Introduction
Spinal scoliosis is a 3-dimensional spine and trunk
deformity that affects millions of individuals worldwide (1,2).
According to its aetiology, it can be divided into the idiopathic,
congenital, degenerative and neuromuscular types, among others
(3–6). However, this classification method
does not reveal more fundamental reasons for scoliosis and has
limited significance. The Lenke classification (7), used by spine surgeons to guide
surgery, only applies to thoracic and lumbar scoliosis, and is only
a morphological classification. Currently, the surgical treatment
of scoliosis mainly relies on the fixation and fusion of spinal
bones with screw-rod systems (8,9). The
operation is challenging, and numerous follow-up problems exist,
including chronic pain, growth retardation of the spine and
fracture of the screw-rod system (10). Therefore, research on the aetiology
of scoliosis is key to the next breakthrough in treatment.
Many studies have focused on the theory of possible
muscular imbalance for scoliosis (11–13).
Considering the close association between the muscle and the nerves
that innervate it, studying the neuromuscular reflex arc cannot be
avoided when exploring the mechanism of muscle imbalance. Spinal
cord injuries such as poliomyelitis and spinal cord tumours
(14,15) can also cause scoliosis. However, the
existing literature includes only a few case reports, and there are
no articles on the clinical features of scoliosis caused by spinal
cord injury. Unlike ependymomas, which are often of central origin
and symmetrically disrupt the spinal cord, astrocytomas are
characterised by a unilateral origin, asymmetric destruction of the
spinal cord, visibility on MRI and the fact that the length of the
spinal cord involved with the astrocytoma is clear; therefore, it
is a suitable carrier for studying the role of muscle imbalance in
scoliosis (16).
The present study analysed cases of scoliosis caused
by astrocytoma in a single research centre, and summarised the
characteristics and pathogenesis of this type of scoliosis. The
incidence of spinal astrocytoma is extremely low, and there is
little associated literature due to the lack of knowledge regarding
scoliosis caused by spinal astrocytomas. To the best of our
knowledge, this study represents the largest sample of patients
with astrocytoma-induced scoliosis currently available for
analysis.
Patients and methods
Patients
The medical records of all patients diagnosed with
spinal astrocytoma at a single research centre (Peking University
Third Hospital, Beijing, China) between January 1990 and October
2022 in both inpatient and outpatient settings were retrospectively
reviewed. The inclusion criteria were as follows: Patients
diagnosed with spinal astrocytoma (including pilocytic astrocytoma,
anaplastic astrocytoma and glioblastoma) according to pathological
examination who underwent spinal X-rays before surgery. Patients
with no clear tumour segments on imaging or medical records were
excluded. Scoliosis was defined as a Cobb angle of >10° for the
two vertebral bodies on the coronal plane of the spine (3).
The following details were obtained from each
medical record: Demographic details, initial side and type of
symptoms, duration of the disease, sagittal tumour location,
presence of scoliosis and astrocytoma pathological grade (World
Health Organisation Neuropathological Classification) (17–22).
In addition, where scoliosis was present, the convex side, end
vertebrae and apical vertebrae of the scoliosis were recorded.
The study was conducted in accordance with the
tenets of the Declaration of Helsinki, and the Ethics Committee of
Peking University Third Hospital (Beijing, China) approved the
study.
Clinical symptom classification and
tumour side inference
Initial clinical symptoms were divided into two main
types: Strength and sensory disturbances. Furthermore, the
patient's symptoms were characterised as unilateral or bilateral.
If the patient's initial symptom was a unilateral sensory
disturbance, the tumour would be on the opposite side of the
sensory disturbance side. On the other hand, if the patient's
initial symptom was unilateral decreased muscle strength, the
tumour would be located on the same side of weakness.
Evaluation of the paraspinal
muscles
If the patient had scoliosis, the cross-sectional
areas of the multifidus and erector spinae muscles on both sides of
the apical level of the scoliosis were assessed using MRI. The
slice thickness was 4 mm, with a 0.1-mm gap between each slice. The
field of view for the scan was 150×163 mm, with 128×256 matrices.
The bilateral cross-sectional areas of the multifidus and erector
spinae muscles at the apical level were measured by outlining the
fascial boundary of the muscle using Image J (ver. 1.3; National
Institute of Health), as described by Shafaq et al (23).
Pathology and diagnosis
All tumours were examined pathologically. This was
consistent with the latest World Health Organisation
Neuropathological Classification at the time of diagnosis (17–22).
Statistical analysis
R4.0.3 statistical software (University of Auckland)
was used for the statistical analysis. For continuous data, the
Shapiro-Wilk normality test was used to determine the normality of
the sample data. If it conformed to the normal distribution, it was
expressed as the mean ± standard deviation, and the comparison
between the two groups was performed using the independent sample
t-test; if it did not conform to the normal distribution, the
median (lowest to highest value) was used, and the Wilcoxon test
was used for comparison between the two groups. A paired sample
t-test was used to assess the cross-sectional area of the
paraspinal muscles on both sides. Categorical data are
statistically described as n (%), and comparisons between groups
were performed using the χ2 test. P<0.05 was used to
indicate a statistically significant difference.
Results
A total of 189 patients (94 men and 95 women) met
the inclusion criteria. The mean patient age was 40.69±14.8 years
(range, 6–84 years), while the mean duration of the disease before
diagnosis was 11.6 months. A total of 119 patients had unilateral
onset and 70 had bilateral onset. Overall, 80 patients had a
sensory impairment and 118 had a motor impairment. The astrocytoma
was located in the cervical spine in 50 patients, in the
cervicothoracic spine in 35 patients, in the thoracic spine in 54
patients, in the thoracolumbar spine in 28 patients and in the
lumbar spine in 14 patients. The tumour invaded the entire length
of the spine in 8 patients. Among all the patients, 57.1% had
scoliosis (Table I).
 | Table I.Patient data summary. |
Table I.
Patient data summary.
| Variables | Value |
|---|
| No. of patients | 189 |
| Mean age ± SD,
years | 40.69±14.8 |
| Sex, n |
|
| Male | 94 |
|
Female | 95 |
| Side of symptoms,
n |
|
|
Unilateral | 119 |
|
Bilateral | 70 |
| Type of symptoms,
n |
|
| Sensory
disturbance | 75 |
| Strength
disturbance | 114 |
| Mean duration of
disease ± SD, months | 11.6±13.6 |
| Tumor sagittal
location, n |
|
|
Cervical | 50 |
|
Cervicothoracic | 35 |
|
Thoracic | 54 |
|
Thoracolumbar | 28 |
|
Lumbar | 14 |
| Full
length | 8 |
| Pathological grade,
n |
|
| 1 | 31 |
| 2 | 116 |
| 3 | 29 |
| 4 | 13 |
| Scoliosis, n |
|
|
Yes | 106 |
| No | 83 |
The patients were divided into two groups according
to whether their initial symptoms were unilateral or bilateral.
There was no statistical difference in the baseline indicators
between the two groups, but the incidence of scoliosis in the
unilateral onset group was significantly higher (Table II).
 | Table II.Comparison of patients with
unilateral and bilateral symptoms. |
Table II.
Comparison of patients with
unilateral and bilateral symptoms.
| Variables | Unilateral
symptoms | Bilateral
symptoms | P-value |
|---|
| No. of
patients | 119 | 70 |
|
| Age, years | 40.08±16.19 | 41.73±12.11 | 0.426 |
| Sex, n |
|
| 0.337 |
|
Male | 56 | 38 |
|
|
Female | 63 | 32 |
|
| Type of symptoms,
n |
|
| 0.194 |
| Sensory
disturbance | 43 | 32 |
|
|
Strength disturbance | 76 | 38 |
|
| Mean duration of
disease ± SD, months | 10.8±11.5 | 13.0±16.5 | 0.240 |
| Tumor sagittal
location, n |
|
| 0.196 |
|
Cervical | 35 | 15 |
|
|
Cervicothoracic | 22 | 13 |
|
|
Thoracic | 38 | 16 |
|
|
Thoracolumbar | 14 | 14 |
|
|
Lumbar | 6 | 8 |
|
| Full
length | 4 | 4 |
|
| Pathological grade,
n |
|
| 0.242 |
| 1 | 23 | 8 |
|
| 2 | 67 | 49 |
|
| 3 | 19 | 10 |
|
| 4 | 10 | 3 |
|
| Scoliosis, n |
|
| 0.012a |
|
Yes | 75 | 31 |
|
| No | 44 | 39 |
|
The details of the patients with scoliosis with
complete information are listed in Table III. The inferred tumour side was
highly consistent with the convex side of scoliosis. According to
classic anatomical studies, in early human embryos, the spinal cord
has the same length as the spine, and each spinal cord segment is
consistent with the corresponding vertebral bone. However, in the
process of growth, the growth rate of the spine is faster than that
of the spinal cord. Therefore, in adults, spinal cord sections do
not precisely correspond to the corresponding vertebral bones
(24). The corresponding rules are
listed in Table IV. The sagittal
position of the astrocytoma and scoliosis end vertebra follow the
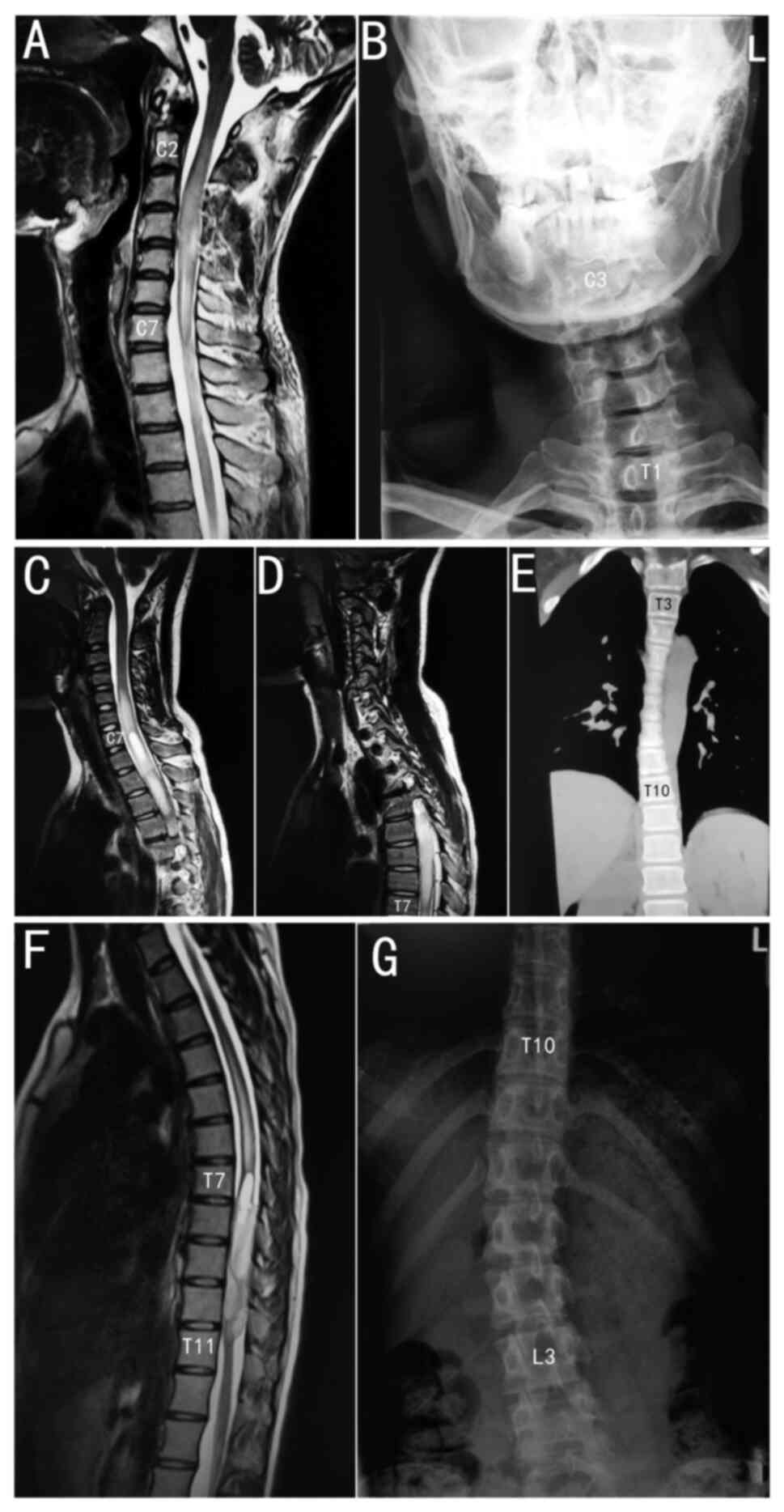
same rules. Three typical cases are shown in Fig. 1: A C2 to C7 segment tumour caused C3
to T1 segment scoliosis, a C7 to T7 segment tumour caused T3 to T10
segment scoliosis and a T7 to T11 segment tumour caused T10 to L3
segment scoliosis. Unlike idiopathic scoliosis, the apical
vertebrae were generally in the middle of the scoliosis, and the
apical vertebrae were more caudal to this scoliosis type. In some
cases, the apical vertebra was the caudal end vertebra.
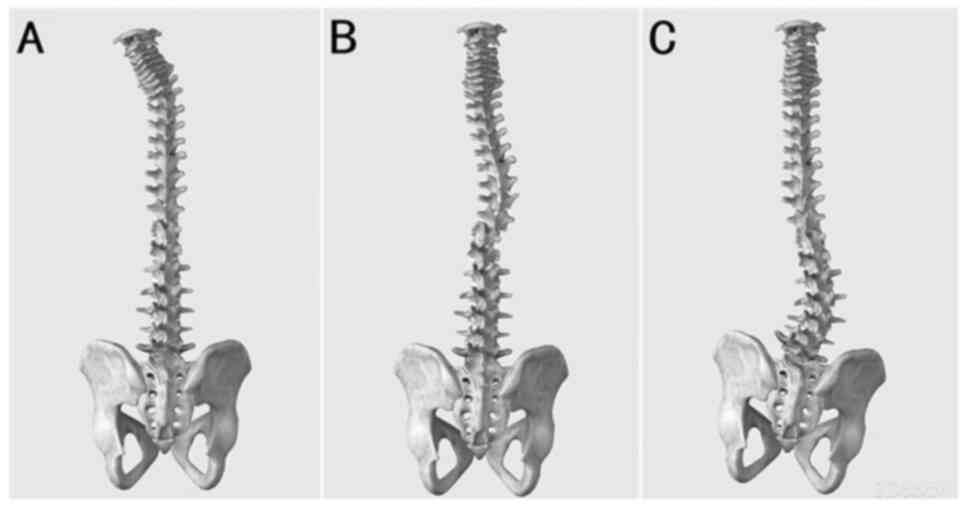
Morphologically, the vertebral bodies of idiopathic scoliosis line
up similarly to a ‘c’ shape, whereas the vertebral bodies of
astrocytoma-induced scoliosis in the present study lined up
similarly to an ‘L’ shape with a larger angle. The morphology of
this scoliosis type in the cervical, thoracic and lumbar spine is
shown in Fig. 2.
 | Table III.Details of tumors and scoliosis in
patients with complete information. |
Table III.
Details of tumors and scoliosis in
patients with complete information.
| Patient no. | Sex | Age, years | Initial symptom
side | Initial symptom
type | Inferred tumor
side | Scoliosis convex
side | Duration of
disease, months | Tumor sagittal
location | Scoliosis end
vertebrae | Scoliosis apical
vertebrae | Pathological
grade |
|---|
| 1 | F | 45 | L | Strength
disorder | L | L | 12 | C0-T1 | C2-T1 | C7, T1 | 4 |
| 2 | M | 40 | L | Strength
disorder | L | L | 2 | C2-6 | C3-T2 | C7 | 3 |
| 3 | F | 20 | R | Sensory
disorder | L | L | 6 | C2-7 | C3-T1 | T1 | 1 |
| 4 | F | 24 | R | Sensory
disorder | L | L | 3 | C3-5 | C4-T2 | C7 | 3 |
| 5 | M | 41 | L | Strength
disorder | L | L | 24 | C3-L2 | T7-L5 | L2-3 | 2 |
| 6 | F | 40 | L | Strength
disorder | L | L | 9 | T10-11 | T10-L4 | L1 | 2 |
| 7 | F | 41 | R | Sensory
disorder | L | L | 36 | T1-2 | C6-T3 | T2-3 | 2 |
| 8 | M | 54 | L | Strength
disorder | L | L | 60 | C5-T2 | / | / | 2 |
| 9 | M | 31 | R | Sensory
disorder | L | L
(cervicothoracic); R (thoracolumbar) | 24 | C6-T5 | C3-T5
(cervicothoracic); T5-L1 (thoracolumbar) | C7, T10 | 1 |
| 10 | M | 60 | R | Strength
disorder | R | R | 36 | C2-T1 | C2-T3 | T1-2 | 2 |
| 11 | F | 18 | R | Strength
disorder | R | R | 2 | T7-11 | T10-L3 | L1-2 | 2 |
| 12 | M | 67 | R | Strength
disorder | R | R | 1 | C3-5 | C2-6 | C4 | 3 |
| 13 | F | 29 | R | Strength
disorder | R | R | 6 | C4-6 | C5-T1 | C6-7 | 2 |
| 14 | F | 12 | L | Sensory
disorder | R | R | 12 | C7-T7 | T3-10 | T8 | 2 |
| 15 | F | 19 | R | Strength
disorder | R | R | 24 | T2-6 | T2-9 | T5 | 2 |
| 16 | F | 45 | L | Sensory
disorder | R | R | 2 | T4-6 | T5-8 | T6-7 | 2 |
| 17 | F | 42 | R | Strength
disorder | R | R | 18 | T5-7 | C6-L2 | T5-6 | 2 |
| 18 | M | 26 | R | Strength
disorder | R | R | 0.5 | T6-10 | T9-L2 | T12, L1 | 1 |
| 19 | M | 40 | R | Strength
disorder | R | L | 6 | C5T1 | C4-T5 | T1-2 | 2 |
| 20 | M | 13 | R | Strength
disorder | R | L | 2 | T11-12 | T12-L5 | L3-4 | 2 |
| 21 | M | 39 | L | Strength
disorder | L | R | 3 | C4T2 | C4-T1 | C7T1 | 1 |
| 22 | M | 49 | L | Strength
disorder | L | R | 12 | T2-5 | T3-T7 | T6 | 3 |
| 23 | M | 41 | Bilateral | Strength
disorder | - | R | 3 | C4-5 | C4-6 | C6 | 1 |
| 24 | M | 13 | Bilateral | Sensory
disorder | - | R (thoracic); L
(thoracolumbar) | 48 | T3-L2 | T3-T11 (thoracic);
T11-L5 (thoracolumbar) | T8, L4 | 1 |
| 25 | M | 21 | Bilateral | Strength
disorder | - | R (thoracic); L
(thoracolumbar) | 60 | T4-11 | T7-T11 (thoracic)
T11-L4 (thoracolumbar) | T9, L4 | 2 |
| 26 | F | 58 | Bilateral | Sensory
disorder | - | R | 12 | C6-T4 | C6-T6 | T4 | 3 |
| 27 | M | 48 | Bilateral | Sensory
disorder | - | L | 4 | T12-L1 | L1-5 | L3 | 2 |
 | Table IV.Association between the location of
the spinal cord segments and the vertebral segments. |
Table IV.
Association between the location of
the spinal cord segments and the vertebral segments.
| Vertebral
segment | Number of segments
difference | Spinal cord
segments |
|---|
| C1-4 | +0 | C1-4 |
| C4-T3 | +1 | C5-T4 |
| T3-6 | +2 | T5-8 |
| T6-9 | +3 | T9-12 |
| T10-12 | Variable | L1-5 |
Of the 106 patients with scoliosis, 12 did not
undergo an MRI scan in the cross-section of the end vertebra, while
the distal vertebral paraspinal muscles of the remaining 94
patients were delineated and analysed. The cross-sectional area of
the multifidus muscle on the convex side of the apical-level
scoliosis was significantly smaller than that on the concave side.
There was no significant difference in the cross-sectional area of
the erector spinae muscles between the convex and concave sides of
the apical vertebrae (Table V).
 | Table V.Cross-sectional area of the deep
paravertebral muscles at the apical vertebrae. |
Table V.
Cross-sectional area of the deep
paravertebral muscles at the apical vertebrae.
| Muscle | Concave side,
mm2 | Convex side,
mm2 | P-value |
|---|
| Multifidus
muscles | 302.5±117.4 | 250.2±103.4 | 0.001a |
| Erector spinae
muscles | 614.8±255.3 | 559.7±237.6 | 0.128 |
Discussion
Scoliosis affects millions of individuals worldwide;
however, the pathogenesis remains unclear (25,26).
Anatomically, the spine consists of vertebral bodies and
intervertebral discs, which are mechanically passive and rigid.
Several muscles, which are mechanically active and retractable
structures, are attached to the spine. Logically, only asymmetrical
contraction of the muscle can lead to spinal curvature. This is the
case with side bending under physiological conditions and should be
the same with scoliosis under pathological conditions. Previous
studies have provided a basis for this hypothesis. Electromyography
shows increased activity on the convex side of the curve (27), and the spine becomes silent when
surgically fused or braced (28).
Histological studies have shown disproportionate slow-twitch vs.
fast-twitch fibres in the paravertebral muscles in cases of
scoliosis (29,30). When assessing how the asymmetrical
activation or weakness of the paravertebral muscles is caused,
research has mainly been focused on the role of the cerebrum, brain
stem and cerebellum (31–34), but no consensus has been reached.
The role of the spinal cord and spinal nerves, which directly
innervate the paraspinal muscles, in muscular imbalance has not yet
been studied.
Spinal astrocytoma is a malignant tumour that occurs
in the spinal cord and causes damage to the neurological function
of the corresponding segment of the spinal cord (35); it is unilateral in origin, visible
on MRI and characterised by a clear length of involvement in the
spinal cord (16). Patients with
spinal astrocytoma have asymmetric damage to the spinal cord, which
can lead to asymmetrical changes in the paraspinal muscles. The
present study investigated the effect of muscular imbalance in
scoliosis by observing scoliosis caused by spinal astrocytomas. In
this study, astrocytomas with unilateral initial symptoms were more
likely to develop scoliosis, and the inferred tumour side was
consistent with the convex side of scoliosis. In addition, the
distal vertebral segments of scoliosis were consistent with the
spinal cord segments (not the vertebral segments) involved in
astrocytomas. This confirms that asymmetrical weakness of the
paraspinal muscles on one side is a cause of scoliosis, and the
cause of muscle weakness is lower motor neuron paralysis due to
spinal cord injury. For the same reason, symmetrical weakness of
the paraspinal muscles on both sides is less likely to cause
scoliosis, similar to bilateral symptoms, although the muscles are
also paralysed. Some patients with initial bilateral symptoms also
had scoliosis as the tumour had asymmetric invasion, which further
contributed to the asymmetric injury of the spinal cord, although
the tumour involved both sides. In some patients, the inferred side
of the tumour was opposite to the convex side of scoliosis, which
may be since the compensatory space in the spinal canal was too
small, and the tumour with a noticeable mass effect directly caused
injury to the contralateral side, although the tumour was located
on the convex side. Furthermore, the present study found that the
cross-sectional areas of the multifidus muscles on the two sides of
the apical vertebrae in patients with scoliosis were different on
MRI, which also provided a basis for the hypothesis that the
scoliosis was caused by atrophy of one side of the muscle, to be
precise, the deep short segment muscle on the side of the tumour. A
previous study showed that in patients with idiopathic scoliosis,
concave-side muscle atrophy is more severe, which is inconsistent
with the findings of the present study (36). This finding suggests that there may
be more than one pathogenesis of scoliosis. Asymmetrical activation
and asymmetrical weakness may be different mechanisms of different
scoliosis types (37).
In summary, the differences between the scoliosis
type presented in the current study and idiopathic scoliosis are as
follows: i) Morphologically, the vertebral bodies of idiopathic
scoliosis line up like a ‘c’ shape (38), whereas the vertebral bodies of
astrocytoma-induced scoliosis line up like an ‘L’ shape. ii) The
apical vertebrae of idiopathic scoliosis are often located in the
middle of the curve (39). By
contrast, the apical vertebrae in astrocytoma-induced scoliosis
tend to be caudal to the curve. iii) Degeneration of the
paravertebral muscles on the concave side of idiopathic scoliosis
is more apparent (21), while
degeneration of the paravertebral muscles on the convex side of
astrocytoma-induced scoliosis is more obvious. These differences
indicate that astrocytoma-induced scoliosis is different from
idiopathic scoliosis. The present study found that the essence of
scoliosis caused by astrocytoma is lower neurone paralysis of the
deep paravertebral muscles caused by spinal cord injury. Given the
difference between idiopathic scoliosis and scoliosis due to spinal
astrocytoma, we believe that idiopathic scoliosis is caused by
excessive contraction of the concave paraspinal muscles, as the
upper neurone spastic paralysis of the concave muscle is caused by
a spinal cord lesion. Of course, this is merely a hypothesis and
requires further evidence.
The present study has a few limitations. First, the
sample size was relatively small. Second, some of the findings were
descriptive studies and not controlled studies. These findings
require further validation in controlled trials with larger sample
sizes.
In conclusion, spinal astrocytomas can cause lower
neuron paralysis of the paraspinal multifidus muscles, which is
innervated by the corresponding spinal cord segment affected by the
tumour, resulting in scoliosis that is convex to the paralysed
side. Astrocytoma-induced scoliosis is a type of scoliosis with
several differences from idiopathic scoliosis.
Acknowledgements
Not applicable.
Funding
This study was supported financially by the National Natural
Science Foundation of China (grant no. 81601200).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YS made substantial contributions to study
conception, designed the study and was involved in drafting the
manuscript. HZ performed the statistical analysis. SBH took the MRI
images and analyzed the data. CLY performed data analysis and
interpretation. QQM repeated the analysis to ensure it was correct.
CCM provided suggestions for research design and reviewed the
article. JY reviewed the article and helped with the data analysis.
All authors read and approved the final manuscript. YS, HZ, SBH,
CLY, QQM and JY confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Peking University Third Hospital (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mccann KS and Kelham SA: Scoliosis. JAAPA.
35:57–58. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heyde CE and Putzier M: Neuromuscular
scoliosis. Orthopade. 50:605–607. 2021.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuznia AL, Hernandez AK and Lee LU:
Adolescent idiopathic scoliosis: Common questions and answers. Am
Fam Physician. 101:19–23. 2020.PubMed/NCBI
|
|
4
|
Yang H, Im G, Zhu C, Osorio C, Masood U,
Zhou C, Yang X, Liu L and Song Y: Unplanned surgery of congenital
scoliosis. Chin Med J (Engl). 135:374–376. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Reuver S, van der Linden PP, Kruyt MC,
Schlosser T and Castelein RM: The role of sagittal pelvic
morphology in the development of adult degenerative scoliosis. Eur
Spine J. 30:2467–2472. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wishart BD and Kivlehan E: Neuromuscular
scoliosis: When, who, why and outcomes. Phys Med Rehabil Clin N Am.
32:547–556. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slattery C and Verma K: Classifications in
Brief: The lenke classification for adolescent idiopathic
scoliosis. Clin Orthop Relat Res. 476:2271–2276. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blevins K, Battenberg A and Beck A:
Management of scoliosis. Adv Pediatr. 65:249–266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mesiti BL: Scoliosis: An overview. Radiol
Technol. 93:55–72. 2021.
|
|
10
|
Oetgen ME, Heyer JH and Kelly SM:
Scoliosis screening. J Am Acad Orthop Surg. 29:370–379. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Acaroglu E, Akel I, Alanay A, Yazici M and
Marcucio R: Comparison of the melatonin and calmodulin in
paravertebral muscle and platelets of patients with or without
adolescent idiopathic scoliosis. Spine (Phila Pa 1976).
34:E659–E663. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stetkarova I, Zamecnik J, Bocek V, Vasko
P, Brabec K and Krbec M: Electrophysiological and histological
changes of paraspinal muscles in adolescent idiopathic scoliosis.
Eur Spine J. 25:3146–3153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riddle HF and Roaf R: Muscle imbalance in
the causation of scoliosis. Lancet. 268:1245–1247. 1955. View Article : Google Scholar
|
|
14
|
Tabibkhooei A, Sadeghipour A and Fattahi
A: Thoracolumbar pilomyxoid astrocytoma concomitant with spinal
scoliosis: A case report and literature review. Surg Neurol Int.
10:2352019. View Article : Google Scholar
|
|
15
|
Zhang D, Fan W, Zhao X, Massicotte EM and
Fan T: Long-level intramedullary spinal cord astrocytoma
complicated with spine scoliosis: Report of two cases. Int J Surg
Case Rep. 79:234–238. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parsa AT, Lee J, Parney IF, Weinstein P,
Mccormick PC and Ames C: Spinal cord and
intradural-extraparenchymal spinal tumors: Current best care
practices and strategies. J Neurooncol. 69:291–318. 2004.
View Article : Google Scholar
|
|
17
|
Zulch KJ: Principles of the new World
Health Organization (WHO) classification of brain tumors.
Neuroradiology. 19:59–66. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kleihues P, Burger PC and Scheithauer BW:
The new WHO classification of brain tumours. Brain Pathol.
3:255–268. 1993. View Article : Google Scholar
|
|
19
|
Kleihues P, Louis DN, Scheithauer BW,
Rorke LB, Reifenberger G, Burger PC and Cavenee WK: The WHO
classification of tumors of the nervous system. J Neuropathol Exp
Neurol. 61:215–225. 226–229. 2002. View Article : Google Scholar
|
|
20
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar
|
|
21
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar
|
|
22
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO Classification of tumors of the
central nervous System: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar
|
|
23
|
Shafaq N, Suzuki A, Matsumura AC, Terai H,
Toyoda H, Yasuda H, Ibrahim M and Nakamura H: Asymmetric
degeneration of paravertebral muscles in patients with degenerative
lumbar scoliosis. Spine (Phila Pa 1976). 37:1398–1406. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Canbay S, Gurer B, Bozkurt M, Comert A,
Izci Y and Baskaya MK: Anatomical relationship and positions of the
lumbar and sacral segments of the spinal cord according to the
vertebral bodies and the spinal roots. Clin Anat. 27:227–233. 2014.
View Article : Google Scholar
|
|
25
|
Zaydman AM, Strokova EL, Kiseleva EV,
Suldina LA, Strunov AA, Shevchenko AI, Laktionov PP and Subbotin
VM: A New look at etiological factors of idiopathic scoliosis:
Neural Crest Cells. Int J Med Sci. 15:436–446. 2018. View Article : Google Scholar
|
|
26
|
Ruwald JM, Eymael RL, Upenieks J, Zhang L,
Jacobs C, Pflugmacher R and Schildberg FA: An overview of the
current state of pediatric scoliosis management. Z Orthop Unfall.
158:508–516. 2020. View Article : Google Scholar
|
|
27
|
Cheung J, Veldhuizen AG, Halberts JP,
Sluiter WJ and Van Horn JR: Geometric and electromyographic
assessments in the evaluation of curve progression in idiopathic
scoliosis. Spine (Phila Pa 1976). 31:322–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsai YT, Leong CP, Huang YC, Kuo SH, Wang
HC, Yeh HC and Lau YC: The electromyographic responses of
paraspinal muscles during isokinetic exercise in adolescents with
idiopathic scoliosis with a Cobb's angle less than fifty degrees.
Chang Gung Med J. 33:540–550. 2010.PubMed/NCBI
|
|
29
|
Yarom R and Robin GC: Studies on spinal
and peripheral muscles from patients with scoliosis. Spine (Phila
Pa 1976). 4:12–21. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawaguchi K, Obayashi J, Koike J, Tanaka
K, Seki Y, Nagae H, Ohyama K, Furuta S, Valsenti G, Pringle KC and
Kitagawa H: Muscle imbalance as a cause of scoliosis: A study in a
fetal lamb abdominal wall defect model. Pediatr Surg Int.
37:1755–1760. 2021. View Article : Google Scholar
|
|
31
|
Carry PM, Duke VR, Brazell CJ, Stence N,
Scholes M, Rousie DL and Hadley MN: Lateral semi-circular canal
asymmetry in females with idiopathic scoliosis. PLoS One.
15:e2324172020. View Article : Google Scholar
|
|
32
|
Antoniadou N, Hatzitaki V, Stavridis S and
Samoladas E: Verticality perception reveals a vestibular deficit in
adolescents with idiopathic scoliosis. Exp Brain Res.
236:1725–1734. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ng A, Pizer B and May P: Congenital spinal
astrocytoma: How favourable is the long-term outcome? Br J
Neurosurg. 14:366–370. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Catanzariti JF, Agnani O, Guyot MA,
Wlodyka-Demaille S, Khenioui H and Donze C: Does adolescent
idiopathic scoliosis relate to vestibular disorders? A systematic
review. Ann Phys Rehabil Med. 57:465–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Biczok A, Strubing FL, Eder JM,
Egensperger R, Schnell O, Zausinger S, Neumann JE, Herms J, Tonn JC
and Dorostkar MM: Molecular diagnostics helps to identify distinct
subgroups of spinal astrocytomas. Acta Neuropathol Commun.
9:1192021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yeung KH, Man G, Shi L, Hui S, Chiyanika
C, Lam TP, Ng B, Cheng J and Chu W: Magnetic resonance
imaging-based morphological change of paraspinal muscles in girls
with adolescent idiopathic scoliosis. Spine (Phila Pa 1976).
44:1356–1363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park Y, Ko JY, Jang JY, Lee S, Beom J and
Ryu JS: Asymmetrical activation and asymmetrical weakness as two
different mechanisms of adolescent idiopathic scoliosis. Sci Rep.
11:175822021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fadzan M and Bettany-Saltikov J:
Etiological Theories of adolescent idiopathic scoliosis: Past and
present. Open Orthop J. 11:1466–1489. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Peng Y, Wang SR, Qiu GX, Zhang JG and
Zhuang QY: Research progress on the etiology and pathogenesis of
adolescent idiopathic scoliosis. Chin Med J (Engl). 133:483–493.
2020. View Article : Google Scholar : PubMed/NCBI
|