Introduction
Glioblastoma multiforme (GBM) is a central nervous
system malignancy and the most common glioma subtype, with very
aggressive and highly infiltrative behavior (1–3). The
incidence of GBM is 12–15% among all intracranial tumors; it is
mainly found in the cerebral hemisphere and originates from the
supratentorial area in 95% of cases (4,5).
Patients with GBM usually have a poor prognosis, with a median
survival time of 12–15 months after diagnosis and a 5-year survival
rate of only 3–5% (6).
The three main signal transduction pathways involved
in GBM are the retinoblastoma pathway, the p53 pathway and the
phosphatidylinositol-3-kinase (PI3K) pathway (7). Activation of the PI3K pathway is
detected in 88% of cancer types and affects the
phosphoinositide-3-kinase regulatory subunit 1 and
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
genes. In signal transduction associated with various cellular
processes, PI3K has essential roles in the regulation of the
metabolism, inflammation, survival, motility and development of
cancer cells (8,9). Stimulating factors such as growth
factors, cytokines, hormones and other factors, including genomic
instability due to excessive non-homologous end joining (NHEJ),
also play a role in activation of the PI3K pathway (10–13).
Furthermore, PI3K induces the activation of Akt, which can inhibit
the activity of various pro-apoptotic proteins, including Bad, Bax
and Bak, and then inhibit the release of cytochrome c from
the mitochondria. As a result, the activity of caspase-3 is
inhibited, and apoptosis does not occur (14–16).
Therefore, high levels of Akt impact the survival and progression
of malignant cells (17,18).
Caspase-3 is an endoprotease with the main role as
an executioner caspase in apoptosis (19). Caspase-3 is activated by caspase-9
and converted to the active form by cleavage at Asp175/Ser176
(19–21). The activation and cleavage of
caspase-3 are crucial for programmed cell death. Several studies
have shown the role of caspase-3 in the regulation of
carcinogenesis in various cancer types, such as breast cancer,
gastric and tongue cancer (22–26).
Therefore, caspase-3 is a good indicator for the evaluation of
cancer progression (19,22).
The apoptosis pathway can be activated by DNA
damage, the most threatening type of which is DNA double-strand
breaks (27,28). Physiological or pathological
processes can cause DNA double-strand breaks to occur.
Physiological processes that cause DNA double-strand breaks
comprise V(D)J recombination, class-switch recombination, meiosis
or DNA replication, in which DNA double-strand breaks occur as a
secondary event during the normal cell cycle (29). DNA double-strand breaks can also
occur due to exposure to ionizing radiation, oxidizing free
radicals, enzymatic processes or cytotoxic agents (30–32).
The physiological response to DNA double-strand
breaks includes the detection of DNA damage, the recruitment of
factors with a role in repairing the damage and the repair process
itself (33). The initial response
when DNA double-strand breaks occur is the phosphorylation of
histone 2AX (H2AX) to form γ-H2AX, which occurs within 10–30 min
after the damage is induced (34,35).
γ-H2AX is a sensitive and early indicator of DNA double-strand
breaks in which the serine amino acid residue at the 139 position
of H2AX is phosphorylated; numerous γ-H2AX molecules are formed in
the damaged area (35–37). γ-H2AX serves as a marker for the
detection of DNA double-strand breaks and genomic instability, and
also indicates the ability of cancer cells to survive (36,38–40).
The mechanism for the repair of DNA double-strand breaks can be
error-prone, and so may induce genomic instability and genetic
mutations that will activate signal transduction, inhibit apoptosis
of the GBM cells, and finally lead to rapid progression and
aggressive behavior in GBM.
To the best of our knowledge, the association
between DNA double-strand breaks and apoptosis in patients with
untreated GBM has not been analyzed previously. Therefore, the
present study evaluated the correlation between γ-H2AX and cleaved
caspase-3, PI3K and Akt in pre-treatment patients with GBM or grade
I glioma with the aim of providing an improved understanding of the
molecular mechanism underlying the survival of GBM cells.
Materials and methods
Ethics approval and patient
consent
The study was approved by the Ethics Committee of
The Faculty of Medicine, Universitas Indonesia (ref. no.
KET-393/UN2.F1/ETIK/PPM.00.02/2020). The patients and/or their
guardians provided written informed consent related to storage and
sharing of data and tissue specimens for future research.
Study design
A total of 26 tumor tissue specimens from patients
with GBM and 7 tumor tissue specimens from patients with grade I
glioma as controls were obtained from Siloam Hospital Lippo Village
(Tangerang, Indonesia) and Mochtar Riady Comprehensive Cancer
Center Siloam Hospital (Jakarta, Indonesia) between January 2013
and December 2016. All tissue specimens were stored at −80°C. This
study included 14 males and 12 females with GBM (mean age, 49
years; age range, 21–63 years), and 4 males and 3 females with
grade I glioma (mean age, 34 years; range, 23–73 years). The
inclusion criteria were a diagnosis of GBM, a World Health
Organization (WHO) grade of IV and WHO grade I glioma based on the
pathological report. None of the patients had received any
treatment before biopsy or surgical resection. The concentrations
of γ-H2AX, total PI3K, total Akt and cleaved caspase-3 were
determined using sandwich enzyme-linked immunosorbent assays
(ELISAs). The ELISA kits used in this study were the Human Gamma
H2AX ELISA Kit (cat. no. MBS167504; MyBioSource, Inc.), the Human
PI3K ELISA Kit (cat. no. CSB-E08417h; Cusabio Technology LLC), the
Akt (Total) Human ELISA Kit (cat. no. KHO0101; Invitrogen; Thermo
Fisher Scientific, Inc.) and the Caspase-3 (Cleaved) Human ELISA
kit (cat. no. KHO1091; Invitrogen, Thermo Fisher Scientific, Inc.).
The research was conducted in the Department of Biochemistry and
Molecular Biology, Faculty of Medicine, Universitas Indonesia
(Jakarta, Indonesia).
Protein isolation
Tumor tissues were homogenized on ice using a micro
homogenizer with 1–2 ml RIPA lysis and extraction buffer (Thermo
Fisher Scientific, Inc.). The extract was incubated on ice for ~15
min and then centrifuged at 14,000 × g for 15 min at room
temperature. The supernatant was collected and the concentration of
γ-H2AX, total PI3K, total Akt and cleaved caspase-3 in the
supernatant was analyzed. The total protein concentration was
determined using a BSA protein assay (MilliporeSigma) with
measurement of the absorbance at 280 nm.
Sandwich ELISA
A 100-µl sample of the supernatant was put into a
microplate well and incubated for 2 h at room temperature, after
which the wells were washed four times with 250 µl wash buffer.
Next, 100 µl antibody from the ELISA kit was added and the wells
were incubated for 1 h at room temperature. After the incubation,
the wells were washed four times using 250 µl wash buffer, 100 µl
streptavidin-HRP was added and the wells were incubated for 30 min
at room temperature. After washing, 100 µl stabilized chromogen was
added, followed by incubation for 30 min at room temperature. Next,
100 µl stop solution was added to each well and the absorbance was
read at 450 nm. The protein concentration was read from the
standard curve. The concentrations of γ-H2AX, total PI3K, total Akt
and cleaved caspase-3 were divided by the total protein
concentration to standardize them.
Statistical analysis
Statistical analysis was performed on the data
obtained, with processing and analysis using SPSS Statistics
version 21.0 (IBM Corp.). All data are presented as the mean ± SD.
Comparative analyses between GBM and grade I glioma were performed
using the independent Student's t-test. The Saphiro-Wilk test was
used to determine the normality of distribution. Correlation
analyses between variables were conducted using Pearson's
correlation test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinicopathological
characteristics
The clinicopathological characteristics of the
patients with GBM and grade I glioma included in the present study
are shown in Table I.
 | Table I.Clinicopathological characteristics
of patients with GBM and grade I glioma. |
Table I.
Clinicopathological characteristics
of patients with GBM and grade I glioma.
|
Characteristics | GBM (n) | Grade I glioma
(n) |
|---|
| Sex |
|
|
|
Male | 14 | 4 |
|
Female | 12 | 3 |
| Age, years |
|
|
|
≤40 | 8 | 5 |
|
>40 | 18 | 2 |
| Tumor size, cm |
|
|
|
<4 | 5 | 1 |
| ≥4 | 15 | 3 |
|
Unknown | 6 | 3 |
| Surgical type |
|
|
| Total
resection | 1 | 3 |
|
Subtotal resection or
biopsy | 19 | 1 |
|
Unknown | 6 | 3 |
| KPS |
|
|
|
<70 | 15 | 0 |
|
≥70 | 11 | 7 |
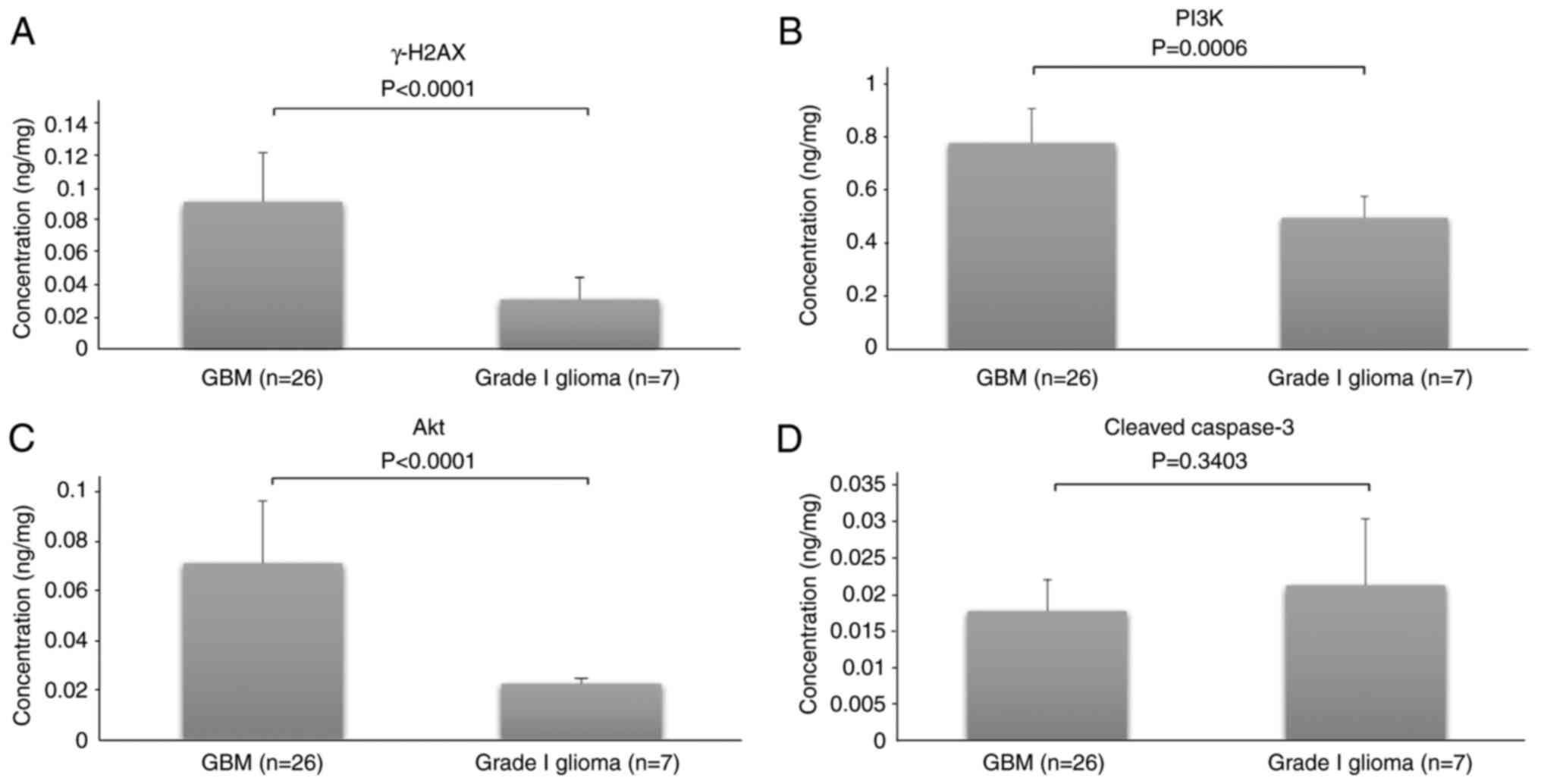
Comparative analysis of γ-H2AX, total
PI3K, total Akt and cleaved caspase-3
The concentrations of γ-H2AX, total PI3K, total Akt
and active (cleaved) caspase-3 were measured to obtain information
based on their known contributions to particular cellular and
molecular mechanisms. Sandwich ELISAs were performed to determine
the levels of γ-H2AX, total PI3K, total Akt and cleaved caspase-3
in the GBM and grade I glioma tissues.
The Saphiro-Wilk test showed that the levels of
γ-H2AX, total PI3K, total Akt and cleaved caspase-3 were normally
distributed. Therefore, a parametric comparative test (independent
Student's t-test) was appropriate. Next, a comparative analysis
between GBM and grade I glioma was conducted and the results are
shown in Fig. 1. In GBM and grade I
glioma, the γ-H2AX concentrations were 0.09±0.03 and 0.03±0.01, the
total PI3K concentrations were 0.77±0.13 and 0.49±0.22, the total
Akt concentrations were 0.07±0.02 and 0.02±0.003, and the cleaved
caspase-3 concentrations were 0.02±0.005 and 0.02±0.009,
respectively.
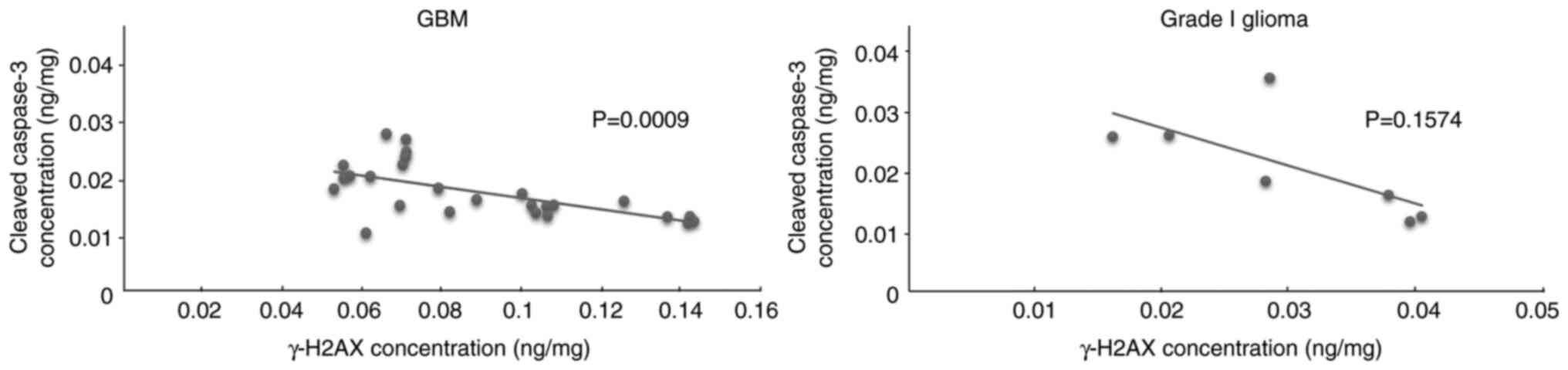
Correlation between γ-H2AX and cleaved
caspase-3
We hypothesized that spontaneous DNA double-strand
breaks might affect the ability of GBM cells to survive through the
inhibition of apoptosis. The correlations between γ-H2AX and
cleaved caspase-3 concentrations in GBM and grade I glioma were
analyzed to investigate this hypothesis using Pearson's correlation
test. The results showed a strong negative correlation (r=−0.61;
P=0.0009) in GBM but no statistically significant correlation in
grade I glioma (Fig. 2).
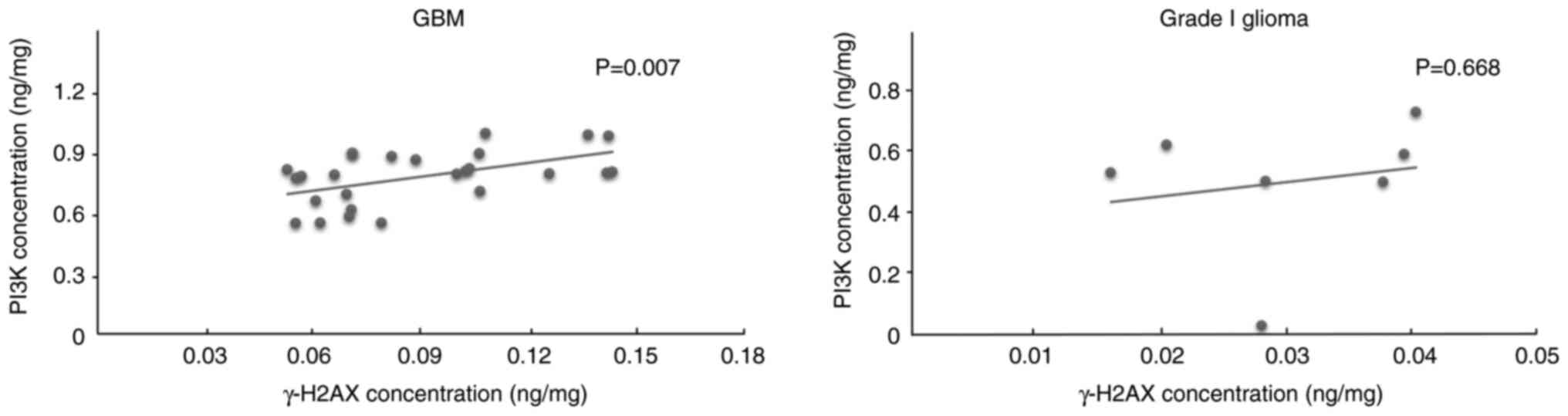
Correlation between γ-H2AX and
PI3K/Akt
To evaluate the PI3K/Akt pathway as a pathway
involved in GBM that may be affected by the DNA double-strand
breaks, the correlations between γ-H2AX and PI3K in GBM and grade I
glioma were analyzed. The results showed a moderate positive
correlation (r=0.52; P=0.007) between γ-H2AX and PI3K in GBM and no
statistically significant correlation in grade I glioma (Fig. 3).
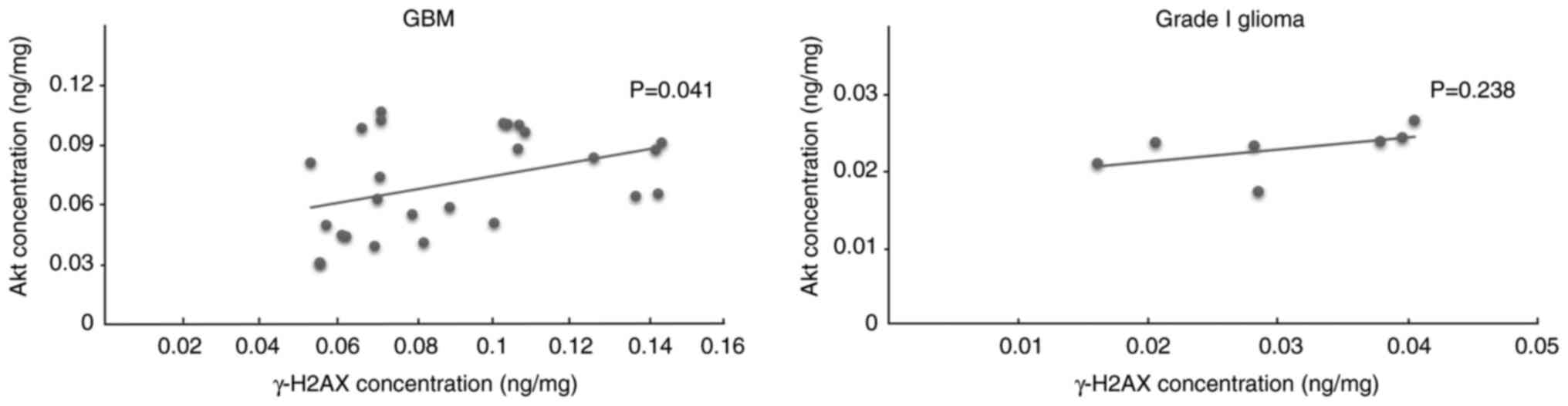
The correlation between γ-H2AX and Akt was also
analyzed. A significant positive correlation was detected between
γ-H2AX and Akt in GBM (r=0.40; P=0.041) but no significant
correlation was found in grade I glioma (Fig. 4).
Discussion
The ability of cancer cells to survive is an
important factor in cancer progression. Apoptosis is a marker of
cancer cell survival that can be induced or inhibited by specific
signal transduction pathways. In the present study, a significant
negative correlation between γ-H2AX and caspase-3 activation in GBM
suggested that elevated levels of DNA double-strand breaks were
associated with reduced apoptosis. This result also suggested that
spontaneous DNA double-strand breaks may contribute to maintaining
the tumorigenicity of GBM cells and enabling them to survive.
Several previous studies have shown that spontaneous DNA
double-strand breaks affect cancer cell survival (41–44).
However, to the best of our knowledge, no study has yet been
performed on the association between the spontaneous DNA
double-strand breaks and the survival of GBM cells.
Spontaneous DNA double-strand breaks are considered
to play a role in the progression of GBM, particularly in the
survival of GBM cells. The repair mechanism is initiated when the
DNA double-strand breaks occur, and includes homologous
recombination or NHEJ (45,46). If excessive damage occurs, this may
cause the repair processes to proceed incompletely and to be
dominated by the NHEJ mechanism, which is considered an error-prone
repair pathway (29). A potential
explanation for this is that the NHEJ mechanism of repair can take
place throughout the cell cycle and does not require any specific
template. Therefore, it can immediately repair the damage. The
occurrence of DNA double-strand breaks is reflected in the
occurrence of erroneous repair mechanisms; if DNA double-strand
breaks occur extensively, then the NHEJ mechanism also occurs
extensively. This may lead to genomic instability that finally
affects the tumorigenicity and aggressive behavior of GBM (10,47,48).
The present study also analyzed the expression of
proteins in the PI3K/Akt signal transduction pathway, which has a
vital role in the survival of cancer cells. The results showed
significant correlations between γ-H2AX and PI3K, as well as
between γ-H2AX and Akt, in GBM. These suggest that spontaneous DNA
double-strand breaks may activate the PI3K/Akt pathway. The
activation of this pathway will suppress the activity of
pro-apoptotic proteins and finally inhibit apoptosis in GBM cells
(14,17). The results of the present study are
consistent with other studies, which have shown that the PI3K/Akt
pathway affects various cellular functions, including apoptosis in
cancer cells (7,9,14).
As controls, these analyses were also performed in
seven tumor tissue specimens from patients with grade I glioma to
investigate the potential correlation between γ-H2AX and cleaved
caspase-3, which should reflect spontaneous DNA double-strand
breaks and apoptosis, respectively. Correlation analyses between
γ-H2AX and PI3K/Akt were also conducted. However, no statistically
significant correlation was observed in grade I glioma. These
findings may strengthen our hypothesis concerning the impact of DNA
double-strand breaks on the inhibition of apoptosis through the
PI3K/Akt pathway in GBM.
There are several limitations to the present study.
First, total PI3K and Akt were examined, which includes both
activated and inactivated forms. The determination of the
phosphorylated (cleaved) forms, phospho (p)-PI3K and p-Akt, would
provide improved information about the role of this pathway,
particularly in the inhibition of apoptosis. A further limitation
is the lack of experimental data supplying information about the
effect of the DNA double-strand breaks on apoptosis through
activation of the PI3K/Akt pathway in GBM. Therefore, further
experimental study is required to support the current hypothesis,
which may be conducted through in vitro experiments using a
GBM cell line. However, activation of the PI3K/Akt pathway can also
stimulate downstream substrates, such as mammalian target of
rapamycin, which can promote cell growth, autophagy and
proliferation (49). Therefore,
further analysis of this pathway is necessary to improve the
understanding of the mechanism of survival and progression of GBM
cells.
In conclusion, to the best of our knowledge, this is
the first study showing a correlation between a high concentration
of γ-H2AX and a marker of reduced apoptosis activation in patients
with GBM. The concentration of γ-H2AX reflects the occurrence of
DNA double-strand breaks that can activate the PI3K/Akt pathway and
affect apoptosis, enabling GBM cells to survive and grow. The
present study provides a new academic insight into the spontaneous
occurrence of DNA double-strand breaks in GBM, and indicates a
potential association between spontaneous DNA double-strand breaks
and apoptosis via the negative correlation between γ-H2AX and
cleaved caspase-3 in patients with GBM prior to treatment. This
result suggests the potential of γ-H2AX as a prognostic biomarker
in the routine clinical practice for GBM patients. However, further
studies are necessary to broaden the understanding of cellular
mechanisms in GBM, particularly the actions of apoptotic and
anti-apoptotic proteins, which may affect the conclusions of the
present study.
Acknowledgements
The authors would like to thank the neurosurgeon Dr
Syaiful Ichwan (Dr Cipto Mangunkusumo Hospital, Jakarta, Indonesia)
for kindly providing four tumor tissue specimens from patients with
GBM for analysis in this study.
Funding
This study was financially supported in part by a PUTI grant
from Universitas Indonesia (contract no.
NKB-577/UN2.RST/HKP.05.00/2020).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CTUB contributed to the concept and design of the
study, data acquisition, statistical analysis, data interpretation.
was involved in drafting and revising the manuscript, and gave
final approval for publication. NSH contributed to study concept
and design, helped with general data analysis and gave final
approval for publication. MS was responsible for the planning and
execution of all research activity, including experiments and
funding acquisition. EJW was responsible for all research activity,
planning, implementation and the provision of research samples. ADH
was involved in visualizing, drafting and revising the manuscript,
and assisted in interpreting the data. All authors participated
sufficiently in the study to take public responsibility for
appropriate portions of the content, and agree to be accountable
for all aspects of the study to ensure that questions related to
the accuracy and integrity of the work are appropriately
investigated and resolved. CTUB and NSH confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Faculty of Medicine, Universitas Indonesia (Jakarta,
Indonesia). The patients and/or their guardians provided written
informed consent related to storage and sharing of data and tissue
specimens for future research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rosell R, de las Peñas R, Balaña C,
Santarpia M, Salazar F, de Aguirre I, Reguart N, Villa S, Wei J,
Ramirez JL, et al: Translational research in glioblastoma
multiforme: Molecular criteria for patient selection. Futur Oncol.
4:219–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mikkelsen VE, Solheim O, Salvesen Ø and
Torp SH: The histological representativeness of glioblastoma tissue
samples. Acta Neurochir (Wien). 163:1911–1920. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohgaki H and Kleihues P: Epidemiology and
etiology of gliomas. Acta Neuropathol. 109:93–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iacob G and Dinca EB: Current data and
strategy in glioblastoma multiforme. J Med Life. 2:386–393.
2009.PubMed/NCBI
|
|
5
|
Nakada M, Kita D, Watanabe T, Hayashi Y,
Teng L, Pyko IV and Hamada J: Aberrant signaling pathways in
glioma. Cancers (Basel). 3:3242–3278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szopa W, Burley TA, Kramer-Marek G and
Kaspera W: Diagnostic and therapeutic biomarkers in glioblastoma:
Current status and future perspectives. Biomed Res Int.
2017:80135752017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cancer Genome Atlas Research Network, .
Comprehensive genomic characterization defines human glioblastoma
genes and core pathways. Nature. 455:1061–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vanhaesebroeck B, Guillermet-Guibert J,
Graupera M and Bilanges B: The emerging mechanisms of
isoform-specific PI3K signalling. Nat Rev Mol Cell Biol.
11:329–341. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moscatello DK, Holgado-Madruga M, Emlet
DR, Montgomery RB and Wong AJ: Constitutive activation of
phosphatidylinositol 3-kinase by a naturally occurring mutant
epidermal growth factor receptor. J Biol Chem. 273:200–206. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suk HS, Hromas R and Lee SH: Emerging
features of DNA double-strand break repair in humans. New Research
Directions in DNA Repair; 2013, View
Article : Google Scholar
|
|
11
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Auger KR, Serunian LA, Soltoff SP, Libby P
and Cantley LC: PDGF-dependent tyrosine phosphorylation stimulates
production of novel polyphosphoinositides in intact cells. Cell.
57:167–175. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruderman NB, Kapeller R, White MF and
Cantley LC: Activation of phosphatidylinositol 3-kinase by insulin.
Proc Natl Acad Sci USA. 87:1411–1415. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crespo S, Kind M and Arcaro A: The role of
the PI3K/AKT/mTOR pathway in brain tumor metastasis. J Cancer
Metastasis Treat. 2:80–89. 2016. View Article : Google Scholar
|
|
15
|
Liu Y, Zheng J, Zhang Y, Wang Z, Yang Y,
Bai M and Dai Y: Fucoxanthin activates apoptosis via inhibition of
PI3K/Akt/mTOR pathway and suppresses invasion and migration by
restriction of p38-MMP-2/9 pathway in human glioblastoma cells.
Neurochem Res. 41:2728–2751. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo SM, Tsai WC, Tsai CK, Chen Y and Hueng
DY: ARID4B knockdown suppresses PI3K/AKT signaling and induces
apoptosis in human glioma cells. Onco Targets Ther. 14:1843–1855.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gonçalves CS, Lourenço T, Xavier-magalhães
A, Pojo M and Costa BM: Mechanisms of aggressiveness in
glioblastoma: Prognostic and potential therapeutic insights.
Evolution of the Molecular Biology of Brain Tumors and the
Therapeutic Implications; 2013
|
|
18
|
Ray SK: Glioblastoma: Molecular Mechanisms
of Pathogenesis and Current Therapeutic Strategies. 1st edition.
Springer; New York, NY: pp. pp4312010
|
|
19
|
Kashyap D, Garg VK and Goel N: Intrinsic
and extrinsic pathways of apoptosis: Role in cancer development and
prognosis. Advances in Protein Chemistry and Structural Biology.
1st edition. Vol 125. Elsevier Inc.; pp. 73–120. 2021, View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brentnall M, Rodriguez-menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Obeng E: Apoptosis (programmed cell death)
and its signals-a review. Braz J Biol. 81:1133–1143. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eskandari E and Eaves CJ: Paradoxical
roles of caspase-3 in regulating cell survival, proliferation, and
tumorigenesis. J Cell Biol. 221:e2022011592022.PubMed/NCBI
|
|
23
|
Yang X, Zhong DN, Qin H, Wu PR, Wei KL,
Chen G, He RQ and Zhong JC: Caspase-3 over-expression is associated
with poor overall survival and clinicopathological parameters in
breast cancer: A meta-analysis of 3091 cases. Oncotarget.
9:8629–8641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang KH, Fang WL, Li AFY, Liang PH, Wu
CW, Shyr YM and Yang MH: Caspase-3, a key apoptotic protein, as a
prognostic marker in gastric cancer after curative surgery. Int J
Surg. 52:258–263. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andressakis D, Lazaris AC, Tsiambas E,
Kavantzas N, Rapidis A and Patsouris E: Evaluation of caspase-3 and
caspase-8 deregulation in tongue squamous cell carcinoma, based on
immunohistochemistry and computerised image analysis. J Laryngol
Otol. 122:1213–1218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin YF, Lai TC, Chang CK, Chen CL, Huang
MS, Yang CJ, Liu HG, Dong JJ, Chou YA, Teng KH, et al: Targeting
the XIAP/caspase-7 complex selectively kills caspase-3-deficient
malignancies. J Clin Invest. 123:3861–3875. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vítor AC, Huertas P, Legube G and de
Almeida SF: Studying DNA double-strand break repair: An
ever-growing toolbox. Front Mol Biosci. 7:242020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khanna KK and Jackson SP: DNA
double-strand breaks: Signaling, repair and the cancer connection.
Nat Genet. 27:247–254. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mladenov E and Iliakis G: The pathways of
double-strand break repair. DNA Repair-On the Pathways to Fixing
DNA Damage and Errors. 2011. View
Article : Google Scholar
|
|
30
|
Srivastava M and Raghavan SC: DNA
double-strand break repair inhibitors as cancer therapeutics. Chem
Biol. 22:17–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mladenov E, Magin S, Soni A and Iliakis G:
DNA double-strand-break repair in higher eukaryotes and its role in
genomic instability and cancer: Cell cycle and
proliferation-dependent regulation. Semin Cancer Biol. 37–38.
51–64. 2016.PubMed/NCBI
|
|
32
|
Swift LH and Golsteyn RM: Genotoxic
anti-cancer agents and their relationship to DNA damage, mitosis,
and checkpoint adaptation in proliferating cancer cells. Int J Mol
Sci. 15:3403–3431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harper JW and Elledge SJ: The DNA damage
response: Ten years after. Mol Cell. 28:739–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Redon C, Pilch D, Rogakou E, Sedelnikova
O, Newrock K and Bonner W: Histone H2A variants H2AX and H2AZ. Curr
Opin Genet Dev. 12:162–169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bonner WM, Redon CE, Dickey JS, Nakamura
AJ, Sedelnikova OA, Solier S and Pommier Y: GammaH2AX and cancer.
Nat Rev Cancer. 8:957–967. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dickey JS, Redon CE, Nakamura AJ, Baird
BJ, Sedelnikova OA and Bonner WM: H2AX: Functional roles and
potential applications. Chromosoma. 118:683–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Redon CE, Nakamura AJ, Martin OA, Parekh
PR, Weyemi US and Bonner WM: Recent developments in the use of
γ-H2AX as a quantitative DNA double-strand break biomarker. Aging
(Albany NY). 3:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sedelnikova OA and Bonner WM: GammaH2AX in
cancer cells: A potential biomarker for cancer diagnostics,
prediction and recurrence. Cell Cycle. 5:2909–2913. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matthaios D, Foukas PG, Kefala M, Hountis
P, Trypsianis G, Panayiotides IG, Chatzaki E, Pantelidaki E, Bouros
D, Karakitsos P and Kakolyris S: γ-H2AX expression detected by
immunohistochemistry correlates with prognosis in early operable
non-small cell lung cancer. Onco Targets Ther. 5:309–314. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gorgoulis VG, Vassiliou LVF, Karakaidos P,
Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA Jr,
Kastrinakis NG, Levy B, et al: Activation of the DNA damage
checkpoint and genomic instability in human precancerous lesions.
Nature. 434:907–913. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu X, Li F, Huang Q, Zhang Z, Zhou L,
Deng Y, Zhou M, Fleenor DE, Wang H, Kastan MB and Li CY:
Self-inflicted DNA double-strand breaks sustain tumorigenicity and
stemness of cancer cells. Cell Res. 27:764–783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ray SK, Patel SJ, Welsh CT, Wilford GG,
Hogan EL and Banik NL: Molecular evidence of apoptotic death in
malignant brain tumors including glioblastoma multiforme:
Upregulation of calpain and caspase-3. J Neurosci Res. 69:197–206.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tirapelli LF, Bolini PH, Tirapelli DP,
Peria FM, Becker AN, Saggioro FP and Carlotti CG Jr: Caspase-3 and
Bcl-2 expression in glioblastoma: An immunohistochemical study. Arq
Neuropsiquiatr. 68:603–607. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
San Filippo J, Sung P and Klein H:
Mechanism of eukaryotic homologous recombination. Annu Rev Biochem.
77:229–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lieber MR, Ma Y, Pannicke U and Schwarz K:
Mechanism and regulation of human non-homologous DNA end-joining.
Nat Rev Mol Cell Biol. 4:712–720. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Turgeon MO, Perry NJS and Poulogiannis G:
DNA damage, repair, and cancer metabolism. Front Oncol. 8:152018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bernstein C, Prasad RA, Nfonsam V and
Bernstei H: DNA damage, DNA repair and cancer. New Research
Directions in DNA Repair; 2013, View
Article : Google Scholar
|
|
49
|
Castedo M, Ferri KF and Kroemer G:
Mammalian target of rapamycin (mTOR): Pro- and anti-apoptotic. Cell
Death Differ. 9:99–100. 2002. View Article : Google Scholar : PubMed/NCBI
|