Introduction
Dendritic cells (DCs) are a major group of antigen
presenting cells (APC). They play a crucial role in the development
of anti-pathogen, anti-cancer and tolerogenic immune responses. DCs
have potential in the treatment of autoimmune diseases and cancer.
One of promising tools to investigate dendritic cells biology are
DC lines such as JAWS II. JAWS II cells are immature DCs isolated
from p53-deficient (p53-/-) C57BL/6 mouse bone marrow cells. Using
murine cell lines is very useful due to high cell yields which
facilitate experiments and allow repeated measurements. JAWS II
cells are being used successfully in DC research on cancer vaccine
delivery (1), anti-tumour effect
(2), immunoparasitological study
(3,4) and others (5–11).
Immunotherapeutic vaccines show a promising weapon in the fight
against cancer, especially when a personalized approach to the
patient's disease is required. DC immunotherapy is very intensively
researched as a form of cancer treatment (12,13).
DC-based immunotherapy is created on functional activity of APC to
develop an effective immune response against cancer with
participation of T cells. In clinical studies, individuals with
diseases like melanoma, breast cancer, prostate cancer, leukemia,
and others are treated using DC cells-based vaccines (14).
The induction of adequate immunological responses is
mostly dependent on the activation status of DCs. Immature,
semi-mature and fully mature cells show different phenotypes and
functions. DCs can induce tolerogenic or immunogenic response
(15). In cancer immunotherapy,
mature DCs with high ability to activate T cells with cytotoxic
activity are expected. Maturation of dendritic cells results in
increased expression of costimulatory e.g. CD40, CD80, CD86 and MHC
molecules, decreased antigen internalization, changes in morphology
of cells, as well as secretion of cytokines and chemokines. They
drive T cell differentiation (16).
Activation and maturation of dendritic cells can be induced by
proinflammatory factors derived from bacteria e.g.
lipopolysaccharide (LPS). Dendritic cells are still mysterious and
can cause experimental difficulties. DC cultivation forms two
fractions-non-adherent and adherent. Adherence as well as
activation status can influence cell potential and properties. Yet
some research conducted on dendritic cell lines does not describe
which fractions of cells have been used. This information can be
crucial in the interpretation of results, especially as human
non-adherent and adherent monocyte-derived dendritic cells (mo-DCs)
show differences in purity, surface markers expression, ability to
process antigen and to stimulate T cells (17). Similarly, adherent and non-adherent
murine bone marrow-derived dendritic cells differ (18).
Here, for the first time we are comparing
non-adherent and adherent cell fractions of the DC line JAWS II.
The study's objectives is to define their characteristics and
differences which is important in the interpretation of the results
of experimental studies on immunotherapy. We determined the
condition, phenotype, antigen uptake capability, signalling
properties and the influence on the activity of T cells of
non-adherent and adherent cell fractions of JAWS II. To determine
the properties of these cells in immature (non-activated) and
activated status, we used LPS as a common DC stimulant.
Materials and methods
JAWS II cells culture
Immature JAWS II cells isolated from p53-deficient
(p53−/−) C57BL/6 murine bone marrow cells were purchased
from the American Type Culture Collection. JAWS II cells were then
cultured in humidified atmosphere at 37ºC and 5% CO2 in
RPMI-1640 medium with L-Glutamine, 20% inactivated FBS, penicillin
(100 U/ml), streptomycin (100 µg/ml) (Biowest) and 5 ng/ml GM-CSF
(PeproTech). The cells were passaged twice a week. JAWS II cells
were cultured at 5×105/ml in 75 cm3 bottles
or 24-well plates for 24 h. The cells were then activated with 2
µg/ml LPS from Escherichia coli 055:B5 (Sigma-Aldrich; Merck KGaA)
for 48 h. The cells were divided into two fractions:
non-adherent-cells suspended in the medium and adherent
cells-adjacent to the substrate. The experiment examined four
groups of cells: immature (non-activated) adherent and non-adherent
JAWS II, as well as LPS-activated adherent and non-adherent cells.
The non-adherent cells were collected and adherent cells removed
with non-enzymatic cell detachment solution. After 48 h of culture,
the cells were collected and washed twice in ice-cold PBS for
analysis on a Muse Cell Analyzer (Merck Millipore). The Muse Count
and Viability Kit provide rapid and reliable determinations of
viability and total cell count. The ratio was calculated by
dividing the number of non-adherent cells by the number of adherent
cells. Apoptosis was examined with the Muse Annexin V & Dead
Cell Assay. Phosphoinositide-3-kinase (Pi3K) and mitogen-activated
protein kinase (MAPK) signal pathway activation was analysed with
the Muse PI3K/MAPK Dual Pathway Activation Kit based on the
percentage of Akt and ERK1/2 activated cells. All procedures were
performed according to the manufacturer's guidelines.
Phenotypic characterisation by flow
cytometry
JAWS II cells were characterised by flow cytometry.
The following fluorochrome-conjugated monoclonal antibodies were
used according to the manufacturer's protocols: CD11c-SB436 (clone
N418; eBioscience), CD40-SB600 (clone 1310; eBioscience), CD80-APC
(clone 16-10A1, eBioscience), CD86-eFluor780 (clone GL1;
eBioscience) and MHC II-FITC (clone NIMR-4; eBioscience). Fixable
Viability Dye (FVD) eFluor 455UV (eBioscience) was used as a vital
dye to exclude dead cells. Briefly, cells were collected, washed
and resuspended in PBS (pH 7.2). Next, the cells (1×106)
were incubated with appropriate monoclonal antibodies for 30 min in
the dark at 4°C. Following this, the cells were washed twice with
Cell Wash (Becton-Dickinson) containing 0.5% bovine serum albumin
(BSA, Biowest) and acquired on a CytoFLEX LX (Beckman Coulter),
calibrated daily using CytoFLEX Daily QC Fluorospheres (Beckman
Coulter). Compensation settings were conducted using single-stained
cells or the VersaComp Antibody Capture Bead Kit (Beckman Coulter).
The following gating strategy was used: A time gate was initially
applied to exclude any electronic noise and artifact. Next, based
on size and granularity, JAWS II cells were gated in a forward
scatter area (FSC-A) vs. side scatter area (SSC-A). Then, doublet
cells were excluded using FSC-A/FSC-height (FSC-H), FSC-A/FSC-Width
and SSC-A/SSC-height (FSC-H) parameters. Within the singlet cell
population, viable JAWS II were gated based on the dim expression
of the Fixable Viability Dye (FVD), followed by expression of
examined markers: CD80, CD86, MHCII or CD40. In all the experiments
at least 100,000 events were analysed for each sample. Positive
staining and the gating strategy was determined by the comparison
to the unstained control and the fluorescence minus one (FMO)
controls. Data was analysed using Kaluza Analysis Software version
2.1 (Beckman Coulter). The results were shown as the percentage of
positively labelled cells and the mean fluorescence intensity (MFI)
calculated by CytoFLEX LX.
Endocytosis assay
To determinate the ability of cells to take up,
fluorescein isothiocyanate-conjugated dextran (FITC-DX 70,000,
Sigma-Aldrich; Merck KGaA) was used. The cells (1×106)
were incubated with FITC-DX (2 mg/ml) for 50 min at 37°C (control
plate at 4°C). At the end of the incubation, the cells were
collected and washed three times by centrifugation at 4°C in PBS (5
min, 300 × g) and re-suspended in 0.1 ml ice-cold PBS (pH 7.2) with
0.5% BSA for flow cytometry analysis.
T cell isolation and mixed lymphocyte
reaction (MLR)
Male C57BL/6 mice were euthanized by cervical
dislocation and their spleens isolated aseptically (n=8). The
spleens were pressed through a nylon cell strainer (BD Falcon) to
produce a single-cell suspension. Erythrocytes in the cell
suspension were depleted with red blood cell (RBC) lysis buffer.
Lymphocytes were purified using a MagniSort Mouse T cell Enrichment
Kit (Thermo Fisher Scientific) according to the manufacturer's
protocol. The purity was determined by flow cytometry (CytoFlex LX,
Beckman Coulter) using the CD3-eFluor506 monoclonal antibody (clone
17A2; eBioscience) post isolation. The T cells were then stained
with Cell Proliferation Dye eFluor 670 (CPD; eBioscience) and
1×106 T cells cocultured with 1×105 adherent
or non-adherent JAWS II cells (ratio 10:1) in 24-well plates for
120 h in the culture medium described above (37°C, 5%
CO2). After culturing, the supernatants were collected
for enzyme-linked immunosorbent assay (ELISA), then the cells were
harvested and labelled with CD3-eFluor506 monoclonal antibody
(clone 17A2; eBioscience) in order to identify T cells. All
procedures were performed according to the manufacturer's
protocols. The cells were prepared for flow cytometry analysis and
analysed as described above.
Cytokine detection in cell
culture
An ELISA method was used to determine IL-6, IL-10,
IL-17A, IFN-γ and TGF-β1 cytokine levels in cell-free supernatants
according to the manufacturer's guidelines (e-Biosciences, Thermo
Fisher Scientific). For the TGF-β1 measurement, the samples were
acidified, and the latent and active cytokine excreted into the
culture medium measured in each sample. The colorimetric reaction
was determined at 450 nm using the Synergy™ H1 Microplate Reader
(BioTek). The mean optical densities (OD) of the triplicate
cultures were compared with the standard curves prepared using
recombinant cytokines.
Statistical analysis
The significance of the differences between two
groups was determined by the Student's unpaired, two-tailed t-test.
The two-way analysis of variance (ANOVA) was used for multiple
group comparisons (GraphPad Software Inc.). The ANOVA was followed
by Tukey's post hoc analyses. Data were expressed as mean ± SD. A
P-value of <0.05 was considered to be statistically
significant.
Results
Viability of adherent and non-adherent
JAWS II cells
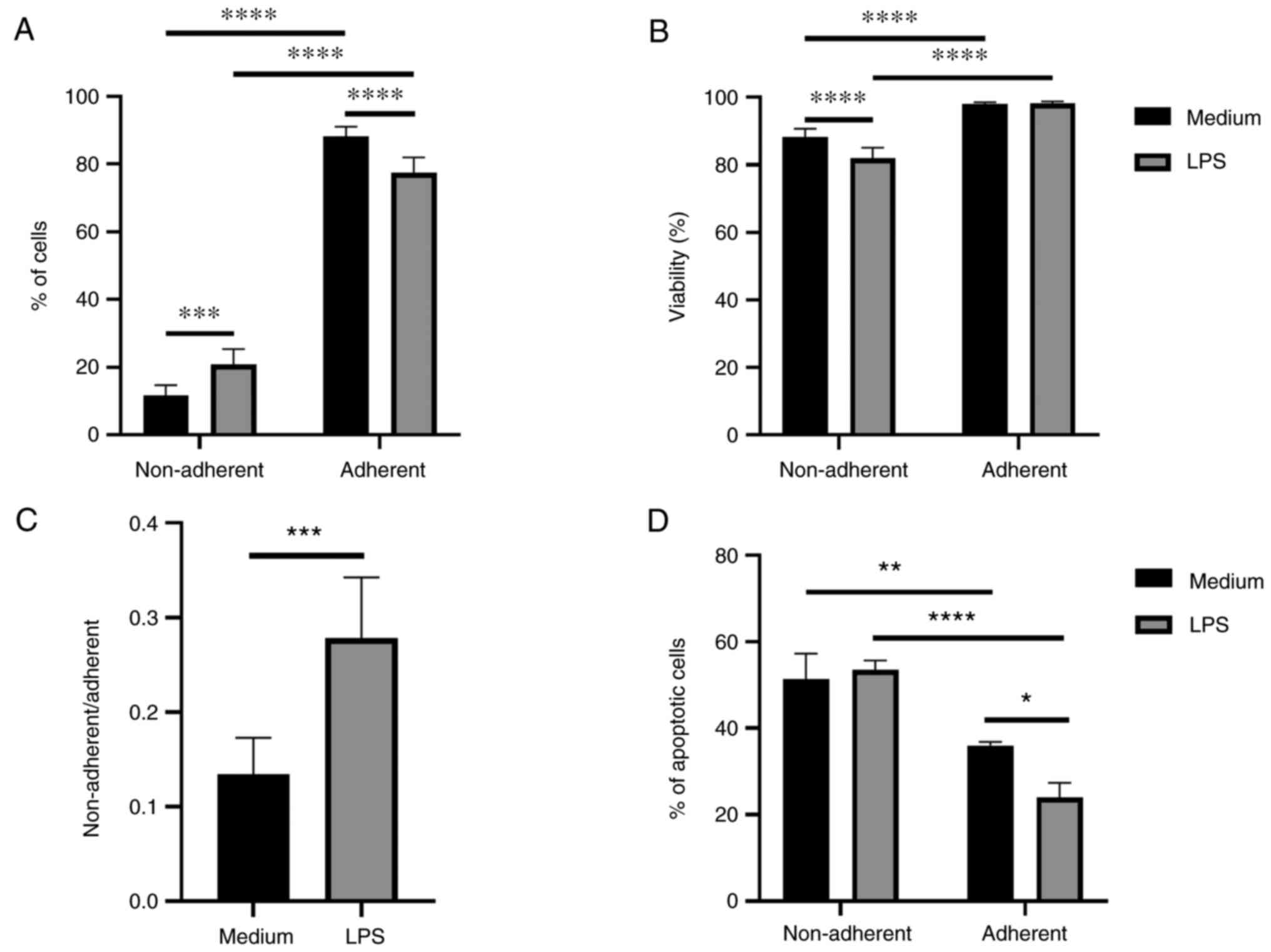
The percentage of cells, viability, and the
percentage of apoptotic cells were investigated in both fractions.
The ratio of the JAWS II cell fractions was calculated as
non-adherent/adherent cells. There were more adherent than
non-adherent cells in both, immature and LPS-activated JAWS II cell
culture. Activation with LPS increased percentage of non-adherent
cells and decreased percentage of cells in adherent fraction
(Fig. 1A). Higher viability of
cells was observed in adherent fraction of immature and LPS
activated cells. A lower viability of cells was observed in the
non-adherent fraction after LPS treatment (Fig. 1B). The ratio of JAWS II cell
fractions was significantly higher after LPS activation compared to
non-activated cells (Fig. 1C). The
percentage of apoptotic cells in the adherent fraction was
decreased in comparison to non-adherent. Apoptosis of adherent JAWS
II cells was even lower after activation with LPS and significantly
reduced compared to non-adherent LPS-activated cells (Fig. 1D).
Differential expression of surface
markers by adherent and non-adherent cells
Adherent and non-adherent cells were phenotyped by
flow cytometry. Immature non-adherent and adherent JAWS II cells
were not significantly phenotypically different. In LPS activated,
both adherent and non-adherent cells had increased MFI values of
the costimulatory molecules CD80 and CD86, compared with immature
cells (Fig. 2E and F). What is
more, higher percentage of CD80+ and CD86+
cells, as well as higher CD80 MFI values were observed in
LPS-activated adherent cells compared to the LPS-activated
non-adherent cells (Fig. 2A, B and
E). The lower percentage of MHC II+ cells but higher
MFI of MHC II was observed in the LPS-activated non-adherent cells
compared to these cells without LPS stimulation (Fig. 2C and G). No significant changes were
observed in the percentage of CD40+ cells and the MFI of
CD40 (Fig. 2D and H). Also, there
were no significant differences in the expression of CD11c (data
not shown).
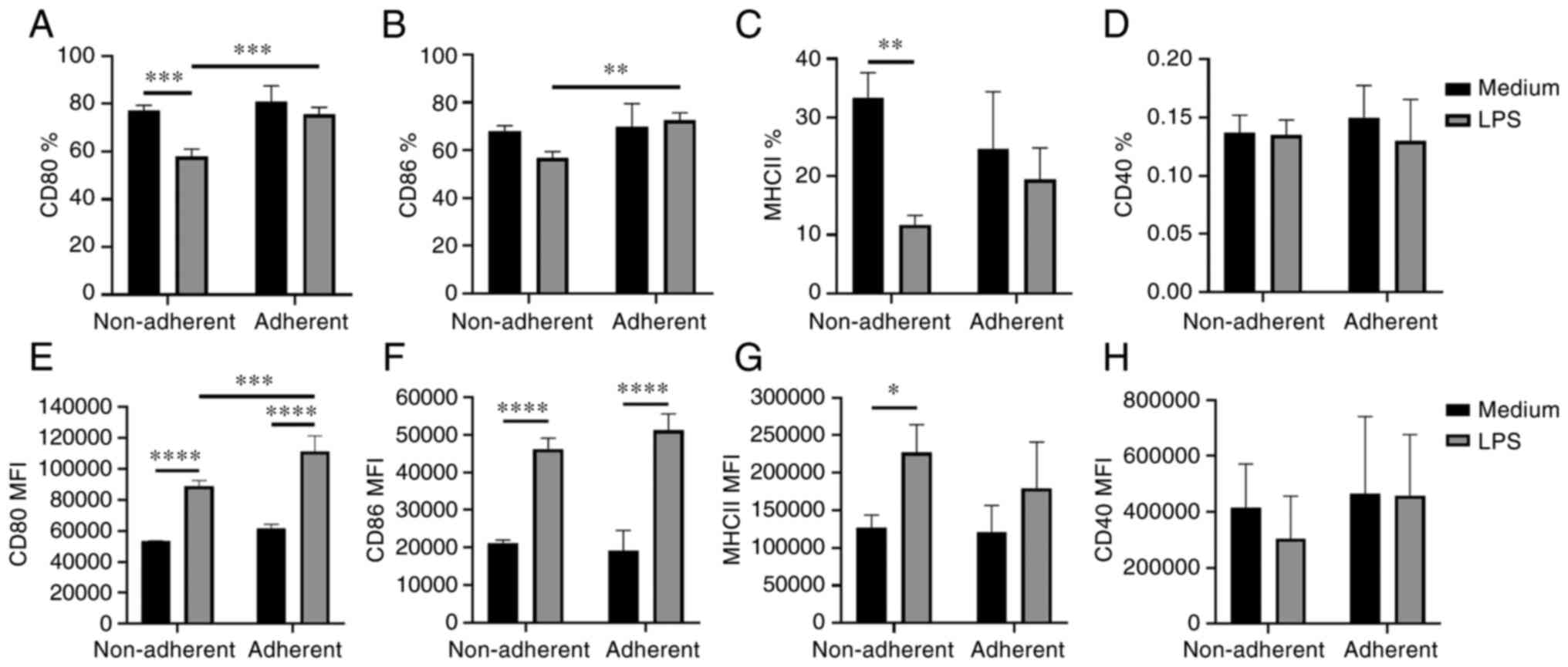 | Figure 2.Phenotype of adherent and non-adherent
JAWS II cells. Surface marker expression was evaluated by flow
cytometry after 48-h stimulation of JAWS II cells with LPS (2
µg/ml). The graphs show the percentage of (A) CD80, (B) CD86, (C)
MHCII, (D) CD40 positive cells, and MFI of (E) CD80, (F) CD86, (G)
MHCII and (H) CD40 ± SD, N=4. The lines indicate significant
differences. *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001. LPS, lipopolysaccharide; MFI, mean fluorescence
intensity; MHC, major histocompatibility complex. |
Immature adherent and non-adherent
JAWS II cells exhibit different endocytosis and signalling
properties
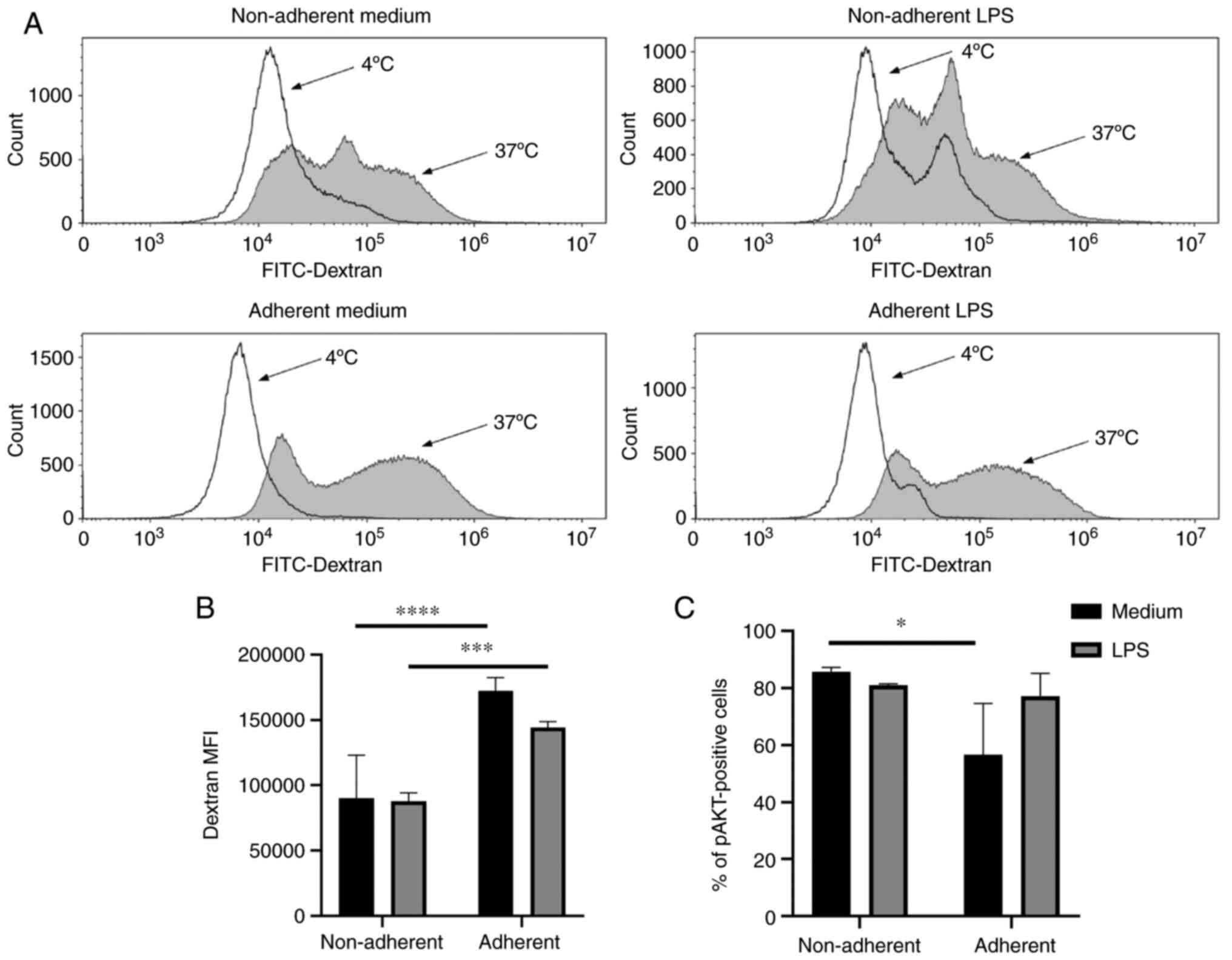
To determine the endocytic activity of JAWS II
cells, which is crucial in antigen presentation, cellular
FITC-dextran uptake was measured by flow cytometry. FITC-dextran
uptake was higher in adherent cells compared to non-adherent cells
in both, immature and LPS-activated JAWS II cells (Fig. 3A and B). To evaluate signalling in
both fractions of JAWS II cells, the MAPK and Pi3K signal pathways
were examined. The immature, non-adherent fraction showed an
increased percentage of pAKT positive cells in comparison with
adherent cells. Activation with LPS did not result in significant
changes in the Pi3K signal pathway (Fig. 3C). No significant changes were
observed in MAPK activation based on the percentage of pERK1/2
positive cells (data not shown).
LPS-activated adherent and
non-adherent JAWS II cells differentially influence T cell
activity
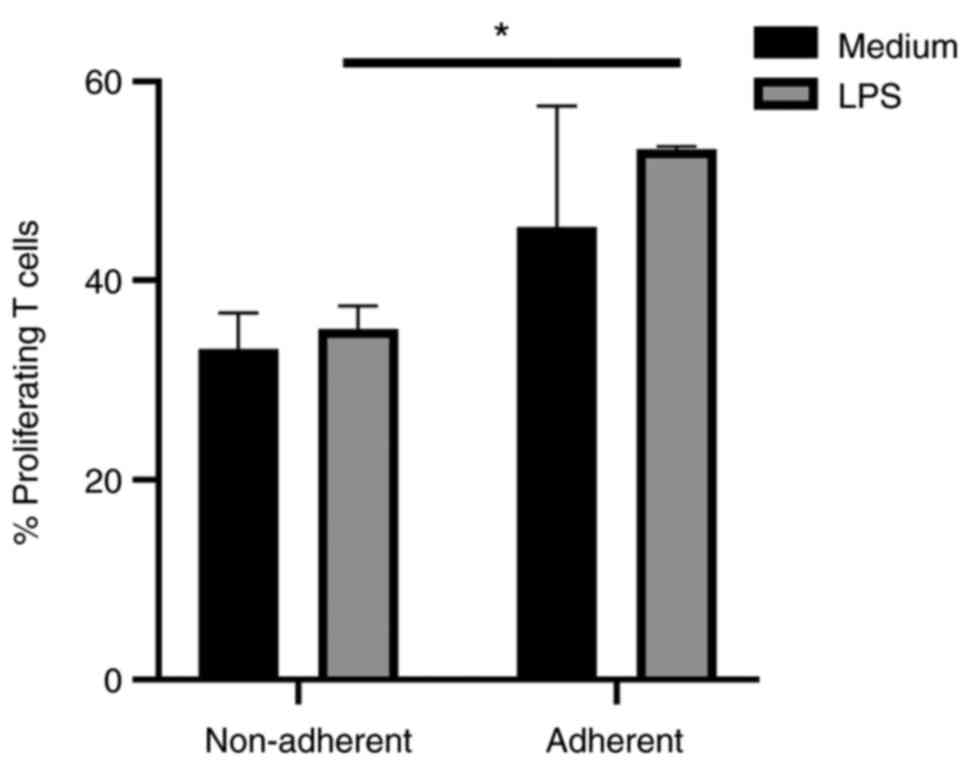
In order to evaluate how non-adherent and adherent
JAWS II cells impact T cells functions, proliferation and cytokine
production were investigated. Unstimulated T cells cultured without
JAWS II cells were used as the negative control, and T cells
stimulated with CD3 and CD28 antibodies were treated as the
positive control. LPS-activated, adherent JAWS II cells induced
stronger proliferation of T cells than non-adherent LPS-activated
JAWS II cells. A similar effect, but not statistically significant,
was observed for immature cells (Fig.
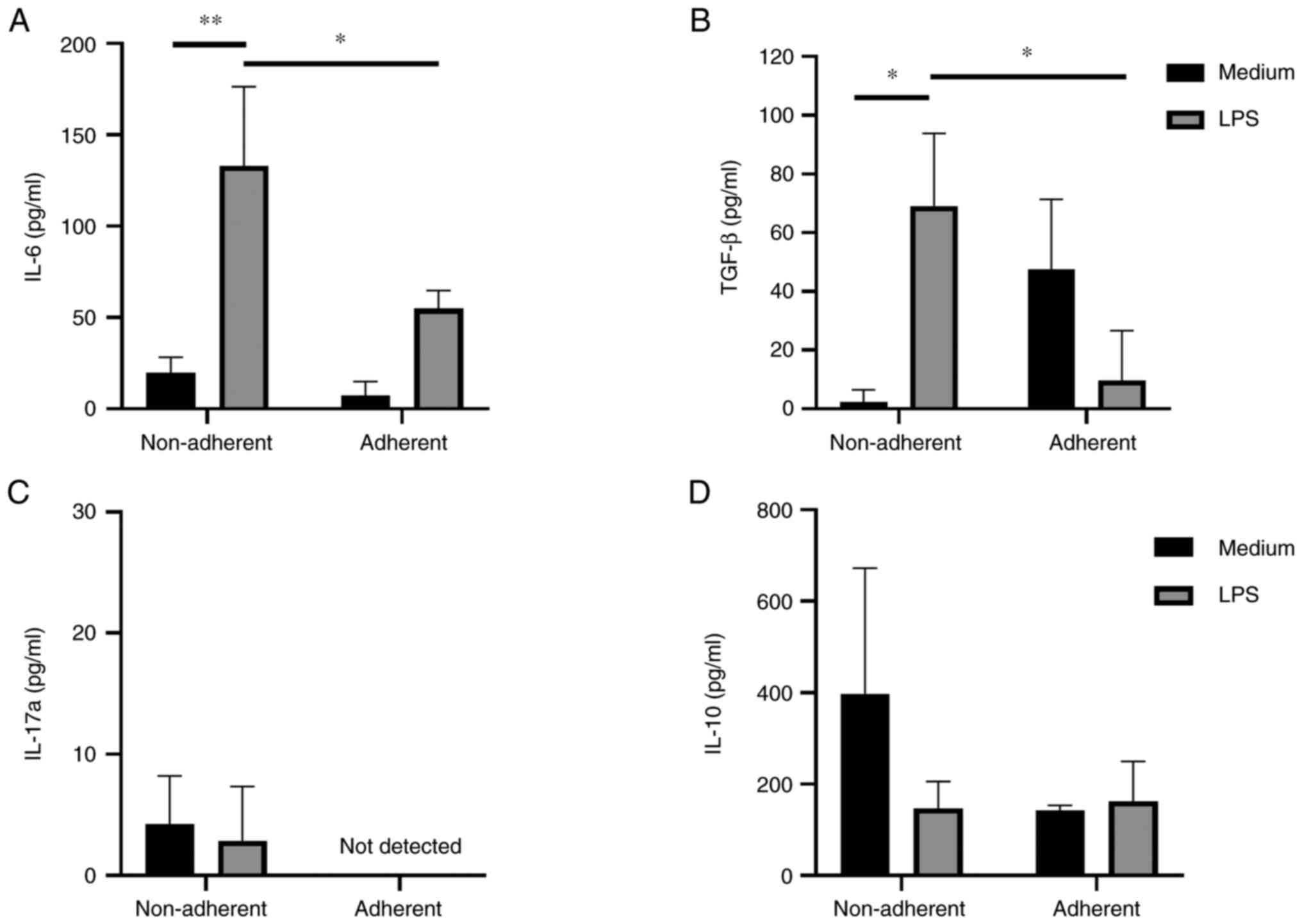
4). LPS-activated non-adherent JAWS II induced significantly
higher level of IL-6 and TGF-β1 than did immature non-adherent
cells. Stimulation of non-adherent cells with LPS resulted in
elevated production of IL-6 and TGF-β1 in comparison to adherent
cells (Fig. 5A and B). IL-17A was
produced in small amounts by non-adherent cells both treated and
not treated with LPS, with no detected production by adherent JAWS
II cells (Fig. 5C). No significant
changes in IL-10 production were observed (Fig. 5D). IFN-γ production was not observed
(data not shown).
Discussion
In the present study, we evaluated the viability,
phenotype and activity of immature and LPS-activated JAWS II cells.
The cells were divided into two fractions: non-adherent (cells
suspended in the medium) and adherent cells (adjacent to the
substrate). The results indicated that these two fractions of JAWS
II cells differs in various ways.
At the beginning we evaluated JAWS II cells
condition. There were more adherent cells than non-adherent cells
and the adherent cells had better viability. However, activation
with LPS resulted in a larger proportion of non-adherent cells in
comparison with unstimulated cells. Next step was to examine the
surface phenotype. The expression of markers associated with T cell
activation (CD80, CD86, MHC II and CD40) was determined. Immature
non-adherent and adherent JAWS II cells were not significantly
phenotypically different. This is in line with previous outcomes on
JAWS II cells (1) and human
monocyte-derived dendritic cells (mo-DCs), where only the
percentage of CD86 positive cells was statistically different
(17).
However, we observed differences in the phenotype
between the two fractions of LPS-activated JAWS II cells. In
LPS-activated JAWS II cells a higher percentages of CD80- and
CD86-positive cells were observed in the adherent fraction. Also,
the MFI of CD80 was increased in LPS-activated adherent JAWS II
cells. These results suggest, that LPS-activated adherent JAWS II
cells may be more effective in antigen presentation and T cells
activation than LPS-activated non-adherent JAWS II cells.
LPS activation of JAWS II resulted in decreased
percentage of CD80- and MHC II-positive cells, but it was
associated with higher expression (MFI) of CD80, CD86, and MHC II
molecules in non-adherent cells. A reduced percentage of cells can
result from several issues, such as the condition of cells in the
LPS environment. However, the marker expression on the cell surface
may be sufficient for effective T cell activation. We have obtained
similar results in our previous work concerning human
monocyte-derived DCs (mo-DCs). After LPS activation, only the MFI
values were significantly higher compared to immature DCs for all
examined receptors: MHC II, CD40, CD80, CD83, CD86, CCR7, TLR2,
TLR4 (19).
With the maturation of DCs, the ability to uptake
antigen decreases. Our studies confirmed that after stimulation
with LPS, cells showed reduced endocytosis, but not statistically
significant. We have observed, that adherent JAWS II cells
exhibited significantly higher endocytosis compared tonon-adherent
cells. This may suggest that adherent cells demonstrate increased
endocytic capacity. Yi and Lu (17)
reported that mature human mo-DCs, adherent cells show higher
antigen uptake than non-adherent cells. We have obtained similar
results.
Our findings demonstrate that there were decreased
numbers of immature adherent cells with activated Pi3K signalling
compared to non-adherent cells. Pi3K signalling plays an important
role in various cellular processes such as proliferation, migration
or antigen presentation. In addition, Pi3K signalling is involved
in the pathogenesis of autoimmune diseases and immunity against
cancer (20,21). The inhibition of Akt phosphorylation
in bone-marrow derived DCs (BMDC) by IL-10 suppresses IKK/NF-κB
activation (22,23).
The crucial role of DCs is antigen presentation and
T cells activation, hence we evaluated proliferation and cytokine
production of T cells isolated from murine spleen with adherent and
non-adherent JAWS II cells. We observed increased proliferation of
T cells cocultured with LPS-activated, adherent DCs in line with
decreased production of IL-6, and TGF-β1, compared to non-adherent
LPS-activated cells. These results confirmed, that LPS-activated
adherent JAWS II cells may induce T cell proliferation. The
experiment with human DCs also showed that adherent cells more
effectively induced proliferation of T cells than non-adherent DC
(17). However, Wang et al
(18) showed that non-adherent
bone-marrow derived DCs (BMDC) more effectively stimulated
proliferation of CD4 T cells isolated from lymph nodes than
adherent cells. The difference in the results could be a
consequence of the source of T cells.
To summarise, adherent immature JAWS II cells show
increased endocytosis and decreased activation of the Pi3K signal
pathway. They also induced increased production of TGF-β1 by T
cells in comparison to non-adherent cells. On the other hand,
adherent, LPS-activated JAWS II cells showed increased expression
of CD80 and CD86 costimulatory molecules, increased endocytosis and
an elevated ability to induce proliferation of T cells in MLR with
decreased production of IL-6, IL17A and TGF-β1 compared to
non-adherent LPS-activated cells.
Choosing the right fraction for cancer immunotherapy
research can be crucial. In immunotherapy of cancer, mature DCs
with great capacity to activate T cells with cytotoxic activity are
expected. Based on our results, LPS-activated adherent JAWS II
cells fraction appears to be more favorable for future DC-vaccine
evaluation against cancers. Such cells showed increased expression
of costimulatory molecules and induced T cells proliferation and
may be more effective in T cell activation.
Non-adherent fraction shows some properties
characteristics for tolerogenic DCs. Tolerogenic DCs are immature
dendritic cells, induce immune tolerance and are able to inhibit T
cell response. Decreased CD80, CD86 and MHC II expression, lower
percentage of proliferating T cells, increased IL-10 production and
activation of the Pi3K signal pathway may indicate tolerogenic
functions of non-adherent immature JAWS II compared to adherent
(24,25). Some results show that DC after
longer exposition to LPS can become more ‘tolerant’ or ‘exhausted’.
This is associated with the production of Th2 cytokines instead of
the development of a pro-inflammatory Th1 immune response (26). Other studies indicate that DC after
long exposure to LPS wait for a signal from T cells, necessary for
their further activity (27).
Heterogenicity of dendritic cells has been discussed
for a long time; however, this problem is not flawlessly described.
Choosing adherent or non-adherent cells is not only relevant for
DCs, but also for other types of cells with potential use in
clinical studies (28). More
attention should be paid to which fraction of DCs is chosen. The
effect of two mixed fractions can be abolished. In addition, a lack
of indication of the used fraction can result in apparently
different results. We showed that the two fractions of JAWS II,
adherent and non-adherent cells, show different properties. In
addition, differences in the phenotype and activity of adherent and
non-adherent cells are more significant after LPS activation.
Therefore, both the adherence and activation status of DCs are
important when planning experiments.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MMC, MM, MK and KDL contributed to the study
conception and design. MMC, MM and KDŁ confirm the authenticity of
all the raw data. MMC performed the experiments, analysed data and
wrote the manuscript. MM performed the experiments, analysed data
and corrected the manuscript. MK performed the experiments and
analysed data. KDŁ analysed the data and corrected the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All methods involving animal studies are in line
with ARRIVE EU guidelines Directive 2010/63/EU on animal
experimentation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BMDC
|
bone marrow-derived dendritic
cells
|
|
DCs
|
dendritic cells
|
|
FITC-DX
|
isothiocyanate-labelled dextran
|
|
GM-CSF
|
granulocyte macrophage-colony
stimulating factor
|
|
MFI
|
mean fluorescence intensity
|
|
MHC II
|
major histocompatibility complex class
II
|
|
MLR
|
mixed lymphocyte reaction
|
|
Mo-DCs
|
monocyte-derived dendritic cells
|
|
RBC
|
red blood cells
|
|
TLR
|
toll like receptor
|
References
|
1
|
Vang KB, Safina I, Darrigues E, Nedosekin
D, Nima ZA, Majeed W, Watanabe F, Kannarpady G, Kore RA, Casciano
D, et al: Modifying dendritic cell activation with plasmonic nano
vectors. Sci Rep. 7:55132017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zapala L, Drela N, Bil J, Nowis D, Basak
GW and Lasek W: Optimization of activation requirements of immature
mouse dendritic JAWSII cells for in vivo application. Oncol Rep.
25:831–840. 2011.PubMed/NCBI
|
|
3
|
Jittimanee S, Wongratanacheewin S,
Kaewraemruaen C and Jittimanee J: Opisthorchis viverrini antigens
up-regulates the expression of CD80 and MHC class II in JAWSII
mouse dendritic cells and promotes IL-10 and TGF-β secretions.
Parasitol Int. 84:1024012021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maruszewska-Cheruiyot M,
Donskow-Łysoniewska K, Piechna K, Krawczak K and Doligalska M: L4
stage Heligmosomoides polygyrus prevents the maturation of
dendritic JAWS II cells. Exp Parasitol. 196:12–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bieńkowska A, Kiernozek E, Kozlowska E,
Zarzycki M and Drela N: Thymus-deriving natural regulatory T cell
generation in vitro: Role of the source of activation signals.
Immunol Lett. 162:199–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eko FO, Mania-Pramanik J, Pais R, Pan Q,
Okenu DM, Johnson A, Ibegbu C, He C, He Q, Russell R, et al: Vibrio
cholerae ghosts (VCG) exert immunomodulatory effect on dendritic
cells for enhanced antigen presentation and induction of protective
immunity. BMC Immunol. 15:5842014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Egger M, Jürets A, Wallner M, Briza P,
Ruzek S, Hainzl S, Pichler U, Kitzmüller C, Bohle B, Huber CG and
Ferreira F: Assessing protein immunogenicity with a dendritic cell
line-derived endolysosomal degradome. PLoS One. 6:e172782011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu Y, Li M, Lu B, Wang X, Chen C and Zhang
M: Corticotropin-releasing factor augments LPS-induced
immune/inflammatory responses in JAWSII cells. Immunol Res.
64:540–547. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mutlu EC, Kaya Ö, Wood M, Mager I, Topkara
KÇ, Çamsarı Ç, Birinci Yildirim A, Çetinkaya A, Acarel D and
Odabaşı Bağcı J: Efficient doxorubicin loading to isolated
dexosomes of immature JAWSII cells: Formulated and characterized as
the bionanomaterial. Materials (Basel). 13:33442020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Safina I, Alghazali KM, Childress L,
Griffin C, Hashoosh A, Kannarpady G, Watanabe F, Bourdo SE, Dings
RPM, Biris AS and Vang KB: Dendritic cell biocompatibility of
ether-based urethane films. J Appl Toxicol. 41:1456–1466. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ssemakalu CC, Ubomba-Jaswa E, Motaung KSCM
and Pillay M: Solar inactivated Vibrio cholerae induces maturation
of JAWS II dendritic cell line in vitro. J Water Health.
18:494–504. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baldin AV, Savvateeva LV, Bazhin AV and
Zamyatnin AA Jr: Dendritic Cells in anticancer vaccination:
Rationale for ex vivo loading or in vivo targeting. Cancers
(Basel). 12:5902020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wculek SK, Cueto FJ, Mujal AM, Melero I,
Krummel MF and Sancho D: Dendritic cells in cancer immunology and
immunotherapy. Nat Rev Immunol. 20:7–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salah A, Wang H, Li Y, Ji M, Ou WB, Qi N
and Wu Y: Insights into dendritic cells in cancer immunotherapy:
From bench to clinical applications. Front Cell Dev Biol.
9:6865442021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lutz MB and Schuler G: Immature,
semi-mature and fully mature dendritic cells: Which signals induce
tolerance or immunity? Trends Immunol. 23:445–449. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dalod M, Chelbi R, Malissen B and Lawrence
T: Dendritic cell maturation: Functional specialization through
signaling specificity and transcriptional programming. EMBO J.
33:1104–1116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi HJ and Lu GX: Adherent and non-adherent
dendritic cells are equivalently qualified in GM-CSF, IL-4 and
TNF-α culture system. Cell Immunol. 277:44–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Dai X, Hsu C, Ming C, He Y, Zhang
J, Wei L, Zhou P, Wang CY, Yang J and Gong N: Discrimination of the
heterogeneity of bone marrow-derived dendritic cells. Mol Med Rep.
16:6787–6793. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Machcińska M, Kotur M, Jankowska A,
Maruszewska-Cheruiyot M, Łaski A, Kotkowska Z, Bocian K and
Korczak-Kowalska G: Cyclosporine A, in contrast to rapamycin,
affects the ability of dendritic cells to induce immune tolerance
mechanisms. Arch Immunol Ther Exp (Warsz). 69:272021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aksoy E, Saveanu L and Manoury B: The
isoform selective roles of PI3Ks in dendritic cell biology and
function. Front Immunol. 9:25742018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Datler H, Vogel A, Kerndl M, Baumgartinger
C, Musiejovsky L, Makivic N, Frech S, Niederreiter B, Haider T,
Pühringer M, et al: PI3K activity in dendritic cells exerts
paradoxical effects during autoimmune inflammation. Mol Immunol.
111:32–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhattacharyya S, Sen P, Wallet M, Long B,
Baldwin AS Jr and Tisch R: Immunoregulation of dendritic cells by
IL-10 is mediated through suppression of the PI3K/Akt pathway and
of IkappaB kinase activity. Blood. 104:1100–1109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cahill CM, Rogers JT and Walker WA: The
role of phosphoinositide 3-kinase signaling in intestinal
inflammation. J Signal Transduct. 2012:3584762012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu J and Wan Y: Tolerogenic dendritic
cells and their potential applications. Immunology. 132:307–314.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Svajger U and Rozman P: Tolerogenic
dendritic cells: Molecular and cellular mechanisms in
transplantation. J Leukoc Biol. 95:53–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Langenkamp A, Messi M, Lanzavecchia A and
Sallusto F: Kinetics of dendritic cell activation: Impact on
priming of TH1, TH2 and nonpolarized T cells. Nat Immunol.
1:311–316. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abdi K, Singh NJ and Matzinger P:
Lipopolysaccharide-activated dendritic cells: ‘Exhausted’ or alert
and waiting? J Immunol. 188:5981–5989. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pittenger MF, Discher DE, Péault BM,
Phinney DG, Hare JM and Caplan AI: Mesenchymal stem cell
perspective: Cell biology to clinical progress. NPJ Regen Med.
4:222019. View Article : Google Scholar : PubMed/NCBI
|