Introduction
In 2016, the World Health Organization revised the
classification of lymphoid tissue tumors, defining mature B-cell
lymphoma based on the morphology into diffuse large B-cell lymphoma
(DLBCL) and Burkitt lymphoma (BL) as high-grade B-cell lymphoma
(HGBL), with MYC and BCL2 and/or BCL6 rearrangements defined as
‘double-hit’ or ‘triple-hit’ lymphomas (DHL/THL) (1). DHL is a highly invasive mature B-cell
lymphoma with rapid progression, easy recurrence, and poor
prognosis (1). In addition, 70–100%
of cases are diagnosed at late stages and >50% exhibit extensive
infiltration, including lymph node invasion, and may also involve
extranodal organs, particularly the bone marrow and the central
nervous system. DHL is not sensitive to conventional treatment with
rituximab plus cyclophosphamide, doxorubicin, vincristine and
prednisone (R-CHOP) and cannot be completely relieved after
treatment or has an easy relapse after remission. Therefore,
accurate diagnosis and treatment of DHL are significant to patient
prognosis. Extranodal primary DHL is rare and double lymphoma
originating from bilateral ovaries is even rarer, accounting for
0.5% of non-Hodgkin's lymphoma and 1.5% of all ovarian tumors
(2). The current study presented a
unique case of a primary DHL in the bilateral ovaries and reviewed
and discussed the clinical history to provide a deeper
understanding of the clinical manifestations, diagnosis and
differential diagnosis of this disease, with the goals of reducing
missed diagnosis and misdiagnosis, contributing to proper clinical
treatment and improving the survival rate of patients.
Case report
A 33-year-old female patient presented to the West
China Second University Hospital, Sichuan University (Sichuan,
China), in January 2020, with the complaint of lower abdominal
distension aggravated during defecation for at least 2 months prior
to admission, but with no obvious inducement of lower abdominal
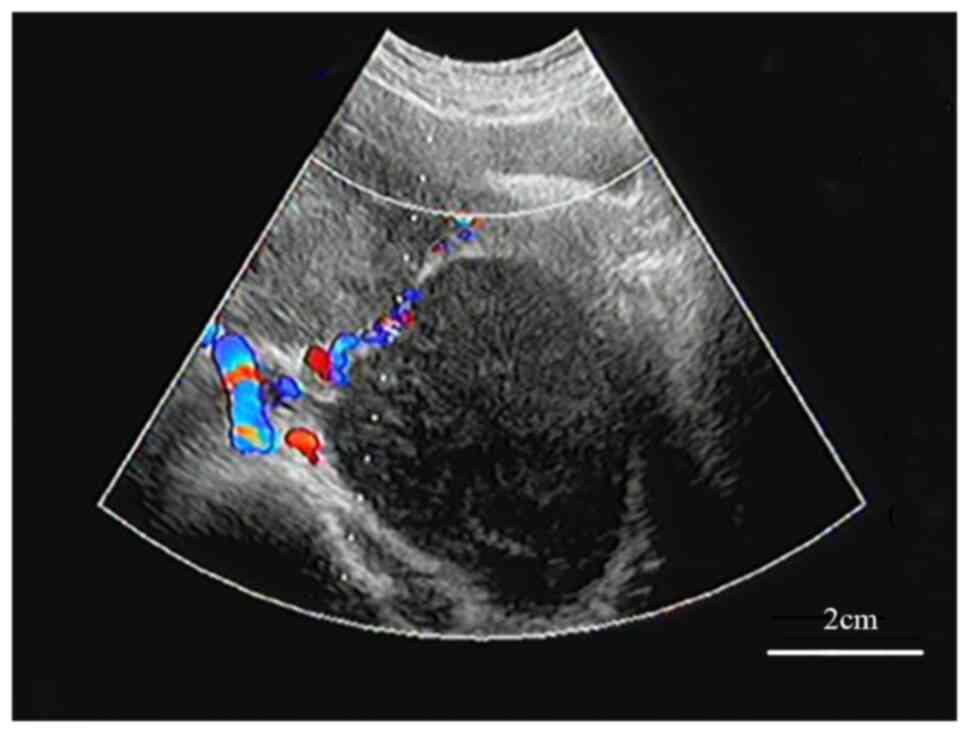
distension. Thus, B-ultrasound was performed locally and indicated
a bilateral adnexal solid mass (Fig.
1). At 20 days prior to admission, the patient developed
abdominal distension, felt abdominal skin tension, palpated an
obvious abdominal mass and occasionally had severe abdominal
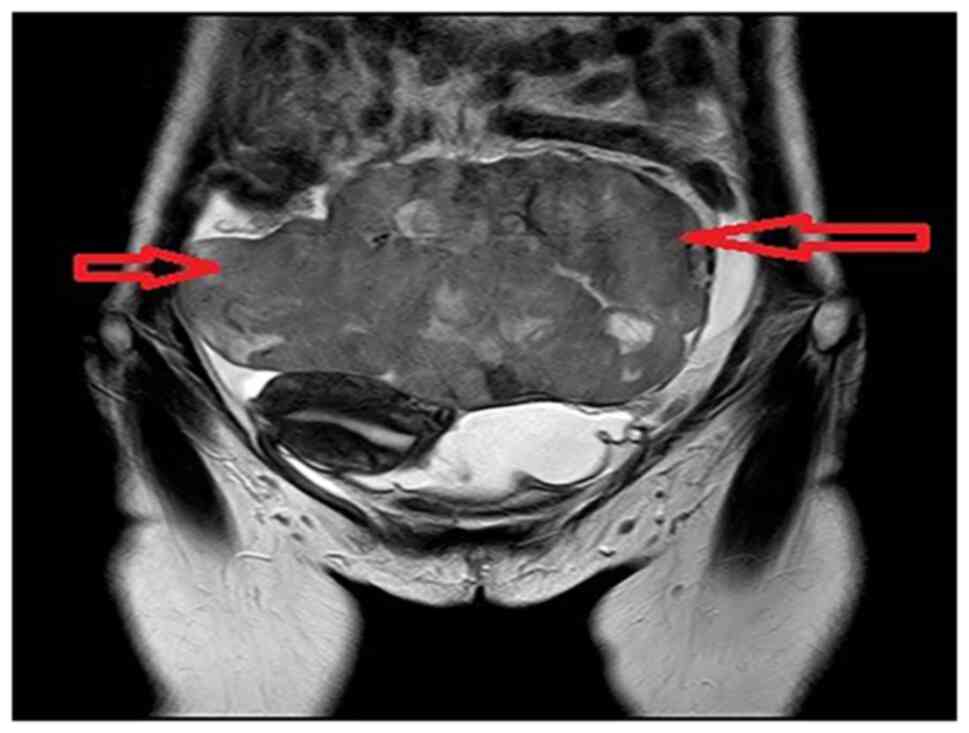
colics. The MRI revealed two huge lobular masses in the pelvis and
abdomen, mainly solid components, and focal liquefaction necrosis
was detected in the mass. The diameter lines of the larger layer
were ~10×17×11.8 and 10.9×9.3×8.9 cm, and a bilateral ‘ovarian
vascular pedicle sign’ was faintly visible. Thus, the possibility
of bilateral ovarian malignant tumors was considered, but the
nature was undetermined (Fig. 2).
In addition, the level of the tumor marker CA125 was raised to 230
U/ml (normal range, <35 U/ml). Seeking further treatment at the
West China Second University Hospital in February 2020, the patient
was admitted and underwent abdominal bilateral appendectomy,
hysterectomy, pelvic lymph node dissection, abdominal aortic lymph
node dissection, momentum resection, appendectomy, intestinal
adhesion lysis and closure of great vessels.
Intraoperatively, one gray and white mass (measuring
19×14×8 cm) was detected in the left adnexa, as well as a partial
cytoplasmic defect. The grayish-yellow solid nature of the luminal
surface was soft and the focal area was hemorrhagic. One
white-gray-red nodule (measuring 15×13.5×4.5 cm) was detected in
the right adnexa, appearing capsular. The section of the nodule was
gray and white, solid and soft, with a focal area of hemorrhage.
Grayish brown non-plastic tissue was observed in various places,
including the side of the uterus serosal surface, parametrial,
posterior uterine wall, the peritoneal reflection of the bladder,
intestinal fat lobes. The tissue was fixed with 4% neutral formalin
(for 24 h at 25°C) and embedded in paraffin, and then 4-µm sections
were prepared that were subjected to hematoxylin and eosin
(H&E) staining (for 8 h at 25°C). H&E indicated that the
cell morphology was similar in all lesions. Tumor cells exhibited
diffuse infiltrative growth, large areas of coagulative necrosis,
cells were medium-sized and round in shape with little cytoplasm,
the nuclear division was easily seen, asterisk phenomenon was
observed and the interstitium had a small amount of vascular and
fibrous tissue (Fig. 3).
Immunohistochemical staining with the EnVision Systems method using
antibodies from Fuzhou Maixin Biotechnology Co., Ltd., was
performed at 37°C for 40 min for all primary antibodies, with the
following results: CD20 (+) (working liquid; cat. no. Kit-0001),
CD79a (+) (working liquid; cat. no. MAB0258), CD3 (−) (working
liquid; cat. no. MAB0740), CD10 (+) (working liquid; cat. no.
MAB0668), Bcl6 (+) (working liquid; cat. no. MAB0746), MUM1 (−)
(working liquid; cat. no. MAB0885), CyclinD1 (−) (working liquid;
cat. no. RMA0541), CD5 (−) (working liquid; cat. no. MAB0827), CD30
(−) (working liquid; cat. no. MAB0868), p53 (+, 70%) (working
liquid; cat. no. MAB0674), MYC (+, 80%) (working liquid; cat. no.
RMA0803), Bcl2 (+, 90%) working liquid; cat. no. MAB0711) and Ki-67
(+, 80%) (1:200; cat. no. RMA0542). The results of fluorescence
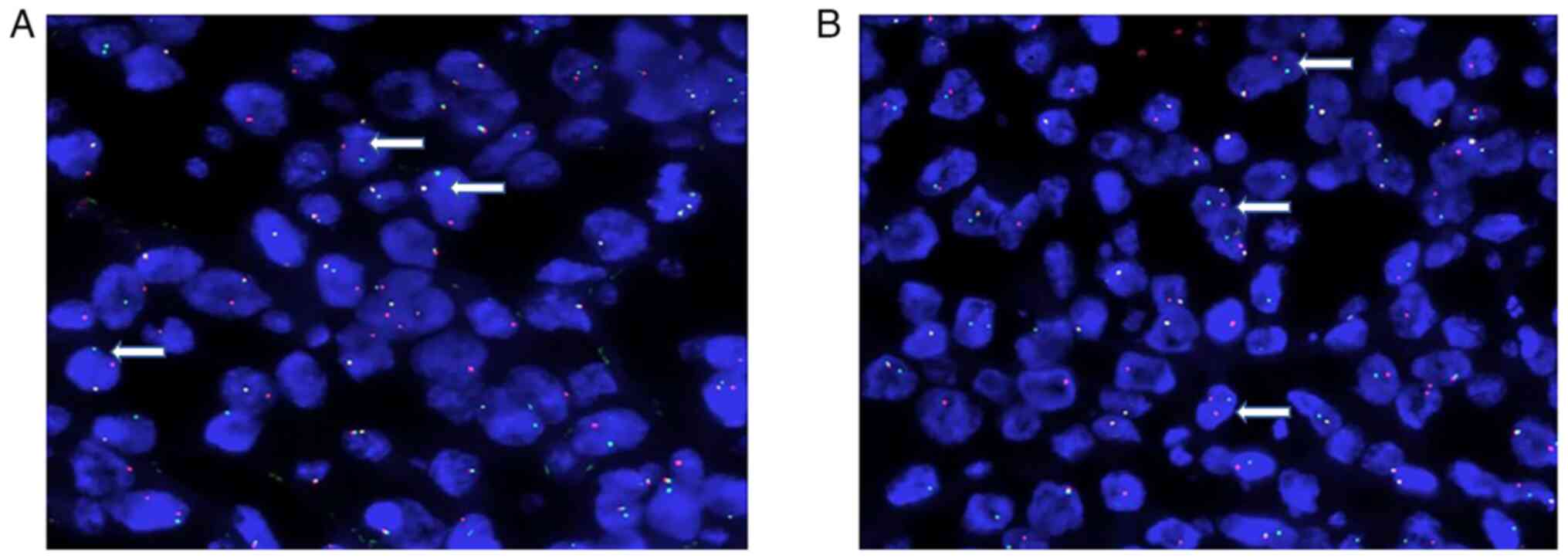
in situ hybridization (FISH) (3) analysis of the original tumor tissue
were as follows: MYC and BCL2 gene translocations (Fig. 4), with no BCL6 gene translocation
and no MYC/IgH fusion detected. The pathologic diagnosis was HGBL
with MYC and BCL2 gene translocations.
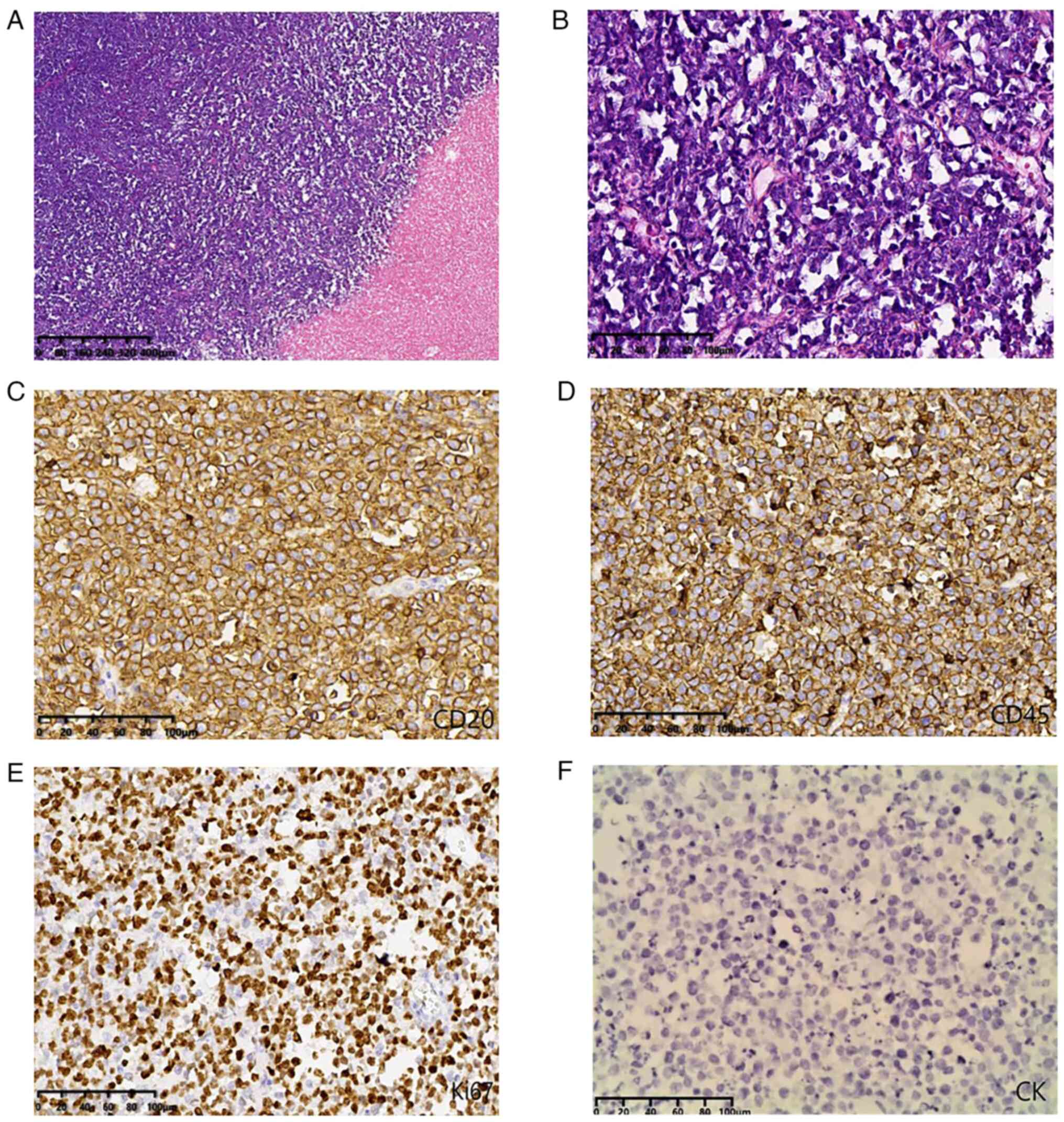 | Figure 3.Histology and immunohistochemical
images. (A) Histological analysis indicated that the tumor cells
exhibited diffuse infiltrative growth, large areas of coagulative
necrosis (magnification, ×10; scale bar, 400 µm; H&E); (B)
cells were medium-sized and round in shape with little cytoplasm,
nuclear division was easily observed, asterisk phenomenon was
present and the interstitium had a small amount of vascular and
fibrous tissue (magnification, ×100; scale bar, 100 µm; H&E).
(C) Tumor cells were positive for CD20 and (D) CD45. (E) The Ki67
labeling index was 80%. (F) Tumor cells were negative for CK.
[(C-F) magnification, ×200; scale bar, 100 µm]. CK,
cytokeratin. |
During the postoperative follow-up, three cycles of
an R-CHOP chemotherapy regimen were administered in the hospital:
600 mg rituximab on day 1 + 1 g cyclophosphamide on day 1 + 4 mg
vindesine on day 1 + 110 mg epirubicin on day 1 + 100 mg prednisone
on days 1–5 each week for 3 weeks, with 21 days as one cycle. The
first follow-up was performed at 3 months after the surgery and
suggested nodular thickening of the pericardium, pleura and
possible metastases on the CT scan of the abdomen. Thereafter, the
patient did not proceed with related treatments. A follow-up visit
was performed every 3 months and the patient died 6 months
later.
Discussion
Primary lymphomas of the female genital tract
account for 0.2-1.1% of all extranodal lymphomas and most cases are
due to secondary disease involvement (4,5). The
ovary is usually involved in 7–30% of secondary disseminated
lymphomas. Primary ovarian lymphoma has a 5-year survival rate of
80%, while it is only 33% for secondary ovarian lymphoma (6). Hence, clarifying the origin of the
lymphoma is crucial. The following diagnostic criteria were
proposed for primary ovarian non-Hodgkin lymphoma: i) At diagnosis,
the lymphoma is clinically confined to the ovary with no evidence
of lymphoma in other tissues but may still be considered primary if
the ovarian lymphoma has spread to immediately adjacent lymph nodes
or has directly invaded adjacent structures; ii) peripheral blood
and bone marrow should not contain any abnormal cells; iii) if
other lymphomatous lesions developed at sites distant from the
ovary, at least a few months would have elapsed between the
appearance of the ovarian and extraovarian lesions (7,8). A
previous study reported lymphoma originating in one ovary (9), but DHL originating in the bilateral
ovaries is rare.
Currently, the main aspect of clinical DHL diagnosis
is based on hematopathological examinations, requiring combined
tissue and cell morphology, immunohistochemistry, genetics and
molecular biology. DHL gene abnormalities detected by FISH are
classified into the MYC gene and the BCL2 or BCL6 gene. MYC gene
rearrangement accompanied by BCL2 or BCL6 gene rearrangement is
required for DHL diagnoses or THL if the MYC gene rearrangement is
accompanied by both BCL2 and BCL6 gene rearrangements. Given the
relatively low incidence of DHL and the high cost of FISH to
complete the examination of three gene rearrangements (MYC, BCL2
and BCL6), immunohistochemistry should be performed in patients
with DLBCL with high MYC and BCL2 protein levels, germinal center
subtype origin and Ki-67 >90%. However, it has been indicated
that this practice may miss certain cases (10). For instance, the Ki-67 was ~80% in
the current case. Of the 108 patients with DHL studied by Li et
al (11), ~84% presented with
Ki-67 >70%, and the distribution of its expression level was
20–100%. Therefore, there are numerous patients with DHL with Ki-67
<90%. Thus, the Ki-67 index is only one reference and the most
reliable diagnostic basis is genetic testing. The histologic
support of the present case was a small round cell malignant tumor,
and CD20 (+), CD79a (+), CD3 (−), CD10 (+), Bcl6 (+), MUM1 (−),
CyclinD1 (−), CD5 (−), CD30 (−), P53 (+, 70%), MYC (+, 80%) and
Bcl2 (+, 90%), suggesting the germinal center-like source, and
DLBCL with double MYC and BCL2 expression. The gene test results
detected the MYC and BCL2 gene translocations, which may be
diagnosed as DHL.
Since DHL occurring in the ovary is not common in
the clinic, it should be distinguished from the following common
ovarian lesions: i) Undifferentiated carcinoma-the tumor is mostly
solid, large in size and frequently accompanied by bleeding and
necrosis. Under the microscope, the tumor cells have a solid
nest-shaped distribution, obvious cell atypia, the nuclear division
is common, a spindle cell area may be seen and immunohistochemical
expression of epithelial markers cytokeratin (CK) and epithelial
membrane antigen (EMA); ii) juvenile granulosa cell tumor-it
usually occurs in adolescent females. It is a cystic, solid mass;
the solid area is gray and yellow. Under the microscope, it is
typically characterized by a diffuse or nodular distribution of
tumor cells, interspersed with follicles of different sizes and
shapes or round, oval shape. Immunohistochemical staining indicates
that the tumor cells express inhibin, calrentin, SF-1, CD99, CD56
and, in certain cases, CK8 or CK18; iii) hypercalcemia small cell
carcinoma-it frequently occurs in young females and may be
accompanied by hypercalcemia. The tumor is large, solid, gray and
yellow in section, and immunohistochemical CK, vimentin,
neuron-specific enolase and EMA are frequently positive; iv) HGBL,
non-specific, with a morphology between DLBCL and BL, high Ki
index, positive CD45 and CD20 on immunohistochemistry, but no
rearrangement of MYC and BCL-2/BCL-6-related genes; v) metastatic
tumors, combined with CT, B-ultrasound and MRI imaging results, no
space-occupying lesions in other systems.
Furthermore, patients with DHL generally have high
LDH levels, late clinical stages, rapid development, high cell
proliferation indexes and are prone to extranodal invasion,
particularly bone marrow and central nervous system involvement.
The international prognostic index score is usually medium or high
risk, the treatment effect is poor and the survival time is
~0.2-1.5 years (1). R-CHOP is a
classic treatment for DLBCL, but its therapeutic effects on DHL are
not ideal, which cannot be completely relieved or relapse occurs
soon after treatment. The patient of the present study did not
undergo gene testing in time after the operation and the effect of
the treatment, according to DLBCL, was poor. However, there is no
unified standard for DHL treatment. R-Hyper-CVAD, R-CODOX-M/IVAC
and R-ECHOP are the most widely used treatments in clinical
practice (12). Studies have
supported using more intensive regimens to treat patients with DHL;
for instance, R-DA-EPOCH rather than R-CHOP (12,13).
However, a retrospective multicenter study of 90 patients with
double expressor lymphoma indicated that DA-EPOCH-R provided no
survival benefit compared to R-CHOP (14). These conflicting results indicate
the requirement for prospective studies. The effects are still
controversial for other treatments, such as autologous
hematopoietic stem cell transplantation. Given the presence of
MYC/BCL-2 and/or BCL6 gene rearrangements in DHL, specific
inhibitors targeting MYC, BCL-2 and BCL-6 have attracted increasing
attention, and immunotherapy and targeted drugs are promising
therapeutic directions (13,15).
One limitation of the present study is that no
further information is available on the patient, as they did not
consent to any further relevant tests, such as fluorodeoxyglucose
positron emission tomography-CT scanning and gene expression
profiling or gene sequencing, which may have been due to economic
or other reasons.
To the best of our knowledge, this rare HGBL
presentation with MYC and BCL2 translocations and unusual
extranodal locations has been reported less frequently in the
ovary. In addition, the case of the present study had involvement
of the bilateral ovaries. Hence, clinicians require awareness of
these aggressive lymphomas that frequently involve extranodal sites
to provide early diagnoses, and the correct multi-agent
chemotherapy may be initiated promptly.
Acknowledgements
Not applicable.
Funding
The Fund of the Basic Research Project of Sichuan Provincial
Science and Technology Department (grant no. 2019YJ0065) and Key
Research and Development Projects of Sichuan Provincial Department
of Science and Technology (grant no. 2022YFS0045) provided
financial support.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YZ and TC drafted the manuscript and conceived the
study. WW and YZ were responsible for the collection and analysis
of case data and literature. TC and WW revised the manuscript and
interpreted the data. WW provided financial support. YZ, TC and WW
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and its
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elharroudi T, Ismaili N, Errihani H and
Jalil A: Primary lymphoma of the ovary. J Cancer Res Ther.
4:195–196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salam DSDA, Thit EE, Teoh SH, Tan SY, Peh
SC and Cheah SC: C-MYC, BCL2 and BCL6 translocation in B-cell
non-Hodgkin lymphoma cases. J Cancer. 11:190–198. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nasioudis D, Kampaktsis PN, Frey M, Witkin
SS and Holcomb K: Primary lymphoma of the female genital tract: An
analysis of 697 cases. Gynecol Oncol. 145:305–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ng SP, Leong CF, Nurismah MI, Shahila T
and Jamil MA: Primary Burkitt lymphoma of the ovary. Med J
Malaysia. 61:363–365. 2006.PubMed/NCBI
|
|
6
|
Crasta JA and Vallikad E: Ovarian
lymphoma. Indian J Med Paediatr Oncol. 30:28–30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H, Lui F and Chen Y: A case of
primary ovary non-Hodgkin's malignant lymphoma. Hunan Yi Ke Da Xue
Xue Bao. 23:5161998.(In Chinese). PubMed/NCBI
|
|
8
|
Fox H, Langley FA, Govan AD, Hill AS and
Bennett MH: Malignant lymphoma presenting as an ovarian tumour: A
clinicopathological analysis of 34 cases. Br J Obstet Gynaecol.
95:386–390. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarina SJ and Song J: A case report and
literature review of ‘double strike’ ovarian diffuse large b-fine.
Gold lymphoma. Chin J PractGynecol Obstet. 38:247–249. 2022.
|
|
10
|
Wei XX, Zhao Q, Li DP and Huang YH: Recent
advances in diagnosis and treatment of double strike lymphoma. J
Exp Hematol. 27:1311–1315. 2019.
|
|
11
|
Li S, Saksena A, Desai P, Xu J, Zuo Z, Lin
P, Tang G, Yin CC, Seegmiller A, Jorgensen JL, et al: Prognostic
impact of history of follicular lymphoma, induction regimen and
stem cell transplant in patients with MYC/BCL2 double hit lymphoma.
Oncotarget. 7:38122–38132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sehn LH and Salles G: Diffuse large B-cell
lymphoma. N Engl J Med. 384:842–858. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhuang Y, Che J, Wu M, Guo Y, Xu Y, Dong X
and Yang H: Altered pathways and targeted therapy in double hit
lymphoma. J Hematol Oncol. 15:262022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
D'Angelo CR, Hanel W, Chen Y, Yu M, Yang
D, Guo L, Karmali R, Burkart M, Ciccosanti C, David K, et al:
Impact of initial chemotherapy regimen on outcomes for patients
with double-expressor lymphoma: A multi-center analysis. Hematol
Oncol. 39:473–482. 2021. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Savage KJ, Johnson NA, Ben-Neriah S,
Connors JM, Sehn LH, Farinha P, Horsman DE and Gascoyne RD: MYC
gene rearrangements are associated with a poor prognosis in diffuse
large B-cell lymphoma patients treated with R-CHOP chemotherapy.
Blood. 114:3533–3537. 2009. View Article : Google Scholar : PubMed/NCBI
|