Introduction
Malignant tumors of the sinonasal tract are
relatively rare, accounting for 3% of all head and neck
malignancies. Adenocarcinomas of various types account for 10–20%
of all primary malignant tumors of the nasal cavity and paranasal
sinuses (1). According to the 4th
edition of the WHO classification, these tumors can be
distinguished into salivary and non salivary types (2) and the latter further divided into
intestinal (ITAC) and non-intestinal type (n-ITAC) (3). n-ITAC is further subdivided into
high-grade (G3) and low-grade (G1) lesions. G3 tumors have an
aggressive course and are usually associated with a poor prognosis,
with a 3-year survival rate of 20% (4–6).
Patients with G3 tumors are 5.4 times more likely to succumb than
those with low-grade tumors (P=0.039) (7). Moreover, due to the lack of specific
symptoms, diagnosis is usually made when the tumor has spread
widely and invaded surrounding tissues (T3 and
T4 diseases) (8), which
makes the treatment more difficult. At present, the options include
open or endoscopic techniques for complete surgical resection,
radical or adjuvant radiotherapy, chemotherapy and targeted
therapy. However, the literature focusing on n-ITAC is inadequate.
Most published series of articles, including homogeneous and
relatively large series, are focused on ITAC, describing
treatments, outcomes and prognostic factors (9). Regarding n-ITAC studies, most studies
include all adenocarcinoma subtypes (10) or focus on low-grade tumors (4) or histological features (11), but lack suggestions for classic
treatment. Therefore, the present study analyzed 12 consecutive
patients with n-ITAC diagnosed in Nanfang Hospital, Southern
Medical University during the last 20 years. The aim of the present
study was to explore the clinical characteristics, outcomes,
prognostic factors and the best treatment strategy for this
particular tumor.
Materials and methods
Patients
The present study was approved by the Ethics
Committee of Nanfang Hospital of Southern Medical University
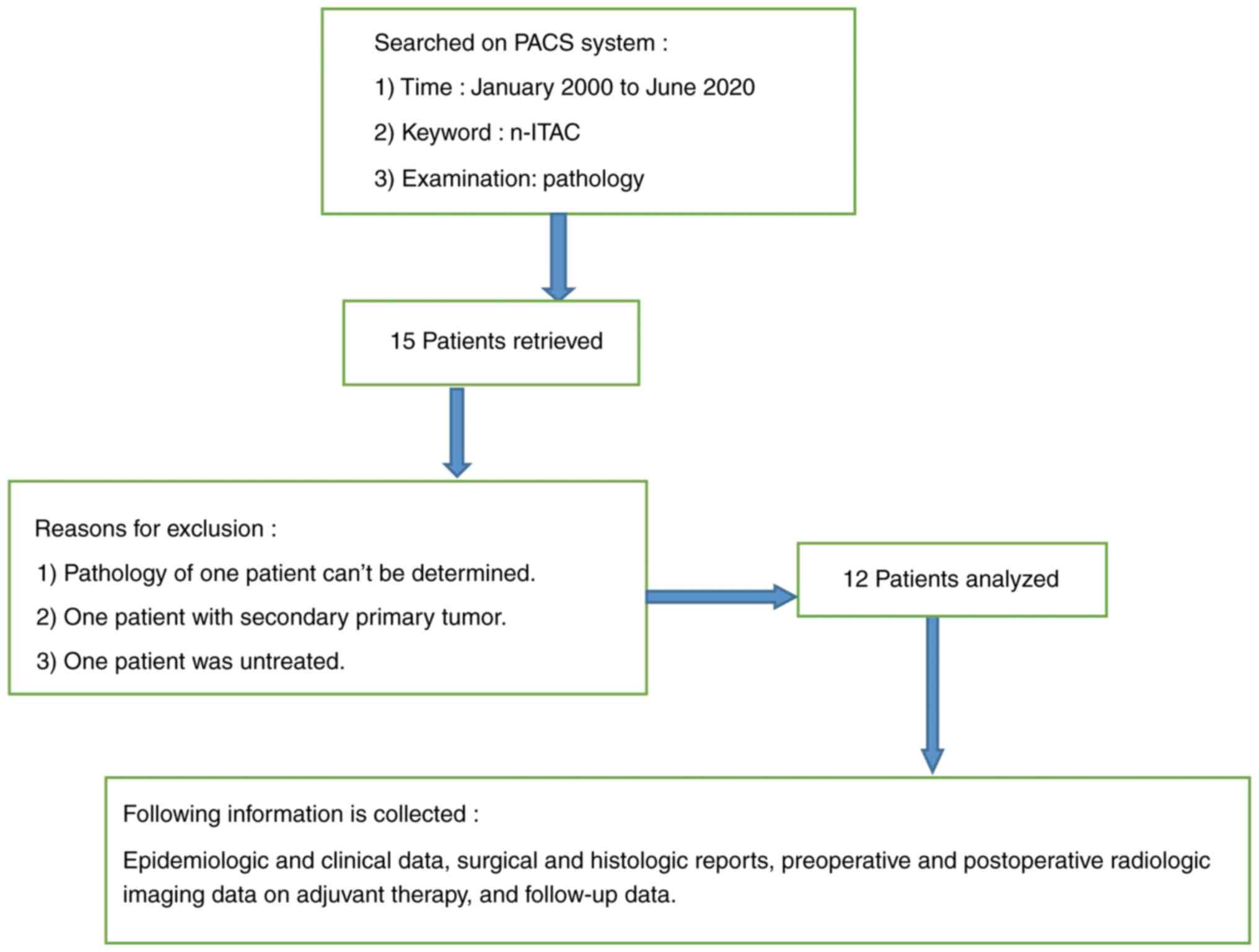
(Guangzhou, China; approval no. NFEC-2022-448). A total of 15
patients with n-ITAC were examined using the PACS imaging system of
Nanfang Hospital; the patients were admitted to Nanfang Hospital of
Southern Medical University between January 2000 and June 2020.
Finally, the images and medical records of 12 patients (excluding
one with two primary tumors, one without pathological diagnosis and
one without treatment) were obtained and reviewed. Of the patients,
10 were men (83.33%) and two were women (16.67%), resulting in a
male-to-female ratio of 5.0. The mean age of the patients was 48
years (range, 33–65 years). Epidemiologic and clinical data,
surgical and histologic reports, preoperative and postoperative
radiologic imaging, data on adjuvant therapy and follow-up data
were reviewed. The specific process is shown in Fig. 1.
The extent of the neoplasm was assessed by clinical,
endoscopic and radiologic examinations, in particular with
multiplanar computed tomography (CT) and contrast enhanced magnetic
resonance imaging. Endoscopic biopsies were performed under local
anesthesia or pathological biopsies were obtained under general
anesthesia. In cases of biopsies performed at an outside
institution, the pathologic slides were reviewed at the Nanfang
Hospital, Southern Medical University. Distant metastasis was
evaluated by bone scan, CT scan and ultrasound. All patients in the
series were retrospectively staged using clinical, radiologic and
histopathologic evaluations according to the 8th edition of the
American Joint Commission on Cancer (AJCC) TNM staging criteria in
2017 (12).
Follow-up and statistical
analysis
Follow-up duration was calculated from the date of
diagnosis of n-ITAC to the date of last follow-up. The following
variables were analyzed with respect to survival: i) patient
factors: Age, sex and smoking history; ii) tumor factors: TNM stage
and tumor site; iii) pathologic factors: Grade (G1/G3) and status
of surgical margins; and iv) treatment: Surgery, radiotherapy,
chemotherapy, targeted therapy or a combination of these
strategies. Overall survival (OS) was calculated from the date of
n-ITAC diagnosis to the date of either mortality or the last
follow-up. OS rates were estimated using the Kaplan-Meier method
and compared using the log-rank test. All statistical analyses were
performed using SPSS version 25.0 software (IBM Corp.). P<0.05
was considered to indicate a statistically significant
difference.
Results
Characteristics of patients with
n-ITAC
The clinicopathologic characteristics of all 12
patients who were diagnosed with n-ITAC are listed in Table I. Of the patients who were included
in the present study, 10 were men (83.33%) and two were women
(16.67%), resulting in a slight male predominance with a
male-to-female ratio of 5.0. The mean age was 48 years (range,
33–65 years). No clear evidence of occupational predisposing
factors was found; five patients were unemployed, three were office
clerks, one was professional and technical, and three patients'
occupations were unknown. Nasal obstruction and nosebleed were the
most frequent symptoms, reported in 10 of 12 patients (83.33%) and
a small number of them were accompanied by blurred vision,
headache, loss of smell, tears, eye and facial swelling. Mass being
found by microscope or macroscopically was reported in two (16.67%)
of 12 patients. Of the patients, 9 (75.0%) exhibited n-ITAC only in
the nasal cavity and one (8.3%) in the nasal cavity and ethmoid
sinus. A total of two patients (16.7%) had lesions in both the
nasal cavity and maxillary sinus. Of the 11 patients (91.7%) who
underwent surgery for n-ITAC, six (54.5%) who underwent resection
had negative margin and five (45.5%) had positive margin. The
tumors were staged according to the 8th edition of the AJCC TNM
classification in 2017, as follows: Six patients with
T1-T3 (50.0%) and 6 patients with
T4 (50.0%). Regarding the grade of differentiation,
seven tumors were at a low grade (58.33%) and 5 were at a high
grade (41.67%).
 | Table I.The clinicopathologic characteristics
of patients with n-ITAC. |
Table I.
The clinicopathologic characteristics
of patients with n-ITAC.
| Clinicopathologic
characteristic | No. of patients
(%) |
|---|
| Sex |
|
|
Male | 10 (83.33) |
|
Female | 2 (16.67) |
| Age (years) |
|
|
Mean/Range | 48/33-65 |
| Smoking |
|
|
Yes | 5 (41.67) |
| No | 7 (58.33) |
| Clinical symptoms
and signs |
|
| Nasal
congestion, Nosebleed | 10 (83.33) |
| Mass founded by
microscopic or macroscopically | 2 (16.67) |
| Affected
sitea |
|
| Nasal
cavity | 11 |
| Ethmoid
sinus | 1 |
|
Maxillary sinus | 2 |
| T
classification |
|
|
T1-T3 | 6 (50.0) |
|
T4 | 6 (50.0) |
| N
classification |
|
|
N0-N1 | 8 (66.67) |
|
N2 | 4 (33.33) |
| TNM
classification |
|
|
I–III | 4 (33.33) |
| IV | 8 (66.67) |
| Type of
surgeryb |
|
|
Endoscopic surgery | 11 (100) |
| Open
surgery | 0 (0) |
| Margin
statusb |
|
|
Positive | 5 (45.5) |
|
Negative | 6 (54.5) |
| Grade |
|
| G1 | 7 (58.33) |
| G3 | 5 (41.67) |
Pathology of n-ITAC
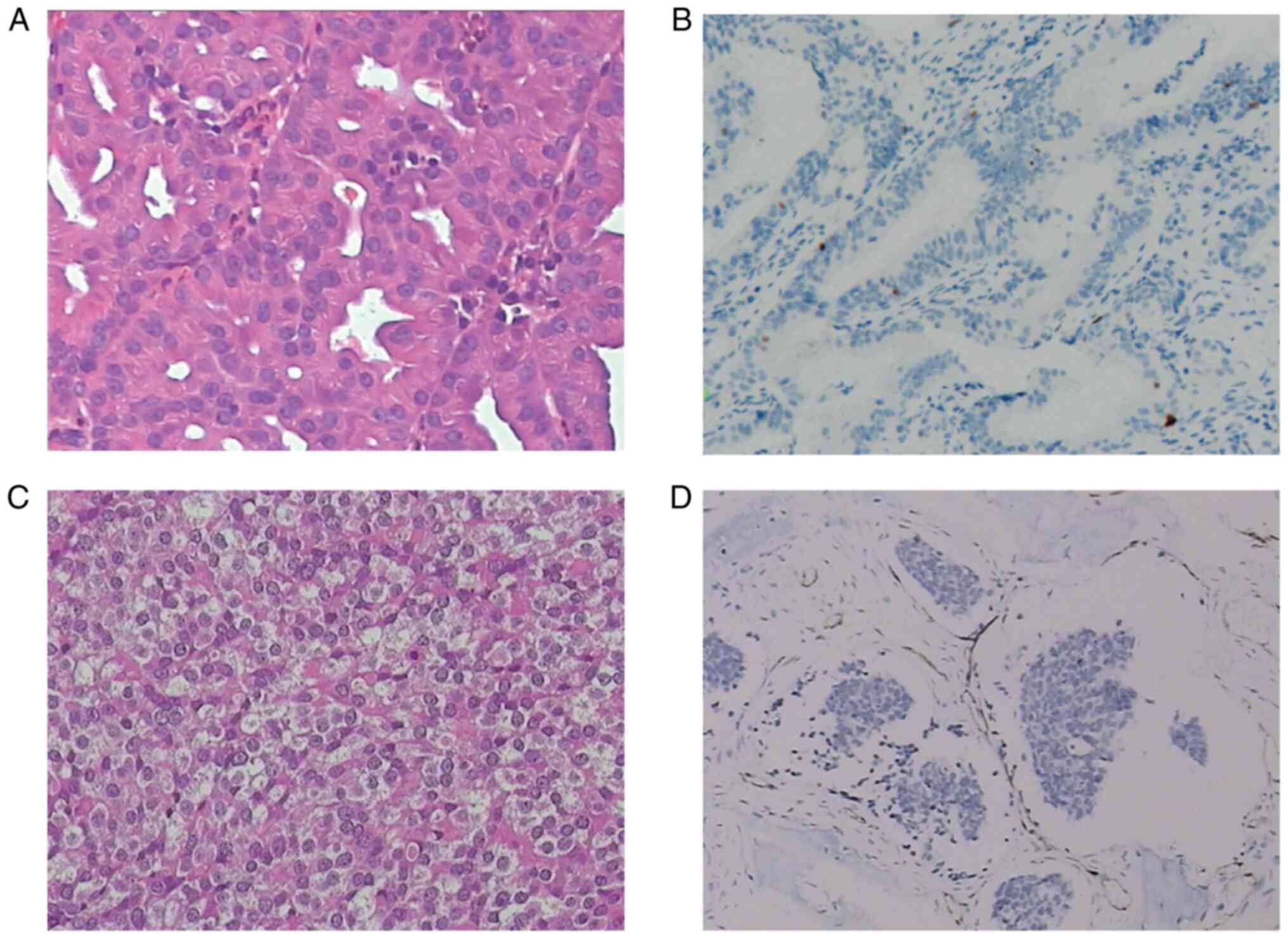
The pathology of non-intestinal adenocarcinoma came
from our patients, including HE staining and immunohistochemistry,
as shown in Fig. 2. Fig. 2A represents a low-grade tumor HE
staining. The cells are uniformly arranged in compact acinar,
back-to-back, confluent glands, cystic space and papillary. They
maintain a tall columnar to cuboidal arrangement without much
layering. The cytoplasm is usually abundant, but the appearance is
variable-basophilic, granular, mucous, eosinophilic and cytolytic.
The nuclear atypical is mild to moderate, with little mitosis.
Fig. 2C shows a high-grade tumor HE
staining, showing significant nuclear polymorphism, nucleolar and
mitotic activity. The signet ring cells and necrosis can often be
seen. Fig. 2B and D show
immunohistochemical results of low-grade and high-grade tumors,
respectively.
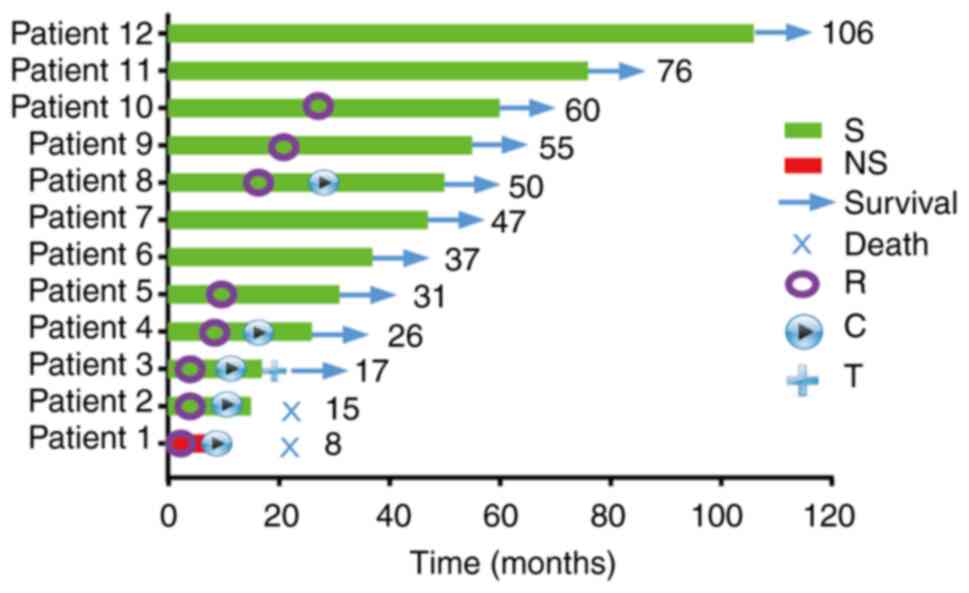
Treatment of patients with n-ITAC
Of the 12 patients, one patient (8.3%) received
concurrent chemo-radiotherapy (platinum 80 mg/m2, q3w)
without surgery. The other 11 (91.7%) were treated surgically. The
surgical method was basically endoscopic endonasal resection (EER).
Of these 11, six (54.5%) achieved gross negative margins (R0) and
five (45.5%) had gross or microscopic positive margins (R+)
(Fig. 3).
Adjuvant treatment included radiation, chemotherapy
and targeted therapy. A total of eight patients (66.7%) underwent
radiation, including five with G3 tumors (one T3 and
four T4) and three with G1 tumors (one T1,
one T3 and one T4). The technique was
intensity-modulated radiotherapy (IMRT; Table II). Besides the primary tumor site,
most patients also received prophylactic irradiation of the
cervical area. The radiation dose for cases without surgery or with
positive margin was relatively high: 66–70 Gy/33F for primary tumor
and 54–64 Gy/30-33F for prophylactic cervical levels; while for
patients with negative margin, the tumor bed was irradiated for 60
Gy/28F and the cervical prophylactic dose was 50 Gy/28F. A total of
five patients received concurrent chemotherapy (platinum 80
mg/m2, q3w). Only one patient received targeted therapy
with cetuximab.
 | Table II.The radiation dose of patients with
n-ITAC. |
Table II.
The radiation dose of patients with
n-ITAC.
| Grade | Margin status | Purpose of
radiation | Radiation dose |
|---|
| G1 | Negative | Adjuvant | PTVp: 60
Gy/28F |
|
|
|
| PTV1: 50
Gy/28F |
| G3 | Negative | Adjuvant | PTVp: 60
Gy/28F |
|
|
|
| PTV1: 50
Gy/28F |
| G3 | NA |
Radical | PTVp: 70
Gy/33F |
| G3 | Positive (R1) | Adjuvant | PTVp: 70
Gy/33F |
|
|
|
| PTVnd:
70 Gy/33F |
|
|
|
| PTV1: 64
Gy/33F |
|
|
|
| PTV2: 59
Gy/33F |
| G3 | Positive (R2) | Adjuvant | PTVp: 70
Gy/33F |
|
|
|
| PTVnd.L:
70 Gy/33F |
|
|
|
| PTV1: 60
Gy/30F |
|
|
|
| PTV2: 54
Gy/30F |
| G3 | Positive (R2) | Adjuvant | PTVp: 70
Gy/33F |
|
|
|
| PTVnd:
66 Gy/33F |
|
|
|
| PTV1: 63
Gy/33F |
Prognostic factors for OS of patients
with n-ITAC
Mean follow-up time was 47 months (range, 17–106
months) and no patients were lost to follow-up. At the time of the
analysis, 10 of 12 patients (83.3%) were alive with no evidence of
disease (NED) and 2 patients (16.7%) succumbed to the disease. One
patient (8.3%) treated with radiotherapy and chemotherapy and whose
tumor was stage T4, grade G3, succumbed to the disease
after 8 months. One patient (8.3%) treated with surgery,
radiotherapy and chemotherapy, whose tumor was T4, grade
G3, and positive surgical margins (R2) succumbed to the disease
after 15 months. Of the five patients with positive incisal margin,
four patients received radiotherapy and the median OS was 50
months.
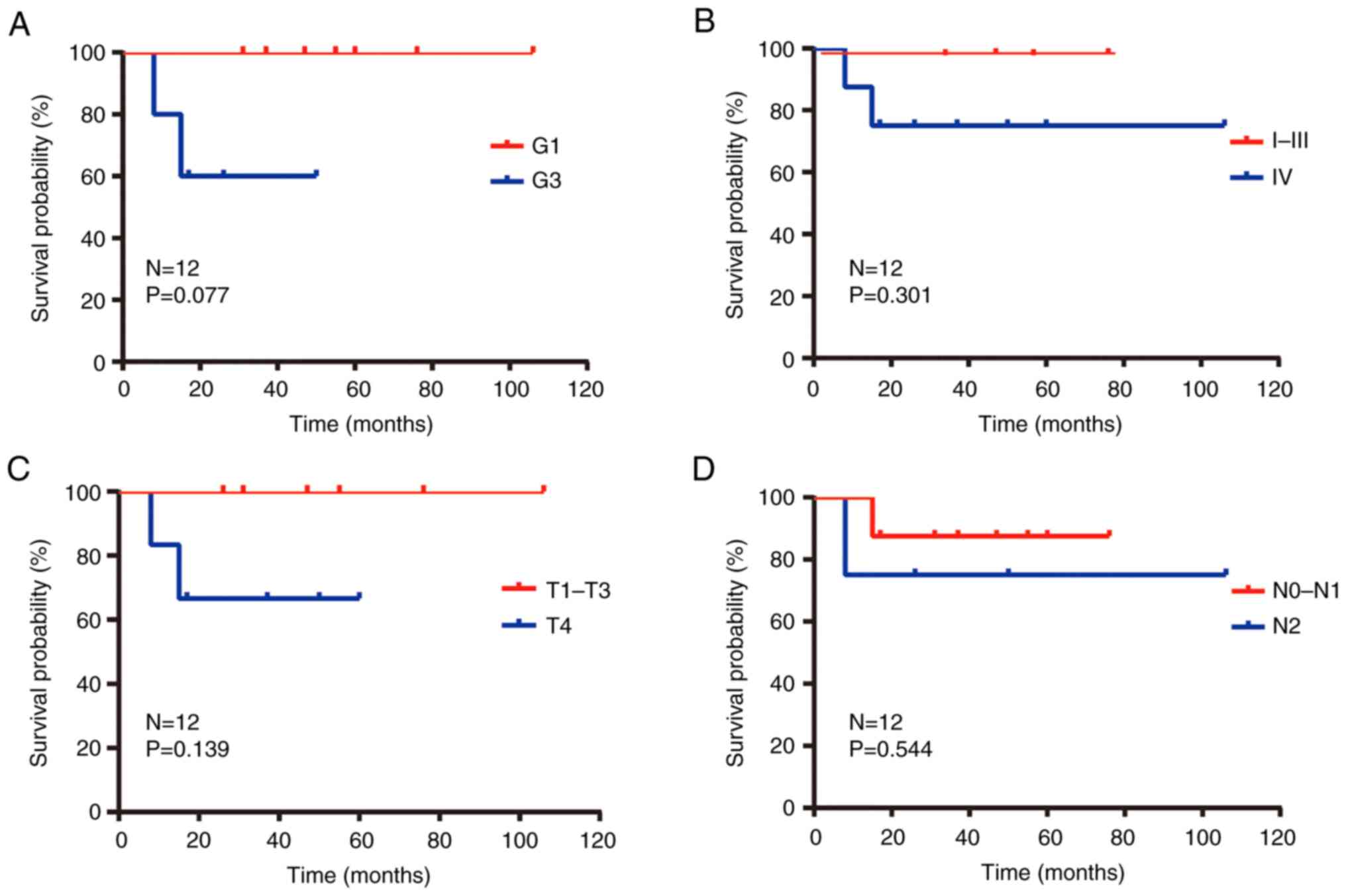
The univariate analysis results showed that grade
and treatment type of n-ITAC tumor were prognostic factors for OS
(Table III). The 1- and 3-year OS
rates were 100 and 85.7% for patients with G1 and were 80.0 and
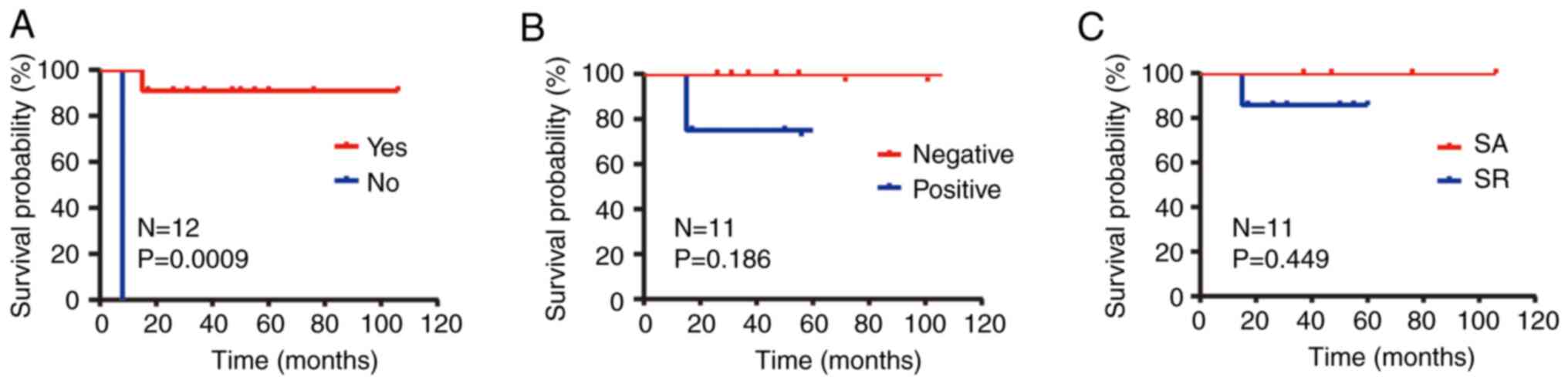
20.0% for patients with G3 (P=0.077; Fig. 4A). In order to analyze the impact of
surgery on the survival of patients, the present study divided the
patients into surgical (n=11) and non-surgical (n=1) groups. The
median follow-up time in the surgical group was 47 months. Of the
patients, 10 are still alive and the longest follow-up period is
106 months. One patient succumbed to the disease with an OS of 15
months. The only patient in the non-surgical group succumbed to the
disease and their OS was 8 months. The 1-year OS rate and 3-year OS
rate of the surgical group were 100 and 63.6%, while the
non-operative group were both 0.0% (P=0.0009; Fig. 5A).
 | Table III.Association of overall survival with
the characteristics of patients with n-ITAC. |
Table III.
Association of overall survival with
the characteristics of patients with n-ITAC.
|
| Survival rate
(%) |
|
|---|
|
|
|
|
|---|
| Characteristic | 1-year | 3-year | P-value |
|---|
| Sex |
|
|
|
|
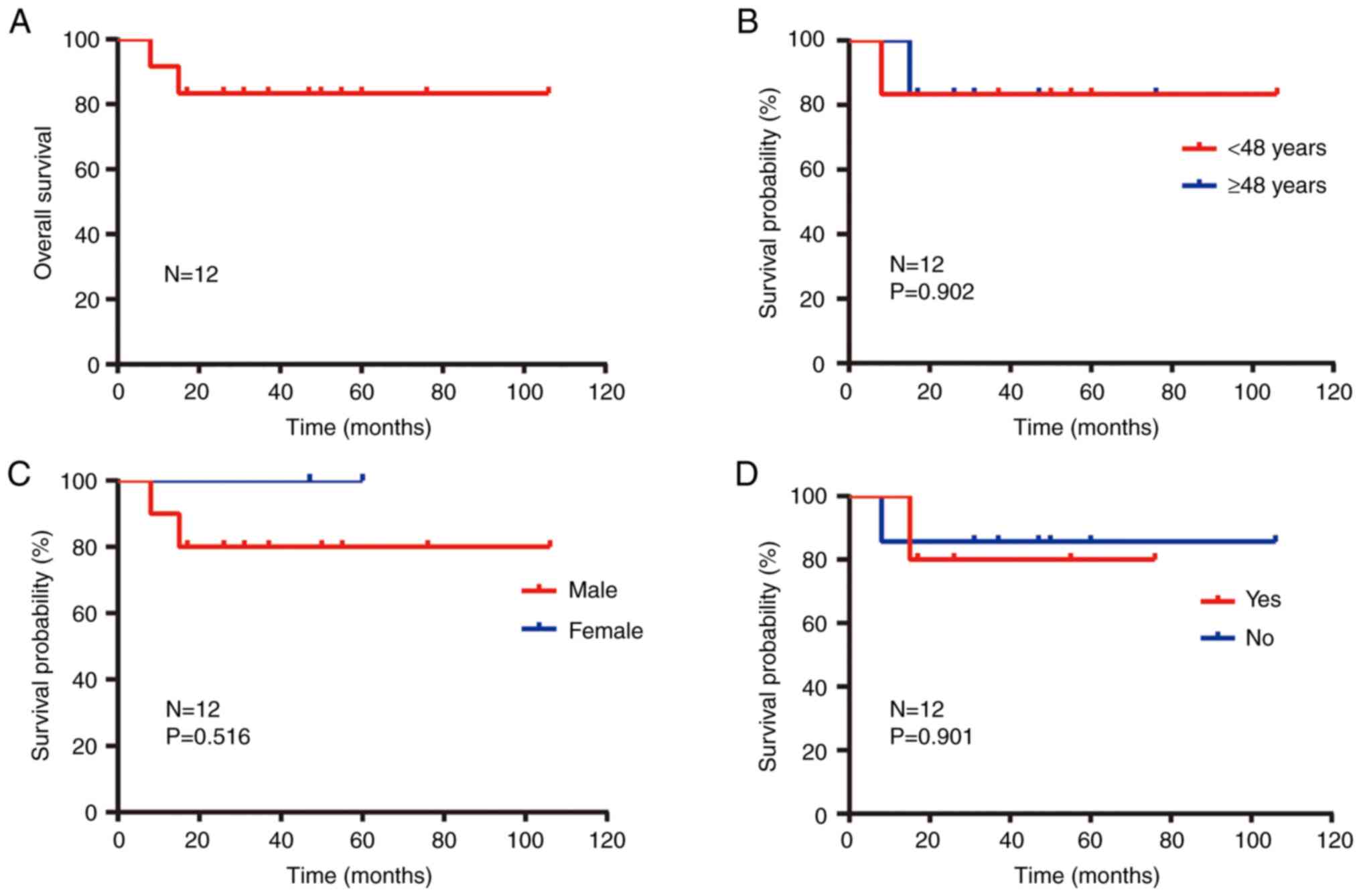
Male | 90.0 | 50.0 | 0.516 |
|
Female | 100.0 | 100.0 |
|
| Age (years) |
|
|
|
|
<48 | 83.3 | 83.3 | 0.902 |
|
≥48 | 100.0 | 33.3 |
|
| Smoking |
|
|
|
|
Yes | 100.0 | 40.0 | 0.901 |
| No | 85.7 | 71.4 |
|
| Surgery |
|
|
|
|
Yes | 100.0 | 63.6 | 0.0009 |
| No | 0 | 0 |
|
| Margin status |
|
|
|
|
Positive | 100.0 | 50 | 0.186 |
|
Negative | 100.0 | 71.4 |
|
| Grade |
|
|
|
| G1 | 100.0 | 85.7 | 0.077 |
| G3 | 80.0 | 20.0 |
|
| T
classification |
|
|
|
|
T1-T3 | 100.0 | 66.7 | 0.139 |
|
T4 | 83.3 | 50.0 |
|
| N
classification |
|
|
|
|
N0-N1 | 100.0 | 62.5 | 0.544 |
|
N2 | 75.0 | 50.0 |
|
| TNM
classification |
|
|
|
|
I–III | 100.0 | 75.0 | 0.301 |
| IV | 87.5 | 50.0 |
|
In order to further analyze the effect of surgery
combined with radiotherapy, the patients were divided into simple
operation group and operation combined with radiotherapy group. The
1- and 3-year OS rates for those not receiving radiation were both
100% and for those receiving radiation were 100 and 42.9% (P=0.449;
Fig. 5C). Using the average values
as cutoff points, patient age was not a significant prognostic
factor for OS. Moreover, the univariate analysis results showed
that sex, smoking, TMN stage, margin status were also not
significantly associated with OS (Fig.
4, Fig. 5, Fig. 6).
Discussion
This retrospective study discussed the clinical
characteristics, outcomes, prognostic factors and treatments of a
uniform cohort of 12 patients with sinonasal n-ITAC. To the best of
the authors' knowledge, there are few reports focusing on n-ITAC
treatments and prognosis.
n-ITAC is a rare malignant tumor, defined as an
adenocarcinoma without the histopathological features of sinus ITAC
or salivary adenocarcinoma (13).
In the past 20 years, only 14 patients have been diagnosed in
Nanfang Hospital, Southern Medical University and 12 patients were
analyzed in the present study. Although it was previously reported
that n-ITAC develops mainly in the maxillary sinus, in the present
study, the most representative site of origin was the nasal cavity
(11, 91.67%). According to the literature (14), the median age was ~60 years old and
the female prevalence was very high. In the present study, there
was male predominance, with a male-female ratio of 5.0, which may
be related to smoking, but there was not have enough evidence to
explain it (P=0.901; Fig. 6D).
There has not been any large-scale study on n-ITAC
due to its low incidence. Choussy et al (15) considered 418 patients of ITAC or
n-ITAC and reported a 5-year OS rate of 64%. Bhayani et al
(16) considered 66 patients, of
whom 31 had n-ITAC and reported a total 5-year OS rate of 65.9%.
Orvidas et al (7) considered
24 patients (58% with n-ITAC) and reported a 5-year OS rate of 58%,
but there are no survival data for n-ITAC in their study. To the
best of the authors' knowledge, the researches of Chen et al
(10), Bignami et al
(14) were the only two researches
that focused only on n-ITAC. In the study of Chen et al
(10), the 5-year disease specific
survival for the nonintestinal type was 71.2%. In the study of
Bignami et al (14), 5-year
OS was 95.2%. The median follow-up time of the present study was 47
months and 1 and 3-year OS rate were 76.9 and 46.2% respectively.
The poor prognosis of our patients was mainly due to the fact that
most of the tumors were of high T stage
(T3-T4). It is reported in the literature
that the clinical outcome of patients with sinonasal carcinomas
remains poor (8,17,18).
The high-grade tumors of nonintestinal sinonasal adenocarcinomas
have an aggressive course and are usually associated with a poor
prognosis, with a 3-year survival rate of merely 20% (4–6).
Orvidas et al (7) reported
that patients with high-grade tumors are 5.4 times more likely to
succumb to any cause than those with low-grade tumors (P=0.039). By
contrast, Choussy et al (15) reported that there was no
statistically significant difference in survival rates between
low-grade and high-grade tumors. However, in the present study the
1 and 3-year OS rates of patients with low-grade tumors were 100
and 85.7%, respectively, significantly higher than those of
patients with high-grade tumors (80.0 and 20.0%; P=0.077; Fig. 4A). It was therefore indicated that
high pathological grade may be an adverse prognostic factor. The
pathological grade is related to the prognosis, but there was no
statistical significance in multivariate analysis, which may be due
to the small number of cases. In Bignami et al (14), the 5-year OS was 100% for
pT1, pT2 and pT3 and 83.3±1.52%
for pT4a and pT4b (P=0.037). However, there
is no significant relationship between N stage and prognosis, which
may be due to the fact that there are lower probability of lymph
node metastasis and a small number of cases in the disease. In the
present study, there was a trend in the survival curve of clinical
stage (Fig. 4B) and T stage
(Fig. 4C), which may be factors
affecting the prognosis of patients. P-value was not statistically
significant and may be related to the number of cases.
The main therapeutic strategy for n-ITAC is radical
surgery with or without adjuvant radiotherapy. Endoscopic
rhinoplasty has recently been promoted as the preferred surgical
treatment for ITAC with correct planning and indications (19), with encouraging results and the
advantage of reducing the incidence. Surgical therapy continues to
be a mainstay for curative treatment. The main controversial point
is whether to add adjuvant therapy after surgery at present. Some
individuals argue that surgery alone is sufficient in patients with
early tumors (16,20,21).
However, Bignami et al (14)
proposed that endoscopic transnasal approach is the preferred
surgical method and surgery combined with postoperative
radiotherapy is the main treatment in cases of high-stage
(T3 and T4) and high-grade tumors (22). Some advocate that postoperative
radiotherapy should be performed in all cases, regardless of stage
or pathological grade, as most cancers of the nasal cavity and
paranasal sinuses present at later stages, often leading to the use
of multimodality treatment (23–26).
Blanch et al (27) proposed
that there was no survival benefit from the addition of
radiotherapy to surgery for early-stage lesions. In the present
study, the OS of the surgery alone group was slightly higher
compared with that of the surgery plus radiotherapy group
(P=0.449). It is known that the indications of RT are high-grade
tumor, positive surgical margin, high T stage and positive lymph
node (14,22). Therefore, the patients in the
combined radiotherapy group had more risk factors than the patients
with operation alone, resulting in a significant bias in the
baseline between the two groups. Unfortunately, no randomly
controlled data were available and this certainly is a common
limitation of the present and other studies. Prospective studies
may be needed in the future to verify the value of radiotherapy.
The above results only suggested that surgery combined with
radiotherapy is also an alternative treatment mode for patients
with high risk factors.
On the mode of radiotherapy, it has been reported in
the literature (15,28–30)
that for ITAC and n-ITAC, the dosage is 30 fractions of 2 Gy, for a
total of 60 Gy, with an extra boost of 6 Gy in case of positive
margins. In the present study, the radiation dose for cases without
surgery or with positive margin was relatively higher: 66–70 Gy/33F
for primary tumor and 54–64 Gy/30-33F for prophylactic cervical
levels; while for patients with negative margin, the dose for tumor
bed was 60 Gy/28F and the cervical prophylactic dose was 50 Gy/28F.
The dose in the present study was higher because it only focused on
n-ITAC, which was more aggressive than ITAC. On the technology of
radiotherapy, it was delivered by IMRT or three-dimensional
radiotherapy, the former provided a local control and survival as
well as a reduced toxicity. In previous studies (16,31,32),
three-dimensional and conformal radiation therapy were the main
technology, while in the present study, all patients received IMRT,
which has the advantage of increasing the target dose without
significantly increasing side effects. With regard to the scope of
exposure, the main controversial point is whether prophylactic
cervical lymph node irradiation should be performed. The patients
in the present study all received prophylactic cervical
irradiation, as suspicious lymph nodes were often seen on images.
Besides, the results of the present study showed no obvious
complications such as cervical lymphedema and fibrosis after
radiotherapy.
Sinus adenocarcinoma often exhibits EGFR
overexpression and mutations that determine the constitutive
activation of the downstream signaling cascade of EGFR are rare,
indicating that these tumors may be good candidates for anti-EGFR
therapy (33). Only one of the
patients in the present study was tested for EGFR, which showed
(++) and the patient was given cetuximab targeted therapy. Since
only one patient received targeted therapy, no clear
recommendations could be given. Since cetuximab has been validated
to be an extremely effective agent in HNSCC and is included in the
guidelines, it was hypothesized that targeted therapy such as
cetuximab might be an effective candidate for treatment of n-TIAC.
Since only a few of the patients in the present study received
chemotherapy, it is not clear whether systemic chemotherapy was
beneficial or not.
The limitations of the present study are as follows:
First, the study was a retrospective analysis and the selection of
patients was biased. Patients received both surgery and
radiotherapy had more potential risk factors at the baseline.
Second, because of the low incidence of n-ITAC, the number of cases
was relatively small. Third, longer follow-up time is needed to
further verify its conclusions.
n-ITAC is a rare type of tumor. Pathological high
grade may be a poor prognostic factor. Surgery is the optimal
treatment and negative surgical margins are the ultimate goal of
benefiting patients. Patients with no surgery, high-grade tumors
and/or positive surgical margins should be given radical
radiotherapy and preventive radiotherapy in the drainage area of
the lymph node at the same time. If the margins are negative, the
radiation dose can be appropriately reduced. Whether combined with
chemotherapy and targeted therapy needs further study.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL, BL, JX, HW, QG, FY, YX, SW, SC, YL, JG and BC
contributed to the study conception and design. JL wrote the
manuscript and collected and analyzed data. BL and BC analyzed data
and critically revised the final manuscript. JX wrote the
manuscript and searched for related literature. HW, QG, FY, YX, SW,
SC, YL and JG searched for related literature. All authors read and
approved the final manuscript. JL and BC confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Nanfang Hospital of Southern Medical University
(approval no. NFEC-2022-448). Due to the retrospective nature of
the study, the committee agreed to exempt patients from informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barnes L: Intestinal-type adenocarcinoma
of the nasal cavity and paranasal sinuses. Am J Surg Pathol.
10:192–202. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leivo I: Sinonasal adenocarcinoma: Update
on classification, immunophenotype and molecular features. Head
Neck Pathol. 10:68–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Naggar AK, Chan JKC, Grandis JR, Takata
T and Slootweg PJ: WHO classification of head and neck tumours. 4th
edition. Lyon; IARC Press: 2017
|
|
4
|
Stelow EB, Jo VY, Mills SE and Carlson DL:
A histologic and immunohistochemical study describing the diversity
of tumors classified as sinonasal high-grade nonintestinal
adenocarcinomas. Am J Surg Pathol. 35:971–980. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heffner DK, Hyams VJ, Hauck KW and
Lingeman C: Low-grade adenocarcinoma of the nasal cavity and
paranasal sinuses. Cancer. 50:312–322. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knegt PP, Ah-See KW, vd Velden LA and
Kerrebijn J: Adenocarcinoma of the ethmoidal sinus complex:
Surgical debulking and topical fluorouracil may be the optimal
treatment. Arch Otolaryngol Head Neck Surg. 127:141–146. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Orvidas LJ, Lewis JE, Weaver AL,
Bagniewski SM and Olsen KD: Adenocarcinoma of the nose and
paranasal sinuses: A retrospective study of diagnosis, histologic
characteristics, and outcomes in 24 patients. Head Neck.
27:370–375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castelnuovo P, Turri-Zanoni M, Battaglia
P, Antognoni P, Bossi P and Locatelli D: Sinonasal malignancies of
anterior skull base: Histology-driven treatment strategies.
Otolaryngol Clin North Am. 49:183–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fiaux-Camous D, Chevret S, Oker N,
TurriZanoni M, Lombardi D, Choussy O, Duprez F, Jorissen M, de
Gabory L, Malard O, et al: Prognostic value of the seventh
AJCC/UICC TNM classification of intestinal-type ethmoid
adenocarcinoma: Systematic review and risk prediction model. Head
Neck. 39:668–678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen MM, Roman SA, Sosa JA and Judson BL:
Predictors of survival in sinonasal adenocarcinoma. J Neurol Surg B
Skull Base. 76:208–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi HR, Sturgis EM, Rashid A, DeMonte F,
Luna MA, Batsakis JG and El-Naggar AK: Sinonasal adenocarcinoma:
Evidence for histogenetic divergence of the enteric and nonenteric
phenotypes. Hum Pathol. 34:1101–1107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
The 8th edition of the American Joint
Commission on Cancer (AJCC) TNM staging criteria in 2017.
https://dia.dakapath.com/home/tumour/view/id/3640.html
|
|
13
|
Barnes L, Eveson JW, Reichart P and
Sidransky D: Pathology and genetics of head and neck tumours. WHO
Classification of Tumours. 3rd edition. Vol. 9. Lyon: IARC Press;
2005, https://ovidsp.dc2.ovid.com/ovid–a/ovidweb.cgi?T=JS&PAGE=fulltext&D=ovft&AN=00003524–200685020–00001&NEWS=N&CSC=Y&CHANNEL=PubMed
|
|
14
|
Bignami M, Lepera D, Volpi L, Lambertoni
A, Arosio A, Pistochini A, Nicolai P and Castelnuovo P: Sinonasal
non-intestinal-type adenocarcinoma: A retrospective review of 22
patients. World Neurosurg. 120:e962–e969. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choussy O, Ferron C, Vedrine PO, Toussaint
B, Liétin B, Marandas P, Babin E, De Raucourt D, Reyt E, Cosmidis
A, et al: Adenocarcinoma of ethmoid: A GETTEC retrospective
multicenter study of 418 cases. Laryngoscope. 118:437–443. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhayani MK, Yilmaz T, Sweeney A, Calzada
G, Roberts DB, Levine NB, DeMonte F, Hanna EY and Kupferman ME:
Sinonasal adenocarcinoma: A 16-year experience at a single
institution. Head Neck. 36:1490–1496. 2014.PubMed/NCBI
|
|
17
|
Robin TP, Jones BL, Gordon OM, Phan A,
Abbott D, McDermott JD, Goddard JA, Raben D, Lanning RM and Karam
SD: A comprehensive comparative analysis of treatment modalities
for sinonasal malignancies. Cancer. 123:3040–3049. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Turner JH and Reh DD: Incidence and
survival in patients with sinonasal cancer: A historical analysis
of population-based data. Head Neck. 34:877–885. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nicolai P, Schreiber A, Bolzoni Villaret
A, Lombardi D, Morassi L, Raffetti E, Donato F, Battaglia P,
Turri-Zanoni M, Bignami M and Castelnuovo P: Intestinal type
adenocarcinoma of the ethmoid: Outcomes of a treatment regimen
based on endoscopic surgery with or without radiotherapy. Head
Neck. 38 (Suppl 1):E996–E1003. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nicolai P, Castelnuovo P, Lombardi D,
Battaglia P, Bignami M, Pianta L and Tomenzoli D: Role of
endoscopic surgery in the management of selected malignant
epithelial neoplasms of the naso-ethmoidal complex. Head Neck.
29:1075–1082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lund VJ, Chisholm EJ, Takes RP, Suárez C,
Mendenhall WM, Rinaldo A, Llorente JL, Terhaard CH, Rodrigo JP,
Maughan E and Ferlito A: Evidence for treatment strategies in
sinonasal adenocarcinoma. Head Neck. 34:1168–1178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bogaerts S, Vander Poorten V, Nuyts S, Van
den Bogaert W and Jorissen M: Results of endoscopic resection
followed by radiotherapy for primarily diagnosed adenocarcinomas of
the paranasal sinuses. Head Neck. 30:728–736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jakobsen MH, Larsen SK, Kirkegaard J and
Hansen HS: Cancer of the nasal cavity and paranasal sinuses.
Prognosis and outcome of treatment. Acta Oncol. 36:27–31. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakata K, Aoki Y, Karasawa K, Nakagawa K,
Hasezawa K, Muta N, Terahara A, Onogi Y, Sasaki Y and Akanuma A:
Analysis of the results of combined therapy for maxillary
carcinoma. Cancer. 71:2715–2722. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harbo G, Grau C, Bundgaard T, Overgaard M,
Elbrønd O, Søgaard H and Overgaard J: Cancer of the nasal cavity
and paranasal sinuses. A clinico-pathological study of 277
patients. Acta Oncol. 36:45–50. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parsons JT, Mendenhall WM, Mancuso AA,
Cassisi NJ and Million RR: Malignant tumors of the nasal cavity and
ethmoid and sphenoid sinuses. Int J Radiat Oncol Biol Phys.
14:11–22. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blanch JL, Ruiz AM, Alos L,
Traserra-Coderch J and Bernal-Sprekelsen M: Treatment of 125
sinonasal tumors: Prognostic factors, outcome, and follow-up.
Otolaryngol Head Neck Surg. 131:973–976. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choussy O, Ferron C, Védrine PO, Toussaint
B, Liétin B, Marandas P, Babin E, De Raucourt D, Reyt E, Cosmidis
A, et al: Role of radiotherapy in the treatment of nasoethmoidal
adenocarcinoma. Arch Otolaryngol Head Neck Surg. 136:143–146. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van Gerven L, Jorissen M, Nuyts S, Hermans
R and Vander Poorten V: Long-term follow-up of 44 patients with
adenocarcinoma of the nasal cavity and sinuses primarily treated
with endoscopic resection followed by radiotherapy. Head Neck.
33:898–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cantù G, Solero CL, Mariani L, Salvatori
P, Mattavelli F, Pizzi N and Riggio E: Anterior craniofacial
resection for malignant ethmoid tumors-a series of 91 patients.
Head Neck. 21:185–191. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dirix P, Vanstraelen B, Jorissen M, Vander
Poorten V and Nuyts S: Intensity-modulated radiotherapy for
sinonasal cancer: Improved outcome compared to conventional
radiotherapy. Int J Radiat Oncol Biol Phys. 78:998–1004. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duprez F, Madani I, Morbée L, Bonte K,
Deron P, Domján V, Boterberg T, De Gersem W and De Neve W: IMRT for
sinonasal tumors minimizes severe late ocular toxicity and
preserves disease control and survival. Int J Radiat Oncol Biol
Phys. 83:252–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Franchi A, Innocenti DR, Palomba A, Miligi
L, Paiar F, Franzese C and Santucci M: Low prevalence of K-RAS,
EGF-R and BRAF mutations in sinonasal adenocarcinomas. Implications
for anti-EGFR treatments. Pathol Oncol Res. 20:571–579. 2014.
View Article : Google Scholar : PubMed/NCBI
|