Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide (1). In 2022, lung cancer is expected to be
the most common cause of death in both men and women in China
(2). Despite precision medicine
having brought novel therapy options and hope to patients with lung
cancer, an early diagnosis is still most important for the
prognosis of these patients. In recent years, low-dose computed
tomography (LDCT) has been used in the early screening for lung
cancer, and it has been demonstrated that this could reduce the
mortality rate from lung cancer. However, accompanying radiation,
misjudgment of lung lesions and further excluded examinations pose
challenges to the success and cost-effectiveness of lung cancer
screening with LDCT (3).
Pathological examinations are also limited by their invasiveness
and false-negative results. Therefore, easy to sample, lower cost
and more effective biomarkers are urgently required for clinical
practice.
Inflammation and immunity impact important steps of
tumourigenesis (4). Various types
of immune and inflammatory cells are frequently present within
tumours; however, they are more easily captured in the peripheral
blood (4). Numerous studies have
demonstrated that haematological biomarkers, including the
neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio
(MLR) and platelet-to-lymphocyte ratio (PLR), exhibit prognostic
value in malignancies such as lung and colorectal cancer (5–7). High
NLR, PLR and MLR values prior to oncotherapy are associated with an
unfavourable prognosis of the disease (8,9). The
nutritional status of patients with cancer can reflect energy
metabolism and indicate disease severity, disease progression and
prognosis (10); this can be
assessed by several blood biochemical, including serum albumin
(11). As one of the negative acute
phase proteins, serum albumin could also reflect the inflammatory
state (12). Some markers that
combine albumin with other hematological indexes, including the
prognostic nutritional index, the albumin-to-alkaline phosphatase
ratio and the C-reactive protein-to-albumin ratio, exhibit
predictive values in the survival prognosis of patients with lung
cancer (6,13,14).
Although these markers are considered to be useful
in tracking and assessing the severity and prognosis of lung
cancer, their utility for early diagnosis and timely treatment is
barely satisfactory.
Novel markers have been explored, identified and
applied to the disease diagnosis and prognostic evaluation.
Monocytes, neutrophils and lymphocytes are part of the body's
immune system. The peripheral absolute cell count of these immune
cells might vary for each individual. Recently, the red blood cell
count was introduced into the individualized evaluation of the
peripheral immune response intensity (15). It has been demonstrated that markers
such as the monocyte to red blood cell count ratio (MRR), the
neutrophil to red blood cell count ratio (NRR) and the lymphocyte
to red blood cell count ratio (LRR) could well reflect the
intensity of circulating immune cells, including monocytes,
neutrophils and lymphocytes (15).
The product of lymphocyte count and albumin concentration (LA) has
been reported as a potential novel prognostic biomarker for stage
II/III rectal cancer (16). The
present study aimed to explore the additional diagnostic value of
these markers in the diagnosis of lung cancer, including in the
early stages.
Materials and methods
Participants
In accordance with the following inclusion and
exclusion criteria, a total of 216 patients newly diagnosed with
lung cancer at the Affiliated Hospital of Jiangsu University
(Zhenjiang, China) between June 2014 and June 2021 were selected,
including 178 patients with non-small cell lung cancer (NSCLC) and
38 patients with SCLC. Additionally, 184 healthy volunteers who
underwent a physical examination were selected as negative
controls. The median age was 61 years (range, 48–78 years) for
patients with lung cancer and 58 years (range, 47–80 years) for the
healthy controls. All patients with lung cancer were newly
diagnosed and previously untreated. These cases were confirmed
using lung tissue biopsy, lymph node biopsy or pleural effusion
cytology. On this basis, chest and abdominal enhanced or
non-enhanced CT or total-body positron emission tomography/CT,
brain magnetic resonance imaging and bone scans were further
performed for the assessment of disease severity. Staging was
performed according to the 8th Edition of the International Union
Against Cancer Lung Cancer Tumor-Node-Metastasis staging criteria
(17). The exclusion criteria for
the patients with lung cancer were as follows: i) Acute and chronic
infectious diseases; ii) hepatic diseases; iii) autoimmune
diseases; iv) haematological diseases; v) diabetes; vi) history of
malignant tumours; and vii) incomplete, inaccessible or obviously
abnormal clinical and laboratory data. All cases were further
classified into early (stage IA-IIIA) and advanced (stage IIIB-IV)
according to the benefit from resectable and potentially resectable
surgery (18–20). Healthy individuals were excluded if
they had acute and chronic infectious diseases, vital organ
diseases, a genetic family history of tumours, inaccessible or
obviously abnormal blood tests, or any suspicious lesions found on
chest CT scan. The present study was approved by the Ethics
Committee of the Affiliated Hospital of Jiangsu University. Oral
informed consent was obtained from all participants included in
this retrospective study.
Laboratory assays
Baseline and clinical characteristics, as well as
laboratory measurements of each eligible individual, were obtained
from the electronic medical record system of the Affiliated
Hospital of Jiangsu University. The latest peripheral blood samples
were collected from patients with an empty stomach in the early
morning before diagnosis and any treatment, with a time span of no
more than 1 week between the two. Routine blood tests were
performed using a SYSMEX XN3000 automated haematology analyzer
(Sysmex Corporation). Serum albumin was detected using a BEKMAN
AU5800 automatic biochemical analyzer (Beckman Coulter, Inc.). The
serum carcinoembryonic antigen (CEA) and cytokeratin 19 fragment
antigen 21-1 (CYFRA21-1) levels were determined using the ABBOTT
ARCHITECT i2000sr (Abbott Pharmaceutical Co., Ltd.) and MAGLUMI X8
Analyzer (Shenzhen New Industry Biological Engineering Co., Ltd.),
respectively, using a chemiluminescence immunoassay with kits from
the corresponding manufacturer (cat. no. 7K68-78; cat. no.
130201013M). The normal range of all indicators was recorded
according to the manufacturer's instructions. The MRR, NRR, LRR and
LA of each group were calculated as follows: MRR was defined as the
monocyte count (×109/l) to red blood cell count
(×1012/l) ratio, NRR was defined as the neutrophil count
(×109/l) to red blood cell count (×1012/l)
ratio, LRR was defined as the lymphocyte count (×109/l)
to red blood cell count (×1012/l) ratio, and LA was
defined as the product of the lymphocyte count (×109/l)
and albumin concentration (g/l).
Statistical analysis
All data were analysed using IBM SPSS Statistics
22.0 (IBM Corp.), MedCalc 20.1.0. (MedCalc Software bvba) and
GraphPad Prism 8.0 (GraphPad Software, Inc.). The
Kolmogorov-Smirnov test was used to evaluate the distribution
characteristics of the data. The continuous variables are presented
as the mean ± (SD) or median (interquartile range). Age is
presented as the median and range. Differences between two groups
were analysed using the unpaired Student's t-test or Mann-Whitney U
test. Comparisons of multiple groups were performed using
Kruskal-Wallis H test with Bonferroni's correction for multiple
post hoc comparisons. Categorical variables are presented as the
number and percentage, and were compared using the χ2
test. Receiver operating characteristic (ROC) curves were used to
evaluate the diagnostic value of markers alone or in combination
for lung cancer. The area under the curve (AUC) values of the
markers were compared using the Z test. Binary logistic regression
analysis was applied for the analysis of the risk factors of lung
cancer. Odds ratios (ORs) and 95% confidence intervals (CIs) were
calculated using logistic regression. Spearman's correlation
analysis was used to assess correlations between peripheral
lymphocyte count and serum albumin concentration. P<0.05 was
considered to indicate a statistically significant difference.
Results
Demographic, clinical and laboratory
characteristics of all participants
A total of 216 patients with newly diagnosed lung
cancer and 184 healthy volunteers were included in the present
study. The former group included 148 patients with adenocarcinoma,
30 patients with squamous cell carcinoma and 38 patients with SCLC.
Among these patients, 60.2% were at the early stages of the disease
(IA-IIIA) and the remaining 39.8% were at the advanced stages
(IIIB-IV). The median age of the patients with lung cancer was 61
years (range, 48–78 years), and males accounted for 57.9%. The
median age of the healthy group was 58 years (range, 47–80 years),
and males accounted for 60.9%. There was no statistical difference
in age or sex composition between the two groups. The comparative
analysis results of the laboratory tests are shown in Table I. White blood cell count, neutrophil
count, monocyte count, CEA and CYFRA21-1 levels were significantly
higher in the patients with lung cancer compared with those in the
controls (all P<0.001), while the red blood cell count,
lymphocyte count and albumin level were significantly lower (all
P<0.001). Compared with those in healthy individuals, the MRR
and NRR were significantly higher (both P<0.001), while the LRR
and LA were significantly lower in patients with lung cancer (both
P<0.05; Table I).
 | Table I.Clinical baseline characteristics of
patients with lung cancer and healthy participants. |
Table I.
Clinical baseline characteristics of
patients with lung cancer and healthy participants.
| Variables | Normal range | Patients with lung
cancer (n=216) | Healthy participants
(n=184) | P-value |
|---|
| Median age (range),
years | - | 61 (48–78) | 58 (47–80) | 0.060 |
| Sex, n (%) |
|
|
| 0.543 |
| Male | - | 125 (57.9) | 112 (60.9) |
|
|
Female | - | 91 (42.1) | 72 (39.1) |
|
| Histopathological
subtype, n (%) |
|
|
|
|
| AC | - | 148 (68.5) | - | - |
|
SCC | - | 30 (13.9) | - | - |
|
SCLC | - | 38 (17.6) | - | - |
| Invasion depth, n
(%) |
|
|
|
|
|
T1+T2 | - | 149 (69.0) | - | - |
|
T3+T4 | - | 67 (31.0) | - | - |
| Lymph node
metastasis, n (%) |
|
|
|
|
| N0 | - | 111 (51.4) | - | - |
|
N1+N2+N3 | - | 105 (48.6) | - | - |
| Distant metastasis,
n (%) |
|
|
|
|
| M0 | - | 154 (71.3) | - | - |
| M1 | - | 62 (28.7) | - | - |
| Clinical stage, n
(%) |
|
|
|
|
|
IA-IIIA | - | 130 (60.2) | - | - |
|
IIIB-IV | - | 86 (39.8) | - | - |
| Laboratory
parameters [median (IQR)] |
|
|
|
|
| WBC
(×109/l) | 3.5-9.5 | 6.3 (5.1-7.5) | 5.6 (4.7-6.4) | <0.001 |
| RBC
(×1012/l) | 4.3-5.8 | 4.4±0.5 | 4.7±0.4 | <0.001 |
|
Neutrophils
(×109/l) | 1.8-6.3 | 3.9 (3.0-4.9) | 3.1 (2.6-4.0) | <0.001 |
|
Monocytes
(×109/l) | 0.1-0.6 | 0.5 (0.4-0.6) | 0.3 (0.3-0.4) | <0.001 |
|
Lymphocytes
(×109/l) | 1.1-3.2 | 1.6 (1.3-2.0) | 1.9 (1.5-2.2) | <0.001 |
| Albumin
(g/l) | 40.00-50.00 | 39.37±3.98 | 44.18±2.28 | <0.001 |
| CEA
(ng/ml) | <5.00 | 3.14
(1.89-5.69) | 1.97
(1.43-2.73) | <0.001 |
|
CYFRA21-1 (ng/ml) | <7.00 | 3.20
(2.40-4.48) | 2.19
(1.69-2.83) | <0.001 |
|
MRR | - | 0.10
(0.08-0.14) | 0.07
(0.06-0.08) | <0.001 |
|
NRR | - | 0.89
(0.68-1.12) | 0.66
(0.55-0.82) | <0.001 |
|
LRR | - | 0.37
(0.30-0.46) | 0.39
(0.32-0.46) | 0.043 |
| LA | - | 65.12
(50.81-76.37) | 81.53
(67.61-97.91) | <0.001 |
Effectiveness of candidate markers in
the diagnosis of lung cancer
The ROC curve was used to evaluate the ability of
the MRR, NRR, LRR and LA to distinguish patients with lung cancer
from healthy individuals. The results revealed that the MRR and LA
had expected diagnostic value for lung cancer, and the MRR had the
higher efficiency. The AUC of the MRR was 0.810 (95% CI,
0.768-0.847), and the sensitivity and specificity were 69.9 and
79.3%, respectively. The diagnostic efficiency of LA was inferior
to MRR, but similar to that of CEA and CYFRA21-1, with an AUC of
0.721 (95% CI, 0.674-0.764), and a sensitivity and specificity of
73.6 and 67.4%, respectively (Fig.
1A; Table II). Due to their
good performance in the ROC curve analysis, MRR and LA were
selected for further analysis. It is widely known that serum CEA
and CYFRA21-1 are common tumour markers for lung cancer (21,22).
The MRR and LA were more sensitive for distinguishing patients with
lung cancer from healthy controls (sensitivity of 69.9 and 73.6%,
respectively), while CEA and CYFRA21-1 had higher specificity
(specificity of 94.0 and 74.5%, respectively). Combined detection
analysis revealed that the MRR could improve the diagnostic
efficacy of CEA and CYFRA21-1. When CEA or CYFRA21-1 was combined
with MRR, the AUC values reached 0.843 and 0.833, respectively,
which was higher than those of CEA (AUC, 0.712) or CYFRA21-1 (AUC,
0.748) alone, with improved sensitivity and specificity
(sensitivity of 73.1 and 68.1%; specificity of 85.3 and 86.4%,
respectively) (Table II). When the
MRR, LA, CEA and CYFRA21-1 were combined for detection, the maximum
diagnostic efficacy and a high sensitivity and specificity were
obtained (AUC, 0.882; sensitivity, 81.9%; specificity, 81.0%;
Fig. 1B; Table II). Subsequently, binary logistic
regression analysis was used to evaluate the risk factors of lung
cancer, and the results showed that reciprocal-transformed MRR (OR,
0.776; 95% CI, 0.723-0.834; P<0.001), LA (OR, 0.969; 95% CI,
0.958-0.981; P<0.001), CEA (OR, 1.226; 95% CI, 1.037-1.449;
P=0.017) and CYFRA21-1 (OR, 1.304; 95% CI, 1.046-1.626; P=0.018)
were all possible risk factors for lung cancer (Table III).
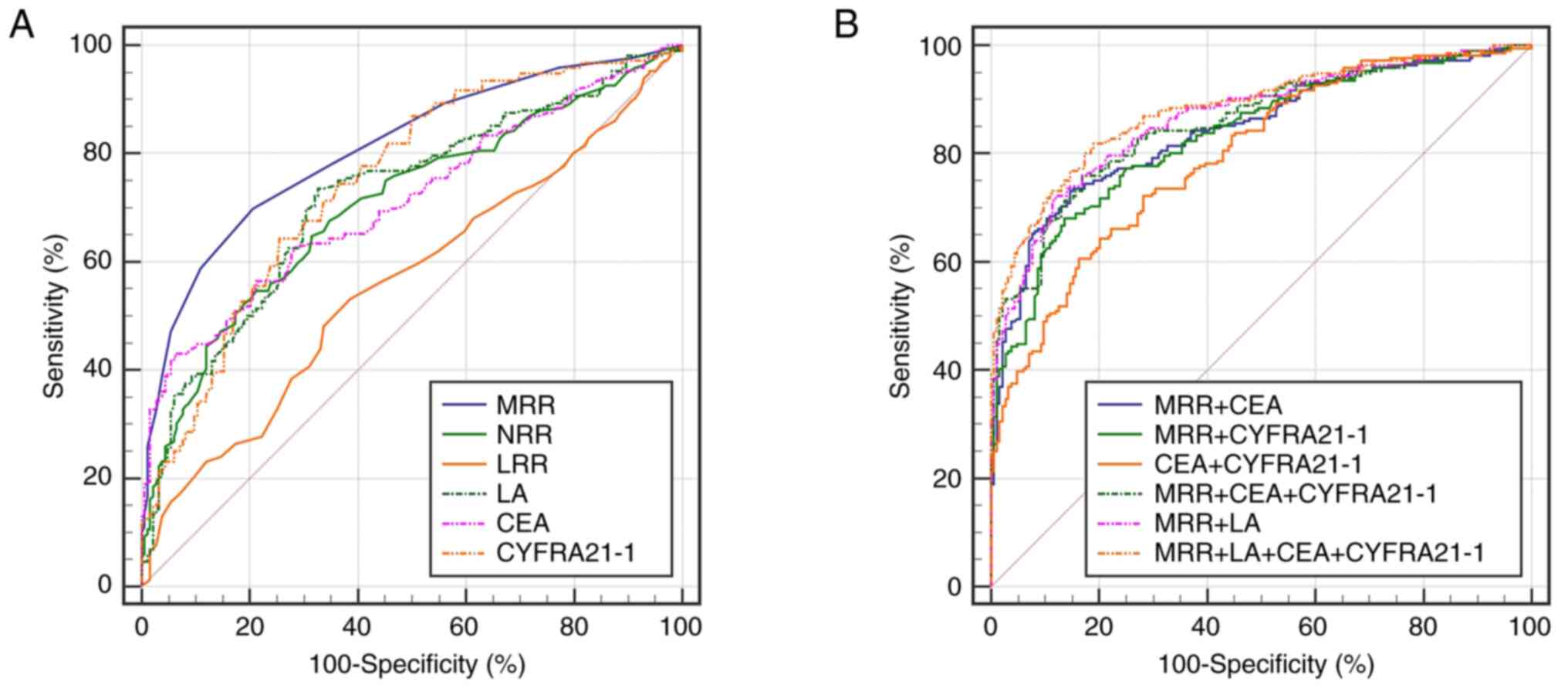 | Figure 1.Receiver operating characteristic
curve analysis of MRR, NRR, LRR, LA, CEA and CYFRA21-1 for the
occurrence of lung cancer. (A) The ability of individual markers to
identify the lung cancer. (B) The ability of combined markers to
identify the lung cancer. MRR, monocyte count to red blood cell
count ratio; NRR, neutrophil count to red blood cell count ratio;
LRR, lymphocyte count to red blood cell count ratio; LA, the
product of lymphocyte count and albumin concentration; CEA,
carcinoembryonic antigen; CYFRA21-1, cytokeratin 19 fragment
antigen 21-1. |
 | Table II.Diagnostic value of MRR, LA, CEA and
CYFRA21-1 alone or in combination in lung cancer. |
Table II.
Diagnostic value of MRR, LA, CEA and
CYFRA21-1 alone or in combination in lung cancer.
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|---|
| Variables | AUC | Cut-off | Sensitivity, % | Specificity, % | Lower limit | Upper limit |
|---|
| MRR | 0.810 a | 0.08 | 69.9 | 79.3 | 0.768 | 0.847 |
| NRR | 0.707a,b | 0.84 | 54.6 | 78.8 | 0.660 | 0.751 |
| LRR | 0.563a,b | 0.37 | 53.2 | 61.4 | 0.513 | 0.612 |
| LA | 0.721a,b | 75.39 | 73.6 | 67.4 | 0.674 | 0.764 |
| CEA | 0.712a,b | 3.80 | 42.6 | 94.0 | 0.665 | 0.756 |
| CYFRA21-1 | 0.748a,b | 2.75 | 64.4 | 74.5 | 0.703 | 0.790 |
| MRR + CEA | 0.843a,b | - | 73.1 | 85.3 | 0.804 | 0.877 |
| MRR +
CYFRA21-1 | 0.833a,b | - | 68.1 | 86.4 | 0.793 | 0.869 |
| CEA +
CYFRA21-1 | 0.796a | - | 60.6 | 83.7 | 0.753 | 0.835 |
| MRR + CEA +
CYFRA21-1 | 0.856a | - | 75.9 | 83.2 | 0.817 | 0.889 |
| MRR + LA | 0.866a | - | 72.2 | 88.0 | 0.828 | 0.897 |
| MRR + LA + CEA +
CYFRA21-1 | 0.882 | - | 81.9 | 81.0 | 0.846 | 0.912 |
 | Table III.Binary logistic regression analysis
of potential risk factors for lung cancer. |
Table III.
Binary logistic regression analysis
of potential risk factors for lung cancer.
| Variable | Β | S.E. | Wald
χ2 | P-value | OR (95% CI) |
|---|
| MRRa | −0.253 | 0.036 | 48.200 | <0.001 | 0.776
(0.723-0.834) |
| LA | −0.031 | 0.006 | 25.741 | <0.001 | 0.969
(0.958-0.981) |
| CEA | 0.204 | 0.085 | 5.716 | 0.017 | 1.226
(1.037-1.449) |
| CYFRA21-1 | 0.265 | 0.113 | 5.558 | 0.018 | 1.304
(1.046-1.626) |
| Constant | 4.375 | 0.937 | 21.789 | <0.001 | - |
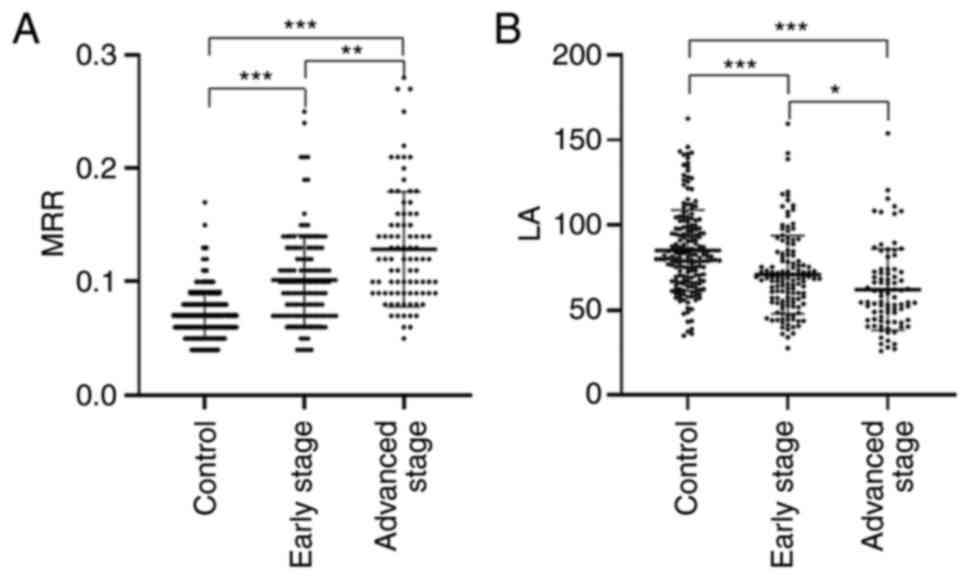
When the levels of MRR and LA were compared among
the control, early stage and advanced stage groups, the advanced
group showed the highest MRR and lowest LA, followed by the early
group and then the control group (P<0.05; Fig. 2A and B). In order to further assess
the ability of candidate diagnostic markers to detect patients with
lung cancer at early stages, the age (P=0.346) and sex composition
(P=0.056) of 184 healthy participants and 130 patients with lung
cancer at stage IA-IIIA were analysed. The ROC curve analysis
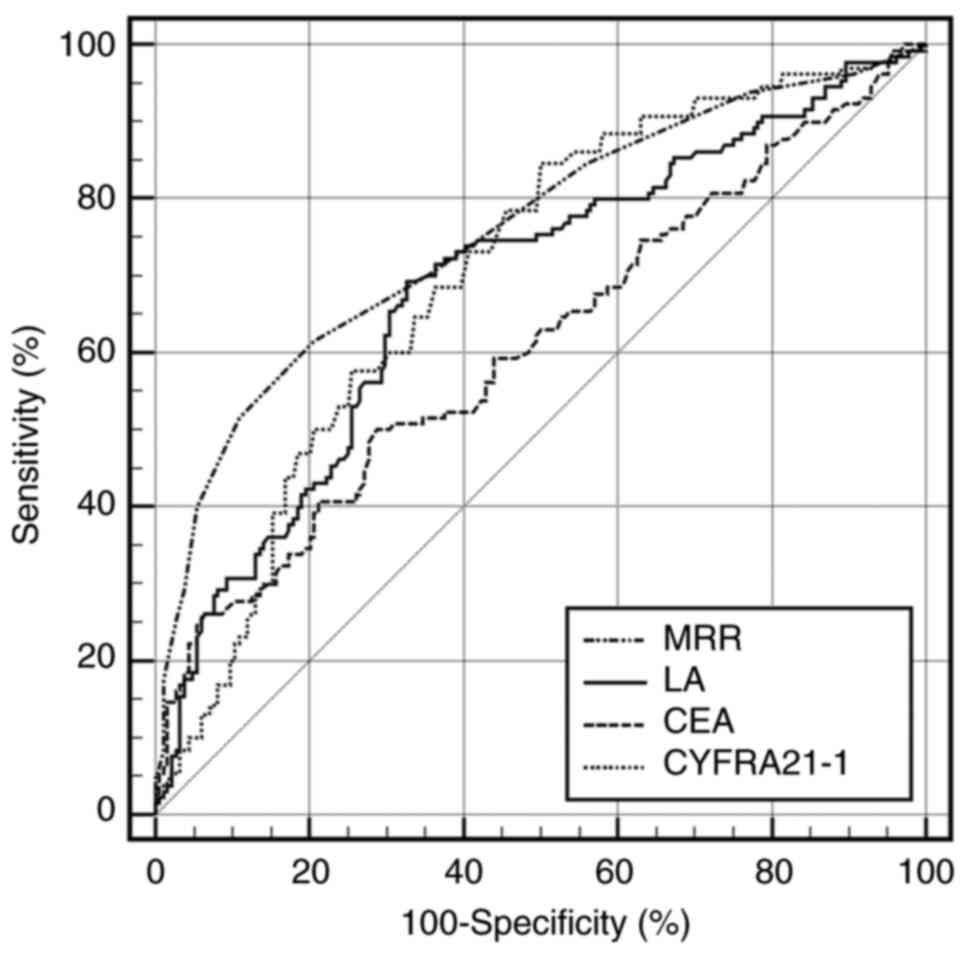
revealed that the ability of MRR in differentiating patients in the
early stages from the healthy controls was stronger than that of
CEA (AUC, 0.761; 95% CI, 0.710-0.807; sensitivity, 62.0%;
specificity, 79.3%; Fig. 3;
Table IV).
 | Table IV.Ability of MRR, LA, CEA and CYFRA21-1
to identify patients with early stage (IA-IIIA) lung cancer. |
Table IV.
Ability of MRR, LA, CEA and CYFRA21-1
to identify patients with early stage (IA-IIIA) lung cancer.
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|---|
| Variables | AUC | Cut-off | Sensitivity
(%) | Specificity
(%) | Lower limit | Upper limit |
|---|
| MRR | 0.761 | 0.08 | 62.0 | 79.3 | 0.710 | 0.807 |
| LA | 0.683 | 75.39 | 69.0 | 67.4 | 0.628 | 0.734 |
| CEA | 0.610a | 2.48 | 50.4 | 71.2 | 0.554 | 0.664 |
| CYFRA21-1 | 0.702 | 2.18 | 84.5 | 50.0 | 0.648 | 0.752 |
Associations among the MRR, LA and
clinical characteristics in patients with lung cancer
The associations among MRR, LA, baseline
characteristics (age and sex) and clinical characteristics
(histopathological subtype, invasion depth, lymph node metastasis,
distant metastasis and clinical stage) were analysed. Age and sex
were identified as potential confounding factors and adjusted for
using binary logistic regression. As shown in Table V, the MRR and LA were closely
related to clinical characteristics in patients with lung cancer.
High MRR levels were significantly related to invasion depth
(P=0.012), regional lymph node metastasis (P=0.003), distant
metastasis (P=0.025) and clinical staging (P=0.002), while LA was
closely related to invasion degree (P<0.001), lymph node
metastasis (P=0.009) and clinical staging (P=0.031). Patients with
different pathological subtypes showed different levels of MRR and
LA. In patients with SCLC, relatively high levels of MRR could be
seen compared with those of patients with NSCLC (P=0.006), while
the levels of LA seem to be lower, but statistically
non-significant (P=0.142). According to the present analysis, MRR
tended to increase as the disease progressed, while LA exhibited
the opposite trend.
 | Table V.Associations between MRR, LA levels
and clinical characteristics in lung cancer. |
Table V.
Associations between MRR, LA levels
and clinical characteristics in lung cancer.
|
Characteristics | n | MRR | P-value | LA | P-value |
|---|
| Sex |
|
| <0.001 |
| 0.018 |
|
Male | 125 | 0.12
(0.09-0.15) |
| 62.22
(44.73-74.13) |
|
|
Female | 91 | 0.09
(0.07-0.11) |
| 68.48
(56.70-82.28) |
|
| Age, years |
|
| 0.002 |
| 0.006 |
|
≤60 | 106 | 0.10
(0.07-0.12) |
| 68.22
(55.01-83.25) |
|
|
>60 | 110 | 0.11
(0.09-0.14) |
| 62.02
(45.92-72.73) |
|
| Histopathological
subtype |
|
| 0.006 |
| 0.142 |
|
NSCLC | 178 | 0.10
(0.07-0.13) |
| 67.43
(53.01-78.78) |
|
|
SCLC | 38 | 0.13
(0.10-0.16) |
| 54.53
(44.10-67.39) |
|
| Invasion depth |
|
| 0.012 |
| <0.001 |
|
T1+T2 | 149 | 0.10
(0.07-0.13) |
| 68.76
(54.66-83.79) |
|
|
T3+T4 | 67 | 0.11
(0.09-0.16) |
| 54.56
(43.08-68.48) |
|
| Lymph node
metastasis |
|
| 0.003 |
| 0.009 |
| N0 | 111 | 0.09
(0.07-0.12) |
| 68.96
(57.90-83.38) |
|
|
N1-N3 | 105 | 0.12
(0.09-0.15) |
| 54.74
(44.26-72.50) |
|
| Distant
metastasis |
|
| 0.025 |
| 0.608 |
| M0 | 154 | 0.10
(0.07-0.13) |
| 66.94
(52.34-77.74) |
|
| M1 | 62 | 0.12
(0.09-0.16) |
| 61.97
(45.66-75.97) |
|
| Clinical
staging |
|
| 0.002 |
| 0.031 |
|
IA-IIIA | 130 | 0.10
(0.07-0.13) |
| 68.70
(55.12-81.47) |
|
|
IIIB-IV | 86 | 0.12
(0.09-0.15) |
| 56.38
(44.17-72.42) |
|
Discussion
Numerous studies have demonstrated that
hematological markers serve an important role in the pathogenesis
and progression of malignancies (23–25).
Lung cancer has the second highest incidence among all cancer types
(1). A delayed diagnosis would
result in disease progression and a poor prognosis. Histological
diagnosis is still considered the gold standard for establishing a
definite diagnosis. However, ideal non-invasive markers are being
sought as an auxiliary method for diagnosis. Sensitivity and
specificity are equally important for such diagnostic markers to
reduce the rates of missed diagnosis and misdiagnosis. Several
novel markers, such as MRR, LRR, NRR and LA, have initially been
studied in neoplastic diseases. Peng et al (26) demonstrated a close association
between MRR and early stage colorectal cancer, and MRR had a better
prognostic value for patients with highly or moderately
differentiated tumours. Wang et al (15) proposed red blood cell count as a
reference index to balance individual differences, and demonstrated
that the MRR, NRR and LRR were independent prognostic factors for
disease-free survival in patients with advanced breast cancer by
reflecting the intensity of the inflammatory immunological
reaction. Furthermore, Yamamoto et al (16) performed a comprehensive assessment
of various circulating inflammatory markers in the prognosis
prediction of patients with stage II/III rectal cancer undergoing
radical resection, and found that LA was associated with overall
survival and recurrence-free survival. Low levels of LA suggested a
poor prognosis of the disease.
To the best of our knowledge, the roles of these
markers in lung cancer remain unclear. The present study aimed to
investigate the diagnostic value of the aforementioned markers
among patients with lung cancer. High MRR and NRR, and low LRR and
LA values were found in patients with newly diagnosed lung cancer.
After adjusting for age and sex in binary logistic regression
analysis, there were significant associations between MRR and
histopathological subtype, lymph node metastasis, distant
metastasis and clinical stage, and between LA and invasion depth,
lymph node metastasis and clinical stage. MRR and LA had the
potential to distinguish patients with lung cancer from the healthy
controls, especially when combined with CEA and CYFRA21-1, which
had a higher sensitivity. However, only the MRR showed an advantage
in the detection of patients with lung cancer at early stages
(IA-IIIA). Additionally, by analysing the present results, it could
be hypothesized that increased MRR, as well as CEA and CYFRA21-1
concentrations, and decreased LA could be risk factors for lung
cancer.
It is widely known that monocytes/macrophages are
involved in tumour progression. Circulating monocytes are highly
adaptive cells, migrating and differentiating with the change of
certain circumstances. Derived from circulating monocytes,
tumour-associated macrophages stimulate tumour cell proliferation,
promote angiogenesis and lymphangiogenesis, and help with tumour
invasion and metastasis, by secreting growth factors, cytokines and
proteases (27). Although the exact
mechanism behind the increase in total circulating monocytes
remains unclear, the activation of the tumour-associated
monocyte-macrophage system can be clearly observed. Regarded as the
monocyte-adjusted red blood cell level, the MRR might be a better
marker than the absolute monocyte count for lung cancer.
As another important type of tumour-related immune
cells, lymphocytes mainly serve a role in immune surveillance and
tumour cytotoxicity, and can interact with tumour-associated
macrophages. Both circulating lymphocytes and tumour-infiltrating
lymphocytes reflect the immune response to the tumour. Decreases in
the circulating lymphocyte count and changes in lymphocyte subsets,
including natural killer (NK) cells, CD4+ T cells and
CD8+ T cells, are observed in most malignant tumours
(28). Compared with those in
healthy controls, the NK cell count and naive
CD4+/CD4+ T lymphocyte numbers are decreased,
and the percentage of activated CD8+ T lymphocytes
(CD38+/HLA-DR+) is increased in multiple
solid tumours, including lung cancer, and these exhibit a
corresponding trend as the disease progresses (29). In the present study, a decrease in
the total count of circulating lymphocytes was also observed, which
might reflect the weakened immune response to the tumour and the
poor prognosis of the patients.
Serum albumin, as another valuable serum marker
reflecting the systemic nutriture and inflammation, has also been
demonstrated to be negatively associated with the risk of lung,
liver and colorectal cancer (30).
It has been reported that low albumin levels are associated with
the poor prognosis in patients with advanced NSCLC treated with
erlotinib (31). Although serum
albumin is not specific to nutritional evaluation, it can partly
reflect protein-energy wasting and the inflammatory response
(32). Previous studies
demonstrated that the lymphocyte levels were influenced by the
nutritional status (33,34). However, an association between
lymphocyte levels and albumin was not observed in the present study
(Fig. S1). Based on the
aforementioned preconditions, we hypothesized that LA could partly
reflect the body's immune and nutritional conditions, and might
have a potential role in distinguishing patients with lung cancer
from healthy individuals.
Based on the aforementioned results, we hypothesized
that the MRR and LA may be potential markers for the clinical
auxiliary diagnosis of lung cancer and assessment of disease
progression. MRR may be a promising marker supplementary to imaging
examinations in screening for lung cancer at early stages. Due to
insufficient sensitivity and specificity, it is inappropriate for
MRR and LA to be used alone as an early-diagnostic marker for lung
cancer. The MRR and LA might be influenced by other factors. It is
therefore essential to augment MRR and LA monitoring with other
biomarkers, including serum tumour markers, in order to decrease
misdiagnoses and missed diagnoses. The changes in these markers may
serve as wake-up calls for clinicians when observed with suspected
lung cancer symptoms. Due to the universality and repeatability of
these indicators, they have the potential to be used as auxiliary
indicators in clinical diagnosis and treatment, especially in areas
without sufficient medical resources.
To the best of our knowledge, this was a novel
attempt to explore the roles of red blood cell-derived markers and
LA in the field of lung cancer. There remain some limitations in
the present study. Firstly, it was a retrospective, single-centre
and single cut-off point study. The small sample size may affect
the reported results. Although there were cut-off values for these
markers in the present study, it is still hard to say that there
are definite thresholds to indicate lung cancer. Larger,
multicentre and prospective studies are required to examine the
efficacy and to learn more about those markers in cases of lung
cancer. Secondly, the present study focused on the comparison and
analysis between the two groups, namely, patients with lung cancer
and healthy individuals. Future studies could be conducted between
benign lung diseases and lung cancer.
In conclusion, MRR and LA could be used as novel
effective auxiliary markers for the diagnosis of patients with lung
cancer, especially when combined with CEA and CYFRA21-1. MMR and LA
could also partly indicate disease progression. As cheap, easily
accessible and effective blood markers, MRR and LA may have
potential value in clinical practice.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the National
Natural Science Foundation of China (no. 81370119).
Availability of data and materials
The datasets used or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XXC and FHQ made substantial contributions to the
conception and design of the work. XXC, STZ, XMY and SCH performed
the data collection and data analysis. XXC interpreted the data,
and was involved in drafting and revision of the manuscript. FHQ
revised the manuscript critically for important intellectual
content and gave final approval of the version to be published.
XXC, STZ, XYM and SCH confirm the authenticity of all the raw data.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethical Review
Committee of Jiangsu University Affiliated Hospital (Zhenjiang,
China). All patients provided oral informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN Estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jonas DE, Reuland DS, Reddy SM, Nagle M,
Clark SD, Weber RP, Enyioha C, Malo TL, Brenner AT, Armstrong C, et
al: Screening for lung cancer with low-dose computed tomography:
Updated evidence report and systematic review for the US preventive
services task force. JAMA. 325:971–987. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W, Xin S and Xu B: Value Research of
NLR, PLR, and RDW in prognostic assessment of patients with
colorectal cancer. J Healthc Eng. 2022:79714152022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mandaliya H, Jones M, Oldmeadow C and
Nordman II: Prognostic biomarkers in stage IV non-small cell lung
cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to
monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and
advanced lung cancer inflammation index (ALI). Transl Lung Cancer
Res. 8:886–894. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garrett C, Becker TM, Lynch D, Po J, Xuan
W, Scott KF and de Souza P: Comparison of neutrophil to lymphocyte
ratio and prognostic nutritional index with other clinical and
molecular biomarkers for prediction of glioblastoma multiforme
outcome. PLoS One. 16:e02526142021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jakubowska K, Koda M, Grudzińska M,
Kańczuga-Koda L and Famulski W: Monocyte-to-lymphocyte ratio as a
prognostic factor in peripheral whole blood samples of colorectal
cancer patients. World J Gastroenterol. 26:4639–4655. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fang T, Wang Y, Yin X, Zhai Z, Zhang Y,
Yang Y, You Q, Li Z, Ma Y, Li C, et al: Diagnostic Sensitivity of
NLR and PLR in early diagnosis of gastric cancer. J Immunol Res.
2020:91460422020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baracos VE, Martin L, Korc M, Guttridge DC
and Fearon KCH: Cancer-associated cachexia. Nat Rev Dis Primers.
4:171052018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z, Pereira SL, Luo M and Matheson
EM: Evaluation of blood biomarkers associated with risk of
malnutrition in older adults: A systematic review and
meta-analysis. Nutrients. 9:8292017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eckart A, Struja T, Kutz A, Baumgartner A,
Baumgartner T, Zurfluh S, Neeser O, Huber A, Stanga Z, Mueller B
and Schuetz P: Relationship of nutritional status, inflammation,
and serum albumin levels during acute illness: A prospective study.
Am J Med. 133:713–722.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie H, Wei L, Tang S and Gan J: Prognostic
value of pretreatment albumin-to-alkaline phosphatase ratio in
cancer: A meta-analysis. Biomed Res Int. 2020:66610972020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang JR, Xu JY, Chen GC, Yu N, Yang J,
Zeng DX, Gu MJ, Li DP, Zhang YS and Qin LQ: Post-diagnostic
C-reactive protein and albumin predict survival in Chinese patients
with non-small cell lung cancer: A prospective cohort study. Sci
Rep. 9:81432019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Wang H, Yin W, Lin Y, Zhou L,
Sheng X, Xu Y, Sha R and Lu J: Novel lymphocyte to red blood cell
ratio (LRR), neutrophil to red blood cell ratio (NRR), monocyte to
red blood cell ratio (MRR) as predictive and prognostic biomarkers
for locally advanced breast cancer. Gland Surg. 8:627–635. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamamoto T, Kawada K, Hida K, Matsusue R,
Itatani Y, Mizuno R, Yamaguchi T, Ikai I and Sakai Y: Combination
of lymphocyte count and albumin concentration as a new prognostic
biomarker for rectal cancer. Sci Rep. 11:50272021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lang-Lazdunski L: Surgery for nonsmall
cell lung cancer. Eur Respir Rev. 22:382–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bograd AJ and Vallières E: Surgery as a
component of multimodality care for known stage IIIA-N2 non-small
cell lung cancer. Semin Respir Crit Care Med. 41:346–353. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wakeam E, Acuna SA, Leighl NB, Giuliani
ME, Finlayson SRG, Varghese TK and Darling GE: Surgery versus
chemotherapy and radiotherapy for early and locally advanced small
cell lung cancer: A propensity-matched analysis of survival. Lung
Cancer. 109:78–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang ZH, Han YW, Liang H and Wang LM:
Prognostic value of serum CYFRA21-1 and CEA for non-small-cell lung
cancer. Cancer Med. 4:1633–1638. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song WA, Liu X, Tian XD, Wang W, Liang CY,
Zhang T, Guo JT, Peng YH and Zhou NK: Utility of squamous cell
carcinoma antigen, carcinoembryonic antigen, Cyfra 21-1 and neuron
specific enolase in lung cancer diagnosis: A prospective study from
China. Chin Med J (Engl). 124:3244–3248. 2011.PubMed/NCBI
|
|
23
|
Giannakeas V, Kotsopoulos J, Cheung MC,
Rosella L, Brooks JD, Lipscombe L, Akbari MR, Austin PC and Narod
SA: Analysis of platelet count and new cancer diagnosis over a
10-year period. JAMA Netw Open. 5:e21416332022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan Q, Liu S, Liang C, Han X and Shi Y:
Pretreatment hematological markers predict clinical outcome in
cancer patients receiving immune checkpoint inhibitors: A
meta-analysis. Thorac Cancer. 9:1220–1230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan SWS, Smith E, Aggarwal R, Balaratnam
K, Chen R, Hueniken K, Fazelzad R, Weiss J, Jiang S, Shepherd FA,
et al: Systemic inflammatory markers of survival in epidermal
growth factor-mutated non-small-cell lung cancer:
Single-institution analysis, systematic review, and meta-analysis.
Clin Lung Cancer. 22:390–407. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng F, Hu D, Lin X, Chen G, Liang B, Li
C, Chen Y, Cui Z, Zhang H, Lin J, et al: The monocyte to red blood
cell count ratio is a strong predictor of postoperative survival in
colorectal cancer patients: The Fujian prospective investigation of
cancer (FIESTA) study. J Cancer. 8:967–975. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Robinson A, Han CZ, Glass CK and Pollard
JW: Monocyte regulation in homeostasis and malignancy. Trends
Immunol. 42:104–119. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wesselius LJ, Wheaton DL, Manahan-Wahl LJ,
Sherard SL, Taylor SA and Abdou NA: Lymphocyte subsets in lung
cancer. Chest. 91:725–729. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang YY, Zhou N, Liu HS, Gong XL, Zhu R,
Li XY, Sun Z, Zong XH, Li NN, Meng CT, et al: Circulating activated
lymphocyte subsets as potential blood biomarkers of cancer
progression. Cancer Med. 9:5086–5094. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Z, Zheng Y, Wu Z, Wen Y, Wang G, Chen
S, Tan F, Li J, Wu S, Dai M, et al: Association between
pre-diagnostic serum albumin and cancer risk: Results from a
prospective population-based study. Cancer Med. 10:4054–4065. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fiala O, Pesek M, Finek J, Racek J,
Minarik M, Benesova L, Bortlicek Z, Sorejs O, Kucera R and Topolcan
O: Serum albumin is a strong predictor of survival in patients with
advanced-stage non-small cell lung cancer treated with erlotinib.
Neoplasma. 63:471–476. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
White JV, Guenter P, Jensen G, Malone A
and Schofield M; Academy of Nutrition and Dietetics Malnutrition
Work Group and A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board
of Directors, : Consensus statement of the Academy of Nutrition and
Dietetics/American society for parenteral and enteral nutrition:
Characteristics recommended for the identification and
documentation of adult malnutrition (undernutrition). J Acad Nutr
Diet. 112:730–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagai M, Noguchi R, Takahashi D, Morikawa
T, Koshida K, Komiyama S, Ishihara N, Yamada T, Kawamura YI, Muroi
K, et al: Fasting-Refeeding impacts immune cell dynamics and
mucosal immune responses. Cell. 178:1072–1087.e14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iyer SS, Chatraw JH, Tan WG, Wherry EJ,
Becker TC, Ahmed R and Kapasi ZF: Protein energy malnutrition
impairs homeostatic proliferation of memory CD8 T cells. J Immunol.
188:77–84. 2012. View Article : Google Scholar : PubMed/NCBI
|