Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide (1). Non-small
cell lung cancer (NSCLC) accounts for ~85% of all histological
types (2). The treatment plan for
patients with advanced NSCLC depends on their genotype, and
treatments include targeted therapy, chemotherapy and immunotherapy
(3). Immune checkpoint inhibitors
(ICIs), such as programmed cell death-1 (PD-1)/programmed cell
death-ligand 1 (PD-L1) or cytotoxic T lymphocyte-associated
antigen-4 (CTLA-4) antibodies, have been approved for the treatment
of patients with advanced NSCLC based on their encouraging efficacy
in a series of clinical trials (4,5).
Although combination with chemotherapy or antiangiogenic agents
improves the clinical outcome of ICIs, a considerable fraction of
patients does not benefit from ICIs (6–8).
Therefore, it is important to identify biomarkers that can be used
to predict the response to ICIs in patients with NSCLC.
At present, PD-L1 expression, tumor mutational
burden (TMB) and microsatellite instability (MSI) status have been
recognized as reliable indicators to predict the response to ICIs
in NSCLC, hepatocellular carcinoma, urothelial carcinoma and other
tumors (9,10). As the most important target of
immunotherapy, the infiltration, T cell receptor repertoire
diversity and gene signatures of CD8+ T cells can also
predict the clinical benefit of ICIs treatment in advanced NSCLC
(11–13). Additionally, several routine blood
parameters, including absolute eosinophil count (14), neutrophil-lymphocyte ratio (15) and lactate dehydrogenase levels
(16), have been found to be
valuable in the prediction of the clinical outcome of ICIs
treatment. However, the role of serum alkaline phosphatase (ALP) in
the prediction of ICIs treatment efficacy remains unclear.
ALP is a glycoprotein that catalyzes hydrolytic and
phospho-transfer reactions. Elevated serum ALP has been reported in
bone and liver-related diseases (17,18).
Additionally, serum ALP has been found to be an independent
prognostic factor in NSCLC, gastric cancer, breast cancer and other
cancer types (19–21). Furthermore, elevated ALP levels have
been associated with bone or liver metastasis in patients with lung
cancer, prostate cancer, breast cancer and other cancer types
(22). In recent studies, bone and
liver metastases have been reported to restrain immunotherapy
efficacy via regulation of the tumor immune microenvironment
(23,24). Therefore, an investigation was
conducted to determine whether ALP could be a predictive indicator
of ICIs treatment efficacy in patients with NSCLC.
In the present study it was found that bone or liver
metastasis was associated with poor prognosis in patients with
NSCLC receiving ICIs. Furthermore, elevated serum ALP levels were
associated with bone and liver metastases, indicating that ALP
levels might be an independent prognostic indicator of ICIs
treatment efficacy in patients with NSCLC.
Patients and methods
Patients and data collection
Between January 2018 and December 2020, patients
with NSCLC receiving ICIs treatment were investigated at the
Institute of Cancer, Xinqiao Hospital, Third Military Medical
University (Chongqing, China). Patients who met the following
criteria were included in the present study: i) Pathologically
diagnosed lung squamous cancer or adenocarcinoma without receiving
an operation; ii) not harboring driver gene mutations, including
EGFR, ALK, ROS1 and MET; iii) receiving ICIs treatment (including
PD-1, PD-L1 or CTLA-4 antibody) for at least 2–3 cycles (6 weeks);
iv) complete image examination of evaluable foci pretreatment and
post-treatment; and v) complete record of pretreatment ALP levels
and complete record of follow-up. Patients with mutations of driver
genes, or without records of pretreatment ALP levels, or receiving
ICIs treatment <6 weeks were excluded. Overall, 143 patients
were included and data regarding their clinical characteristics
[age, sex, histology type, TNM stage (according to the 8th AJCC
staging system) (25), metastatic
sites, treatment lines (first-line or ≥2nd line) and treatment
methods] were collected from medical records. The treatment methods
included ICIs monotherapy or combined therapy with other treatments
(chemotherapy, anti-angiogenic agents or both). The present study
was approved by the Ethics Committee of Xinqiao Hospital, Third
Military Medical University (approval no. AMUWEC20210386;
Chongqing, China). All patients or their legal representatives gave
written informed consent for the treatment and inquiries related to
the present study.
ALP detection and cut-off value
selection
ALP was detected using the endpoint
spectrophotometric method in the peripheral blood of patients <1
week before treatment with ICIs, and the ALP level data were
collected from medical records. The normal level of ALP is between
0 and 110 U/l (26). In the present
study, 110 U/l, which is the normal upper limit of ALP used at
Xinqiao Hospital, was selected as the cut-off value to divide
patients into high and low ALP level groups.
Follow-up evaluation and definition of
progression-free survival (PFS)
Patients were evaluated during follow-up by CT of
the lungs and abdomen or MRI of the brain. The response to ICIs
treatment was classified according to iRECIST criteria (27) as complete response (iCR), partial
response (iPR), stable disease or progressive disease. Treatment
response was evaluated every 6 weeks.
The ORR was defined as the proportion of patients
with response of iPR and iCR. PFS was defined as the time from the
beginning of immunotherapy until recurrence of disease or death due
to any cause. Telephone follow-up was performed every 6 months, and
the last follow-up date was August 31, 2021.
Statistical analysis
The ALP level data are presented as the median and
interquartile range. The mPFS data are presented as Kaplan-Meier
curves. The composition of patients or response rate data are
presented as n (%), n or the proportion. The results of univariate
and multivariate Cox regression analyses are presented as the
hazard ratio and 95% CI. The difference in ALP levels was analyzed
using an unpaired Student's t-test or one-way ANOVA with a
Bonferroni post hoc test. The associations between ALP levels and
clinical characteristics were analyzed using a χ2 test
and Fisher's exact test if the expected count was <5. The
percentages of clinical response of patients to ICIs treatment in
two groups were compared using a χ2 test. Kaplan-Meier
survival curves and the log-rank test were used to estimate mPFS,
and univariate and multivariate Cox regression analyses were used
to determine the associations between clinicopathological factors
and survival. A forest plot was used to show the results of
multivariate Cox regression analysis using Prism GraphPad 9.0
software (GraphPad Software, Inc.). All statistical analyses were
conducted using SPSS 20.0 software (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 143 patients with NSCLC receiving ICIs
were included, and their baseline characteristics are presented in
Table I. There were 61 (42.7%)
older patients (age ≥60 years), and the mean age was 57.26±9.86
years (range, 35–76 years). Most patients were male (79.0%) and had
stage IV disease (87.4%). In addition, 51.7% of patients had
squamous cancer, 59.4% of patients received first-line ICIs
treatment, and 45.5% of patients were treated with combination
therapy of ICIs and other treatments. The median follow-up time was
30.2 months (range, 2.7-52.4 months).
 | Table I.Baseline characteristics of 143
patients with lung cancer. |
Table I.
Baseline characteristics of 143
patients with lung cancer.
|
Characteristics | No. (%) |
|---|
| Age, years |
|
|
≥60 | 61 (42.7) |
|
<60 | 82 (57.3) |
| Sex |
|
|
Male | 113 (79.0) |
|
Female | 30 (21.0) |
| Histology type |
|
| SC | 74 (51.7) |
| AD | 69 (48.3) |
| TNM stage |
|
|
III | 18 (12.6) |
| IV | 125 (87.4) |
| Metastatic
sites |
|
| Bone
only | 45 (31.4) |
| Liver
only | 10 (7.0) |
| Both
bone and liver | 17 (11.9) |
| Neither
bone or liver | 71 (49.7) |
| Treatment
lines |
|
|
1st | 85 (59.4) |
|
≥2nd | 58 (40.6) |
| Combined
therapy |
|
|
Yes | 65 (45.5) |
| No | 78 (54.5) |
Bone and liver metastases predict poor
prognosis of patients with NSCLC receiving ICIs
Previous preclinical and clinical evidence has
demonstrated that bone or liver metastases can restrain
immunotherapy efficacy (23,24).
Therefore, the predictive value of bone or liver metastasis in the
prognosis of patients with NSCLC receiving ICIs was investigated.
There was a significant association between the response and bone
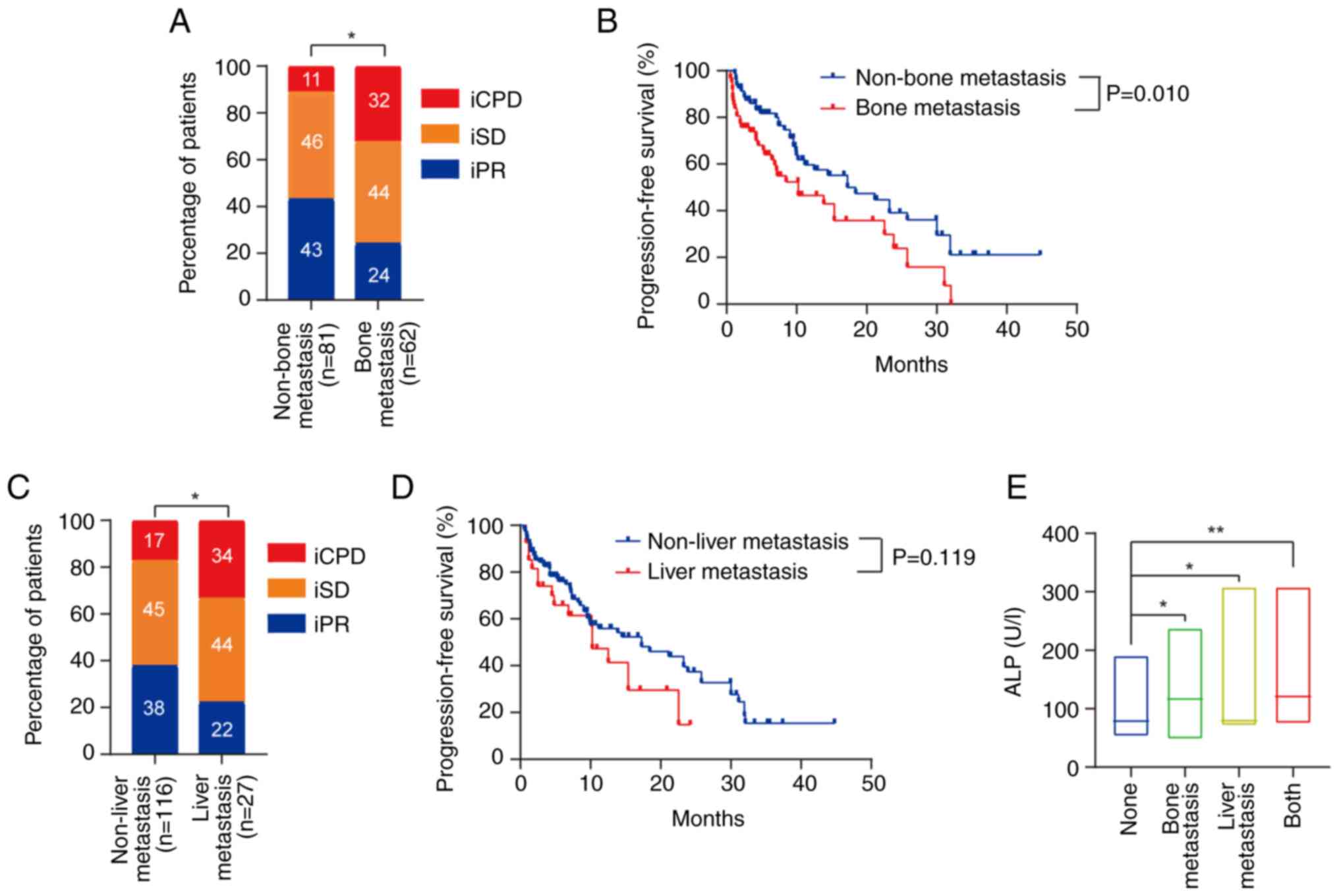
metastasis (P<0.05; Fig. 1A).
Furthermore, bone metastasis was associated with a shorter mPFS
time in patients with NSCLC receiving ICIs treatment (10.2 vs. 17.3
months; P=0.010; Fig. 1B).
Similarly, there was also a significant association between the
response and liver metastasis (P<0.05; Fig. 1C). However, liver metastasis was not
associated with mPFS time (P=0.119; Fig. 1D).
Elevated ALP levels are associated
with bone and liver metastases in patients with NSCLC
ALP has been recognized as a valuable marker for
skeletal and hepatobiliary disorders, including bone and liver
metastases in patients with cancer (22). Therefore, the relationship between
ALP levels and bone or liver metastasis in patients with NSCLC was
investigated. ALP levels were higher in patients with bone or liver
metastasis than in those without (119.6 or 103.6 vs. 83.3 U/l,
respectively; P<0.05; Fig. 1E).
Furthermore, the ALP levels of patients with both bone and liver
metastases were higher compared with the ‘None’ group (135.7 vs.
83.3 U/l; P<0.01; Fig. 1E). In
addition, higher levels of ALP were also associated with bone or
liver metastasis, but not with age, sex, stage and other clinical
characteristics (Table II).
 | Table II.Association between clinical
variables and serum ALP levels. |
Table II.
Association between clinical
variables and serum ALP levels.
|
| Pretreatment ALP
levels |
|
|---|
|
|
|
|
|---|
|
Characteristics | ≥110 U/l, n | <110 U/l, n | P-value |
|---|
| Age, years |
|
| 0.809 |
|
≥60 | 16 | 45 |
|
|
<60 | 23 | 59 |
|
| Sex |
|
| 0.402 |
|
Male | 29 | 84 |
|
|
Female | 10 | 20 |
|
| Histology type |
|
| 0.931 |
| SC | 21 | 53 |
|
| AD | 18 | 51 |
|
| Stage |
|
| 0.100 |
|
III | 2 | 16 |
|
| IV | 37 | 88 |
|
| Bone
metastasis |
|
| <0.001 |
|
Yes | 34 | 28 |
|
| No | 5 | 76 |
|
| Liver
metastasis |
|
| 0.036 |
|
Yes | 10 | 17 |
|
| No | 29 | 87 |
|
| Bone and liver
metastasis |
|
| <0.001 |
|
Both | 10 | 7 |
|
|
Neither | 4 | 67 |
|
| Treatment
lines |
|
| 0.945 |
|
1st | 23 | 62 |
|
|
≥2nd | 16 | 42 |
|
| Combined
therapy |
|
| 0.304 |
|
Yes | 15 | 50 |
|
| No | 24 | 54 |
|
Elevated ALP levels are an independent
prognostic indicator for patients with NSCLC receiving ICIs
To determine the predictive value of ALP, the ALP
levels in responders and non-responders to ICIs treatment were
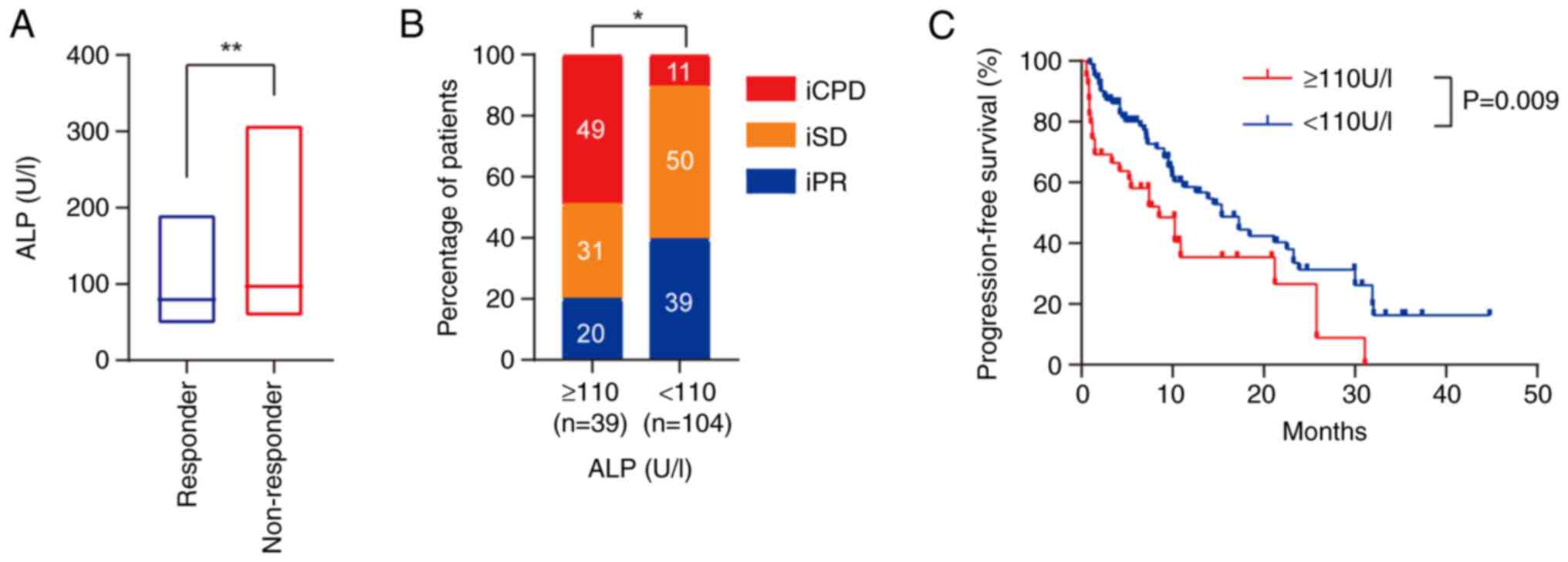
first compared. It was found that the levels of ALP were higher in
non-responders than in responders (110.1 vs. 88.0 U/l; P<0.01;
Fig. 2A). There was a significant
association between the response and ALP levels (P<0.05;
Fig. 2B). Furthermore, high ALP
levels were associated with shorter mPFS (8.5 vs. 15.4 months;
P=0.009; Fig. 2C). Additionally,
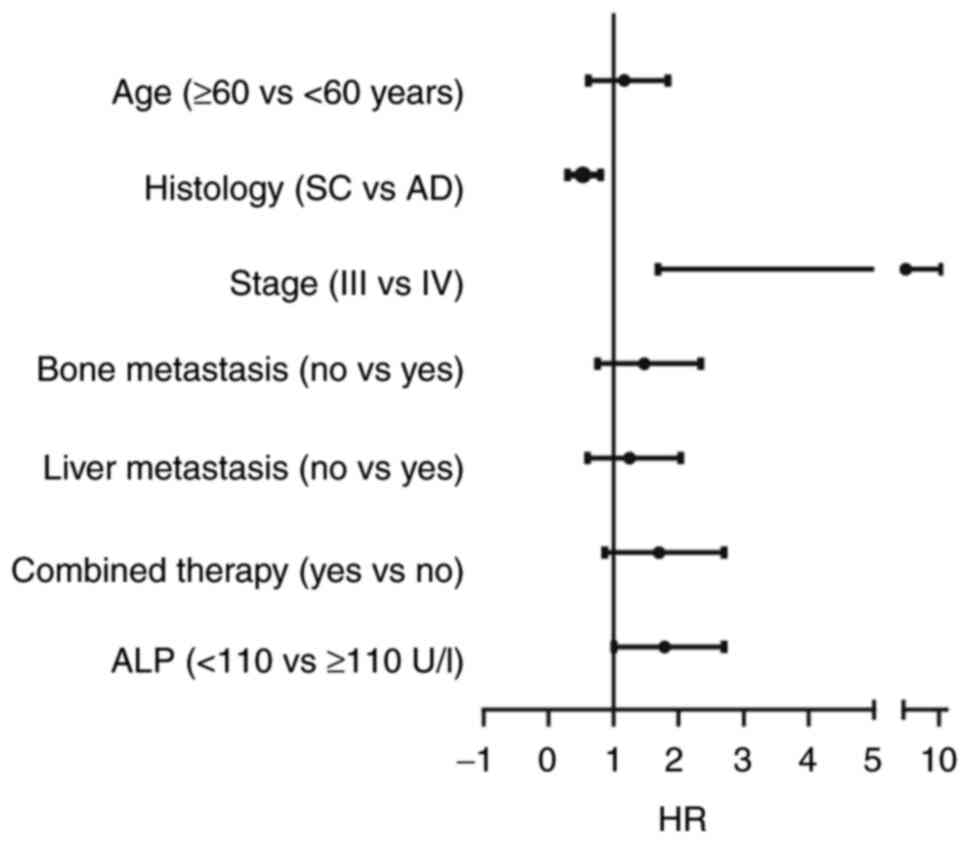
multivariate analysis revealed that ALP level, stage and histology
type were independent prognostic indicators for mPFS in the present
study (Table III). Patients with
high ALP levels or stage IV had a shorter mPFS than those with low
ALP levels or stage III, while patients with squamous cancer had a
longer mPFS than those with adenocarcinoma (Fig. 3). However, associations between bone
metastasis and mPFS were not observed in the multivariate analysis
(Table III).
 | Table III.Univariate and multivariate analyses
of different parameters for progression-free survival. |
Table III.
Univariate and multivariate analyses
of different parameters for progression-free survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 1.274 | 0.731-2.219 | 0.417 |
|
|
|
| Age (≥60 vs. <60
years) | 1.028 | 0.653-1.617 | 0.903 |
|
|
|
| Histology (SC vs.
AD) | 1.402 | 0.889-2.209 | 0.134 | 0.511 | 0.302-0.863 | 0.012 |
| Stage (III vs.
IV) | 0.343 | 0.195-0.603 | 0.006 | 3.104 | 1.213-7.944 | 0.018 |
| Bone metastasis (no
vs. yes) | 0.563 | 0.349-0.907 | 0.010 | 1.174 | 0.856-2.641 | 0.103 |
| Liver metastasis
(no vs. yes) | 0.658 | 0.356-1.217 | 0.119 |
|
|
|
| Treatment lines
(1st vs. ≥2nd) | 0.898 | 0.548-1.472 | 0.662 |
|
|
|
| Combined therapy
(yes vs. no) | 1.453 | 0.804-2.262 | 0.199 |
|
|
|
| ALP (<110 vs.
≥110 U/l) | 1.838 | 1.609-3.191 | 0.009 | 1.856 | 1.030-3.343 | 0.040 |
Discussion
Immunotherapy, especially ICIs treatment, has been
one of the most promising treatment approaches in NSCLC (4,5). ICIs
monotherapy or combined therapy has been approved as a first-line
treatment and has improved the prognosis of patients with advanced
lung cancer (28,29). Although the ORR has been increased
by combined therapy independent of the PD-L1 level measured, not
all patients benefit from the ICIs treatment, and this is termed a
so-called primary resistance (30).
Numerous studies have demonstrated the mechanisms of
primary resistance in ICIs treatment (31–33),
which include the absence of tumor-specific antigens,
downregulation of antigen presentation molecules and suppression of
T cell infiltration. Emerging evidence has indicated that bone and
liver metastases might induce primary resistance to immunotherapy
by regulating the immune microenvironment (23,24).
Subgroup analysis of clinical trials revealed that patients with
prostate cancer and bone metastasis exhibited primary resistance to
ICIs treatment (34).
Mechanistically, bone metastatic loci could release TGF-β to
restrain Th1 lineage development to further inhibit antitumor
immunity (34). Furthermore, a
recent study reported that the presence of liver metastasis is
associated with poor response to immunotherapy in patients with
melanoma or NSCLC, which is consistent with the findings of the
present study (23). An animal
experiment revealed that liver metastasis could recruit
immunosuppressive macrophages to promote antigen-specific T cell
apoptosis, resulting in a systemic loss of T cells and diminished
immunotherapy efficacy (23).
However, a recent meta-analysis reported that patients with lung
cancer with and without liver metastasis obtained comparable
efficacy after ICIs treatment (35). Therefore, prospective studies might
be required to further identify the relationship between liver
metastasis and the efficacy of ICIs treatment.
Primary resistance cannot be completely explained by
currently recognized biomarkers, such as PD-L1 expression, MSI
status or TMB level. Thus, studies are increasingly focusing on
other biomarkers to predict the response to ICIs treatment
(14,36). As aforementioned, elevated serum ALP
levels have been demonstrated to be associated with bone and
liver-related diseases. In the present study, it was found that
high levels of serum ALP were associated with bone or liver
metastasis in patients with NSCLC, which is consistent with
previous studies (22,37). Although numerous studies have
demonstrated that a high ALP level predicted poor prognosis in
various types of cancer, such as NSCLC, gastric cancer and breast
cancer (19–21), there is little research on the
relationship between serum ALP levels and efficacy of ICIs
treatment. In the present study, serum ALP levels were identified
as an independent prognostic indicator of ICIs treatment in
patients with NSCLC. Recently, it was also reported that high
levels of serum ALP predicted poor prognosis in patients with
HER-2-negative gastric cancer receiving immunotherapy (38), which might support the findings of
the present study. However, the cut-off value of ALP was not the
same in the previous study and the present study (225 U/l in the
previous study and 110U/l in the present study). The cut-off value
in the present study was based on the normal upper limit of ALP,
and was consistent with another study (26). Therefore, 110 U/l might be a more
suitable cut-off value. There are four isoenzymes of ALP in human
serum, including tissue non-specific, germ cell, placental and
intestinal ALP, but the standard test could not detect the tissue
non-specific isoenzymes, which account for 26–34% of total ALP
(22). Thus, whether the
undetectable isoenzymes of ALP could also predict the efficacy of
ICIs treatment remains to be further studied.
As aforementioned, bone or liver metastasis, and
high ALP levels were associated with poor prognosis in patients
with NSCLC receiving ICIs treatment in the present study. However,
in the present study, it was found that only high ALP levels,
rather than bone or liver metastasis, were an independent
prognostic factor using multivariate analysis, rather than bone or
liver metastasis. This difference indicated that other factors
affecting ALP levels, such as benign liver and bone diseases, might
also restrain the efficacy of ICIs treatment. Furthermore, it was
found that higher ALP levels were associated with a shorter PFS
time in patients without bone metastasis (data not shown). However,
this hypothesis should be verified in further studies.
There were a number of limitations in the present
study. Firstly, this was a retrospective study in a single center
with a small number of cases. Secondly, the lack of overall
survival data might affect the reliability of the conclusions made.
Thirdly, the PD-L1 expression, TMB and MSI status in patients might
also affect the outcomes of ICIs treatment (9,10),
which should be explored further in the future.
In conclusion, serum ALP levels might be an
independent predictor of the response to ICIs treatment in patients
with NSCLC. Due to a number of limitations of the present study,
prospective studies are required to determine the actual prognostic
significance of ALP levels in patients with NSCLC.
Acknowledgements
Not applicable.
Funding
The study was supported by the National Nature Science
Foundation of China (nos. 82003006 and 82102878) and Nature Science
Foundation of Hainan Province (nos. 821QN384 and 818QN322).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL and JY conceived and designed the study. KY and
TS developed the methodology. TY, JC and SF acquired the data. TY
and JC confirmed the authenticity of all the raw data. TY and JC
analyzed and interpreted the data. TY and JC wrote the original
draft. JY and FL reviewed and revised the manuscript. FL and JY
supervised the study. TY, JC and TS were involved in funding
acquisition. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xinqiao Hospital, Third Military Medical University (Chongqing,
China). All patients or their legal representatives gave written
informed consent for the treatment and inquiries related to this
study.
Patient consent for publication
The patients provided written informed consent for
publication of any associated data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALP
|
alkaline phosphatase
|
|
CTLA-4
|
cytotoxic T lymphocyte-associated
antigen-4
|
|
ICIs
|
immune checkpoint inhibitors
|
|
MSI
|
microsatellite instability
|
|
NSCLC
|
non-small cell lung cancer
|
|
ORR
|
objective response rate
|
|
PD-1
|
programmed cell death-1
|
|
PD-L1
|
programmed cell death-ligand 1
|
|
PFS
|
progression-free survival
|
|
TMB
|
tumor mutational burden
|
References
|
1
|
Barta JA, Powell CA and Wisnivesky JP:
Global epidemiology of lung cancer. Ann Glob Health. 85:82019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li T, Kung HJ, Mack PC and Gandara DR:
Genotyping and genomic profiling of non-small-cell lung cancer:
Implications for current and future therapies. J Clin Oncol.
31:1039–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for First-Line Treatment
of Metastatic Nonsquamous NSCLC. N Engl J Med. 378:2288–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Updated analysis of KEYNOTE-024: Pembrolizumab versus
platinum-based chemotherapy for advanced non-small-cell lung cancer
with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol.
37:537–546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rodriguez-Abreu D, Powell SF, Hochmair MJ,
Gadgeel S, Esteban E, Felip E, Speranza G, De Angelis F, Dómine M,
Cheng SY, et al: Pemetrexed plus platinum with or without
pembrolizumab in patients with previously untreated metastatic
nonsquamous NSCLC: Protocol-specified final analysis from
KEYNOTE-189. Ann Oncol. 32:881–895. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paz-Ares L, Vicente D, Tafreshi A,
Robinson A, Soto Parra H, Mazières J, Hermes B, Cicin I,
Medgyasszay B, Rodríguez-Cid J, et al: A randomized,
placebo-controlled trial of pembrolizumab plus chemotherapy in
patients with metastatic squamous NSCLC: Protocol-Specified final
analysis of KEYNOTE-407. J Thorac Oncol. 15:1657–1669. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Socinski MA, Nishio M, Jotte RM, Cappuzzo
F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D,
Moro-Sibilot D, Thomas CA, et al: IMpower150 final overall survival
analyses for atezolizumab plus bevacizumab and chemotherapy in
first-line metastatic nonsquamous NSCLC. J Thorac Oncol.
16:1909–1924. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yarchoan M, Albacker LA, Hopkins AC,
Montesion M, Murugesan K, Vithayathil TT, Zaidi N, Azad NS, Laheru
DA, Frampton GM and Jaffee EM: PD-L1 expression and tumor
mutational burden are independent biomarkers in most cancers. JCI
Insight. 4:e1269082019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang L, Chang M, Chang HM and Chang F:
Microsatellite instability: A predictive biomarker for cancer
immunotherapy. Appl Immunohistochem Mol Morphol. 26:e15–e21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X,
Li Z, Traugh N, Bu X, Li B, et al: Signatures of T cell dysfunction
and exclusion predict cancer immunotherapy response. Nat Med.
24:1550–1558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hurkmans DP, Kuipers ME, Smit J, van
Marion R, Mathijssen RHJ, Postmus PE, Hiemstra PS, Aerts JGJV, von
der Thüsen JH and van der Burg SH: Tumor mutational load, CD8(+) T
cells, expression of PD-L1 and HLA class I to guide immunotherapy
decisions in NSCLC patients. Cancer Immunol Immunother. 69:771–777.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han J, Duan J, Bai H, Wang Y, Wan R, Wang
X, Chen S, Tian Y, Wang D, Fei K, et al: TCR repertoire diversity
of peripheral PD-1(+)CD8(+) T cells predicts clinical outcomes
after immunotherapy in patients with non-small cell lung cancer.
Cancer Immunol Res. 8:146–154. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soyano AE, Dholaria B, Marin-Acevedo JA,
Diehl N, Hodge D, Luo Y, Manochakian R, Chumsri S, Adjei A, Knutson
KL and Lou Y: Peripheral blood biomarkers correlate with outcomes
in advanced non-small cell lung Cancer patients treated with
anti-PD-1 antibodies. J Immunother Cancer. 6:1292018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Diem S, Schmid S, Krapf M, Flatz L, Born
D, Jochum W, Templeton AJ and Früh M: Neutrophil-to-Lymphocyte
ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic
markers in patients with non-small cell lung cancer (NSCLC) treated
with nivolumab. Lung Cancer. 111:176–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Banna GL, Signorelli D, Metro G, Galetta
D, De Toma A, Cantale O, Banini M, Friedlaender A, Pizzutillo P,
Garassino MC and Addeo A: Neutrophil-to-lymphocyte ratio in
combination with PD-L1 or lactate dehydrogenase as biomarkers for
high PD-L1 non-small cell lung cancer treated with first-line
pembrolizumab. Transl Lung Cancer Res. 9:1533–1542. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siddique A and Kowdley KV: Approach to a
patient with elevated serum alkaline phosphatase. Clin Liver Dis.
16:199–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vimalraj S: Alkaline phosphatase:
Structure, expression and its function in bone mineralization.
Gene. 754:1448552020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Namikawa T, Ishida N, Tsuda S, Fujisawa K,
Munekage E, Iwabu J, Munekage M, Uemura S, Tsujii S, Tamura T, et
al: Prognostic significance of serum alkaline phosphatase and
lactate dehydrogenase levels in patients with unresectable advanced
gastric cancer. Gastric Cancer. 22:684–691. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen B, Dai D, Tang H, Chen X, Ai X, Huang
X, Wei W and Xie X: Pre-treatment serum alkaline phosphatase and
lactate dehydrogenase as prognostic factors in triple negative
breast cancer. J Cancer. 7:2309–2316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li D, Yu H and Li W: Albumin-to-alkaline
phosphatase ratio at diagnosis predicts survival in patients with
metastatic non-small-cell lung cancer. Onco Targets Ther.
12:5241–5249. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ðokic-Lisanin M, Pantovic V, Jovanovic Z,
Samardz G and Jurisic V: Values of alkaline phosphathase and their
isoenzyme profiles in patients with cancer in respect to bone and
liver metastasis. Arch Oncol. 21:14–16. 2013. View Article : Google Scholar
|
|
23
|
Yu J, Green MD, Li S, Sun Y, Journey SN,
Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, et al: Liver
metastasis restrains immunotherapy efficacy via macrophage-mediated
T cell elimination. Nat Med. 27:152–164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Landi L, D'Inca F, Gelibter A, Chiari R,
Grossi F, Delmonte A, Passaro A, Signorelli D, Gelsomino F, Galetta
D, et al: Bone metastases and immunotherapy in patients with
advanced non-small-cell lung cancer. J Immunother Cancer.
7:3162019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He S, Wang Y, Peng H, Yang L, Chen H,
Liang S, Lu L and Chen Y: Pretreatment alkaline phosphatase and
epstein-barr virus DNA predict poor prognosis and response to
salvage radiotherapy in patients with nasopharyngeal carcinoma and
metachronous bone-only metastasis. J Cancer. 8:417–424. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pai-Scherf L, Blumenthal GM, Li H,
Subramaniam S, Mishra-Kalyani PS, He K, Zhao H, Yu J, Paciga M,
Goldberg KB, et al: FDA Approval Summary: Pembrolizumab for
treatment of metastatic non-small cell lung cancer: First-Line
therapy and beyond. Oncologist. 22:1392–1399. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saxena P, Singh PK, Malik PS and Singh N:
Immunotherapy alone or in combination with chemotherapy as
first-line treatment of non-small cell lung cancer. Curr Treat
Options Oncol. 21:692020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary, adaptive, and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Isazadeh A, Hajazimian S, Garshasbi H,
Shadman B, Baghbanzadeh A, Chavoshi R, Taefehshokr S, Farhoudi
Sefidan Jadid M, Hajiasgharzadeh K and Baradaran B: Resistance
mechanisms to immune checkpoints blockade by monoclonal antibody
drugs in cancer immunotherapy: Focus on myeloma. J Cell Physiol.
236:791–805. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bai R, Chen N, Li L, Du N, Bai L, Lv Z,
Tian H and Cui J: Mechanisms of cancer resistance to immunotherapy.
Front Oncol. 10:12902020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li YZ and Zhang HM: Recent advances in
primary resistance mechanisms against immune checkpoint inhibitors.
Curr Opin Oncol. 34:95–106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiao S, Subudhi SK, Aparicio A, Ge Z, Guan
B, Miura Y and Sharma P: Differences in tumor microenvironment
dictate T helper lineage polarization and response to immune
checkpoint therapy. Cell. 179:1177–1190. –e13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qin BD, Jiao XD, Liu J, Liu K, He X, Wu Y,
Ling Y, Duan XP, Qin WX, Wang Z and Zang YS: The effect of liver
metastasis on efficacy of immunotherapy plus chemotherapy in
advanced lung cancer. Crit Rev Oncol Hematol. 147:1028932020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McKean WB, Moser JC, Rimm D and
Hu-Lieskovan S: Biomarkers in precision cancer immunotherapy:
Promise and challenges. Am Soc Clin Oncol Educ Book. 40:e275–e291.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ramaswamy G, Rao VR, Krishnamoorthy L,
Ramesh G, Gomathy R and Renukadevi D: Serum levels of bone alkaline
phosphatase in breast and prostate cancers with bone metastasis.
Indian J Clin Biochem. 15:110–113. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu J, Yang S, Wang J, Zhang Q, Zhao L,
Zhang D, Yu D, Jin M, Ma H, Liu H, et al: Blood alkaline
phosphatase predicts prognosis of patients with advanced
HER2-negative gastric cancer receiving immunotherapy. Ann Transl
Med. 9:13162021. View Article : Google Scholar : PubMed/NCBI
|