With the development of high-throughput sequencing
technology and biotransformation technology, and the decreasing
cost of sequencing, the understanding of the microbiome has been
deepened. The homology and diversity of microorganisms at target
sites can be known through sequence comparison and statistical
analysis (1). The dynamic balance
and stability of the microbiome is an important indicator of the
stability of human internal environments (2). Common gynecological cancer types
include cervical caner, ovarian and endometrial cancer, which occur
in women at all ages, but mostly in postmenopausal women (3,4). The
vagina is a structure with open physiological features that allow
easy invasion and colonization by microbes. Invading pathogenic
microbes can disrupt the ecological balance of the vagina and cause
inflammation, which can lead to the risk of cancer (5,6).
Recently, more and more studies have focused on the impact of
vaginal microorganisms on gynecological malignancies. The present
review first discusses the vaginal microbial environment to
elucidate the vaginal steady state and the role of lactobacilli and
their metabolites in maintaining stability. Most studies are
limited to phenomenological findings and there is a lack of
research on the underlying mechanisms, and no method for the early
screening of gynecological malignancies is widely recognized. In
addition, the results obtained vary depending on the sampling
method, the geographical location of the subjects and the clinical
indicators. The present review pooled the latest research advances
and compared the consistency and differences between studies in
order to obtain more generalized results. Moreover, the microbes
discovered recently in cancerous tissues and their role in tumor
immunity may be a key to future cancer treatment (7), but the research is still in its
infancy and further study is required to confirm the importance of
these microbes. Tumor tissue microbes are in direct contact with
cancer cells and immune cells (8).
Microbes are not only the ‘cause’ of gynecologic cancer, but also
the ‘consequence’ of a series of adverse effects brought about by
cancer. The current review presents the association between
microbes and gynecological malignancies in terms of cancer
susceptibility, marker prediction, subsequent treatment and
prognosis, and the disorders and changes of microbes due to
gynecological carcinogenesis from both cause and consequence
perspectives. The aim is to elucidate the association between
gynecological malignancies and microbes in vaginal and tumor
tissues, and to provide a theoretical basis for future cancer
interventions and in-depth studies using flora.
Lactobacilli and related metabolites adversely
affect the growth and survival of cervical cancer cells. A study
has shown that different concentrations of lactobacilli supernatant
can inhibit the activity of cervical cancer cells by regulating the
expression of genes (32).
Motevaseli et al (33) also
found that lactobacilli supernatant and normal vaginal lactobacilli
had cytotoxic effects on cervical tumor cells, but not on normal
cervical epithelial cells, and were not affected by pH and lactic
acid in the vaginal environment. Moreover, the exopolysaccharides
secreted by the L. gasseri strains (G10 and H15), which were
isolated from the vagina of healthy women, could both inhibit the
proliferation of cervical cancer cells and also affect apoptosis
(34). Palma et al (35) showed that long-term (6 months) use
of Lactobacillus rhamnosus BMX 54 was twice as likely to
resolve HPV-associated cytological abnormalities than short-term
use (3 months). Based on this feature, vaginal
Lactobacillus-like probiotic supplementation for patients
with cervical cancer may be a future aid to delay the progression
of cervical cancer.
The decline of the dominance of lactobacilli is
associated to the occurrence of gynecological diseases. The
decreased abundance of lactobacilli facilitates the invasion of
pathogenic bacteria and promotes the development of sexually
transmitted diseases, and precancerous inflammation and lesions. It
has been revealed that South African women with a low vaginal
lactobacilli abundance are more than four times more likely to be
infected with human immunodeficiency virus than other women
(36). This phenomenon is
associated with interference of immune regulation by high-risk
flora (36). In turn, local
inflammation and infection caused by pathogen invasion and lesions
can affect the abundance of lactobacilli, further causing
microenvironmental dysregulation and promoting disease
deterioration (37). Using 16S
rRNA-seq, Borgogna et al (38) found that women with HPV infections
had a lower lactobacilli content and lost the dominance of vaginal
flora compared to women who were HPV−. An increase in
the abundance of bacteria associated with bacterial vaginosis,
provides favorable conditions for the development of precancerous
lesions (39).
The detection of cancer biomarkers (e.g., protein
molecules and microRNA) can be used for cancer diagnosis (40), drug administration (41) and prognosis prediction (42), and its potential role has important
implications for controlling the cancer burden. A 2019 study showed
that proinflammatory cytokines (IL-6 and TNFα), apoptosis-related
proteins [soluble (s)Fas, sFas ligand (sFasL) and TNF-related
apoptosis-inducing ligand], growth and angiogenesis factors
(hepatocyte growth factor, stem cell factor and vascular
endothelial growth factor) and others (α-fetoprotein and
osteopontin), were elevated in the local cervicovaginal
microenvironment of patients with cervical cancer, and were
negatively correlated with the abundance of lactobacilli and
positively correlated with vaginal pH (43). Changes in lactobacilli and the
acidic environment they create may cause activation of pathways
related to inflammation and apoptosis within the vaginal cervical
epithelium, acting as a pro-cancer factor (44). Additionally, lactobacilli can
regulate the vaginal microecological system and exogenous
lactobacilli supplementation can reverse the dysregulation to some
extent (45,46). One study displayed reduced Nugent
scores and improvement in vaginal dysbiosis by oral administration
of a pertinent lactobacilli strain mixture (Lactobacillus
crispatus LbV 88, Lactobacillus gasseri LbV 150N,
Lactobacillus jensenii LbV 116 and Lactobacillus
rhamnosus LbV96) (47). Other
researchers have found that vaginal microbiome transplantation can
improve dysbiosis when transplanting vaginal microorganisms from
healthy women to treat bacterial vaginosis (48). However, the feasibility and safety
of vaginal microbiome transplantation cannot be determined, as the
number of subjects in which this has been performed is too small
and the potential risks remain elusive. The exploration of the
beneficial effects of vaginal lactobacilli will contribute to the
future development of vaginal targeted probiotic products, the
macroscopic control of vaginal flora through flora transplantation
and the modification of vaginal antibiotics.
HPV infection. High-risk HPV (hrHPV), such as HPV-16
and HPV-18, are recognized oncogenic factors in cervical cancer
(49). Although most hrHPV
infections are spontaneously cleared (50), persistent infection is capable of
causing cervical intraepithelial neoplasia, which can eventually
lead to cancer (51). Firstly,
linear discriminant analysis effect size analysis showed that
increased vaginal microbial diversity is strongly associated with
HPV infections (52), and
Fusobacteria, including Sneathia spp., may be microbial
markers associated with HPV infections (Table I) (53). In addition, HPV infections also
cause fluctuations in the diversity and abundance of vaginal
microorganisms (25). The
composition and functions of vaginal microorganisms are altered in
women with hrHPV infections (54),
and Pseudomonas is more likely to be detected in hrHPV+
patients and patients with cervical cancer than in HPV- subjects
(55). In addition, the
pathogenicity of different HPV types varies significantly, with
hrHPV being carcinogenic and low-risk HPV tending to cause only
benign lesions (56). Not only
that, but the incidence of cervical cancer likewise varies among
different types of hrHPV infection (57). Huang et al (58) found that combinations of
Oribacterium, Lachnobacterium and Thermus among the cervicovaginal
microbiota were more likely to be associated with HPV-16, while
combinations of Motilibacter were more likely to be associated with
HPV-52, and combinations of Litorilinea and Paludibaculum, and the
absence of L. iners, were more likely to be associated with HPV-58.
It was predicted that cervicovaginal flora may also be associated
with specific types of HPV infection, which in turn may affect
carcinogenesis (58). Artificial
interventions with cervicovaginal microbes may affect the cervical
cancer burden by suppressing hrHPV infections. In addition, the
varying cervicovaginal microbiota have differences in the
persistence and elimination rate of HPV in vivo (59). Moreover, CST IV, dominated by
bacteria associated with bacterial vaginosis, is a risk factor for
the persistence of HPV, and Atopobium spp. may be a microbial
marker for the persistence of HPV (60). In addition, a 16S RNA-seq analysis
of 28 women with persistent HPV infection and 30 women with HPV
clearance showed the proportion of bifidobacteria and lactobacilli
may play a role in clearing HPV to a certain extent (61). It has been speculated that
inflammation may be involved in the interaction between
microorganisms and HPV infections. Lv et al (62) compared the cervicovaginal microbiota
of hrHPV- and hrHPV+ subjects and found that Ureaplasma
urealyticum, Ureaplasma parvum, Chlamydia trachomatis and
Trichomonas vaginalis were risk factors for hrHPV infections. In
addition, it was hypothesized that microbes promoted hrHPV
infections by triggering inflammation and thereby affecting the
protective effect of the immune system. Other similar studies
indicated that this conjecture was correct (23,63).
However, there is no definite conclusion about the underlying
mechanism.
Control of precancerous lesions can prevent them
from deteriorating to cervical cancer. A decrease in the dominance
of lactobacilli and an increase in the cervicovaginal microbial
diversity facilitate the development of cervical cancer (64–68).
Researchers adjusted the dysregulated cervicovaginal flora by
excising the precancerous lesions. For vaginal flora, resection of
the cervical intraepithelial neoplasia (CIN) had no ameliorative
effect (69), whereas, for cervical
microorganisms, resection of the CIN using a loop electrosurgical
excision procedure (70) decreased
the cervical microbial diversity and restored the lactobacilli
dominance. In terms of treatment, chemotherapy efficacy varies
among patients (30). One study
revealed significantly lower vaginal microbial α diversity and
enrichment of Bacteroides in patients with non-responding
cervical cancer than in patients with significant responses to
platinum drugs (30), suggesting
that manual intervention of vaginal-cervical microorganisms may
facilitate the responsiveness of patients to chemotherapy and
enhance the therapeutic effect. The accurate control of microbial
changes and roles at the CIN stage plays an important role in the
construction of future non-invasive early screening models to
reduce global cervical cancer mortality disparities and reduce the
disease burden.
The regional difference in cervical cancer incidence
is associated with regional financial level (71). Early screening for cervical cancer
is an effective way to reduce the global burden of cervical cancer.
Therefore, using microbial markers to identify patients in the
early stages is one of the current research focuses and is
important in cervical cancer prevention and treatment. Previous
studies found that Sneathia was a hallmark of patients with
HPV+ SILs and that it was enriched in all women with
precancerous lesions, cervical cancer and abnormal vaginal pH
(67,72). The abundance of Fusobacterium
was significantly higher in advanced cervical cancer compared with
that in the early stages, and F. necrophorum was observed
only in cervical cancer (72). In
addition, according to a Korean study, significant abundance
variations were found in vaginal Lactobacillus and
Gardnerella in women with cervical cancer compared with that
in healthy women, while Streptococcus abundance in women
with CIN was significantly different from that in healthy women
(73). Thus, it is speculated that
fusobacteria, Sneathia and Streptococcus may be
microbial markers of precancerous lesions and cervical cancer, and
may promote the development of cervical cancer via maintenance of
an immunosuppressive microenvironment by increasing the levels of
relevant cytokines in vivo. The aforementioned exploration
has limitations, such as small sample sizes and no assessment of
environmental factors. However, it provides a relatively reliable
direction for early screening and treatment of cervical cancer in
the future. Moreover, due to different study focuses and research
methods, some differences exist in the prediction of cervical
cancer biomarkers. However, in general, it is a consensus among
scholars that a microbial environment with a higher proportion of
anaerobic bacteria and a lower proportion of lactobacilli is more
prone to HPV infection.
Ovarian cancer is a severe threat to women's health,
as due to the lack of symptoms in the early stage and the
deficiency of obvious screening effects, the majority of cases are
already in the terminal stages when diagnosed (74). Therefore, although the incidence of
ovarian cancer is lower than that of cervical and endometrial
cancer, the mortality rate ranks top among the gynecological
malignancies (75). Due to
differences in sequencing methods, inclusion populations and
analytical methods, there is some variation in the identification
of microbial markers. However, in general, the dominance of the
vaginal microorganism lactobacilli is lower in patients with
ovarian cancer than in healthy women and is accompanied by a rise
in diversity (76,77).
The studies on vaginal flora have indicated that
bilateral salpingectomy reduces the risk of ovarian cancer by 42 to
78%, which is more protective than bilateral tubal ligation (13 to
41% risk reduction) (78). It is
hypothesized that a salpingo-oophorectomy or ligation reduces the
exposure of the ovary to microorganisms, thus reducing the risk of
ovarian cancer (79,80). Regular physical examination and
bilateral tubal ligation for fertile women with high risks of
ovarian cancer may help reduce the short-term incidence of ovarian
cancer. In addition, cervicovaginal microbes with <50%
lactobacilli are significantly associated with ovarian cancer and
known risk factors, such as age or BRCA1 germline mutations
(76). However, more experiments in
this area are needed to further validate such ideas and action
mechanisms.
The sensitivity of patients to platinum drugs is the
key to the outcomes of ovarian cancer treatment. Jacobson et
al (81) concluded that the
vaginal microbiota of patients with ovarian cancer was
statistically associated with platinum sensitivity. Vaginal flora
with a predominance of Escherichia was likely in patients
with platinum-resistant tumors. According to these findings, we
hypothesize that artificially aligning a patient's vaginal flora to
platinum-sensitive flora through probiotics, vaginal flora
transplantation and other methods could improve the efficacy of
platinum drugs and prolong the platinum-free interval. However,
this speculation needs to be verified by more in-depth studies in
the future.
Alterations in the microorganism and
microenvironment have been demonstrated to associate with
carcinogenesis in a variety of cancer types, such as lung,
gastrointestinal and skin cancer (82). Although the vaginal microbiome
associated with endometrial cancer has been less studied, the
correlation between the two cannot be denied. First, epidemiology
shows that endometrial cancer is more frequent in postmenopausal
women (83), and changes in vaginal
microbiology in postmenopausal women (e.g., a lower proportion of
Lactobacillus and a higher proportion of Prevotella
and Gardnerella) (84–86)
may be involved in endometrial carcinogenesis, which in turn
affects its incidence. Second, scholars are now beginning to accept
the presence of microorganisms inside the uterus, and evidence
points to the possibility that vaginal microorganisms may rise
during uterine peristalsis and sperm transport through the cervical
canal (87–89). With advances in next-generation
sequencing technology, the characterization of the in utero
microbiome is becoming increasingly clear. Although the biomass of
microorganisms in the uterus is low (90–92),
bacteria, viruses (such as adeno-associated virus, human herpes
viruses and human cytomegalovirus) (93–95),
Chlamydia (Chlamydia trachomatis) (96,97),
Mycoplasma (Mycoplasma hominis) (98) and fungus (Candida albicans)
(99) have been detected. In 2015,
Mitchell et al (91)
evaluated the endometrial flora of 58 patients by quantitative PCR,
among which L. iners, Prevotella and L. crispatus
were the most common. However, a 2017 16s rRNA-seq analysis showed
that Lactobacillus, Pseudomonas, Acinetobacter and
Vagococcus were the most abundant genera in the endometrial
environment (92). Some scholars
believe that vaginal microorganisms may be the source of abnormal
microbial composition in the uterus. Evidence for this speculation
was provided in a study by Walther-António et al (100). The study included 16S RNA-seq
experiments on collected samples from the vagina, cervix, fallopian
tubes, ovaries, peritoneum and urine, and found a significant
correlation between the microbial composition of the vagina,
cervix, fallopian tubes and ovaries in patients with endometrial
cancer (100). Since the vagina is
the only channel through which the aforementioned organs are
connected to the outside, it is scientific and relevant to study
the microbial environment within the organs of gynecological cancer
and the tumor tissue, starting with the vaginal flora. Firmicutes,
spirochaetes, actinobacteria, bacteroidetes and proteobacteria in
patients with endometrial cancer were significantly increased
compared with those in controls; the presence of
Porphyromonas and Atopobium vaginae at a vaginal pH
of >5, with a sensitivity and specificity of 100 and 60%,
respectively, for the diagnosis of endometrial cancer (100), provides a potentially effective
way to diagnose endometrial cancer. In another study a few years
later, Porphyromonas was also used as a biomarker for
endometrial cancer; female menopausal status, body mass index and
vaginal pH were demonstrated to influence the vaginal microbial
composition, with the three factors independently increasing
microbial diversity (101).
Cancer is a malignant lesion caused by multiple
pathological factors, in which microbiome changes may be a
combination of bacterial, viral and other alterations. Despite the
association between HPV and endometrial cancer being hypothesized
as early as 1991 (102), there is
general agreement that there is little association between HPV
infection and common endometrial cancer (103,104), with only one study belatedly
opposing this (105). The study by
Abu-Lubad et al (105) used
polymerase chain reaction to detect HPV DNA in 144
formaldehyde-fixed paraffin-embedded tissues and found that the
infection rate of the endometrial cancer group was higher. However,
no definite conclusions can be drawn. Notably, however, some
studies have found associations between endometrial cancer and
hepatitis B virus infections (106), viral antigens and RNAs of measles
viruses (107). Jiang et al
(106) analyzed the statuses of
HBV serum markers in 398 women with endometrial cancer and compared
them with those of 788 healthy women, and found that the hepatitis
B surface antigen-positive rate and the hepatitis B carrier rate in
women with endometrial cancer were both significantly higher than
those in the controls. In addition, Benharroch et al
(107) used immunohistochemistry
to find the presence of measles virus antigen in tumor cells in 72%
of patients with endometrial cancer. Although this does not prove a
causal association between endometrial cancer and the virus, all
such findings provide a new direction for endometrial cancer
research, and the etiology of endometrial cancer may be
complex.
There is a relatively clear understanding of the
microbes within the human environment that are in close contact
with the outside world. However, there are questions with regard to
whether microbes exist within the tumor tissues that form later in
life, and whether the microbes have similar effects to vaginal
intestinal microbes on host health and tumor development. Due to
the superior geographic location of bacteria within tumor tissues,
studying such microbes may play a key role in future tumor
control.
Microbes do exist in tumor tissues, and this fact
was supported by a microbiome analysis of 1,526 samples from 7
tumor types, with some differences in the microbial composition of
various tumor types (8). Nejman
et al (109) used 5R 16S
rRNA-seq technology to detect the microbiome in breast, lung,
ovarian and pancreatic cancer, melanoma, and bone and brain tumors,
and found that there were significant differences in microbial
diversity and richness, among which breast cancer samples had the
highest microbial diversity, higher than in neighboring tissues and
healthy subjects. No higher bacterial load was found in ovarian
cancer than in adjacent tissues. Moreover, the dominant bacteria of
different cancer types were also different. For example, firmicutes
and bacteroidetes phyla were dominant in colorectal cancer, while
proteobacteria dominated in pancreatic cancer. In general,
proteobacteria, firmicutes, actinobacteria, bacteroidetes,
fusobacteria and cyanobacteria occupied a high proportion in the
aforementioned seven types of cancer. Intratumoral microbes were
mainly found within tumor cells, in the periphery and interior of
tumor tissues, and in the surrounding blood vessels, most of which
were localized in cancer cells and immune cells (109), and their secretions could act
directly. In addition, the microbiome in the same tumor tissue
differs at different sites in different stages and staging
(110). The role of tumor microbes
in tumors can be divided into two aspects; the presence of related
microbes affect the proliferation and apoptosis of cancer cells,
expression of biomarkers (111),
gene expression levels and control of immune cell activity
(111,112), thus affecting cancer immunity,
treatment and prognosis (113)
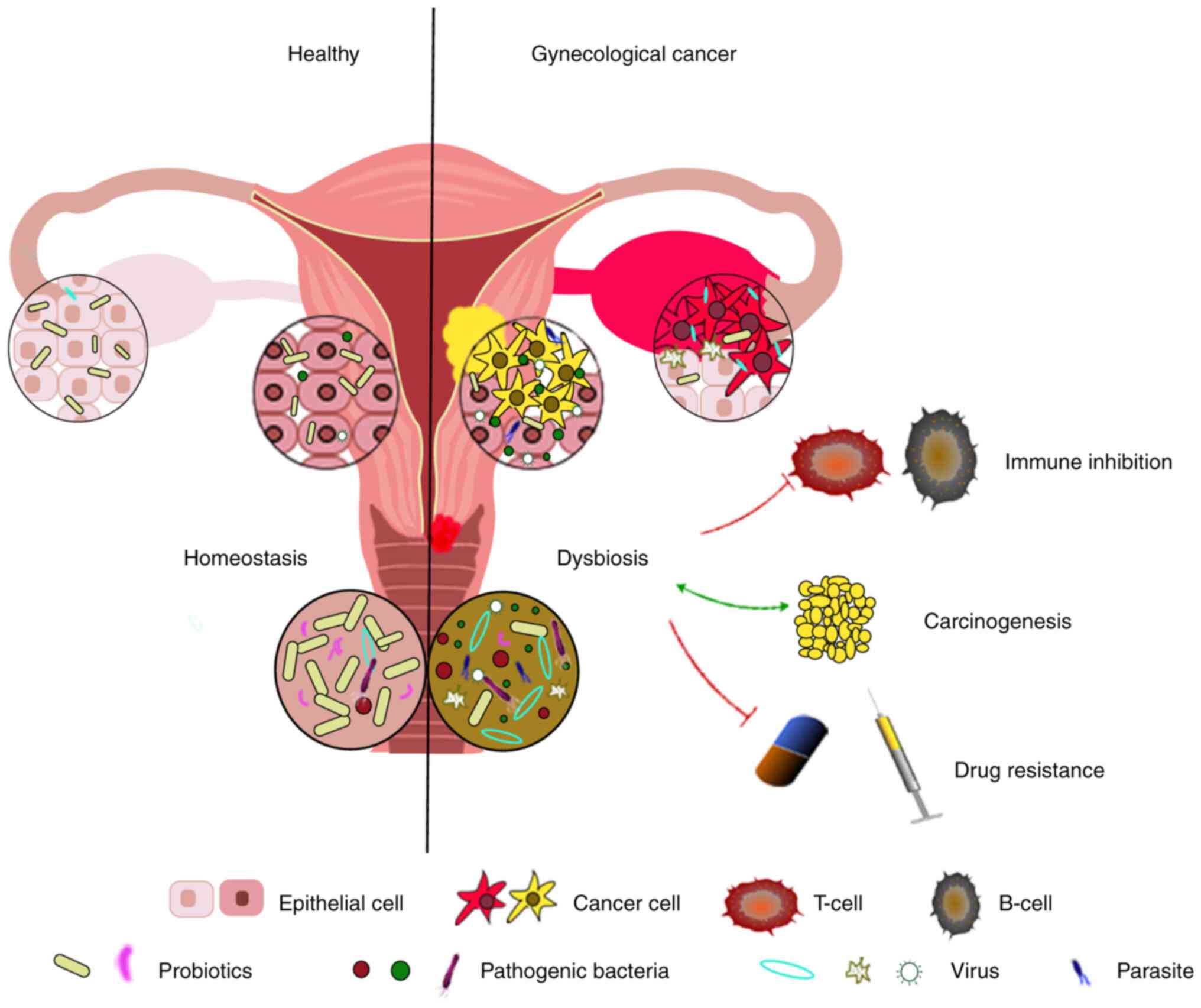
(Fig. 1). Microorganisms within
tumor tissues may be associated with inflammation. For example,
Kostic et al (114)
revealed marked enrichment of fusobacterium in colorectal cancer
tissues through genomic and histological analyses, and hypothesized
that these microbes might be involved in tumorigenesis via
inflammation-mediated mechanisms.
The source of microbes within the tumors also
deserves consideration. Various types of human flora are known to
play a role in the production and development of cancers, so we
speculate that intestinal, vaginal and other human flora may be the
main source of microbes within the tumors. In a study exploring the
microbial origin of pancreatic cancer tissues, the bacterial DNA
profiles of both pancreatic and non-pancreatic cancer tissues were
found to be similar to those of duodenal tissues, suggesting that
bacteria present in the pancreas might migrate from the intestine
to the pancreas (115). Due to the
structural and functional connectivity of the reproductive organs,
we speculate that the source of microorganisms within gynecological
malignancies may be closely associated with the vagina. However,
few studies have been conducted. Research on the presence and role
of tumor tissue microbes is still in its infancy, and most
speculations have not been confirmed. Future research on tumor
microbes should focus on the source and role of microbes to improve
the tumor microenvironment and achieve tumor control by
understanding the relevant mechanisms.
The presence and variability of microorganisms have
been found in ovarian cancer samples. The viruses, bacteria, fungi
and parasites in ovarian cancer samples and the viral integration
sites in the host genomes of tumor samples (116) may play a role in the development
of cancer. The relevance of viruses, Chlamydia and
Mycoplasma, among others, to ovarian carcinogenesis is
controversial. Some scholars believe that despite the low
percentage of detection in ovarian cancer samples, there are still
differences in species diversity and composition compared with
healthy samples and this may be involved in ovarian cancer
progression in concert with other oncogenic factors (117–120). By contrast, the results of a 2010
study showed that Chlamydia trachomatis, Mycoplasma genitalium,
Neisseria gonorrhoeae, HPV and polyomavirus were not detectable
in either benign diseases, borderline tumors or ovarian cancer
samples (121). The bacterial
composition of ovarian cancer tissues has been found to be
different from that of non-cancerous tissues. Zhou et al
(122) found that ovarian cancer
tissues had a significantly lower diversity and abundance of
microflora, and a higher ratio of proteobacteria/firmicutes than
normal tissues, suggesting that changes in the microbial
composition might be associated with the development of ovarian
cancer. Not only is there a significant difference in the diversity
and composition of the microbiomes between women with and without
cancer (122,123), but the flora of different parts of
the upper genital tract of women with ovarian cancer are also
significantly different. A recent study that collected tissue flora
of the proximal fallopian tube, fimbriae and ovaries in women with
ovarian cancer found significant differences between the
microbiomes of the different sites through high throughput
sequencing of the 16S gene in the V1-V3 region (124). In terms of the early prediction of
ovarian cancer, one study indicated a significant decrease in
Lactococcus in ovarian cancer tissue, which may be a
potential biomarker for identifying the disease. However, due to
the lack of studies targeting the ovarian microbiota, the role of
microbes in the ovaries remains ambiguous.
Scholars used to regard the uterus as a sterile
environment, but current research points to the existence of
microbiota in the uterus as well. Studies on the uterine flora are
still in their initial stages, so there are different views on the
composition and variation of microbes in the uterus. Some scholars
believe that lactobacilli are most abundant in the endometrium
(125,126). However, others hold the opposing
view that microbes in the uterus are dominated by Acinetobacter,
Pseudomonas, Cloacibacterium and Comamonadaceae, while
lactobacilli are less common (21).
Carcinogenesis may be able to cause local microbiome variation.
Wang et al (127) revealed
that endometrial cancer tissues differed significantly from the
non-cancerous fraction of the microbiome, accompanied by elevated α
diversity and enrichment of Prevotella, Atopobium, Anaerococcus,
Dialister, Porphyromonas and Peptoniphilus, similar to
the dysbiotic state of the vaginal flora (127). The differences in results are
related to the method of tissue sampling and the magnitude of
contamination potential, the scientific nature of which needs to be
confirmed by numerous studies. Dysbiosis of the intrauterine flora
may be associated with inflammation and may impact the development
of cancer. Lu et al (128)
demonstrated that dysbiosis of the endometrial flora and
inflammatory factors in patients with endometrial cancer was
associated with possible Micrococcus. Additionally, it has
been suggested that the specific presence of Atopobium
vaginae and Porphyromonas somerae can target endometrial
cells to express pro-inflammatory cytokines and chemokines
(129), which potentially provides
a reference to further explore the mechanism between endometrial
flora and inflammatory response. Furthermore, viruses that invade
and parasitize the uterus may have oncogenic effects. A study on
the association between the endometrium and human mammary tumor
viruses showed that 23.2% of human mammary tumor viruses and
proteins were detected in patients with endometrial cancer, while
none were detected in the normal endometrium (130). It is hypothesized that human
mammary tumor viruses influence endometrial development. However,
the exact mechanism is unknown. In terms of biomarker prediction,
scholars indicated Pelomonas and Prevotella
enrichment in cancerous tissues, found Prevotella to be
associated with elevated serum D-dimer and fibrin degradation
products, and hypothesized that the microbial marker of
Prevotella, along with D-dimer and fibrin degradation
products, might predict endometrial cancer (131). However, this study has the problem
of a small sample size. There is still a great lack of research on
microbes in the uterus. Choosing the correct sampling enables a
study to determine the microbial composition of the normal
endometrium, then compare it with that of diseased endometrial
samples for analysis, and finally investigate the mechanisms by
which microorganisms within tumor tissues affect endometrial
cancer, thus providing a unique direction for preventing and
treating endometrial cancer.
In summary, the associations between vaginal
microbes and gynecological malignancies are broadly divided into
three aspects: i) The interaction of vaginal flora with infection
by pathogenic microorganisms such as HPV and Chlamydia
trachomatis; ii) the significance of landmark microorganisms
for the early prediction of gynecological malignancies; and iii)
the interaction of vaginal microbes with the treatment effect and
prognosis of gynecological malignancies. Vaginal microbes exist in
almost all stages of the development and control of gynecological
malignancies. Additionally, the presence and role of microorganisms
within the tumor should not be ignored, especially their link with
tumor immunity. Further research should focus on the connection
between the tumor microbiome of gynecological malignancies and the
composition and structure of vaginal microbes, to study the
microbial composition at the level of the reproductive tract as a
whole and make macroscopic regulation of the microecological
stability of the reproductive tract to promote the prevention and
treatment of gynecological cancer. Finally, strengthening the
popularization of knowledge about gynecological malignant tumors
and other diseases to enable the majority of women to understand
the risk of gynecological diseases deeply, avoid risks in daily
life, take physical examinations on time and improve their
awareness of vaccinations related to gynecological diseases, will
play a huge role in reducing the incidence of gynecological cancer
in the future. In further studies, analyses should be conducted in
larger sample sizes and more complex populations, taking into
account the differences in clinical indicators of the women tested
and focusing on the dynamic changes in cancer occurrence, in order
to grasp the overall disease development and a general trend of
microbiome changes in gynecological cancer. In addition, the
development of personalized screening and treatment should also be
on the agenda, and through the study of population differences,
screening and treatment plans can be formulated more precisely and
with better results.
Not applicable.
This study was supported by Shenyang Breast Cancer Clinical
Medical Research Center (grant no. 2020-48-3-1), LiaoNing
Revitalization Talents Program (grant no. XLYC1907160), Beijing
Medical Award Foundation (grant nos. YXJL-2020-0941-0752 and
CORP-239-N27), Wu Jieping Medical Foundation (grant no.
320.6750.2020-12-21,320.6750.2020-6-30) and the Fundamental
Research Funds for the Central Universities (grant nos. 2022029 and
2022030).
Not applicable.
JX and TS conceived and designed the review. MH and
NW wrote the draft of the paper. WH and MB created the figure and
edited the manuscript. All authors read and approved the final
manuscript and submission of this manuscript. Data authentication
is not applicable.
Not applicable.
Not applicable.
MB is employed by Liaoning Microhealth
Biotechnology Co., Ltd.
|
1
|
Johnson JS, Spakowicz DJ, Hong BY,
Petersen LM, Demkowicz P, Chen L, Leopold SR, Hanson BM, Agresta
HO, Gerstein M, et al: Evaluation of 16S rRNA gene sequencing for
species and strain-level microbiome analysis. Nat Commun.
10:50292019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rinninella E, Raoul P, Cintoni M,
Franceschi F, Miggiano GAD, Gasbarrini A and Mele MC: What is the
healthy gut microbiota composition? A changing ecosystem across
age, environment, diet, and diseases. Microorganisms. 7:142019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Einstein MH, Levine NF and Nevadunsky NS:
Menopause and cancers. Endocrinol Metab Clin North Am. 44:603–617.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu Y, Sun W, Liu H and Zhang D: Age at
menopause and risk of developing endometrial cancer: A
meta-analysis. Biomed Res Int. 2019:85841302019.PubMed/NCBI
|
|
5
|
Liang Y, Chen M, Qin L, Wan B and Wang H:
A meta-analysis of the relationship between vaginal microecology,
human papillomavirus infection and cervical intraepithelial
neoplasia. Infect Agent Cancer. 14:292019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gillet E, Meys JF, Verstraelen H, Verhelst
R, De Sutter P, Temmerman M and Vanden Broeck D: Association
between bacterial vaginosis and cervical intraepithelial neoplasia:
Systematic review and meta-analysis. PLoS One. 7:e452012012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sepich-Poore GD, Zitvogel L, Straussman R,
Hasty J, Wargo JA and Knight R: The microbiome and human cancer.
Science. 371:eabc45522021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poore GD, Kopylova E, Zhu Q, Carpenter C,
Fraraccio S, Wandro S, Kosciolek T, Janssen S, Metcalf J, Song SJ,
et al: Microbiome analyses of blood and tissues suggest cancer
diagnostic approach. Nature. 579:567–574. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Witkin SS, Linhares IM and Giraldo P:
Bacterial flora of the female genital tract: function and immune
regulation. Best Pract Res Clin Obstet Gynaecol. 21:347–354. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lidbeck A and Nord CE: Lactobacilli and
the normal human anaerobic microflora. Clin Infect Dis. 16 (Suppl
4):S181–S187. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chee WJY, Chew SY and Than LTL: Vaginal
microbiota and the potential of Lactobacillus derivatives in
maintaining vaginal health. Microb Cell Fact. 19:2032020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilson JD, Lee RA, Balen AH and Rutherford
AJ: Bacterial vaginal flora in relation to changing oestrogen
levels. Int J Std Aids. 18:308–311. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barrientos-Duran A, Fuentes-Lopez A, de
Salazar A, Plaza-Diaz J and Garcia F: Reviewing the composition of
vaginal microbiota: Inclusion of nutrition and probiotic factors in
the maintenance of eubiosis. Nutrients. 12:4192020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ravel J, Gajer P, Abdo Z, Schneider GM,
Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO,
et al: Vaginal microbiome of reproductive-age women. Proc Natl Acad
Sci USA. 108 Suppl 1 (Suppl 1):S4680–S4687. 2011. View Article : Google Scholar
|
|
15
|
Gajer P, Brotman RM, Bai G, Sakamoto J,
Schutte UM, Zhong X, Koenig SS, Fu L, Ma ZS, Zhou X, et al:
Temporal dynamics of the human vaginal microbiota. Sci Transl Med.
4:132ra522012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Gregorio PR, Parolin C, Abruzzo A,
Luppi B, Protti M, Mercolini L, Silva JA, Giordani B, Marangoni A,
Nader-Macías MEF and Vitali B: Biosurfactant from vaginal
Lactobacillus crispatus BC1 as a promising agent to
interfere with Candida adhesion. Microb Cell Fact. 19:1332020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung H, Ehlers MM, Peters RPH, Lombaard H,
Redelinghuys MJ, Bezuidenhoudt JE and Kock MM: Growth forms of
Gardnerella spp. and Lactobacillus spp. On vaginal
cells. Front Cell Infect Microbiol. 10:712020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aldunate M, Tyssen D, Johnson A, Zakir T,
Sonza S, Moench T, Cone R and Tachedjian G: Vaginal concentrations
of lactic acid potently inactivate HIV. J Antimicrob Chemother.
68:2015–2025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamarelle J, Thiebaut ACM, de Barbeyrac B,
Bebear C, Ravel J and Delarocque-Astagneau E: The vaginal
microbiota and its association with human papillomavirus, Chlamydia
trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium
infections: A systematic review and meta-analysis. Clin Microbiol
Infect. 25:35–47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Witkin SS and Linhares IM: Why do
lactobacilli dominate the human vaginal microbiota? BJOG.
124:606–611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kyrgiou M, Mitra A and Moscicki AB: Does
the vaginal microbiota play a role in the development of cervical
cancer? Transl Res. 179:168–182. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ilhan ZE, Laniewski P, Thomas N, Roe DJ,
Chase DM and Herbst-Kralovetz MM: Deciphering the complex interplay
between microbiota, HPV, inflammation and cancer through
cervicovaginal metabolic profiling. EBioMedicine. 44:675–690. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vornhagen J, Armistead B, Santana-Ufret V,
Gendrin C, Merillat S, Coleman M, Quach P, Boldenow E, Alishetti V,
Leonhard-Melief C, et al: Group B streptococcus exploits vaginal
epithelial exfoliation for ascending infection. J Clin Invest.
128:1985–1999. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scillato M, Spitale A, Mongelli G,
Privitera GF, Mangano K, Cianci A, Stefani S and Santagati M:
Antimicrobial properties of Lactobacillus cell-free
supernatants against multidrug-resistant urogenital pathogens.
Microbiologyopen. 10:e11732021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Qiu X, Wang W, Li D, Wu A, Hong Z,
Di W and Qiu L: Human papillomavirus infection and cervical
intraepithelial neoplasia progression are associated with increased
vaginal microbiome diversity in a Chinese cohort. BMC Infect Dis.
20:6292020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mitra A, MacIntyre DA, Marchesi JR, Lee
YS, Bennett PR and Kyrgiou M: The vaginal microbiota, human
papillomavirus infection and cervical intraepithelial neoplasia:
what do we know and where are we going next? Microbiome. 4:582016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Onderdonk AB, Delaney ML and Fichorova RN:
The human microbiome during Bacterial Vaginosis. Clin Microbiol
Rev. 29:223–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mitchell C and Marrazzo J: Bacterial
vaginosis and the cervicovaginal immune response. Am J Reprod
Immunol. 71:555–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Doerflinger SY, Throop AL and
Herbst-Kralovetz MM: Bacteria in the vaginal microbiome alter the
innate immune response and barrier properties of the human vaginal
epithelia in a species-specific manner. J Infect Dis.
209:1989–1999. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Xiao R, Huang J, Qin X, Hu D, Guo
E, Liu C, Lu F, You L, Sun C and Chen G: The diversity of vaginal
microbiota predicts neoadjuvant chemotherapy responsiveness in
locally advanced cervical cancer. Microb Ecol. 84:302–313. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Klatt NR, Cheu R, Birse K, Zevin AS,
Perner M, Noël-Romas L, Grobler A, Westmacott G, Xie IY, Butler J,
et al: Vaginal bacteria modify HIV tenofovir microbicide efficacy
in African women. Science. 356:938–945. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang KD, Xu DJ, Wang BY, Yan DH, Lv Z and
Su JR: Inhibitory effect of vaginal Lactobacillus
supernatants on cervical cancer cells. Probiotics Antimicrob
Proteins. 10:236–242. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Motevaseli E, Shirzad M, Akrami SM,
Mousavi AS, Mirsalehian A and Modarressi MH: Normal and tumour
cervical cells respond differently to vaginal lactobacilli,
independent of pH and lactate. J Med Microbiol. 62((Pt 7)):
1065–1072. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sungur T, Aslim B, Karaaslan C and Aktas
B: Impact of Exopolysaccharides (EPSs) of Lactobacillus
gasseri strains isolated from human vagina on cervical tumor
cells (HeLa). Anaerobe. 47:137–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Palma E, Recine N, Domenici L, Giorgini M,
Pierangeli A and Panici PB: Long-term Lactobacillus
rhamnosus BMX 54 application to restore a balanced vaginal
ecosystem: A promising solution against HPV-infection. BMC Infect
Dis. 18:132018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gosmann C, Anahtar MN, Handley SA,
Farcasanu M, Abu-Ali G, Bowman BA, Padavattan N, Desai C, Droit L,
Moodley A, et al: Lactobacillus-Deficient cervicovaginal
bacterial communities are associated with increased HIV Acquisition
in Young South African Women. Immunity. 46:29–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kalia N, Singh J and Kaur M: Microbiota in
vaginal health and pathogenesis of recurrent vulvovaginal
infections: A critical review. Ann Clin Microbiol Antimicrob.
19:52020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Borgogna JC, Shardell MD, Santori EK,
Nelson TM, Rath JM, Glover ED, Ravel J, Gravitt PE, Yeoman CJ and
Brotman RM: The vaginal metabolome and microbiota of cervical
HPV-positive and HPV-negative women: A cross-sectional analysis.
BJOG. 127:182–192. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wei ZT, Chen HL, Wang CF, Yang GL, Han SM
and Zhang SL: Depiction of vaginal microbiota in women with
high-risk human papillomavirus infection. Front Public Health.
8:5872982021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Uttley L, Whiteman BL, Woods HB, Harnan S,
Philips ST and Cree IA; Early Cancer Detection Consortium, :
Building the evidence base of blood-based biomarkers for early
detection of cancer: A rapid systematic mapping review.
EBioMedicine. 10:164–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Papageorgis P, Ozturk S, Lambert AW,
Neophytou CM, Tzatsos A, Wong CK, Thiagalingam S and Constantinou
AI: Targeting IL13Ralpha2 activates STAT6-TP63 pathway to suppress
breast cancer lung metastasis. Breast Cancer Res. 17:982015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fang L, Lu W, Choi HH, Yeung SC, Tung JY,
Hsiao CD, Fuentes-Mattei E, Menter D, Chen C, Wang L, et al:
ERK2-Dependent phosphorylation of CSN6 is critical in colorectal
cancer development. Cancer Cell. 28:183–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Laniewski P, Cui H, Roe DJ, Barnes D,
Goulder A, Monk BJ, Greenspan DL, Chase DM and Herbst-Kralovetz MM:
Features of the cervicovaginal microenvironment drive cancer
biomarker signatures in patients across cervical carcinogenesis.
Sci Rep. 9:73332019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Seta F, Campisciano G, Zanotta N, Ricci
G and Comar M: The vaginal community state types microbiome-immune
network as key factor for bacterial vaginosis and aerobic
vaginitis. Front Microbiol. 10:24512019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Recine N, Palma E, Domenici L, Giorgini M,
Imperiale L, Sassu C, Musella A, Marchetti C, Muzii L and Benedetti
Panici P: Restoring vaginal microbiota: Biological control of
bacterial vaginosis. A prospective case-control study using
Lactobacillus rhamnosus BMX 54 as adjuvant treatment against
bacterial vaginosis. Arch Gynecol Obstet. 293:101–107. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
De Alberti D, Russo R, Terruzzi F, Nobile
V and Ouwehand AC: Lactobacilli vaginal colonisation after oral
consumption of Respecta((R)) complex: A randomised controlled pilot
study. Arch Gynecol Obstet. 292:861–867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
de Vrese M, Laue C, Papazova E, Petricevic
L and Schrezenmeir J: Impact of oral administration of four
Lactobacillus strains on Nugent score-systematic review and
meta-analysis. Benef Microbes. 10:483–496. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lev-Sagie A, Goldman-Wohl D, Cohen Y,
Dori-Bachash M, Leshem A, Mor U, Strahilevitz J, Moses AE, Shapiro
H, Yagel S and Elinav E: Vaginal microbiome transplantation in
women with intractable bacterial vaginosis. Nat Med. 25:1500–1504.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Molijn A, Jenkins D, Chen W, Zhang X,
Pirog E, Enqi W, Liu B, Schmidt J, Cui J, Qiao Y, et al: The
complex relationship between human papillomavirus and cervical
adenocarcinoma. Int J Cancer. 138:409–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ho GY, Bierman R, Beardsley L, Chang CJ
and Burk RD: Natural history of cervicovaginal papillomavirus
infection in young women. N Engl J Med. 338:423–428. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Holly EA: Cervical intraepithelial
neoplasia, cervical cancer, and HPV. Annu Rev Public Health.
17:69–84. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Norenhag J, Du J, Olovsson M, Verstraelen
H, Engstrand L and Brusselaers N: The vaginal microbiota, human
papillomavirus and cervical dysplasia: A systematic review and
network meta-analysis. BJOG. 127:171–180. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee JE, Lee S, Lee H, Song YM, Lee K, Han
MJ, Sung J and Ko G: Association of the vaginal microbiota with
human papillomavirus infection in a Korean twin cohort. PLoS One.
8:e635142013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang Q, Wang Y, Wei X, Zhu J, Wang X, Xie
X and Lu W: The alterations of vaginal microbiome in HPV16
infection as identified by shotgun metagenomic sequencing. Front
Cell Infect Microbiol. 10:2862020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang Z, Li T, Zhang D, Zong X, Bai H, Bi
H and Liu Z: Distinction between vaginal and cervical microbiota in
high-risk human papilloma virus-infected women in China. BMC
Microbiol. 21:902021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Egawa N and Doorbar J: The low-risk
papillomaviruses. Virus Res. 231:119–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vanska S, Luostarinen T, Lagheden C,
Eklund C, Kleppe SN, Andrae B, Sparén P, Sundström K, Lehtinen M
and Dillner J: Differing age-specific cervical cancer incidence
between different types of human papillomavirus: Implications for
predicting the impact of elimination programs. Am J Epidemiol.
190:506–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang X, Li C, Li F, Zhao J, Wan X and
Wang K: Cervicovaginal microbiota composition correlates with the
acquisition of high-risk human papillomavirus types. Int J Cancer.
143:621–634. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Brotman RM, Shardell MD, Gajer P, Tracy
JK, Zenilman JM, Ravel J and Gravitt PE: Interplay between the
temporal dynamics of the vaginal microbiota and human
papillomavirus detection. J Infect Dis. 210:1723–1733. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Di Paola M, Sani C, Clemente AM, Iossa A,
Perissi E, Castronovo G, Tanturli M, Rivero D, Cozzolino F,
Cavalieri D, et al: Characterization of cervico-vaginal microbiota
in women developing persistent high-risk Human Papillomavirus
infection. Sci Rep. 7:102002017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mei L, Wang T, Chen Y, Wei D, Zhang Y, Cui
T, Meng J, Zhang X, Liu Y, Ding L and Niu X: Dysbiosis of vaginal
microbiota associated with persistent high-risk human papilloma
virus infection. J Transl Med. 20:122022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lv P, Zhao F, Xu X, Xu J, Wang Q and Zhao
Z: Correlation between common lower genital tract microbes and
high-risk human papillomavirus infection. Can J Infect Dis Med
Microbiol. 2019:96781042019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Torcia MG: Interplay among vaginal
microbiome, immune response and sexually transmitted viral
infections. Int J Mol Sci. 20:2262019. View Article : Google Scholar
|
|
64
|
Mitra A, MacIntyre DA, Lee YS, Smith A,
Marchesi JR, Lehne B, Bhatia R, Lyons D, Paraskevaidis E, Li JV, et
al: Cervical intraepithelial neoplasia disease progression is
associated with increased vaginal microbiome diversity. Sci Rep.
5:168652015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mitra A, MacIntyre DA, Ntritsos G, Smith
A, Tsilidis KK, Marchesi JR, Bennett PR, Moscicki AB and Kyrgiou M:
The vaginal microbiota associates with the regression of untreated
cervical intraepithelial neoplasia 2 lesions. Nat Commun.
11:19992020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tango CN, Seo SS, Kwon M, Lee DO, Chang HK
and Kim MK: Taxonomic and functional differences in cervical
microbiome associated with cervical cancer development. Sci Rep.
10:97202020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wu S, Ding X, Kong Y, Acharya S, Wu H,
Huang C, Liang Y, Nong X and Chen H: The feature of cervical
microbiota associated with the progression of cervical cancer among
reproductive females. Gynecol Oncol. 163:348–357. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Łaniewski P, Barnes D, Goulder A, Cui H,
Roe DJ, Chase DM and Herbst-Kralovetz MM: Linking cervicovaginal
immune signatures, HPV and microbiota composition in cervical
carcinogenesis in non-Hispanic and Hispanic women. Sci Rep.
8:75932018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mitra A, MacIntyre DA, Paraskevaidi M,
Moscicki AB, Mahajan V, Smith A, Lee YS, Lyons D, Paraskevaidis E,
Marchesi JR, et al: The vaginal microbiota and innate immunity
after local excisional treatment for cervical intraepithelial
neoplasia. Genome Med. 13:1762021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang H, Lu J, Lu Y, Cai Q, Liu H and Xu
C: Cervical microbiome is altered in cervical intraepithelial
neoplasia after loop electrosurgical excision procedure in China.
Sci Rep. 8:49232018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global Cancer Statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Audirac-Chalifour A, Torres-Poveda K,
Bahena-Roman M, Tellez-Sosa J, Martinez-Barnetche J,
Cortina-Ceballos B, López-Estrada G, Delgado-Romero K,
Burguete-García AI, Cantú D, et al: Cervical microbiome and
cytokine profile at various stages of cervical cancer: A pilot
study. PLoS One. 11:e01532742016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kang GU, Jung DR, Lee YH, Jeon SY, Han HS,
Chong GO and Shin JH: Potential association between vaginal
microbiota and cervical carcinogenesis in Korean Women: A cohort
study. Microorganisms. 9:2942021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Carlson KJ, Skates SJ and Singer DE:
Screening for ovarian cancer. Ann Intern Med. 121:124–132. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Coburn SB, Bray F, Sherman ME and Trabert
B: International patterns and trends in ovarian cancer incidence,
overall and by histologic subtype. Int J Cancer. 140:2451–2460.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Nené NR, Reisel D, Leimbach A, Franchi D,
Jones A, Evans I, Knapp S, Ryan A, Ghazali S, Timms JF, et al:
Association between the cervicovaginal microbiome, BRCA1 mutation
status, and risk of ovarian cancer: A case-control study. Lancet
Oncol. 20:1171–1182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Morikawa A, Kawabata A, Shirahige K,
Akiyama T, Okamoto A and Sutani T: Altered cervicovaginal
microbiota in premenopausal ovarian cancer patients. Gene.
811:1460832022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ely LK and Truong M: The role of
opportunistic bilateral salpingectomy vs tubal occlusion or
ligation for ovarian cancer prophylaxis. J Minim Invasive Gynecol.
24:371–378. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cibula D, Widschwendter M, Majek O and
Dusek L: Tubal ligation and the risk of ovarian cancer: Review and
meta-analysis. Hum Reprod Update. 17:55–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yoon SH, Kim SN, Shim SH, Kang SB and Lee
SJ: Bilateral salpingectomy can reduce the risk of ovarian cancer
in the general population: A meta-analysis. Eur J Cancer. 55:38–46.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jacobson D, Moore K, Gunderson C, Rowland
M, Austin R, Honap TP, Xu J and Warinner C: Shifts in gut and
vaginal microbiomes are associated with cancer recurrence time in
women with ovarian cancer. PeerJ. 9:e115742021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wong-Rolle A, Wei HK, Zhao C and Jin C:
Unexpected guests in the tumor microenvironment: Microbiome in
cancer. Protein Cell. 12:426–435. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Amant F, Moerman P, Neven P, Timmerman D,
Van Limbergen E and Vergote I: Endometrial cancer. Lancet Oncol.
366:491–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kim S, Seo H, Rahim MA, Lee S, Kim YS and
Song HY: Changes in the microbiome of vaginal fluid after menopause
in Korean Women. J Microbiol Biotechnol. 31:1490–1500. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shardell M, Gravitt PE, Burke AE, Ravel J
and Brotman RM: Association of vaginal microbiota with signs and
symptoms of the genitourinary syndrome of menopause across
reproductive stages. J Gerontol A Biol Sci Med Sci. 76:1542–1550.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Brotman RM, Shardell MD, Gajer P, Fadrosh
D, Chang K, Silver MI, Viscidi RP, Burke AE, Ravel J and Gravitt
PE: Association between the vaginal microbiota, menopause status,
and signs of vulvovaginal atrophy. Menopause. 21:450–458. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Suarez SS and Pacey AA: Sperm transport in
the female reproductive tract. Hum Reprod Update. 12:23–37. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hansen LK, Becher N, Bastholm S, Glavind
J, Ramsing M, Kim CJ, Romero R, Jensen JS and Uldbjerg N: The
cervical mucus plug inhibits, but does not block, the passage of
ascending bacteria from the vagina during pregnancy. Acta Obstet
Gynecol Scand. 93:102–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zervomanolakis I, Ott HW, Hadziomerovic D,
Mattle V, Seeber BE, Virgolini I, Heute D, Kissler S, Leyendecker G
and Wildt L: Physiology of upward transport in the human female
genital tract. Ann N Y Acad Sci. 1101:1–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Garcia-Grau I, Simon C and Moreno I:
Uterine microbiome-low biomass and high expectationsdagger. Biol
Reprod. 101:1102–1114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mitchell CM, Haick A, Nkwopara E, Garcia
R, Rendi M, Agnew K, Fredricks DN and Eschenbach D: Colonization of
the upper genital tract by vaginal bacterial species in nonpregnant
women. Am J Obstet Gynecol. 212:611e1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen C, Song X, Wei W, Zhong H, Dai J, Lan
Z, Li F, Yu X, Feng Q, Wang Z, et al: The microbiota continuum
along the female reproductive tract and its relation to
uterine-related diseases. Nat Commun. 8:8752017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Furukawa T, Jisaki F, Sakamuro D, Takegami
T and Murayama T: Detection of human cytomegalovirus genome in
uterus tissue. Arch Viro. 135:265–277. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tobiasch E, Rabreau M, Geletneky K,
Laruë-Charlus S, Severin F, Becker N and Schlehofer JR: Detection
of adeno-associated virus DNA in human genital tissue and in
material from spontaneous abortion. J Med Viro. 44:215–222. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Komaroff AL, Rizzo R and Ecker JL: Human
Herpesviruses 6A and 6B in reproductive diseases. Front Immunol.
12:6489452021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Callan T, Woodcock S and Huston WM:
Ascension of Chlamydia is moderated by uterine peristalsis and the
neutrophil response to infection. PLoS Comput Biol.
17:e10093652021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Paavonen J, Aine R, Teisala K, Heinonen
PK, Punnonen R, Lehtinen M, Miettinen A and Grönroos P: Chlamydial
endometritis. J Clin Pathol. 38:726–732. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Moreno I, Cicinelli E, Garcia-Grau I,
Gonzalez-Monfort M, Bau D, Vilella F, De Ziegler D, Resta L,
Valbuena D and Simon C: The diagnosis of chronic endometritis in
infertile asymptomatic women: A comparative study of histology,
microbial cultures, hysteroscopy, and molecular microbiology. Am J
Obstet Gynecol. 218:602e1–e16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Smith JR, Wells C, Jolly M, Shah P, Savage
M, Reginald P and Kitchen VS: Is endometrial infection with Candida
albicans a cause of recurrent vaginal thrush? Genitourin Med.
69:295–296. 1993.PubMed/NCBI
|
|
100
|
Walther-António MR, Chen J, Multinu F,
Hokenstad A, Distad TJ, Cheek EH, Keeney GL, Creedon DJ, Nelson H,
Mariani A and Chia N: Potential contribution of the uterine
microbiome in the development of endometrial cancer. Genome Med.
8:1222016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Walsh DM, Hokenstad AN, Chen J, Sung J,
Jenkins GD, Chia N, Nelson H, Mariani A and Walther-António MRS:
Postmenopause as a key factor in the composition of the endometrial
cancer microbiome (ECbiome). Sci Rep. 9:192132019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Milde-Langosch K, Becker G and Löning T:
Human papillomavirus and c-myc/c-erbB2 in uterine and vulvar
lesions. Virchows Arch A Pathol Anat Histopathol. 419:479–485.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yang HJ, Liu VW, Tsang PC, Yip AM, Ng TY,
Cheung AN and Ngan HY: Comparison of human papillomavirus DNA
levels in gynecological cancers: Implication for cancer
development. Tumour Biol. 24:310–316. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Olesen TB, Svahn MF, Faber MT,
Duun-Henriksen AK, Junge J, Norrild B and Kjaer SK: Prevalence of
human papillomavirus in endometrial cancer: A systematic review and
meta-analysis. Gynecol Oncol. 134:206–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Abu-Lubad MA, Jarajreh DA, Helaly GF,
Alzoubi HM, Haddadin WJ, Dabobash MD, Albataineh EM, Aqel AA and
Alnawaiseh NA: Human papillomavirus as an independent risk factor
of invasive cervical and endometrial carcinomas in Jordan. J Infect
Public Health. 13:613–618. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Jiang XF, Tang QL, Zou Y, Xu L, Zeng H,
Chi C, Jiang JR and Zhang BZ: Does HBV infection increase risk of
endometrial carcinoma? Asian Pac J Cancer Prev. 15:713–716. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Benharroch D, Klinkovich I, Piura B,
Shaco-Levy R and Gopas J: Evidence of measles virus antigens and
RNA in endometrial cancer. Eur J Obstet Gynecol Reprod Biol.
147:206–209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Tsementzi D, Pena-Gonzalez A, Bai J, Hu
YJ, Patel P, Shelton J, Dolan M, Arluck J, Khanna N, Conrad L, et
al: Comparison of vaginal microbiota in gynecologic cancer patients
pre- and post-radiation therapy and healthy women. Cancer Med.
9:3714–3724. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Nejman D, Livyatan I, Fuks G, Gavert N,
Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E,
et al: The human tumor microbiome is composed of tumor
type-specific intracellular bacteria. Science. 368:973–980. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Tzeng A, Sangwan N, Jia M, Liu CC, Keslar
KS, Downs-Kelly E, Fairchild RL, Al-Hilli Z, Grobmyer SR and Eng C:
Human breast microbiome correlates with prognostic features and
immunological signatures in breast cancer. Genome Med. 13:602021.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ma J, Gnanasekar A, Lee A, Li WT, Haas M,
Wang-Rodriguez J, Chang EY, Rajasekaran M and Ongkeko WM: Influence
of intratumor microbiome on clinical outcome and immune processes
in prostate cancer. Cancers (Basel). 12:25242020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Pushalkar S, Hundeyin M, Daley D,
Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres
LE, et al: The pancreatic cancer microbiome promotes oncogenesis by
induction of innate and adaptive immune suppression. Cancer Discov.
8:403–416. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Gnanasekar A, Castaneda G, Iyangar A,
Magesh S, Perez D, Chakladar J, Li WT, Bouvet M, Chang EY and
Ongkeko WM: The intratumor microbiome predicts prognosis across
gender and subtypes in papillary thyroid carcinoma. Comput Struct
Biotechnol J. 19:1986–1997. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kostic AD, Gevers D, Pedamallu CS, Michaud
M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, et
al: Genomic analysis identifies association of Fusobacterium with
colorectal carcinoma. Genome Res. 22:292–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Del Castillo E, Meier R, Chung M, Koestler
DC, Chen T, Paster BJ, Charpentier KP, Kelsey KT, Izard J and
Michaud DS: The microbiomes of pancreatic and duodenum tissue
overlap and are highly subject specific but differ between
pancreatic cancer and noncancer subjects. Cancer Epidemiol
Biomarkers Prev. 28:370–383. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Banerjee S, Tian T, Wei Z, Shih N, Feldman
MD, Alwine JC, Coukos G and Robertson ES: The ovarian cancer
oncobiome. Oncotarget. 8:36225–36245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang PP, Zhou L, Cao JS, Li YP, Zeng Z,
Sun N, Shen L, Zhu HY, Ruan Y, Zha WT, et al: Possible epithelial
ovarian cancer association with HPV18 or HPV33 Infection. Asian Pac
J Cancer Prev. 17:2959–2964. 2016.PubMed/NCBI
|
|
118
|
Hassan ZK, Hafez MM, Kamel MM and Zekri
AR: Human papillomavirus genotypes and methylation of CADM1, PAX1,
MAL and ADCYAP1 genes in epithelial ovarian cancer patients. Asian
Pac J Cancer Prev. 18:169–176. 2017.PubMed/NCBI
|
|
119
|
Shanmughapriya S, Senthilkumar G,
Vinodhini K, Das BC, Vasanthi N and Natarajaseenivasan K: Viral and
bacterial aetiologies of epithelial ovarian cancer. Eur J Clin
Microbiol Infect Dis. 31:2311–2317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Jonsson S, Oda H, Lundin E, Olsson J and
Idahl A: Chlamydia trachomatis, Chlamydial heat shock protein 60
and anti-chlamydial antibodies in women with epithelial ovarian
tumors. Transl Oncol. 11:546–551. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Idahl A, Lundin E, Elgh F, Jurstrand M,
Moller JK, Marklund I, Lindgren P and Ottander U: Chlamydia
trachomatis, Mycoplasma genitalium, Neisseria gonorrhoeae, human
papillomavirus, and polyomavirus are not detectable in human tissue
with epithelial ovarian cancer, borderline tumor, or benign
conditions. Am J Obstet Gynecol. 202:71e1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhou B, Sun C, Huang J, Xia M, Guo E, Li
N, Lu H, Shan W, Wu Y, Li Y, et al: The biodiversity composition of
microbiome in ovarian carcinoma patients. Sci Rep. 9:16912019.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang Q, Zhao L, Han L, Fu G, Tuo X, Ma S,
Li Q, Wang Y, Liang D, Tang M, et al: The differential distribution
of bacteria between cancerous and noncancerous ovarian tissues in
situ. J Ovarian Res. 13:82020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Brewster WR, Burkett WC, Ko EM, Bae-Jump
V, Nicole McCoy A and Keku TO: An evaluation of the microbiota of
the upper reproductive tract of women with and without epithelial
ovarian cancer. Gynecol Oncol Rep. 42:1010172022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Moreno I, Codoñer FM, Vilella F, Valbuena
D, Martinez-Blanch JF, Jimenez-Almazán J, Alonso R, Alamá P, Remohí
J, Pellicer A, et al: Evidence that the endometrial microbiota has
an effect on implantation success or failure. Am J Obstet Gynecol.
216:684–703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Kyono K, Hashimoto T, Nagai Y and Sakuraba
Y: Analysis of endometrial microbiota by 16S ribosomal RNA gene
sequencing among infertile patients: a single-center pilot study.
Reprod Med Biol. 17:297–306. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wang L, Yang J, Su H, Shi L, Chen B and
Zhang S: Endometrial microbiota from endometrial cancer and paired
pericancer tissues in postmenopausal women: Differences and
clinical relevance. Menopause. 29:1168–1175. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lu W, He F, Lin Z, Liu S, Tang L, Huang Y
and Hu Z: Dysbiosis of the endometrial microbiota and its
association with inflammatory cytokines in endometrial cancer. Int
J Cancer. 148:1708–1716. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Caselli E, Soffritti I, D'Accolti M, Piva
I, Greco P and Bonaccorsi G: Atopobium vaginae and porphyromonas
somerae induce proinflammatory cytokines expression in endometrial
cells: A possible implication for endometrial cancer? Cancer Manag
Res. 11:8571–8575. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Deligdisch L, Marin T, Lee AT, Etkind P,
Holland JF, Melana S and Pogo BG: Human mammary tumor virus (HMTV)
in endometrial carcinoma. Int J Gynecol Cancer. 23:1423–1428. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Li C, Gu Y, He Q, Huang J, Song Y, Wan X
and Li Y: Integrated analysis of microbiome and transcriptome data
reveals the interplay between commensal bacteria and fibrin
degradation in endometrial cancer. Front Cell Infect Microbiol.
11:7485582021. View Article : Google Scholar : PubMed/NCBI
|