Introduction
Submucosal tumours (SMTs) are a kind of lesion that
originates below the mucosal layer (1). The incidence of gastric SMTs (G-SMTs)
is lower than that of gastric mucosal tumours, and the related
clinical symptoms appear later (2).
G-SMTs generally do not produce clinical symptoms in the early
stage of onset. Most of them are found by physical examination or
for other reasons (3). Most G-SMTs
are benign, and only some are malignant (4,5). The
most common type of SMT is a gastrointestinal stromal tumour
(GIST), followed by leiomyoma and ectopic pancreas (6). All GISTs have the potential for
malignant transformation, with 10–30% becoming malignant tumours,
so it is of great significance to diagnose and treat them early
(7–9). GISTs are the most common mesenchymal
tumours in the gastrointestinal tract and are widely located,
especially in the stomach (10). In
GISTs, endoscopic ultrasonography (EUS) showed hypoechoic lesions
in the muscularis propria (MP) of the stomach (11). At present, the clinical treatment of
G-SMTs, including GISTs, is mainly surgical resection (12).
With the development of endoscopic technology, early
carcinoma of the digestive tract and some SMTs can now be resected
endoscopically (13,14). In recent years, endoscopic
submucosal dissection (ESD) has been widely used to treat
gastrointestinal tumours, including SMTs (15–17).
The feasibility, safety, and effectiveness of endoscopic therapy
have been proven by many studies (18–22).
ESD can completely remove gastric lesions with a diameter smaller
than 3 cm, which is conducive to postoperative recovery and reduces
body damage (23). If the tumours
originate deep in the MP or adhere to the serosa layer, perforation
and incomplete resection more easily occur during ESD (24,25).
In this case, exposed endoscopic full-thickness resection
(Eo-EFTR), derived from ESD, is used to address the problem and
reduces the incidence of residual tumours (26,27).
Based on ESD, Eo-EFTR is a treatment method involving completely
removing the tumours by actively manufacturing digestive tract
perforations and then closing the perforation sites (28). Eo-EFTR effectively removes the
tissue of SMTs, reduces the risk of recurrence, and does not
increase the incidence of complications (29,30).
The effect of surgical resection is easily affected
by the location and size of the tumours (31). For example, the gastric fundus is a
difficult area in which to operate because of its peculiar
anatomical location. The lesions in the gastric fundus are
difficult to access with the front end of the endoscope, especially
when they have extraluminal growth (32–34).
Therefore, our study selected a method called clip- and
snare-assisted traction to promote the resection of SMTs. Clip- and
snare-assisted traction is performed by clamping the edge of the
cut lesion mucosa with the snare device and metallic clip and then
pushing or pulling the snare device to achieve traction and expose
the submucosa, providing a better surgical field of vision
(35). Clip- and snare-assisted
traction technology can effectively shorten the operation time and
reduce complications (36).
Therefore, our study recorded and reported 20
consecutive cases to explore the safety and effectivity of Eo-EFTR
with clip- and snare-assisted traction for G-SMTs in the
fundus.
Materials and methods
Study design and patients
This study was designed as a single-arm,
retrospective, case-series study. Data from 20 patients who
underwent EUS and abdominal computed tomography (CT) before
undergoing Eo-EFTR for SMTs in the gastric fundus at the First
Affiliated Hospital of Soochow University from April 2018 to
December 2021 were retrospectively enrolled. The median age of the
patients was 58 (50–68) years.
The patient inclusion criteria were as follows: i)
G-SMTs arising from the muscularis propria (MP) layer, which were
confirmed by EUS; ii) abdominal CT before endoscopic resection
showed no sign of lymph node involvement or distant metastasis;
iii) the location of the SMTs was in the gastric fundus; and iv)
Eo-EFTR with clip- and snare-assisted traction was chosen to resect
the tumours.
Patients who met any of the following criteria were
excluded: i) Metastatic disease revealed on EUS or abdominal CT;
ii) continuous use of anticoagulants or coagulation disorders; iii)
severe cardiopulmonary dysfunction; iv) anaesthesia allergy; and v)
lack of informed consent.
The following data were extracted: Sex, age, lesion
characteristics (size, location, and origin of tumours), operating
time (from submucosal injection to the accomplishment of the wound
suture), en bloc resection (that is, complete resection
without tumour rupture or bleeding), R0 resection (that is, the
tumours are removed completely without disruption of the tumour
capsule, and the lateral and vertical margins were negative), the
success rate of the procedure, surgical conversion, intraoperative
complication, pro-operative complication, hospital stay after the
procedure, pathology, National Institutes of Health (NIH)
classification of GISTs, and follow-up period.
All of our patients underwent Eo-EFTR by an
experienced endoscopist. This study was approved by the Ethics
Committee of the First Affiliated Hospital of Soochow University
[ethical approval number: (2022) No. 384], and it was performed
following The Helsinki Declaration. The requirement for informed
consent was waived due to the retrospective nature of the
study.
Endoscopic equipment and
accessories
Full-thickness resection was performed by employing
a standard single-channel endoscope (GIT-H290, Olympus). A
transparent cap (D-201-11304; Olympus) was attached to the front of
the endoscope. An IT knife (KD-611 L; Olympus, Tokyo, Japan) and a
Dual Knife (KD-655 L; Olympus) were used for incision and
dissection. A clip (ROCC-D-26-195, Microtech Nanjing, China) and a
snare (Snare Master; Olympus; Japan) were used to assist in the
traction of lesions. A high frequency generator (ICC-200, ERBE,
Erbe Elektromedizin GmbH, Tübingen, Germany) and hot biopsy forceps
(FD-410LR, Olympus) were used to achieve intraoperative
haemostasis. Other equipment consisted of injection needles
(NM-4L-1, Olympus), endoloops (LeCampTM, Changzhou, China), and
carbon dioxide insufflation (Olympus).
Preoperative evaluation and
procedures
All patients underwent a preoperative evaluation to
identify contraindications for Eo-EFTR. EUS was performed to
identify the depth of invasion and the risk of malignant
transformation. Abdominal enhanced CT was performed to exclude
lymph node involvement and distant metastasis before Eo-EFTR, in
which case patients were converted to surgery.
Conventional examinations were performed to evaluate
the health condition of the patients, such as electrocardiograms,
routine blood tests, liver and kidney function tests, serum
electrolyte assessments, etc.
All patients underwent procedures under monitored
anaesthesia care with endotracheal intubation.
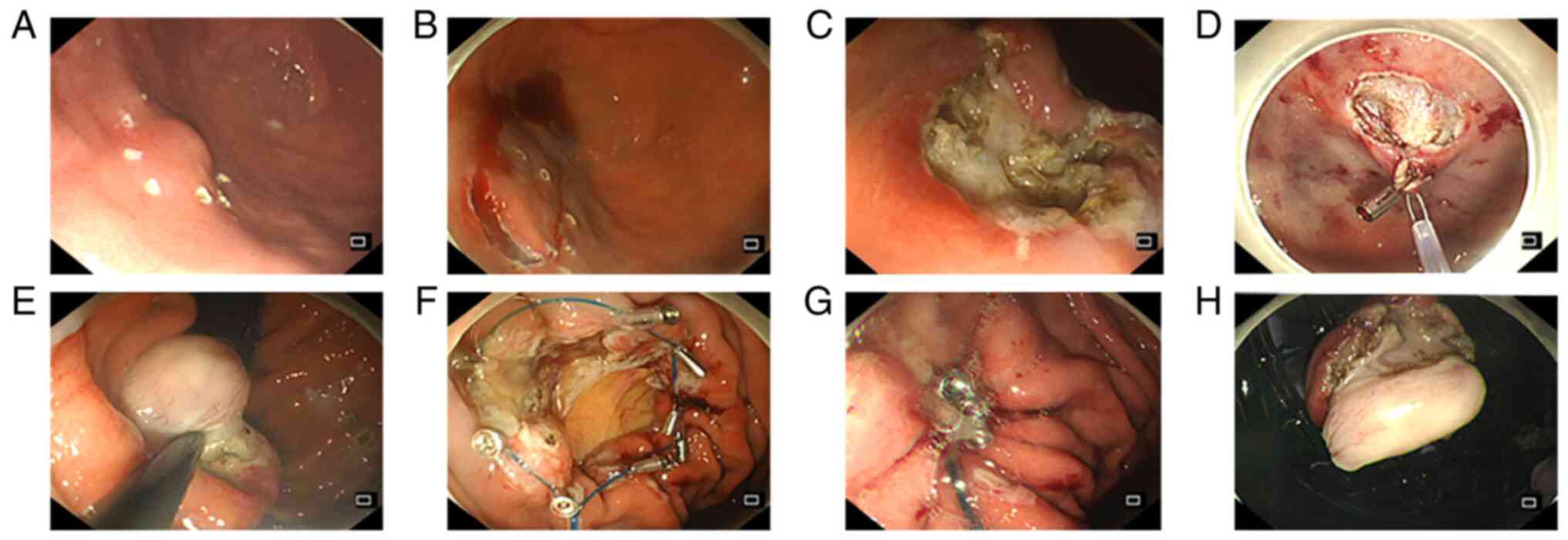
The Eo-EFTR procedure involved the following
consecutive steps (Fig. 1): (a)
Marking: The edge of the lesion was marked with a Dual Knife; (b)
Injection: A mixed solution (including indigo carmine, 1:2,000
epinephrine, and normal saline) was injected submucosally; (c)
Incision: An incision was made in the mucosal and submucosal layers
along the marked points by a Dual knife. (d) Clip- and
snare-assisted traction: i) The gastroscope was withdrawn from the
body, and the snare was opened and placed on the front end of the
transparent cap of the gastroscope. ii) The gastroscope was used to
bring the snare into the gastric cavity. iii) A metallic clip was
inserted into the gastroscopic biopsy hole, and the snare was
grabbed by the metallic clip to trap the tumour. iv) The snare was
tightened, the tumour was pushed or pulled upwards, and the
surgical field was exposed at the edge of the lesion after
traction. (e) Resection: The MP and serosa around the tumour were
resected with an IT knife. (f) After complete resection, the tumour
was removed from the stomach with the help of the tightened snare.
(g) Defect closure: When the diameter of the perforation was
relatively small (<1 cm), metallic clips were used to repair the
defect. In other cases of a larger wound or perforation, the
purse-string suture technique was used instead. The specific
operation method of purse-string suture: The metal clip fixes the
nylon rope along the perforation edge, then uses the nylon rope to
surround the wound to be closed, and finally tightens the nylon
rope to make the gastric wall mucosa around the wound converge to
the perforation centre to close the perforation. (h) Haemostasis:
Throughout the procedure, adequate haemostasis was ensured as soon
as a bleeding spot or active bleeding was detected. (i) Finally,
the lesions were fixed and sent for pathological examination.
Postoperative management and
follow-up
The patients were monitored with electrocardiography
on the day of surgery, and oxygen was administered if necessary.
The patients fasted for 24–48 h after the operation. During the
fasting period, they were given intravenous fluids, including
antibiotics, proton pump inhibitors (PPIs), haemostasis and
nutritional support. Gastrointestinal decompression was
administered to reduce the stimulation of digestive juice in
response to the lesions. If there was no significant discomfort,
the gastric tube was removed, and a liquid diet was started.
Patients gradually returned to a normal diet as tolerated. The
patients were monitored for bleeding, perforation, fever, abdominal
pain, and other complications through observation of clinical
symptoms and laboratory examinations. The patients were discharged
when no obvious symptoms or complications were present. Gastroscopy
and abdominal CT were performed regularly after the operation to
monitor wound healing in cases of tumour recurrence or
metastasis.
Results
Patient characteristics
The patients' characteristics are listed in Table I. There were 20 patients undergoing
Eo-EFTR for SMTs in the gastric fundus at the First Affiliated
Hospital of Soochow University from April 2018 to December 2021.
Among these 20 patients, there were 12 males and 8 females, with a
median age of 58 years. In the 20 patients, the lesions were
located in the gastric fundus. All tumours originated from the deep
part of the MP or adhered to the serosa layer, and some of them
showed partial extraluminal growth. The median diameter of the
lesions was 1.0 cm (range 0.3-2.0 cm). Of the 20 patients, the
indications for endoscopic resection included EUS features of
irregular edges (1/20), short-term enlargement of the tumours
(1/20), patients' preference (5/20), and EUS features of
heterogeneous echoes (13/20).
 | Table I.Patient characteristics (n=20). |
Table I.
Patient characteristics (n=20).
| Variable | Value |
|---|
| Median age, years
(range) | 58 (50–68) |
| Sex, n |
|
|
Male/female | 12/8 |
| Median tumour
diameter, cm (range) | 1.0 (0.3-2.0) |
| Tumour location,
n |
|
| Gastric
fundus | 20 |
| Origin of tumours,
n |
|
|
Muscularis propria | 20 |
| Indication for
resection, n |
|
|
Short-term enlargement of the
tumour | 1 |
| EUS
features of irregular edges | 1 |
| Patient
preference | 5 |
| EUS
features of heterogeneous echoes | 13 |
| Pathology, n |
|
|
GIST | 19 |
|
Leiomyoma | 1 |
| NIH classification
of GISTs, n |
|
| Very
low | 16 |
|
Low | 2 |
|
Intermediate | 1 |
|
High | 0 |
Operation-related data
The procedure-associated data are shown in Table II. The average operation time was
62.90 min (range 25–130 min). Haemorrhaging was effectively treated
during the operation, and there was no severe bleeding due to the
high-frequency generator, metallic clips, and hot biopsy forceps.
Intraoperative perforations were closed via the purse-string suture
technique or simple metallic clips, with 11 cases treated with
clips and the rest treated with the purse-string suture technique.
All endoscopic resections were performed successfully, and none of
them were converted to open surgery. The en bloc resection
rate was 100%. None the tumours resected during the operation fell
into the abdominal cavity, which can lead to a risk of tumour
dissemination. No pneumoperitoneum occurred during the procedure.
The average length of hospital stay after the procedure was 5.2
days (range 4–9 days).
 | Table II.Procedure-associated data for
patients (n=20). |
Table II.
Procedure-associated data for
patients (n=20).
| Variable | Value |
|---|
| En bloc
resection, % | 100 |
| R0 resection,
% | 100 |
| Surgical
conversion, % | 0 |
| Intraoperative
complications, n (%) |
|
| Active
perforation | 20 (100) |
| Severe
haemorrhage | 0 (0) |
|
Pneumoperitoneum | 0 (0) |
| Tumour
falling into the abdominal cavity | 0 (0) |
| Perforation repair
method, n (%) |
|
|
Conventional metallic clip
closure | 11 (55) |
|
Purse-string suturing | 9 (45) |
| Postoperative
complications, n (%) |
|
|
Abdominal pain | 2 (10) |
|
Fever | 7 (35) |
| Delayed
perforation | 0 (0) |
| Delayed
haemorrhage | 0 (0) |
|
Abdominal abscess | 0 (0) |
|
Peritonitis | 0 (0) |
| Average procedure
time, min (range) | 62.9 (25–130) |
| Average hospital
stay after procedure, days (range) | 5.2 (4–9) |
| Average follow-up
period, months (range) | 15.3 (6–45) |
| Local recurrence,
% | 0 (0) |
| Residual/Metastatic
tumour, % | 0 (0) |
Complications
Delayed perforation, delayed haemorrhage, fistula,
abdominal abscess or peritonitis were not observed after the
procedure. After the procedure, 2 patients developed abdominal pain
and fever, 5 patient developed fever only, and all of them returned
to normal after conservative treatment (Table II).
A 68-year-old male developed median abdominal pain
and bloating, along with fever. The maximum body temperature was
38.0°C. Mild tenderness over the left upper abdomen was found on
physical examination. Thereafter, abdominal CT was performed to
exclude delayed perforation and peritonitis and revealed mild
pneumoperitoneum and hydrothorax. After administering
anti-infection (imipenem), spasm relieving (magnesium sulphate),
and acid suppression (esomeprazole) treatments, his symptoms were
resolved completely. The patient was discharged on the seventh day
after the procedure. Other patients who developed mild abdominal
pain or fever were treated with symptomatic treatment.
Pathology
The postoperative pathology showed GISTs in 19
patients, and leiomyoma in one patient (Table I; Fig.
2). The R0 resection rate was 100% (Table II). 19 GISTs had a very low risk or
a low risk of recurrence because no more than 5 mitoses were seen
per 50 high-power fields (HPF). One GIST showed 8 mitoses per 50
HPF, indicating an intermediate risk of recurrence (Table I).
Follow-up
Each patient underwent endoscopic surveillance after
Eo-EFTR. The average follow-up time was 15.3 months (range 6–45
months). The wound healed well, and no local recurrence or residual
tumour was observed. Abdominal CT is not performed routinely except
for in medium- and high-risk patients. The medium-risk patient aged
55 was examined by CT every six months, and no metastatic tumours
were found (Table II).
Discussion
Most G-SMTs are identified by chance with endoscopy
because of atypical symptoms (37).
G-SMTs include GISTs, leiomyomas, calcifying fibroma lipoma,
ectopic pancreas, and so on (38).
Among these, GISTs are the most common mesenchymal tissue-derived
tumours in the gastrointestinal tract (39). Nevertheless, GISTs have a certain
probability of malignant transformation (7). It is estimated that the annual
incidence of GIST is approximately 11–15 per million individuals
(40,41). Therefore, early diagnosis and
treatment are quite significant for GISTs. According to the
National Comprehensive Cancer Network (NCCN) guidelines, EUS-guided
fine-needle aspiration biopsy is the preferred method to use in the
diagnosis of GISTs owing to the risk of tumour haemorrhage or
rupture with other methods, such as endoscopic biopsy and
hollow-core needle biopsy (42).
GISTs can usually be successfully diagnosed based on
histopathological morphology, immunohistochemistry, and molecular
biology (43,44). The treatment for GISTs includes
surgical treatment, drug treatment, and endoscopic treatment
(43). When the diameter is ≥2 cm,
surgical resection with or without targeted therapy is usually the
first choice for GISTs. When the GIST diameter is <2 cm, the
NCCN guidelines recommend active follow-up if there is no sign of
high-risk malignant transformation (45). Studies in Japan and Europe suggest
that once a GIST is confirmed histologically, resection should be
performed regardless of the diameter (43,46,47).
With the development of endoscopic technology,
endoscopic surgery has gradually begun to be applied to the
treatment of SMTs such as GISTs. Most studies have shown that the
en bloc resection rate for endoscopic resection of tumours
originating from the MP can reach up to 96–100% (34,48–51).
Compared with surgery, endoscopic treatment has the advantages of
equivalent curative effects, less trauma, rapid recovery, and less
impact on organ function (52). A
retrospective study comparing the efficacy of surgery and
endoscopic treatment showed that EFTR has the advantage of less
blood loss, shorter bowel function restoration time, and lower
hospital costs. The lower en bloc resection rate and higher
tumour capsule rupture rate of EFTR should be notable (48,53).
To date, the main indications for endoscopic treatment of GISTs
include i) GISTs with tumour enlargement in a short time and a
strong willingness for endoscopic treatment; ii) preoperative
evaluation excluding lymph nodes or distant metastasis; and iii)
low-risk GISTs with diameters between 2 and 5 cm (54). However, when the tumour diameter is
less than 2 cm, regular follow-up may increase the economic burden
and anxiety. Once the tumour suddenly increases, the opportunity
for timely treatment may be lost, resulting in increased risks.
Therefore, patients who cannot be followed up regularly may choose
elective endoscopic resection (55). The commonly used endoscopic
treatments for GISTs include ESD, submucosal tunnelling endoscopic
resection, EFTR and laparoscopic and endoscopic cooperative surgery
(56–58). EFTR should be selected when EUS and
abdominal enhanced CT identify that the tumour originates from the
MP adhering to the serosa layer or with the exophytic growth
pattern (27,59). Therefore, 20 cases of gastric SMTs
in the fundus originating from the MP were treated with Eo-EFTR.
Due to the need for retroflexion of the endoscope when tumours are
located in the gastric fundus, the gastroscope has difficulty
getting close to the deep part of lesions, which increases the
difficulty of complete resection (36,60,61).
Given this, plenty of auxiliary traction options have been
developed for use during endoscopic treatment, including the
clip-with-line method, snare traction, clip-snare traction,
grasping forceps traction, transparent cap traction, the suture
loop needle-T tag tissue anchors method, the robot-assisted method,
and magnetic anchor technology (62).
The advantages of the clip- and snare-assisted
traction featured in our study consist of the following: i) Simple
device: Clips and snares are common devices that are available in
most hospitals. ii) Widespread application: Clip- and
snare-assisted traction can be used in multiple parts of the
gastrointestinal tract. For difficult parts, the surgical field of
vision can be effectively expanded by applying auxiliary traction
to improve the success rate of the operation. iii) Flexible
traction: Different directions can be selected by pushing or
pulling the snare. The snare can also be used to adjust the
traction force to meet the different needs of the treatment
(36,63).
However, there are also some knacks and pitfalls in
the clip- and snare-assisted traction. First, the operation of
clip- and snare-assisted traction is difficult, which requires
endoscopists to have rich experience and competent operative
ability. Second, the traction force is affected by the hardness of
the snare, and the softer snare is not easy to change the direction
of the tumour during the operation. Finally, excessive traction
force or clamping too little gastric mucosa will easily cause the
titanium clip to fall off from the mucosa, consuming the operation
time and increasing mucosal damage (64).
Compared with traditional ESD, EFTR involves an
iatrogenic perforation. The larger the postoperative wound is, the
slower the wound healing. Therefore, it is critical to effectively
close the lesion defect (65). In
this study, two methods were used to repair the perforation. When
the perforation was <1 cm, conventional metallic clip closure
was used. When the defect was ≥1 cm, purse-string suturing was
selected. The advantages of purse-string suturing are as follows:
i) It is suitable for perforations with a relatively large
diameter. ii) It is easily controlled. iii) The spacing between
metallic clips is more than 5 mm, which can reduce the need for
additional clips. iv) By tightening the nylon rope, mucosal
aggregation can promote wound closure without leaving gaps and
prevent the leakage of gastrointestinal contents into the abdominal
cavity (66,67). There are other methods of defect
closure after Eo-EFTR, such as over-the-scope clips (OTSCs), which
are suitable for closing larger defects after Eo-EFTR than
endoscopic purse-string sutures, but the equipment is expensive and
limited to lesions <3 cm, resulting in limitations to their
clinical application (68–73). Omental patches, fibrin glue,
endoscopic puncture suture devices, and the overstitch system are
also used to repair therapeutic perforations (74–77).
The operation time in our study was 62.9 min (range
25–130 min). Tan et al reported that the mean procedure time
for conventional EFTR (n=32) for GISTs was 69.1±27.0 min (78). In addition, Hu et al showed a
procedure time of 130.6±51.9 min in the traditional EFTR group
(n=20) (61), indicating that our
study had a shorter operative time than other studies that did not
use traction. Li et al found that the mean time for EFTR
assisted by dental floss and a haemoclip for G-SMTs in the fundus
was 44.2±24.4 min (79). In a
retrospective study consisting of 13 patients treated with
thread-traction-assisted EFTR, the mean procedure time was
71.9±30.5 min (80). Effective
traction methods can reduce the difficulty of endoscopic surgery,
reduce the operation time to a certain extent, and reduce the risk
of complications, while the efficacy and safety of surgery are also
affected by the experience of the endoscopist and the size and
location of the lesions (81,82).
The main complications of EFTR were delayed bleeding
and perforation. Related studies have reported that complications
after EFTR also include peritonitis, abdominal abscess,
subcutaneous emphysema, and mediastinal emphysema (59). Granata A et al reported that
the pooled estimates for overall delayed bleeding and delayed
perforation were 0.14 and 0.14%, respectively (83). Appropriate haemostasis measures and
defect repair are important means to preventing postoperative
bleeding and perforations. Additionally, the time of resumption of
a normal diet, gastrointestinal decompression, and the use of PPIs
and antibiotics also have a certain impact on the occurrence of
postoperative complications (84).
When conservative treatment is ineffective, endoscopic exploration
should be carried out in a timely manner to effectively treat
bleeding points or perforation sites. If endoscopic treatment is
ineffective, further surgery is required (59). Most studies reported no major
complications in EFTR (51,61,73,85).
None of our patients experienced delayed perforation or
haemorrhage. In a study reported by Tan et al where 32
patients with tumours originating from the MP were treated with
EFTR, delayed bleeding was seen in 1 patient, and abdominal pain
with low-grade fever was seen in 4 patients (78). Another study including 192 patients
by Li et al (79) reported
that pneumoperitoneum was seen in 7 (3.6%) patients, hydrothorax
was seen in 6 (3.1%) patients, and post-EFTR electrocoagulation
syndrome was seen in 18 (9.4%). No significant pneumoperitoneum
occurred in our study. The reasons may be: i) The use of carbon
dioxide insufflation during the operation could reduce the
incidence of pneumoperitoneum; ii) The exposure time of abdominal
cavity is controlled by endoscopists. iii) During the exposure of
the patient's abdominal cavity, the endoscopy physician will try to
reduce the gas injection to avoid excessive gas entering the
abdominal cavity. In our study, after the endoscopic suture, the
patients' abdomen will be palpated to assess the abdominal tension.
If the tension is judged to be high, a Veress needle will be used
for decompression.
The postoperative pathology of the 19 patients was
GISTs, including 18 cases with mitotic images <5/50 HPF and 1
case with mitotic images of 8/50 HPF. Medium- and high-risk
patients are advised to undergo additional treatment, such as
molecular targeted therapy or additional surgery, according to the
guidelines (43). The NIH grading
standard divides the risk of postoperative recurrence into four
grades by considering the size, location, and mitotic image of the
patient's tumour (86). In our
study, 16 cases were very low risk, 2 cases were low risk, and 1
case was medium risk. The patient with a moderate risk was given
imatinib targeted therapy and followed up regularly. The last
follow-up showed that the tumour had healed well without signs of
recurrence or distant metastasis.
The success rate, complete resection rate, and R0
resection rate in this study were 100%. There was no conversion to
surgery, and there were no serious adverse events or complications
during or after the operation. During postoperative follow-up, all
patients healed well without recurrence or distant metastasis. Our
results show the feasibility, safety, and effectiveness of Eo-EFTR
with clip- and snare-assisted traction for G-SMTs in the fundus.
Most of the studies compared the effectiveness of EFTR and surgery,
showing the advantages of EFTR, such as a shorter operation time
and faster cure time (53,87). Some studies have indicated that when
the lesions are too large (more than 3 cm) or there are
contraindications (distant metastasis), the recurrence rate and
complete resection rate of endoscopic treatment are lower than
those of surgery (53). At this
time, surgery is a more appropriate choice.
This study had several limitations. First of all, it
is a single-centre and retrospective study, which may cause
selection bias and retrospective bias. Secondly, this study was
descriptive and lacked a control group. Due to the difficulty of
traditional Eo-EFTR in the gastric fundus, it is difficult to
collect cases in the control group. So more data need to be further
collected in the future. Moreover, the object of this study are the
G-SMTs, which does not involve gastric cancer cases. If possible,
auxiliary traction can be applied to the endoscopic resection of
gastric cancers in the future, and the effectiveness and safety of
endoscopic therapy combined with auxiliary traction in gastric
cancer cases can be evaluated. Finally, a prospective or
retrospective study with a larger sample size is needed to further
confirm the feasibility and efficacy of Eo-EFTR with clip- and
snare-assisted traction for G-SMTs in the fundus, as the sample
size of this study is small.
After this study, we will conduct a retrospective
case-control study to compare the traditional and assisted traction
Eo-EFTR and identify the specific advantages (such as less
operative time and complications) of traction methods in Eo-EFTR
through data analysis. In addition, we will also learn and study
other traction methods, such as multiple clips- and snare-assisted
traction, clip-with-line method, etc. Through the collection of
data, the characteristics of different traction methods will be
compared to find a more suitable and effective traction method for
Eo-EFTR.
In conclusion, Eo-EFTR with clip- and snare-assisted
traction appears to be a relatively safe and effective treatment
for gastric SMTs in the fundus. The traction method can shorten the
operative time to a certain extent and don't increase the incidence
of complications.
Acknowledgments
Not applicable.
Funding
This study was funded by the National Natural Science Foundation
of China (grant no. 81900508) and the Natural Science Foundation of
Jiangsu Province (grant no. BK20190172).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LN and XG confirm the authenticity of all the raw
data. All persons who meet authorship criteria are listed as
authors, and all authors certify that they have participated
sufficiently in the work to take public responsibility for the
content. LN, XL, CY, XG, CW and AW participated in the conception,
design and data acquisition. LN, CZ and GX were involved with data
analysis and interpretation. LN and CW wrote the draft. XG, XL and
AW revised the final manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the First Affiliated Hospital of Soochow University [ethical
approval number: (2022) No. 384]. The requirement for informed
consent was waived due to the retrospective nature of the
study.
Patient consent for publication
Written informed consent for publication was
obtained from all participants in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang J, Huang K, Ding S, Wang Y, Nai T,
Huang Y and Zhou L: Clinical applicability of various treatment
approaches for upper gastrointestinal Submucosal tumors.
Gastroenterol Res Pract. 2016:94306522016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song JH, Kim SG, Chung SJ, Kang HY, Yang
SY and Kim YS: Risk of progression for incidental small
subepithelial tumors in the upper gastrointestinal tract.
Endoscopy. 47:675–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gaspar JP, Stelow EB and Wang AY: Approach
to the endoscopic resection of duodenal lesions. World J
Gastroenterol. 22:600–617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ikehara H, Li Z, Watari J, Taki M, Ogawa
T, Yamasaki T, Kondo T, Toyoshima F, Kono T, Tozawa K, et al:
Histological diagnosis of gastric submucosal tumors: A pilot study
of endoscopic ultrasonography-guided fine-needle aspiration biopsy
vs mucosal cutting biopsy. World J Gastrointest Endosc.
7:1142–1149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nishida T, Goto O, Raut CP and Yahagi N:
Diagnostic and treatment strategy for small gastrointestinal
stromal tumors. Cancer. 122:3110–3118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishida T, Kawai N, Yamaguchi S and
Nishida Y: Submucosal tumors: Comprehensive guide for the diagnosis
and therapy of gastrointestinal submucosal tumors. Dig Endosc.
25:479–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fletcher CD: The evolving classification
of soft tissue tumours-an update based on the new 2013 WHO
classification. Histopathology. 64:2–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dematteo RP, Gold JS, Saran L, Gonen M,
Liau KH, Maki RG, Singer S, Besmer P, Brennan MF and Antonescu CR:
Tumor mitotic rate, size, and location independently predict
recurrence after resection of primary gastrointestinal stromal
tumor (GIST). Cancer. 112:608–615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
von Mehren M and Joensuu H:
Gastrointestinal stromal tumors. J Clin Oncol. 36:136–143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang D, Ding Q, Cao L, Feng X, Zhang Z, Lu
P, Ji X, Li L, Tian D and Liu M: Clinical outcomes of endoscopic
treatment for gastric gastrointestinal stromal tumors: A
single-center study of 240 cases in China. Scand J Gastroenterol.
57:996–1004. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo J, Liu Z, Sun S, Wang S, Ge N, Liu X,
Wang G and Liu W: Endosonography-assisted diagnosis and therapy of
gastrointestinal submucosal tumors. Endosc Ultrasound. 2:125–133.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Capkinoglu E, Durak E, Acar N, Acar T,
Kamer E and Haciyanli M: Comparison of laparoscopic and open
resections for gastric gastrointestinal stromal tumors (GISTs). Ann
Ital Chir. 11:S0003469X2203682X20222022.
|
|
13
|
Ebrahim A, Leeds SG, Clothier JS and Ward
MA: Endoscopic submucosal dissection of a gastric mass. Proc (Bayl
Univ Med Cent). 32:629–630. 2019.PubMed/NCBI
|
|
14
|
Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon
T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P,
Bialek A, et al: Endoscopic submucosal dissection: European society
of gastrointestinal endoscopy (ESGE) guideline. Endoscopy.
47:829–854. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hulagu S, Senturk O, Aygun C, Kocaman O,
Celebi A, Konduk T, Koc D, Sirin G, Korkmaz U, Duman AE, et al:
Endoscopic submucosal dissection for premalignant lesions and
noninvasive early gastrointestinal cancers. World J Gastroenterol.
17:1701–1709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi N, Takeuchi Y, Ohata K, Igarashi
M, Yamada M, Kodashima S, Hotta K, Harada K, Ikematsu H, Uraoka T,
et al: Outcomes of endoscopic submucosal dissection for colorectal
neoplasms: Prospective, multicenter, cohort trial. Dig Endosc.
34:1042–1051. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He Z, Sun C, Zheng Z, Yu Q, Wang T, Chen
X, Cao H, Liu W and Wang B: Endoscopic submucosal dissection of
large gastrointestinal stromal tumors in the esophagus and stomach.
J Gastroenterol Hepatol. 28:262–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi ZP, Shi Q, Liu JZ, Yao LQ, Xu MD, Cai
SL, Li B, Take I, Zhang YQ, Chen WF, et al: Efficacy and safety of
endoscopic submucosal dissection for submucosal tumors of the colon
and rectum. Gastrointest Endosc. 87:540–548.e1. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang HY, Zeng X, Bai SY, Pu K, Zheng Y, Ji
R, Guo QH, Guan QL, Wang YP and Zhou YN: The safety and efficacy of
endoscopic submucosal dissection for treating early oesophageal
carcinoma: A meta-analysis. Ann R Coll Surg Engl. 102:702–711.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Li B, Wang S, Yang B, Zhu L, Ma S,
Wu J, He Q, Zhao J, Zheng Z, et al: Efficacy and safety of
endoscopic submucosal dissection for gastrointestinal
neuroendocrine tumors: A 10-year data analysis of Northern China.
Scand J Gastroenterol. 54:384–389. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Watanabe D, Hayashi H, Kataoka Y,
Hashimoto T, Ichimasa K, Miyachi H, Tanaka S and Toyonaga T:
Efficacy and safety of endoscopic submucosal dissection for
non-ampullary duodenal polyps: A systematic review and
meta-analysis. Dig Liver Dis. 51:774–781. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akintoye E, Obaitan I, Muthusamy A, Akanbi
O, Olusunmade M and Levine D: Endoscopic submucosal dissection of
gastric tumors: A systematic review and meta-analysis. World J
Gastrointest Endosc. 8:517–532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhagat VH, Kim M and Kahaleh M: A review
of endoscopic full-thickness resection, submucosal tunneling
endoscopic resection, and endoscopic submucosal dissection for
resection of subepithelial lesions. J Clin Gastroenterol.
55:309–315. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meier B, Schmidt A and Caca K: Endoscopic
full-thickness resection. Internist (Berl). 57:755–762. 2016.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsu WH, Wu TS, Hsieh MS, Kung YM, Wang YK,
Wu JY, Yu FJ, Kuo CH, Su YC, Wang JY, et al: Comparison of
endoscopic submucosal dissection application on mucosal tumor and
subepithelial tumor in stomach. J Cancer. 12:765–770. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD,
Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW and Liu JZ: Endoscopic
full-thickness resection without laparoscopic assistance for
gastric submucosal tumors originated from the muscularis propria.
Surg Endosc. 25:2926–2931. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai MY, Martin Carreras-Presas F and Zhou
PH: Endoscopic full-thickness resection for gastrointestinal
submucosal tumors. Dig Endosc. 30 (Suppl 1):S17–S24. 2018.
View Article : Google Scholar
|
|
28
|
CASGE Technology Committee, . Aslanian HR,
Sethi A, Bhutani MS, Goodman AJ, Krishnan K, Lichtenstein DR,
Melson J, Navaneethan U, Pannala R, et al: ASGE guideline for
endoscopic full-thickness resection and submucosal tunnel
endoscopic resection. VideoGIE. 4:343–350. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai M, Zhou P, Lourenco LC and Zhang D:
Endoscopic Full-thickness Resection (EFTR) for Gastrointestinal
Subepithelial Tumors. Gastrointest Endosc Clin N Am. 26:283–295.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brewer Gutierrez OI, Akshintala VS,
Ichkhanian Y, Brewer GG, Hanada Y, Truskey MP, Agarwal A, Hajiyeva
G, Kumbhari V, Kalloo AN, et al: Endoscopic full-thickness
resection using a clip non-exposed method for gastrointestinal
tract lesions: A meta-analysis. Endosc Int Open. 8:E313–E325. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abe N, Takeuchi H, Ohki A, Hashimoto Y,
Mori T and Sugiyama M: Comparison between endoscopic and
laparoscopic removal of gastric submucosal tumor. Dig Endosc. 30
(Suppl 1):S7–S16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu J, Zheng M, Jiao T, Wang Y and Lu X:
Transcardiac tunneling technique for endoscopic submucosal
dissection of gastric fundus tumors arising from the muscularis
propria. Endoscopy. 46:888–892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duan TY, Tan YY, Wang XH, Lv L and Liu DL:
A comparison of submucosal tunneling endoscopic resection and
endoscopic full-thickness resection for gastric fundus submucosal
tumors. Rev Esp Enferm Dig. 110:160–165. 2018.PubMed/NCBI
|
|
34
|
Li B, Chen T, Qi ZP, Yao LQ, Xu MD, Shi Q,
Cai SL, Sun D, Zhou PH and Zhong YS: Efficacy and safety of
endoscopic resection for small submucosal tumors originating from
the muscularis propria layer in the gastric fundus. Surg Endosc.
33:2553–2561. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshida N, Doyama H, Ota R and Tsuji K:
The clip-and-snare method with a pre-looping technique during
gastric endoscopic submucosal dissection. Endoscopy. 46 (Suppl
1):E611–E612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu J, Jiao T, Li Y, Zheng M and Lu X:
Facilitating retroflexed endoscopic full-thickness resection
through loop-mediated or rope-mediated countertraction (with
videos). Gastrointest Endosc. 83:223–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim GH: Endoscopic resection of
subepithelial tumors. Clin Endosc. 45:240–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li DM, Ren LL and Jiang YP: Long-term
outcomes of endoscopic resection for gastric subepithelial tumors.
Surg Laparosc Endosc Percutan Tech. 30:187–191. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Joensuu H, Hohenberger P and Corless CL:
Gastrointestinal stromal tumour. Lancet. 382:973–983. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Goettsch WG, Bos SD, Breekveldt-Postma N,
Casparie M, Herings RM and Hogendoorn PC: Incidence of
gastrointestinal stromal tumours is underestimated: Results of a
nation-wide study. Eur J Cancer. 41:2868–2872. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Santos L, Jin C, Gazarkova T, Thornell A,
Norlen O, Saljo K and Teneberg S: Characterization of
glycosphingolipids from gastrointestinal stromal tumours. Sci Rep.
10:193712020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
von Mehren M, Randall RL, Benjamin RS,
Boles S, Bui MM, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane
JM, et al: Soft tissue sarcoma, version 2.2018, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
16:536–563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Casali PG, Abecassis N, Aro HT, Bauer S,
Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG,
Brodowicz T, et al: Gastrointestinal stromal tumours: ESMO-EURACAN
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 29 (Suppl 4):iv2672018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Corless CL, Barnett CM and Heinrich MC:
Gastrointestinal stromal tumours: Origin and molecular oncology.
Nat Rev Cancer. 11:865–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
von Mehren M, Randall RL, Benjamin RS,
Boles S, Bui MM, Conrad EU III, Ganjoo KN, George S, Gonzalez RJ,
Heslin MJ, et al: Soft tissue sarcoma, version 2.2016, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
14:758–786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nishida T, Hirota S, Yanagisawa A, Sugino
Y, Minami M, Yamamura Y, Otani Y, Shimada Y, Takahashi F and Kubota
T; GIST Guideline Subcommittee, : Clinical practice guidelines for
gastrointestinal stromal tumor (GIST) in Japan: English version.
Int J Clin Oncol. 13:416–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cao H and Wang M: Similarities and
differences in diagnosis and treatment of gastrointestinal stromal
tumors between China, Japan and Korea: From expert consensus to
cooperation prospect. Zhonghua Wei Chang Wai Ke Za Zhi. 22:812–819.
2019.(In Chinese). PubMed/NCBI
|
|
48
|
Zhao Y, Pang T, Zhang B, Wang L, Lv Y,
Ling T, Zhang X, Huang Q, Xu G and Zou X: Retrospective comparison
of endoscopic full-thickness versus laparoscopic or surgical
resection of small (</=5 cm) gastric gastrointestinal stromal
tumors. J Gastrointest Surg. 24:2714–2721. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jain D, Desai A, Mahmood E and Singhal S:
Submucosal tunneling endoscopic resection of upper gastrointestinal
tract tumors arising from muscularis propria. Ann Gastroenterol.
30:262–272. 2017.PubMed/NCBI
|
|
50
|
Zhu H, Shi D, Song H, Zhou M, Sun D, Li R
and Zhao Y: Snare-assisted endoscopic resection of gastric
subepithelial tumors originating from the muscularis propria layer:
A multicenter study. Surg Endosc. 34:3827–3832. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ge N, Hu JL, Yang F, Yang F and Sun SY:
Endoscopic full-thickness resection for treating small tumors
originating from the muscularis propria in the gastric fundus: An
improvement in technique over 15 years. World J Gastrointest Oncol.
11:1054–1064. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhu H, Zhao S, Jiao R, Zhou J, Zhang C and
Miao L: Comparison of endoscopic versus laparoscopic resection for
gastric gastrointestinal stromal tumors: A preliminary
meta-analysis. J Gastroenterol Hepatol. 35:1858–1868. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu S, Zhou X, Yao Y, Shi K, Yu M and Ji
F: Resection of the gastric submucosal tumor (G-SMT) originating
from the muscularis propria layer: Comparison of efficacy,
patients' tolerability, and clinical outcomes between endoscopic
full-thickness resection and surgical resection. Surg Endosc.
34:4053–4064. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou P, Zhong Y and Li Q: Chinese
Consensus on Endoscopic Diagnosis and Management of
Gastrointestinal Submucosal Tumor (Version 2018). Zhonghua Wei
Chang Wai Ke Za Zhi. 21:841–852. 2018.(In Chinese). PubMed/NCBI
|
|
55
|
Yu C, Liao G, Fan C, Yu J, Nie X, Yang S
and Bai J: Long-term outcomes of endoscopic resection of gastric
GISTs. Surg Endosc. 31:4799–4804. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ahmed M: Recent advances in the management
of gastrointestinal stromal tumor. World J Clin Cases. 8:3142–3155.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim SY and Kim KO: Management of gastric
subepithelial tumors: The role of endoscopy. World J Gastrointest
Endosc. 8:418–424. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
An W, Sun PB, Gao J, Jiang F, Liu F, Chen
J, Wang D, Li ZS and Shi XG: Endoscopic submucosal dissection for
gastric gastrointestinal stromal tumors: A retrospective cohort
study. Surg Endosc. 31:4522–4531. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ren Z, Lin SL, Zhou PH, Cai SL, Qi ZP, Li
J and Yao LQ: Endoscopic full-thickness resection (EFTR) without
laparoscopic assistance for nonampullary duodenal subepithelial
lesions: Our clinical experience of 32 cases. Surg Endosc.
33:3605–3611. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shi Q, Li B, Qi ZP, Yao LQ, Xu MD, Cai SL,
Sun D, Zhou PH and Zhong YS: Clinical values of dental floss
traction assistance in endoscopic full-thickness resection for
submucosal tumors originating from the muscularis propria layer in
the gastric fundus. J Laparoendosc Adv Surg Tech A. 28:1261–1265.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hu J, Ge N, Wang S, Guo J, Liu X, Wang G
and Sun S: Direct endoscopic full-thickness resection for
submucosal tumors with an intraluminal growth pattern originating
from the muscularis propria layer in the gastric fundus. BMC
Gastroenterol. 20:702020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gu L, Wu Y, Yi J and Liu XW: Current
status and research advances on the use of assisted traction
technique in endoscopic full-thickness resection. Zhonghua Wei
Chang Wai Ke Za Zhi. 24:1122–1128. 2021.(In Chinese). PubMed/NCBI
|
|
63
|
Tian X, Shi B and Chen WQ: Modified
endoscopic full-thickness resection of gastric stromal tumor
originating from the muscularis Propria layer. J Gastrointest
Oncol. 11:461–466. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang Q, Cai JQ, Wang Z, Xiao B and Bai Y:
Snare combined with endoscopic clips in endoscopic resection of
gastric submucosal tumor: A method of tumor traction. Endosc Int
Open. 7:E1150–E1162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Granata A, Martino A, Amata M, Ligresti D
and Traina M: Gastrointestinal exposed endoscopic full-thickness
resection in the era of endoscopic suturing: A retrospective
single-center case series. Wideochir Inne Tech Maloinwazyjne.
16:321–328. 2021.PubMed/NCBI
|
|
66
|
Inayat F, Aslam A, Grunwald MD, Hussain Q,
Hurairah A and Iqbal S: Omental patching and purse-string
endosuture closure after endoscopic full-thickness resection in
patients with gastric gastrointestinal stromal tumors. Clin Endosc.
52:283–287. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang Y, Wang X, Xiong G, Qian Y, Wang H,
Liu L, Miao L and Fan Z: Complete defect closure of gastric
submucosal tumors with purse-string sutures. Surg Endosc.
28:1844–1851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Weiland T, Fehlker M, Gottwald T and
Schurr MO: Performance of the OTSC System in the endoscopic closure
of iatrogenic gastrointestinal perforations: A systematic review.
Surg Endosc. 27:2258–2274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang W, Liu CX, Niu Q, Wang AL, Shi N, Ma
FZ and Hu YB: OTSC assisted EFTR for the treatment of GIST: 40
cases analysis. Minim Invasive Ther Allied Technol. 31:238–245.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tang SJ, Naga YM, Wu R and Zhang S:
Over-the-scope clip-assisted endoscopic full thickness resection: A
video-based case series. Surg Endosc. 34:2780–2788. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pawlak KM, Raiter A, Kozlowska-Petriczko
K, Szelemej J, Petriczko J, Wojciechowska K and
Wiechowska-Kozlowska A: Optimal endoscopic resection technique for
selected gastric GISTs. The endoscopic suturing system combined
with ESD-a new alternative? J Clin Med. 9:17762020.PubMed/NCBI
|
|
72
|
Mori H, Kobara H, Nishiyama N and Masaki
T: Current status and future perspectives of endoscopic
full-thickness resection. Dig Endosc. 30 (Suppl 1):S25–S31. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jain D, Mahmood E, Desai A and Singhal S:
Endoscopic full thickness resection for gastric tumors originating
from muscularis propria. World J Gastrointest Endosc. 8:489–495.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Guo J, Sun B, Sun S, Liu X, Wang S, Ge N,
Wang G and Liu W: Endoscopic puncture-suture device to close
gastric wall defects after full-thickness resection: A porcine
study. Gastrointest Endosc. 85:447–450. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sun B, Guo J, Ge N, Sun S, Wang S, Liu X,
Wang G and Feng L: Endoscopic ultrasound-guided puncture suture
device versus metal clip for gastric defect closure after
endoscopic full-thickness resection: A randomized, comparative,
porcine study. Endosc Ultrasound. 5:263–268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sigmon DF, Tuma F, Kamel BG and Cassaro S:
Gastric Perforation. StatPearls. Treasure Island; FL: 2022
|
|
77
|
Seehawong U, Morita Y, Nakano Y, Iwasaki
T, Krutsri C, Sakaguchi H, Sako T, Takao T, Tanaka S, Toyonaga T,
et al: Successful treatment of an esophageal perforation that
occurred during endoscopic submucosal dissection for esophageal
cancer using polyglycolic acid sheets and fibrin glue. Clin J
Gastroenterol. 12:29–33. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tan Y, Tang X, Guo T, Peng D, Tang Y, Duan
T, Wang X, Lv L, Huo J and Liu D: Comparison between submucosal
tunneling endoscopic resection and endoscopic full-thickness
resection for gastric stromal tumors originating from the
muscularis propria layer. Surg Endosc. 31:3376–3382. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li B, Shi Q, Qi ZP, Yao LQ, Xu MD, Lv ZT,
Yalikong A, Cai SL, Sun D, Zhou PH, et al: The efficacy of dental
floss and a hemoclip as a traction method for the endoscopic
full-thickness resection of submucosal tumors in the gastric
fundus. Surg Endosc. 33:3864–3873. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li J, Meng Y, Ye S, Wang P and Liu F:
Usefulness of the thread-traction method in endoscopic
full-thickness resection for gastric submucosal tumor: A
comparative study. Surg Endosc. 33:2880–2885. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Abe S, Wu SYS, Ego M, Takamaru H,
Sekiguchi M, Yamada M, Nonaka S, Sakamoto T, Suzuki H, Yoshinaga S,
et al: Efficacy of current traction techniques for endoscopic
submucosal dissection. Gut Liver. 14:673–684. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zheng S, Ali FS, Zhang J, Zhao L and Liu
B: Endoscopic traction techniques. Am J Gastroenterol. 116:862–866.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Granata A, Martino A, Ligresti D,
Tuzzolino F, Lombardi G and Traina M: Exposed endoscopic
full-thickness resection without laparoscopic assistance for
gastric submucosal tumors: A systematic review and pooled analysis.
Dig Liver Dis. 54:729–736. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Jian G, Tan L, Wang H, Lv L, Wang X, Qi X,
Le M, Tan Y and Liu D: Factors that predict the technical
difficulty during endoscopic full-thickness resection of a gastric
submucosal tumor. Rev Esp Enferm Dig. 113:35–40. 2021.PubMed/NCBI
|
|
85
|
Huang J, Xian XS, Huang LY, Zhang B, Wu CR
and Cui J: Endoscopic full-thickness resection for gastric
gastrointestinal stromal tumor originating from the muscularis
propria. Rev Assoc Med Bras (1992). 64:1002–1006. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Joensuu H: Risk stratification of patients
diagnosed with gastrointestinal stromal tumor. Hum Pathol.
39:1411–1419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang H, Feng X, Ye S, Wang J, Liang J, Mai
S, Lai M, Feng H, Wang G and Zhou Y: A comparison of the efficacy
and safety of endoscopic full-thickness resection and
laparoscopic-assisted surgery for small gastrointestinal stromal
tumors. Surg Endosc. 30:3357–3361. 2016. View Article : Google Scholar : PubMed/NCBI
|