Introduction
Lenvatinib (LEN) is a multitarget tyrosine kinase
inhibitor that can target vascular endothelial growth factor
receptors 1, 2, and 3, fibroblast growth factor receptors 1 through
4, platelet-derived growth factor receptor α, rearranged during
transfection (RET), and stem cell factor receptor (KIT). Moreover,
LEN also inhibits the formation of vessel-like luminal structures
of vascular endothelial cells induced by VEGF and FGF. The
recommended dose of LEN is usually 12 mg/day for those weighing ≥60
kg and 8 mg/day for those weighing <60 kg, administered orally
once a day.
LEN is a standard therapeutic agent for
hepatocellular carcinoma, but the high incidence of adverse events
(AEs) is a problematic aspect of LEN treatment (1–4), since
these AEs may necessitate treatment discontinuation. Rash and
diarrhea are common side effects of tyrosine kinase inhibitor (TKI)
therapy administered to patients with non-small cell lung cancer
(5). The most common any-grade AEs
of LEN are hypertension (42%), diarrhoea (39%), decreased appetite
(34%), decreased weight (31%), and fatigue (30%) (2). Iwamoto reported that the incidence
rates of any grade and grade ≥3 AEs were 82.1 and 49.6%. Fatigue is
the most important AE because it can result in dose reduction and
discontinuation (4).
Although the LEN dose can be tapered in cases with
severe AEs, the lowest dose is 4 mg/day. Therefore, in clinical
practice, if the dose is to be further reduced for treatment
continuation, modified administration methods such as taking the
drug for 5 days with 2-day intervals (weekends off) may have to be
applied. Iwamoto et al reported the tolerability and
therapeutic effects of weekends off administration (4), and in their study, of the 30 patients
who received weekends off LEN, 66.7% had tolerable AEs. Thus,
weekends off administration significantly prolongs the
administration period and survival (4). In actual clinical practice, other
modified administration methods such as administration every other
day may also be performed depending on the patient's condition.
However, the benefit of administration every other day has not been
reported. Therefore, analyses of LEN dosage and reasons for
discontinuation due to AEs and clarifying the usefulness of these
administration methods will facilitate AE management in the
future.
The purpose of this study is to clarify the
therapeutic efficacy and tolerability of LEN administered with a
modified dosing method such as alternate day dosing due to AEs.
Patients and methods
Patients and evaluations
A total of 66 patients treated with LEN as the
first-line treatment for hepatocellular carcinoma at Ogaki
Municipal Hospital (Ogaki, Japan) between April 2018 and January
2022 were retrospectively evaluated. The LEN-administered patients
were divided into those who completed treatment with the standard
administration method (standard LEN) and those who changed from the
standard administration method to a modified method in the middle
of treatment [modified LEN (weekends off/alternate days)]. We
analysed patient characteristics, treatment duration, AEs, relative
dose intensity (RDI), and LEN-related reasons for discontinuation
over the treatment duration. The data were analysed using
electronic charts and pharmacy service records. AEs were evaluated
according to the Common Terminology Criteria for Adverse Events,
version 5.0 (6), and the most
severe grades during chemotherapy were reported. Personal
information was protected in the aggregated data. This study was
approved by the Institutional Review Board of Ogaki Municipal
Hospital (Ogaki, Japan; approval number: 20221124-16). The need for
informed consent was waived because of the retrospective nature of
the study.
Treatment protocol
The dose of LEN was based on body weight: the
initial dose was 12 mg/day for those weighing ≥60 kg and 8 mg/day
for those weighing <60 kg. During 28-day cycles, dose adjustment
was allowed for lenvatinib based on adverse events with reduction
to 8 mg or 4 mg per day, 4 mg every other day, and interruption
(2,7). For patients with Child-Pugh B disease,
the initial dose was reduced from 12 to 8 mg once daily. In
patients experiencing unacceptable drug-related AEs, the LEN dose
was reduced or treatment was interrupted in accordance with the
instructions provided by the manufacturer. Dose reduction or
temporary interruption of LEN was maintained until the AEs resolved
to grade 1 or 2. When continuing administration at a reduced dose,
the dose was reduced to 20, 14, 10, 8, or 4 mg once daily.
Statistical analysis
Between-group comparisons were performed using the
F-test. Kruskal-Wallis test, Fisher's exact probability
tests were used to compare patient characteristics, AEs and reasons
for discontinuation. Fisher's PLSD were used to compare RDI.
Kaplan-Meier and log-rank tests followed by the Bonferroni
correction were used to compare treatment durations. Differences
were considered statistically significant at P<0.05. All
analyses were performed using EZR software (version 1.30, Saitama
Medical Center, Jichi Medical University, Saitama, Japan), which is
a graphical user interface for R software (The R Foundation for
Statistical Computing, Vienna, Austria) (8).
Results
Patient characteristics
The standard and modified LEN groups (weekends
off/alternate days) included 48 and 18 (6/12) patients,
respectively. Patient characteristics are summarised in Table I. Patient background in weight and
body surface area did differ significantly between the standard,
modified LEN (weekends off), and modified LEN (alternate days)
groups.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Standard LEN | Modified LEN
(weekends off) | Standard LEN
(alternate day) | P-value |
|---|
| Patients, n | 48.0 | 6.0 | 12.0 |
|
| Age, years |
|
|
|
|
| Median
(range) | 73.0 (48.0–86.0) | 76.0 (74.0–87.0) | 76.0 (69.0–88.0) | 0.441a |
| Sex, n |
|
|
|
|
| Male | 43.0 | 5.0 | 11.0 | 0.811b |
|
Female | 5.0 | 1.0 | 1.0 |
|
| Height, cm |
|
|
|
|
| Median
(range) | 161.0
(144.0–173.0) | 162.0
(158.0–166.0) | 160.0
(158.0–182.0) | 0.950a |
| Weight, kg |
|
|
|
|
| Median
(range) | 60.0 (41.0–98.0) | 55.0 (40.0–79.0) | 69.0 (54.0–84.0) | 0.028a,c |
| Body surface area,
kg/m2 |
|
|
|
|
| Median
(range) | 1.6 (1.2–2.0) | 1.6 (1.4–1.9) | 1.7 (1.6–2.1) | 0.043a,c |
| Creatinine clearance,
ml/min |
|
|
|
|
| Median
(range) | 69.7
(35.3–131.6) | 56.9 (37.7–73.6) | 68.0 (39.0–93.9) | 0.320a |
| Cause of
hepatocellular carcinoma, n |
|
|
| 0.590b |
| Hepatitis
B virus | 7.0 | 0.0 | 2.0 |
|
| Hepatitis
C virus | 16.0 | 4.0 | 3.0 |
|
| Non-B
non-C | 25.0 | 2.0 | 7.0 |
|
| Performance status,
n |
|
|
| 0.936b |
| 0 | 31.0 | 4.0 | 7.0 |
|
| 1 | 15.0 | 2.0 | 4.0 |
|
| 2 | 2.0 | 0.0 | 1.0 |
|
| Past history of
transcatheter arterial chemoembolization, n |
|
|
|
|
| Yes | 38.0 | 5.0 | 10.0 | 0.930b |
| Child-Pugh, n |
|
|
|
|
| A | 29.0 | 4.0 | 6.0 | 0.709b |
| B | 19.0 | 2.0 | 6.0 |
|
Reasons for discontinuation
Discontinuations due to AEs in the modified LEN
group (1 of 18 patients) were fewer than those in the standard LEN
group (16 of 48 patients, P=0.022). Discontinuations due to
progressive disease (PD) in the modified LEN group (9 of 18
patients) were more than those in the standard LEN group (11 of 48
patients, P=0.032). The reasons for discontinuation in the standard
and modified LEN groups are summarised in Table II. The two groups showed no
significant differences in deterioration in performance status, or
deterioration of condition.
 | Table II.Reasons for discontinuation at
lenvatinib treatment. |
Table II.
Reasons for discontinuation at
lenvatinib treatment.
| Events | Modified LEN
(n=18) | Standard LEN
(n=48) | P-value |
|---|
| Adverse events | 1 | 16 | 0.022a |
| Progressive
disease | 9 | 11 | 0.032a |
| Deterioration in
performance status | 2 | 8 | 0.566 |
| Deterioration of
condition | 3 | 6 | 0.660 |
| Others | 2 | 4 | 0.704 |
| Ongoing | 1 | 3 | 0.916 |
AEs leading to treatment
discontinuation and the doses at the time of discontinuation
The AEs leading to treatment discontinuation and the
LEN doses at the time of discontinuation are summarised in Table III. In the modified LEN group,
treatment was discontinued due to abnormal urinary protein levels
during administration of alternate day dosing at 4 mg/day (1 case).
In the standard LEN group, 10 of the 16 patients (62.5%) who
discontinued treatment due to AEs were receiving LEN at 4 mg/day.
The reasons for discontinuation in the standard LEN group when
receiving 4 mg/day were fatigue (three cases), anorexia (four
cases), abnormal urinary protein levels (two cases),
nausea/vomiting (one case), fullness (one case), disorientation
(one case), and hoarseness (one case). On the other hand, six of
the 16 patients (37.5%) discontinued treatment while receiving 8
mg/day or 12 mg/day.
 | Table III.Adverse events and dose leading to
treatment discontinuation during LEN treatment. |
Table III.
Adverse events and dose leading to
treatment discontinuation during LEN treatment.
| A, Modified
LEN |
|---|
|
|---|
| Dose | Adverse events |
|---|
| Alternate-day
dosing with 4 mg/day | Urinary
protein |
|
| B, Standard
LEN |
|
| Dose | Adverse
events |
|
| 12 mg/day every
day | Rash |
| 8 mg/day every
day | Stomachache |
| 8 mg/day every
day | Anorexia |
| 8 mg/day every
day |
Hypothyroidism/slight fever/rash |
| 8 mg/day every
day | Nausea |
| 8 mg/day every
day |
Anorexia/fatigue |
| 4 mg/day every
day |
Fullness/fatigue |
| 4 mg/day every
day | Urinary
protein |
| 4 mg/day every
day |
Fatigue/anorexia |
| 4 mg/day every
day |
Anorexia/hoarseness |
| 4 mg/day every
day |
Nausea/vomiting |
| 4 mg/day every
day | Urinary
protein |
| 4 mg/day every
day | Anorexia |
| 4 mg/day every
day | Anorexia |
| 4 mg/day every
day | Disorientation |
| 4 mg/day every
day | Fatigue |
The effects of lenvatinib treatment on
the treatment duration
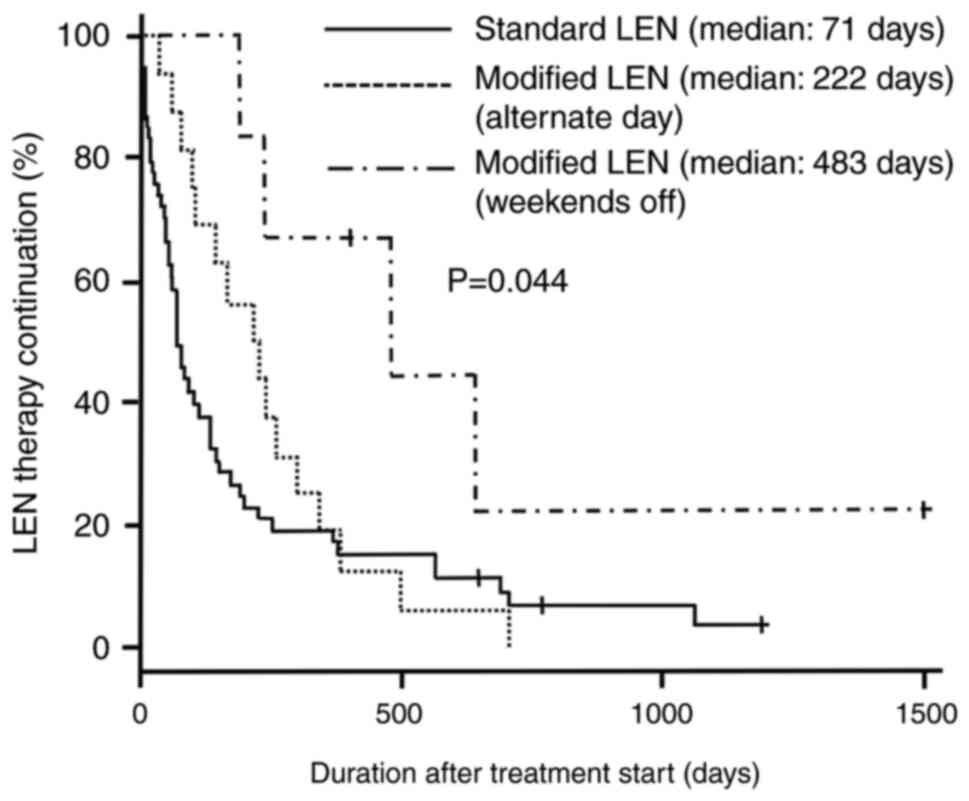
Kaplan-Meier survival curves according to the
duration of treatment with LEN for all patients are shown in
Fig. 1. The median treatment
duration for patients in the standard LEN (n=48), modified LEN
(weekends off, n=6), and modified LEN (alternate days, n=12) groups
was 71 (95% confidence interval [CI]: 55–134), 483 (95% CI:
193–644), and 222 (95% CI: 98–303) days, respectively (log-rank
test, P=0.044).
RDI of lenvatinib in the treatment
groups
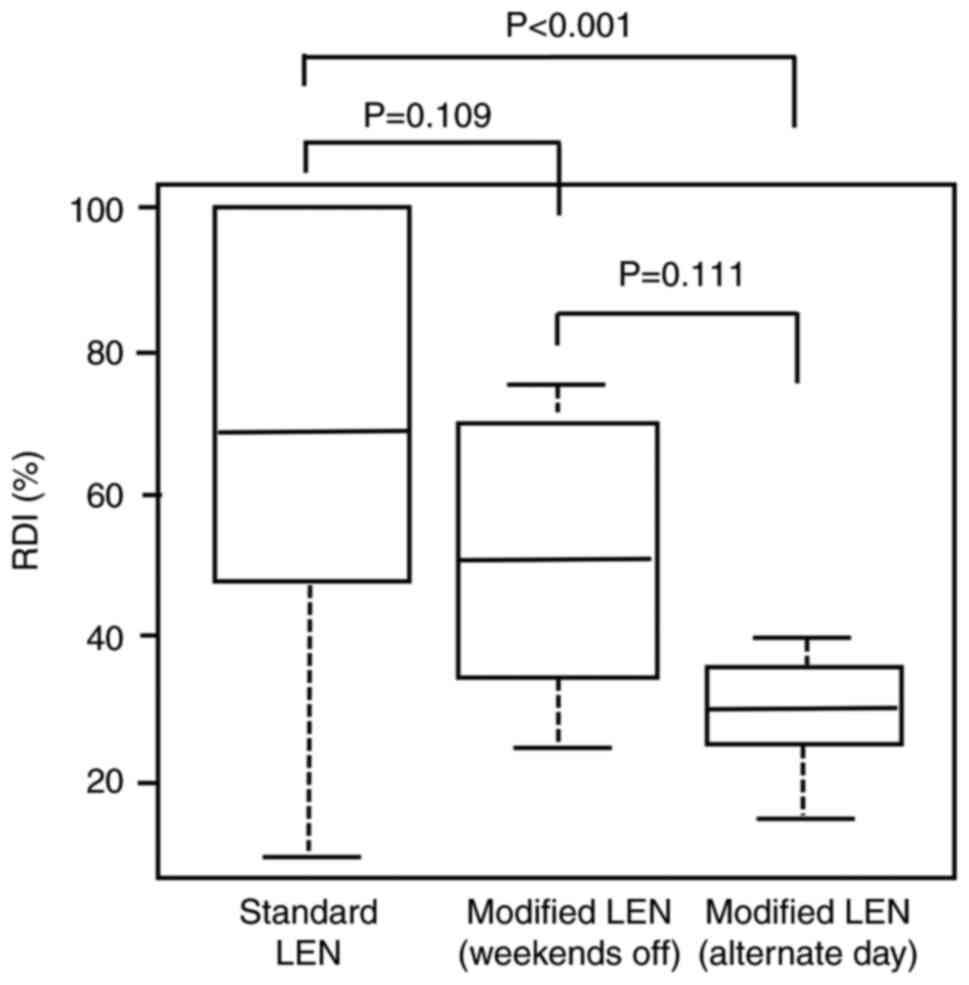
The RDI of LEN for the standard LEN and modified LEN
groups are shown in Fig. 2. The
median RDI in the standard LEN (n=48), modified LEN (weekends off,
n=6), and modified LEN (alternate days, n=12) groups was 68.5% (95%
CI: 46.3–100%), 50.0% (95% CI: 32.5–71.2%), and 30.0% (95% CI:
25.0–35.0%), respectively. The median RDI in the modified LEN
(alternate days) group was lower than that in the standard LEN
group (P<0.001).
Analysis of the effects depending on
age and sex
Kaplan-Meier survival curves by age (65 years) or
sex by duration of treatment with LEN are as follows. The median
duration of treatment for patients aged over 65 years (n=57) and
under 65 years (n=9) groups were 136 (95% CI: 80–193) and 49 (95%
CI: 9–241) days, respectively (log-rank test, P=0.072). The median
duration of treatment for patients in female (n=7) and male (n=59)
groups were 66.5 (95% CI: 28–98) and 136.0 (95% CI: 80–196) days,
respectively (log-rank test, P=0.466) (Fig. 3).
Discussion
This study clarified the therapeutic effects and
tolerability of AEs with modified LEN administration methods such
as alternate day administration. Modification of the administration
method ensured fewer AE-related treatment discontinuations.
However, weekends off dosing showed in a longer treatment duration
than standard dosing, whereas alternate day dosing showed no
difference from standard dosing.
In the REFLECT study, fatigue and anorexia
frequently occurred from the beginning of administration (2). Among AEs leading to discontinuation of
LEN, Iwamoto et al reported that fatigue was the most
important because it led to dose reduction and discontinuation
(4). Moreover, Katsuragawa et
al reported that in patients taking LEN for hepatocellular
carcinoma, fatigue and anorexia are often responsible for
discontinuation of administration (9). Similar to these reports, the present
study showed that fatigue and anorexia were the most common AEs
that led to discontinuation. The number of discontinuations due to
AEs in the modified LEN group was lower (1 of 18) than that in the
standard LEN group (16 of 48), suggesting that AE-related
discontinuations can be prevented by devising an appropriate
administration method. Modified LEN had more discontinuations due
to PD than standard LEN. This may be caused by the increased PD
discontinuations due to reduced discontinuations because of the
side effects with modified LEN.
In our analyses of the AEs leading to treatment
discontinuation and the doses at the time of discontinuation, one
patient in the modified LEN group discontinued treatment due to AEs
despite receiving 4 mg every other day. In contrast, 10 of the 16
patients (62.5%) in the standard LEN group who discontinued
treatment due to AEs were administered 4 mg/day. Thus, in the
standard LEN group, if treatment continuation was difficult due to
AEs at a dose of 4 mg/day, treatment could be continued by
modifying the dosing method, e.g., by administering 4 mg every
other day. In the standard LEN group, the remaining six of the 16
patients (37.5%) who discontinued treatment due to LEN were
receiving treatment at 8 mg or 12 mg. Thus, it is conceivable that
treatment could be continued by further dose reduction or drug
interruption. This finding highlights the importance of AE
management, including prompt dose reduction and changes in the
administration method. However, in terms of treatment duration,
alternate day dosing resulted in fewer treatment withdrawals due to
AEs than standard dosing, but the treatment duration remained the
same. However, weekends off dosing resulted in a significantly
longer treatment duration than standard dosing, indicating that
alternate day administration was insufficient in terms of efficacy.
Iwamoto et al reported that the weekend administration
method significantly extends the administration period and survival
rate (4).
In our assessments of the RDI of LEN during the
treatment period, the RDI in the alternate day dosing group was
lower than that in standard administration group. However, no
difference in RDI was observed between standard dosing and weekends
off dosing. To derive the optimal therapeutic benefit of LEN in
anticancer drug therapy, adequately manage of AEs during LEN
therapy and maintain RDI is important (10). In addition, a higher RDI is
associated with better overall survival and progression-free
survival as factors affecting the continuation of LEN treatment
(11–14). In this study, there was no
difference in treatment duration between alternate-day
administration and standard administration, suggesting that the RDI
results indicate that the therapeutic effect is low if the RDI is
below a certain level. Based on these findings, we consider that if
the RDI is too low, the treatment duration cannot be prolonged.
However, for sorafenib, a multikinase inhibitor similar to LEN, the
presence of HFS or diarrhoea may be relevant factors predicting
longer overall survival in patients with advanced hepatocellular
carcinoma (15–17). Therefore, early identification and
control of these reactions are important for continued sorafenib
therapy (15–17). Specifically, we considered that even
if the RDI is high, if treatment is discontinued because of AEs, it
will lead to a shortened duration of treatment and may affect
survival.
To facilitate the management of AEs in patients
receiving LEN for liver cancer, we would like to emphasise some
aspects of the study: First, the dose of LEN was tapered due to
AEs, but the lowest dose was maintained at 4 mg. Therefore, in the
clinical setting, patients should be explained that AEs can be
tolerated and treatment can be continued by devising a modified
dosing method such as weekends off dosing. Second, Ogushi et
al reported that Child-Pugh B patients require dose reduction
due to grade 2 AEs and show less tolerability to these AEs than
Child-Pugh A patients (3).
Therefore, special care should be taken in the management of
Child-Pugh B patients.
The study limitations included the relatively short
observation period, the limited sample, and the retrospective,
single-centre nature of the study. Because of the small sample
size, this study may not have had sufficient statistical power to
yield accurate estimates. In some cases, we were unable to capture
information on tumour size, so we were unable to analyse treatment
response (PD, stable disease, partial response). Future studies
should aim to address this limitation. Moreover, we recommend
calculating the effective RDI when determining the administration
method. Although overall survival (OS) was not examined in this
study, it would be desirable to clarify the relationship between
reasons for treatment discontinuation and OS.
In the future, to demonstrate the therapeutic
effects of LEN, adequate management of AEs by appropriately
modifying the timing and duration of treatment will be an important
consideration. Among the management approaches, devising an
administration method to prevent treatment discontinuation owing to
AEs is considered useful.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MK contributed to the study design, collected and
provided the data, was the principal author of the report and was
the guarantor of the article and all data. MG, SY, HA, EU and TY
contributed to the study design, reviewed the article, and
supervised the drafting of the report and submission process. All
authors approved the final version of the manuscript. MG, SY and HA
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of Ogaki Municipal Hospital (Ogaki, Japan; approval
no. 20221124-16). The requirement for obtaining informed consent
was waived due to the retrospective study design.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AE
|
adverse event
|
|
CI
|
confidence interval
|
|
LEN
|
lenvatinib
|
|
RDI
|
relative dose intensity
|
References
|
1
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:6387472014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ogushi K, Chuma M, Uojima H, Hidaka H,
Numata K, Kobayashi S, Hirose S, Hattori N, Fujikawa T, Nakazawa T,
et al: Safety and efficacy of lenvatinib treatment in child-pugh A
and B patients with unresectable hepatocellular carcinoma in
clinical practice: A multicenter analysis. Clin Exp Gastroenterol.
13:385–396. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwamoto H, Suzuki H, Shimose S, Niizeki T,
Nakano M, Shirono T, Okamura S, Noda Y, Kamachi N, Nakamura T, et
al: Weekends-Off lenvatinib for unresectable hepatocellular
carcinoma improves therapeutic response and tolerability toward
adverse events. Cancers (Basel). 12:10102020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Obradovic J, Todosijevic J and Jurisic V:
Side effects of tyrosine kinase inhibitors therapy in patients with
non-small cell lung cancer and associations with EGFR
polymorphisms: A systematic review and meta-analysis. Oncol Lett.
25:622022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
US Department Of Health and Human
Services, . Common terminology criteria for adverse events (CTCAE)
version 5.0. United States: National Cancer Institute; 2017,
https://ctep.Cancer.Gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf
|
|
7
|
Tamai T, Hayato S, Hojo S, Suzuki T,
Okusaka T, Ikeda K and Kumada H: Dose finding of lenvatinib in
subjects with advanced hepatocellular carcinoma based on population
pharmacokinetic and exposure-response analyses. J Clin Pharmacol.
57:1138–1147. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katsuragawa M, Oshita K, Yamashita Y,
Ishizuka N, Matsuki Y, Okubo R, Shibanami A and Hiura K: Usefulness
of outpatient pharmacist interventions for Lenvatinib-treated
patients. Iryoyakugaku. 47:623–630. 2021.
|
|
10
|
Takahashi A, Moriguchi M, Seko Y, Ishikawa
H, Yo T, Kimura H, Fujii H, Shima T, Mitsumoto Y, Ishiba H, et al:
Impact of relative dose intensity of early-phase lenvatinib
treatment on therapeutic response in hepatocellular carcinoma.
Anticancer Res. 39:5149–5156. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sasaki R, Fukushima M, Haraguchi M, Miuma
S, Miyaaki H, Hidaka M, Eguchi S, Matsuo S, Tajima K, Matsuzaki T,
et al: Response to lenvatinib is associated with optimal
relativedose intensity in hepatocellular carcinoma: Experience in
clinical settings. Cancers (Basel). 11:17692019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kirino S, Tsuchiya K, Kurosaki M, Kaneko
S, Inada K, Yamashita K, Osawa L, Hayakawa Y, Sekiguchi S, Okada M,
et al: Relative dose intensity over the first four weeks of
lenvatinib therapy is a factor of favorable response and overall
survival in patients with unresectable hepatocellular carcinoma.
PLoS One. 15:e02318282020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ono A, Aikata H, Yamauchi M, Kodama K,
Ohishi W, Kishi T, Ohya K, Teraoka Y, Osawa M, Fujino H, et al:
Circulating cytokines and angiogenic factors based signature
associated with the relative dose intensity during treatment in
patients with advanced hepatocellular carcinoma receiving
lenvatinib. Ther Adv Med Oncol. 12:17588359209220512020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Havrilesky LJ, Reiner M, Morrow PK, Watson
H and Crawford J: A review of relative dose intensity and survival
in patients with metastatic solid tumors. Crit Rev Oncol Hematol.
93:203–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Otsuka T, Eguchi Y, Kawazoe S, Yanagita K,
Ario K, Kitahara K, Kawasoe H, Kato H and Mizuta T; Saga Liver
Cancer Study Group, : Skin toxicities and survival in advanced
hepatocellular carcinoma patients treated with sorafenib. Hepatol
Res. 42:879–886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho JY, Paik YH, Lim HY, Kim YG, Lim HK,
Min YW, Gwak GY, Choi MS, Lee JH, Koh KC, et al: Clinical
parameters predictive of outcomes in sorafenib-treated patients
with advanced hepatocellular carcinoma. Liver Int. 33:950–957.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Costanzo GG, de Stefano G, Tortora R,
Farella N, Addario L, Lampasi F, Lanza AG, Cordone G, Imparato M
and Caporaso N: Sorafenib off-target effects predict outcomes in
patients treated for hepatocellular carcinoma. Future Oncol.
11:943–951. 2015. View Article : Google Scholar : PubMed/NCBI
|