Introduction
As a prevalent gynecological tumor, cervical cancer
(CC) endangers the health and lives of women due to its high
mortality rates and poor prognoses (1). In 2020, there were 604,127 new cases
of CC and 341,831 CC-associated deaths worldwide (2). Determining the pathogenesis is of
importance in the timely diagnosis and management of CC. Previous
evidence has shown that persistent high-risk human papillomavirus
(hrHPV) infection can induce the carcinogenesis of CC (3). Furthermore, the interactions between
oncogenes, and their association with hrHPV infections have been
widely analyzed (4). Nevertheless,
the exact molecular mechanisms involved in the progression and
metastasis of CC remain largely unclear. Therefore, it is
imperative to explore the pathogenesis and molecular mechanisms
underlying the development of CC in order to identify effective
diagnostic markers and therapeutic targets to improve its
prognosis.
HPV encodes E6 and E7 proteins, which target TP53
and retinoblastoma proteins, respectively, and thus, disrupts
cell-cycle progression, prevents apoptosis and enables the
potentially transformed cells to replicate (5). Cell cycle progression from
G1 to S-phase is closely regulated by cyclin-dependent
kinases (CDKs), such as CDK4/6 (6).
CDK4/6 can be controlled via their subunits, including cyclin D and
cyclin-dependent kinase inhibitors, which include the CDK
interacting protein/kinase inhibitory protein and inhibitors of
cyclin-dependent kinases (INK4) families (7). Cyclin dependent kinase inhibitor 2D
(CDKN2D; also known as p19Ink4d), a member of the INK4
family, functions as a key negative regulator of G1/S
transition (7). Aberrant CDKN2D
expression has been demonstrated to contribute to the
differentiation block and abnormal proliferation of malignant cells
in multiple types of cancer, including pancreatic cancer,
hepatocellular carcinoma and leukemia (8–10).
However, to the best of our knowledge, it is unclear whether CDKN2D
is involved in CC cell cycle progression and proliferation.
Long noncoding RNAs (lncRNAs) are non-coding RNAs
>200 nucleotides in length. A previous study has demonstrated
that lncRNAs are potentially involved in the development of
malignant tumors (11). In
particular, dysregulation of lncRNA expression can result in
abnormally activated genes and pathways that trigger malignant
tumor development (12,13). Additionally, lncRNAs are promising
prognostic or diagnostic markers (14,15).
The promoting effects of lncRNA SNHG1 and DLG1-AS1 on the malignant
cellular behaviors of CC have been validated in previous studies
(16,17).
The aim of the present study was to explore the
roles of LINC01012 in CC. Public databases were analyzed using
bioinformatic methods, and in vitro and in vivo
experiments were performed to reveal the potential mechanism of
LINC01012 in CC. The oncogenic role of LINC01012 might provide
novel therapeutic targets for patients with CC.
Materials and methods
Data extraction and sample
collection
The GSE75132 dataset (18), containing data for 20 female
patients with persistent HPV16-infection and progression to
cervical intraepithelial neoplasia grade 3 or CC (CIN3+), 10 female
patients with persistent HPV16-infection without progression, and
11 HPV-negative female patients with normal cervix, was obtained
from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi).
Differentially expressed genes between groups were screened by
analyzing the GSE75132 dataset with packages ‘limma’ (version
3.54.0; https://bioconductor.org/packages/release/bioc/html/limma.html)
(19) in R (version 4.0.4 for
Windows 10; http://mran.microsoft.com/snapshot/2021-03-21/bin/windows/base/).
In addition, data on LINC01012 expression and the clinical data of
304 patients with CC were obtained from The Cancer Genome Atlas
(TCGA)-cervical squamous cell carcinoma and endocervical
adenocarcinoma (CESC; http://portal.gdc.cancer.gov/). A total of 304
patients were stratified into low- or high-expression groups
according to the auto-selected best cut-off value of LINC0102 mRNA
level calculated using X-tile software (version 3.6.1; http://x-tile.software.informer.com/)
(20). Afterwards, Kaplan-Meier
(KM) analysis for overall survival was performed and the log-rank
test was used to compare the prognosis between high- and
low-expression of LINC0102 mRNA groups using R packages ‘survival’
(version 3.5.0; http://CRAN.R-project.org/package=survival) (21) and ‘survminer’ (version 0.4.8;
http://CRAN.R-project.org/package=survminer) (22). Cervical cytology samples were
obtained from 50 female patients with CC (median age, 57 years; age
range, 39–65 years), 50 female patients with cervical
intra-epithelial neoplasia grade 3 (CIN 3; (median age, 53 years;
age range, 49–59 years) and 50 female patients with uterine myoma
but normal cervix aged (median, 55 years; age range, 18–57 years)
undergoing surgery at The First Affiliated Hospital of Nanjing
Medical University (Nanjing, China) between January 2019 and
January 2021. The cytology samples were used to examine LINC01012
expression by RT-qPCR. The inclusion criteria were: Histologically
confirmed aforementioned diagnosis. Patients were excluded if they
had other co-existing tumors or if they received previous
chemotherapy or radiation.
The study was conducted in accordance with the
Declaration of Helsinki (as revised in 2013) and approved by the
Ethics Committee of First Affiliated Hospital of Nanjing Medical
University (approval no. 2019-SR-373; Nanjing, China). All
participants provided written informed consent. The animal
experiments were performed under a project license (approval no.
F201844) granted by the Project of Maternal and Child Health of
Jiangsu, in compliance with national and international guidelines,
and approved by the Animal Care and Use Institutional Review Board
of Nanjing Medical University (Nanjing, China). Additionally, the
experiments were performed in accordance with the Animal Research:
Reporting of In Vivo Experiments (ARRIVE) reporting
checklist (23).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from human CC cell lines
(HeLa and SiHa) and cervical cytology samples using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Next, cDNA was synthesized by reverse transcription using a
PrimeScript™ II 1st Strand cDNA synthesis kit (Takara Bio, Inc.)
according to the manufacturer's protocol. RT-qPCR was then
performed using SYBR Premix Ex Taq (Takara Bio, Inc.) according to
the manufacturer's protocol. The thermocycling conditions were as
follows: denaturation at 95°C for 10 min, followed by 45 cycles at
95°C for 30 sec and 68°C for 30 sec. U6 was used as the
housekeeping gene. The relative mRNA level was determined using the
comparative cycle threshold 2−ΔΔCq method (24). The sequences of the primers were:
LINC01012 forward, 5′-AAAAATCGGGAGGTGACGGG-3′ and reverse,
5′-GCTTCCCGATGCACCTTTTC-3′; CDKN2D forward,
5′-AGTCCAGTCCATGACGCAG-3′ and reverse, 5′-ATCAGGCACGTTGACATCAGC-3′;
and U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′.
Cell culture and transfection
Human CC cell lines (HeLa and SiHa) obtained from
American Type Culture Collection were cultivated in DMEM (HyClone;
Cytiva) containing 10% FBS (HyClone; Cytiva), 100 U/ml penicillin
and 100 µg/ml streptomycin (HyClone; Cytiva) in a humidified cell
culture chamber with 5% CO2 at 37°C. The cells were
passaged when cells reached 80% confluences and the medium was
replaced every 2–3 days.
Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) and pGPU6/GFP/Neo vector (Shanghai
GenePharma Co. Ltd.) were used to transfect sh-LINC01012, sh-CDKN2D
and non-targeting short hairpin RNA (sh-NC; all Shanghai GenePharma
Co., Ltd.). The interval between infection and subsequent
experimentation was 48 h. Briefly, cells were seeded in a 6-well
plate at a density of 2×105 cells per well, and the cell
culture medium was replaced with antibiotic-free medium at 12 h
prior to transfection. To transfect the cells, 2.5 µg transfection
plasmid and 4 µl Lipofectamine® 2000 were separately
dissolved in 200 µl OPTI-MEM for 5 min at room temperature, gently
mixed, placed at room temperature for 20 min and added into the
cell-containing medium. After 6 h of transfection at 37°C, the
medium was replaced with fresh medium. Subsequently, the cells were
cultivated for 48 h at 37°C and collected for in vitro
experiments. The sequences of the transfection vectors were as
follows: sh-LINC01012, 5′-GAACAATGTCAGATTGCAA-3′; sh-CDKN2D,
5′-GGTTATGTATCAGAAGAGA-3′; and sh-NC,
5′-UUCUCCGAACGUGUCACGUTT-3′.
5-ethynyl-2′-deoxyuridine (EdU) assay
and colony formation analysis
For the EdU assay, cells were seeded in a 96-well
plate at a density of 3×103 cells per well and cultured
overnight at 37°C. Subsequently, the cells were stained with 50
µmol/l EdU solution (Guangzhou RiboBio Co., Ltd.) for 2 h at 37°C,
fixed in 4% paraformaldehyde for 30 min and incubated with 2 mg/ml
glycine for 5 min (both at room temperature). After washing once
for 20 min in 0.5% Triton X-100 at room temperature, cells were
labeled with 1X Apollo solution at room temperature for 30 min.
Following washing three times for 10 min with PBS and 4% methanol
at room temperature, nuclei were labeled with Hoechst 33342
solution at room temperature for 30 min in the dark. Images of
EdU-stained cells (red), Hoechst33342-stained nuclei (blue) and
merged images were captured under a fluorescence microscope at a
magnification of ×100 and analyzed using ImageJ software
(ij153-win-java8; National Institutes of Health). The EDU-positive
cell ratio was calculated using the following formula: Mean EDU
positive cell numbers in each group/mean EDU positive cell numbers
in sh-NC group.
For colony formation analysis, the HeLa and SiHa
cells transfected with sh-LINC01012 or sh-NC for 48 h at 37°C were
seeded in a 6-well plate at a density of 1×103 cells per
well and cultivated for 14 days. When colonies reached a size of
>50 cells, the cells were washed in PBS, fixed in 4%
paraformaldehyde for 15 min at room temperature and dyed for 20 min
in 0.1% crystal violet at room temperature. Images of colonies
(definition, diameter of 0.3-1.0 mm) were captured under a light
microscope for counting using ImageJ software (ij153-win-java8;
National Institutes of Health). The relative number of colonies was
calculated using the following formula: Mean colony number in each
group/mean colony number in sh-NC group.
Transwell migration assay
Transwell inserts (pore size, 8 µm; Corning, Inc.)
without Matrigel-coating were used for the Transwell migration
assay. The HeLa and SiHa cells (4×104 cells/well)
transfected with sh-LINC01012, or sh-LINC01012 and sh-CDKN2D, or
sh-NC were seeded in each insert with FBS-free DMEM. The inserts
were placed in a 24-well plate with 500 µl DMEM supplemented with
10% FBS as an attractant in each lower chamber. After culture for
24 h at 37°C, the cells that migrated to the lower surface of the
membrane were fixed in 4% paraformaldehyde for 15 min and dyed in
0.1% crystal violet solution (Beyotime Institute of Biotechnology)
for 20 min (both at room temperature). After PBS washing and air
drying, images of migratory cells were captured under a light
microscope. The numbers of migrated cells in five random fields per
sample were counted. The relative migrated cell ratio was
calculated using the following formula: Mean migrated cell numbers
in each group/mean migrated cell numbers in sh-NC group.
In vivo experiments
Eight female BALB/c nude mice (aged 5 weeks and
weighed 16 g) were provided by Shanghai SLAC Laboratory Animal Co.,
Ltd. The animal experiments were approved by the Animal Care and
Use Institutional Review Board of Nanjing Medical University
(Nanjing, China) and carried out following the ARRIVE checklist.
The mice were housed in a pathogen-free animal facility with a 12-h
light-dark cycle at a temperature of 20–24°C and a relative
humidity of 50% and randomly assigned to the control group (sh-NC
group) or sh-LINC01012 group (4 mice per group). The mice had free
access to water and food. Animal health and behavior were monitored
daily. Animal welfare was taken into consideration, including
efforts to minimize suffering and distress, and use of analgesics
according to the National Institutes of Health Guidelines for the
Care and Use of Laboratory Animals (25). SiHa cells stably transfected with
sh-NC or sh-LINC01012 were harvested and resuspended in PBS at
2×107 cells/ml. Cell suspension (100 µl;
2×106 cells in total) was injected subcutaneously into
the left axilla of the mice. Tumor size was measured every 3 days
and tumor volumes were calculated using the following formula: 0.5
× length × width2. The mice were euthanized after
anesthesia at 3 weeks after injection and the maximum diameter of
tumor was measured. All mice were administered vaporized isoflurane
(inhaled) for anesthesia at a concentration of 2.0% for induction
and 1.0% for maintenance. Afterwards, the mice were sacrificed by
rapid cervical dislocation. The mice with relaxed muscles were
judged as dead when no breathing and no nerve reflex were observed.
The xenograft tumors were harvested for further study including
H&E and immunohistochemistry (IHC) staining.
H&E and IHC analysis
The xenograft tumors were fixed in 4% formaldehyde
solution for 48 h at room temperature, then washed in distilled
water for 45 sec, dehydrated with gradient ethanol (50, 70, 80, 95
and 100% respectively; 5 min each) at room temperature, embedded in
paraffin at room temperature and cut into 4-µm-thick sections. The
sections were deparaffinized in xylene for 5 min at room
temperature, gradually hydrated using a descending alcohol series
(100% for 3 min; 95, 70 and 50% for 3 min each) at room temperature
and then washed in distilled water at room temperature. Afterwards,
some of the sections for each sample were used for H&E
staining, the others were for the IHC assay. For H&E staining,
the slices were stained with hematoxylin for 5 min and with eosin
for 3 min (both at room temperature). The pathological alterations
of the tumors were observed under a light microscope.
For the IHC assay, the sections were incubated in 3%
H2O2 solution in methanol at room temperature
for 10 min to block endogenous peroxidase activity and washed with
PBS for 5 min. Antigen retrieval was performed by incubation in 300
ml 10 mM citrate buffer with pH 6.0 and heating to 93°C for 10 min.
The slides were cooled naturally at room temperature for 20 min and
rinsed with PBS for 5 min. Antigen blocking was conducted by adding
100 µl FBS (10%) onto the slides and incubation at room temperature
for 1 h. The slides were rinsed with PBS for 2 min. IHC analysis
was carried out using KI-67 (1:800; sc-23900; Santa Cruz
Biotechnology, Inc.) and E-cadherin (1:50; sc-8426; Santa Cruz
Biotechnology, Inc.) primary antibodies to measure cellular
proliferation and adhesion. Briefly, the sections were subsequently
incubated with diluted primary antibodies overnight at 4°C and then
washed with PBS. Afterwards, the slices were washed with PBS for 5
min and treated with peroxidase-conjugated secondary antibodies
(1:1,000; PV-9000; OriGene Technologies, Inc.) for 2 h at 37°C and
washed three times with PBS, 5 min each. After treatment with
3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich; Merck
KGaA) solution at room temperature for 30 min, the sections were
washed for 5 min and counter-stained with hematoxylin for 1 min at
room temperature. Finally, the slides were dehydrated four times
using alcohol (95, 95, 100 and 100%; 5 min each), soaked three
times in xylene solution and sealed with a coverslip. Samples were
observed and images were captured under a light microscope
(magnification, ×100; Olympus Corporation) and analyzed with ImageJ
software (ij153-win-java8; National Institutes of Health).
Western blotting
Total protein was extracted from cells with RIPA
lysis buffer (Beyotime Institute of Biotechnology) on ice. After
measurement of the protein concentration using the BCA method,
equal amounts of protein samples (20 µg/lane) were separated by 10%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes
(Millipore Sigma). The membranes were blocked in 5% skimmed milk
for 1 h at room temperature, and immunoblotted with primary
antibodies, including anti-CDKN2D (1:1,000; 10272-2-AP; Proteintech
Group, Inc.) and anti-GAPDH (1:1,000; 10494-1-AP; Proteintech
Group, Inc.) antibodies, at 4°C overnight, and HRP-conjugated
secondary antibody (1:1,000, cat. no. sc271984; Santa Cruz
Biotechnology, Inc.) at room temperature for 1 h. Protein detection
was achieved using an enhanced chemiluminescence detection kit
(Millipore Sigma) and analyzed with ImageJ software
(ij153-win-java8; National Institutes of Health).
Statistical analysis
The differentially expressed genes (|log2 fold
change|>1 and false discovery rate <0.05) were identified
using packages ‘limma’ (version 3.54.0; http://bioconductor.org/packages/release/bioc/html/limma.html)
(19) in R (version 4.0.4 for
Windows 10; http://mran.microsoft.com/snapshot/2021-03-21/bin/windows/base/).
Pearson's correlation analysis was performed on LINC01012 and
CDKN2D expression levels in TCGA-CESC dataset using R software.
Kaplan-Meier (KM) survival analysis and the log-rank test were
conducted with R packages to assess the prognostic potential of
LINC01012 in CC using a RNA-Seq and clinical dataset (TCGA-CESC).
The R packages ‘survival’ (version 3.5.0; http://CRAN.R-project.org/package=survival)
(21) and ‘survminer’ (version
0.4.8; http://CRAN.R-project.org/package=survminer) (22) were employed for KM analysis.
All experiments were independently carried out at
least three times, with samples in triplicate. SPSS 24.0 software
(IBM Corp.) and GraphPad Prism 5.0 software (GraphPad Software;
Dotmatics) were used for statistical analyses. Data are presented
as the mean ± SD. Differences between groups were analyzed using
unpaired Student's t-test or a Mann-Whitney U test. Comparisons
among multiple groups were performed by one-way ANOVA followed by
Scheffe's or Dunnett's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Upregulation of LINC01012 in CC and
its prognostic significance
Through bioinformatics analysis of the GSE75132
dataset from the GEO database, differentially expressed LINC01012
was identified to be upregulated in persistent HPV16-infection and
progression to CIN3+ samples compared with persistent
HPV16-infection without progression and HPV-negative samples
(Fig. 1A), and was also
demonstrated to be upregulated in cervical cytology samples of CC
and CIN 3 compared with the normal samples (Fig. 1B). A total of 304 patients with CC
from TCGA were assigned into low- or high-expression groups
according to the best cutoff value (−0.58) of LINC01012 mRNA level
calculated using X-tile software. The association between LINC01012
expression and clinicopathologic characteristics is shown in
Table SI, which showed that high
LINC01012 expression in patients with CC was positively associated
with advanced pathologic TNM stage. Furthermore, KM survival
analysis using TCGA-CESC dataset revealed that high LINC01012
expression was associated with an unfavorable prognosis of CC
(P=0.01; log-rank test; Fig. 1C).
Therefore, we hypothesized that LINC01012 might participate in the
progression of CC and a series of experiments were designed to
investigate this hypothesis.
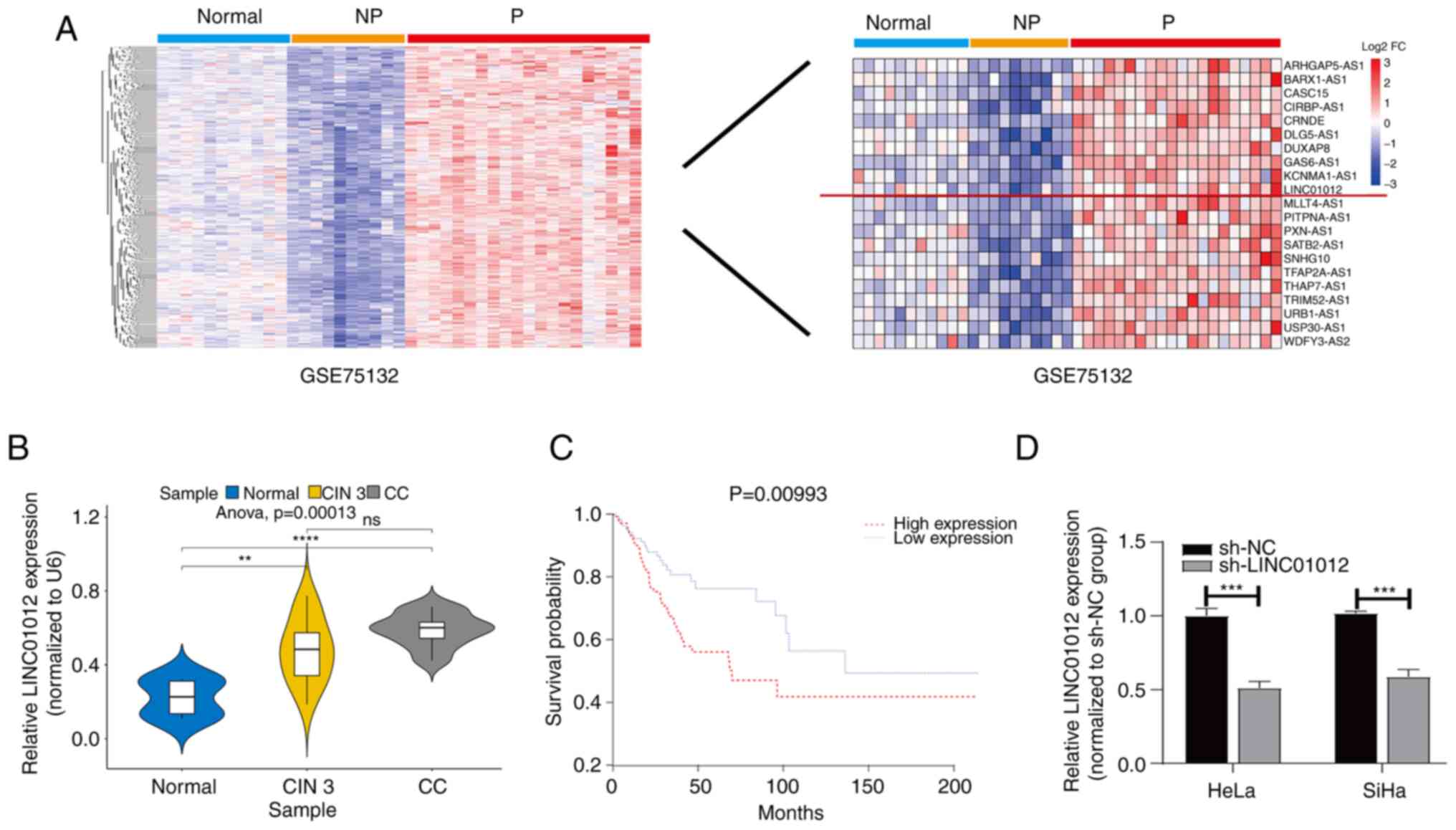 | Figure 1.LINC01012 expression is upregulated
in CC and its high expression is associated with an unfavorable
prognosis. (A) Differentially expressed genes were identified using
the ‘limma’ R package (based on |log2 fold change|>1 and a false
discovery rate <0.05 threshold) in the GSE75132 dataset, and
LINC01012 was identified to be upregulated in 20 samples from
female patients with persistent HPV16-infection and progression to
CIN 3+ compared with in samples from 10 female patients with
persistent HPV16-infection without progression and 11 HPV-negative
female patients with normal cervix (normal group). (B) Comparison
of LINC01012 levels in cervical cytology samples from patients at
The First Affiliated Hospital of Nanjing Medical University
(Nanjing, China) with CC and CIN 3 with those with uterine myoma
but normal cervix (normal group) (n=50 per group). Comparisons
among multiple groups were performed by one-way ANOVA followed by
Scheffe's post hoc test. **P<0.001, ***P<0.001 vs. normal
group. (C) Kaplan-Meier survival analysis and log-rank test of
LINC01012 expression in CC using data from The Cancer Genome Atlas.
(D) LINC01012 levels in HeLa and SiHa cells transfected with sh-NC
or sh-LINC01012. Differences between groups were analyzed using
unpaired Student's t-test. ***P<0.001 vs. sh-NC group. CC,
cervical cancer; CIN 3+, cervical intraepithelial neoplasia grade 3
or cervical cancer; CIN 3, cervical intra-epithelial neoplasia
grade 3; NP, persistent HPV16-infection without progression; ns,
not significant; P, persistent HPV16-infection and progression to
CIN 3+; log2 FC, log2 fold change; sh-NC, negative control short
hairpin RNA; sh-LINC01012, LINC01012 short hairpin RNA. |
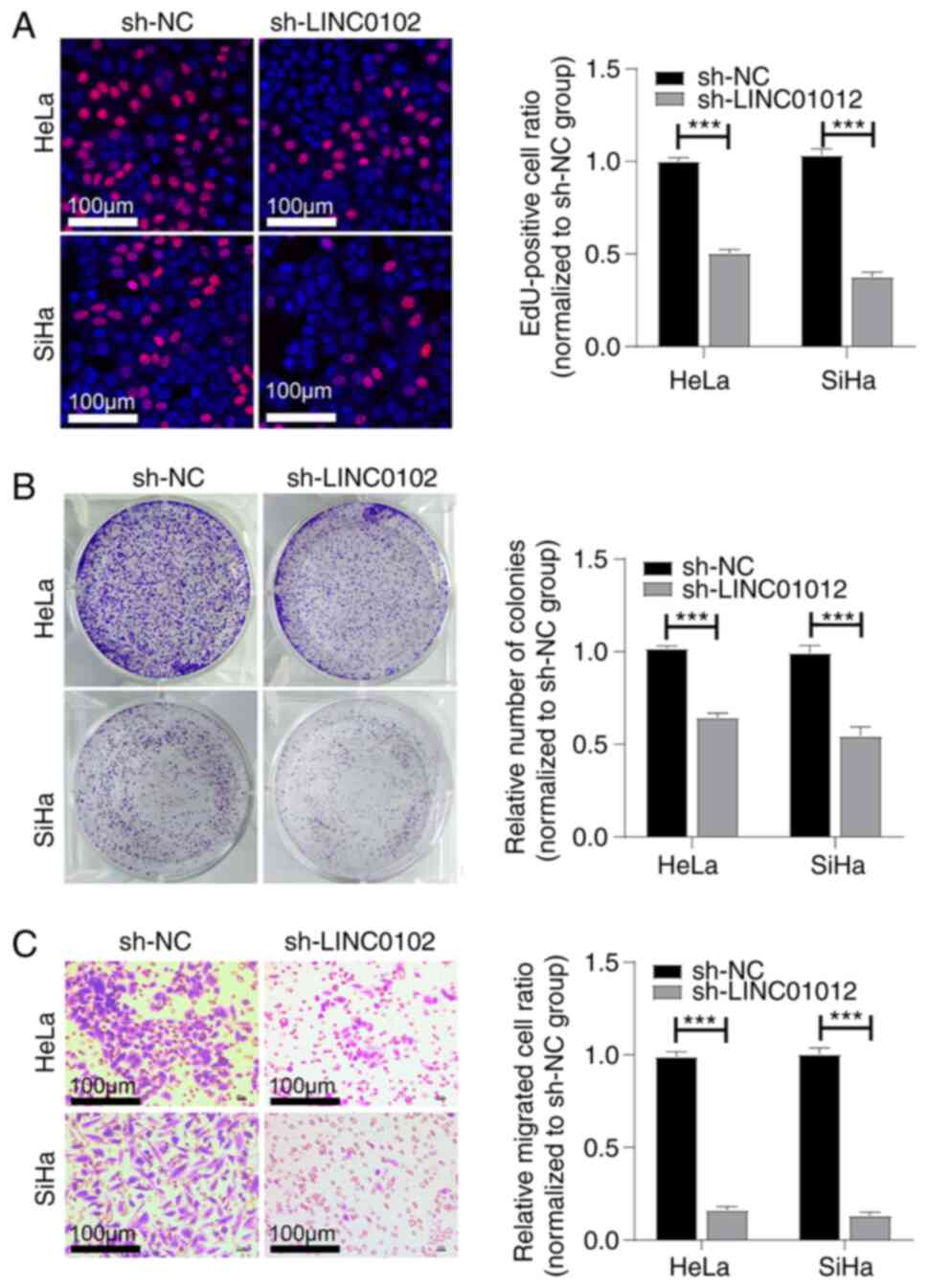
LINC01012 knockdown suppresses
proliferation and migration of CC cells in vitro
To explore the potential functions of LINC01012 in
CC cells, LINC01012 was initially silenced by transfection of
sh-LINC01012 in HeLa and SiHa cells and the transfection efficacy
was validated by RT-qPCR (Fig. 1D).
A series of functional experiments were then conducted to assess
the biological functions of LINC01012 in CC cells. Transfection of
sh-LINC01012 significantly reduced the EdU-positive cell ratio
(Fig. 2A) and colony ratio both
normalized to sh-NC group in CC cells (Fig. 2B), which indicated that LINC01012
knockdown suppressed cell proliferation and colony formation. In
addition, the migration of CC cells was also inhibited following
LINC01012 knockdown (Fig. 2C), as
indicated by a Transwell migration assay.
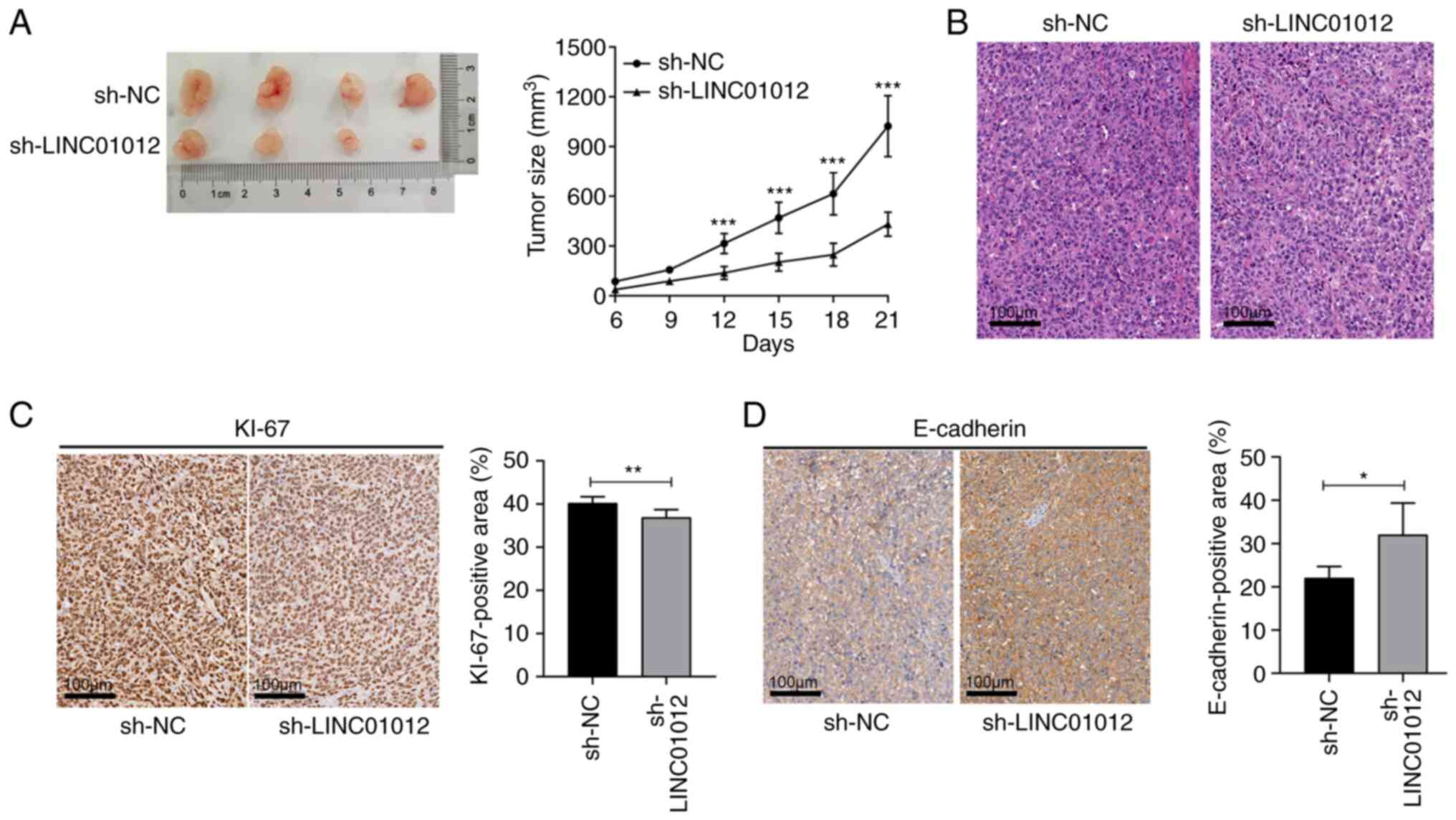
LINC01012 regulates tumor growth of CC
in vivo
A subcutaneous xenograft model was constructed to
validate the biological function of LINC01012 in vivo.
Consistent with the in vitro results, silencing LINC01012 by
transfection with sh-LINC01012 significantly slowed down tumor
growth as shown by comparing tumor volume at 3 weeks after
injection in the sh-LINC01012 group with that in the sh-NC group
(0.94±0.17 vs. 0.41±0.07 cm3, respectively; Fig. 3A). Fig.
3B shows the pathological features of the xenograft tumor
examined using H&E staining under a light microscope.
Furthermore, IHC assays confirmed that silencing LINC01012
decreased KI67 expression and increased E-cadherin expression
(Fig. 3C and D), indicating that
LINC01012 knockdown inhibited the proliferation and promoted the
adhesion of CC in vivo. Taken together, the aforementioned
data demonstrated that LINC01012 could facilitate tumor growth by
enhancing the proliferation and migration of CC cells, which
further validated the oncogenic roles of LINC01012 in CC.
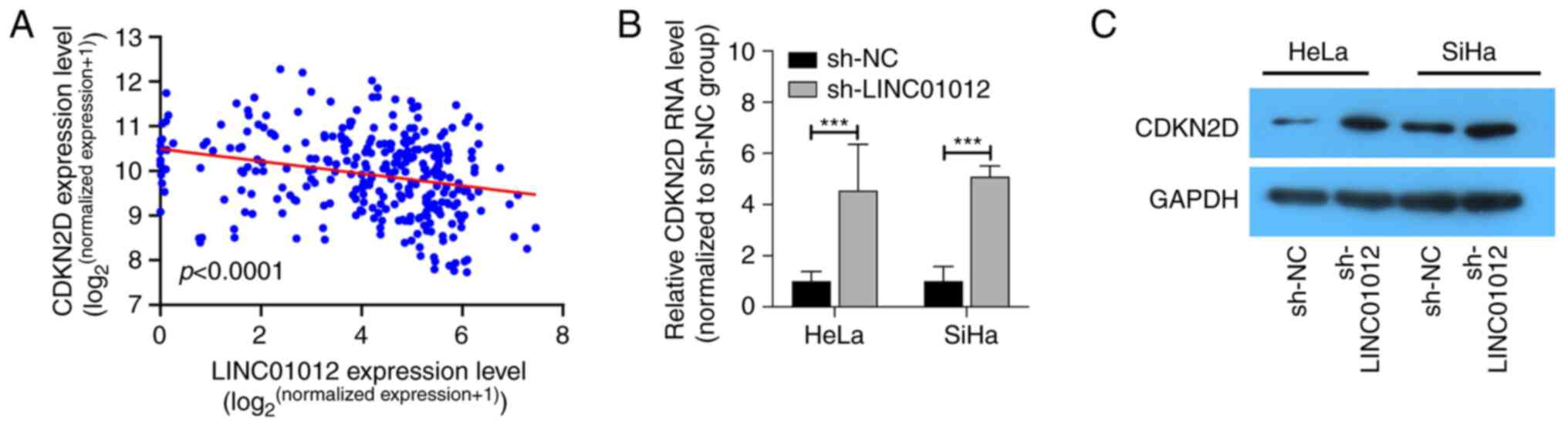
LINC01012 negatively regulates CDKN2D
expression
By analyzing potential target genes regulated by
LINC01012 in TCGA, CDKN2D was identified to be negatively weakly
correlated with LINC01012 (R=−0.19; P<0.001; Fig. 4A). Furthermore, the RNA and protein
levels of CDKN2D were found to be upregulated in CC cells
transfected with sh-LINC01012 (Fig. 4B
and C), suggesting that LINC01012 could negatively regulate
CDKN2D.
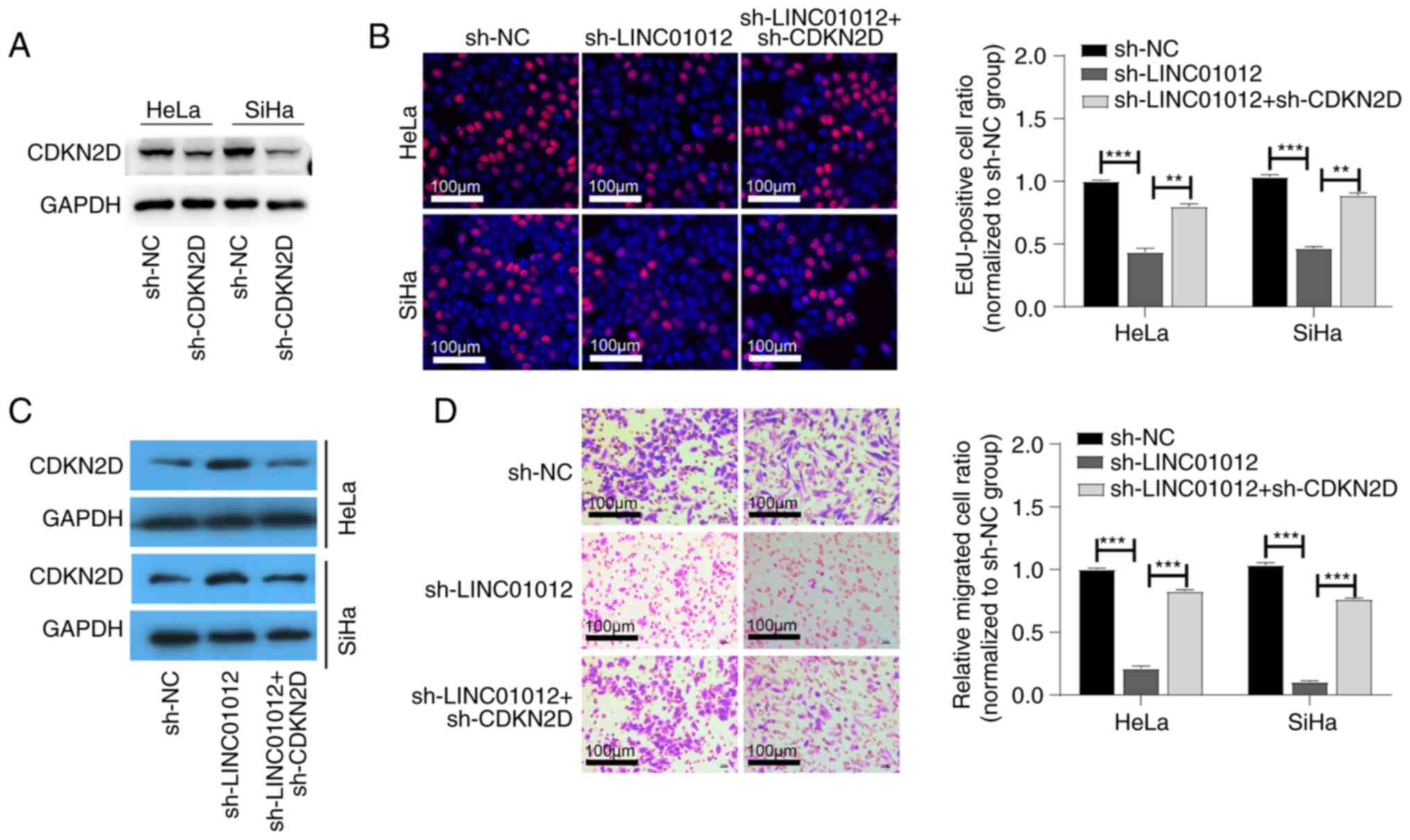
LINC01012 promotes proliferation and
migration of CC cells by negatively regulating CDKN2D
sh-CDKN2D was used to further investigate whether
CDKN2D serves a role in the effect of LINC0102 on CC development.
The transfection efficiency is shown in Fig. S1. The protein levels of CDKN2D were
also found to be downregulated in CC cells transfected with
sh-CDKN2D (Fig. 5A). The protein
expression levels of CDKN2D in HeLa and SiHa cells were upregulated
after sh-LINC0102 transfection, which was partially reversed by
co-transfection of sh-CDKN2D and sh-LINC01012 (Fig. 5C). The EdU-positive cell ratio was
higher in CC cells with co-transfection of sh-LINC01012 and
sh-CDKN2D compared with in those only with LINC01012 knockdown
(Fig. 5B). The inhibited migration
of CC cells following sh-LINC01012 transfection was consistently
reversed by the knockdown of both LINC01012 and CDKN2D (Fig. 5D). These findings indicated that
LINC01012 accelerated proliferation and migration of CC cells by
downregulating CDKN2D.
Discussion
CC ranked fourth in terms of incidence and mortality
among female malignancies worldwide in 2020 (2), and its pathogenesis remains
incompletely understood. Although therapeutic strategies for CC,
such as surgery, radiotherapy and chemotherapy, are continuously
being improved, the 5-year survival rate of patients with advanced
CC remains <50% worldwide in 2020 (2,26).
Therefore, there is an urgent need to further explore the
pathogenesis and identify novel target genes for the clinical
treatment of CC.
lncRNAs are non-coding RNAs of >200 nucleotides
that do not encode proteins (27).
With the rapid progression in high-throughput sequencing techniques
and bioinformatics analysis, a growing number of lncRNAs have
emerged as functional regulators involved in various types of human
cancer (28–30). lncRNAs participate in cancer
development by promoting cell proliferation, migration and invasion
(31), and have been revealed to
exert pivotal roles by regulating target gene expression through
cis- or trans-regulation (32).
To the best of our knowledge, the present study was
the first to reveal that LINC01012 expression was upregulated in CC
cytology samples. Survival analysis based on data obtained from
TCGA revealed that high LINC01012 expression was associated with
unfavorable prognosis of CC. In vitro experiments
demonstrated that LINC01012 knockdown in CC cells significantly
suppressed cell proliferation and migration. Consistent with the
in vitro results, LINC01012 was found to promote tumor
growth by increasing proliferation and migration of CC cells in a
subcutaneous xenograft model.
Further analysis of TCGA data demonstrated that
CDKN2D expression was negatively correlated with LINC01012 in CC
specimens. Therefore, it was hypothesized that CDKN2D was involved
in LINC01012-mediated oncogenesis. CDKN2D, located on chromosome
19p13, is a negative regulator of cell cycle progression (7). Its encoded protein is a member of the
INK4 family and can form a stable complex with CDK4 or CDK6, thus
preventing the activation of CDKs and regulating G1
phase progression (7). As a
tumor-suppressor gene, CDKN2D is closely linked to tumor
development by negatively regulating cell cycle progression
(33–35). The in vitro assays performed
in the present study demonstrated that the protein levels of CDKN2D
were upregulated following LINC01012 knockdown in CC cells.
Furthermore, the inhibited proliferation and migration of CC cells
after LINC01012 knockdown were partially reversed by
co-transfection with sh-LINC01012 and sh-CDKN2D. These findings
indicated that LINC01012 accelerated the proliferation and
migration of CC cells by downregulating CDKN2D expression. Taken
together, the present findings demonstrated that LINC01012 could
facilitate CC progression by targeting CDKN2D. The underlying
mechanisms of LINC01012 regulating CDKN2D have not been
investigated in the present study. Multiple signaling pathways have
been revealed to be associated with tumorigenesis and progression
of breast cancer, bladder cancer and CC. Among the pathways, the
PI3K/Akt/mTOR signaling pathway has been demonstrated to be one of
the most frequently activated signaling pathways (36). The specific signaling pathway
downstream of LINC01012 and CDKN2D will be further explored in
future studies. HeLa and SiHa cell lines were employed to explore
the function and potential mechanism of LINC01012 in vitro.
Nevertheless, other cells lines, including C33A, CaSki, HT-3 and
human cervical epithelial cells, should be studied to further
verify the conclusions.
In summary, to the best of our knowledge, the
present study was the first to demonstrate that LINC01012 was
upregulated in cervical cytology samples of CC and CIN 3 compared
with in normal cervical tissues, and it was negatively associated
with the overall survival of patients with CC. Upregulated
LINC01012 expression in CC may stimulate the proliferation and
migration of cancer cells both in vitro and in vivo,
thus promoting the progression of CC by downregulating CDKN2D. The
results of the present study indicated that LINC01012 may serve as
a novel target for the management of CC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Ms. Zhiwei Zhang
(Department of Medical English, Nanjing Medical University,
Nanjing, China) for their language editing assistance.
Funding
The present study was supported by Project of Maternal and Child
Health of Jiangsu, P.R. China (grant no. F201844).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL conceived and designed the study. KZ, XN and XM
performed the experiments, collected the data, and drafted and
reviewed the manuscript. RS and JQ contributed to the analysis and
interpretation of the data. All authors reviewed the final
manuscript and agreed to be accountable for all aspects of the work
in ensuring that questions related to the accuracy or integrity of
any part of the work are appropriately investigated and resolved.
CL, KZ, XN and XM confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki (as revised in 2013). The study was
approved by the Ethics Committee of First Affiliated Hospital of
Nanjing Medical University (approval no. 2019-SR-373; Nanjing,
China) and written informed consent was obtained from all
individual participants. The animal experiments were performed
under a project license (approval no. F201844) granted by the
Project of Maternal and Child Health of Jiangsu, in compliance with
national and international guidelines, and approved by the Animal
Care and Use Institutional Review Board of Nanjing Medical
University (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crosbie EJ, Einstein MH, Franceschi S and
Kitchener HC: Human papillomavirus and cervical cancer. Lancet.
382:889–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoppe-Seyler K, Bossler F, Braun JA,
Herrmann AL and Hoppe-Seyler F: The HPV E6/E7 oncogenes: Key
factors for viral carcinogenesis and therapeutic targets. Trends
Microbiol. 26:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bertoli C, Skotheim JM and de Bruin RA:
Control of cell cycle transcription during G1 and S phases. Nat Rev
Mol Cell Biol. 14:518–528. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suryadinata R, Sadowski M and Sarcevic B:
Control of cell cycle progression by phosphorylation of
cyclin-dependent kinase (CDK) substrates. Biosci Rep. 20:243–255.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu H, Gao Y, Wang Y, Liang C, Zhang Z and
Chen Y: LncRNA CRNDE promotes the progression and angiogenesis of
pancreatic cancer via miR-451a/CDKN2D axis. Transl Oncol.
14:1010882021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee HA, Chu KB, Moon EK and Quan FS:
Histone deacetylase inhibitor-induced CDKN2B and CDKN2D contribute
to G2/M cell cycle arrest incurred by oxidative stress in
hepatocellular carcinoma cells via forkhead box M1 suppression. J
Cancer. 12:5086–5098. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Jin W, Jia X, Luo R, Tan Y, Zhu X,
Yang X, Wang X and Wang K: Transcriptional repression of CDKN2D by
PML/RARα contributes to the altered proliferation and
differentiation block of acute promyelocytic leukemia cells. Cell
Death Dis. 5:e14312014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hosseini ES, Meryet-Figuiere M,
Sabzalipoor H, Kashani HH, Nikzad H and Asemi Z: Dysregulated
expression of long noncoding RNAs in gynecologic cancers. Mol
Cancer. 16:1072017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hosono Y, Niknafs YS, Prensner JR, Iyer
MK, Dhanasekaran SM, Mehra R, Pitchiaya S, Tien J, Escara-Wilke J,
Poliakov A, et al: Oncogenic role of THOR, a conserved
cancer/testis long non-coding RNA. Cell. 171:1559–1572.e20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mondal T, Juvvuna PK, Kirkeby A, Mitra S,
Kosalai ST, Traxler L, Hertwig F, Wernig-Zorc S, Miranda C, Deland
L, et al: Sense-antisense lncRNA pair encoded by locus 6p22.3
determines neuroblastoma susceptibility via the USP36-CHD7-SOX9
regulatory axis. Cancer Cell. 33:417–434.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C, Wang S, Xing Z, Lin A, Liang K, Song
J, Hu Q, Yao J, Chen Z, Park PK, et al: A ROR1-HER3-lncRNA
signalling axis modulates the Hippo-YAP pathway to regulate bone
metastasis. Nat Cell Biol. 19:106–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin A, Hu Q, Li C, Xing Z, Ma G, Wang C,
Li J, Ye Y, Yao J, Liang K, et al: The LINK-A lncRNA interacts with
PtdIns(3,4,5)P3 to hyperactivate AKT and confer
resistance to AKT inhibitors. Nat Cell Biol. 19:238–251. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Yang Y, Li L, Liu Y, Geng P, Li G
and Song H: LncRNA SNHG1 enhances cell proliferation, migration,
and invasion in cervical cancer. Biochem Cell Biol. 96:38–43. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rui X, Xu Y, Huang Y, Ji L and Jiang X:
LncRNA DLG1-AS1 promotes cell proliferation by competitively
binding with miR-107 and up-regulating ZHX1 expression in cervical
cancer. Cell Physiol Biochem. 49:1792–1803. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Manawapat-Klopfer A, Thomsen LT, Martus P,
Munk C, Russ R, Gmuender H, Frederiksen K, Haedicke-Jarboui J,
Stubenrauch F, Kjaer SK and Iftner T: TMEM45A, SERPINB5 and
p16INK4A transcript levels are predictive for development of
high-grade cervical lesions. Am J Cancer Res. 6:1524–1536.
2016.PubMed/NCBI
|
|
19
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Terry MT and Patricia MG: Modeling
survival data: Extending the cox model. Springer; New York: ISBN
0-387-98784-3; pp. 261–287. 2000
|
|
22
|
Kassambara A, Kosinski M and Biecek P:
Drawing survival curves using ‘ggplot2’ [r package survminer
version 0.4.8]. 2020.
|
|
23
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. PLoS Biol. 18:e30004102020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington, DC: 2011
|
|
26
|
Liontos M, Kyriazoglou A, Dimitriadis I,
Dimopoulos MA and Bamias A: Systemic therapy in cervical cancer: 30
Years in review. Crit Rev Oncol Hematol. 137:9–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu W, Wang Z, Liu L, Yang Z, Liu S, Ma Z,
Liu Y, Ma Y, Zhang L, Zhang X, et al: LncRNA Malat1 inhibition of
TDP43 cleavage suppresses IRF3-initiated antiviral innate immunity.
Proc Natl Acad Sci USA. 117:23695–23706. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma Y, Yang Y, Wang F, Moyer MP, Wei Q,
Zhang P, Yang Z, Liu W, Zhang H, Chen N, et al: Long non-coding RNA
CCAL regulates colorectal cancer progression by activating
Wnt/β-catenin signalling pathway via suppression of activator
protein 2α. Gut. 65:1494–1504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang ZW, Chen JJ, Xia SH, Zhao H, Yang
JB, Zhang H, He B, Jiao J, Zhan BT and Sun CC: Long intergenic
non-protein coding RNA 319 aggravates lung adenocarcinoma
carcinogenesis by modulating miR-450b-5p/EZH2. Gene. 650:60–67.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chi Y, Wang D, Wang J, Yu W and Yang J:
Long non-coding RNA in the pathogenesis of cancers. Cells.
8:10152019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu G, Niu F, Humburg BA, Liao K, Bendi S,
Callen S, Fox HS and Buch S: Molecular mechanisms of long noncoding
RNAs and their role in disease pathogenesis. Oncotarget.
9:18648–18663. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Felisiak-Golabek A, Dansonka-Mieszkowska
A, Rzepecka IK, Szafron L, Kwiatkowska E, Konopka B, Podgorska A,
Rembiszewska A and Kupryjanczyk J: p19(INK4d) mRNA and protein
expression as new prognostic factors in ovarian cancer patients.
Cancer Biol Ther. 14:973–981. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zang WQ, Yang X, Wang T, Wang YY, Du YW,
Chen XN, Li M and Zhao GQ: MiR-451 inhibits proliferation of
esophageal carcinoma cell line EC9706 by targeting CDKN2D and
MAP3K1. World J Gastroenterol. 21:5867–5876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bartkova J, Thullberg M, Rajpert-De Meyts
E, Skakkebaek NE and Bartek J: Lack of p19INK4d in human testicular
germ-cell tumours contrasts with high expression during normal
spermatogenesis. Oncogene. 19:4146–4150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alzahrani AS: PI3K/Akt/mTOR inhibitors in
cancer: At the bench and bedside. Semin Cancer Biol. 59:125–132.
2019. View Article : Google Scholar : PubMed/NCBI
|