Introduction
Colon cancer is a highly aggressive malignancy of
the digestive system that carries a poor prognosis; according to
the results of the National Center for Health Statistics, the
number of new cases of colon cancer is 104,610, and the number of
deaths is 53,200 in the United States (1). It constitutes the third leading cause
of cancer diagnosis and mortality in both males and females,
despite the reduced mortality rates in females in recent decades,
and reduced overall incidence and mortality rates due to colon
cancer screening (2). Traditional
surgery, radiotherapy and chemotherapy primarily target tumor cells
in the proliferative phase, but have limited killing effects on
cells in the non-proliferative phase, which leads to metastasis,
recurrence, and drug resistance (3). Cancer stem cells (CSCs) may be
responsible for these poor clinical outcomes (4,5).
CSCs are a self-renewing subpopulation of neoplastic
cells that promote tumorigenesis, proliferation and growth
(6,7). Originally discovered in leukemia, CSCs
have been identified and isolated from solid tumors, such as those
of the prostate, brain, colorectum, pancreas and breast (8). CSC isolation and identification
methods include fluorescence-activated cell sorting (FACS),
magnetic-activated cell sorting (MACS) and serum-free medium (SFM)
cultures (9). FACS separates CSCs
via flow cytometry using cell surface markers; however, it is
inefficient, expensive, and can injure cells. MACS relies on the
labeling of cells with monoclonal antibodies tagged with magnetic
beads, which are then sorted using a strong magnetic field;
however, this technique produces small-scale cell yields of low
purity that are inadequate to meet experimental requirements. CSCs
survive and proliferate in serum-free suspension culture
environment, but cancer cells cannot (10,11).
Therefore, the SFM suspension culture method can effectively expand
and enrich CSCs. Advantages include high yields, improved cell
viability, ease of operation and low cost (9,12).
In the study of CSCs derived from breast cancer
(13), prostate cancer (14), and endometrial cancer (15), researchers have applied the method
of SFM suspension culture to enrich CSCs. The obtained CSCs were
further sorted and purified for subsequent research, or directly
used in the follow-up experiments, allowing studies on CSCs to be
carried out successfully.
In the present study of colon CSCs, SFM suspension
culture protocols were used to effectively obtain CSCs. In order to
maintain the integrity of signaling pathways in CSCs, epidermal
growth factor (EGF) and basic fibroblast growth factor (bFGF) were
added to the culture medium. However, the concentration to be used
for the supplementation of these two growth factors remains a
controversial issue; some researchers have used 10 ng/ml EGF + 10
ng/ml bFGF in SFM suspension culture (16,17),
whereas others have used 20 ng/ml EGF + 10 ng/ml bFGF (18,19),
and others still have used only a single growth factor (20). The selection of the concentrations
of the two growth factors is not very rigorous. Another important
aspect is that the culture time required for enriching CSCs by SFM
suspension culture is controversial. Certain groups have opted for
a culture time of 7–10 days (21–24),
whereas others have reported using cultures of up to 14 days
(25,26), or even >28 days (27). Insufficient time for suspension
culture may lead to the incomplete formation of cell spheroids.
Conversely, a prolonged culture time will weaken cell stemness and
induce abnormal differentiation of spheroid cells.
Although the abovementioned culture strategies can
enrich CSCs, the efficiency of their enrichment remains to be
studied, particularly as variable serum-free suspension culture
strategies produce dissimilar enrichment efficiencies. Insulin can
participate in the metabolism and stemness of CSCs through EGF/EGF
receptor pathways, making CSCs more malignant (28). Previous studies reported adding
insulin to SFM to promote the proliferation of CSCs and maintain
stemness (27,29). The currently preferred effective SFM
suspension culture strategy is to add insulin, cell culture
additive B27 and other basic nutrients to DMEM/F12, followed by the
addition of EGF and bFGF (30).
However, to our knowledge, there have been no systematic studies
into the effects of culture medium composition, EGF and bFGF
concentrations, or culture time on CSC enrichment. Consequently,
the aim of the present study was to determine the optimal EGF and
bFGF composition strategy and culture time for the serum-free
suspension method. An optimized, efficient and reliable enrichment
strategy that generates high yields of CSCs may facilitate
preclinical drug development and other areas of basic and clinical
research.
Materials and methods
Materials
The human colon cancer DLD-1 cell line was obtained
from the American Type Culture Collection. DMEM/F12 and RPMI 1640
media were purchased from HyClone (Cytiva). EGF and bFGF were
purchased from PeproTech, Inc. Fetal bovine serum (FBS) and bovine
serum albumin (BSA) were purchased from Amresco, LLC. Insulin was
purchased from MilliporeSigma. SFM supplement factor B27 was
purchased from Invitrogen (Thermo Fisher Scientific, Inc.).
CD133-phycoerythrin (PE; cat. no. 130-098-826), CD44-FITC
(130-095-195) were purchased from Miltenyi Biotec GmbH.
Corresponding isotype control antibodies Mouse IgG1κ Isotype
Control PE (12-4714-41) and FITC (cat. no. 11-4714-41) were
purchased from eBioscience. PrimeScript™ RT Master Mix
kits were purchased from Takara Biotechnology Co., Ltd.
Phosphate-buffered saline (PBS), crystal violet dye and trypsin
were purchased from Wuhan Boster Biological Technology, Ltd. PCR
primers were synthesized by Invitrogen (Thermo Fisher Scientific,
Inc.). The BD FACSCanto II Flow Cytometry Instrument was purchased
from ACEA Bioscience, Inc. (Agilent Technologies, Inc.), and the
PCR thermocycler was purchased from Roche Diagnostics. Sevoflurane
was purchased from MilliporeSigma. BALB/C-Nu/Nu mice were purchased
from Beijing HFK Bioscience Co., Ltd.
Experimental group design
SFM cultures were prepared by combining DMEM/F12
with 10% BSA, 5 mg/ml insulin, B27 cell culture additive (1:50),
100 U/ml streptomycin, 0.1 mg/ml penicillin and variable
concentrations of growth factors. A total of nine experimental
groups were prepared using different combinations of EGF and bFGF:
Group 1 (G1), 5 ng/ml EGF; G2, 5 ng/ml bFGF; G3, 5 ng/ml EGF + 5
ng/ml bFGF; G4, 10 ng/ml EGF; G5, 10 ng/ml bFGF; G6, 10 ng/ml EGF +
10 ng/ml bFGF; G7, 20 ng/ml EGF; G8, 20 ng/ml bFGF; and G9, 20
ng/ml EGF + 20 ng/ml bFGF.
DLD-1 monolayer cell culture
After thawing, DLD-1 cells adhered in a monolayer in
RPMI 1640 medium containing 10% FBS. The medium was changed every 2
days. When cells reached the logarithmic growth phase, they were
digested with EDTA-containing trypsin for 4 min at 37°C, followed
by the addition of 4 ml RPMI-1640 and transfer to a centrifuge
tube. After centrifugation at 111.8 × g for 5 min with 4°C, the
supernatant was discarded, and cells were resuspended in new
medium. Finally, cells were cultivated in a new culture dish
according to the number of cells. All cell cultures were maintained
in a humidified incubator at 37°C and 5% CO2.
Suspension culture of DLD-1 spherical
cells
Adherent DLD-1 cells were cultured to the
logarithmic growth phase, and then cultured in DMEM/F12 for 24 h at
37°C. Cultures underwent trypsin digestion for 4 min to create
single-cell suspensions in DMEM/F12, followed by cell counting. A
volume of 20 ml of the G1-G9 culture media preparations were added
to super slip plastic culture flasks, followed by the addition of
3×104 cells to each experimental group flask. Cultures
were then incubated with the addition of 2 ml of the corresponding
group medium daily at 37°C and 5% CO2. Follow-up tests
were performed at days 10, 20 and 30.
Flow cytometric analysis
Suspended cell spheroids were cultured to days 10,
20 and 30, and then were subjected trypsin digestion and
quantification. Single-cell suspensions were obtained in PBS.
Aliquots of 5×105 cells were rinsed twice with PBS,
followed by the addition of staining buffer blocking solution (pH
7.2, containing 2% FBS and 0.4% BSA) and storage at 4°C for 1 h.
The suspension was centrifuged (251.55 × g for 5 min with 4°C) and
the supernatant was discarded. Subsequently, the cells were treated
with the addition of 25 µl staining buffer and 1.25 µl of either
anti-CD133 antibodies, anti-CD44 antibodies, or a mixture of both
anti-CD133 and anti-CD44 antibodies (all 1:50), and protected from
light at 4°C for 30 min. Finally, the supernatant was discarded
after centrifugation (251.55 × g for 5 min with 4°C). CSCs were
then suspended in 500 µl PBS and detected via flow cytometry. The
data were analyzed using FlowJo software version 10.0 (BD
Bioscience, ACEA Bioscience, Inc.).
RT-quantitative (q)PCR analysis
RNA was extracted from spheroids with chloroform and
precipitated with absolute ethanol at days 10, 20 and 30. mRNA was
then reverse-transcribed into cDNA (37°C for 15 min, 85°C for 5
min, 4°C for 5 min). GAPDH was used as an internal reference.
PrimeScript™ RT Master Mix kit and TB Green®
Premix Ex Taq™ (Tli RNaseH Plus; cat. no. RR420A; both
from Takara Biotechnology Co., Ltd.) were used according to the
manufacturer's instructions to detect the mRNA expression level in
each sample using the 2−ΔΔCq method (31). PCR was executed according to the
follows: 1 cycle of pre-incubation at 95°C for 10 min, followed by
40 cycles of amplification for 10 sec at 95°C, 10 sec at 60°C, 10
sec at 72°C, then 1 cycle of melting at 95°C for 10 sec, at 65°C
for 60 sec, at 97°C for 1 sec, and cooling at 50°C for 30 sec. The
primer sequences used are presented in Table I.
 | Table I.Primer sequences of spheroid cell
genes analyzed via reverse transcription-quantitative PCR. |
Table I.
Primer sequences of spheroid cell
genes analyzed via reverse transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| GAPDH | F:
GGGGAGCCAAAAGGGTCATCATCT |
|
| R:
GACGCCTGCTTCACCACCTTCTTG |
| KLF4 | F:
CGAACCCACACAGGTGAGAA |
|
| R:
TACGGTAGTGCCTGGTCAGTTC |
| Lgr5 | F:
CTCTTCCTCAAACCGTCTGC |
|
| R:
GATCGGAGGCTAAGCAACTG |
| CD44 | F:
CTGCCGCTTTGCAGGTGTA |
|
| R:
CATTGTGGGCAAGGTGCTATT |
| CD133 | F:
ACCGACTGAGACCCAACATC |
|
| R:
GACCGCAGGCTAGTTTTCAC |
| E-cadherin | F:
GCCCTGCCAATCCCGATGAAA |
|
| R:
GGGGTCAGTATCAGCCGCT |
| Vimentin | F:
GCTTCAGAGAGAGGAAGCCGAAAA |
|
| R:
CCGTGAGGTCAGGCTTGGAAA |
| Wnt-3a | F:
AGCTACCCGATCTGGTGGTC |
|
| R:
CAAACTCGATGTCCTCGCTAC |
Sphere-forming assay
A total of 100 spheroid cells were collected from
each group and cultured in 24-well plates with 1 ml of the
corresponding group medium for 14 days at 37°C. The number of
spheroids in each group was then counted in three fields of view
under a light microscope at ×100 magnification for statistical
analysis. Count three times for each sample.
Colony formation assay
Spheroid cells were trypsinized, suspended and
counted. A total of 500 spheroid cells were collected from each
group and seeded in 6-well plates with 5 ml RPMI-1640 medium
containing 10% FBS for 7 days at 37°C. Cells were fixed with 75%
ethanol solution at 37°C for 15 min and stained crystal violet
alcohol solution (Shangbao Biological Company) at 37°C for 30 min.
Colonies (>3 cells) counted manually under a light microscope at
40× magnification for statistical analysis. The experiments was
performed three times.
Subcutaneous tumorigenesis in nude
mice
All animal experiments were approved by the Ethical
Committee of Huazhong University of Science and Technology
(approval no. S255). A total of 81 female nude mice (age, 4–6
weeks; weight, 15–21 g) were randomly divided into 27 groups (n=3
mice/group), and housed in a specific pathogen-free environment
(18–26°C; humidity, 40–50%, 12/12-h light/dark cycle and free
access to food and water) for 1 week to facilitate adaption to the
environment. Spheroid cells were trypsinized, collected and
counted. Suspensions of 1×105 cells in 200 µl
physiological saline were prepared for later use. According to the
grouping of nude mice, the corresponding cells were injected
subcutaneously using a 1-ml syringe, and the injection tracks were
clamped for 20 sec to prevent leakage. Nude mice were observed
daily to evaluate their general condition and changes in body
weight. After 30 days, sevoflurane (5%) was used to anesthetize the
nude mice via breathing inhalation, then nude mice were sacrificed
via cervical dislocation; after confirming the onset of rigor
mortis, the tumors were harvested and measured, and tumor volumes
were calculated using the following formula: Volume=length ×
width2 × 0.5.
Statistical analysis
SPSS 25.0 (IBM Corp) was used for statistical
analysis, and GraphPad Prism 7 (GraphPad Software, Inc.) was used
for graphing of results. The normal distributions of data were
presented as the mean ± SD, and one-way analysis of variance and
Tukey's post hoc tests were used to compare statistical differences
between each group. P<0.05 was considered to indicate a
statistically significant difference. All experiments were
performed ≥3 times.
Results
Proliferation of adherent cells and
suspension culture spheroids
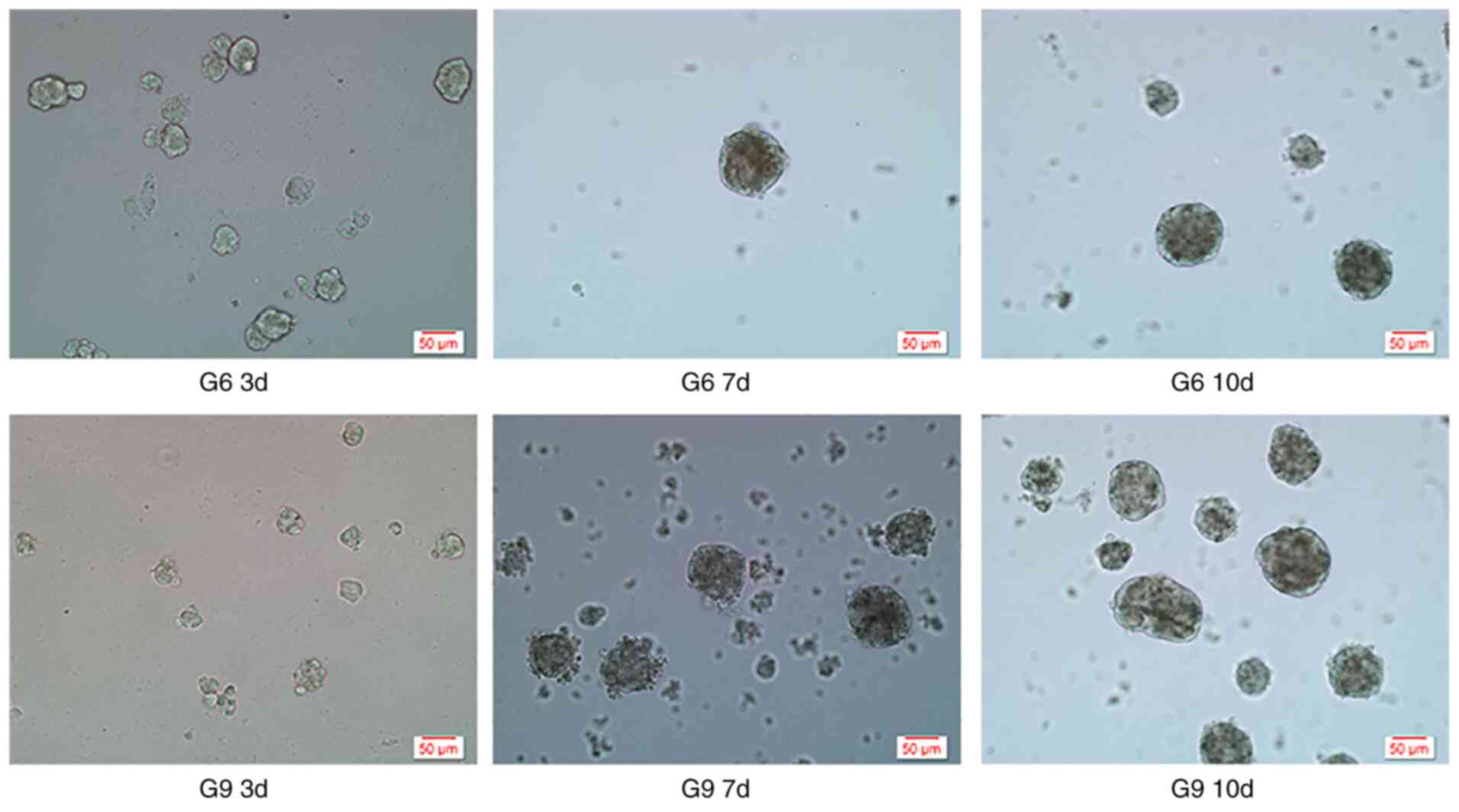
Adherent cells were arranged individually and grew
close to the bottom of the culture flasks. Cells in each group
began to assume a spheroid morphology on day 3, and tended to be
suspended in the culture medium as a single oval unit at day 7,
after which the volume of cell spheroids gradually increased. There
were no intergroup differences in spheroid volume (Fig. 1). The suspension cultures started to
produce stable spheroids after the seventh day of culture.
Flow cytometry
Flow cytometry revealed the percentages of
CD133+, CD44+ and
CD133+CD44+ double-positive spheroid cells of
the nine groups at days 10, 20 and 30 of culture (Table II). The percentages of
CD133+ and CD44+ cells were highest in G9 at
30 days, and were significantly elevated compared with the other
combinations (F=123.554 and 99.528, respectively, P<0.001). The
percentage of CD133+ cells in the G9 group at day 30 was
2.558±0.085%, which was similar to the results for G7 at day 10, 20
and 30, and G8 at day 10 and 30 (P>0.999, P=0.686, P>0.999,
P=0.282 and 0.818, respectively); however, the percentage of
CD44+ cells was 72.990±2.188%, which was higher compared
with in any other group. The percentage of
CD133+CD44+ double-positive spheroid cells in
G9 at day 30 was second only to G3 at day 10 and G6 at day 20 and
G9 at day 30, and G9 at day 30 was significantly higher compared
with other combinations (except G3 at day 10 and G6 at day 20)
(F=57.897, P<0.001). The difference between G3 at day 10 and G6
at day 20 was not statistically significant (P=0.574). These
results suggested that the G9 suspension-cultured spheroids at 30
days of culture exhibited more prominent CSC characteristics than
those grown under the other experimental conditions.
 | Table II.Surface marker expression in spheroid
cells. |
Table II.
Surface marker expression in spheroid
cells.
| A, Proportion of
CD133+ cells |
|---|
|
|---|
| Group | 10 days (%) | 20 days (%) | 30 days (%) |
|---|
| G1 | 0.227±0.124 | 0.127±0.049 | 0.317±0.050 |
| G2 | 0.140±0.040 | 0.693±0.163 | 0.507±0.051 |
| G3 | 0.593±0.091 | 0.590±0.053 | 1.363±0.060 |
| G4 | 0.035±0.005 | 0.720±0.061 | 0.597±0.040 |
| G5 | 0.840±0.110 | 0.090±0.027 | 0.195±0.005 |
| G6 | 0.177±0.015 | 1.223±0.105 | 0.217±0.023 |
| G7 | 2.470±0.149 | 2.277±0.047 | 0.336±0.507 |
| G8 | 2.210±0.297 | 1.087±0.006 | 2.300±0.131 |
| G9 | 1.820±0.046 | 1.030±0.035 | 2.558±0.085 |
|
| B, Proportion of
CD44+ cells |
|
| Group | 10 days
(%) | 20 days
(%) | 30 days
(%) |
|
| G1 | 20.263±0.738 | 54.620±1.992 | 22.400±2.256 |
| G2 | 37.237±0.240 | 46.813±4.772 | 16.087±2.222 |
| G3 | 36.097±0.415 | 38.027±2.720 | 13.043±4.142 |
| G4 | 38.717±2.546 | 37.453±0.645 | 21.107±0.230 |
| G5 | 4.687±0.075 | 57.383±2.799 | 21.817±1.569 |
| G6 | 31.523±3.441 | 42.490±5.059 | 17.013±5.169 |
| G7 | 20.553±3.572 | 14.013±1.544 | 24.870±1.946 |
| G8 | 36.020±2.017 | 25.470±2.647 | 17.920±1.991 |
| G9 | 41.723±2.430 | 22.147±2.870 | 72.990±2.188 |
|
| C, Proportion of
CD133+CD44+ cells |
|
| Group | 10 days
(%) | 20 days
(%) | 30 days
(%) |
|
| G1 | 0.267±0.108 | 0.173±0.021 | 0.910±0.114 |
| G2 | 0.027±0.012 | 0.843±0.131 | 0.177±0.040 |
| G3 | 1.643±0.225 | 0.750±0.147 | 0.347±0.160 |
| G4 | 0.193±0.038 | 0.493±0.023 | 0.217±0.032 |
| G5 | 0.127±.038 | 0.273±0.035 | 0.087±0.032 |
| G6 | 0.287±0.047 | 1.597±0.237 | 0.173±0.055 |
| G7 | 0.387±0.091 | 0.353±0.047 | 0.950±0.053 |
| G8 | 0.603±0.055 | 0.557±0.100 | 0.617±0.047 |
| G9 | 1.020±0.046 | 0.407±0.121 | 1.257±0.144 |
RT-qPCR
RT-qPCR detected the mRNA expression of stemness
genes [Krüppel-like factor 4 (KLF4), leucine-rich repeat-containing
G protein-coupled receptor 5 (Lgr5), CD44, CD133],
epithelial-mesenchymal transition (EMT) genes (E-cadherin,
Vimentin) and Wnt/β-catenin pathway genes (Wnt-3a; Fig. 2). The mRNA expression levels of KLF4
(Fig. 2A), Lgr5 (Fig. 2B), CD44 (Fig. 2C), CD133 (Fig. 2D), Vimentin (Fig. 2E) and Wnt-3a (Fig. 2F) were significantly altered between
the culture groups (F=22.682, 25.401, 3.272, 7.852, 13.331 and
17.445, respectively, P<0.001). with the highest expression
values most commonly observed in G9 at day 30. Comparing the
spheroids of each group with G9 at day 30, CD44 expression in G2 at
day 20, G3 at days 20 and 30, G5 at day 20, G7 at day 30, and G9 at
days 10 and 20 was similar (P=0.218, 0.830, 0.069, 0.162, 0.123,
0.060 and 0.133, respectively; Fig.
2C). There were no significant differences in CD133 expression
between G9 at day 30 and G3 at day 20, G8 at days 20, G8 at days 30
(P=0.876, 0.304 and 0.664, respectively; Fig. 2D). There were no significant
differences in Vimentin expression between G3 at day 30 and G9 at
day 30 (P=0.350; Fig. 2E). Wnt-3a
mRNA expression was similar between G9 at day 30 and G2, 3, 4 and 6
at day 20 (P=0.339, 0.235, 0.09 and 0.198; Fig. 2F). E-cadherin expression was lowest
in the G9 group at day 30 (F=10.851; P<0.001), but was not
significantly different compared with 15 other culture conditions
(Fig. 2G). Increased expression of
Wnt-3a indicated activation of the Wnt/β-catenin signaling pathway,
whereas high expression of Vimentin and low expression of
E-cadherin indicated that EMT had occurred, and was accompanied
with substantially increased expression of stemness genes. In
summary, these results indicated that cell spheroids exhibited the
characteristics of CSCs. Comprehensive analysis of the data showed
that G9 at day 30 exhibited the most upregulated expression of
stemness genes and the lowest expression of E-cadherin, indicative
of the most characteristic gene expression of CSCs (32,33).
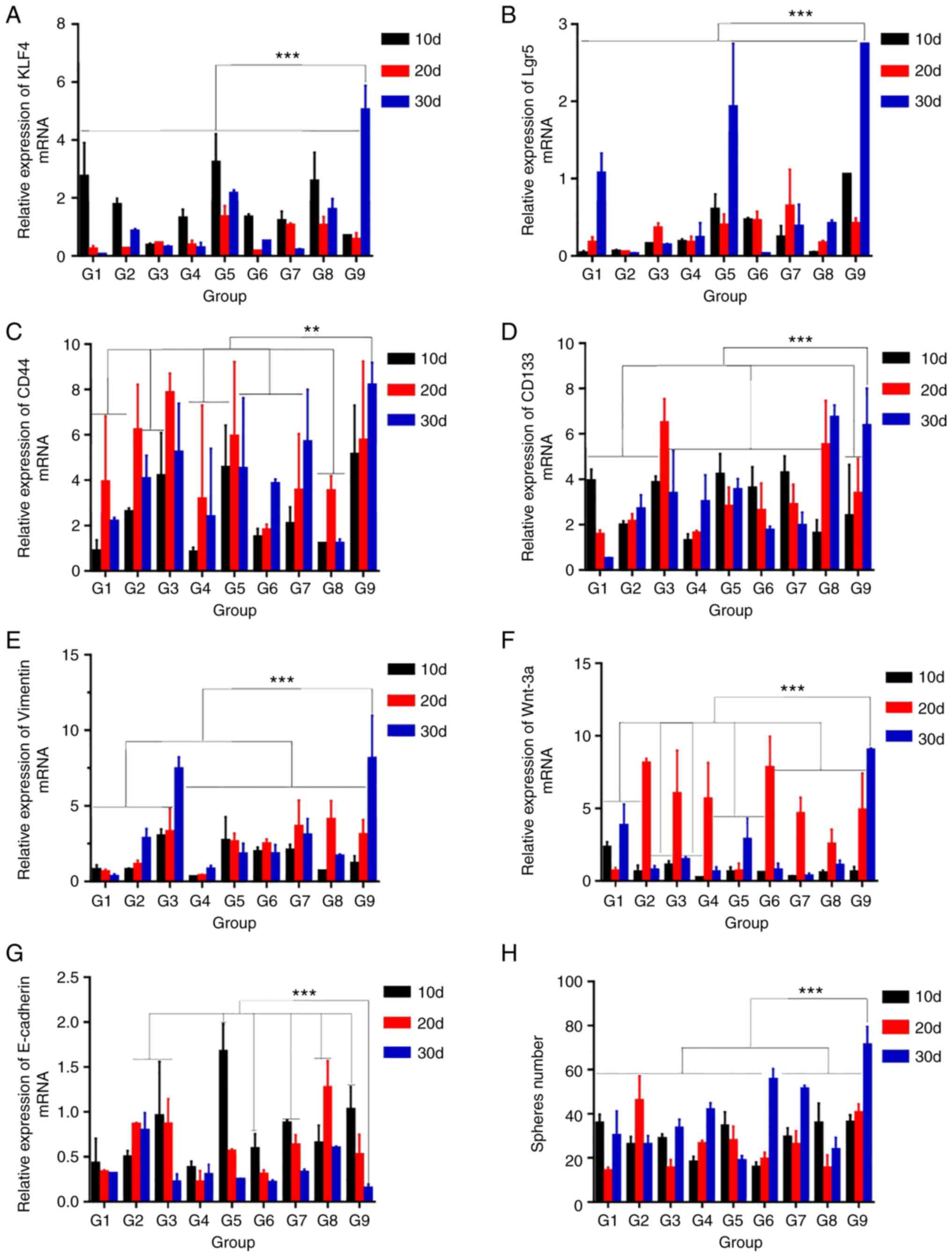 | Figure 2.Reverse transcription-quantitative
PCR to detect mRNA expression and sphere-forming ability of
spheroid cells. All data are presented as the mean ± SD of three
independent experiments. mRNA expression of (A) KLF4, (B) Lgr5, (C)
CD44, (D) CD133, (E) Vimentin, (F) Wnt-3a and (G) E-cadherin. GAPDH
was used as an internal reference. (H) Results of a sphere-forming
assay. **P<0.01, ***P<0.001. G, group; d, day; KLF4,
Krüppel-like factor 4; Lgr5, leucine-rich repeat-containing G
protein-coupled receptor 5. |
Sphere-forming assay
Self-renewal ability is a landmark feature of CSCs,
which is closely associated with cancer occurrence, development,
recurrence and treatment resistance (34). The purpose of the sphere-forming
assay is to test the self-renewal ability of spheroid cells. The
sphere count was significantly different between the different
conditions (F=19.147, P<0.001; Fig.
2H), with the highest yield of spheroids in the G9 condition
from day 30. Of note, the difference between G6 and G9 from day 30
was not statistically significant (P=0.116). These results
suggested that G9 spheroid cells cultured for 30 days exhibited the
most efficient self-renewal capacity.
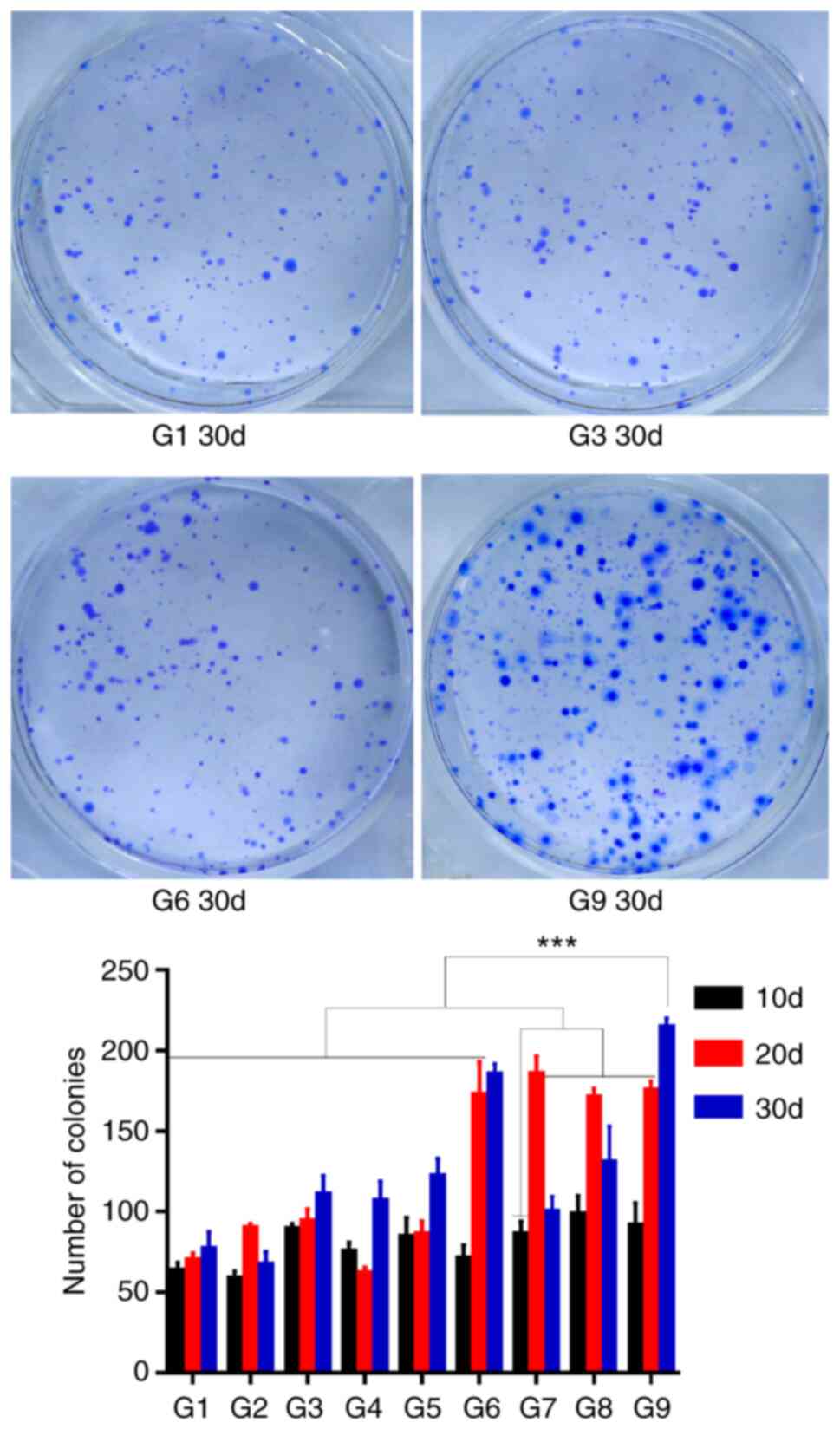
Colony formation assay
To test in vitro tumorigenicity, colony
formation assays were performed for each experimental group
(Fig. S1). Spheroid cells can
redifferentiate into adherent cells. The number of colonies was
significantly different between the different conditions (F=60.767,
P<0.001), with the largest number of colonies observed for G9
cells cultured for 30 days. However, there was no statistically
significant difference between G9 from day 30 compared with G6 from
day 30 or G7 from day 20 (P=0.119 and 0.131; Fig. 3). Therefore, the results suggested
that G9 spheroid cells cultured for 30 days exhibited the most
prominent in vitro tumorigenic capacity.
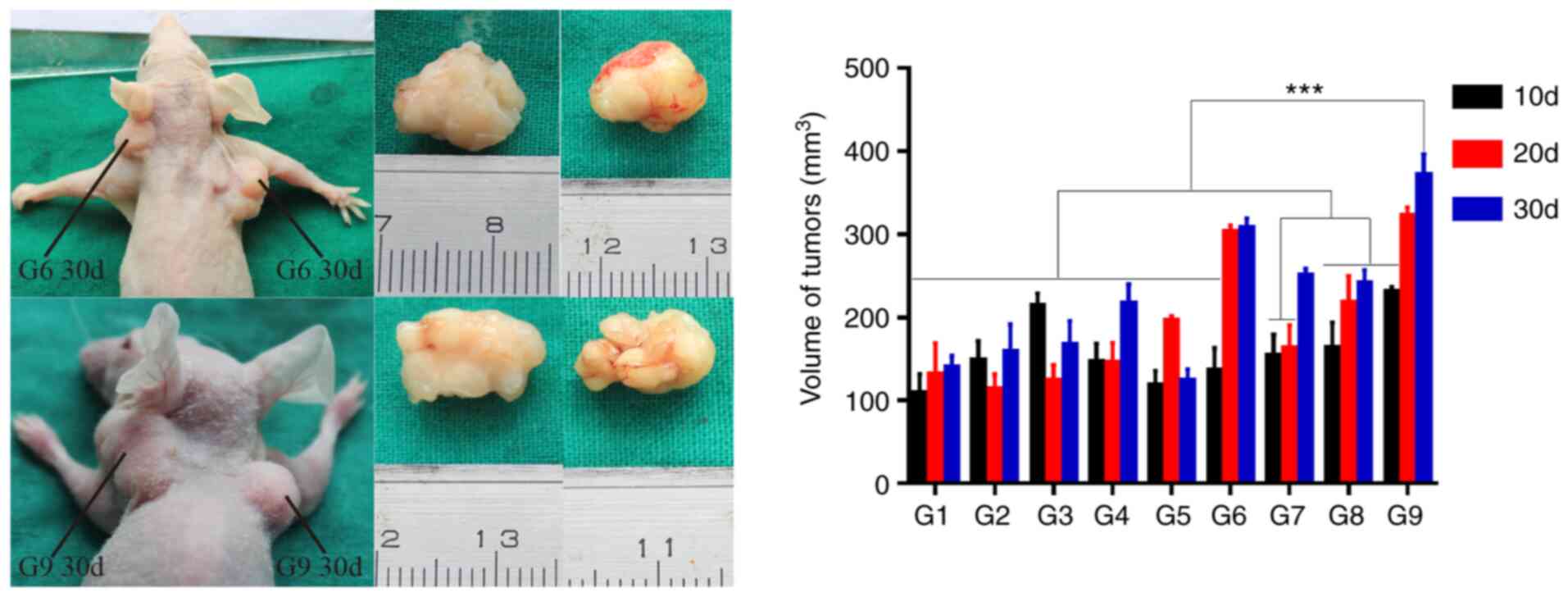
Subcutaneous tumorigenesis in nude
mice
In vivo tumorigenesis was analyzed by using
the tumor cell xenograft formation assay (Fig. S2). G9 at day 30 promoted the
largest tumor volume, which was significantly increased compared
with other combinations (F=12.539, P<0.001; Fig. 4). However, there were no
statistically significant differences between G9 from day 30
compared with G6 from days 20 and 30, G7 from day 30 or G9 from day
20 (P=0.892, 0.940. 0.053 and 0.997, respectively). It is therefore
proposed that G9 spheroid cells cultured for 30 days exhibited the
most potent in vivo tumorigenicity.
Discussion
Increasing evidence suggests that targeting and
selective killing of CSCs to prevent tumor metastasis, recurrence
and drug resistance may improve the prognosis of colon cancer
(3,35). Annett and Robson (36) reported that therapeutic candidates
that specifically target CSCs are now entering clinical trials.
Gupta et al (37) reported
that monoclonal antibodies that target surface markers on colon
CSCs effectively treat colorectal cancer in preclinical in
vivo models. Jahanafrooz et al (3) proposed that the combined targeting of
CSCs and the tumor microenvironment (TME) may potentially lead to
successful colon cancer eradication. Therefore, it is proposed that
targeted therapy directed against colon CSCs will open novel
opportunities for colon cancer treatment. However, a prerequisite
for all supporting research is the procurement of sufficient and
viable colon CSCs.
Acquisition and identification of colon CSCs is
essential to targeted therapy research. Obtaining large quantities
of high-purity colon CSCs has become a major challenge, due to
their scarcity and difficult detection (38). FACS and MACS are traditional methods
of obtaining CSCs, but are limited by low cell yields, reduced
cellular viability and cost. By incorporating technological
advances, suspension culture has become a complementary method to
FACS or MACS, and has been widely recognized as a means to
circumvent the aforementioned disadvantages (12). Spheroid cells enriched by SFM
suspension culture have prominent characteristics of CSCs, and can
either be used directly or purified via FACS or MACS prior to
CSC-related research (39). CD133,
CD44 (40), high aldehyde
dehydrogenase activity (41) and
Lgr5 (42,43) are markers of colon CSCs. Combined
surface markers can accurately identify and facilitate the
isolation of CSCs from spheroid cells (43).
Currently, the most prevalent strategy for enriching
CSCs with SFM suspension culture utilizes serum-free suspension
medium with various concentrations and combinations of EGF and
bFGF, and incubation times ranging from 7 to 15 days (15,44,45).
Suspension culture time is crucial for the expression of CSC
characteristics. Excessive culture time not only increases costs
and the risk of culture contamination, but also increases the risk
of in vitro culture variation and attenuation of CSC
characteristics (46,47). Insufficient culture time produces
enriched spheroid cells with diminished CSC characteristics
(48). Culture times of either 7 or
14 days are favored in current suspension culture strategies, but
there is no empirical validation that these are optimal time
intervals (21,22). The present study observed that
spheroid cell volume and stability did not change with increasing
culture time after the seventh day of culture. However, PCR and
flow cytometry results showed that the expression of various genes
in spheroid cells were higher at 30 days compared with at 10 or 20
days. Moreover, the results of the sphere-forming and colony
formation assays demonstrated that spheroid cells at 30 days
exhibited more evident CSC characteristics. It is therefore
proposed that CSC characteristics gradually increase through
proliferation and accumulation of spheroid cells in suspension
culture with increased culture time.
Colon CSCs simulate a CSC niche, where the TME
maintains the CSC population and modulates the occurrence and
development of cancer (49–51). Ahmed et al (52) reported that the chemoresistance,
metastasis and recurrence of CSCs are regulated by the TME. Lenos
et al (49) proposed that
CSCs are regulated by cytokines, chemokines and stem cell factors
in the TME. Consequently, the TME determines CSC characteristics
and capabilities. In vitro serum-free suspension culture
simulates the TME, as EGF and bFGF are among the most important
factors affecting the TME; during CSC enrichment, EGF regulates
proliferation, apoptosis and EMT via the EGF signaling pathway
(28,53), whereas bFGF promotes self-renewal
and maintains stemness (34,54).
Varying concentrations of EGF and bFGF have been used previously to
enrich CSCs in suspension culture, but to our knowledge, the
optimization of growth factor concentrations and culture times has
not been addressed. The present study comprehensively analyzed
multiple experimental conditions, and found that G9 spheroids after
30 days exhibited the most proficient self-renewal, in vivo
and in vitro tumorigenicity, and characteristic expression
of stemness-associated genes compared with the other groups.
Although it is possible that higher concentrations of EGF and bFGF
may be more effective and require investigation in future studies,
it was determined that a 30-day incubation using 20 ng/ml EGF + 20
ng/ml bFGF medium supplement was the most conducive method for CSC
enrichment compared with other strategies.
Next, the mechanisms underlying the effects of EGF
and bFGF combination on CSC enrichment were explored. Activation of
the Wnt/β-catenin pathway promotes EMT, and Wnt signaling
contributes to the maintenance of CSC populations (55). In addition, EMT serves important
roles in CSC metastasis, tumorigenesis, self-renewal and drug
resistance (56–58). The Wnt/β-catenin pathway and EMT
should be considered when identifying or isolating CSCs. The
present study showed that Wnt/β-catenin pathway activation and EMT
occurred in all groups of spheroid cells. It is worth mentioning
that the expression levels of Wnt/β-catenin pathway-(Wnt-3a) and
EMT-associated (Vimentin) genes were highest and the expression of
E-cadherin was lowest in the G9 condition (20 ng/ml EGF + 20 ng/ml
bFGF) after 30 days of culture compared with the other groups. It
is proposed that the results observed for G9 after 30 days of
culture are at least partly explained by the activation of ETM and
the Wnt/β-catenin pathway due to the combination of EGF and
bFGF.
Nonetheless, the present study has several
shortcomings. The suspension culture method was not applied to a
variety of cell lines and primary cells, and the study did not
evaluate enrichment after 30 days. In addition, the underlying
molecular mechanisms have not been explored completely. Such issues
require further investigation.
In summary, the present study reported that SFM
suspension cultures can effectively enrich tumor stem cells using
the DLD-1 cell line, and that a 30-day culture using medium
supplemented with a combination of 20 ng/ml EGF + 20 ng/ml bFGF
presented the best enrichment efficiency due to activation of EMT
and the Wnt/β-catenin pathway.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Foundation of the National
Natural Science Foundation of China (grant no. 81402444), the
Bureau of Science and Technology Nanchong City (grant no.
18SXHZ0460); the Department of Science and Technology of Sichuan
province (grant no. 2017JY0170), the 2019 National Natural Science
Foundation Pre-research of North Sichuan Medical College (grant no.
CBY-19YZ06), the Scientific Research of the Affiliated Hospital of
North Sichuan Medical College (grant no. 2019ZD007) and the
Research and Development Project of North Sichuan Medical College
(grant no. CBY17-A-YB53).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GZ, XL and XZ conducted the cell and animal
experiments, the analysis of experimental data, and the writing and
revision of the manuscripts. WY and WL contributed to the cell
experiments. YF and QX contributed to the subcutaneous tumor
formation experiments in nude mice. JL, SJ and ZL made substantial
contributions to the experimental design, and analysis and
interpretation of data. ZL was involved in drafting the manuscript
and revising it critically for important intellectual content. GZ
and ZL confirmed the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Ethical
Committee of Huazhong University of Science and Technology
(approval no S255; Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katona BW and Weiss JM: Chemoprevention of
colorectal cancer. Gastroenterology. 158:368–388. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jahanafrooz Z, Mosafer J, Akbari M,
Hashemzaei M, Mokhtarzadeh A and Baradaran B: Colon cancer therapy
by focusing on colon cancer stem cells and their tumor
microenvironment. J Cell Physiol. 235:4153–4166. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agnoletto C, Corrà F, Minotti L,
Baldassari F, Crudele F, Cook WJJ, Di Leva G, d'Adamo AP, Gasparini
P and Volinia S: Heterogeneity in circulating tumor cells: The
relevance of the stem-cell subset. Cancers (Basel). 11:4832019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dwivedi AR, Thakur A and Kumar V,
Skvortsova I and Kumar V: Targeting cancer stem cells pathways for
the effective treatment of cancer. Curr Drug Targets. 21:258–278.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyoshi N, Mizushima T, Doki Y and Mori M:
Cancer stem cells in relation to treatment. JPN J Clin Oncol.
49:232–237. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Najafi M, Mortezaee K and Ahadi R: Cancer
stem cell (a)symmetry & plasticity: Tumorigenesis and therapy
relevance. Life Sci. 231:1165202019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Najafi M, Farhood B and Mortezaee K:
Cancer stem cells (CSCs) in cancer progression and therapy. J Cell
Physiol. 234:8381–8395. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cammareri P, Lombardo Y, Francipane MG,
Bonventre S, Todaro M and Stassi G: Isolation and culture of colon
cancer stem cells. Methods Cell Biol. 86:311–324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mather JP: In vitro models. Stem Cells.
30:95–99. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu YT, Wang CY, Pang SY, Lei CY, Luo Y
and Tan WL: A modified method by differential adhesion and
serum-free culture medium for enrichment of cancer stem cells. J
Cancer Res Ther. 14 (Suppl):S421–S426. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rai N, Singh AK, Singh SK, Gaurishankar B,
Kamble SC, Mishra P, Kotiya D, Barik S, Atri N and Gautam V: Recent
technological advancements in stem cell research for targeted
therapeutics. Drug Deliv Transl Res. 10:1147–1169. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li W, Li Y, Cui Y, Li S, Zhu Y, Shang C,
Song G, Liu Z, Xiu Z, Cong J, et al: Anti-tumour effects of a dual
cancer-specific oncolytic adenovirus on breast cancer stem cells. J
Cell Mol Med. 25:666–676. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ying C, Xiao B, Qin Y, Wang BR, Liu XY,
Wang RW, Fang L, Yan H, Zhou XM and Wang YG: GOLPH2-regulated
oncolytic adenovirus, GD55, exerts strong killing effect on human
prostate cancer stem-like cells in vitro and in vivo. Acta
Pharmacol Sin. 39:405–414. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laranjo M, Carvalho MJ, Serambeque B,
Alves A, Marto CM, Silva I, Paiva A and Botelho MF: Obtaining
cancer stem cell spheres from gynecological and breast cancer
tumors. J Vis Exp. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian C, Lang T, Qiu J, Han K, Zhou L, Min
D, Zhang Z and Qi D: SKP1 promotes YAP-mediated colorectal cancer
stemness via suppressing RASSF1. Cancer Cell Int. 20:5792020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Farace C, Pisano A, Griñan-Lison C,
Solinas G, Jiménez G, Serra M, Carrillo E, Scognamillo F, Attene F,
Montella A, et al: Deregulation of cancer-stem-cell-associated
miRNAs in tissues and sera of colorectal cancer patients.
Oncotarget. 11:116–130. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Wang MH, Zhu JY, Xie CF, Li XT, Wu
JS, Geng SS, Han HY and Zhong CY: TAp63α targeting of Lgr5 mediates
colorectal cancer stem cell properties and sulforaphane inhibition.
Oncogenesis. 9:892020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Wang X, Zhang Q, Zhu JY, Li Y, Xie
CF, Li XT, Wu JS, Geng SS, Zhong CY and Han HY:
(−)-Epigallocatechin-3-gallate inhibits colorectal cancer stem
cells by suppressing Wnt/β-catenin pathway. Nutrients. 9:5722017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wen Y, Xiong X, Zaytseva YY, Napier DL,
Vallee E, Li AT, Wang C, Weiss HL, Evers BM and Gao T:
Downregulation of SREBP inhibits tumor growth and initiation by
altering cellular metabolism in colon cancer. Cell Death Dis.
9:2652018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho YH, Ro EJ, Yoon JS, Mizutani T, Kang
DW, Park JC, Il Kim T, Clevers H and Choi KY: 5-FU promotes
stemness of colorectal cancer via p53-mediated WNT/β-catenin
pathway activation. Nat Commun. 11:53212020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo H, Zhang B, Nairn AV, Nagy T, Moremen
KW, Buckhaults P and Pierce M: O-Linked N-acetylglucosamine
(O-GlcNAc) expression levels epigenetically regulate colon cancer
tumorigenesis by affecting the cancer stem cell compartment via
modulating expression of transcriptional factor MYBL1. J Biol Chem.
292:4123–4137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JH, Kim YH, Shim S, Kim A, Jang H,
Lee SJ, Park S, Seo S, Jang WI, Lee SB and Kim MJ:
Radiation-activated PI3K/AKT pathway promotes the induction of
cancer stem-like cells via the upregulation of SOX2 in colorectal
cancer. Cells. 10:1352021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo P, Wang J, Gao W, Liu X, Wu S, Wan B,
Xu L and Li Y: Salvianolic acid B reverses multidrug resistance in
nude mice bearing human colon cancer stem cells. Mol Med Rep.
18:1323–1334. 2018.PubMed/NCBI
|
|
25
|
Wang L, Huang X, Zheng X, Wang X, Li S,
Zhang L, Yang Z and Xia Z: Enrichment of prostate cancer stem-like
cells from human prostate cancer cell lines by culture in
serum-free medium and chemoradiotherapy. Int J Biol Sci. 9:472–479.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sanches JGP, Song B, Zhang Q, Cui X,
Yabasin IB, Ntim M, Li X, He J, Zhang Y, Mao J, et al: The role of
KDM2B and EZH2 in regulating the stemness in colorectal cancer
through the PI3K/AKT pathway. Front Oncol. 11:6372982021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mirzaei H, Salehi H, Sahebkar A, Avan A,
Jaafari MR, Namdar A, Rezaei A and Mirzaei HR: Deciphering
biological characteristics of tumorigenic subpopulations in human
colorectal cancer reveals cellular plasticity. J Res Med Sci.
21:642016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen M, Sharma A, Lin Y, Wu Y, He Q, Gu Y,
Xu ZP, Monteiro M and Gu W: Insluin and epithelial growth factor
(EGF) promote programmed death ligand 1(PD-L1) production and
transport in colon cancer stem cells. BMC Cancer. 19:1532019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu QQ, Li CM, Fu LN, Wang HL, Tan J, Wang
YQ, Sun DF, Gao QY, Chen YX and Fang JY: Enterotoxigenic
bacteroides fragilis induces the stemness in colorectal cancer via
upregulating histone demethylase JMJD2B. Gut Microbes.
12:17889002020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shiraiwa K, Matsuse M, Nakazawa Y, Ogi T,
Suzuki K, Saenko V, Xu S, Umezawa K, Yamashita S, Tsukamoto K and
Mitsutake N: JAK/STAT3 and NF-κB signaling pathways regulate cancer
stem-cell properties in anaplastic thyroid cancer cells. Thyroid.
29:674–682. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leng Z, Tao K, Xia Q, Tan J, Yue Z, Chen
J, Xi H, Li J and Zheng H: Krüppel-like factor 4 acts as an
oncogene in colon cancer stem cell-enriched spheroid cells. PLoS
One. 8:e560822013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Munro MJ, Wickremesekera SK, Peng L, Tan
ST and Itinteang T: Cancer stem cells in colorectal cancer: A
review. J Clin Pathol. 71:110–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kozovska Z, Gabrisova V and Kucerova L:
Colon cancer: cancer stem cells markers, drug resistance and
treatment. Biomed Pharmacother. 68:911–916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Otte J, Dizdar L, Behrens B, Goering W,
Knoefel WT, Wruck W, Stoecklein NH and Adjaye J: FGF signalling in
the self-renewal of colon cancer organoids. Sci Rep. 9:173652019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Castagnoli L, De Santis F, Volpari T,
Vernieri C, Tagliabue E, Di Nicola M and Pupa SM: Cancer stem
cells: Devil or savior-looking behind the scenes of immunotherapy
failure. Cells. 9:5552020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Annett S and Robson T: Targeting cancer
stem cells in the clinic: Current status and perspectives.
Pharmacol Ther. 187:13–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gupta R, Bhatt LK, Johnston TP and
Prabhavalkar KS: Colon cancer stem cells: Potential target for the
treatment of colorectal cancer. Cancer Biol Ther. 20:1068–1082.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Garza Treviño EN, González PD, Valencia
Salgado CI and Martinez Garza A: Effects of pericytes and colon
cancer stem cells in the tumor microenvironment. Cancer Cell Int.
19:1732019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fong CY, Peh GS, Gauthaman K and Bongso A:
Separation of SSEA-4 and TRA-1-60 labelled undifferentiated human
embryonic stem cells from a heterogeneous cell population using
magnetic-activated cell sorting (MACS) and fluorescence-activated
cell sorting (FACS). Stem Cell Rev Rep. 5:72–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsunekuni K, Konno M, Haraguchi N, Koseki
J, Asai A, Matsuoka K, Kobunai T, Takechi T, Doki Y, Mori M and
Ishii H: CD44/CD133-positive colorectal cancer stem cells are
sensitive to trifluridine exposure. Sci Rep. 9:148612019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Toledo-Guzmán ME, Hernández MI,
Gómez-Gallegos ÁA and Ortiz-Sánchez E: ALDH as a stem cell marker
in solid tumors. Curr Stem Cell Res Ther. 14:375–388. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fumagalli A, Oost KC, Kester L, Morgner J,
Bornes L, Bruens L, Spaargaren L, Azkanaz M, Schelfhorst T,
Beerling E, et al: Plasticity of Lgr5-negative cancer cells drives
metastasis in colorectal cancer. Cell Stem Cell. 26:569–578.e7.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Leng Z, Xia Q, Chen J, Li Y, Xu J, Zhao E,
Zheng H, Ai W and Dong J: Lgr5+CD44+EpCAM+ strictly defines cancer
stem cells in human colorectal cancer. Cell Physiol Biochem.
46:860–872. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Olejniczak A, Szaryńska M and Kmieć Z: In
vitro characterization of spheres derived from colorectal cancer
cell lines. Int J Oncol. 52:599–612. 2018.PubMed/NCBI
|
|
45
|
Olejniczak-Kęder A, Szaryńska M, Wrońska
A, Siedlecka-Kroplewska K and Kmieć Z: Effects of 5-FU and
anti-EGFR antibody in combination with ASA on the spherical culture
system of HCT116 and HT29 colorectal cancer cell lines. Int J
Oncol. 55:223–242. 2019.PubMed/NCBI
|
|
46
|
Prasetyanti PR and Medema JP: Intra-tumor
heterogeneity from a cancer stem cell perspective. Mol Cancer.
16:412017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kuşoğlu A and Biray Avcı Ç: Cancer stem
cells: A brief review of the current status. Gene. 681:80–85. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim B, Seo Y, Kwon JH, Shin Y, Kim S, Park
SJ, Park JJ, Cheon JH, Kim WH and Il Kim T: IL-6 and IL-8, secreted
by myofibroblasts in the tumor microenvironment, activate HES1 to
expand the cancer stem cell population in early colorectal tumor.
Mol Carcinog. 60:188–200. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lenos KJ, Miedema DM, Lodestijn SC, Nijman
LE, van den Bosch T, Romero Ros X, Lourenço FC, Lecca MC, van der
Heijden M, van Neerven SM, et al: Stem cell functionality is
microenvironmentally defined during tumour expansion and therapy
response in colon cancer. Nat Cell Biol. 20:1193–1202. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gur-Cohen S, Yang H, Baksh SC, Miao Y,
Levorse J, Kataru RP, Liu X, de la Cruz-Racelis J, Mehrara BJ and
Fuchs E: Stem cell-driven lymphatic remodeling coordinates tissue
regeneration. Science. 366:1218–1225. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
van der Heijden M and Vermeulen L: Stem
cells in homeostasis and cancer of the gut. Mol Cancer. 18:662019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ahmed N, Escalona R, Leung D, Chan E and
Kannourakis G: Tumour microenvironment and metabolic plasticity in
cancer and cancer stem cells: Perspectives on metabolic and immune
regulatory signatures in chemoresistant ovarian cancer stem cells.
Semin Cancer Biol. 53:265–281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang X, Bandyopadhyay S, Araujo LP, Tong
K, Flores J, Laubitz D, Zhao Y, Yap G, Wang J, Zou Q, et al:
Elevating EGFR-MAPK program by a nonconventional Cdc42 enhances
intestinal epithelial survival and regeneration. JCI Insight.
5:e1359232020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mossahebi-Mohammadi M, Quan M, Zhang JS
and Li X: FGF signaling pathway: A key regulator of stem cell
pluripotency. Front Cell Dev Biol. 8:792020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Clara JA, Monge C, Yang Y and Takebe N:
Targeting signalling pathways and the immune microenvironment of
cancer stem cells-a clinical update. Nat Rev Clin Oncol.
17:204–232. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kahn M: Wnt signaling in stem cells and
cancer stem cells: A tale of two coactivators. Prog Mol Biol Transl
Sci. 153:209–244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Najafi M, Mortezaee K and Majidpoor J:
Cancer stem cell (CSC) resistance drivers. Life Sci.
234:1167812019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Saltanatpour Z, Johari B, Alizadeh A,
Lotfinia M, Majidzadeh-A K, Nikbin B and Kadivar M: Enrichment of
cancer stem-like cells by the induction of epithelial-mesenchymal
transition using lentiviral vector carrying E-cadherin shRNA in
HT29 cell line. J Cell Physiol. 234:22935–22946. 2019. View Article : Google Scholar : PubMed/NCBI
|