang WY and Jiang B: JAK2/STAT3 signaling pathway activation
mediates tumor angiogenesis by upregulation of VEGF and bFGF in
non-small-cell lung cancer. Lung Cancer. 73:366–374. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kujawski M, Kortylewski M, Lee H, Herrmann
A, Kay H and Yu H: Stat3 mediates myeloid cell-dependent tumor
angiogenesis in mice. J Clin Invest. 118:3367–3377. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Molek P, Strukelj B and Bratkovic T:
Peptide phage display as a tool for drug discovery: Targeting
membrane receptors. Molecules. 16:857–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Agu CA, Klein R, Schwab S, König-Schuster
M, Kodajova P, Ausserlechner M, Binishofer B, Bläsi U, Salmons B,
Günzburg WH and Hohenadl C: The cytotoxic activity of the
bacteriophage lambda-holin protein reduces tumour growth rates in
mammary cancer cell xenograft models. J Gene Med. 8:229–241. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
David M, Ribeiro J, Descotes F, Serre CM,
Barbier M, Murone M, Clézardin P and Peyruchaud O: Targeting
lysophosphatidic acid receptor type 1 with Debio 0719 inhibits
spontaneous metastasis dissemination of breast cancer cells
independently of cell proliferation and angiogenesis. Int J Oncol.
40:1133–1141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chaudhary PK and Kim S: An insight into
GPCR and G-proteins as cancer drivers. Cells. 10:32882021.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bar-Shavit R, Maoz M, Kancharla A, Nag JK,
Agranovich D, Grisaru-Granovsky S and Uziely B: G protein-coupled
receptors in cancer. Int J Mol Sci. 17:13202016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yang J, Mapelli C, Wang Z, Sum CS, Hua J,
Lawrence RM, Ni Y and Seiffert DA: An optimized agonist peptide of
protease-activated receptor 4 and its use in a validated
platelet-aggregation assay. Platelets. 33:979–986. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hu Y, Ma A, Lin S, Yang Y and Hong G:
Novel peptide screened from a phage display library antagonizes the
activity of CC chemokine receptor 9. Oncol Lett. 14:6471–6476.
2017.PubMed/NCBI
|
|
70
|
Nickho H, Younesi V, Aghebati-Maleki L,
Motallebnezhad M, Majidi Zolbanin J, Movassagh Pour A and Yousefi
M: Developing and characterization of single chain variable
fragment (scFv) antibody against frizzled 7 (Fzd7) receptor.
Bioengineered. 8:501–510. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pavlovic Z, Adams JJ, Blazer LL, Gakhal
AK, Jarvik N, Steinhart Z, Robitaille M, Mascall K, Pan J, Angers
S, et al: A synthetic anti-frizzled antibody engineered for
broadened specificity exhibits enhanced anti-tumor properties.
MAbs. 10:1157–1167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tobia C, Chiodelli P, Nicoli S, Dell'era
P, Buraschi S, Mitola S, Foglia E, van Loenen PB, Alewijnse AE and
Presta M: Sphingosine-1-phosphate receptor-1 controls venous
endothelial barrier integrity in zebrafish. Arterioscler Thromb
Vasc Biol. 32:e104–e116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dąbrowska K, Kaźmierczak Z, Majewska J,
Miernikiewicz P, Piotrowicz A, Wietrzyk J, Lecion D, Hodyra K,
Nasulewicz-Goldeman A, Owczarek B and Górski A: Bacteriophages
displaying anticancer peptides in combined antibacterial and
anticancer treatment. Future Microbiol. 9:861–869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hart SL, Knight AM, Harbottle RP, Mistry
A, Hunger HD, Cutler DF, Williamson R and Coutelle C: Cell binding
and internalization by filamentous phage displaying a cyclic
Arg-Gly-Asp-containing peptide. J Biol Chem. 269:12468–12474. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kantoch M and Mordarski M: Binding of
bacterial viruses by cancer cells in vitro. Postepy Hig Med Dosw.
12:191–192. 1958.PubMed/NCBI
|
|
76
|
Porayath C, Salim A, Palillam Veedu A,
Babu P, Nair B, Madhavan A and Pal S: Characterization of the
bacteriophages binding to human matrix molecules. Int J Biol
Macromol. 110:608–615. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lehti TA, Pajunen MI, Skog MS and Finne J:
Internalization of a polysialic acid-binding Escherichia coli
bacteriophage into eukaryotic neuroblastoma cells. Nat Commun.
8:19152017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kantoch M: Studies on phagocytosis of
bacterial viruses. Arch Immunol Ther Exp. 6:63–84. 1958.PubMed/NCBI
|
|
79
|
Bloch H: Experimental investigation on the
relationships between bacteriophages and malignant tumors. Arch
Virol. 1:481–496. 1940.(In German).
|
|
80
|
Szczaurska-Nowak K, Dabrowska K, Celka M,
Kurzepa A, Nevozhay D, Wietrzyk J, Switala-Jelen K, Syper D,
Pozniak G, Opolski A, et al: Antitumor effect of combined treatment
of mice with cytostatic agents and bacteriophage T4. Anticancer
Res. 29:2361–2370. 2009.PubMed/NCBI
|
|
81
|
Dabrowska K, Skaradziński G, Jończyk P,
Kurzepa A, Wietrzyk J, Owczarek B, Zaczek M, Switała-Jeleń K,
Boratyński J, Poźniak G, et al: The effect of bacteriophages T4 and
HAP1 on in vitro melanoma migration. BMC Microbiol. 9:132009.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kurzepa-Skaradzinska A, Skaradzinski G,
Weber-Dabrowska B, Zaczek M, Maj T, Slawek A, Switalska M,
Maciejewska M, Wietrzyk J, Rymowicz W and Gorski A: Influence of
bacteriophage preparations on migration of HL-60 leukemia cells in
vitro. Anticancer Res. 33:1569–1574. 2013.PubMed/NCBI
|
|
83
|
Merril CR, Friedman TB, Attallah AF, Geier
MR, Krell K and Yarkin R: Isolation of bacteriophages from
commercial sera. In Vitro. 8:91–93. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Eriksson F, Tsagozis P, Lundberg K, Parsa
R, Mangsbo SM, Persson MA, Harris RA and Pisa P: Tumor-specific
bacteriophages induce tumor destruction through activation of
tumor-associated macrophages. J Immunol. 182:3105–3111. 2009.
View Article : Google Scholar : PubMed/NCBI
|