Introduction
Although modern medicine has advanced greatly,
bladder cancer (BC) still occurs in an increasing number of people,
with 573,278 cases in 2020 globally, while the number of deaths has
not reduced either, with 212,536 cases in this same year (1,2).
Sarcomatoid carcinoma is a rare type of BC that has both
mesenchymal and epithelial characteristics (3); however, it is essentially an
epithelial-derived cancer (4–6).
Sarcomatoid carcinoma can be found in tumours of every system, and
its presence means that metastases occur quickly, even after
radical surgery (7,8). The median survival time for bladder
sarcomatoid carcinoma ranges from 13.4 to 18.5 months (9,10). It
is reported that this histopathological type is not sensitive to
systemic treatment, such as chemotherapy, and the pathological
response to chemotherapy is often not optimistic (11). Therefore, a number of studies
analyzing prognostic factors and treatment efficacy have excluded
sarcomatoid carcinoma to reduce selection bias (12–14).
Despite this, some patients with sarcomatoid
carcinoma can still survive for a long time or even be cured.
Identification of these individuals can help with the
implementation of more aggressive medical treatment. To the best of
our knowledge, to date, few studies have focused on sarcomatoid
carcinoma of the bladder (11,15).
One study found that lower stages of the cancer may be associated
with better survival time (15).
Smaller tumour diameter, lower percentage of sarcomatoid
components, absence of lymph node invasion and distant metastasis,
and absence of tumour necrosis are associated with a prolonged
survival time, although this has been found in sarcomatoid
carcinoma of organs other than the bladder (16–19).
To the best of our knowledge, prognostically relevant variables in
sarcomatoid carcinoma of the bladder have not been
investigated.
The purpose of the present study was to summarise
the clinicopathological features and prognosis of sarcomatoid
carcinomas of the bladder, and to confirm the prognostic value of
the proportion of sarcomatoid components.
Patients and methods
Patients
The present study included 38 patients with
sarcomatoid carcinoma of the bladder and 76 patients with
non-sarcomatoid bladder cancer who were treated at the Department
of Urology, The Affiliated Hospital of Qingdao University (Qingdao,
China), between August 2010 and May 2021. The inclusion criteria
were as follows: i) pathologically diagnosed surgical specimens;
and ii) pT staging ≥T1. Patients with carcinosarcoma and sarcoma,
which are two pathological types that differ from sarcomatoid
carcinoma, were excluded. All patients diagnosed with other cancer
types at the same time were excluded. The patients underwent
radical cystectomy or transurethral resection, performed by
experienced urologists, and volunteered to participate in this
study. The Affiliated Hospital of Qingdao University Ethics
Committee approved the study (approval number, AHQU-MAL 20210110).
All methods were performed in accordance with the relevant
guidelines and regulations, such as the Declaration of
Helsinki.
Patient details, such as age at diagnosis, sex, body
mass index, smoking status, alcohol consumption, diagnosis of
diabetes mellitus, presence of hypertension, type of surgery, and
documentation of adjuvant regimens of chemotherapeutic agents and
immune checkpoint inhibitors, were obtained from the medical
records or telephone follow-up. Imaging data were reviewed to
determine whether distant metastases were present at the time of
surgery. The pathology reports included immunohistochemical
information, proportion of sarcomatoid components, pathological T
stage, regional lymph node invasion, tumour necrosis,
lymphovascular invasion and vascular tumour thrombus. Pathological
staging was on the basis of the eighth edition of the TNM staging
criteria of the Union for International Cancer Control (20). Lymphovascular invasion implied the
invasion of endothelium-lined spaces of microvessels and lymphatics
(21). The subgroup analysis was
performed, and the patients were divided into two groups, namely,
muscle-invasive BC and non-muscle-invasive BC, according to whether
the tumour invaded the muscle.
Follow-up
Patients were followed up every 3 months for 2 years
and annually thereafter. Recurrence or metastasis was detected
using computed tomography and/or magnetic resonance imaging. The
primary outcomes were cancer-specific survival (CSS) and overall
survival (OS), defined as death from BC and all causes,
respectively, or the end of follow-up. Another outcome was
recurrence-free survival (RFS), defined as disease recurrence,
newly detected metastasis or death from all causes.
Propensity score matching
To balance the confounders of sarcomatoid and
non-sarcomatoid BC, propensity score matching was applied. The
logistic regression model was performed according to age, sex and
pathological T stage. The matching algorithm was 1:2 nearest
neighbour matching with a 0.1 calliper width.
Statistical analysis
The Wilcoxon rank-sum test, χ2 test and
Fisher's exact test were used to compare the continuous and
categorical variables of the patients. The Kaplan-Meier method was
used to obtain survival rates and the log-rank test was used to
compare survival curves. The association between the sarcomatoid
components and other variables and the survival outcomes were
calculated by Cox proportional hazard regression models and
summarised using hazard ratios (HRs) and 95% confidence intervals
(CIs). Collinearity diagnosis was used to test for
multi-collinearity. The optimal cut-off value of the proportion of
sarcomatoid components for prognostic value was determined through
the receiver operating characteristic curve. Statistical analysis
was performed using R 3.5.0 software (R Foundation) and SPSS
version 26.0 (IBM Corp.). A two-sided P-value of <0.05 was
considered to indicate a statistically significant difference.
Results
Baseline characteristics
A total of 38 patients with sarcomatoid carcinomas
of the bladder were identified from the hospital database. Another
76 patients with non-sarcomatoid BC were selected as the control
group after matching by propensity score. The median follow-up time
was 46 months. The patients with sarcomatoid and non-sarcomatoid
carcinoma of the bladder were mostly male (76 vs. 78%,
respectively; P=0.875). The median age at diagnosis was 72 years
(range, 65–79 years) and 71 years (range, 64–76 years),
respectively (P=0.658). The majority of patients with stage T1 BC
underwent transurethral resection of the bladder (88 vs. 84%,
respectively; P>0.999). By contrast, most patients with
muscle-invasive BC underwent radical cystectomy (82 vs. 96%,
respectively; P=0.090). Muscle-invasive BC accounted for the
majority of sarcomatoid and non-sarcomatoid carcinomas (58 vs. 58%,
respectively; P=0.921) (data not shown). The proportion of patients
with regional lymph node invasion (11 vs. 11%, respectively;
P>0.999) and lymphovascular invasion (13 vs. 26%, respectively;
P=0.109) was low. A sarcomatoid component proportion of >50% was
observed in 45% of the patients with sarcomatoid carcinoma. A
single patient with sarcomatoid carcinoma used tyrosine kinase
inhibitor anlotinib after the discovery of lung metastases (data
not shown). Patient characteristics after matching were not
significantly different and are listed in Table I.
 | Table I.Patient characteristics of the
sarcomatoid and non-sarcomatoid groups. |
Table I.
Patient characteristics of the
sarcomatoid and non-sarcomatoid groups.
| Feature | Sarcomatoid
(n=38) | Non-sarcomatoid
(n=76) | P-value |
|---|
| Median age (IQR),
years | 72 (65–79) | 71 (64–76) | 0.658 |
| Male, n (%) | 29 (76) | 59 (78) | 0.875 |
| Median BMI (IQR) | 25.2 (21.8-27.5) | 23.7 (20.8-26.0) | 0.336 |
| Hypertension, n
(%) | 12 (32) | 25 (33) | 0.888 |
| Diabetes mellitus, n
(%) | 6 (16) | 11 (14) | 0.924 |
| Smoking, n (%) | 16 (42) | 37 (49) | 0.545 |
| Alcohol, n (%) | 6 (16) | 32 (42) | 0.005 |
| ECOG ≥1, n (%) | 7 (18) | 10 (13) | 0.457 |
| Type of surgery, n
(%) |
|
|
|
| Radical
cystectomy | 20 (53) | 47 (62) | 0.346 |
|
Transurethral resection | 18 (47) | 29 (38) |
|
| Median tumor size
(IQR), cm | 3.5 (2.6-4.4) | 3.0 (2.1-4.5) | 0.356 |
| pT stage, n
(%) |
|
|
|
|
pT1 | 16 (42) | 32 (42) | 0.921 |
|
pT2 | 10 (26) | 21 (28) |
|
|
pT3 | 9 (24) | 18 (24) |
|
|
pT4 | 3 (8) | 5 (7) |
|
| pN1, n (%) | 4 (11) | 8 (11) | >0.999 |
| LVI, n (%) | 5 (13) | 20 (26) | 0.109 |
| Coagulative tumor
necrosis, n (%) | 8 (21) | 6 (8) | 0.086 |
| Multifocal, n
(%) | 8 (21) | 24 (32) | 0.238 |
Prognosis and independent prognostic
factors
A total of 11 patients with sarcomatoid carcinoma
and 9 with non-sarcomatoid carcinoma died of cancer during the
follow-up. Significant differences were found between the
sarcomatoid and non-sarcomatoid carcinoma groups in terms of 1-year
CSS rate (80.2 vs. 100%, respectively), 3-year CSS rate (73.4 vs.
96.4%, respectively) and 5-year CSS rate (69.4 vs. 86.9%,
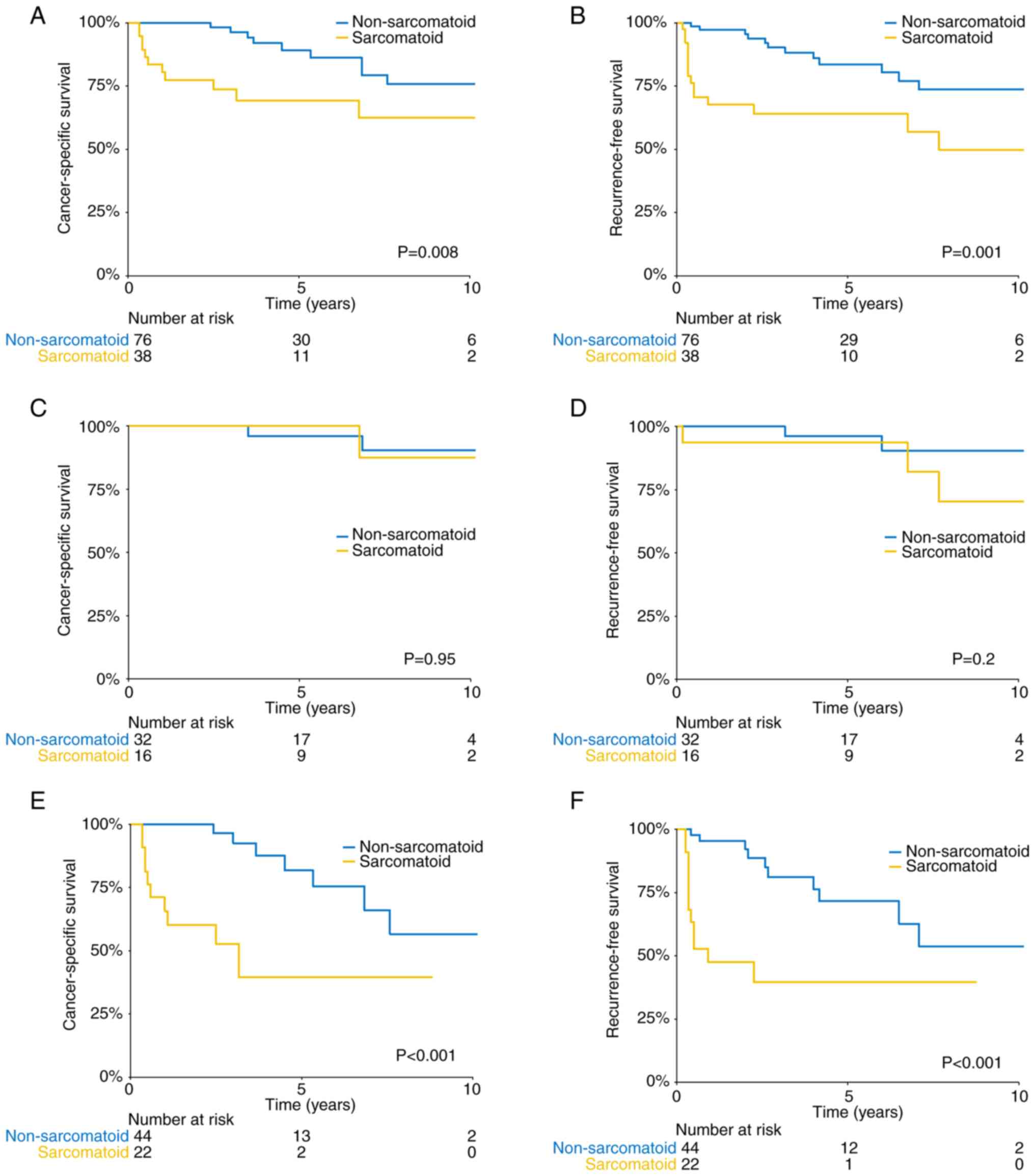
respectively) (log-rank P=0.008) (Fig.
1A). After stratification by depth of invasion,
non-muscle-invasive BC with sarcomatoid carcinoma components did
not confer a worse CSS rate (5-year CSS rate: 100 vs. 91.8%,
respectively; log-rank P>0.05) (Fig.
1C). Among the patients with muscle-invasive BC, sarcomatoid
carcinomas had a significantly worse median CSS (38 vs. >120
months, respectively), with a 5-year CSS rate of 39.1% compared
with 81.8% for non-sarcomatoid carcinomas (log-rank P<0.001)
(Fig. 1E). A total of 15 patients
with sarcomatoid BC and 12 with non-sarcomatoid BC experienced
recurrence. The common metastatic sites of sarcomatoid carcinoma of
the bladder were the bones (n=4), lungs (n=3), liver (n=2) and
pelvic cavity (n=2). The 5-year RFS rates of the sarcomatoid and
non-sarcomatoid carcinoma groups were 64.1 and 83.6%, respectively
(log-rank P=0.001) (Fig. 1B). The
5-year RFS rates with and without sarcomatoid differentiation were
93.8 and 96.2%, respectively, in the non-muscle-invasive subgroup
(Fig. 1D). The patients with
sarcomatoid carcinoma had a worse median RFS time (11 vs. >120
months) in the muscle-invasive subgroup (Fig. 1F).
On multivariate analysis, larger tumour diameter, pT
stage ≥T2, regional lymph node invasion and sarcomatoid carcinoma
were independent prognostic factors for cancer-specific death. pT
stage ≥T2, regional lymph node invasion and sarcomatoid carcinoma
increased the risk of disease recurrence in the patients with BC.
Independent predictors of OS were BMI, tumor size, pT stage and
sarcomatoid carcinoma. Multivariate analysis showed that
sarcomatoid carcinoma of the bladder was significantly associated
with worse CSS (HR, 6.19; 95% CI, 2.31-16.6; P<0.001), RFS (HR,
4.44; 95% CI, 2.03-9.71; P<0.001) and OS (HR, 3.04; 95% CI,
1.39-6.66; P=0.005) (Table
II).
 | Table II.Univariate and multivariate Cox
regression analyses of all patients for CSS, RFS and OS. |
Table II.
Univariate and multivariate Cox
regression analyses of all patients for CSS, RFS and OS.
|
| CSS | RFS | OS |
|---|
|
|
|
|
|
|---|
|
| Univariate |
Multivariatea | Univariate |
Multivariatea | Univariate |
Multivariateb |
|---|
|
|
|
|
|
|
|
|
|---|
| Feature | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (per 10-year
increase) | 0.98
(0.58-1.65) | 0.870 |
| - | 0.94
(0.60-1.47) | 0.762 |
| - | 1.32
(0.88-1.99) | 0.221 |
| - |
| Sex (male vs.
female) | 0.62
(0.23-1.73) | 0.367 |
| - | 0.79
(0.32-1.95) | 0.624 |
| - | 0.83
(0.36-1.92) | 0.702 |
| - |
| ECOG (≥1 vs.
0) | 1.28
(0.37-4.34) | 0.697 |
| - | 1.17
(0.40-3.37) | 0.801 |
| - | 1.45
(0.60-3.49) | 0.371 |
| - |
| Hypertension
(yesvs. no) | 0.36
(0.11-1.21) | 0.098 |
| - | 0.58
(0.23-1.42) | 0.224 |
| - | 0.50
(0.22-1.15) | 0.052 |
| - |
| DM (yes vs.
no) | 2.33
(0.85-6.37) | 0.010 |
| - | 1.50
(0.57-3.95) | 0.437 |
| - | 1.39
(0.58-3.35) | 0.537 |
| - |
| Smoking (yes vs.
no) | 0.88
(0.37-2.10) | 0.772 |
| - | 0.78
(0.37-1.65) | 0.477 |
| - | 1.20
(0.61-2.36) | 0.571 |
| - |
| Alcohol (yes vs.
no) | 0.58
(0.22-1.49) | 0.256 |
| - | 0.89
(0.41-1.93) | 0.751 |
| - | 0.71
(0.35-1.45) | 0.326 |
| - |
| BMI (per
5-kg/m2 increase) | 0.96
(0.54-1.73) | 0.868 |
| - | 1.35
(0.82-2.28) | 0.278 |
| - | 1.00
(0.42-2.41) | 0.026 | 0.54
(0.32-0.94) | 0.030 |
| Surgery (RC vs.
TUR) | 3.42
(0.43-27.11) | 0.212 |
| - | 3.20
(1.34-7.66) | 0.010 |
| - | 3.43
(1.58-7.43) | 0.002 |
| - |
| Tumor size (>3.5
vs. ≤3.5 cm) | 3.57
(1.46-8.72) | 0.005 | 2.64
(1.01-6.89) | 0.047 | 3.08
(1.43-6.65) | 0.004 |
| 0.101 | 3.42
(1.72-6.80) | <0.001 | 2.59
(1.28-5.26) | 0.008 |
| pT stage (≥T2 vs.
T1) | 5.93
(1.96-17.03) | 0.002 | 6.50
(1.92-22.08) | 0.003 | 4.71
(1.87-11.75) | 0.001 | 4.73
(1.74-12.91) | 0.002 | 4.71
(2.10-10.55) | <0.001 | 4.85 (2.02- | <0.001 |
| pN stage (N1 vs.
N0) | 5.83
(1.97-17.21) | 0.001 | 3.48
(1.13-10.75) | 0.032 | 7.35
(3.04-17.84) | <0.001 | 5.58
(2.20-14.18) | <0.001 | 4.79
(2.00-11.50) | <0.001 |
| 0.125 |
| LVI (yes vs.
no) | 1.41
(0.46-4.31) | 0.544 |
| - | 1.37
(0.54-3.46) | 0.458 |
| - | 2.43
(1.14-5.20) | 0.018 |
| 0.786 |
| Necrosis (yes vs.
no) | 2.21
(0.64-7.62) | 0.210 |
| - | 2.48
(0.93-6.59) | 0.074 |
| - | 2.36
(0.90-6.15) | 0.079 |
| - |
| Multifocal (yes vs.
no) | 1.38
(0.57-3.33) | 0.474 |
| - | 1.29
(0.59-2.79) | 0.455 |
| - | 0.84
(0.40-1.76) | 0.638 |
| - |
| Sarcomatoid
differentiation (yes vs. no) | 2.89
(1.23-6.82) | 0.020 | 6.19
(2.31-16.62) | <0.001 | 2.98
(1.42-6.27) | 0.004 | 4.44
(2.03-9.71) | <0.001 | 1.59
(0.81-3.12) | 0.156 | 3.04
(1.39-6.66) | 0.005 |
Analysis of the sarcomatoid
component
Among the patients with sarcomatoid carcinoma, 11
had pure sarcomatoid carcinomas and 27 had mixed component
carcinomas (sarcomatoid and non-sarcomatoid carcinoma). No
significant difference in pathological stage (P=0.5), CSS and RFS
was observed between the two groups, but both groups had higher CSS
and RFS rates than the non-sarcomatoid group (Fig. S1A and B). According to the receiver
operating characteristic curve, 45% sarcomatoid components was
identified as the optimal cut-off score (Fig. S1C). The area under the curve was
0.68. Finally, 50% was used for the comparison of sarcomatoid
components to achieve higher specificity. No significant
differences in characteristics were found between patients with a
sarcomatoid component >50% and patients with a sarcomatoid
component ≤50% (Table SI), with
the exception that tumours were larger in patients with a
sarcomatoid component >50% (4 vs. 3 cm; P=0.009). The 5-year CSS
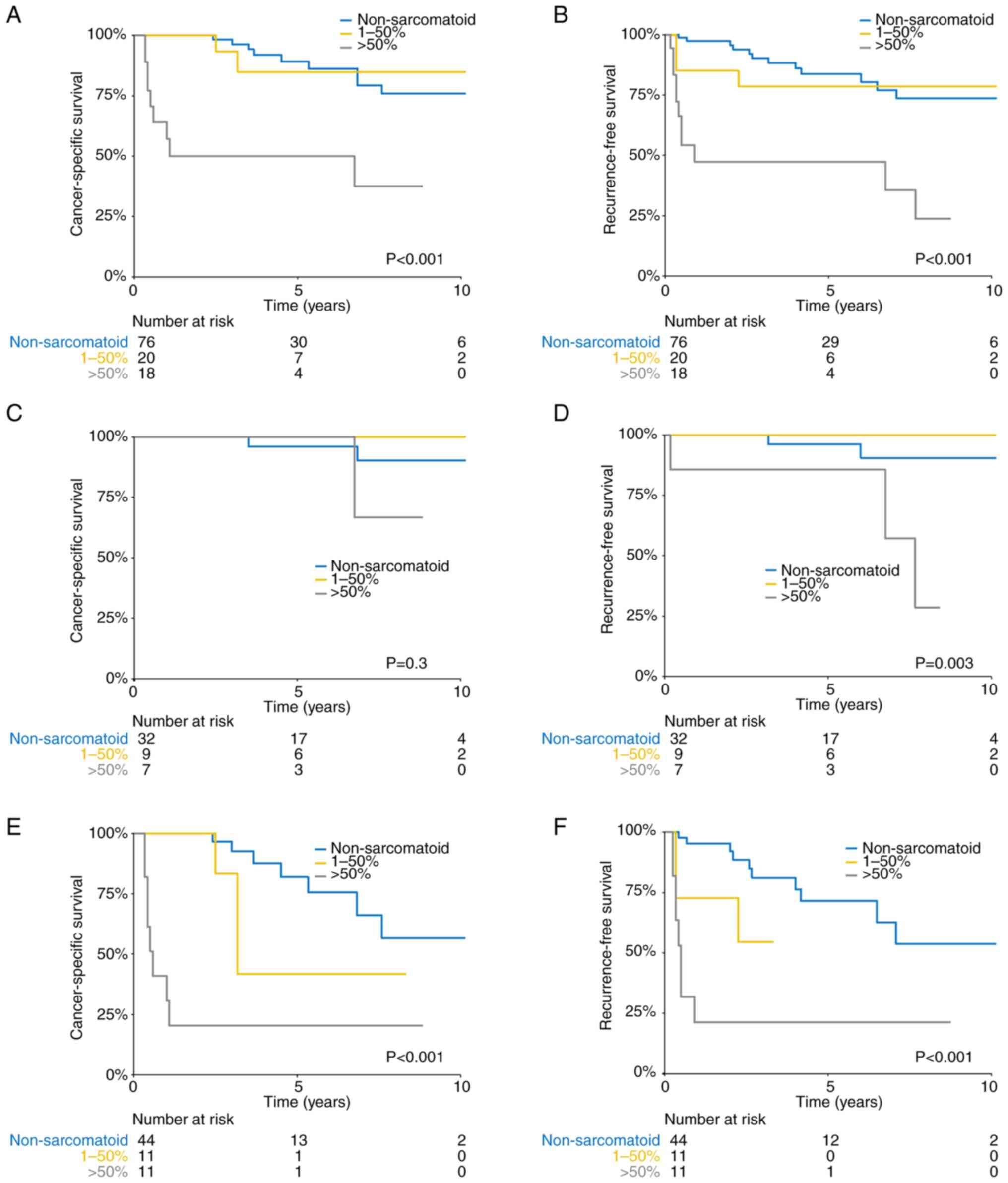
and RFS rates for patients with a sarcomatoid component >50%
were lower than those for patients with a low proportion (≤50%) of
sarcomatoid component and patients with non-sarcomatoid BC (CSS:
53.5 vs. 80.6 vs. 89.2%, respectively; log-rank P<0.001;
Fig. 2A; RFS: 47.4 vs. 78.5 vs.
83.6%, respectively; log-rank P<0.001; Fig. 2B). In the subgroup of
muscle-invasive BC, patients with a sarcomatoid component >50%
similarly had worse 5-year CSS and RFS rates (CSS: 22.9 vs. 37.9
vs. 81.8%, respectively; log-rank P<0.001; Fig. 2E; RFS: 23.3 vs. ns (indicating <5
years of follow-up) vs. 71.6%, respectively; log-rank P<0.001;
Fig. 2F). In the
non-muscle-invasive BC subgroup, in the >50% and ≤50%
sarcomatoid component, and non-sarcomatoid groups, there was no
significant difference in CSS (100.0 vs. 100.0 vs. 96.0%,
respectively; log-rank P=0.3; Fig.
2C) but there was a significant difference in RFS (85.7 vs.
100.0 vs. 96.2%, respectively; log-rank P=0.003; Fig. 2D). Multivariate analysis showed that
a sarcomatoid component >50%, pT stage ≥T2 and tumour size
>3.5 cm predicted cancer-specific death. Independent predictors
of recurrence were a higher proportion of sarcomatoid component,
higher T stage, regional lymph node invasion and larger tumour
size. Tumour T stage, tumour size, and Eastern Cooperative Oncology
Group score were predictors of OS (Table III). No multi-collinearity was
observed between variables in all regression analyses.
 | Table III.Univariate and multivariate Cox
regression analyses of patients with sarcomatoid carcinoma for CSS,
RFS and OS. |
Table III.
Univariate and multivariate Cox
regression analyses of patients with sarcomatoid carcinoma for CSS,
RFS and OS.
|
| CSS | RFS | OS |
|---|
|
|
|
|
|
|---|
|
| Univariate |
Multivariatea | Univariate |
Multivariatea | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|
|
|---|
| Feature | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (per 10-year
increase) | 0.70
(0.42-1.16) | 0.168 |
| - | 0.80
(0.51-1.28) | 0.354 |
| - | 0.90
(0.55-1.48) | 0.678 |
| - |
| Gender (male vs.
female) | 0.38
(0.11-1.30) | 0.124 |
| - | 0.41
(0.14-1.22) | 0.110 |
| - | 0.53
(0.17-1.72) | 0.293 |
| - |
| ECOG (≥1 vs.
0) | 1.40
(0.29-6.66) | 0.673 |
| - | 1.71
(0.47-6.23) | 0.417 |
| - | 3.13
(0.93-10.49) | 0.07 | 5.77
(1.26-26.5) | 0.024 |
| Hypertension (yes
vs. no) | 0.45
(0.10-2.10) | 0.310 |
| - | 0.72
(0.23-2.26) | 0.569 |
| - | 0.32
(0.07-1.45) | 0.140 |
| - |
| DM (yes vs.
no) | 5.14
(1.48-17.81) | 0.010 |
| 0.478 | 2.96
(0.92-9.48) | 0.068 |
| 0.419 | 3.49
(1.08-11.29) | 0.037 |
| 0.762 |
| Smoking (yes vs.
no) | 1.16
(0.35-3.81) | 0.810 |
| - | 0.69
(0.24-2.01) | 0.494 |
| - | 1.38
(0.48-3.98) | 0.557 |
| - |
| Alcohol (yes vs.
no) | 1.05
(0.22-4.90) | 0.955 |
| - | 1.10
(0.30-3.97) | 0.888 |
| - | 0.73
(0.16-3.30) | 0.681 |
| - |
| BMI (per
5-kg/m2 increase) | 0.83
(0.42-1.65) | 0.588 |
| - | 1.26
(0.64-2.49) | 0.511 |
| - | 0.60
(0.33-1.10) | 0.096 |
| 0.122 |
| Surgery (RC vs.
TUR) | 4.33
(1.11-16.93) | 0.035 |
| - | 2.15
(0.72-6.41) | 0.169 |
| - | 4.28
(1.30-14.08) | 0.017 |
| - |
| Tumor size (>3.5
vs. ≤3.5 cm) | 9.25
(1.97-43.50) | 0.005 | 10.60
(1.65-67.48) | 0.013 | 3.88
(1.29-11.70) | 0.016 | 5.59
(1.46-21.39) | 0.012 | 5.20
(1.60-16.88) | 0.006 | 4.47
(1.17-18.7) | 0.029 |
| pT stage (≥T2 vs.
T1) | 14.2
(1.74-115.04) | 0.013 | 17.93
(1.76-182.89) | 0.015 | 6.01
(1.54-23.36) | 0.010 | 4.71
(1.05-21.11) | 0.043 | 8.81
(1.90-40.84) | 0.006 | 10.50
(1.96-56.1) | 0.006 |
| pN stage (N1 vs.
N0) | 6.95
(1.64-29.46) | 0.009 | 3.48
(1.13-10.75) | 0.182 | 7.44
(2.18-25.37) | 0.001 | 9.41
(1.58-56.02) | 0.014 | 4.85
(1.24-19.01) | 0.023 |
| 0.510 |
| LVI (yes vs.
no) | 0.041
(0.00-127.18) | 0.436 |
| - | 0.50
(0.06-3.82) | 0.500 |
| - | 0.62
(0.08-4.76) | 0.647 |
| - |
| Necrosis (yes vs.
no) | 1.31
(0.28-6.14) | 0.729 |
| - | 1.30
(0.36-4.60) | 0.689 |
| - | 1.73
(0.47-6.31) | 0.407 |
| - |
| Multifocal (yes vs.
no) | 1.35
(0.36-5.10) | 0.658 |
| - | 1.16
(0.37-3.65) | 0.800 |
| - | 1.45
(0.46-4.65) | 0.528 |
| - |
| Sarcomatoid
proportion (>50 vs. 1–50%) | 4.57
(1.21-17.33) | 0.025 | 5.18
(1.18-22.82) | 0.030 | 2.99
(1.02-8.78) | 0.046 | 4.35
(1.07-17.66) | 0.040 | 3.08
(1.03-9.24) | 0.045 | 4.03
(0.82-19.91) | 0.087 |
Discussion
Sarcomatoid carcinoma is a rare pathological type of
BC, accounting for 0.07-2.4% of BCs (22,23).
In the present study, 38 out of 5,196 patients with BC had final
pathological confirmation of sarcomatoid carcinoma, representing
~0.73% of the cases. To the best of our knowledge, this is the
first study to compare sarcomatoid and non-sarcomatoid carcinoma of
the bladder using propensity score matching and assessing the
prognostic value of the proportion of sarcomatoid components.
The prognosis of sarcomatoid carcinoma of the
bladder remains controversial, with some studies concluding that
the sarcomatoid component was not significantly associated with
survival (10,23–26)
and others arguing for a markedly increased risk of death (11,27).
Most of these studies were from the SEER database, case series, and
studies with a too short follow-up time (<6 months) or a too
small number of cases (<20) (10,23–27),
which resulted in a high risk of bias. There was an interaction
between sarcomatoid differentiation and advanced stage. Therefore,
the worse prognosis in sarcomatoid carcinoma compared with
non-sarcomatoid carcinoma might be explained by a higher proportion
of patients with muscle invasion, invasion outside the bladder and
regional lymph node invasion (11,28).
The present study matched sarcomatoid and non-sarcomatoid carcinoma
well for pathological T stage by propensity score matching, and
found that sarcomatoid carcinoma conferred worse survival time and
exhibited more aggressive behaviour. After being adjusted by T
stage, N stage and tumour size, the same conclusion was still
reached. Results were consistent with a study that investigated
patients with sarcomatoid carcinoma at the Memorial Sloan Kettering
Cancer Center (11). After
stratification for T stage, the results were the same for
muscle-invasive sarcomatoid carcinoma. Due to the limited number of
patients, the prognostic value of sarcomatoid carcinoma in
non-muscle invasive BC should be studied in a larger sample.
The 5-year CSS rate for patients with sarcomatoid
carcinoma in the Affiliated Hospital of Qingdao University was
higher than that in previous studies (37–64%) (10,23–26).
This may be due to the different inclusion criteria in the previous
studies. Inclusion of patients who underwent radical cystectomy or
muscle invasion meant a higher stage, or inclusion of patients who
also had carcinosarcoma resulted in a significantly worse prognosis
compared with that for patients with sarcomatoid carcinoma. In the
present multivariate analysis of patients with bladder sarcomatoid
carcinoma, the proportion of sarcomatoid components, pathological T
stage and tumour size were predictors of cancer-specific death.
Diamantopoulos et al (15)
found that American Joint Committee on Cancer stage and an age ≥85
years increased the risk of cancer-specific death. Sui et al
(9) found that stage ≥T3 and
Charlson Comorbidity Index ≥1 were prognostic factors for all-cause
mortality. The proportion of sarcomatoid components of the bladder
has not been reported to be of prognostic value and, to the best of
our knowledge, the present study showed for the first time that a
high proportion of sarcomatoid components is an independent
prognostic factor. Reporting on the percentage of sarcomatoid
carcinoma components is recommended to improve risk stratification
and predict survival of patients with BC.
Currently, no uniform consensus guidelines are
available for the standard treatment of patients with sarcomatoid
carcinoma (29,30). In the present study, patients with
non-muscle-invasive BC mostly underwent transurethral resection
(87.5%), while radical cystectomy was the main treatment option for
muscle-invasive BC (81.8%). The present study showed no significant
survival benefit from radical cystectomy for non-muscle-invasive BC
presenting with sarcomatoid carcinoma components. Therefore, stage
T1 bladder sarcomatoid carcinoma is less aggressive, and additional
radical cystectomy will only increase the likelihood of surgical
complications, with no evidence of a survival benefit. No patients
with sarcomatoid carcinoma received neoadjuvant chemotherapy. A
total of 2 patients were treated with tyrosine kinase inhibitors
and radiotherapy, respectively, only after the discovery of lung
metastases. Previous studies have found no significant survival
benefit from neoadjuvant or adjuvant chemotherapy in patients with
sarcomatoid differentiated urothelial carcinoma of the bladder
(9,10,31,32).
In the study by Almassi et al (11) consisting of 131 patients with
sarcomatoid carcinoma, it was concluded that there was no
significant difference in the reduced recurrence rate with
neoadjuvant chemotherapy. As muscle-invasive bladder sarcomatoid
carcinoma has a worse prognosis and insensitivity to chemotherapy,
more treatment options should be explored.
The present study had several limitations. The study
was a single-centre, retrospective study, and patients' baseline
characteristics were imbalanced. Propensity score matching only
controlled for partially important confounders; therefore, partial
selection bias still existed. As sarcomatoid carcinoma is rare, the
sample size of this study was inevitably small. Therefore, further
prospective study in a large-scale population should be undertaken
to validate the results.
In conclusion, the present study showed that
patients with BC containing sarcomatoid components had poorer
survival and higher recurrence rates than those with
non-sarcomatoid carcinoma of the bladder. No difference in
prognosis was found between patients with pure sarcomatoid
components and those with mixed sarcomatoid components. A
sarcomatoid carcinoma component accounting for >50% of a tumour
is a predictor of death and recurrence, and can be used as a
valuable cut-off point. Pathologists should report the proportion
of sarcomatoid carcinoma components, and patients with sarcomatoid
carcinoma >50% should be brought to the attention of
clinicians.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was partly funded by the Natural Science Foundation
of Shandong Province (grant no. ZR2021MH354), the Medical and
Health Research Program of Qingdao (grant no. 2021-WJZD170) and the
Clinical Medicine + X Project of The Affiliated Hospital of Qingdao
University (grant no. QDFY+X2021029).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
GZ and LS conceived and designed the study. SL, YY
and ZW collected and analyzed the data. SL and LS wrote the
manuscript. LS revised the statistical analysis and revised the
manuscript. All authors have read and approved the final
manuscript. GMZ, SL, YY, JCM and LJS confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
The study was performed in accordance with the
relevant guidelines and regulations of the Declaration of Helsinki.
The study protocol was approved by the Ethics Committee of The
Affiliated Hospital of Qingdao University (Qingdao, China). All
patients involved in the present study provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lobo N, Shariat SF, Guo CC, Fernandez MI,
Kassouf W, Choudhury A, Gao J, Williams SB, Galsky MD, Taylor JA
III, et al: What is the significance of variant histology in
urothelial carcinoma? Eur Urol Focus. 6:653–663. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng L, Zhang S, Alexander R, Maclennan
GT, Hodges KB, Harrison BT, Lopez-Beltran A and Montironi R:
Sarcomatoid carcinoma of the urinary bladder: The final common
pathway of urothelial carcinoma dedifferentiation. Am J Surg
Pathol. 35:e34–e46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spiess PE, Tuziak T, Tibbs RF, Bassett R,
Tamboli P, Brown GA, Grossman HB, Ayala AG and Czerniak B:
Pseudosarcomatous and sarcomatous proliferations of the bladder.
Hum Pathol. 38:753–761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sung MT, Wang M, MacLennan GT, Eble JN,
Tan PH, Lopez-Beltran A, Montironi R, Harris JJ, Kuhar M and Cheng
L: Histogenesis of sarcomatoid urothelial carcinoma of the urinary
bladder: Evidence for a common clonal origin with divergent
differentiation. J Pathol. 211:420–430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanfrancesco J, McKenney JK, Leivo MZ,
Gupta S, Elson P and Hansel DE: Sarcomatoid urothelial carcinoma of
the bladder: Analysis of 28 cases with emphasis on
clinicopathologic features and markers of epithelial-to-mesenchymal
transition. Arch Pathol Lab Med. 140:543–551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bansal A, Kumar N and Sharma SC:
Sarcomatoid variant of urothelial carcinoma of the urinary bladder.
J Cancer Res Ther. 9:571–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sui W, Matulay JT, Onyeji IC, Theofanides
MC, James MB, RoyChoudhury A, Wenske S and DeCastro GJ:
Contemporary treatment patterns and outcomes of sarcomatoid bladder
cancer. World J Urol. 35:1055–1061. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berg S, D'Andrea D, Vetterlein MW, Cole
AP, Fletcher SA, Krimphove MJ, Marchese M, Lipsitz SR, Sonpavde G,
Noldus J, et al: Impact of adjuvant chemotherapy in patients with
adverse features and variant histology at radical cystectomy for
muscle-invasive carcinoma of the bladder: Does histologic subtype
matter? Cancer. 125:1449–1458. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Almassi N, Vertosick EA, Sjoberg DD, Wong
NC, Huang C, Pietzak EJ, Cha EK, Donahue TF, Dalbagni G, Bochner
BH, et al: Pathological and oncological outcomes in patients with
sarcomatoid differentiation undergoing cystectomy. BJU Int.
129:463–469. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Advanced Bladder Cancer (ABC)
Meta-analysis Collaborators Group, : Adjuvant chemotherapy for
muscle-invasive bladder cancer: A systematic review and
meta-analysis of individual participant data from randomised
controlled trials. Eur Urol. 81:50–61. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khaled HM, Shafik HE, Zabhloul MS, Ghoneim
M, Saber RA, Manie M, Enein HA, Megeed HA, Mansur O, Sherbini ME,
et al: Gemcitabine and cisplatin as neoadjuvant chemotherapy for
invasive transitional and squamous cell carcinoma of the bladder:
Effect on survival and bladder preservation. Clin Genitourin
Cancer. 12:e233–e240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams SB, Shan Y, Jazzar U, Mehta HB,
Baillargeon JG, Huo J, Senagore AJ, Orihuela E, Tyler DS, Swanson
TA and Kamat AM: Comparing survival outcomes and costs associated
with radical cystectomy and trimodal therapy for older adults with
muscle-invasive bladder cancer. JAMA Surg. 153:881–889. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Diamantopoulos LN, Korentzelos D,
Alevizakos M, Wright JL, Grivas P and Appleman LJ: Sarcomatoid
urothelial carcinoma: A population-based study of clinicopathologic
characteristics and survival outcomes. Clin Genitourin Cancer.
20:139–147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding L, Bi ZF, Yuan H, Zhao XH, Guan XD,
Yao HR and Liu YM: Sarcomatoid carcinoma in the head and neck: A
population-based analysis of outcome and survival. Laryngoscope.
131:E489–e499. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park I, Cho YM, Lee JL, Ahn JH, Lee DH,
Song C, Hong JH, Kim CS and Ahn H: Prognostic factors of metastatic
renal cell carcinoma with extensive sarcomatoid component. J Cancer
Res Clin Oncol. 139:817–827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ro JY, Ayala AG, Sella A, Samuels ML and
Swanson DA: Sarcomatoid renal cell carcinoma: Clinicopathologic. A
study of 42 cases. Cancer. 59:516–526. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang BY, Thompson RH, Lohse CM, Leibovich
BC, Boorjian SA, Cheville JC and Costello BA: A novel prognostic
model for patients with sarcomatoid renal cell carcinoma. BJU Int.
115:405–411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. Eighth
edition. John Wiley & Sons, Inc.; New York, NY, USA: pp.
204–207. 2017
|
|
21
|
Belsante M, Darwish O, Youssef R, Bagrodia
A, Kapur P, Sagalowsky AI, Lotan Y and Margulis V: Lymphovascular
invasion in clear cell renal cell carcinoma-association with
disease-free and cancer-specific survival. Urol Oncol.
32:30.e23–e38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torenbeek R, Blomjous CE, de Bruin PC,
Newling DW and Meijer CJ: Sarcomatoid carcinoma of the urinary
bladder. Clinicopathologic analysis of 18 cases with
immunohistochemical and electron microscopic findings. Am J Surg
Pathol. 18:241–249. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wright JL, Black PC, Brown GA, Porter MP,
Kamat AM, Dinney CP and Lin DW: Differences in survival among
patients with sarcomatoid carcinoma, carcinosarcoma and urothelial
carcinoma of the bladder. J Urol. 178:2302–2306; discussion 2307.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Monn MF, Kaimakliotis HZ, Pedrosa JA, Cary
KC, Bihrle R, Cheng L and Koch MO: Contemporary bladder cancer:
Variant histology may be a significant driver of disease. Urol
Oncol. 33:18.e15–18.e20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moschini M, Dell'Oglio P, Luciano R,
Gandaglia G, Soria F, Mattei A, Klatte T, Damiano R, Shariat SF,
Salonia A, et al: Incidence and effect of variant histology on
oncological outcomes in patients with bladder cancer treated with
radical cystectomy. Urol Oncol. 35:335–341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robinson SP, Farooq A, Laniado M and
Motiwala H: The demographic features, clinical outcomes, prognosis
and treatment options for patients with sarcomatoid carcinoma of
the urinary bladder: A single centre experience. Int Braz J Urol.
44:45–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patel SG, Weiner AB, Keegan K and Morgan
T: Oncologic outcomes in patients with nonurothelial bladder
cancer. Indian J Urol. 34:39–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Gillaspie C, Kunadharaju R, Talmon
GA and Enke C: Sarcomatoid urothelial carcinoma: A single cancer
center experience. World J Oncol. 2:175–180. 2011.PubMed/NCBI
|
|
29
|
Malla M, Wang JF, Trepeta R, Feng A and
Wang J: Sarcomatoid carcinoma of the urinary bladder. Clin
Genitourin Cancer. 14:366–372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Veskimäe E, Espinos EL, Bruins HM, Yuan Y,
Sylvester R, Kamat AM, Shariat SF, Witjes JA and Compérat EM: What
is the prognostic and clinical importance of urothelial and
nonurothelial histological variants of bladder cancer in predicting
oncological outcomes in patients with muscle-invasive and
metastatic bladder cancer? A European association of urology muscle
invasive and metastatic bladder cancer guidelines panel systematic
review. Eur Urol Oncol. 2:625–642. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu L, Ai Q, Cheng Q, Ma X, Wang B, Huang
Q, Li X, Zhang P, Liu K, Zhao X, et al: Sarcomatoid variant
urothelial carcinoma of the bladder: A systematic review and
meta-analysis of the clinicopathological features and survival
outcomes. Cancer Cell Int. 20:5502020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vetterlein MW, Wankowicz SAM, Seisen T,
Lander R, Löppenberg B, Chun FK, Menon M, Sun M, Barletta JA,
Choueiri TK, et al: Neoadjuvant chemotherapy prior to radical
cystectomy for muscle-invasive bladder cancer with variant
histology. Cancer. 123:4346–4355. 2017. View Article : Google Scholar : PubMed/NCBI
|