Introduction
Endometrial cancer (EC) is the most common
gynecologic malignancy worldwide, with an incidence estimated at
5.9% and continuing to rise (1,2). EC
ranks as the fourth most frequently occurring cancer among females
and exerts a high psychological and physical burden on patients
(3). The standard treatment for
metastatic endometrial carcinoma includes chemotherapy based on
platinum drugs and hormone therapy (4). Although EC is usually diagnosed at an
early stage and the prognosis is generally good, some patients
experience metastatic or recurrent EC, and their 5-year survival
rate is only 10–20% (5). Therefore,
the discovery of novel drugs for the treatment of EC is
required.
Platycodin D (PD), a triterpenoid saponin extracted
from the roots of Platycodin grandiflorum, has been reported
to possess anticancer effects in several cancer cell lines,
including lung, gastric and prostate cancer cells (6). PD has been shown to suppress the tumor
growth of breast cancer cells in vitro and in vivo
via the inhibition of mouse double minute 2 homolog and mutated p53
in MDA-MB-231 cells (7). In
addition, PD has been reported to inhibit the proliferation,
migration and invasion of MDA-MB-231 human breast cancer cells via
the suppression of EGF-induced activation of the EGFR, MAPK and
PI3K/Akt signaling pathways (8).
Furthermore, PD markedly impacts the proliferation of prostate
cancer cells via the induction of apoptosis and cell cycle arrest
(9). However, the mechanisms
underlying the effects of PD on EC cells are yet to be fully
elucidated.
A preliminary analysis performed for the present
study using the ENCORI database (10), revealed that the α2A-adrenergic
receptor (ADRA2A) is downregulated in EC tissues, and the low
expression of ADRA2A is associated with poor outcomes in patients
with EC. In addition, a previous study showed that ADRA2A
expression is markedly downregulated in cervical cancer tissue and
cell lines, while its overexpression suppresses the proliferation,
migration and invasion of cervical cancer cells. Moreover, the
study also demonstrated that ADRA2A overexpression promotes cell
aging and apoptosis, and decreases the phosphorylation levels of
PI3K, Akt and mTOR in cervical cancer cells (11). Therefore, it is hypothesized that
ADRA2A may block PI3K/Akt signaling pathways in other diseases.
Notably, the SwissTargetPrediction webtool (12) predicts that PD targets ADRA2A, and
therefore may regulate its expression. However, whether ADRA2A
inhibits the proliferation, migration and invasion of EC cells
remains to be fully elucidated. Therefore, the present study aimed
to investigate the effects and underlying mechanisms of PD and the
roles of ADRA2A in the proliferation, migration and invasion of EC
cells, in particular, whether they involve the PI3K/Akt signaling
pathway.
Materials and methods
Bioinformatics tools
The ENCORI database (https://starbase.sysu.edu.cn/index.php) was used to
detect ADRA2A expression in endometrial cancer tissues. The
SwissTargetPrediction webtool (http://www.swisstargetprediction.ch/) predicted the
interaction between PD and ADRA2A.
Cell culture
The human endometrial stromal cell (HESCs; cat. no.
CRL-4003) line and RL95-2 human EC cell line were obtained from the
American Type Culture Collection. The ESCs were cultured in a 1:1
mixture of DMEM/F12 (Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 0.1 mg/ml streptomycin (Beyotime Institute of
Biotechnology) in a T25 culture flask with 5% CO2 and
95% air at 37°C. The RL95-2 cells were grown in DMEM/F12 containing
10% FBS, 100 U/ml penicillin and 0.1 mg/ml streptomycin at 37°C in
a humidified atmosphere containing 5% CO2. PD was
purchased from Shanghai Yuanye Bio-Technology Co., Ltd.
Cell Counting Kit-8 (CCK-8) assay
ESCs and RL95-2 cells were seeded into 96-well
plates at a density of 4×104 cells/well and incubated
for 24 h at 37°C. Following cell attachment, 5, 10, 20 and 40 µM PD
solution was added into each well. Untreated cells served as a
control. Subsequently, the plate was incubated for 24, 48 and 72 h
at 37°C, and 10 µl CCK-8 reagent (Beyotime Institute of
Biotechnology) was added to each well. A microplate reader was used
to measure the optical density at 450 nm.
Cell transfection
Short hairpin (sh)RNA vectors with a plasmid
backbone of pRNA-U6.1 targeting ADRA2A (sh-ADRA2A-1,
5′-GGATCAAGACATAAGTAAA-3′ and sh-ADRA2A-2,
5′-GCTCAAGATTCAAGATACA-3′) and a negative control (sh-NC,
5′-CCGGCAACAAGATGAAGAGCACCAACTC-3′) were synthesized by
ChemicalBook. RL95-2 cells were transfected with 100 nM sh-ADRA2A
or sh-NC at 37°C for 48 h. All transfection experiments were
carried out using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) following the manufacturer's
protocol. Cells were collected for subsequent experiments at 48 h
post-transfection.
Colony formation assay
RL95-2 cells in the logarithmic growth phase were
cultured in culture dishes (diameter, 60 mm) at a concentration of
1×103 cells/100 µl at 37°C for 10 days with or without
5, 10 or 20 µM PD. Subsequently, the medium was removed and the
cells were rinsed in PBS. The cells were then fixed with methanol
for 15 min at room temperature and stained with Giemsa for 10 min
at room temperature. Clusters containing >50 cells were
identified as a colony. The colonies were counted manually and
images captured under a microscope (Olympus Corporation). All
experiments were performed in triplicate.
Western blotting
Total proteins were obtained from RL95-2 cells
treated with or without 5, 10 or 20 µM PD for 24 h, or from ESCs by
lysis using cold RIPA lysis buffer (Beyotime Institute of
Biotechnology). Protein concentration was determined using a BCA
Protein Assay kit (Beijing Solarbio Science & Technology Co.,
Ltd.) and protein samples (20 µg per lane) were separated on 10%
gels by SDS-PAGE (Thermo Fisher Scientific, Inc.), transferred onto
PVDF membranes (MilliporeSigma) and incubated for 1 h at room
temperature with 5% skimmed milk. The membranes were then incubated
at 4°C overnight with the following primary antibodies: Ki67
(1:1,000; ab92742), proliferating cell nuclear antigen (PCNA;
1:1,000; ab92552), MMP2 (1:1,000; ab92536), MMP9 (1:1,000;
ab76003), ADRA2A (1:1,000; ab85570), phospho (p)-PI3K (1:1,000;
ab182651), p-Akt (1:1,000; ab192623), PI3K (1:1,000; ab191606), Akt
(1:1,000; ab179463) and GAPDH (1:2,500; ab9485), all from Abcam.
After washing with TBST (0.1% Tween), the membranes were incubated
with Goat Anti-Rabbit IgG H&L (HRP) secondary antibody
(1:2,000; ab6721; Abcam) at 37°C for 1 h. The immunoreactive
protein bands were visualized using an enhanced chemiluminescence
detection system (Amersham; Cytiva) according to the manufacturer's
instructions. Subsequently, protein brands were observed using
ImageJ (v1.8.0; National Institutes of Health) and quantified by
densitometry (QuantityOne 4.5.0 software; Bio-Rad Laboratories,
Inc.). GAPDH served as an internal reference.
Wound healing assay
RL95-2 cells treated with 5, 10 or 20 µM
concentrations of PD were seeded into 6-well plates (500
cells/well) and cultured until 90% confluence was reached. Five
scratches were made in each well using a 200-µl pipette tip. The
wells were washed with PBS three times to remove the detached cells
from the wound. Subsequently, following the application of
serum-free medium, the cells were incubated at 37°C in 5%
CO2 for 24 h. Cell migration was evaluated using a light
microscope (Olympus Corporation). The relative migration rate
(%)=(wound width at 0 h-wound width at 48 h)/wound width at 0 h
×100.
Transwell assay
RL95-2 cells were suspended at a final concentration
of 2×105 cells/ml. DMEM/F12 (200 µl) was placed into the
upper compartment of a Transwell chamber, precoated with Matrigel
(Sigma-Aldrich; Merck KGaA) at 37°C for 30 min, following treatment
with 5, 10 or 20 µM concentrations of PD. Subsequently, medium
supplemented with 10% FBS was added to the lower chamber. Following
24 h of incubation at 37°C, a cotton swab was used to remove the
non-invasive cells. The remaining invaded cells were fixed with 4%
paraformaldehyde for 5 min at room temperature and stained using
0.1% crystal violet for 20 min at room temperature. A light
microscope (Olympus Corporation) was used to observe and count the
stained cells.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from RL95-2 cells treated
with or without 5, 10 or 20 µM PD for 24 h, and from ESCs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) following the manufacturer's protocol. Subsequently, cDNA was
obtained by reverse transcription of the extracted RNA using a
PrimeScript™ RT reagent kit (cat. no. RR037Q; Takara Bio, Inc.).
The temperature protocol was 15 min at 37°C, 5 sec at 85°C and 30
min at 4°C. qPCR was conducted using a SYBR green qPCR kit (Takara
Bio, Inc.) in a Roche LightCycler®96 system (Roche
Diagnostics GmbH) following the manufacturer's protocol. The primer
sequences for PCR were as follows: ADRA2A:
5′-ATCCTGGCCTTGGGAGAGAT-3′ (forward) and 5′-TCTCAAAGCAGGTCCGTGTC-3′
(reverse); GAPDH: 5′-GGGAAACTGTGGCGTGAT-3′ (forward) and
5′-GAGTGGGTGTCGCTGTTGA-3′ (reverse). The PCR thermocycling program
was 95°C for 3 min, followed by 35 cycles of denaturation at 95°C
for 30 sec, annealing at 60°C for 30 sec and extension at 72°C for
1 min. A final extension step at 72°C for 7 min was performed in
each qPCR assay. The 2−ΔΔCq method was used for
quantification (13).
MTT assay
An MTT assay was carried out to assess the
proliferation of EC cells. Briefly, RL95-2 cells were seeded into
96-well plates (2×103/well) for 24, 48 and 72 h
incubation with or without 20 µM PD. Following incubation, 10 µl
MTT (5 mg/ml) was added to each well and incubation was continued
for a further 4 h. Subsequently, the culture medium was discarded,
150 µl DMSO (Thermo Fisher Scientific, Inc.) was added and the
plates were shaken for 10 min for full dissolution. The absorbance
was read at 490 nm using a Microplate Autoreader (Omega Bio-Tek,
Inc.).
Statistical analysis
All experiments were repeated three times, with
three replicates each time. Statistical analysis was carried out
using SPSS 19.0 statistical software (IBM Corp). Data are presented
as the mean ± standard deviation. One-way ANOVA followed by
Bonferroni's post hoc test was used to assess differences among
multiple groups, and an unpaired Student's t-test was used to
analyze differences between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
PD inhibits the viability of EC
cells
The effects of PD on the viability of ESCs and
RL95-2 cells treated with different concentrations of PD for 24 h
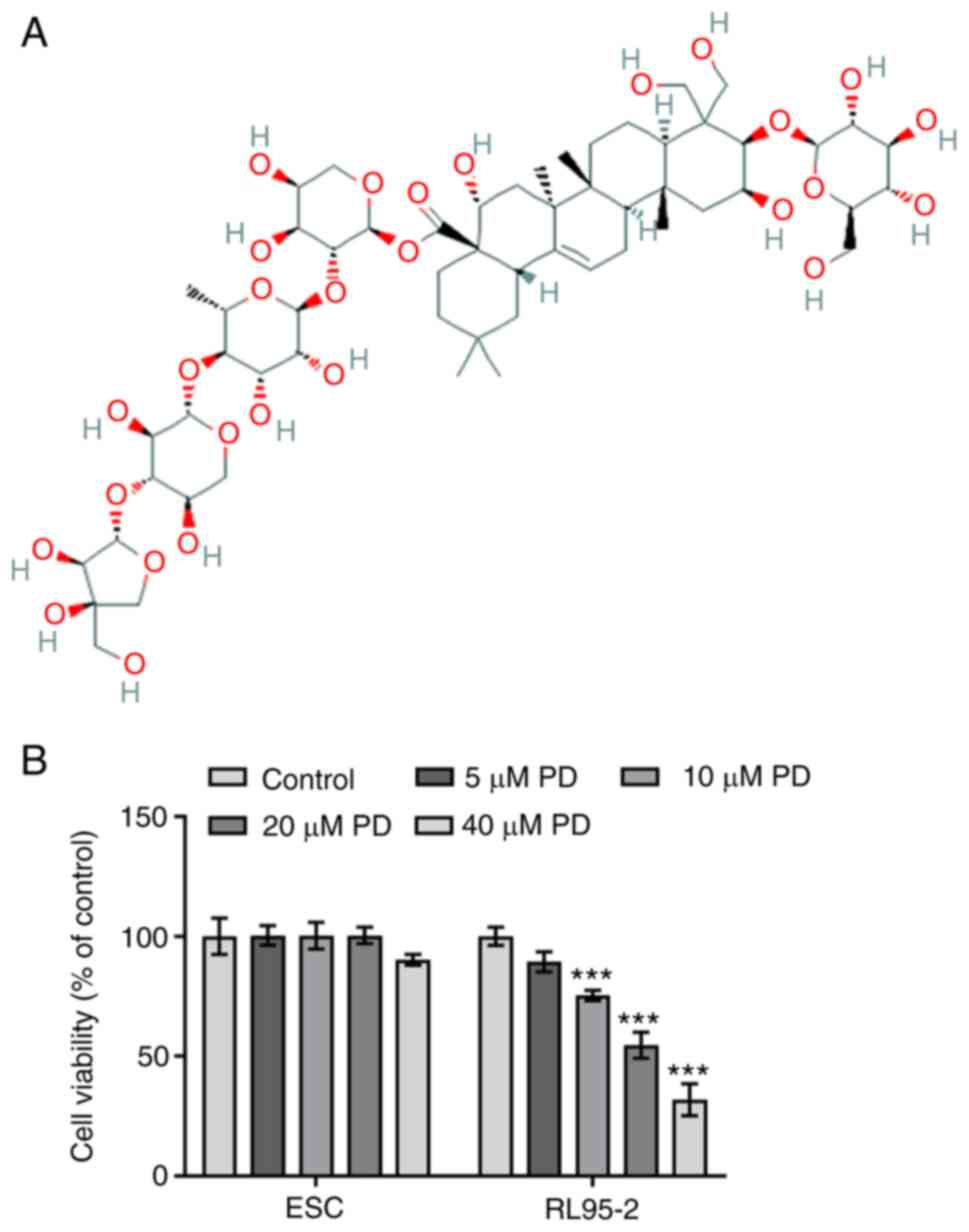
were investigated. The chemical structure of PD is shown in
Fig. 1A. No change was observed in
the viability of ESCs treated with 5, 10 and 20 µM PD, while the
viability of ESCs treated with 40 µM PD was slightly decreased
compared with that of the untreated control, as displayed in
Fig. 1B. The results indicate that
40 µM PD exerted an inhibitory effect on normal endometrial cell
viability. However, the viability of RL95-2 cells treated with
higher concentrations of PD (10–40 µM) was significantly reduced
compared with that of the untreated control group. These results
indicate that various concentrations of PD inhibited the viability
of EC cells. On the basis of these results, 5, 10 and 20 µM
concentrations of PD were selected for use in subsequent
experiments.
PD suppresses the proliferation of EC
cells
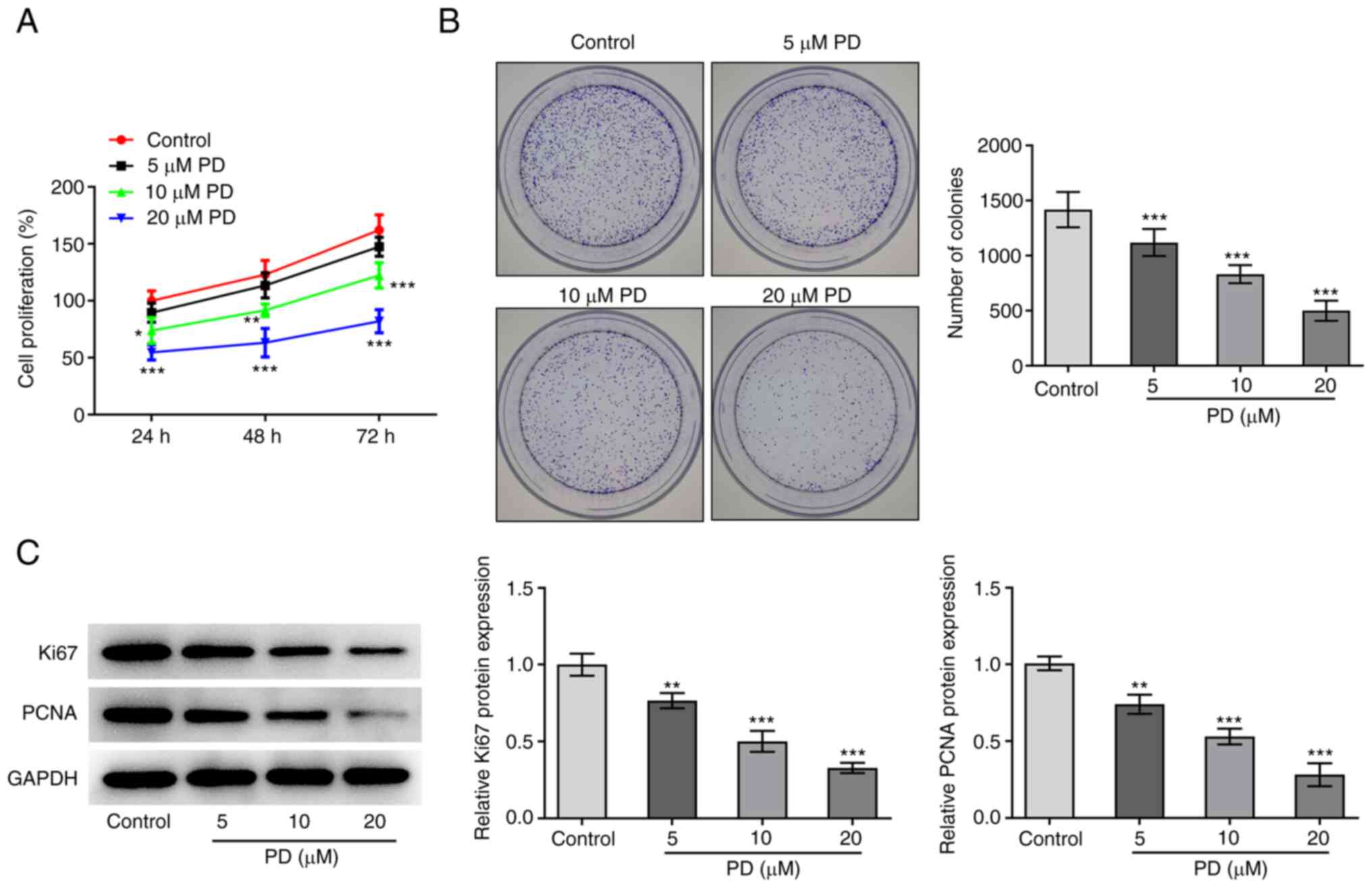
To verify the inhibitory effect of PD on the
proliferation of EC cells, CCK-8 and colony formation assays were
performed and the expression levels of Ki67 and PCNA were examined
in PD-treated EC cells. The results demonstrate that in comparison
with the control group, cell proliferation decreased as the PD
concentration increased, and the reduction in proliferation was
maintained over a prolonged time period (Fig. 2A). Moreover, the results of the
colony formation assay reveal that the number of colonies gradually
decreased as the PD concentration increased (Fig. 2B). In addition, the western blotting
results shown in Fig. 2C
demonstrate that the expression levels of Ki67 and PCNA also
decreased following treatment with PD. Collectively, the
aforementioned results indicate that PD exerted a significant
inhibitory effect on EC proliferation.
PD inhibits the migration and invasion
of EC cells
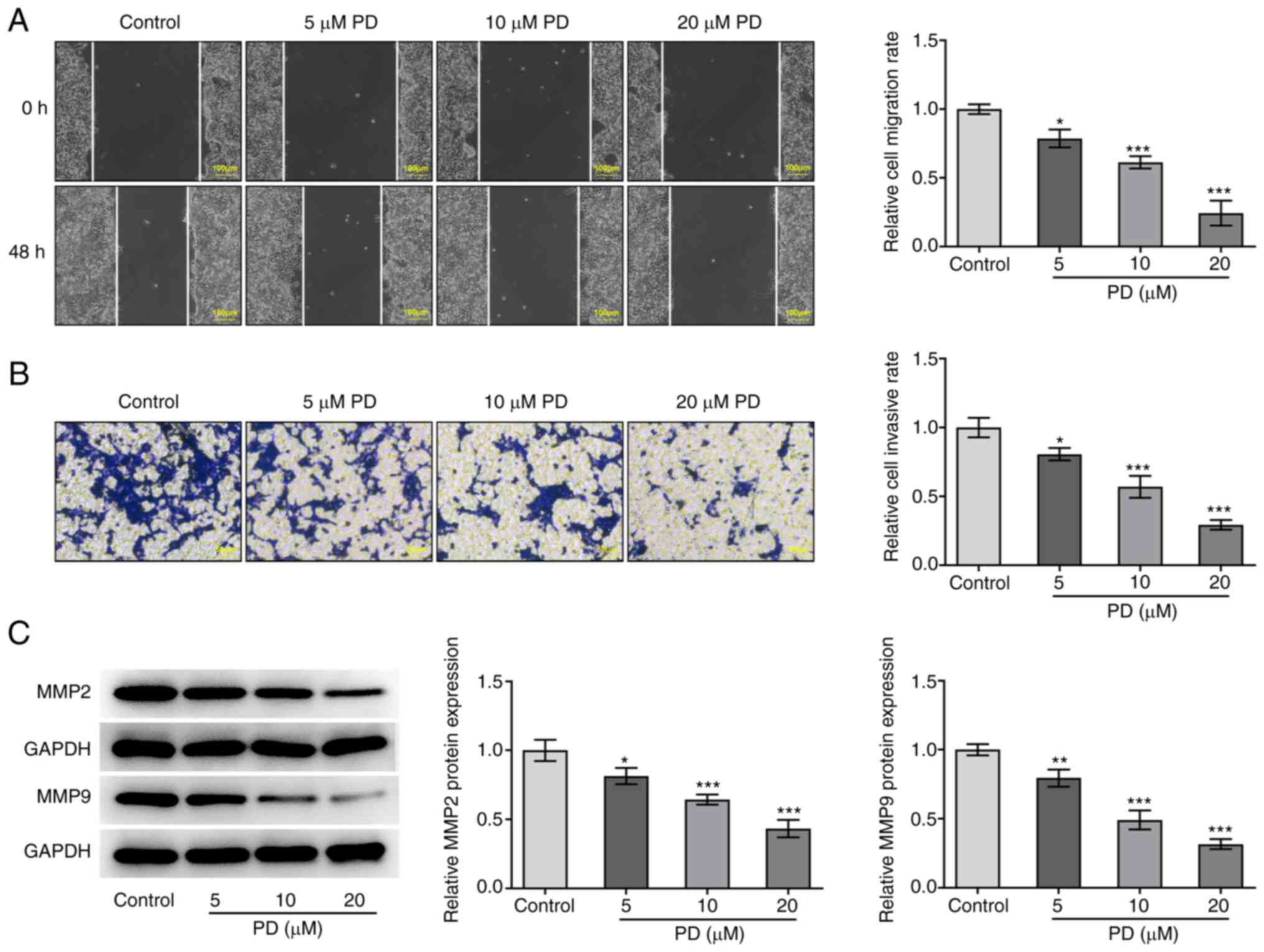
The effect of PD on EC cell migration and invasion
was evaluated using wound healing and Transwell assays,
respectively, and the protein expression levels of MMP2 and MMP9 in
EC cells were detected following PD treatment. As displayed in
Fig. 3A and B, treatment with PD
significantly reduced the migration and invasion of the EC cells.
Moreover, the expression levels of MMP2 and MMP9 were reduced by PD
compared with those in the control group, as shown in Fig. 3C. These results demonstrate that PD
inhibited the migration and invasion of EC cells.
PD increases the expression of ADRA2A
in EC cells
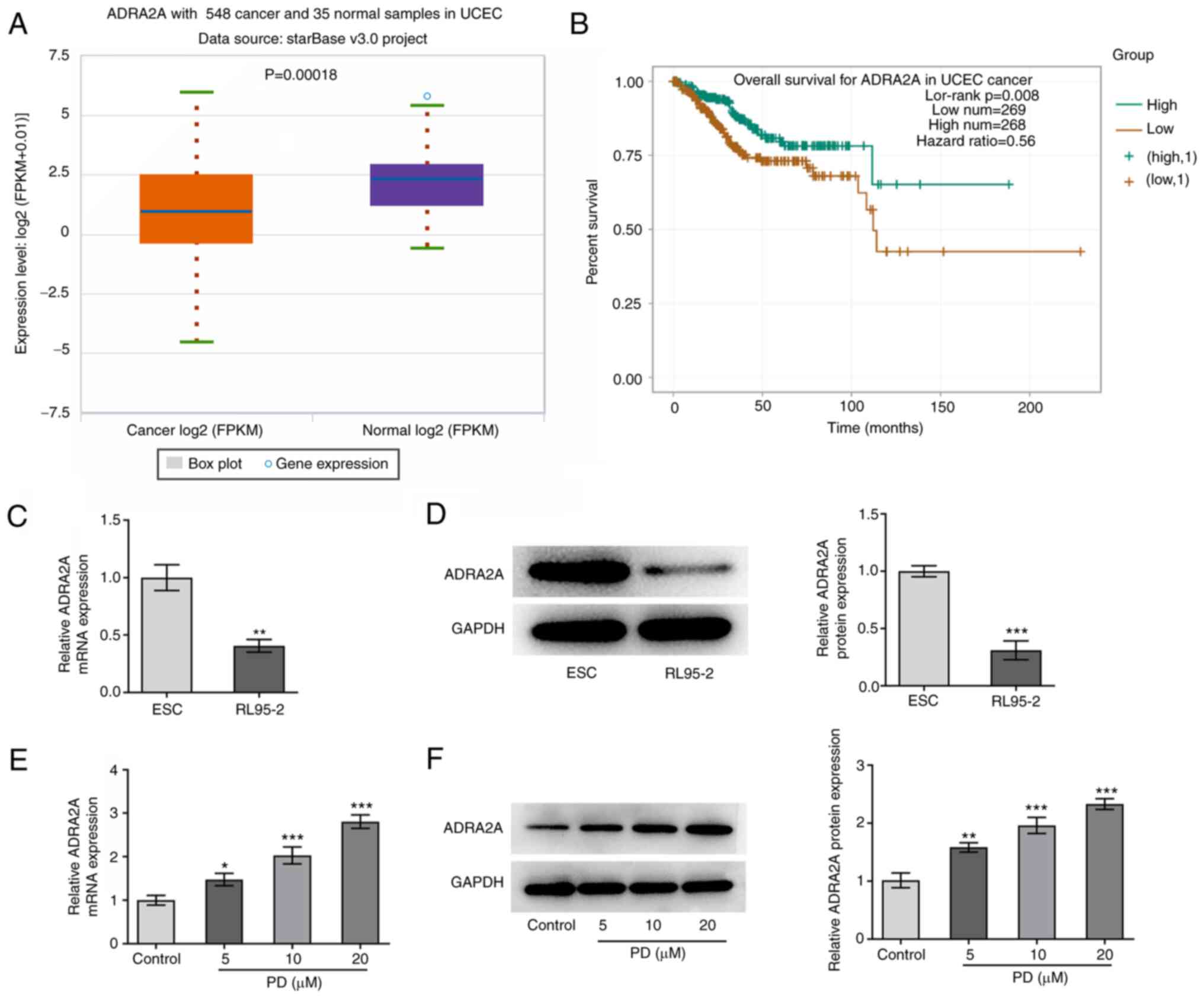
Gene expression data obtained from the ENCORI
database demonstrate that ADRA2A is expressed at a lower level in
EC patient samples compared with normal samples, and low expression
levels of ADRA2A are associated with poorer overall survival in
patients with EC (Fig. 4A and B).
The effect of PD treatment on the expression of ADRA2A in EC cells
was then investigated. The RT-qPCR and western blotting results in
Fig. 4C and D demonstrate that the
expression of ADRA2A in RL-95 cells was markedly reduced compared
with that in healthy ESCs. As displayed in Fig. 4E and F, the treatment of RL-95 cells
with PD significantly increased the expression levels of ADRA2A
compared with those in the untreated control group. These findings
indicate that PD increases ADRA2A expression in EC cells.
PD inhibits the proliferation,
invasion and migration of EC cells via the upregulation of ADRA2A
expression
The ADRA2A, Ki67, PNCA, MMP2 and MMP9 expression
levels and cell proliferation, colony formation, invasion and
migration abilities of EC cells were determined following PD
treatment, to assess whether PD exerts its effects on EC cells via
the upregulation of ADRA2A expression. As displayed in Fig. 5A and B, ADRA2A expression in EC
cells was significantly decreased following transfection with
shADRA2A-1 and −2. Moreover, the expression levels of ADRA2A were
decreased to a higher extent following transfection with
sh-ADRA2A-1 compared with sh-ADRA2A-2. Consequently, sh-ADRA2A-1
was selected for use in subsequent experiments, along with 20 µM
PD. The results of MTT assays demonstrate that cell proliferation
following treatment with PD was markedly reduced compared with that
of the untreated control (Fig. 5C),
and the PD-induced reduction in cell proliferation was
significantly attenuated by sh-ADRA2A-1 in the PD + sh-ADRA2A-1
group. The results of the colony formation assay were consistent
with this, and demonstrate that while treatment with PD reduced the
number of colonies, the PD-induced reduction in colony formation
was attenuated in the PD + sh-ADRA2A-1 group (Fig. 5D). In addition, the protein
expression levels of Ki67 and PCNA were significantly decreased in
the PD group compared with the control group, and these reductions
were attenuated in the PD + sh-ADRA2A-1 group, as displayed in
Fig. 5E. The migration and invasion
of EC cells treated with PD were also significantly reduced
compared with those of the untreated control, and significantly
increased in the PD + sh-ADRA2A-1 transfection group compared with
the PD group (Fig. 5F and G).
Moreover, the knockdown of ADRA2A attenuated the reduction in MMP2
and MMP9 expression in EC cells that was induced by PD treatment
(Fig. 5H). Collectively, the
aforementioned results indicate that PD inhibited the
proliferation, invasion and migration of EC cells via the
upregulation of ADRA2A expression.
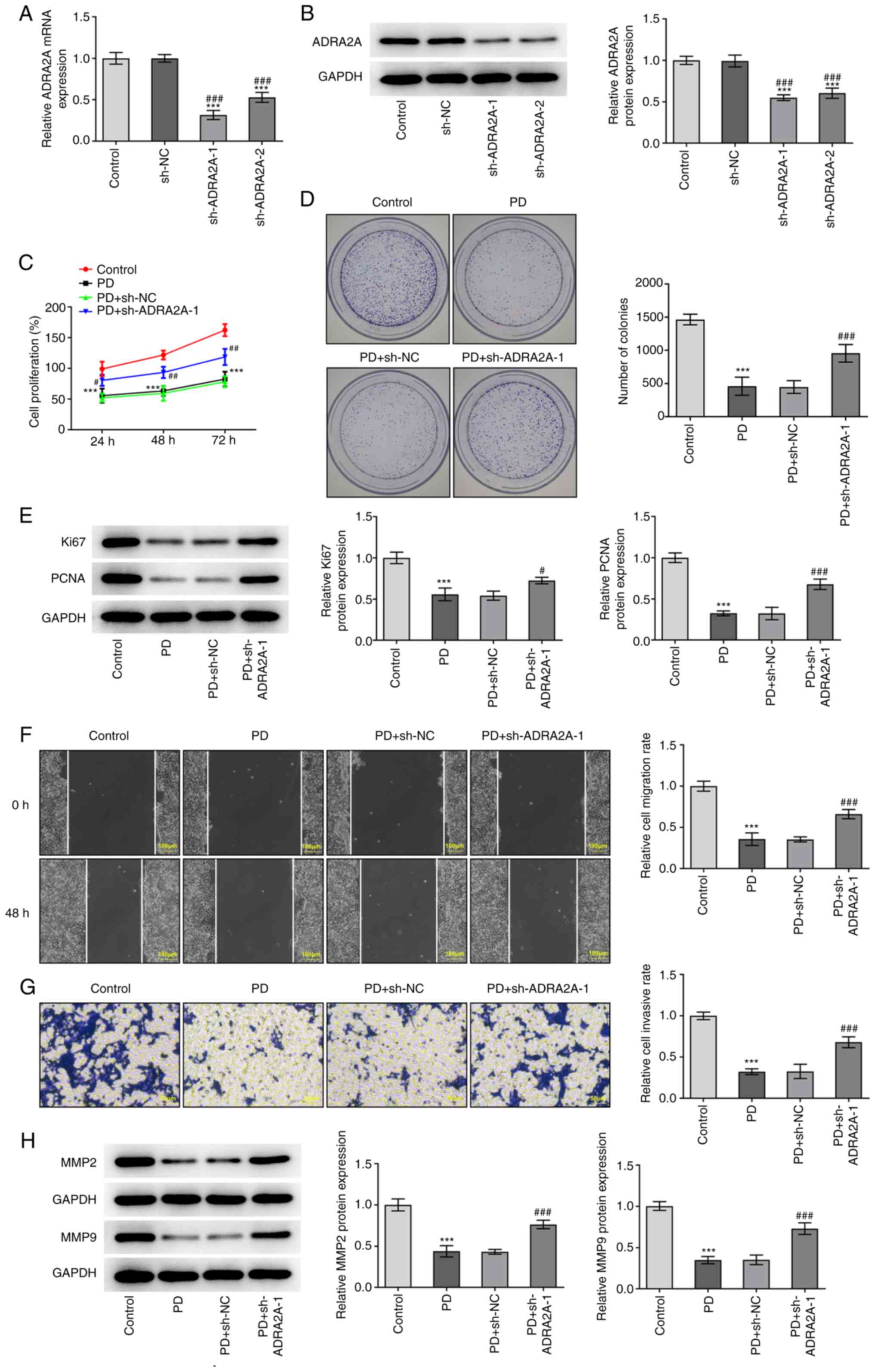 | Figure 5.PD inhibits the proliferation,
invasion and migration of RL95-2 cells via the upregulation of
ADRA2A expression. (A) RT-qPCR and (B) western blot assays were
carried out to detect the expression of ADRA2A in RL95-2 cells
after transfection with sh-ADRA2A-1 and sh-ADRA2A-2. (C) Cell
proliferation was evaluated by MTT assay after transfection with
sh-ADRA2A-1. (D) The colony formation of RL95-2 cells following
transfection with sh-ADRA2A-1 was investigated. (E) The protein
levels of Ki67 and PCNA were examined by western blot assay after
treatment with PD and/or transfection with sh-ADRA2A-1. (F) Cell
migration and (G) invasion were measured using wound healing and
Transwell assays, respectively, after treatment with PD and/or
transfection with sh-ADRA2A-1. Wound healing assay magnification,
×100; Transwell assay magnification, ×200. (H) Western blotting was
carried out to assess the expression of MMP2 and MMP9 in RL95-2
cells treated with PD for 24 h after transfection with sh-ADRA2A-1.
Data are expressed as the mean ± SD. ***P<0.001 vs. control.
#P<0.05, ##P<0.01,
###P<0.001 vs. PD + sh-NC. PD, platycodin D; ADRA2A,
α2A-adrenergic receptor; RT-qPCR, reverse
transcription-quantitative PCR; sh, short hairpin; NC, negative
control; PCNA, proliferating cell nuclear antigen. |
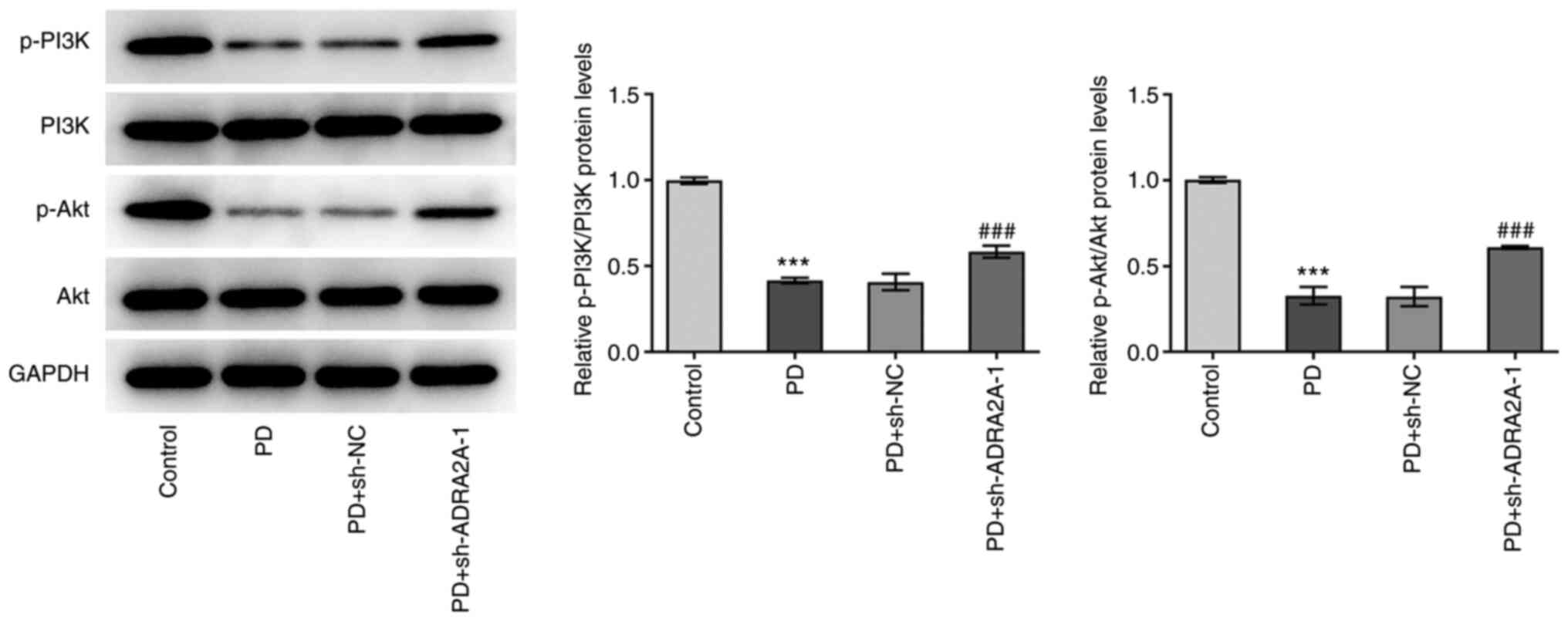
PD blocks the PI3K/Akt signaling
pathway via the upregulation of ADRA2A expression
To clarify the effects of PD on the PI3K/Akt
signaling pathway, the phosphorylation levels of PI3K and Akt were
investigated by western blotting. As shown in Fig. 6, the p-PI3K/PI3K and p-Akt/Akt
ratios in EC cells treated with PD were significantly decreased
compared with those in the control cells, while transfection with
sh-ADRA2A-1 significantly attenuated the PD-induced changes in the
p-PI3K and p-Akt levels. These findings demonstrate that PD blocked
activation of the PI3K/Akt signaling pathway via the upregulation
of ADRA2A expression.
Discussion
EC is regarded as one of the most serious diseases
in females, with rising incidence and mortality rates (14), and a poor prognosis with regard to
recurrence or metastasis (15).
Despite advances in the biological understanding of EC, numerous
aspects of the current treatment options remain controversial,
including the surgical assessment of the lymph nodal status in EC,
which may determine the specific therapeutic method, and the
selection of patients for adjuvant radiation or chemotherapy, which
may impact the therapeutic effect and prognosis of the patients
(16). A previous study
demonstrated that PD may serve as an effective antitumor drug
(17). However, the antitumor
effect of PDs in EC has not been fully elucidated. In the present
study, the inhibitory effects of PD on the viability,
proliferation, invasion and migration of EC cells were explored. In
addition, the present study demonstrated that ADRA2A expression
levels were lower in EC cells than in normal ESCs. The results of
various assays in the present study indicated that PD suppressed
the proliferation, invasion and migration of EC cells via the
upregulation of ADRA2A expression. Moreover, the results confirmed
that ADRA2A blocks the PI3K/Akt signaling pathway and reduces its
activation. These findings indicate that PD and the expression
levels of ADRA2A have an association with EC.
The results of previous studies have shown that PD
has antitumor effects in several types of cancer, including lung
(18), gastric (19) and bladder cancer (17). PD has also been reported to target
cancer cells by inducing apoptosis, arresting the cell cycle and
inhibiting angiogenesis, invasion and metastasis via multiple
signaling pathways (20). For
example, the results of a previous study indicated that PD exerts
antitumor effects by inducing apoptosis, suppressing invasion and
migration, and arresting the cell cycle of gallbladder cancer cells
(21). Moreover, the results of
another study demonstrated that PD inhibited cell proliferation and
induced apoptosis in BEL-7402 human hepatocellular carcinoma cells
(22). A study by Wu et al
(23) demonstrated that PD
inhibited the proliferation and migration of various multiple
myeloma cell lines by activating the NF-κB and Janus kinase 2/STAT3
pathways. In another study, Zhang et al (24) demonstrated that PD exerted
inhibitory effects on the growth and invasion of human oral
squamous cell carcinoma via inactivation of the NF-κB pathway. The
results of the present study revealed that the viability of EC
cells was decreased following treatment with PD. In addition, high
expression levels of the proliferation marker Ki67 are associated
with reduced EC-specific survival (25), and the present study detected
reduced protein expression levels of Ki67 and PCNA in EC cells
following treatment with PD, which indicated that PD has the
ability to inhibit cell proliferation in EC.
In a previous study, PD was shown to markedly
downregulate the expression levels of MMP2 and MMP9 in rats with
cardiac hypertrophy (26). The
expression levels of MMP2 and MMP9 in EC cells were measured in the
present study, and the results demonstrated that they were
decreased following treatment with PD, as were the invasion and
migration of the cells. The aforementioned results indicate the
ability of PD to inhibit the invasion and migration of EC
cells.
Analyses conducted using the ENCORI database
revealed that the expression of ADRA2A is downregulated in the
tumor tissues of patients with EC, and low ADRA2A expression is
associated with a poor prognosis. In the present study, the
expression of ADRA2A was observed to be downregulated in EC cells
compared with ESCs. However, the expression levels of ADRA2A in EC
cells were elevated following PD treatment, which suggests that
ADRA2A is a target gene that is regulated by PD. This finding is in
accordance the predicted binding between PD and ADRA2A obtained
using the SwissTargetPrediction webtool. In addition, a previous
study reported that the downregulation of ADRA2A gene expression in
prostate cancer was associated with aggregation, proliferation and
migration (27). Moreover, another
study identified that high ADRA2A expression is associated with the
inhibition of tumor cell proliferation (28). Consistent with these previous
findings, the present study demonstrated that the proliferation,
invasion and migration of EC cells were reduced following PD
treatment, and these effects were attenuated following ADRA2A
knockdown, which suggests that PD inhibits the proliferation,
invasion and migration of EC cells via the upregulation of ADRA2A
expression.
As reported, PD and ADRA2A are pivotal regulators of
the PI3K/AKT pathway in tumors (8,10,29).
PI3K/AKT signaling is a growth-regulating cellular signaling
pathway, the activation of which is increased in numerous human
cancers (30). It may also
participate in the apoptosis and migration of tumor cells (31). Notably, the PI3K/Akt signaling
pathway is a key mechanism by which the growth, migration,
proliferation and metabolism of mammalian cells are controlled
(32). For example, microRNA-936
has been shown to promote the proliferation and invasion of gastric
cancer cells by downregulating fibroblast growth factor 2
expression and activating the PI3K/Akt signaling pathway (33), while TRIM29 has been reported to
promote the progression of thyroid carcinoma via activation of the
PI3K/Akt signaling pathway (34).
The western blotting results in the present study demonstrated that
the protein levels of p-PI3K and p-Akt were significantly reduced
following PD treatment; however, these PD-induced reductions in
phosphorylation levels were increased following ADRA2A knockdown,
which suggests that ADRA2A regulates the PI3K/Akt signaling
pathway. Collectively, these findings suggest that PD inhibits the
PI3K/Akt signaling pathway via the upregulation of ADRA2A
expression. Notably, the results of the present study show that
ADRA2A knockdown only partially reversed the antitumor effects of
PD, suggesting that other PD-mediated antitumor pathways may exist
in EC. Thus, other potential PD-mediated mechanisms will be
investigated in the future. For example, a previous study
demonstrated that NLRC5 promotes cell migration and invasion in EC
via activation of the PI3K/Akt signaling pathway (35). Therefore, this is a specific
mechanism that requires further investigation with regard to the
effects of PD treatment on EC cells.
In conclusion, the results of the present study
suggest that PD inhibits the proliferation, migration and invasion
of EC cells via the upregulation of ADRA2A and inhibition of the
PI3K/Akt signaling pathway.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZN and ZDa designed the study, drafted and revised
the manuscript. ZN, ZDa, DS, PQ and ZL performed the experiments.
QP and ZDe analyzed the data and searched the literature. All
authors read and approved the final manuscript. ZN and ZDe confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Passarello K, Kurian S and Villanueva V:
Endometrial cancer: An overview of pathophysiology, management, and
care. Semin Oncol Nurs. 35:157–165. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banz-Jansen C, Helweg LP and Kaltschmidt
B: Endometrial cancer stem cells: Where do we stand and where
should we go? Int J Mol Sci. 23:34122022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McAlpine JN, Temkin SM and Mackay HJ:
Endometrial cancer: Not your grandmother's cancer. Cancer.
122:2787–2798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Emons G and Vordermark D: Adjuvant
treatment for endometrial cancer. Curr Opin Oncol. 31:404–410.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Boer SM, Powell ME, Mileshkin L,
Katsaros D, Bessette P, Haie-Meder C, Ottevanger PB, Ledermann JA,
Khaw P, D'Amico R, et al: Adjuvant chemoradiotherapy versus
radiotherapy alone in women with high-risk endometrial cancer
(PORTEC-3): Patterns of recurrence and post-hoc survival analysis
of a randomised phase 3 trial. Lancet Oncol. 20:1273–1285. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han Y, Jin SW, Lee GH, Choi JH, Lee HS,
Chung YC, Jeong HG and Lee KY: Stimulatory effects of platycodin D
on osteoblast differentiation. J Cell Biochem. 120:13085–13094.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong Y, Lu ZL, Wang JJ, Zhou R, Guo J, Liu
J, Sun HL, Wang H, Song W, Yang J and Xu HX: Platycodin D, a
metabolite of platycodin grandiflorum, inhibits highly metastatic
MDA-MB-231 breast cancer growth in vitro and in vivo by targeting
the MDM2 oncogene. Oncol Rep. 36:1447–1456. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chun J and Kim YS: Platycodin D inhibits
migration, invasion, and growth of MDA-MB-231 human breast cancer
cells via suppression of EGFR-mediated Akt and MAPK pathways. Chem
Biol Interact. 205:212–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou R, Lu Z, Liu K, Guo J, Liu J, Zhou Y,
Yang J, Mi M and Xu H: Platycodin D induces tumor growth arrest by
activating FOXO3a expression in prostate cancer in vitro and in
vivo. Curr Cancer Drug Targets. 14:860–871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang W, Guo X and Dan H: α2A-adrenergic
receptor inhibits the progression of cervical cancer through
blocking PI3K/AKT/mTOR pathway. Onco Targets Ther. 13:10535–10546.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
StarBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Daina A, Michielin O and Zoete V:
SwissTargetPrediction: Updated data and new features for efficient
prediction of protein targets of small molecules. Nucleic Acids
Res. 47:W357–W364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu KH and Broaddus RR: Endometrial cancer.
N Engl J Med. 383:2053–2064. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Post CCB, Westermann AM, Bosse T,
Creutzberg CL and Kroep JR: PARP and PD-1/PD-L1 checkpoint
inhibition in recurrent or metastatic endometrial cancer. Crit Rev
Oncol Hematol. 152:1029732020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brooks RA, Fleming GF, Lastra RR, Lee NK,
Moroney JW, Son CH, Tatebe K and Veneris JL: Current
recommendations and recent progress in endometrial cancer. CA
Cancer J Clin. 69:258–279. 2019.PubMed/NCBI
|
|
17
|
Chen D, Chen T, Guo Y, Wang C, Dong L and
Lu C: Platycodin D (PD) regulates LncRNA-XIST/miR-335 axis to slow
down bladder cancer progression in vitro and in vivo. Exp Cell Res.
396:1122812020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang MY, Jiang XM, Xu YL, Yuan LW, Chen
YC, Cui G, Huang RY, Liu B, Wang Y, Chen X and Lu JJ: Platycodin D
triggers the extracellular release of programed death Ligand-1 in
lung cancer cells. Food Chem Toxicol. 131:1105372019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng Y, Fan JY, Xiong J, Lou Y and Zhu Y:
MiR-34a enhances the susceptibility of gastric cancer to platycodin
d by targeting survivin. Pathobiology. 86:296–305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan M, Maryam A, Zhang H, Mehmood T and
Ma T: Killing cancer with platycodin D through multiple mechanisms.
J Cell Mol Med. 20:389–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Zhai T, Hei Z, Zhou D, Jin L, Han
C and Wang J: Effects of platycodin D on apoptosis, migration,
invasion and cell cycle arrest of gallbladder cancer cells. Oncol
Lett. 20:3112020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li T, Xu XH, Tang ZH, Wang YF, Leung CH,
Ma DL, Chen XP, Wang YT, Chen Y and Lu JJ: Platycodin D induces
apoptosis and triggers ERK- and JNK-mediated autophagy in human
hepatocellular carcinoma BEL-7402 cells. Acta Pharmacol Sin.
36:1503–1513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu D, Zhang W, Chen Y, Ma H and Wang M:
Platycodin D inhibits proliferation, migration and induces
chemosensitization through inactivation of the NF-κB and
JAK2/STAT3 pathways in multiple myeloma cells. Clin Exp Pharmacol
Physiol. 46:1194–1200. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Z, Zhao M, Zheng W and Liu Y:
Platycodin D, a triterpenoid saponin from Platycodon grandiflorum,
suppresses the growth and invasion of human oral squamous cell
carcinoma cells via the NF-κB pathway. J Biochem Mol
Toxicol. 31:219342017. View Article : Google Scholar
|
|
25
|
Sivalingam VN, Latif A, Kitson S, McVey R,
Finegan KG, Marshall K, Lisanti MP, Sotgia F, Stratford IJ and
Crosbie EJ: Hypoxia and hyperglycaemia determine why some
endometrial tumours fail to respond to metformin. Br J Cancer.
122:62–71. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin YC, Lin YC, Kuo WW, Shen CY, Cheng YC,
Lin YM, Chang RL, Padma VV and Huang CY and Huang CY: Platycodin D
reverses pathological cardiac hypertrophy and fibrosis in
spontaneously hypertensive rats. Am J Chin Med. 46:537–549. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Urbinati G, Ali HM, Rousseau Q, Chapuis H,
Desmaele D, Couvreur P and Massaad-Massade L: Antineoplastic
effects of siRNA against TMPRSS2-ERG junction oncogene in prostate
cancer. PLoS One. 10:e01252772015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rivero EM, Martinez LM, Bruque CD,
Gargiulo L, Bruzzone A and Luthy IA: Prognostic significance of α-
and β2-adrenoceptor gene expression in breast cancer patients. Br J
Clin Pharmacol. 85:2143–2154. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu C, Sun G, Yuan G, Wang R and Sun X:
Effects of platycodin D on proliferation, apoptosis and PI3K/Akt
signal pathway of human glioma U251 cells. Molecules.
19:21411–21423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uko NE, Guner OF, Matesic DF and Bowen JP:
Akt pathway inhibitors. Curr Top Med Chem. 20:883–900. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng H and Liu JF: Studies on the
relationship between P13K/AKT signal pathway-mediated MMP-9 gene
and lung cancer. Eur Rev Med Pharmacol Sci. 21:753–759.
2017.PubMed/NCBI
|
|
32
|
Pompura SL and Dominguez-Villar M: The
PI3K/AKT signaling pathway in regulatory T-cell development,
stability, and function. J Leukoc Biol.
22:10.1002/JLB.2MIR0817–349R. 2018.
|
|
33
|
Chen ZF, Wang J, Yu Y and Wei W:
MicroRNA-936 promotes proliferation and invasion of gastric cancer
cells by down-regulating FGF2 expression and activating P13K/Akt
signaling pathway. Eur Rev Med Pharmacol Sci. 24:6707–6715.
2020.PubMed/NCBI
|
|
34
|
Xu J, Li Z, Su Q, Zhao J and Ma J: TRIM29
promotes progression of thyroid carcinoma via activating P13K/AKT
signaling pathway. Oncol Rep. 37:1555–1564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fan Y, Dong Z, Shi Y, Sun S, Wei B and
Zhan L: NLRC5 promotes cell migration and invasion by activating
the PI3K/AKT signaling pathway in endometrial cancer. J Int Med
Res. 48:3000605209253522020. View Article : Google Scholar : PubMed/NCBI
|