Context
Available therapeutic modalities have the potential
to cure patients with locally advanced non-small cell lung cancer
(NSCLC). However, given the aggressive nature of current
treatments, proper staging remains the cornerstone for designing
the optimal management strategy. More accurate, easy to perform
staging procedures and the diversity of treatment modalities have
made medical decisions more challenging. The advancement of
clinical and pathological descriptions of the disease, coupled with
the multitude of targeted, precision and personalized medication
strategies, have raised the patients' (and the treating
oncologists') hopes for prolonged survival time. The medical
professionals involved in the management of these patients are
under increased pressure to provide accurate diagnosis to inform
clinical management and best possible patient care. This obligation
is further accentuated in the case of locally advanced
(non-metastatic) disease, in which medical decisions are quite
literally a matter of life and death. Members of the Lebanese
Society of Medical Oncology and the Lebanese Pulmonary Society have
convened to discuss and lay down a set of recommendations that
govern NSCLC screening, staging, clinical management and follow-up,
in an attempt to share their collective experience and unify
medical care for patients with locally advanced NSCLC.
Screening and diagnosis
NSCLC is a heterogeneous disease that might remain
asymptomatic while invading the lung and adjacent tissues,
eventually progressing and spreading to distant sites, thus
compromising response to treatment and prognosis. Whilst chances
for a complete remission or a cure are highest in early-stage
cancers, survival rates decrease when the cancer is locally
advanced, or even further when it has metastasized (1). Therefore, the earlier a lung lesion is
discovered, the better the medical outcome. In addition, survival
rate among patients with NSCLC is also enhanced by individual
factors, such as young age, stage IIIA versus stage IIIB or IIIC,
and concurrent chemoradiation, as well as other conventional
prognostic factors, such as metabolic activity of the tumor and
biomarkers of cell cycle regulation and apoptosis (2,3).
Glucose avidity (high uptake) on the positron-emission tomography
(PET) scan is indicative of metabolic activity and is associated
with a 2.18-fold lower chance for patient survival from the disease
(3). Biomarkers of interest in
stage III NSCLC with unfavorable prognostic value include KRAS,
p53, EGFR, VEGF, c-erbB-2 (or HER-2), Bcl-2, Ki-67, microvessel
density and aneuploidy (3).
Lung cancer screening can be performed on specific
population strata at higher risk for developing malignant pulmonary
lesions, including those with a family history of lung cancer,
workers in chemical plants and older adults with previous exposure
to carcinogens or with respiratory disease history, in addition to
active personal or second-hand smokers (1,4,5).
Lung cancer risk prediction tools can be used to
identify individuals at high-risk for lung cancer and select them
for screening (6). In particular,
recently updated lung cancer guidelines recommend using risk models
to refer ever-smokers for screening (7). The different risk prediction models
are all based on the risk stratification approach, and were shown
to yield comparable results (8). In
addition to age and smoking status (pack years and quit time), the
Prostate, Lung, Colorectal and Ovarian 2012 risk model
(PLCOm2012) addresses other risk factors, such as the
presence of chronic pulmonary diseases, family history of cancer
(in general and lung cancer in particular) and socioeconomic status
(1,6). Other tools can also be used depending
on the physician's preference and on the regulations laid down by
governmental agencies, such as the Ministry of Public Health. These
models include the Bach model (9),
the Lung Cancer Death Risk Assessment Tool (10,11),
and the Liverpool Lung Project (12), which together with the
PLCOm2012 have proven accurate in predicting lung cancer
risk in ever-smokers (8,13). NSCLC, specifically lung
adenocarcinoma, can affect never-smokers; however, tobacco smoking
remains the major contributor to lung cancer (14), given the malignant genomic landscape
that characterizes smokers (15,16).
These mutations shape the tumor microenvironment and are more
abundant in metastases than in primary tumors (14). Advanced lung cancer is associated
with poor prognosis.
Based on the available evidence, the European
Society of Radiology and the European Respiratory Society recommend
systematic lung cancer screening where possible and when supported
by the local healthcare system and infrastructure (17).
Diagnosing and staging lung cancer
According to international recommendations, a
low-dose chest computerized tomography (CT) scan is the preferred
tool for lung tumor detection (1,18). For
lung nodules, a CT scan or PET/CT scan of the chest and abdomen
using the glucose analogue 18fluorodeoxyglucose
(18F-FDG) with intravenous contrast will confirm the
diagnosis of a lung tumor, inform on the number of masses, the size
of the primary tumor, the degree of invasion of the mediastinum and
chest wall, as well as on the presence of abdominal lesions
(19). The patient should then
undergo pathological analyses to determine the exact type of lung
cancer, followed by clinical staging.
Staging procedures include non- or minimally
invasive methods, as well as invasive non-surgical and surgical
methods. Minimally or non-invasive staging methods include CT or
PET/CT scan with the glucose analogue 18F-FDG, and
magnetic resonance imaging (MRI), which is mostly for brain
evaluation. The PET/CT scan is superior to the simple chest CT scan
owing to its high sensitivity for the detection of pathological
lymph nodes (especially those >1 cm in their shortest dimension)
and distant metastases. For pathological and molecular profiling
purposes, a tumor biopsy can be obtained using minimally invasive
techniques, such as bronchoscopy-guided, endoscopic ultrasound
(EUS)-guided or endobronchial ultrasound (EBUS)-guided fine-needle
aspiration (FNA) or core-needle biopsy (CNB), transthoracic
CT-guided FNA or CNB and transbronchial FNA or CNB (20–22).
Although minimally or non-invasive staging methods are preferred,
they might present some pitfalls, which warrant careful
interpretation of imaging results for accurate staging. For
instance, while conventional MRI and CT scanning might have similar
accuracies in detecting chest wall invasion, bone destruction is
best assessed by CT scan, whereas lymphangitic carcinomatosis and
pleural invasion are best assessed by MRI (23), particularly by respiratory
functional MRI (24). In fact, the
CT scan technology might not allow evaluating the exact depth of
pleural invasion, but functional MRI is laborious and expensive
with relatively poor spatial resolution (24).
In the absence of pathological lymph nodes showing
on the PET/CT scan, but in cases in which the disease presents as a
central tumor or a peripheral tumor with a diameter >3 cm,
occult N2 lymph nodes might be present, thus warranting exploration
of the mediastinum and lymph node dissection (25). Mediastinal lymph node involvement is
important for prognosis of stage III disease; therefore,
preoperative mediastinal staging is necessary (25,26).
In NSCLC, not all N2 cases are the same (26). In particular, mono-station versus
multi-station lymph node involvement, bulky versus non-bulky
disease, and adherence to central airways, all entail very
different implications in terms of treatment (resectable versus
unresectable) and prognosis (26).
Needle techniques allow the confirmation of mediastinal involvement
in accessible lymph node stations, including paratracheal,
posterior tracheal, sub-carinal, hilar, interlobar and lobar lymph
nodes (26). EBUS-guided puncture
can be supplemented by EUS to assess paratracheal, sub-carinal,
paraesophageal and pulmonary ligament lymph nodes (26). When sampled lymph nodes are sent to
the pathology laboratory, it is important to indicate the number of
sampled lymph nodes and their site of origin, as fragmenting them
will make quantification obsolete (27).
If the patient's status motivates pleural
exploration, a thoracentesis with or without needle pleural biopsy
(commonly referred to as pleural tap) is usually the first
procedure performed to diagnose exudative pleural effusion and
determine its etiology (malignant or not) by differential and
cytopathological analysis (28).
More advanced techniques (such as pleuroscopy) or newer techniques
(such as narrow band imaging, infrared or auto-fluorescence
thoracoscopy, and video-assisted thoracic surgery) are also
available (29). These techniques
are used when more extensive sampling of tissue or advanced
procedures are needed, such as stapled lung biopsy, resection of
pulmonary nodules, lobectomy, pericardial window and lower nodal
stations examination (30).
However, exploratory thoracoscopy should not exceed 1% of
procedures (31).
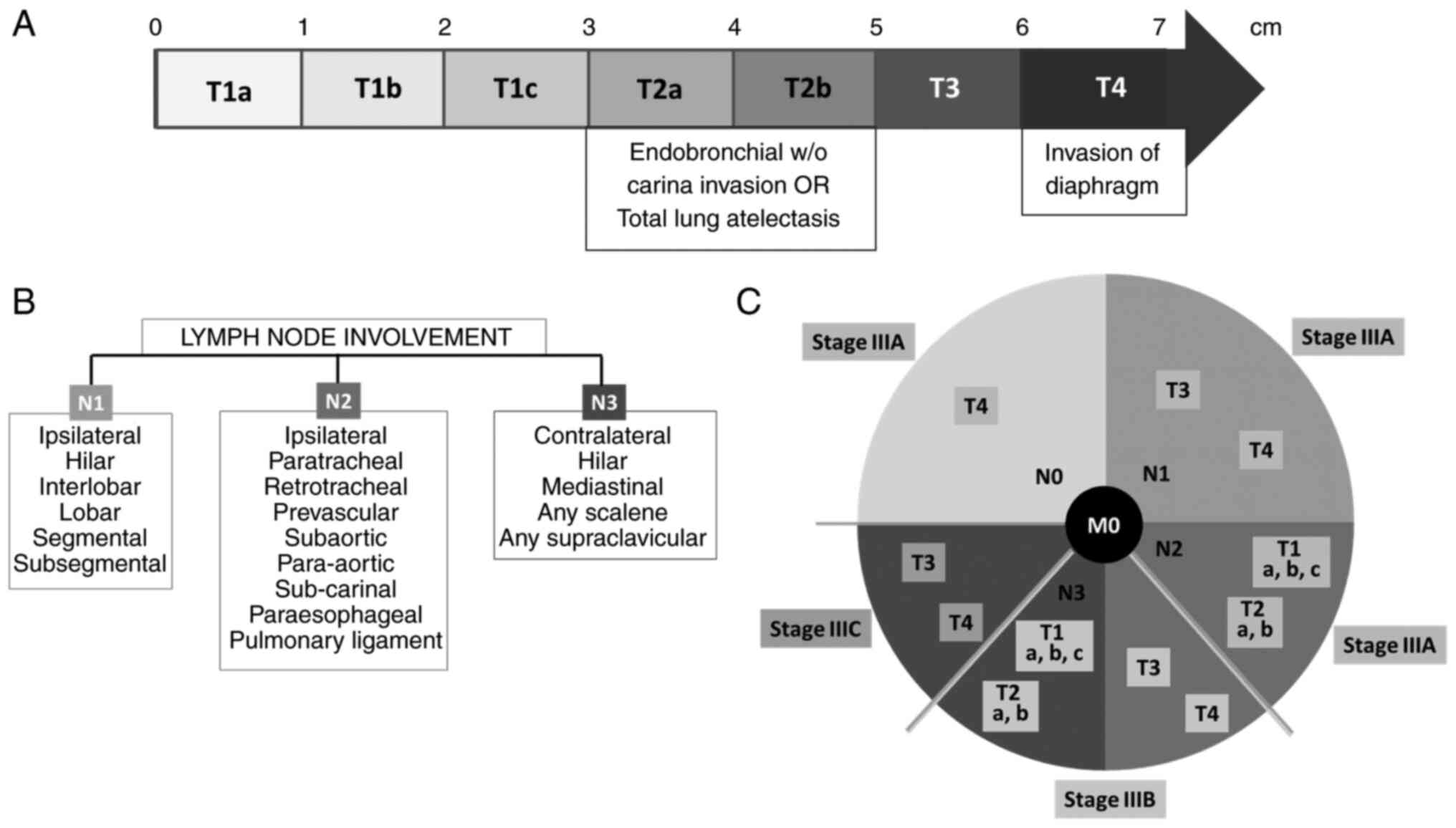
Tumor staging encompasses three descriptors: Tumor
size (T), involved lymph nodes (N) and presence of metastatic
lesions (M), known as the TNM classification. Stage III NSCLC is a
locally advanced lung cancer, with no evidence of distant
metastatic lesions (M0). Tumor size is one of the most important
tumor parameters (32,33), it is reported based on the CT scan
in the projection that gives the greatest dimension. Fig. 1A presents the classification of
NSCLC tumors, according to their size. When establishing nodal
involvement, a PET/CT scan has the highest sensitivity in detecting
enlarged lymph nodes and determining their dimensions (32), compared with either imaging
technique alone (34). N1 disease
refers to the involvement of one ipsilateral hilar nodal station;
N2 disease refers to the involvement of one or multiple ipsilateral
mediastinal nodal stations, with or without N1 (35); and N3 indicates contralateral lymph
node malignancy (Fig. 1B). Lymph
nodes have been mapped into zones and stations according to the TNM
classification seventh edition (36,37),
which has been maintained in the TNM classification eighth edition
(38). Fig. 1C associates the T and N components
of TNM classification to determine if the cancer is stage IIIA,
IIIB or IIIC, according to the currently applicable TNM
classification.
Cardiopulmonary assessment
The patient should be clinically evaluated by a
cardiologist and a pulmonologist to determine their surgical risk
(39). Cardiologic assessment
starts with a checkup of the cardiac function and a revision of any
chronically prescribed medications. The Cardiac Risk Index is an
algorithm developed in 1999 and revised in 2013, which can help
calculate a cardiac risk score in patients considered for thoracic
surgery (39,40). The Revised Cardiac Risk Index
tailored for patients undergoing thoracic surgery is free and is
available online and may help identify patients at risk of cardiac
complications following lung tumor resection. Patients who score
<1.5 points are considered low-risk and additional assessments
may not be necessary 40).
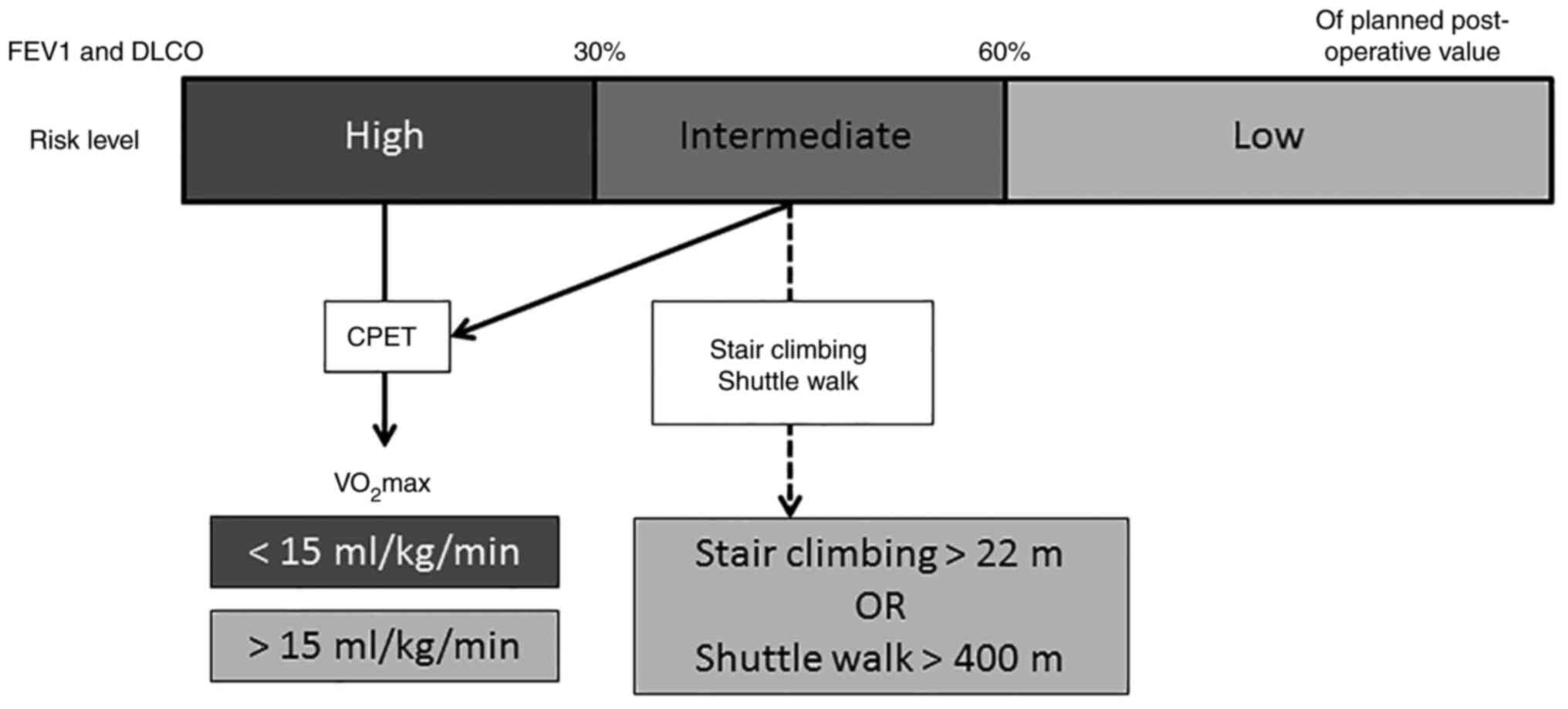
Pulmonary function assessment helps predict
tolerance to general anesthesia and post-operative pulmonary
function (41,42). The maximum expiratory volume in the
first second of forced expiration (FEV1) and the diffusion capacity
of the lung for carbon monoxide (DLCO) can be calculated (43). Post-operative FEV1 and DLCO levels
can be estimated by quantitative perfusion scintigraphy. If both
values are >60% of their planned post-operative level, then the
patient can safely proceed to surgery (43). However, if either FEV1 or DLCO is
<30% of the planned post-operative value, then cardiopulmonary
exercise testing (CPET) should be performed (39,43).
Fig. 2 visually illustrates the
proposed pre-operative lung assessment flow.
Tools to evaluate clinical profiles and
performance of patients
The Karnofsky Performance Status (KPS) measures the
ability of patients with cancer to perform ordinary tasks, and
scores range from 0 (unable) to 100 (very able) (44). The Eastern Cooperative Oncology
Group (ECOG) score helps quantify a patients' performance status
(45), and ranges from 0 (full
functionality) to 5 (death). Either the KPS or the ECOG score can
be used for assessing performance and to inform treatment decisions
(46). Response Evaluation Criteria
In Solid Tumors is a set of published guidelines to help evaluate
the patient's response to treatment, using imaging techniques
(47–49). The Patient-Reported Outcomes
Measurement Information System® is another tool that
allows the evaluation of clinical profile and performance from the
patient's standpoint (50). This
set of person-centered questions relies on technology,
psychometrics, and qualitative and cognitive health-related
outcomes to describe the medical, mental and social health of
individuals.
Treatment and management of stage III
NSCLC
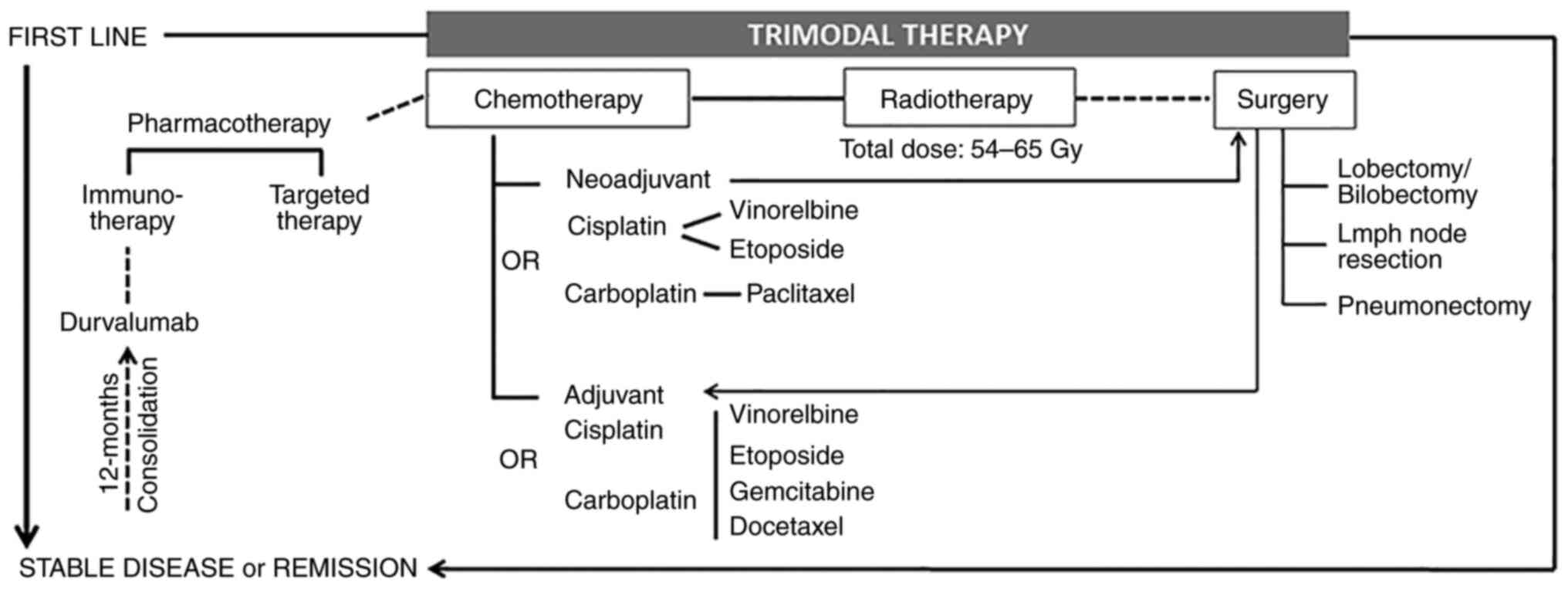
Trimodal therapy
A trimodal therapy combining radiotherapy and
chemotherapy followed by surgery is currently a widely accepted
approach for the management of stage III NSCLC (51), even in the case of surgically
challenging tumors such as superior sulcus or Pancoast's tumors
(52). It is strongly recommended
to hold a multidisciplinary board meeting to evaluate tumor
resectability according to size, location, lymph node involvement
and prognosis, as well as the patient's general status and
cardiopulmonary function (37). A
multidisciplinary team approach to lung cancer management has been
widely recognized to improve patient outcomes (53,54),
and has been endorsed by the International Association for the
Study of Lung Cancer, the American Thoracic Society and the
European Respiratory Society (55).
Adjuvant chemotherapy was shown to offer substantial
improvement in the management of resectable stage IIIA NSCLC
(56). If the tumor presents an
EGFR mutation, targeted adjuvant therapy with EGFR tyrosine kinase
inhibitors (TKIs) might improve disease-free survival (57). The Food and Drug Administration has
recently approved the EGFR-TKI osimertinib for adjuvant therapy in
patients with exon 19 deletions or exon 21 L858R mutations
(58). In patients with
unresectable stage IIIA or with stage IIIB or IIIC NSCLC, the
current standard treatment is definitive or palliative concurrent
chemoradiotherapy (59,60), especially for larger tumors (>7
cm) (61,62). In patients for whom concomitant
chemoradiotherapy is not recommended (e.g., unacceptable weight
loss, large irradiation surface), sequential chemotherapy followed
by radiation therapy can be used (63). Treatment should be followed by CT
scan within 3 weeks (preferably 2 weeks) of the last radiation
dose.
If none of these options (surgery or
chemoradiotherapy) is medically acceptable, other therapeutic
modalities might be available. For instance, in 2019, after being
exclusively indicated for metastatic stage IV NSCLC, the use of
pembrolizumab was extended to cover first-line treatment for
patients with stage III NSCLC ineligible for surgical resection or
definitive chemoradiation (64).
Immunotherapy with the programmed death-ligand 1 inhibitor,
durvalumab, is used as maintenance treatment for 12 months when the
disease is stable following chemoradiotherapy, as it was reported
to prolong progression-free and overall survival (65,66).
Indeed, a previous review presented evidence on promising potential
of durvalumab as consolidation therapy for patients with
unresectable stage III NSCLC who have completed two cycles of
platinum-based chemotherapy with concurrent radiotherapy (67).
Surgical resection
A locally advanced lung tumor is resectable if
surgery is sufficient to achieve complete tumor removal and if the
patient can tolerate the surgery (e.g., absence of severe cardiac
problems, adequate pulmonary reserve). Usually, patients with
single-station N2 disease or multi-station N1 disease are
considered candidates for surgery, which offers a potential cure,
whereas multi-station N2 disease confers a worse prognosis and
surgical resection should be discouraged (68). A pneumonectomy is indicated when the
tumor crosses the major fissure, extending to more than one lobe or
to the hilum (69). Tumors that
have invaded vital mediastinal structures are unresectable, with
the following exceptions: i) Invasion of the carina and 3–4 cm of
the trachea; ii) minimal left atrial invasion; iii) extension into
the intrapericardial portions of the pulmonary arteries; iv)
invasion of the aortic adventitia or the superior vena cava; and v)
those limited to the vertebral body and amenable to en bloc
resection (69). Invasion of the
chest wall, in addition to mediastinal involvement, indicates poor
prognosis and surgical resection of the primary lung nodule(s)
becomes obsolete. The integrated PET/CT scan is the best available
imaging technique to evaluate mediastinum or chest wall
involvement, despite a 20–25% possibility of false-positive or
false-negative results (37).
Intraoperative staging is also important to
determine the benefits of surgical resection and to describe
previously undetected lesions (70). A thoracotomy will help determine
whether the tumor is peripheral or central, which lymph node
stations are affected and whether the tumor has crossed the fissure
(37). If a lobectomy is likely to
provide complete removal of the lung lesion(s), it should be
performed in addition to hilar and mediastinal lymph node
dissection (51,71).
Radiation therapy
Radiation therapy is rarely prescribed as
monotherapy, given its limited benefit in terms of prognosis
(51). However, patients with stage
III NSCLC who are unsuitable for chemoradiotherapy are offered
radiation therapy alone (72).
Pre-operative radiotherapy in addition to induction chemotherapy
might not improve the overall outcome of the surgery and should be
decided on a case-by-case basis (73). Patients who are likely to undergo
radiation therapy should be assessed for the risk of lung toxicity
secondary to radiation (e.g., radiation-induced lung injury or
radiation pneumonitis). The mean lung dose and the volume of
healthy lungs receiving ≥20 Gy radiation doses are good indicators
of the risk of radiation-induced lung injury, mainly grade 2
radiation pneumonitis (43,74,75).
Radiation doses
A meta-analysis and systematic review published in
2019 described radiation therapy protocols given to patients
concomitantly with or following chemotherapy (62). Table
I proposes radiation dosage for patients with stage III NSCLC.
Palliative radiation therapy is given at a dose of 10 Gy in a
single fraction or 16 Gy in two fractions at 1-week intervals.
 | Table I.Radiation doses for patients with
stage III non-small cell lung cancer. |
Table I.
Radiation doses for patients with
stage III non-small cell lung cancer.
| Radiation
therapy | Total radiation
dose, Gy | Dose per daily
fractiona,
Gy/fraction |
|---|
| Radical | 60–66 | 2 |
| Sequential
(accelerated) | 60–66 | 3 |
| Pre-operative
(induction) | 45–54 | 1.8–2.0 |
| Post-operative | 50–54 | 1.8–2.0 |
Radiation volumes
The radiation oncology team should define the
following radiation volumes for each patient: i) Gross tumor volume
for primary tumors and affected lymph nodes; ii) clinical target
volume (CTV) for three-dimensional margins of the disease
(post-operative CTV should include the bronchial stump, the
subcarinal, ipsilateral and contralateral hilar, and paratracheal
nodal stations); and iii) planning target volume, which is a CTV
that takes into account tumor movement and patient positioning.
Image-guided radiation therapy, or more advanced technologies,
should be used whenever available (76,77).
Chemotherapy
Chemotherapy can be used as neoadjuvant or adjuvant
therapy, or as the main treatment for patients ineligible for
surgery; it is generally given with radiotherapy. The most commonly
used chemotherapeutic agents in NSCLC are: i) Platinum-based
cisplatin and carboplatin, which induce DNA damage and inhibit its
replication, leading to cell death; ii) paclitaxel and docetaxel,
which stabilize microtubules, blocking mitosis; iii) vinorelbine,
which prevents the formation of mitotic spindle; and iv)
gemcitabine, etoposide and pemetrexed, which interfere with DNA
synthesis. These agents are most commonly used in combination. The
gold standard for chemotherapy in unresectable stage III NSCLC is
doublet chemotherapy: Cisplatin with vinorelbine/etoposide, or
carboplatin with paclitaxel (78,79).
Patients with potentially resectable tumors might benefit from
pre-operative induction therapy, which aims at destroying
metastatic and nodal lesions undetected by imaging and at reducing
the extent of the surgery (43).
Surgical resection aims to achieve complete tumor removal, which
includes microscopically confirmed clear resection margins,
mediastinal lymph node removal, absence of extracapsular invasion
of lymph nodes and absence of tumor cells in the most distal lymph
node resected (72,80).
Rescue or salvage surgery refers to surgical
resection following chemotherapy and/or radiotherapy, and it can be
performed in patients who have responded exceptionally well to
therapy and for whom surgery might pose a potential cure (72). This mainly occurs when the tumor was
judged unresectable at diagnosis (81). A previous meta-analysis presented
evidence that neoadjuvant chemotherapy or chemoradiotherapy would
improve prognosis compared with definitive treatment alone
(82). Fig. 3 presents a treatment algorithm for
stage III NSCLC, which can serve as a visual guide for physicians
and patients. Adverse events should be closely monitored by a
multidisciplinary team and, in particular, radiation-related
pneumonitis should be followed-up by an expert pulmonologist.
Post-treatment management
Restaging
Video-assisted mediastinoscopy allows for a highly
specific, sensitive and accurate staging and restaging of NSCLC
(83). A PET/CT scan can be useful
to determine a positive response to treatment (leading to disease
down-staging) or disease recurrence (leading to disease up-staging)
(84). Taking into account patient
safety and risk minimization without compromising clinical
accuracy, optimal restaging would combine a PET/CT scan with
cytopathological analysis of biopsies obtained by EBUS or
EUS-guided needle aspiration.
Functional assessment
Pulmonary function should be assessed after surgical
resection to determine FEV1 and DLCO. Some patients may require
post-operative pulmonary rehabilitation, which should be decided on
a case-by-case basis; cardiac assessment might also be required for
some patients. For patients prescribed adjuvant chemotherapy, liver
and kidney function should also be evaluated before starting
treatment, with kidney function (i.e. serum creatinine levels)
checked before each chemotherapeutic cycle.
Follow-up of patients with stage III
NSCLC
Patients whose pharmacological treatment and/or
surgical resection are considered to have achieved complete
remission and are ‘cancer-free’ should undergo a CT scan every 6
months in the first 2 years after treatment, and then yearly
afterwards for 5 years, with a PET/CT prescribed at the 1-year
milestone (85).
Signs of relapse or disease
progression
Some patients with stage III NSCLC who are in
remission might show early signs of relapse, such as a slightly
enlarged lymph node (>1 cm), fatigue or bone pain. According to
the organ preference of metastasis, lung tumors tend to metastasize
to bone, brain and adrenal tissues (75). Recent-onset fatigue should prompt an
evaluation of the patient's cardiopulmonary function to check for
malignant pleural or pericardial effusion. Onset of bone pain
should prompt evaluation of skeletal lesions and the initiation of
treatment to prevent further damage or bone embrittlement and
fracture. Patients who relapse or show signs of disease progression
might benefit from immunotherapy or a course of chemotherapy with
or without radiation.
Table II provides a
summary of recommendations by the Lebanese panel of experts, for
the diagnosis and management of stage III non-small cell lung
cancer.
 | Table II.Summary of recommendations for the
diagnosis and management of stage III non-small cell lung
cancer. |
Table II.
Summary of recommendations for the
diagnosis and management of stage III non-small cell lung
cancer.
| Clinical
activity |
Recommendations |
|---|
| Diagnosis and
staging | Screen individuals
at risk of lung cancer with a low-dose CT scan and if lung cancer
is suspected, perform a chest and abdomen CT scan with
contrast. |
|
| Start with
non-invasive techniques: PET/CT scan and/or MRI. If needed, recur
to more invasive EBUS and EUS-guided, transthoracic or
transbronchial FNA or CNB to obtain a biopsy. |
|
| If lung cancer is
confirmed, assess mediastinal involvement and lymphadenopathy. |
|
| Use the currently
applicable TNM classification to precisely define the lung cancer
stage that will inform treatment. |
|
| For patients who
might be treated for curative purposes, perform a brain MRI to rule
out brain lesions. |
| Management of stage
III NSCLC | Eligible patients
should undergo trimodal therapy: Chemotherapy, radiation therapy
and surgical excision of the tumor. |
|
| Unresectable tumors
[stages IIIA (N2), IIIB and IIIC] should be treated by concurrent
or sequential chemoradiation. |
|
| Patients with
non-progressive stage IIIA (N2), IIIB or IIIC cancer should be
given durvalumab for 12 months (consolidation therapy). Durvalumab
is best initiated within 42 days of the last radiation dose. |
|
| Targeted therapies
might be prescribed, according to cytopathology results and the
patient's general status and prognosis. |
|
| While
anti-neoplastic treatment should be defined and prescribed by a
clinical oncologist with experience in lung cancer, decisions about
tumor resectability should involve the surgeon and functional
assessment of the lungs should be performed by a pulmonologist. The
decision on the management of stage III NSCLC should be performed
at the level of the multidisciplinary team. |
|
| Immune-related
adverse events should be managed by a multidisciplinary team; in
particular, patients who develop radiation-related pneumonitis
should be quickly referred to an expert pulmonologist. |
|
| When a patient is
considered cured or in remission, clinical evaluation and follow-up
CT scans should be performed every 6 months during the first 2
years after the cancer is cleared, and then once a year for up to 5
years, with a PET/CT scan at the 1-year milestone. |
Conclusion
Diagnosis and management of stage III NSCLC have
evolved in the past decade, presenting patients and physicians with
several tools for staging and several therapeutic options. While
this joint statement aims at providing guidance and closing some
gaps in the management of locally advanced lung cancer in Lebanon,
we underscore the importance of designing the therapeutic approach
on a patient-by-patient basis, considering the clinical,
demographic and socioeconomic profiles. A multidisciplinary team
meeting is recommended in the management of lung cancer to decide
on the best possible treatment. It should therefore be an
obligation to stay updated on novel therapies and treatment
modalities, which are continuously being refined and fine-tuned, to
keep up with the highest level of evidence-based medicine.
Acknowledgements
The authors would like to acknowledge Dr Jessica
Saliba and Dr Rana Abdel-Samad for their support in literature
reviewing and writing of this joint statement.
Funding
This work was supported by an unrestricted grant from
AstraZeneca. Funding also covered writing support from the contract
research organization KBP-Biomak.
Availability of data and materials
Not applicable.
Authors' contribution
ZAB and NB were involved in manuscript outline,
writing and editing. WAS, HA, JB, PB, HC, GD, FEK, FF, HG, MG, GJ,
FN, RN, MR, GT, AT, MW and PY critically contributed to the
drafting of sections falling within their expertise, reviewed and
corrected the manuscript. Data authentication is not applicable.
All authors approved the current version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Balata H, Fong KM, Hendriks LE, Lam S,
Ostroff JS, Peled N, Wu N and Aggarwal C: Prevention and early
detection for NSCLC: Advances in thoracic oncology 2018. J Thorac
Oncol. 14:1513–1527. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Urvay SE, Yucel B, Erdis E and Turan N:
Prognostic factors in stage III non-small-cell lung cancer
patients. Asian Pac J Cancer Prev. 17:4693–4697. 2016.PubMed/NCBI
|
|
3
|
Berghmans T, Paesmans M and Sculier JP:
Prognostic factors in stage III non-small cell lung cancer: A
review of conventional, metabolic and new biological variables.
Ther Adv Med Oncol. 3:127–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Center for Chronic Disease
Prevention, Health Promotion (US) Office on Smoking and Health, .
The health consequences of smoking-50 years of progress: A report
of the surgeon general. Centers for Disease Control and Prevention
(US); Atlanta (GA): 2014
|
|
5
|
Rahal Z, El Nemr S, Sinjab A, Chami H,
Tfayli A and Kadara H: Smoking and lung cancer: A geo-regional
perspective. Front Oncol. 7:1942017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tammemägi MC: Selecting lung cancer
screenees using risk prediction models-where do we go from here.
Transl Lung Cancer Res. 7:243–253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer Network
(2023), . NCCN Guidelines Version 1.2023 - Non-Small Cell Lung
Cancer, Updates. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdfFebruary
1–2023
|
|
8
|
Kumar V, Cohen JT, van Klaveren D,
Soeteman DI, Wong JB, Neumann PJ and Kent DM: Risk-targeted lung
cancer screening: A cost-effectiveness analysis. Ann Intern Med.
168:161–169. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cronin KA, Gail MH, Zou Z, Bach PB,
Virtamo J and Albanes D: Validation of a model of lung cancer risk
prediction among smokers. J Natl Cancer Inst. 98:637–640. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
National Cancer Institute DoCEG, . Lung
cancer risk assessment tool. https://analysistools.cancer.gov/lungCancerRiskAssessment/#/February
2–2023
|
|
11
|
Katki HA, Kovalchik SA, Berg CD, Cheung LC
and Chaturvedi AK: Development and validation of risk models to
select ever-smokers for CT lung cancer screening. JAMA.
315:2300–2311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raji OY, Duffy SW, Agbaje OF, Baker SG,
Christiani DC, Cassidy A and Field JK: Predictive accuracy of the
liverpool lung project risk model for stratifying patients for
computed tomography screening for lung cancer: A case-control and
cohort validation study. Ann Intern Med. 157:242–250. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katki HA, Kovalchik SA, Petito LC, Cheung
LC, Jacobs E, Jemal A, Berg CD and Chaturvedi AK: Implications of
nine risk prediction models for selecting ever-smokers for computed
tomography lung cancer screening. Ann Intern Med. 169:10–19. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding L, Getz G, Wheeler DA, Mardis ER,
McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan
MB, et al: Somatic mutations affect key pathways in lung
adenocarcinoma. Nature. 455:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kauczor HU, Bonomo L, Gaga M, Nackaerts K,
Peled N, Prokop M, Remy-Jardin M, von Stackelberg O and Sculier JP:
ESR/ERS white paper on lung cancer screening. Eur Respir J.
46:28–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gierada DS, Black WC, Chiles C, Pinsky PF
and Yankelevitz DF: Low-dose CT screening for lung cancer: Evidence
from 2 decades of study. Radiol Imaging Cancer. 2:e1900582020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Covington MF, Koppula BR, Fine GC, Salem
AE, Wiggins RH, Hoffman JM and Morton KA: PET-CT in clinical adult
oncology: II. Primary thoracic and breast malignancies. Cancers
(Basel). 14:26892022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta RK, Naran S, Lallu S and Fauck R:
The diagnostic value of fine needle aspiration cytology (FNAC) in
the assessment of palpable supraclavicular lymph nodes: A study of
218 cases. Cytopathology. 14:201–207. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stigt JA, Boers JE and Boomsma MF:
Ultrasound-guided tissue core biopsies in supraclavicular lymph
nodes in patients with suspected thoracic malignancies.
Respiration. 90:412–415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muthu V, Sehgal IS, Dhooria S, Prasad KT,
Gupta N, Aggarwal AN and Agarwal R: Endobronchial ultrasound-guided
transbronchial needle aspiration: Techniques and challenges. J
Cytol. 36:65–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng SH and Yang ST: The new 8th TNM
staging system of lung cancer and its potential imaging
interpretation pitfalls and limitations with CT image
demonstrations. Diagn Interv Radiol. 25:270–279. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akata S, Kajiwara N, Park J, Yoshimura M,
Kakizaki D, Abe K, Hirano T, Ohira T, Tsuboi M and Kato H:
Evaluation of chest wall invasion by lung cancer using respiratory
dynamic MRI. J Med Imaging Radiat Oncol. 52:36–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao SJ, Kim AW, Puchalski JT, Bramley K,
Detterbeck FC, Boffa DJ and Decker RH: Indications for invasive
mediastinal staging in patients with early non-small cell lung
cancer staged with PET-CT. Lung Cancer. 109:36–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Flury DV, Minervini F and Kocher GJ:
Heterogeneity of stage IIIA non-small cell lung cancer-different
tumours, different nodal status, different treatment, different
prognosis: A narrative review. Curr Chall ThoracSurg. 4:132020.
View Article : Google Scholar
|
|
27
|
Kutob L and Schneider F: Lung cancer
staging. Surg Pathol Clin. 13:57–71. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karkhanis VS and Joshi JM: Pleural
effusion: Diagnosis, treatment, and management. Open Access Emerg
Med. 4:31–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anevlavis S and Froudarakis ME: Advances
in pleuroscopy. Clin Respir J. 12:839–847. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moisiuc FV and Colt HG: Thoracoscopy:
Origins revisited. Respiration. 74:344–355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Foucault C, Mordant P, Grand B, Achour K,
Arame A, Dujon A, Barthes FL and Riquet M: Unexpected extensions of
non-small-cell lung cancer diagnosed during surgery: Revisiting
exploratory thoracotomies and incomplete resections. Interact
Cardiovasc Thorac Surg. 16:667–672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rami-Porta R, Call S, Dooms C, Obiols C,
Sánchez M, Travis WD and Vollmer I: Lung cancer staging: A concise
update. Eur Respir J. 51:18001902018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Travis WD, Asamura H, Bankier AA, Beasley
MB, Detterbeck F, Flieder DB, Goo JM, MacMahon H, Naidich D,
Nicholson AG, et al: The IASLC lung cancer staging project:
Proposals for coding T categories for subsolid nodules and
assessment of tumor size in part-solid tumors in the forthcoming
eighth edition of the TNM classification of lung cancer. J Thorac
Oncol. 11:1204–1223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shim SS, Lee KS, Kim BT, Chung MJ, Lee EJ,
Han J, Choi JY, Kwon OJ, Shim YM and Kim S: Non-small cell lung
cancer: Prospective comparison of integrated FDG PET/CT and CT
alone for preoperative staging. Radiology. 236:1011–1019. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shien K, Toyooka S, Soh J, Okami J,
Higashiyama M, Kadota Y, Maeda H, Hayama M, Chida M, Funaki S, et
al: Clinicopathological characteristics and lymph node metastasis
pathway of non-small-cell lung cancer located in the left lingular
division. Interact Cardiovasc Thorac Surg. 20:791–796. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brierley J.D..Asamura H, van Eycken E and
Rous Brian: TNM Atlas. 7th edition. Wiley-Blackwell; New Jersey,
US: pp. p4482021
|
|
37
|
Van Schil PE, Balduyck B, De Waele M,
Hendriks JM, Hertoghs M and Lauwers P: Surgical treatment of
early-stage non-small-cell lung cancer. EJC suppl. 11:110–122.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Van Schil PE, Rami-Porta R and Asamura H:
The 8th TNM edition for lung cancer: A critical
analysis. Ann Transl Med. 6:872018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brunelli A, Kim AW, Berger KI and
Addrizzo-Harris DJ: Physiologic evaluation of the patient with lung
cancer being considered for resectional surgery: Diagnosis and
management of lung cancer, 3rd ed: American college of chest
physicians evidence-based clinical practice guidelines. Chest.
143:e166S–e190S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee TH, Marcantonio ER, Mangione CM,
Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss
R, Ho KK, et al: Derivation and prospective validation of a simple
index for prediction of cardiac risk of major noncardiac surgery.
Circulation. 100:1043–1049. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saraswat V: Effects of anaesthesia
techniques and drugs on pulmonary function. Indian J Anaesth.
59:557–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ntima NO and Lumb AB: Pulmonary function
tests in anaesthetic practice. BJA Edu. 19:206–211. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Majem M, Hernández-Hernández J,
Hernando-Trancho F, de Dios NR, Sotoca A, Trujillo-Reyes JC,
Vollmer I, Delgado-Bolton R and Provencio M: Multidisciplinary
consensus statement on the clinical management of patients with
stage III non-small cell lung cancer. Clin Transl Oncol. 22:21–36.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Péus D, Newcomb N and Hofer S: Appraisal
of the Karnofsky performance status and proposal of a simple
algorithmic system for its evaluation. BMC Med Inform Decis Mak.
13:722013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chow R, Bruera E, Temel JS, Krishnan M, Im
J and Lock M: Inter-rater reliability in performance status
assessment among healthcare professionals: An updated systematic
review and meta-analysis. Supportive Care Cancer. 28:2071–2078.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Karmakar A, Kumtakar A, Sehgal H, Kumar S
and Kalyanpur A: Interobserver variation in response evaluation
criteria in solid tumors 1.1. Acad Radiol. 26:489–501. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lalchandani UR, Sahai V, Hersberger K,
Francis IR and Wasnik AP: A radiologist's guide to response
evaluation criteria in solid tumors. Curr Probl Diagn Radiol.
48:576–585. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bevans M, Ross A and Cella D:
Patient-reported outcomes measurement information system (PROMIS):
Efficient, standardized tools to measure self-reported health and
quality of life. Nurs Outlook. 62:339–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kunitoh H and Suzuki K: How to evaluate
the risk/benefit of trimodality therapy in locally advanced
non-small-cell lung cancer. Br J Cancer. 96:1498–1503. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rusch VW, Giroux DJ, Kraut MJ, Crowley J,
Hazuka M, Johnson D, Goldberg M, Detterbeck F, Shepherd F, Burkes
R, et al: Induction chemoradiation and surgical resection for
non-small cell lung carcinomas of the superior sulcus: Initial
results of Southwest oncology group trial 9416 (Intergroup Trial
0160). J Thoracic Cardiovasc Surg. 121:472–483. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rivas-Perez H and Nana-Sinkam P:
Integrating pulmonary rehabilitation into the multidisciplinary
management of lung cancer: A review. Respir Med. 109:437–442. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cortes AA, Urquizu LC and Cubero JH:
Adjuvant chemotherapy in non-small cell lung cancer:
State-of-the-art. Transl Lung Cancer Res. 4:191–197.
2015.PubMed/NCBI
|
|
56
|
Pennell NA, Neal JW, Chaft JE, Azzoli CG,
Janne PA, Govindan R, Evans TL, Costa DB, Wakelee HA, Heist RS, et
al: SELECT: A phase II trial of adjuvant erlotinib in patients with
resected epidermal growth factor receptor-mutant non-small-cell
lung cancer. J Clin Oncol. 37:97–104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
U.S. Food and drug administration (FDA), .
FDA approves osimertinib as adjuvant therapy for non-small cell
lung cancer with EGFR mutations. FDA. 2020.
|
|
58
|
Furuse K, Fukuoka M, Kawahara M, Nishikawa
H, Takada Y, Kudoh S, Katagami N and Ariyoshi Y: Phase III study of
concurrent versus sequential thoracic radiotherapy in combination
with mitomycin, vindesine, and cisplatin in unresectable stage III
non-small-cell lung cancer. J Clin Oncol. 17:2692–2699. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jeremic B, Shibamoto Y, Acimovic L and
Milisavljevic S: Hyperfractionated radiation therapy with or
without concurrent low-dose daily carboplatin/etoposide for stage
III non-small-cell lung cancer: A randomized study. J Clin Oncol.
14:1065–1070. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Strøm HH, Bremnes RM, Sundstrøm SH,
Helbekkmo N and Aasebø U: Poor prognosis patients with inoperable
locally advanced NSCLC and large tumors benefit from palliative
chemoradiotherapy: A subset analysis from a randomized clinical
phase III trial. J Thorac Oncol. 9:825–833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hung MS, Wu YF and Chen YC: Efficacy of
chemoradiotherapy versus radiation alone in patients with
inoperable locally advanced non-small-cell lung cancer: A
meta-analysis and systematic review. Medicine (Baltimore).
98:e161672019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Auperin A, Le Pechoux C, Rolland E, Curran
WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus
R, et al: Meta-analysis of concomitant versus sequential
radiochemotherapy in locally advanced non-small-cell lung cancer. J
Clin Oncol. 28:2181–2190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
U.S. Food and drug administration (FDA), .
FDA expands pembrolizumab indication for first-line treatment of
NSCLC (TPS ≥1%). FDA. 2019.
|
|
64
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et
al: Durvalumab after chemoradiotherapy in stage III non-small-cell
lung cancer. N Engl J Med. 377:1919–1929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et
al: Overall survival with durvalumab after chemoradiotherapy in
stage III NSCLC. N Engl J Med. 379:2342–2350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Botticella A, Mezquita L, Le Pechoux C and
Planchard D: Durvalumab for stage III non-small-cell lung cancer
patients: Clinical evidence and real-world experience. Ther Adv
Respir Dis. 13:17534666198855302019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lee JG, Lee CY, Park IK, Kim DJ, Cho SH,
Kim KD and Chung KY: The prognostic significance of multiple
station N2 in patients with surgically resected stage IIIA N2
non-small cell lung cancer. J Korean Med Sci. 23:604–608. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Quint LE: Lung cancer: Assessing
resectability. Cancer Imaging. 4:15–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Biancosino C, Krüger M, Vollmer E and
Welker L: Intraoperative fine needle aspirations-diagnosis and
typing of lung cancer in small biopsies: Challenges and
limitations. Diagn Pathol. 11:592016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu T, Chen Z, Dang J and Li G: The role
of surgery in stage I to III small cell lung cancer: A systematic
review and meta-analysis. PLoS One. 13:e02100012018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Suzuki S and Goto T: Role of surgical
intervention in unresectable non-small cell lung cancer. J Clin
Med. 9:38812020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jeremic B, Casas F, Dubinsky P,
Gomez-Caamano A, Cihoric N, Videtic G and Latinovic M: Combined
modality therapy in stage IIIA non-small cell lung cancer: Clarity
or confusion despite the highest level of evidence? J Radiat Res.
58:267–272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dehing-Oberije C, De Ruysscher D, van
Baardwijk A, Yu S, Rao B and Lambin P: The importance of patient
characteristics for the prediction of radiation-induced lung
toxicity. Radiother Oncol. 91:421–426. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang W, Xu Y, Schipper M, Matuszak MM,
Ritter T, Cao Y, Haken RK and Kong FM: Effect of normal lung
definition on lung dosimetry and lung toxicity prediction in
radiation therapy treatment planning. Int J Radiat Oncol Biol Phys.
86:956–963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
De Ruysscher D, Faivre-Finn C, Moeller D,
Nestle U, Hurkmans CW, Le Péchoux C, Belderbos J, Guckenberger M
and Senan S: European organization for research and treatment of
cancer (EORTC) recommendations for planning and delivery of
high-dose, high precision radiotherapy for lung cancer. Radiother
Oncol. 124:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Keall PJ, Mageras GS, Balter JM, Emery RS,
Forster KM, Jiang SB, Kapatoes JM, Low DA, Murphy MJ, Murray BR, et
al: The management of respiratory motion in radiation oncology
report of AAPM task group 76. Med Phys. 33:3874–3900. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Naito Y, Kubota K, Nihei K, Fujii T, Yoh
K, Niho S, Goto K, Ohmatsu H, Saijo N and Nishiwaki Y: Concurrent
chemoradiotherapy with cisplatin and vinorelbine for stage III
non-small cell lung cancer. J Thorac Oncol. 3:617–622. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liang J, Bi N, Wu S, Chen M, Lv C, Zhao L,
Shi A, Jiang W, Xu Y, Zhou Z, et al: Etoposide and cisplatin versus
paclitaxel and carboplatin with concurrent thoracic radiotherapy in
unresectable stage III non-small cell lung cancer: A multicenter
randomized phase III trial. Ann Oncol. 28:777–783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Rami–Porta R, Wittekind C and Goldstraw P;
International Association for the Study of Lung Cancer (IASLC)
Staging Committee, : Complete resection in lung cancer surgery:
Proposed definition. Lung Cancer. 49:25–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sonobe M, Yutaka Y, Nakajima D, Hamaji M,
Menju T, Ohsumi A, Chen-Yoshikawa TF, Sato T and Date H: Salvage
surgery after chemotherapy or chemoradiotherapy for initially
unresectable lung carcinoma. Ann Thorac Surg. 108:1664–1670. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xu XL, Dan L, Chen W, Zhu SM and Mao WM:
Neoadjuvant chemoradiotherapy or chemotherapy followed by surgery
is superior to that followed by definitive chemoradiation or
radiotherapy in stage IIIA (N2) nonsmall-cell lung cancer: A
meta-analysis and system review. Onco Targets Ther. 9:845–853.
2016.PubMed/NCBI
|
|
82
|
Lardinois D, Schallberger A, Betticher D
and Ris HB: Postinduction video-mediastinoscopy is as accurate and
safe as video-mediastinoscopy in patients without pretreatment for
potentially operable non-small cell lung cancer. Ann Thorac Surg.
75:1102–1106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Castello A, Rossi S and Lopci E: 18F-FDG
PET/CT in restaging and evaluation of response to therapy in lung
cancer: State of the art. Curr Radiopharm. 13:228–237. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Dane B, Grechushkin V, Plank A, Moore W
and Bilfinger T: PET/CT vs. non-contrast CT alone for surveillance
1-year post lobectomy for stage I non-small-cell lung cancer. Am J
Nucl Med Mol Imaging. 3:408–416. 2013.PubMed/NCBI
|
|
85
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|